Abstract
Peroxiredoxins are ubiquitous enzymes which protect cells against oxidative stress. The first step of catalysis is common to all peroxiredoxins and results in oxidation of a conserved peroxidatic cysteine residue to sulfenic acid. This forms an intermolecular disulfide bridge in the case of 2-Cys peroxiredoxins, which is a substrate for the thioredoxin system. 1-Cys Prx's contain a peroxidatic cysteine but do not contain a second conserved cysteine residue, and hence the identity of the in vivo reduction system has been unclear. Here, we show that the yeast mitochondrial 1-Cys Prx1 is reactivated by glutathionylation of the catalytic cysteine residue and subsequent reduction by thioredoxin reductase (Trr2) coupled with glutathione (GSH). This novel mechanism does not require the usual thioredoxin (Trx3) redox partner of Trr2 for antioxidant activity, although in vitro assays show that the Trr2/Trx3 and Trr2/GSH systems exhibit similar capacities for supporting Prx1 catalysis. Our data also indicate that mitochondria are a main target of cadmium-induced oxidative stress and that Prx1 is particularly required to protect against mitochondrial oxidation. This study demonstrates a physiological reaction mechanism for 1-Cys peroxiredoxins and reveals a new role in protection against mitochondrial heavy metal toxicity.
All aerobic organisms are exposed to reactive oxygen species (ROS) during the course of normal aerobic metabolism or following exposure to radical-generating compounds. ROS cause wide-ranging damage to macromolecules, which can result in genetic degeneration, physiological dysfunction, and eventual cell death (18, 19). Sulfhydryls play a key role in the response to oxidative stress, regulated primarily by the glutathione (GSH)/glutaredoxin and thioredoxin systems (9, 19, 42, 44). These redox systems were originally identified as hydrogen donors for ribonucleotide reductase, but they also act upon metabolic enzymes that form a disulfide as part of their catalytic cycle. They have proposed roles in diverse processes, including protein folding and regulation, reduction of dehydroascorbate, repair of oxidatively damaged proteins, and sulfur metabolism (20, 42). Glutaredoxins and thioredoxins are structurally and functionally conserved. Despite this considerable functional overlap, they are differentially regulated. The oxidized disulfide form of thioredoxin is reduced directly by NADPH and thioredoxin reductase, whereas glutaredoxin is reduced by GSH using electrons donated by NADPH via GSH reductase. The two systems are therefore thermodynamically linked, as each uses NADPH as a source of reducing equivalents.
During respiration, mitochondria are the primary source of ROS in the cell, and complex III of the respiratory chain is responsible for approximately 80% of ROS production (4, 5). Mitochondrial ROS cause wide-ranging damage to various cellular organelles and tissues and have been implicated in a number of disease processes. Not surprisingly, therefore, mitochondrial thiols are major ROS targets, a fact which is exacerbated by the relatively alkaline pH of mitochondria. Hence, redox regulation is critical for numerous mitochondrial functions. For example, GSH deficiency in mammalian cells leads to widespread mitochondrial damage (29), and yeast strains lacking GSH are unable to grow by respiration due to an accumulation of oxidative damage to mitochondrial DNA (24). GSH is synthesized in the cytosol and must be transported into mitochondria via an active energy-requiring process (7, 17). Oxidized GSH (GSSG) formed in the mitochondrial matrix is unable to exit this compartment and must be reduced by GSH reductase (29, 34). Mitochondrial and cytosolic forms of GSH reductase are both encoded by a nuclear gene via alternative start site selection in a mechanism that is conserved in mammalian cells (35).
Yeast, like other eukaryotes, contains a complete mitochondrial thioredoxin system, comprising a thioredoxin (TRX3) and a thioredoxin reductase (TRR2) (39). This system has been implicated in protection against oxidative stress generated during respiratory metabolism. However, the mitochondrial thioredoxin reductase was found to have an antioxidant role independent of thioredoxin since mutants deleted for TRR2 are sensitive to oxidative stress, in contrast to trx3 mutants, which are unaffected in oxidant resistance (39, 48). The redox state of Trx3 is maintained in a reduced form in wild-type cells, but surprisingly is unaffected by the loss of the mitochondrial thioredoxin reductase (48). We have shown that unlike cytoplasmic thioredoxins, the redox state of mitochondrial Trx3 can be buffered by the GSSG/2GSH redox couple, since Trr2 and Glr1 are required to maintain the redox state of Trx3. The requirement for the yeast mitochondrial thioredoxin is as yet unknown, since it is dispensable for growth under respiratory conditions and under conditions of oxidative stress. In contrast, mammalian mitochondrial Trx2 is required for normal development of the mouse embryo, and a lack of Trx2 results in embryonic lethality (33).
The human mitochondrial thioredoxin system is important for the detoxification of ROS through the activity of mitochondrial peroxiredoxin (Prx3) (6). Similarly, yeast contains a single mitochondrial peroxiredoxin (Prx1, also called mTpx) which functions for protection against oxidative stress (39). Peroxiredoxins are ubiquitous thiol-specific proteins that have multiple functions for stress protection as antioxidants and molecular chaperones and in the regulation of signal transduction. They are divided into the 1-Cys and 2-Cys Prx's, based on the number of cysteine residues directly involved in catalysis (52). Typical 2-Cys peroxiredoxins contain two redox-active Cys residues that are directly involved in enzyme activity. During catalysis, the peroxidatic cysteine is oxidized to a sulfenic acid, which condenses with the resolving cysteine to form a disulfide. This disulfide is reduced by thioredoxin. Yeast Prx1 is a member of the 1-Cys family of Prx's (39). 1-Cys Prx's contain a peroxidatic cysteine but do not contain a resolving cysteine residue. Since 1-Cys peroxiredoxins cannot, therefore, form a disulfide, the cysteine sulfenic acid generated by a reaction with peroxides is thought to be reduced by a thiol-containing electron donor, but this reaction mechanism is poorly understood (41, 52). Yeast mitochondrial Prx1 is active as a peroxidase, but surprisingly thioredoxin was shown to be able to function as an electron donor in an in vitro enzyme assay (39). However, another study suggested that thioredoxin is a poor electron donor for Prx1 compared with other yeast 2-Cys Prx's (36). In this current study, we have characterized the antioxidant role of Prx1 and provide the first in vivo evidence that GSH, and not thioredoxin, is the physiological electron donor for a 1-Cys peroxiredoxin. We also show that Prx1 is required for cadmium toxicity, identifying mitochondria as a target of cadmium-induced oxidative stress.
MATERIALS AND METHODS
Yeast strains and growth conditions.
The Saccharomyces cerevisiae strains used in this study were isogenic derivatives of W303 (MATa ura3-52 leu2-3 leu2-112 trp1-1 ade2-1 his3-11 can1-100). Strains deleted for the mitochondrial thioredoxin (trx3::kanMX4), mitochondrial thioredoxin reductase (trr2::HIS3), GSH reductase (glr1::TRP1), and γ-glutamylcysteine synthetase (gsh1::LEU2) have been described previously (15, 49). Strains deleted for PRX1 were constructed using a one-step PCR amplification protocol that replaced its entire open reading frame with the KanMX4 gene (1).
Strains were grown in rich YEPD medium (2% [wt/vol] glucose, 2% [wt/vol] Bacto peptone, 1% [wt/vol] yeast extract) or minimal SD medium (0.17% [wt/vol] yeast nitrogen base without amino acids, 5% [wt/vol] ammonium sulfate, 2% [wt/vol] glucose) supplemented with appropriate amino acids and bases. Cells were grown on lactate medium as nonfermentable carbon sources (2% [vol/vol] dl-lactic acid, 0.3% [wt/vol] yeast extract, 0.05% [wt/vol] glucose, 0.05% [wt/vol] CaCl2, 0.05% [wt/vol] NaCl, 0.06% [wt/vol] MgCl2, 0.1% [wt/vol] KH2PO4, 0.1% [wt/vol] NH4Cl, adjusted to pH 5.5 with NaOH). For anaerobic growth conditions, medium was supplemented with 0.1% (vol/vol) Tween 80 and 30 mg/liter ergosterol, and plates were maintained in an anaerobic jar containing a gas-generating kit (Oxoid). Stress sensitivity was determined by growing cells to stationary phase and then diluting and spotting them onto agar plates containing various concentrations of oxidants. Viability was determined by growing cells to exponential phase and treating them with cadmium for 1 h. Aliquots of cells were diluted in fresh YEPD medium and plated in triplicate on YEPD plates to obtain viable counts after 3 days of growth.
Plasmids.
For overexpression studies, multicopy plasmids containing GLR1, TRR2, TRX3, and PRX1 were constructed in plasmid pRS426 (8). Single-copy plasmids (pRS416) containing wild-type PRX1 were also constructed, and mutant versions of Prx1 (prx1::C38S, prx1::C91S) were made by using the QuikChange method (Stratagene).
