Abstract
Eukaryotic 18S rRNA processing is mediated by the small subunit (SSU) processome, a machine comprised of the U3 small nucleolar RNP (U3 snoRNP), tUTP, bUTP, MPP10, and BMS1/RCL1 subcomplexes. We report that the human SSU processome is a dynamic structure with the recruitment and release of subcomplexes occurring during the early stages of ribosome biogenesis. A novel 50S U3 snoRNP accumulated when either pre-rRNA transcription was blocked or the tUTP proteins were depleted. This complex did not contain the tUTP, bUTP, MPP10, and BMS1/RCL1 subcomplexes but was associated with the RNA-binding proteins nucleolin and RRP5 and the RNA helicase DBP4. Our data suggest that the 50S U3 snoRNP is an SSU assembly intermediate that is likely recruited to the pre-rRNA through the RNA-binding proteins nucleolin and RRP5. We predict that nucleolin is only transiently associated with the SSU processome and likely leaves the complex not long after 50S U3 snoRNP recruitment. The nucleolin-binding site potentially overlaps that of several other key factors, and we propose that this protein must leave the SSU processome for pre-rRNA processing to occur.
In eukaryotes, the 18S, 5.8S, and 28S (25S in yeast) ribosomal RNAs (rRNAs) are transcribed as a single precursor molecule by RNA polymerase I that is processed by both endo- and exonucleolytic cleavages (12, 17) in the nucleolus. The production of each ribosome requires 80 ribosomal proteins and more than 150 additional factors (including exonucleases, endonucleases, chaperones, helicases, annealing factors, and small nucleolar RNPs [snoRNPs]). Ribosome biogenesis is a major consumer of energy in the cell (36); is regulated relative to development, cell growth, the cell cycle, and stress; and is upregulated in the majority of transformed cells (33).
The production of 18S rRNA involves the removal of the 5′ external transcribed spacer (5′ETS) and internal transcribed spacer 1 by cleavages at sites A′, A1, and A2. This is mediated by the SSU processome, a complex that contains the U3 snoRNA and more than 40 additional proteins (reviewed in references 12 and 17). In higher eukaryotes, the nucleolus contains three subcompartments, namely, the fibrillar center (FC), the dense fibrillar component (DFC), and the granular component (GC) (18, 31). Pre-rRNA transcription occurs on the border between the FC and DFC, and processing intermediates migrate through the nucleolar compartments in vectorial fashion (28). The initial cleavages in the 5′ETS (A′) and 3′ETS occur in the DFC, while the removal of the core 5′ETS sequence (A1 cleavage) takes place subsequently in the GC. The U3 snoRNP is found throughout the nucleolus and cycles between the DFC and the GC as part of the SSU processome (8, 15). Indeed, the ability of the U3 snoRNA to localize to the GC correlates with its ability to be recruited to the SSU processome and to participate in the A′ cleavage event (15).
The U3 snoRNP is present in the cell as a 12S monoparticle comprised of the U3 snoRNA, the core box C/D snoRNP proteins (15.5K [Snu13p], NOP56, NOP58, and fibrillarin [Nop1p]), and the U3-specific hU3-55K protein (Rrp9p) (15, 38) and as a subcomplex of the SSU processome. The 5′ end of the U3 snoRNA base pairs with sequences in the 5′ETS and 18S rRNA (reviewed in reference 17). The SSU processome contains several other subcomplexes, including the MPP10, tUTP, bUTP, and BMS1/RCL1 complexes (see reference 17 and references therein). The MPP10 complex contains the M-phase phosphoprotein MPP10, and the two annealing factors IMP3 and IMP4 (9, 13, 40). The BMS1/RCL1 complex is comprised of the GTPase BMS1, which binds the U3 snoRNA in vitro, and the RNA cyclase-like protein RCL1 (2, 19, 20, 39). The tUTP complex (comprising tUTP4, tUTP5, tUTP10, tUTP15, and tUTP17, as well as tUTP8 and tUTP9 in yeast) associates with ribosomal DNA, appears important for pre-rRNA transcription, and is predicted to function in the cotranscriptional recruitment of the SSU processome to the pre-rRNA (7, 23, 30). The bUTP complex (comprising PWP2, UTP6, UTP12, UTP13, UTP18, and UTP21) is important for U3 snoRNP recruitment to the SSU processome in yeast (23, 29). The SSU processome contains at least 25 additional factors, including several RNA helicases (e.g., DBP4, HAS1, and DHR1) and RNA-binding proteins (e.g., RRP5 and MRD1) (17).
Much remains unknown about the assembly of the SSU processome and the regulation of the early processing steps. The majority of the work analyzing SSU processome composition and function has been performed with Saccharomyces cerevisiae, and surprisingly, little is known about this process in higher eukaryotes (6). Several RNA-binding proteins, including RRP5, RRP7, and MRD1, have been found in the yeast SSU processome (6, 12, 17). It is, however, unclear what factors specifically recognize the pre-rRNA and coordinate the assembly of the SSU processome. In higher eukaryotes, nucleolin binds an evolutionarily conserved motif (ECM) at the A′ site in the 5′ETS and stimulates U3 snoRNP recruitment to the pre-rRNA in vitro (10). The individual U3 snoRNA-pre-rRNA base-pairing sequences are not essential for the integration of the snoRNP into the SSU processome (15). This suggests that nucleolin plays a key role in U3 snoRNP recruitment. However, the mechanism by which nucleolin recruits the U3 snoRNP to the pre-RNA is unclear. In this paper, we have characterized the human SSU processome and identified a 50S U3 snoRNP that contains nucleolin, RRP5, and DBP4. We propose that this complex is a novel SSU processome assembly intermediate that requires the pre-rRNA and the tUTP proteins for incorporation into the processing complex.
MATERIALS AND METHODS
RNA interference (RNAi).
A small interfering RNA (siRNA) duplex targeting RPA135 (sense, CCACCUAACUGCCGUAUGUTT, and antisense, AACAUACGGCAGUUAGGUGGTT) was electroporated into HeLa SS6 cells. Sixty hours after transfection, the cells were either harvested for extract preparation or were fixed and analyzed by either in situ hybridization or immunofluorescence as described previously (26). The GL2 siRNA (targets luciferase) was used as a control in these experiments (4). The depletion of tUTP10 and tUTP4 was performed as described previously (30).
Antibodies.
Most antibodies were raised in rabbits using peptide epitopes. The peptides used were PWP2 (CKQRGTKRSLDPLGSE and CATPAEEEKTGKVKYS), UTP12 (CEPDPKKIKGSSPGIQ and CPGETQGDSYFTGKKT), BMS1 (CSKPSQVSSGQKLGPQ and GKVPKDRRRPAVIREC), PP10 (CKLKSGKSSRNLKYKD and CDDLQENEDNKQHKES), UTP13 (CEPFYKGGKAQLDQTG and CAAAPTPWETHKGALP), RRP5 (MANLEESFPRGGTRKC), and MRD1 (CLQDTPSEPMEKDPAE and RAETEKPANQKEPTTC).
Anti-hU3-55K (14) and -tUTP10 (30) antibodies have been previously described. Nucleolin antibodies were purchased from Abcam and anti-DHR1 (DHX37), -HAS1 (DDX18), and -DBP4 (DDX10) antibodies were from Bethyl Laboratories. Fibrillarin antibody (72B9) was provided by Michael Pollard and Ger Pruijn.
Extract preparation, glycerol gradient analysis, and immunoprecipitation.
Nucleolar extracts were prepared and analyzed by immunoprecipitation as described previously (37) from nuclear pellets after the production of nuclear extract. Whole-cell extracts were prepared and analyzed by glycerol gradient centrifugation as described previously (15). It is important to note that all gradients in the same panel of each figure were run in parallel. The snRNPs (e.g., U1 snRNP) and ribosomes present in the extracts or 30S and 50S purified Escherichia coli ribosomal subunits, which were run on parallel gradients, were used as size markers (15). To inhibit RNA polymerase I transcription, the cells were incubated for 2 h (or shorter, if stated) with 100 ng/ml actinomycin D (ActD) prior to extract preparation. The proteins present in the gradient fractions were precipitated using trichloroacetic acid before being separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE) and transferred to a nitrocellulose membrane for Western blotting.
RNA analysis.
S1 nuclease protection assays were performed using an oligonucleotide complementary to and overlapping the A′ cleavage site as described previously (30). For analysis of large pre-rRNAs, RNA samples were separated on an agarose gel, transferred to a membrane by capillary blotting, and hybridized with probes specific for the 5′ETS (30). Nucleotides 1 to 339 and 1146 to 1534, amplified by PCR, were used as probes for the 5′ETS leader and core sequences, respectively.
RESULTS
Characterization of the human SSU processome.
In order to characterize the formation and function of the SSU processome, it was important to first define the complex in HeLa cell extracts. We have demonstrated that the U3 snoRNP exists in the cell in two distinct complexes, the 12S U3 snoRNP monoparticle and the 80S complex predicted to represent the SSU processome (15). We were next interested in determining whether, under our conditions, these complexes also contained pre-rRNA. Nucleolar extracts were first separated by glycerol gradient centrifugation. Northern blot analysis of RNA extracted from individual fractions was used to determine the positions of the 12S (fractions 1 to 5) and 80S (fractions 11 to 19) complexes in the gradient (Fig. 1A). We next identified the fractions containing pre-rRNA by S1 mapping using a 5′- labeled antisense probe spanning the A′ cleavage site in the 5′ETS (30). S1 mapping was chosen for this analysis, as it enables the simultaneous analysis of transcribed pre-rRNAs and those transcripts still undergoing transcription that would be missed by Northern hybridization. This is especially important in higher eukaryotes as the A′ cleavage is proposed to occur cotranscriptionally (24). Protected DNA fragments were separated by denaturing PAGE and detected by autoradiography. The major signals, corresponding to both the uncleaved 5′ETS and the core 5′ETS cleaved at A′, comigrated with the U3 snoRNA in the 80S complex (Fig. 1B, fractions 11 to 19), consistent with earlier predictions that this represents the SSU processome. A second peak, corresponding to the 20S to 30S complex on the gradient, was observed for the uncleaved pre-rRNA (Fig. 1B, fractions 4 to 8). This peak did not significantly overlap with that of the 12S U3 snoRNP, and we could not reliably coprecipitate the pre-rRNA in the 20S to 30S complex with any of the antibodies tested (see below; data not shown). It is possible that this may represent aberrant transcripts undergoing, or waiting to undergo, degradation. Further work is, however, required before any conclusions can be drawn about the functional significance of this complex.
FIG. 1.
Characterization of the human SSU processome. HeLa cell nucleolar extracts were separated by glycerol gradient centrifugation, the gradient was fractionated, and RNA was isolated from each fraction. (A) The RNA was separated on an 8% polyacrylamide/7 M urea gel and analyzed by Northern blot hybridization using a probe for the U3 snoRNA. (B) The RNA isolated from the gradient fractions was analyzed by S1 nuclease mapping using a probe spanning the A′ cleavage site. The protected fragments were separated on a 10% polyacrylamide/7 M urea gel and revealed by autoradiography. The peak positions of the 12S and 80S U3 snoRNP complexes are indicated at the top of the panels. The fraction numbers are indicated at the bottom. The position of the S1 nuclease signal corresponding to the uncleaved (U) and A′-processed (P) pre-rRNA is indicated on the right of the panel. The top and bottom of the gradient are indicated. (C) Nucleolar extract was separated by glycerol gradient centrifugation, and the fractions corresponding to the 12S and 80S U3 complexes (as indicated on the left) were pooled and then immunoprecipitated using protein-specific antibodies. Copurifying RNAs were isolated and analyzed by Northern blot hybridization using a U3-specific probe. The antibody used is indicated at the top of each lane (α). Input, 10% of the starting material; Beads, protein A-Sepharose in the absence of antibody.