Purification of recombinant proteins and enzyme assays.
PRX1 and TRX3 were amplified by PCR and cloned into the pBAD expression vector (Invitrogen), and TRR2 was amplified and cloned into the pET23b expression vector (Novagen). Proteins were purified using Ni-nitrilotriacetic acid His bind resin (BugBuster; Novagen) and protein purity checked on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels. Thioredoxin peroxidase activity was assayed in a reaction mixture containing 250 μM NADPH, 0.1 μM thioredoxin reductase, 4 μM thioredoxin, and 0.5 μM Prx1 in 50 mM HEPES, pH 7.0. Reactions were started by the addition of 500 μM hydrogen peroxide, and the A340 decrease followed for 2 min.
MS analysis.
Recombinant Prx1 (250 μM) in 50 mM NH4HCO3-HCl, pH 7.5, was reduced by incubation with 1.0 mM dithiothreitol (DTT) for 10 min. DTT was removed using size exclusion centrifugation filters (Microcon; Millipore) prior to incubation with 2.5 mM GSSG at room temperature for 4 h. Intact masses were determined by electrospray ionization-mass spectrometry (ESI-MS) on an Applied Biosystems 4000 Q-Trap with a Waters NanoAcquity chromatography system. The mass accuracy was better than 100 ppm. Peptides were analyzed by matrix-assisted laser desorption ionization-time of flight MS (MALDI-TOF MS) on a Bruker Ultraflex II MS. The instrument was calibrated externally with peptide standard II from Bruker, resulting in a mass accuracy of 100 ppm in the range of up to 5,000 Da. The sulfenic acid form of Prx1, Cys91, was detected by incubating Prx1 (250 μM) with 500 μM H2O2 for 10 min. Glutathionylation was analyzed following incubation with 500 μM H2O2 and 1.0 mM GSH for 4 h. Reduction of glutathionylated Cys91 was analyzed by incubation of Prx1 (250 μm) with 2.5 mM GSSG to promote glutathionylation, combined with incubation with 0.5 μM Trr2 and 1.0 mM GSH as indicated above at room temperature for 4 h. All reaction mixtures contained 600 μM NADPH.
Protein and GSH analysis.
Protein extracts were electrophoresed under reducing or nonreducing conditions on SDS-PAGE minigels and electroblotted onto polyvinylidene difluoride membranes (Amersham Pharmacia Biotech). Bound antibody (anti-Prx1, anti-Tsa1) was visualized by using enhanced chemiluminescence (Amersham Pharmacia Biotech). The redox state of Prx1 and Tsa1 was measured by covalent modification with the thiol-reactive probe 4-acetamido-4′maleimidyldystilbene-2,2′-disulfonic acid (AMS; Molecular Probes) as described previously (47). For subcellular fractionation, yeast cells were grown aerobically to mid-log phase in lactate medium to promote respiration. Extracts were separated into mitochondrial and postmitochondrial supernatant fractions by cells conversion to spheroplasts and gentle lysis by Dounce homogenization, followed by differential centrifugation (10, 13). Cell fractionation was verified by Western blot analysis using mitochondrial-specific (anti-Tim10) and cytosolic-specific (anti-Trx1) antibodies. GSH and GSSG levels were determined as described previously (16).
RESULTS
Prx1 promotes tolerance to oxidative stress conditions.
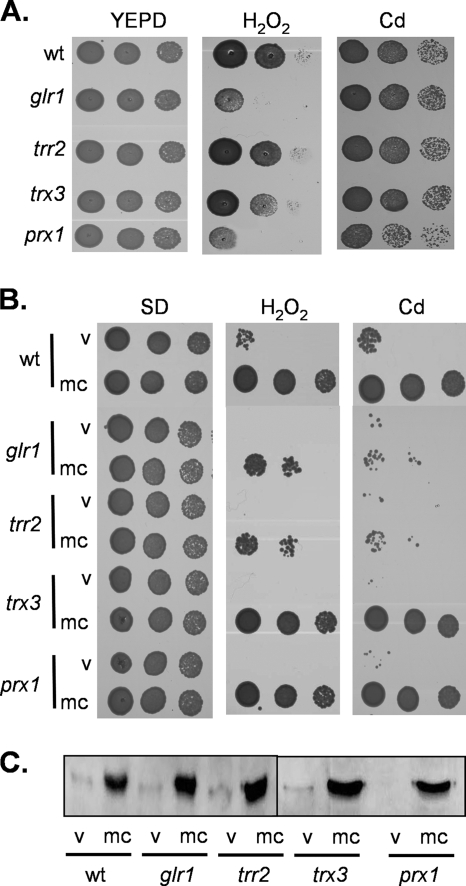
We initially constructed a mutant deleted for PRX1 to confirm its sensitivity to oxidative stress conditions. Strains were grown to stationary phase and spotted onto YEPD plates containing various concentrations of oxidants. This analysis confirmed the sensitivity of the prx1 mutant to 4 mM H2O2, which was comparable to that of a glr1 mutant (Fig. 1A). In comparison, mutants lacking the genes for mitochondrial thioredoxin (trx3) or thioredoxin reductase (trr2) were unaffected in their sensitivities to H2O2 under these conditions. It should be noted that the trr2 mutant does display oxidant sensitivity at higher concentrations of H2O2 (38, 48). Interestingly, the prx1 mutant was found to be more sensitive to cadmium stress than the wild type and the glr1, trr2, and trx3 mutants (Fig. 1A). Cadmium sulfate was used for these experiments, but similar results were obtained with cadmium chloride (data not shown). This sensitivity to a heavy metal appears to be specific for cadmium since the prx1 mutant was unaffected in resistance to other metals, including chromium and iron (data not shown). To further confirm the role of Prx1 in oxidant tolerance, we examined whether overexpression of PRX1 increases oxidant resistance. Strains were transformed with a multicopy plasmid containing PRX1 and spotted onto SD medium lacking uracil to select for the plasmid. This required different concentrations of oxidants to be used since yeast strains display greater sensitivity to H2O2 and greater resistance to cadmium on SD versus YEPD media. Overexpression of PRX1 was found to significantly increase the H2O2 and cadmium resistance of both a wild-type and prx1 mutant strain (Fig. 1B). The requirement for a mitochondrial Prx to protect against cadmium stress was unexpected; we therefore examined the role of Prx1 in protecting against mitochondrial cadmium toxicity.
FIG. 1.
Prx1 is required for resistance to oxidative stress. (A) A mutant lacking PRX1 is sensitive to oxidative stress. Cultures of the wild-type (wt) and the glr1, trr2, trx3, and prx1 mutant strains were grown to stationary phase, and the A600 was adjusted to 1, 0.1, or 0.01 before strains were spotted onto plates containing various concentrations of oxidants. Growth was monitored after 3 days of incubation at 30°C. Results are shown for plates containing no oxidant (YEPD), 4.0 mM H2O2, and 5 μM cadmium. (B) Overexpression of PRX1 increases resistance to oxidative stress. The wild-type and glr1, trr2, trx3, and prx1 mutant strains containing pRS426 (v) or mcPRX1 (mc) were tested for stress sensitivity on SD plates containing 15 μM cadmium or 0.5 mM hydrogen peroxide. (C) Western blot analysis of Prx1 with the strains described above confirmed overexpression of Prx1.
Prx1 protects against cadmium-induced mitochondrial oxidative stress.
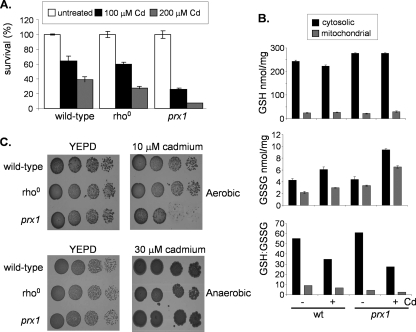
We examined whether sensitivity to cadmium is a common phenotype of strains affected in mitochondrial function. A range of mitochondrial mutants, including [rho0] mutants that lack mitochondrial DNA, were examined by using spot tests, but all showed a tolerance similar to that of the wild-type strain (data not shown). The apparent sensitivity of the prx1 mutant to cadmium on agar plates may arise either due to cell death or due to an arrest in the cell cycle, preventing growth. To differentiate between these possibilities, we examined the viability of the prx1 mutant following cadmium stress. The wild type and the prx1 and [rho0] strains were grown to exponential phase and treated with 100 μM or 200 μM cadmium for 1 h. The 100 μM cadmium treatment resulted in a similar loss of viability in the wild-type and [rho0] strains, and sensitivity to 200 μM cadmium was slightly higher in the [rho0] strain than in the wild type (Fig. 2A). In contrast, the prx1 mutant was sensitive to both concentrations of cadmium, with less than 10% viability remaining following the 200 μM treatment compared with 40% viability in the wild-type strain. These data indicate that mitochondrial Prx1 is required to protect against cell killing caused by exposure to cadmium, but this is not a general phenotype of mutants affected in mitochondrial function.