We were next interested in identifying components of the key subunits of the human SSU processome. In addition to the characterized antibodies that recognize tUTP10 and hU3-55K (14, 30), we also raised antibodies against human homologues of SSU processome components UTP12 and PWP2 (both bUTP proteins), BMS1, and MPP10. We tested the association of these proteins with either the 12S or 80S pooled peak fractions by immunoprecipitation using the protein-specific antibodies. Coprecipitated RNAs were analyzed by Northern hybridization using a U3-specific probe. Anti-hU3-55K antibodies coprecipitated the U3 snoRNA from both the 12S and 80S complexes (Fig. 1C). This protein is a stable component of the U3 snoRNP, appears to be only present in U3 snoRNP complexes (13), and as such is recognized as a marker for the snoRNP. Antibodies raised against BMS1, MPP10, tUTP10, PWP2, and UTP12 coprecipitated the U3 snoRNA present in the 80S complex. However, these antibodies did not coprecipitate the U3 snoRNA in the 12S fractions (Fig. 1C). This indicates that these proteins, which have been characterized as U3 snoRNP proteins, are only associated with the U3 snoRNA as part of the SSU processome.
A 50S U3 snoRNP complex accumulates in the absence of RNA polymerase I transcription.
The SSU processome is predicted to assemble around the nascent pre-rRNA. To determine whether this is the case, we characterized the U3 snoRNP complexes present in HeLa cells in the absence of pre-rRNA transcription. To achieve this, RNA polymerase I activity was inhibited using either ActD (0.1 μg/ml) for 2 h or siRNA duplexes to deplete the RNA polymerase I subunit RPA135 in HeLa cells. Sixty hours after transfection, the cells were harvested and specific protein depletion determined by Western blotting (Fig. 2A). Pre-rRNA levels in both the ActD and siRNA-treated cells were monitored by Northern blotting using probes specific for the 5′ETS leader (detects 47S) and 5′ETS core (detects 47S, 45S, and 30S) sequences. Pre-rRNA intermediates were readily detected in RNA samples from untreated cells and those transfected with the control siRNA. In contrast, none of the intermediates could be detected in RNA derived from ActD-treated cells (Fig. 2B). In addition, a fivefold decrease in the levels of the pre-rRNA intermediates was seen in cells depleted of RPA135. As a control for loading, we also compared the levels of mature rRNAs in all RNA samples (Fig. 2B).
FIG. 2.
Accumulation of a 50S U3 snoRNP. (A) HeLa cells were transfected with either control siRNAs (targeting firefly luciferase) or siRNAs targeting RPA135 (ΔRPA135). Protein levels in the transfected cells were determined by Western blot analysis. The protein targeted is indicated above each panel. The antibodies used are indicated on the left. Proteins derived from equal numbers of cells were loaded. (B) Total RNA from equal numbers of control and treated cells was separated by agarose gel electrophoresis and then analyzed by ethidium bromide staining (bottom panel) and Northern blot hybridization using probes specific for the 5′ETS leader (top panel) and core sequences (middle panel). The positions of the mature and pre-rRNAs are indicated on the left. The cell treatment is indicated at the top. (C) HeLa cells were treated with ActD for various amounts of time (indicated on the right of the respective panel) and extracts prepared and then separated by glycerol gradient centrifugation. RNA was isolated from each fraction and analyzed by Northern hybridization using a U3 snoRNA-specific probe. The top and bottom of the gradients are indicated. The positions of the 12S, 50S, and 80S peaks are indicated. (D) HeLa cells were transfected with either control siRNAs or siRNAs targeting RPA135 (indicated on the right of the respective panel). Extracts were prepared from the transfected cells and the U3 snoRNA distribution characterized by glycerol gradient centrifugation and Northern blot analysis as described for panel C. (E) HeLa cells were transfected with either a control duplex or siRNAs targeting tUTP4 (ΔUTP4) or tUTP10 (ΔUTP10). Extracts were prepared from the transfected cells and separated by glycerol gradient centrifugation, and the U3 snoRNA distribution was analyzed as described for panel C. The protein targeted is indicated on the right. The positions of the various complexes are indicated at the top of the panels.
To examine the effects of inhibiting RNA polymerase I transcription on U3 snoRNP complexes, extracts were prepared from untreated cells and cells treated with ActD and separated by glycerol gradient centrifugation. The distribution of the U3 snoRNA in the gradients was analyzed by Northern hybridization. At each of the time points after the addition of ActD, the U3 snoRNA was present in a 12S complex (Fig. 2C). The 80S U3 snoRNP-containing complex disappeared over time and a novel 50S complex appeared. The 50S U3 snoRNP showed the same sedimentation behavior as purified E. coli 50S ribosome subunits that were run on a parallel gradient (data not shown). The loss of the 80S complex probably represents the processing complexes, which were present in the cell when ActD was added, being chased through to completion. Interestingly, the ratio between the smaller 12S complex and the larger (50S to 80S) complexes was not affected by ActD treatment. The peaks for the 12S monoparticle and larger U3 snoRNP complex were consistently spread over fewer fractions in the extracts derived from ActD-treated cells compared to those for the complexes in the control extracts, also suggesting changes to their conformation/composition.
We next analyzed the U3 snoRNP in cells electroporated with either control siRNAs or siRNAs targeting RPA135. Extracts were prepared from cells 60 h after transfection and the U3 complexes analyzed by glycerol gradient centrifugation as described above. Control cell extracts contained both the 12S and 80S U3 snoRNP complexes (Fig. 2D). In contrast, extracts derived from RPA135-depleted cells contained 12S and 50S U3 snoRNP complexes. As seen with the ActD treatment, the peaks for the two U3 complexes were also repeatedly spread over fewer fractions in cells where RNA polymerase I activity is compromised than in the control cells. Importantly, two different means of inhibiting RNA polymerase I, either ActD treatment or RNAi, both resulted in the loss of the 80S SSU processome and the accumulation of a novel 50S U3 snoRNP complex.