FIG. 2.
Prx1 protects against cadmium-induced mitochondrial oxidative stress. (A) A prx1 mutant shows a reduced ability to survive cadmium stress. The wild-type strain and the [rho0] and prx1 mutant strains were grown to exponential phase in YEPD medium and treated with 100 or 200 μM cadmium for 1 h. Percent survival is expressed relative to that of untreated cultures. (B) Regulation of GSH metabolism in response to cadmium stress. The wild-type (wt) strain and the prx1 mutant strain were grown to exponential phase and treated with 100 μM cadmium for 1 h. The levels of reduced (GSH) and oxidized (GSSG) glutathiones were determined in cytosolic and mitochondrial fractions. Values shown are the means of three independent determinations and are expressed as nanomoles/milligram of protein. (C) Anaerobic growth conditions rescue cadmium toxicity. The wild-type strain and the [rho0] and prx1 mutant strains were spotted onto YEPD plates containing cadmium and grown under aerobic or anaerobic conditions.
Cadmium is a highly toxic metal and a well-established human carcinogen, but its exact mechanism of action is uncertain at present. It may cause toxicity through depletion of GSH and by binding to sulfhydryl groups. Alternatively, it may displace iron and copper from various cytoplasmic and membrane proteins, contributing to oxidative stress via Fenton reactions (50). We therefore tested whether cadmium exposure alters cytoplasmic or mitochondrial GSH levels. The relative levels of reduced GSH were significantly lower in crude mitochondrial fractions than in the postmitochondrial supernatant, but it did not significantly differ between the wild type and the prx1 mutant (Fig. 2B). Little or no alteration in GSH levels was observed in response to a treatment with 100 μM cadmium for 1 h, indicating that cadmium toxicity does not appear to arise due to GSH depletion. The relative levels of GSSG were comparable in mitochondrial and cytosolic fractions. This resulted in a lowered mitochondrial redox ratio (GSH/GSSG) compared with the cytosol (Fig. 2B), consistent with previous observations describing the oxidizing environment of mitochondria (35). Cadmium stress elevated both cytosolic and mitochondrial GSSG levels in the wild-type and prx1 mutant strains. This was most pronounced in the prx1 mutant and resulted in approximately 22% of the mitochondrial GSH pool being present in the oxidized form compared with 11% in the wild-type strain. To confirm that oxidative stress accounts for the toxicity of cadmium, we examined cadmium tolerance in the absence of oxygen (Fig. 2C). Anaerobic growth conditions were found to significantly decrease cadmium toxicity. For example, the wild-type and [rho0] and prx1 mutant strains were able to grow on plates containing 30 μM cadmium under anaerobic conditions, a concentration which completely prevented the growth of all strains under aerobic conditions. Additionally, the prx1 mutant displayed a tolerance to cadmium similar to that of the wild-type strain under anaerobic conditions (Fig. 2C). Taken together, these data indicate that the cadmium sensitivity of the prx1 mutant does not arise due to depletion of GSH but appears to be mediated by an oxidative stress.
Prx1 requires Trr2 and the GSH system, but not Trx3, to promote oxidant resistance.
Yeast mitochondrial Prx1 has been shown to act as a peroxidase in vitro, but unusually for a 1-Cys Prx, thioredoxin was shown to function as an electron donor (39). To examine the requirement for reductants in vivo, we examined the ability of Prx1 to promote oxidant tolerance in mutants lacking components of the mitochondrial redox systems. We reasoned that if Trx3 and Trr2 function as an electron donor system for Prx1, then they should be required for Prx1-mediated oxidant resistance. Surprisingly, however, TRR2 but not TRX3 was found to be required for cadmium and hydrogen peroxide resistance promoted by overexpression of PRX1 (Fig. 1B). Western blot analysis was used to confirm that PRX1 is overexpressed to a similar level in wild-type and mutant strains (Fig. 1C). These data are unexpected, since they indicate that mitochondrial thioredoxin reductase is needed for Prx1 activity, but there is no requirement for the mitochondrial thioredoxin.
To test whether there is a requirement for GSH in Prx1 function, we examined oxidant tolerance in a glr1 mutant lacking GSH reductase, which is unable to recycle GSSG to the reduced form (14). Interestingly, Prx1 was unable to promote cadmium or peroxide resistance in the glr1 mutant compared with that in the wild-type strain (Fig. 1B). Thus, thioredoxin reductase and GSH reductase are both required for Prx1 to be able to promote oxidant tolerance. A recent study suggested that ascorbate may be the physiological reductant for 1-Cys Prx's (31). However, the relevance of ascorbate to the yeast oxidative stress response is unclear since yeast contains a C-5 analogue, erythroascorbate, which may have limited importance as an antioxidant (43). We therefore examined a strain deleted for ALO1, encoding d-arabinono-1,4-lactone oxidase, which catalyzes the final step in erythroascorbate biosynthesis. Prx1-mediated resistance was comparable in the wild-type and alo1 strains, indicating that erythroascorbate does not appear to be required for Prx1 function in vivo (data not shown).
Requirement for Prx1 cysteine residues in antioxidant activity.
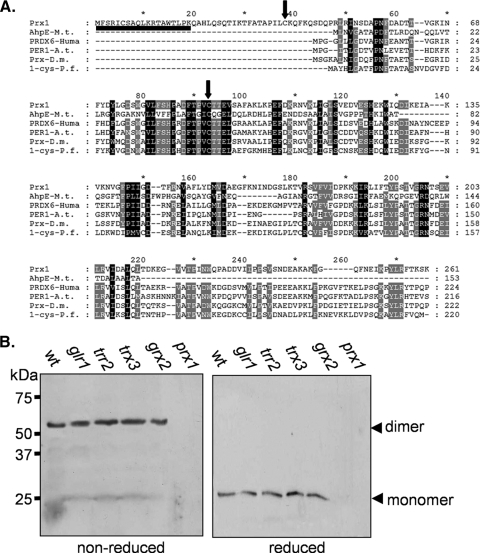
Mature Prx1 contains two cysteine residues which might be important for enzyme function. Cys91 is the peroxidatic cysteine residue, which is highly conserved in 1-Cys peroxiredoxins from bacterial, plant, and mammalian species (Fig. 3A). Nascent Prx1 contains two other Cys residues. The first Cys residue will be cleaved as part of the mitochondrial targeting sequence, leaving a single nonperoxidatic Cys residue (Cys38) in the mature protein. Cys38 is part of a long amino-terminal extension in Prx1 which is not conserved in other 1-Cys peroxiredoxins (Fig. 3A). Interestingly, Cys38 has been shown to be required for the disulfide-bonded dimer form of purified Prx1 in vitro (39), and we therefore examined whether Cys38 disulfide bond formation occurs in vivo. A predominant band of approximately 60-kDa was detected in the wild-type strain under nonreducing conditions, consistent with the expected molecular size of a dimer (Fig. 3B). This disulfide-bonded form was shifted to the monomeric size in response to reducing conditions. Loss of GLR1, TRR2, TRX3, or GRX2 did not affect the disulfide-bonded form of Prx1 (Fig. 3B). Similarly, no alteration was observed following oxidant treatments (data not shown), indicating that Prx1 is present predominantly in cells in an intermolecular disulfide-bonded form.
FIG. 3.
Prx1 forms an intermolecular disulfide bond. (A) Alignment of the amino acid sequences of Prx1 with other 1-Cys peroxiredoxins. The amino acid sequences of Prx1 and 1-Cys peroxiredoxins from Mycobacterium tuberculosis (AhpE-M.t.), humans (PRD6-Huma), Arabidopsis thaliana (PER1-A.t.), Drosophila melanogaster (Prx-D.m.), and Plasmodium falciparum (1-cys-P.f.) were aligned using ClustalW2 (http://www.ebi.ac.uk/Tools/clustalw2/index.html) and displayed using GeneDoc (http://www.nrbsc.org/). Sequences are aligned for maximal homology with dashes being used to denote gaps introduced for maximal alignment. Residues which are conserved in all sequences are boxed with black shading, and residues which are conserved in at least five out of six sequences are boxed with gray shading. Arrows denote the two cysteine residues (Cys38 and Cys91) in mature Prx1, and the mitochondrial targeting sequence is underlined. (B) Prx1 is present in a disulfide-bonded form in wild-type and redox mutant cells. Western blot analysis of Prx1 is shown for the wild-type strain and the glr1, trr2, trx3, grx2, and prx1 mutant strains grown to exponential phase. Proteins were separated using reducing or nonreducing SDS-PAGE, and the dimer and monomer sizes of Prx1 are indicated.
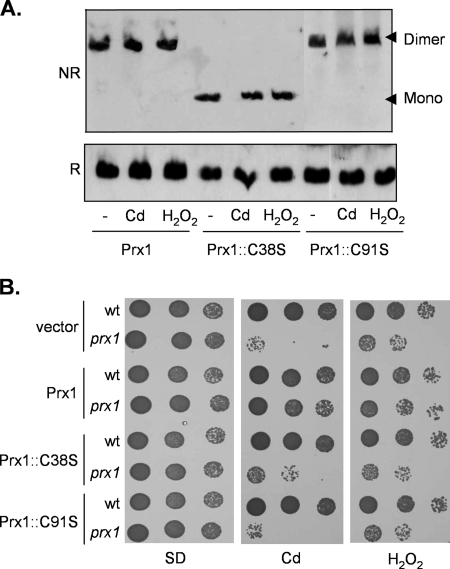
We next constructed mutant versions of Prx1 in which Cys38 and Cys91 were replaced with Ser residues to test the requirements for these Cys residues in vivo. Formation of the disulfide-bonded form of the prx1::C91S mutant was still observed under normal growth conditions and following exposure to cadmium and hydrogen peroxide stress (Fig. 4A). In contrast, no disulfide-bonded form was observed in the prx1::C38S mutant under normal or oxidative stress conditions. These data indicate that Cys38 is essential for intermolecular disulfide bond formation and that Cys91 is unable to form an intermolecular disulfide bond even under oxidative stress conditions. We confirmed that both cysteine residues are essential for Prx1 activity by examining oxidant tolerance. Single-copy PRX1 rescued the sensitivity of the prx1 mutant to cadmium and H2O2, whereas prx1::C91S and prx1::C38S mutants were unable to promote oxidant tolerance (Fig. 4B).
FIG. 4.
Prx1 Cys residues are required for antioxidant function. (A) Cys38 is required for disulfide bond formation. Western blot analysis of Prx1 is shown for the prx1 mutant containing wild-type PRX1 on a single-copy vector (Prx1) or cysteine mutant versions of PRX1 (Prx1::C38S and Prx1::C91S). Strains were grown to exponential phase and treated with 1 mM hydrogen peroxide or 100 μM cadmium for 1 h. Proteins were separated using reducing (R) or nonreducing (NR) SDS-PAGE. mono, monomer. (B) Cys38 and Cys91 are required for Prx1 antioxidant function. Sensitivity to oxidants was determined by spotting the strains described above in panel A onto SD plates containing 10 μM cadmium or 0.3 mM H2O2.
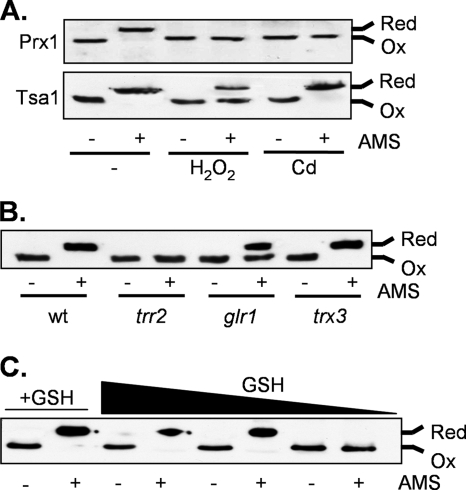
Our data indicate that Prx1 is present in vivo with Cys38, forming an intermolecular disulfide bond; we therefore examined the oxidation state of Cys91 in Prx1. The cellular oxidation state was preserved by rapidly treating cells with trichloroacetic acid, which protonates free thiol groups. Extracts were reacted with the thiol-specific probe AMS. AMS alkylates cysteine residues in a free SH state but not in an oxidized state, increasing their relative molecular masses which can be detected by SDS-PAGE and Western blot analysis (47). The migration of all Prx1 from the wild-type strain was decreased following treatment with AMS, indicating that the vast majority is present in the reduced form (Fig. 5A). Exposure to cadmium or hydrogen peroxide shifted the Prx1 redox balance to an oxidized state. Cys91 in Prx1 is therefore maintained in a reduced state under normal growth conditions but can be oxidized, consistent with its role as the peroxidatic cysteine residue. In comparison, Tsa1, which is the major yeast cytosolic peroxiredoxin, was also present in the reduced form during normal growth conditions and was partially oxidized in response to peroxide treatment (Fig. 5A). However, the redox state of Tsa1 was unaffected following cadmium exposure, suggesting that mitochondrial Prx1 plays a specific role in mediating cadmium tolerance.
FIG. 5.
Redox state analysis of Prx1. Proteins were precipitated with trichloroacetic acid and free thiols modified by reaction with AMS. Fully oxidized (Ox) and fully reduced (Red) proteins are indicated. (A) Prx1 is oxidized in response to oxidative stress. The wild-type (wt) strain was grown to exponential phase in SD medium and treated with 1 mM hydrogen peroxide or 100 μM cadmium for 1 h. Prx1 and Tsa1 were detected using specific antibodies. (B) The redox state of Prx1 is shifted to a more oxidized form in trr2 and glr1 mutants, but there is no apparent requirement for Trx3. (C) Prx1 becomes oxidized in response to GSH depletion. The gsh1 mutant was grown overnight in YEPD medium and washed with distilled water to remove exogenous GSH. The gsh1 mutant accumulates intracellular GSH from an external medium such as YEPD, which means that the growth of a gsh1 mutant on medium lacking GSH is entirely dependent on the size of the inoculum used, owing to the intracellular GSH accumulated during pregrowth on YEPD medium. The gsh1 mutant was inoculated into SD medium at different initial cellular concentrations (A600 of 0.03, 0.01, and 0.005), and growth continued for 19 h. The black arrow indicates decreasing concentrations of available GSH. As a control, the gsh1 mutant was incubated with 0.1 mM GSH (+GSH) at a starting concentration of 0.001 (lanes 1 and 2).
Analysis of the redox state of Prx1 revealed that it is fully oxidized in a trr2 mutant, whereas it is unaffected by the loss of TRX3 (Fig. 5B). Additionally, the loss of GLR1 shifted the oxidation state of Prx1 such that the reduced and oxidized forms were present at approximately equal levels. To further test the requirement for GSH, the Prx1 redox state was examined under conditions which deplete the available pool of GSH. Strains lacking GSH1 are viable but require a source of exogenous GSH for growth. For example, the gsh1 mutant grows normally on YEPD medium which contains approximately 0.5 mM GSH (24). The gsh1 mutant was therefore pregrown in YEPD medium, washed to remove exogenous GSH, and inoculated at different initial cell densities in minimal medium. In the absence of GSH, oxidized Prx1 was detected in the gsh1 mutant as the size of the inoculum was decreased (Fig. 5C). In comparison, the gsh1 mutant grown in minimal medium containing GSH maintained Prx1 in the reduced form. These data further confirm that Trr2 and the GSH system, but not Trx3, are required to maintain functional Prx1.
Glutathionylation of the Prx1 peroxidatic Cys residue.
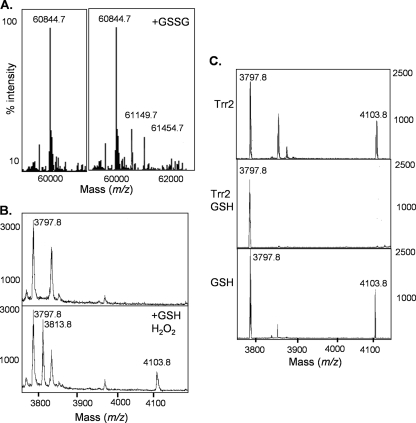
Our data indicate that GSH appears to be required for Prx1 antioxidant function in yeast cells. Given that reduction of mammalian 1-Cys-Prx (Prdx6) occurs through glutathionylation of the oxidized catalytic cysteine residue (28, 40), we tested whether Prx1 can be similarly glutathionylated. Prx1 was purified in order to characterize its reaction mechanism in vitro. Recombinant Prx1 analyzed by ESI-MS was predominantly detected as a dimeric form, which shifted to the monomeric form following DTT treatment, indicating the formation of a disulfide-bonded dimer (data not shown). Incubation of Prx1 with a 10-fold molar excess of GSSG resulted in two new peaks with a mass shift of 305 and 610 Da, respectively (Fig. 6A). This is consistent with the addition of one or two GSH molecules, confirming that both Cys91 residues in the purified Prx1 dimer can be glutathionylated. To confirm that the peroxidatic Cys residue of Prx1 is the target of glutathionylation, the recombinant protein was subjected to tryptic digestion and analyzed by MALDI-TOF MS. The tryptic peptide encompassing Cys91 was found to show a mass increase consistent with modification by glutathionylation, whereas no glutathionylation of Cys38 could be detected (data not shown).
FIG. 6.