The depletion of the tUTP proteins results in the accumulation of the 50S U3 snoRNP.
The 50S U3 snoRNP could be an assembly intermediate that is recruited to the pre-rRNA during the formation of the SSU processome. Previous evidence, from work performed with yeast, suggests that the tUTP proteins direct the cotranscriptional assembly of the SSU processome and could possibly function directly in the recruitment of the U3 snoRNP. Therefore, we next investigated whether the 50S U3 snoRNP accumulated in the absence of the tUTP proteins. HeLa cells were depleted of either tUTP4 or tUTP10 using RNAi, as described previously (30). The loss of either tUTP10 or tUTP4 was previously shown to result in a clear block in the A′, A1, and A2 cleavages. Of particular interest is the depletion of tUTP4 which, in contrast to the loss of tUTP10, did not inhibit pre-rRNA transcription (30). The depletion of either tUTP protein, however, did not result in the loss of pre-rRNA intermediates in the cell. Whole-cell extracts were prepared from siRNA-treated cells and characterized by glycerol gradient centrifugation. Northern blot analysis of the resultant fractions revealed that the U3 snoRNP was present in 12S and 80S complexes in cells treated with the control siRNA. In extracts prepared from both tUTP4- and tUTP10-depleted cells, however, a 50S U3 snoRNP was present (Fig. 2E). The loss of tUTP10 resulted in severe reductions in 80S SSU processome levels consistent with the effect on pre-rRNA accumulation and processing (30). The loss of tUTP4 resulted in a reduction in the levels of the 80S SSU processome and the appearance of the 50S U3 snoRNP (Fig. 2E). This suggests that the tUTP proteins are important for the efficient recruitment of the U3 snoRNP into the SSU processome.
The 50S U3 snoRNP does not contain the tUTP, bUTP, MPP10, or BMS1/RCL1 subcomplexes or the pre-rRNA.
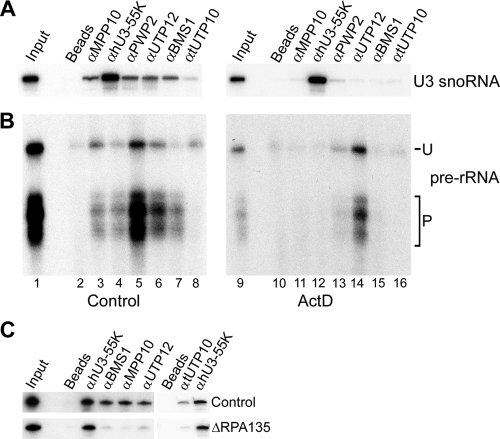
Having identified the 50S U3 snoRNP, a putative SSU processome intermediate, we were interested in determining which SSU processome components were associated with this complex. Extracts derived from untreated or ActD-treated cells were therefore analyzed by immunoprecipitation using antibodies that recognized hU3-55K, tUTP10, PWP2, UTP12, MPP10, and BMS1. The coprecipitated RNAs were analyzed by Northern hybridization using a probe specific for the U3 snoRNA as described above. This revealed that anti-hU3-55K antibodies efficiently coprecipitated the U3 snoRNA from extracts derived from untreated and ActD-treated cells (Fig. 3A). Antibodies that recognize tUTP10, PWP2, UTP12, MPP10, and BMS1 coprecipitated significant levels of the U3 snoRNA from the control cell extracts (Fig. 3A). In contrast, these antibodies coprecipitated little or no U3 snoRNA from the ActD-treated cell extracts. These key SSU processome components were therefore not associated with the 50S U3 snoRNP.
FIG. 3.
Characterization of the 50S U3 snoRNP complex. (A and B) Extracts from ActD-treated (right panels) or control cells (left panels) were analyzed by immunoprecipitation. Copurifying RNAs were isolated and analyzed by either Northern blot hybridization using a U3-specific probe (A) or S1 mapping using a probe spanning the A′ cleavage site (B). The antibody used is indicated at the top of each lane (α). The position of the S1 nuclease signal corresponding to the uncleaved (U) and A′-processed (P) pre-rRNA is indicated to the right of the panel. (C) Cells transfected with siRNAs targeting RPA135 or firefly luciferase (Control) were analyzed by immunoprecipitation and the associated RNAs analyzed by Northern blot hybridization using a U3-specific probe. The antibody used is indicated at the top of each lane. Input, 10% of the starting material; Beads, protein A-Sepharose in the absence of antibody.
We next analyzed the association of SSU processome components with the U3 snoRNP in cells depleted of the RNA polymerase I subunit RPA135. Extracts from cells electroporated with either the control duplex or an siRNA designed to deplete RPA135 were analyzed by immunoprecipitation and copurifying U3 snoRNA levels determined by Northern hybridization as described above. This revealed a 5- to 10-fold reduction in the association of BMS1, MPP10, UTP12, and tUTP10 with the U3 snoRNA in extracts derived from RPA135-depleted cells compared to those isolated from control cells (Fig. 3C). This implies that the 50S complexes present in cells where transcription has been blocked by either ActD or the depletion of RNA polymerase I by RNAi are not associated with the major SSU processome subunits. In contrast, hU3-55K was associated with the U3 snoRNA present in extracts derived from both the control and RPA135-depleted cells.