Glutathionylation of Prx1. (A) Analysis of recombinant Prx1 by ESI-MS revealed that it was predominantly present in a dimeric form (60,844.7 Da). Incubation with a 10-fold molar excess of GSSG (+GSSG) resulted in two new peaks with a mass shift of 305 Da, consistent with the addition of one (61,149.7 Da) or two (61,454.7 Da) GSH molecules to the dimer. (B) MALDI-TOF MS analysis is shown for Prx1. The ion at 3,797.8 m/z corresponds to the Cys91 containing peptide. Peptides containing the glutathionylated (4,103.8 m/z) and the sulfenic acid form (SOH) (3,813.8 m/z) of Cys91 could be detected, following incubation of Prx1 with H2O2 and GSH. (C) Trr2 can reduce glutathionylated Cys91 only in the presence of GSH. Prx1 was glutathionylated by incubation with a 10-fold molar excess of GSSG. The ion at 3,797.8 m/z corresponds to the Cys91 containing peptide and at 4,103.8 m/z to the glutathionylated form. The addition of Trr2 and 1.0 mM GSH reduced the glutathionylated form of Cys91 (middle), whereas Trr2 or GSH alone was unable to reduce Prx1.
Glutathionylation of Prx1 may occur through oxidation of the peroxidatic Cys residue and reaction with GSH. We therefore examined the oxidation state of Cys91 following a treatment with 0.1 mM H2O2 for 10 min. MALDI-TOF MS analysis revealed a product with a mass increase of 16 Da, consistent with results from the addition of an oxygen atom and formation of cysteine sulfenic acid (Cys-SOH) (data not shown). To confirm that the sulfenic acid form Cys91 could be glutathionylated, Prx1 was incubated with hydrogen peroxide and reduced GSH. This resulted in the detection of the cysteine sulfenic acid and glutathionylated forms of the Cys91 peptide, suggesting a reaction mechanism in which the oxidized peroxidatic cysteine residue becomes modified by mixed disulfide formation with GSH (Fig. 6B).
Thioredoxin peroxidase activity of Prx1.
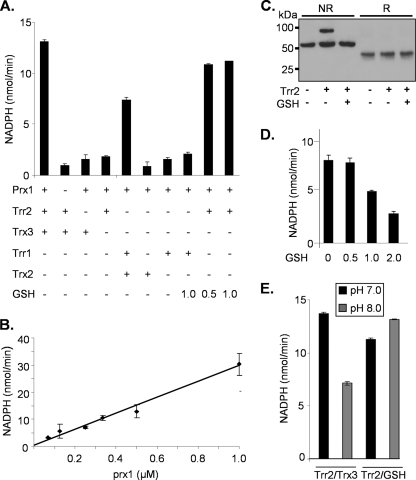
Given the unexpected finding that Trr2, but not Trx3, is required for Prx1 function in yeast cells, we established an in vitro assay for Prx1 peroxidase activity. The mitochondrial thioredoxin system (Trx3 and Trr2) was purified and compared with the cytoplasmic thioredoxin system (Trx2 and Trr1). The reactivity of the thioredoxin systems was first confirmed using insulin. Insulin is often used as a model substrate to determine the protein disulfide reductase activities of thioredoxins, and the yeast mitochondrial and cytoplasmic systems were found to exhibit comparable activities (data not shown). The ability of Prx1 to reduce H2O2 was initially tested using purified Prx1 in a reaction comprising Prx1, Trx3, Trr2, NADPH, and H2O2. Reaction conditions were essentially the same as described in Pedrajas et al., and peroxidase activity was followed by the decrease in A340 due to the oxidation of NADPH (39). In an assay using 500 μM hydrogen peroxide, the reaction velocity was approximately 13 nmol/min, comparable with the reaction velocity of approximately 20 nmol/min described in reference 39. Omission of Trx3 or Trr2 from the reaction abrogated Prx1 activity, indicating a requirement for the complete thioredoxin system. Thus, despite the fact that we could find no requirement for Trx3 to maintain Prx1 antioxidant activity in yeast cells, the mitochondrial thioredoxin system could support enzyme activity in vitro. The cytoplasmic thioredoxin system (Trx2 and Trr1) was also able to support the peroxidase activity of Prx1, albeit at approximately 50% of the rate of the mitochondrial system (Fig. 7A).
FIG. 7.
Peroxidase activity of Prx1 (A) The peroxidase activity of Prx1 was measured in vitro with purified proteins. Reaction mixtures contained NADPH (250 μM), thioredoxin reductase (0.1 μM), thioredoxin (4 μM), Prx1 (0.5 μM), and GSH, as indicated. Reactions were started by the addition of 500 μM hydrogen peroxide, followed by a decrease in A340 attributable to the oxidation of NADPH. (B) Catalytic activity of Prx1. The reaction mixture containing Trr2 and 1.0 mM GSH was repeated using different amounts of Prx1. Reaction velocity (nanomoles NADPH oxidized/minute) was found to show strict linearity with Prx1 concentration, confirming the catalytic activity of Prx1 in this reaction system. (C) Formation of a disulfide-bonded interaction between Prx1 and Trr2. The reaction mixtures described in the legend to Fig. 6C were analyzed by nonreducing (NR) and reducing (R) SDS-PAGE. Western blot analysis using anti-Prx1 confirmed that Prx1 was detected at a size corresponding to a dimer. The addition of Trr2 shifted the molecular mass of Prx1, confirming the formation of a Prx1-Trr2 reaction intermediate. Prx1 was shifted to its monomeric size under reducing conditions, confirming the formation of a disulfide-bonded intermediate. (D) GSH competes with thioredoxin in attacking the Cys-SOH group of Prx1. Prx1 enzyme activity was measured with Trr1/Trx2 in the presence of increasing concentrations of GSH to compare the efficiency of thioredoxin versus GSH attack on the Cys-SOH of Prx1. Increasing concentrations of GSH (0.5 to 2 mM) were found to inhibit Trr1/Trx2-dependent Prx1 activity, consistent with the idea that GSH competes with thioredoxin and inhibits Prx1 activity. (E) The Trr2/GSH reduction system is better suited to maintaining Prx1 enzyme activity at the physiological pH found in mitochondria. Prx1 enzyme activity was measured at pH 8.0 to more accurately reflect the higher mitochondrial pH. The reaction mixture containing Trr2 and Trx3 was inhibited by approximately 50% at pH 8.0 compared with that at pH 7.0, whereas the reaction mixture containing Trr2 and GSH was unaffected by the higher pH.
Our in vivo data indicate that Trr2 and GSH are required for Prx1 antioxidant activity. To mimic a possible Prx1 peroxidase system in vitro, increasing concentrations of GSH were added to a reaction mixture containing Trr2 in the absence of Trx3 (Fig. 7A). GSH added at concentrations of 0.5 to 1.0 mM was found to stimulate a Trr2-dependent reaction such that Prx1 peroxidase activity was detected at a rate comparable to that of reaction mixtures containing Trx3. In contrast to the mitochondrial Trr2, cytoplasmic Trr1 was unable to support Prx1 peroxidase activity in a reaction mixture containing GSH in the absence of thioredoxin. The mitochondrial Prx1 is therefore able to use GSH to reduce hydrogen peroxide in a reaction coupled with thioredoxin reductase and NADPH. The reduction of H2O2 was followed by the use of different amounts of Prx1 in a reaction mixture containing Trr2 and 1.0 mM GSH (Fig. 7B). Reaction velocity was found to show strict linearity with Prx1 concentration, confirming the catalytic activity of Prx1 in this reaction system.
To address the reaction mechanism whereby Trr2 is able to promote GSH-dependent Prx1 peroxidase activity, we tested whether Trr2 can reduce glutathionylated Prx1. Prx1 was glutathionylated by incubation with GSSG, and MALDI-TOF MS was used to identify the tryptic peptide encompassing Cys91 (Fig. 6C). The addition of Trr2 alone was unable to reduce the glutathionylated peptide. In contrast, when Trr2 was added with 1.0 mM GSH, glutathionylated Cys91 was completely reduced. As a control, we confirmed that GSH alone is also unable to reduce glutathionylated Cys91 (Fig. 6C). These data suggest that Trr2 can reduce glutathionylated Prx1 in a reaction requiring GSH. To test this mechanism further, we examined the ability of Trr2 to form a disulfide-bonded interaction with Prx1. We reasoned that if Trr2 reduces glutathionylated Prx1, we may be able to trap a reaction intermediate between Trr2 and Prx1 by omitting GSH from the reaction mixture. This was investigated by analyzing the reaction mixtures described for Fig. 6C by using nonreducing SDS-PAGE. Prx1 was detected at a size of approximately 60 kDa, corresponding to the dimeric form as expected (Fig. 7C). Incubation with Trr2 resulted in a mass shift consistent with the formation of a disulfide-bonded reaction intermediate between Prx1 and Trr2. This intermediate was not detected if GSH was included in the reaction mixture. We confirmed that the interaction between Trr2 and Prx1 was stabilized by a disulfide bond since Prx1 was completely shifted to its monomeric size under reducing conditions (Fig. 7C).
Taken together, our data indicate that the yeast mitochondrial 1-Cys is reactivated by glutathionylation of its catalytic cysteine residue, followed by reduction by Trr2 coupled with GSH. This mechanism does not require the usual thioredoxin (Trx3) redox partner of Trr2 for antioxidant activity, although in vitro assays show that the Trr2/Trx3 and Trr2/GSH systems exhibit similar capacities for supporting Prx1 catalysis (Fig. 7A). To compare the efficiency of thioredoxin versus GSH attack on the Cys-SOH of Prx1, we examined Prx1 enzyme activity with Trr1/Trx2 in the presence of increasing concentrations of GSH (Fig. 7D). We reasoned that since cytoplasmic Trr1 is unable to reduce the glutathionylated form of Prx1, it would enable us to directly compare thioredoxin and GSH in the same reaction. Increasing concentrations of GSH (0.5 to 2 mM) were found to inhibit Trr1/Trx2-dependent Prx1 activity, consistent with the idea that Prx1 becomes stuck in the glutathionylated form, which cannot be reduced by the cytoplasmic Trr1. Unfortunately, the activity of Trr1 with Trx3 was too poor to be able to directly compare the abilities of Trx3 and GSH to attack the Prx1-SOH group. Nevertheless, these data indicate that GSH competes with thioredoxin in attacking the Cys-SOH group of Prx1.
An alternative explanation as to why the thioredoxin system (Trr2/Trx3) does not maintain Prx1 activity in vivo is that it is inefficient at the relatively alkaline pH found in mitochondria. We therefore examined Prx1 enzyme activity at pH 8.0 to more accurately reflect the higher mitochondrial pH (25). The reaction mixture containing Trr2 and Trx3 was inhibited by approximately 50% at pH 8.0 compared with that at pH 7.0 (Fig. 7E). In contrast, the reaction mixture containing Trr2 and GSH was unaffected by the higher pH. Thus, the Trr2/GSH reduction system may be better suited to maintaining Prx1 enzyme activity at the physiological pH found in mitochondria.
DISCUSSION
Peroxiredoxins are ubiquitous enzymes with well-characterized roles as peroxidases. The first step of catalysis is common to all peroxiredoxins and results in oxidation of the reactive peroxidatic cysteine residue to an SOH intermediate. This can be attacked by another cysteine residue to form an intermolecular disulfide bridge in the case of 2-Cys peroxiredoxins. This disulfide is usually reduced by thioredoxin regenerating active peroxiredoxin which can participate in another round of enzyme activity. 1-Cys Prx's contain a peroxidatic cysteine but do not contain a resolving cysteine residue. The cysteine sulfenic acid generated by reaction with peroxides is thought to be reduced by a thiol-containing electron donor, but this is poorly understood. Reduction of mammalian 1-Cys-Prx (Prdx6) occurs through glutathionylation of the oxidized catalytic cysteine residue. This involves heterodimerization with GSH transferase (πGST), followed by GSH-mediated reduction and enzyme reactivation (27). πGST is thought to serve as a source of activated GSH catalyzing glutathionylation of the oxidized peroxidatic cysteine residue, and GSH is the reductant in this mechanism which regenerates the active 1-Cys peroxiredoxin (28, 40). More recently, ascorbate has been proposed to function as a reductant for 1-Cys peroxiredoxins from diverse species (31). Using purified enzymes, ascorbate was shown to display a similar catalytic efficiency as thioredoxin for the reduction of Prx1, and it was proposed that the ascorbate-dependent reduction of cysteine sulfenic acid may be as important as thiol-mediated reduction for 1-Cys peroxiredoxins. However, our current study found that there is no in vivo requirement for erythroascorbate to maintain the yeast 1-Cys peroxiredoxin.
Up until now, studies aimed at identifying the in vivo reductant of 1-Cys peroxiredoxins have been lacking. This is important because the high reactivity and promiscuity of electron transfer reactions can result in misleading findings using highly purified cell-free systems. Pedrajas et al. (39) reported that the mitochondrial thioredoxin system is the physiological electron donor for Prx1. This was unexpected since 1-Cys peroxiredoxins are not thought to form a disulfide bond as part of their catalytic cycle which could act as a substrate for thioredoxin reduction. The first indication that thioredoxin may not be the physiological electron donor for Prx1 came from observations that prx1 and trr2 mutants are sensitive to oxidants, whereas the loss of TRX3 does not affect oxidant sensitivity (38, 39, 48). This pattern of oxidant sensitivity would not be expected if both Trr2 and Trx3 were serving in a reduction system to maintain the antioxidant activity of Prx1, and it raises the question of the true identity of the physiological reductant for Prx1. We previously showed that Trr2 and Glr1 have an overlapping function in the mitochondrial response to oxidative stress (48). Based on our current yeast cell studies, we now propose that this common function is maintaining the antioxidant activity of Prx1. This is based on two lines of evidence. First, the GSH system and Trr2 are both required for Prx1-mediated oxidative stress resistance. Second, Trr2 and GSH are required to maintain Prx1 in a reduced active form since the peroxidatic Cys residue of Prx1 becomes oxidized in trr2, glr1, and gsh1 mutants, whereas there is no apparent requirement for Trx3. GSH therefore appears to reduce Prx1 in an antioxidant reaction which requires thioredoxin reductase (Trr2).
We have performed Prx1 peroxidase assays using purified Prx1 and thioredoxin system components. In agreement with Pedrajas et al. (39), mitochondrial Trr2 and Trx3 were able to support thioredoxin peroxidase activity with hydrogen peroxide as a substrate. These data highlight the importance of extending the findings made in vitro to a whole-cell model. Despite the finding that Trx3 can act as a reductant for Prx1 in vitro, there does not appear to be any requirement for the mitochondrial thioredoxin to maintain Prx1 activity in yeast cells. We cannot at this stage rule out that Trx3 plays some role in maintaining Prx1 activity under particular growth conditions, but it does not appear to be required during oxidative stress. The finding that a thioredoxin can reduce cysteine sulfenic acid is in agreement with the observation that thioredoxin can act as a reducing agent for mammalian methionine sulfoxide reductase (23). Mammalian MsrB2 and MsrB3 form a sulfenic acid intermediate as part of their reaction mechanism but lack a resolving cysteine residue with which to form a disulfide bond. Thus, the formation of an Msr-thioredoxin disulfide-bonded intermediate may be analogous to the reaction mechanism of Trx3 with Prx1. This raises the question as to why the mitochondrial thioredoxin does not appear to support Prx1 activity in vivo. Our enzyme assays were performed at a neutral pH, and one possibility is that the thioredoxin system (Trr2/Trx3) is inefficient at the relatively alkaline pH found in mitochondria. In agreement with this idea, thioredoxin-dependent Prx1 peroxidase activity was reduced by approximately 50% when the assay was performed at the physiological pH of mitochondria. Additionally, the relative concentrations of the components used in the in vitro enzyme assays may not accurately reflect their mitochondrial concentrations, such that Trx3 may not normally be present in sufficient quantities in mitochondria to reduce Prx1.
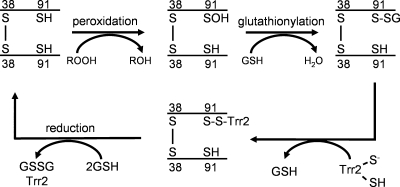
Our enzyme assays confirmed that Trr2 and GSH alone are sufficient to act as an electron donor system for Prx1, since the addition of physiological concentrations of GSH to a reaction lacking Trx3 was found to promote Prx1 peroxidase activity. Based on the formation of a Prx1-Trr2 reaction intermediate, we propose the mechanism shown in Fig. 8. Prx1 was detected in cells with Cys38, forming an intermolecular disulfide bond. The peroxidatic Cys91 residue is present in the reduced form unless cells are subjected to oxidative stress. Under oxidative stress conditions, MS analysis revealed that Cys91 could undergo glutathionylation. The exposure of cells to hydroperoxides results in oxidation of the peroxidatic cysteine residue (Cys91), leading to the formation of the sulfenic acid form. Direct comparison of thioredoxin and GSH revealed that GSH efficiently attacks the sulfenic acid intermediate, resulting in the formation of glutathionylated Prx1. Following glutathionylation, this mixed disulfide is a substrate for reduction by Trr2, forming a Prx1-Trr2 disulfide-bonded intermediate. The Prx1-Trr2 intermediate is then reduced by GSH, leading to the regeneration of active Prx1.
FIG. 8.
Proposed reaction mechanism for the redox cycle of Prx1. Prx1 is shown as a Cys38 disulfide-bonded dimer, but native Prx1 may be present in cells in other oligomeric forms. Reduction of hydrogen peroxide results in oxidation of the peroxidatic cysteine residue (Cys91) to the sulfenic acid form. Following glutathionylation, the mixed disulfide form is a substrate for reduction by Trr2 in a reaction that requires GSH.