The ActD treatment of cells results in a loss of full-length pre-rRNA. This inhibitor primarily affects transcription elongation, and short partial pre-rRNA transcripts are produced in ActD-treated cells (16). We were therefore interested in whether these shorter transcripts were associated with SSU processome components. The RNAs isolated from the control and ActD-treated cells were analyzed by S1 mapping using a probe spanning the A′ cleavage site. This probe detected both the shorter transcripts in ActD-treated cells and the longer pre-rRNAs present in the control cells. An analysis of the RNAs coprecipitated from the control cell extracts revealed that hU3-55K, MPP10, PWP2, and UTP12 were associated with both the unprocessed and core 5′ETS sequences processed at A′ at levels significantly higher than those seen with protein A Sepharose beads alone (Fig. 3B). This suggests that the majority of the SSU processome components bind before the initial A′ cleavage and remain associated for the later processing steps at sites A1 and A2. Anti-tUTP10 antibodies preferentially coprecipitated the unprocessed pre-rRNA from the control cell extracts. In contrast, antibodies recognizing the GTPase BMS1 preferentially copurified the pre-rRNA cleaved at A′ but not the unprocessed pre-rRNA. Taken together, the data suggest a dynamic involvement of key factors with the SSU processome at different stages during ribosome biogenesis.
We next analyzed the association of SSU processome components with the short pre-rRNAs present in extracts derived from ActD-treated cells. Anti-MPP10, -hU3-55K, and -tUTP10 antibodies coprecipitated only background levels of the short pre-rRNAs (Fig. 3B, compare lanes 11, 12, 15, and 16 to lane 10). In contrast, antibodies that recognize UTP12, and to a lesser extent PWP2, coprecipitated both species of pre-rRNA present in the extracts (Fig. 3B, lane 14). This indicated that the two bUTP proteins were associated with the short pre-rRNAs independently of the U3 snoRNP, tUTP, and MPP10 subcomplexes. Interestingly, some cleavage at A′ occurs in the shorter transcripts even without the stable formation of the SSU processome (Fig. 3B; compare lanes 1 and 9). The proportion of the pre-rRNA cleaved at A′ in the ActD-treated cells was often significantly lower than that seen for the pre-rRNAs present in the control cells, suggesting the less-efficient processing of the shorter pre-rRNAs.
The 50S U3 snoRNP is found in the FC and the DFC in the nucleolus.
The U3 snoRNP is found throughout the nucleolus, and during ribosome biogenesis, this complex moves from the DFC to the GC as part of the SSU processome (8, 15). In contrast, the majority of snoRNPs localize to, and function in, the DFC. If, as our data suggest, the 50S U3 snoRNP is an SSU processome assembly intermediate, the complex would be expected to primarily localize to the DFC, the site of SSU processome formation. HeLa cells were electroporated with either the control siRNA or an siRNA targeting RPA135 and then grown on coverslips for 60 h. The transfected cells were analyzed by fluorescent in situ hybridization using probes specific for the U3 and U8 snoRNAs. The U8 snoRNA is naturally found in the FC and DFC, where it participates in the early stages of 28S and 5.8S processing. In the control cells, the U3 snoRNA localized throughout the nucleolus, while U8 was restricted to the DFC and FC. In cells depleted of RPA135, the U3 snoRNA was no longer found in the GC and colocalized with the U8 snoRNA in the FC and DFC (Fig. 4A). This therefore implies that as previously predicted (8, 15), the U3 snoRNP requires active ribosome biogenesis to localize to the GC. Since approximately half of the U3 snoRNA is present in the 50S complex in cells depleted of RPA135, we can also conclude that this novel U3 snoRNP complex is also present in the DFC and FC, where, as an SSU processome assembly intermediate, it would be ready to dock onto newly transcribed pre-rRNA. Importantly, the only noticeable difference observed between cells treated with the control and RPA135 siRNAs was the fact that the nucleoli appeared more regular and rounder upon the loss of the RNA polymerase I subunit. Therefore, this approach has enabled the characterization of nucleoli in which transcription has been inhibited, or severely reduced, without the dramatic effects that ActD has on nucleolar structure. Interestingly, a small fraction of the cells did show a cap-like organization of the nucleolus reminiscent of that seen with ActD treatment (Fig. 4B). The number of cells showing nucleolar disruption increased with longer incubation (>72 h) after transfection with the siRNA duplexes.
FIG. 4.
Subnucleolar localization of the 50S U3 snoRNP. HeLa cells were transfected with either control siRNAs or siRNAs targeting RPA135 (ΔRPA135) (as indicated above each set of images) and then analyzed by either fluorescent in situ hybridization using probes specific for the U3 and/or U8 snoRNAs (A and B) or immunofluorescence using protein-specific antibodies (C). The localization of the individual RNAs or proteins is shown in the left two panels of each set. A color overlay of the two images, with DAPI staining (blue), is shown in the right panels (merge). In the merged images, fibrillarin (Fib) and U8 are colored red, and U3 and the other proteins are shown in green. The identity of the protein/RNA detected is indicated in each panel. Images of nucleoli from a single cell are shown. Nucl, nucleolin.
We were next interested in investigating whether other SSU processome components localize to the DFC and FC in cells depleted of RPA135. The knockdown and control cells were analyzed by immunofluorescence using antibodies specific for MPP10, BMS1, UTP13, and nucleolin. In each case, anti-fibrillarin antibodies were used as a comparison and to highlight the DFC. In the control cells, BMS1, UTP13, nucleolin, and MPP10 were found throughout the nucleolus (Fig. 4C). BMS1 and MPP10 appeared to be more concentrated in the GC in many cells (data not shown) as reported previously for GFP-tagged MPP10 (25). The localization profiles of these two proteins did not significantly alter when RPA135 was depleted. Note the round nucleoli in the knockdown cells indicative of RPA135 depletion. After the depletion of RPA135, the bUTP protein UTP13 was still found throughout the nucleolus, although the levels in the GC appeared reduced with the protein concentrated in the DFC, and a significant proportion of the signal was seen in the nucleoplasm. The depletion of RPA135 resulted in the localization of a significant proportion of nucleolin in the nucleoplasm with the remaining nucleolar signal present at the outer rim of this nuclear body. Taken together, the data indicate that the localization profiles of BMS1 and MPP10 were not dependent on active ribosome biogenesis and naturally localized to the GC. In contrast, the localization of the U3 snoRNA, nucleolin, and, to a lesser extent, UTP13 were altered when RNA polymerase I activity was blocked.