It is not clear at present whether this mechanism may be conserved in other 1-Cys peroxiredoxins. The Cys38 residue is found in an N-terminal extension which is not conserved in other 1-Cys peroxiredoxins. However, the main structural constraint on this model is that GSH can access the oxidized peroxidatic cysteine residue of the 1-Cys peroxiredoxin. Glutathionylation has been reported for peroxiredoxins from diverse species, suggesting that this modification is a common feature of this class of enzymes (11, 12, 30, 32, 37, 45). The reaction mechanism also depends on the ability of thioredoxin reductase to reduce the mixed disulfide formed between Prx1 and GSH. It has long been known that thioredoxin reductases are not specific for thioredoxins and for example, mammalian enzymes show broad substrate specificity and are able to reduce diverse low-molecular-weight substrates (2). There is a precedent for the reduction of a glutathionylated protein by thioredoxin reductase since human TrxR2 can reduce the GSH/glutaredoxin intermediate which is formed in the reduction of glutathionylated substrates by Grx2 (22). Unlike those of yeasts, however, mammalian thioredoxin reductases are selenoproteins which display higher reactivity and broader substrate specificity than their cysteine-containing homologues in lower eukaryotes. This may explain why the covalent interaction between Prx1 and Trr2 cannot be resolved by the Trr2 active site cysteine but requires additional reductant input from GSH. Yeast mitochondrial Trr2 and cytosolic Trr1 share 84% sequence identity, including conserved FAD- and NADPH-binding sites (38). However, only the mitochondrial isoform was able to reduce the Prx1-GSH mixed disulfide. Deglutathionylation activity, therefore, appears to be specific to the mitochondrial form of the enzyme, which may not be surprising given that mitochondria are particularly exposed to ROS and hence modification of mitochondrial proteins by glutathionylation is of particular importance in this organelle (21).
Our data indicate that mitochondria are a particular target of cadmium-induced oxidative stress and that Prx1 is required to protect against mitochondrial cadmium toxicity. Cadmium is a highly toxic metal and a well-established human carcinogen. It is capable of entering cells via the same transport systems used by essential heavy metals. Once inside the cell, a main mechanism for toxicity is thought to be through the depletion of GSH and binding to sulfhydryl groups (50). However, at the concentrations of cadmium used in this current study, there was no effect on the levels of free GSH either in the cytosol or mitochondria. Rather, cadmium exposure was found to elevate the cellular concentrations of GSSG, lowering the GSH redox ratio in both organelles. Thus, analogous to its role with hydrogen peroxide toxicity, Prx1 appears to protect cells against cadmium-induced oxidative stress. Growth in the absence of oxygen rescued cadmium toxicity in wild-type and prx1 mutant cells, confirming the role of ROS in cadmium stress. Cadmium can displace iron and copper from cellular proteins, increasing the levels of unbound free or chelated copper and iron ions, causing an oxidative stress via Fenton reactions (51). Since mitochondria play a key role in iron metabolism as they are the site of iron-sulfur cluster assembly, this may make them particularly sensitive to cadmium exposure. In agreement with this idea, heavy metals, including cadmium, have been shown to promote mitochondrial ROS production and eventual cell death (3).
This is the first confirmation that GSH plays a direct role in peroxide detoxification in yeasts. Based on extensive biochemical analyses, the thioredoxin system and not the GSH system had been thought to play the main role in peroxide detoxification (46). This is because the major peroxidase enzymes described in this organism, including peroxiredoxins (Tsa1, Tsa2, Ahp1, nTpx) and GSH peroxidase-like enzymes (Gpx1-3), all appear to depend on the thioredoxin system for reduction. However, this idea is now complicated by our current finding which indicates that GSH and thioredoxin reductase have an overlapping role in providing reducing activity for the peroxide scavenging activity of a mitochondrial peroxiredoxin. There is increasing evidence that the thioredoxin and GSH/glutaredoxin redox systems have overlapping functions, and extensive deletion analyses in yeast have identified considerable genetic redundancy in the various systems (26, 46). The reason for this apparent functional redundancy in highly conserved systems remains an important unanswered question, but it is very likely that further overlapping activities remain to be identified.
Acknowledgments
This work was supported by the Wellcome Trust.
We are grateful to David Knight and Emma-Jane Keevil (University of Manchester) for MS analysis. We thank Neil Bulleid (Manchester) for critical reading of the manuscript.
Footnotes
Published ahead of print on 30 March 2009.
REFERENCES
- 1.Baudin, A., O. Ozier-Kalogeropoulos, A. Danouel, F. Lacroute, and C. Cullin. 1993. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 213329-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker, K., S. Gromer, R. H. Schirmer, and S. Müller. 2000. Thioredoxin reductase as a pathophysiological factor and drug target. Eur. J. Biochem. 2676118-6125. [DOI] [PubMed] [Google Scholar]
- 3.Belyaeva, E. A., D. Dymkowska, M. R. Wieckowski, and L. Wojtczak. 2008. Mitochondria as an important target in heavy metal toxicity in rat hepatoma AS-30D cells. Toxicol. Appl. Pharmacol. 23134-42. [DOI] [PubMed] [Google Scholar]
- 4.Boveris, A., and E. Cadenas. 1982. Production of superoxide radicals and hydrogen peroxide in mitochondria, p. 15-30. In L. W. Oberley (ed.), Superoxide dismutases, vol. 2. CRC Press, Boca Raton, FL. [Google Scholar]
- 5.Chance, B., H. Sies, and A. Boveris. 1979. Hydroperoxide metabolism in mammalian organs. Physiol. Rev. 59527-605. [DOI] [PubMed] [Google Scholar]
- 6.Chen, Y., J. Cai, T. J. Murphy, and D. P. Jones. 2002. Overexpressed human mitochondrial thioredoxin confers resistance to oxidant-induced apoptosis in human osteosarcoma cells. J. Biol. Chem. 27733242-33248. [DOI] [PubMed] [Google Scholar]
- 7.Chen, Z., and L. H. Lash. 1998. Evidence for mitochondrial uptake of glutathione by dicarboxylate and 2-oxoglutarate carriers. J. Pharmacol. Exp. Ther. 285608-618. [PubMed] [Google Scholar]
- 8.Christianson, T. W., R. S. Sikorski, M. Dante, J. H. Shero, and P. Hieter. 1992. Multifunctional yeast high-copy-number shuttle vectors. Gene 110119-122. [DOI] [PubMed] [Google Scholar]
- 9.Demple, B. 1998. A bridge to control. Science 2791655-1656. [DOI] [PubMed] [Google Scholar]
- 10.Diekert, K., A. I. de Kroon, G. Kispal, and R. Lill. 2001. Isolation and subfractionation of mitochondria from the yeast Saccharomyces cerevisiae. Methods Cell Biol. 6537-51. [DOI] [PubMed] [Google Scholar]
- 11.Fratelli, M., H. Demol, M. Puype, S. Casagrande, I. Eberini, M. Salmona, V. Bonetto, M. Mengozzi, F. Duffieux, E. Miclet, A. Bachi, J. Vandekerckhove, E. Gianazza, and P. Ghezzi. 2002. Identification by redox proteomics of glutathionylated proteins in oxidatively stressed human T lymphocytes. Proc. Natl. Acad. Sci. USA 993505-3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fratelli, M., H. Demol, M. Puype, S. Casagrande, P. Villa, I. Eberini, J. Vandekerckhove, E. Gianazza, and P. Ghezzi. 2003. Identification of proteins undergoing glutathionylation in oxidatively stressed hepatocytes and hepatoma cells. Proteomics 31154-1161. [DOI] [PubMed] [Google Scholar]
- 13.Glick, B. S., and L. A. Pon. 1995. Isolation of highly purified mitochondria from Saccharomyces cerevisiae. Methods Enzymol. 260213-223. [DOI] [PubMed] [Google Scholar]
- 14.Grant, C. M., L. P. Collinson, J.-H. Roe, and I. W. Dawes. 1996. Yeast glutathione reductase is required for protection against oxidative stress and is a target gene for yAP-1 transcriptional regulation. Mol. Microbiol. 21171-179. [DOI] [PubMed] [Google Scholar]
- 15.Grant, C. M., F. H. MacIver, and I. W. Dawes. 1996. Glutathione is an essential metabolite required for resistance to oxidative stress in the yeast Saccharomyces cerevisiae. Curr. Genet. 29511-515. [DOI] [PubMed] [Google Scholar]
- 16.Grant, C. M., G. Perrone, and I. W. Dawes. 1998. Glutathione and catalase provide overlapping defenses for protection against hydrogen peroxide in the yeast Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 253893-898. [DOI] [PubMed] [Google Scholar]
- 17.Griffith, O. W., and A. Meister. 1985. Origin and turnover of mitochondrial glutathione. Proc. Natl. Acad. Sci. USA 824668-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gutteridge, J. M. C. 1993. Free radicals in disease processes: a compilation of cause and consequence. Free Radic. Res. Commun. 19141-158. [DOI] [PubMed] [Google Scholar]
- 19.Halliwell, B., and J. M. C. Gutteridge. 1989. Free radicals in biology and medicine, 2nd ed. Oxford University Press, Oxford, United Kingdom.
- 20.Holmgren, A. 1989. Thioredoxin and glutaredoxin systems. J. Biol. Chem. 26413963-13966. [PubMed] [Google Scholar]
- 21.Hurd, T. R., N. J. Costa, C. C. Dahm, S. M. Beer, S. E. Brown, A. Filipovska, and M. P. Murphy. 2005. Glutathionylation of mitochondrial proteins. Antioxid. Redox Signal. 7999-1010. [DOI] [PubMed] [Google Scholar]
- 22.Johansson, C., C. H. Lillig, and A. Holmgren. 2004. Human mitochondrial glutaredoxin reduces S-glutathionylated proteins with high affinity accepting electrons from either glutathione or thioredoxin reductase. J. Biol. Chem. 2797537-7543. [DOI] [PubMed] [Google Scholar]
- 23.Kim, H. Y., and J. R. Kim. 2008. Thioredoxin as a reducing agent for mammalian methionine sulfoxide reductases B lacking resolving cysteine. Biochem. Biophys. Res. Commun. 371490-494. [DOI] [PubMed] [Google Scholar]
- 24.Lee, J.-C., M. J. Straffon, T.-Y. Jang, C. M. Grant, and I. W. Dawes. 2001. The essential and ancillary role of glutathione in Saccharomyces cerevisiae: studies with a grande gsh1 disruptant strain. FEMS Yeast Res. 157-65. [DOI] [PubMed] [Google Scholar]
- 25.Llopis, J., J. M. McCaffery, A. Miyawaki, M. G. Farquhar, and R. Y. Tsien. 1998. Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc. Natl. Acad. Sci. USA 956803-6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.López-Mirabal, H. R., and J. R. Winther. 2008. Redox characteristics of the eukaryotic cytosol. Biochim. Biophys. Acta 1783629-640. [DOI] [PubMed] [Google Scholar]
- 27.Manevich, Y., S. I. Feinstein, and A. B. Fisher. 2004. Activation of the antioxidant enzyme 1-CYS peroxiredoxin requires glutathionylation mediated by heterodimerization with pi GST. Proc. Natl. Acad. Sci. USA 1013780-3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manevich, Y., and A. B. Fisher. 2005. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radic. Biol. Med. 381422-1432. [DOI] [PubMed] [Google Scholar]
- 29.Meister, A. 1995. Mitochondrial changes associated with glutathione deficiency. Biochim. Biophys. Acta 127135-42. [DOI] [PubMed] [Google Scholar]
- 30.Michelet, L., M. Zaffagnini, H. Vanacker, P. Le Marechal, C. Marchand, M. Schroda, S. D. Lemaire, and P. Decottignies. 2008. In vivo targets of S-thiolation in Chlamydomonas reinhardtii. J. Biol. Chem. 28321571-21578. [DOI] [PubMed] [Google Scholar]
- 31.Monteiro, G., B. B. Horta, D. C. Pimenta, O. Augusto, and L. E. Netto. 2007. Reduction of 1-Cys peroxiredoxins by ascorbate changes the thiol-specific antioxidant paradigm, revealing another function of vitamin C. Proc. Natl. Acad. Sci. USA 1044886-4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noguera-Mazon, V., J. Lemoine, O. Walker, N. Rouhier, A. Salvador, J.-P. Jacquot, J.-M. Lancelin, and I. Krimm. 2006. Glutathionylation induces the dissociation of 1-Cys d-peroxiredoxin non-co-valent homodimer. J. Biol. Chem. 28131736-31742. [DOI] [PubMed] [Google Scholar]
- 33.Nonn, L., R. R. Williams, R. P. Erickson, and G. Powis. 2003. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol. Cell. Biol. 23916-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olafsdottir, K., and D. J. Reed. 1988. Retention of oxidized glutathione by isolated rat liver mitochondria during hydroperoxide treatment. Biochim. Biophys. Acta 964377-382. [DOI] [PubMed] [Google Scholar]
- 35.Outten, C. E., and V. C. Culotta. 2004. Alternative start sites in the S. cerevisiae GLR1 gene are responsible for mitochondrial and cytosolic isoforms of glutathione reductase. J. Biol. Chem. 2797785-7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park, S. G., M.-K. Cha, W. Jeong, and I.-H. Kim. 2000. Distinct physiological functions of thiol peroxidase isoenzymes in Saccharomyces cerevisiae. J. Biol. Chem. 2755723-5732. [DOI] [PubMed] [Google Scholar]
- 37.Pauwels, F., B. Vergauwen, F. Vanrobaeys, B. Devreese, and J. J. Van Beeumen. 2003. Purification and characterization of a chimeric enzyme from Haemophilus influenzae Rd that exhibits glutathione-dependent peroxidase activity. J. Biol. Chem. 27816658-16666. [DOI] [PubMed] [Google Scholar]
- 38.Pedrajas, J. R., E. Kosmidou, A. Miranda-Vizuete, J.-A. Gustafsson, A. P. H. Wright, and G. Spyrou. 1999. Identification and functional characterization of a novel mitochondrial thioredoxin system in Saccharomyces cerevisiae. J. Biol. Chem. 2746366-6373. [DOI] [PubMed] [Google Scholar]
- 39.Pedrajas, J. R., A. Miranda-Vizuete, N. Javanmardy, J.-A. Gustafsson, and G. Spyrou. 2000. Mitochondria of Saccharomyces cerevisiae contain one-conserved cysteine type peroxiredoxin with thioredoxin peroxidase activity. J. Biol. Chem. 27516296-16301. [DOI] [PubMed] [Google Scholar]
- 40.Ralat, L. A., S. A. Misquitta, Y. Manevich, A. B. Fisher, and R. F. Colman. 2008. Characterization of the complex of glutathione S-transferase pi and 1-cysteine peroxiredoxin. Arch. Biochem. Biophys. 474109-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhee, S. G., H. Z. Chae, and K. Kim. 2005. Peroxiredoxins: a historical overview and speculative preview of novel mechanisms and emerging concepts in cell signaling. Free Radic. Biol. Med. 381543-1552. [DOI] [PubMed] [Google Scholar]
- 42.Rietsch, A., and J. Beckwith. 1998. The genetics of disulfide bond metabolism. Annu. Rev. Gen. 32163-184. [DOI] [PubMed] [Google Scholar]
- 43.Spickett, C. M., N. Smirnoff, and A. R. Pitt. 2000. The biosynthesis of erythroascorbate in Saccharomyces cerevisiae and its role as an antioxidant. Free Radic. Biol. Med. 28183-192. [DOI] [PubMed] [Google Scholar]
- 44.Stewart, E. J., F. Aslund, and J. Beckwith. 1998. Disulfide bond formation in the Escherichia coli cytoplasm: an in vivo role reversal for the thioredoxins. EMBO J. 175543-5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sullivan, D. M., N. B. Wehr, M. M. Fergusson, R. L. Levine, and T. Finkel. 2000. Identification of oxidant-sensitive proteins: TNF-alpha; induces protein glutathiolation. Biochemistry 3911121-11128. [DOI] [PubMed] [Google Scholar]
- 46.Toledano, M. B., C. Kumar, N. Le Moan, D. Spector, and F. Tacnet. 2007. The system biology of thiol redox system in Escherichia coli and yeast: differential functions in oxidative stress, iron metabolism and DNA synthesis. FEBS Lett. 5813598-3607. [DOI] [PubMed] [Google Scholar]
- 47.Trotter, E. W., and C. M. Grant. 2003. Non-reciprocal regulation of the redox state of the glutathione/glutaredoxin and thioredoxin systems. EMBO Rep. 4184-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trotter, E. W., and C. M. Grant. 2005. Overlapping roles of the cytoplasmic and mitochondrial redox regulatory systems in the yeast Saccharomyces cerevisiae. Eukaryot. Cell 4392-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trotter, E. W., and C. M. Grant. 2002. Thioredoxins are required for protection against a reductive stress in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 46869-878. [DOI] [PubMed] [Google Scholar]
- 50.Valko, M., H. Morris, and M. T. Cronin. 2005. Metals, toxicity and oxidative stress. Curr. Med. Chem. 121161-1208. [DOI] [PubMed] [Google Scholar]
- 51.Valko, M., C. J. Rhodes, J. Moncol, M. Izakovic, and M. Mazur. 2006. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 1601-40. [DOI] [PubMed] [Google Scholar]
- 52.Wood, Z. A., E. Schroder, J. R. Harris, and L. B. Poole. 2003. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem. Sci. 2832-40. [DOI] [PubMed] [Google Scholar]