Association of nucleolin, RRP5, and DBP4 with the 50S U3 snoRNP.
Our data indicate that the 50S U3 snoRNP is an SSU processome assembly intermediate that is recruited to the newly transcribed pre-rRNA in the DFC. We were therefore interested in identifying other proteins associated with the 50S complex. Nucleolin is an RNA-binding protein shown to function in U3 snoRNP recruitment to the pre-rRNA, and presumably SSU processome formation, and is therefore a candidate component of the 50S complex (10). Immunoprecipitation experiments revealed that antinucleolin antibodies weakly coprecipitated U3 snoRNA from control cell extracts. The levels of U3 snoRNA precipitated by the antinucleolin antibodies was always higher than that seen for the control (beads alone) but always significantly lower than that seen for anti-hU3-55K (Fig. 5A). A 5- to 10-fold increase in the coprecipitation of the U3 snoRNA by antinucleolin antibodies was seen in extracts derived from ActD-treated cells compared to that in the control cell extracts. We next analyzed whether nucleolin was associated with the U3 monoparticle or the larger complexes. Extracts from the control or ActD-treated cells were separated by glycerol gradient centrifugation, the peaks corresponding to the small 12S and large (80S or 50S) U3 complexes pooled and immunoprecipitated with anti-nucleolin and anti-hU3-55K antibodies. Northern blot analysis revealed that nucleolin was associated with the SSU processome/80S complex but not the 12S U3 monoparticle in the control cells (Fig. 5B). However, the weak level of coprecipitation observed with antinucleolin antibodies suggests that this protein was either only weakly associated or simply bound to a small subfraction of the larger U3 complex. Antinucleolin antibodies clearly coprecipitated the 50S U3 snoRNP, but not the 12S complex, in extracts derived from ActD-treated cells. This therefore indicated that nucleolin, a protein required for the efficient recruitment of the U3 snoRNP into the SSU processome, was a stable component of the 50S U3 snoRNP complex.
FIG. 5.
Association of nucleolin, RRP5, and DBP4 with the 50S U3 snoRNP. (A) Extracts prepared from control and ActD-treated cells (indicated on right) were analyzed by immunoprecipitation. Copurifying RNAs were isolated and analyzed by Northern blot hybridization using a U3-specific probe. (B) Extracts prepared from control and ActD-treated cells were separated by glycerol gradient centrifugation. The regions of the gradient corresponding to the 12S and 80S regions from control cell extracts and the 12S and 50S regions from ActD-treated cell extracts (indicated on the right) were analyzed by immunoprecipitation and U3 snoRNA levels analyzed by Northern blot hybridization. (C) Nucleolar extract was separated by glycerol gradient centrifugation, and the fractions corresponding to the 12S and 80S U3 complexes (as indicated on the right) were pooled and then immunoprecipitated using protein-specific antibodies. Copurifying RNAs were isolated and U3 snoRNA levels determined by Northern hybridization. (D and E) Extracts from ActD-treated or control cells (as indicated) were analyzed by immunoprecipitation. Copurifying RNAs were isolated and U3 snoRNA levels determined by Northern blot hybridization (D) or S1 mapping using a probe spanning the A′ cleavage site (E). The position of the S1 nuclease signal corresponding to the uncleaved (U) and A′-processed (P) pre-rRNA is indicated to the right of the panel. The antibody used is indicated at the top of each lane (α). Input, 10% of the starting material; Beads, protein A-Sepharose in the absence of antibody.
Having identified the RNA-binding protein nucleolin as a component of the 50S U3 snoRNP, we next screened for the association of other potential RNA-binding/interacting proteins. The RNA-binding proteins MRD1 and RRP5 have been linked to early pre-rRNA processing events and are associated with U3 snoRNP in S. cerevisiae (reviewed in reference 17). The RNA helicases DHR1 and HAS1 have been linked to U3 snoRNP recruitment and release, respectively, and DBP4 is involved in early pre-rRNA processing steps (reviewed in reference 17). As a comparison in these experiments, we also included an antibody that recognized the bUTP protein UTP13. Anti-UTP13, -MRD1, -RRP5, -HAS1, -DBP4, and -DHR1 antibodies efficiently coprecipitated the U3 snoRNA from gradient fractions containing the 80S U3 snoRNP but not the 12S U3 snoRNP (Fig. 5C). In contrast, and as seen earlier, antibodies that recognize hU3-55K efficiently coprecipitated the U3 snoRNP from both the 12S and 80S complexes. We next compared the association of these proteins with the U3 snoRNP in extracts derived from control and ActD-treated cells. Anti-MRD1, -HAS1, and -DHR1 antibodies coprecipitated the U3 snoRNA from control cell extracts but not from those derived from ActD-treated cells (Fig. 5D). In comparison to the control cell extracts, a fivefold-lower coprecipitation of the U3 snoRNA by anti-UTP13 antibodies was seen from ActD-treated cell extracts. A low-level coprecipitation of the U3 snoRNA in ActD-treated cell extracts was also seen using anti-PWP2 antibodies (Fig. 3A), suggesting either a weak association or that the bUTP proteins are bound to a subpopulation of the 50S complex. In contrast, antibodies that recognize hU3-55K, RRP5, and DBP4 coprecipitated the U3 snoRNA from both the control and ActD-treated cell extracts. This therefore implies that RRP5 and DBP4, but not MRD1, HAS1, and DHR1, are components of the 50S U3 snoRNP.
We next analyzed whether any of these proteins were associated with either the natural pre-rRNAs or the short, premature transcripts present in normal or ActD-treated cells, respectively. Extracts derived from the control and ActD-treated cells were immunoprecipitated as described above and the isolated RNAs analyzed by S1 mapping using a probe spanning the A′ cleavage site. This revealed that the anti-hU3-55K, -MRD1, -HAS1, -DHR1, -UTP13, -DBP4, and -RRP5 antibodies efficiently coprecipitated both the unprocessed and A′-processed pre-rRNAs from the control cell extracts (Fig. 5E). This confirms that these proteins are components of the SSU processome. An analysis of the RNAs coprecipitated from the extracts derived from ActD-treated cells revealed that MRD1, HAS1, RRP5, and DBP4 were not associated with the short pre-rRNA transcripts present in these cells. In contrast, anti-UTP13 antibodies, like the antibodies raised against the other bUTP proteins, coprecipitated both species of pre-rRNA.
The association of nucleolin, DBP4, and RRP5 with the 50S U3 snoRNP suggested that these proteins would be present in 50S complexes in ActD-treated cells. To test this, we separated extracts from the control and ActD-treated cells by glycerol gradient centrifugation and then analyzed the fractions by Western blotting using antibodies specific for hU3-55K, RRP5, DBP4, and nucleolin. The U3 snoRNA was also extracted from the individual fractions and analyzed as described above, to serve as a marker for the 12S, 50S, and 80S U3 snoRNP complexes. Western blot analysis revealed that hU3-55K peaked in 12S (Fig. 6A, fractions 2 to 5) and 80S (fractions 11 to 20) complexes in a profile almost identical to that seen for the U3 snoRNA. In extracts derived from the ActD-treated cells, hU3-55K was present in 12S (Fig. 6B, fractions 2 to 5) and 50S (fractions 11 to 15) complexes. RRP5 was present in a 40S peak (Fig. 6A; fractions 10 to 12) in the control cell extracts, although a weaker complex was present in a 20S (fractions 5 to 7) complex and some RRP5 was seen in the 80S region of the gradient. After treatment with ActD, RRP5 was primarily found in a 50S complex. DBP4 was present in 20S (Fig. 6A, fractions 5 to 7), 40S (fractions 10 to 12), and 80S complexes in the control cell extracts. After the addition of ActD, DBP4 was found in a 30S (fractions 6 to 8) and a 50S complex. Nucleolin was seen throughout the gradient but was primarily present in a 40S complex in the control cell extracts. After the treatment with ActD, nucleolin was mainly found at the top of the gradient, although some was still present in the 50S region. The data shows that hU3-55K, nucleolin, DBP4, and RRP5 comigrate with the 50S U3 snoRNP in a glycerol gradient after ActD treatment. It was clear, however, from the Western analysis that with the exception of hU3-55K, only a fraction of each of these proteins was present in U3 snoRNP-containing complexes. Indeed, based on relative abundance, only a small percentage of nucleolin (up to 10% of the nucleolar mass) can be associated with the U3 snoRNP (∼200,000 copies/cell).
FIG. 6.
Gradient analysis of RRP5, nucleolin, and DBP4. Extracts were prepared from control HeLa cells (A) or cells treated with ActD (B) and then separated by glycerol gradient centrifugation. RNA was isolated from each fraction and analyzed by Northern hybridization using a U3 snoRNA-specific probe (top panel). Proteins were separated by sodium dodecyl sulfate-PAGE and analyzed by Western blotting (bottom four panels). The top and bottom of the gradients are indicated. The positions of the 12S, 50S, and 80S peaks are indicated. The protein detected is indicated to the right of each panel. Where necessary, an arrow marks the specific signal.
DISCUSSION
In this work, we have used antibodies against key human SSU processome components to learn more about 18S pre-rRNA processing in higher eukaryotes. We found that components of the tUTP, bUTP, MPP10, and BMS1/RCL1 subcomplexes, as well as MRD1, RRP5, DBP4, HAS1 and DHR1 were all associated with the U3 snoRNA only as part of the SSU processome in normal cells. Components of the bUTP and MPP10 complexes, MRD1, RRP5, DHR1, HAS1, DBP4, and the U3 snoRNP were associated with both uncleaved and A′-processed pre-rRNA. This suggests that these components are involved in the early and later stages of pre-rRNA processing when the 5′ETS core is removed. The early and late stages of 5′ETS removal take place in the DFC and GC, respectively (28). These proteins/complexes were present throughout the nucleolus consistent with their involvement in both phases of processing. The tUTP proteins were only associated with pre-rRNA that had yet to be cleaved at A′, suggesting that they dissociate prior to or during this initial cleavage. Consistent with their involvement in just the early processing steps, the tUTP proteins were previously shown to be preferentially found in the DFC and FC (30). Conversely, BMS1 was predominantly associated with pre-rRNA cleaved at A′, suggesting it associated after this cleavage event. This protein was found throughout the nucleolus and in some cells appeared biased toward the GC (data not shown). Taken together, our data suggest a dynamic restructuring of the SSU processome relative to the cleavage at A′. This may either reflect the removal of the 5′ETS leader sequence and/or the change in subnucleolar localization. Interestingly, in S. cerevisiae the tUTP proteins remain associated with the pre-rRNA after the initial A0 cleavage (1, 3, 7). Even though many of the SSU processome components are evolutionarily highly conserved, the initial cleavage in yeast (A0) and humans (A′) occurs in different regions of the 5′ETS (17). It is therefore possible that differences in protein binding and release reflect that different sites are used for the initial cleavage in the two systems.
We found that depleting RNA polymerase I subunit RPA135 did not, at 60 h after transfection, result in major structural changes to the nucleolus. Interestingly, after longer time points (e.g., 72 or 90 h) the nucleoli showed signs of nucleolar disruption, suggesting that this change in nucleolar morphology is due to a block in RNA polymerase I activity (Fig. 4B). The use of the 60-h time point enabled the analysis of the localization of rRNA processing factors when ribosome biogenesis was significantly reduced. In the absence of RPA135, there was a significant reduction in U3 snoRNA levels in the GC. This is consistent with the notion that the localization of the U3 snoRNA to the GC is dependent on active ribosome biogenesis (8, 15). The effect of RPA135 depletion on UTP13 localization was not as severe, and MPP10 and BMS1 were not affected. This suggested that not all SSU processome components shuttle between the DFC and GC during ribosome biogenesis and that some factors naturally localize to the GC. Each of these factors could, however, be associated with other complexes in the nucleolus and therefore localize to the GC independently of ribosome biogenesis.
The SSU processome is predicted to assemble cotranscriptionally onto the nascent pre-rRNA. We found that blocking transcription, using two different approaches, resulted in the accumulation of a novel 50S U3 snoRNP. This complex also accumulated when the tUTP proteins tUTP10 and tUTP4 were depleted using RNAi. The loss of either of the tUTP proteins resulted in a severe block in 18S pre-rRNA processing (30). This therefore implies that the tUTP proteins are important for the recruitment of the 50S U3 snoRNP into the SSU processome. The two tUTP proteins differ in their importance for U3 recruitment to the SSU processome and pre-rRNA transcription (30). This may reflect the importance of the individual proteins in the function of the tUTP complex.
The 50S U3 snoRNP is a large complex that will contain significantly more components than those identified in this work. This may include components, such as nucleolin, that are not stably associated with the SSU processome and that may function specifically in U3 snoRNP recruitment into the processing complex. The novel U3 snoRNP accumulated in the absence of pre-rRNA transcription and when the tUTP proteins, factors involved early in SSU processome function and linked to U3 recruitment, were depleted. The 50S U3 snoRNP was also associated with key SSU processome components (RRP5 and DBP4) and the fact that it accumulates in the DFC and FC, where the SSU processome is assembled. We cannot completely rule out the possibility that the novel U3 snoRNP is a breakdown/recycling product. The association of the 50S complex with nucleolin, a protein predicted to be involved in U3 recruitment and therefore SSU processome formation, strongly suggests that this novel U3 snoRNP is an SSU processome assembly intermediate. There appears to be little detectable 50S U3 snoRNP in normal cells, suggesting that this complex may be a rate-limiting or regulatory step in the rate of pre-rRNA processing. It is possible that the cell controls pre-rRNA processing by regulating the amount of U3 snoRNP in the larger complex.
Nucleolin, the most abundant nucleolar protein, is involved in many processes, and an excess of this protein blocks pre-rRNA processing in Xenopus sp. oocytes (32). Therefore, the association of nucleolin with the U3 snoRNP may help regulate the interaction of this protein with the pre-rRNA. Furthermore, nucleolin likely functions to dock the 50S complex onto the pre-rRNA and the U3 snoRNA sequences that base pair with the 5′ETS, which are not required for SSU processome formation and serve to fine tune the organization of the processing machinery and to mark the A′ processing site. The docking of the 50S complex onto the pre-rRNA is likely also assisted by the RNA-binding protein RRP5. Indeed, this protein was recently shown to be essential for 18S rRNA production in human cells (35). DBP4 is linked to the release/unwinding of a subset of snoRNAs, most notably the U14 snoRNA (22, 27), and could also be involved in the unwinding of pre-rRNA sequences to allow modification and processing. A large U3 complex has been described in plants that contains nucleolin, the U14 snoRNA, and RNA polymerase I (34). Furthermore, a transcription and processing complex was found in yeast that contained SSU processome factors and RNA polymerase I proteins (5). We did not find the transcription factor UBF nor the U14, U17 and U8 snoRNAs in the 50S U3 snoRNP (data not shown), suggesting that the human 50S U3 snoRNP is distinct from the plant and yeast complexes.
Several of the factors are predicted to bind in and around the ECM in the pre-rRNA (Fig. 7). This includes the U3 snoRNP, nucleolin, the MPP10 subcomplex, and the A′ endonuclease. Nucleolin is a 70-kDa protein with four RRM motifs and would be predicted to cover the A′ processing site and/or the U3 snoRNA base-paring sequence (11). The MPP10 complex binds the “hinge” region of the U3 snoRNA, probably when this is base paired to the pre-rRNA (9, 15, 41). The close proximity of the nucleolin-, U3 snoRNP-, and MPP10-binding sites on the 5′ETS suggests that some of these interactions with the pre-rRNA may be mutually exclusive. Indeed, the low levels of nucleolin associated with the SSU processome suggests that this protein is only transiently associated with this complex. We propose that nucleolin functions to dock the 50S U3 snoRNP complex onto the pre-rRNA. Once the SSU processome is formed, nucleolin is released, thus enabling the binding of other factors, such as the A′ endonuclease, to the ECM.
FIG. 7.
Schematic representation of factors associated with the ECM in the 5′ETS. The 5′ETS including the A′ cleavage site (arrows, as determined in reference 21), with nucleolin, the U3 snoRNP, and the MPP10 complex, is represented schematically. The sequence of the A′ cleavage sites, nucleolin binding site (white text on a black background), and two U3 snoRNA base-pairing sequences is shown. The remaining sequence is represented by a black line. The U3 snoRNA is represented by a black line with the hinge sequences, which base pair to the 5′ETS, presented. The MPP10 complex, nucleolin, and the U3 snoRNP protein component are denoted by the blue, red, and gray shapes, respectively.
Acknowledgments
We thank Ger Pruijn and Michael Pollard for the generous gift of antibodies. Thanks are also due to Claudia Schneider, Jeremy Brown, and Sander Granneman for critically reading the manuscript.
This work was supported by the MRC and the Royal Society.
Footnotes
Published ahead of print on 30 March 2009.
REFERENCES
- 1.Bernstein, K. A., J. E. Gallagher, B. M. Mitchell, S. Granneman, and S. J. Baserga. 2004. The small-subunit processome is a ribosome assembly intermediate. Eukaryot. Cell 31619-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Billy, E., T. Wegierski, F. Nasr, and W. Filipowicz. 2000. Rcl1p, the yeast protein similar to the RNA 3′-phosphate cyclase, associates with U3 snoRNP and is required for 18S rRNA biogenesis. EMBO J. 192115-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dez, C., M. Dlakic, and D. Tollervey. 2007. Roles of the HEAT repeat proteins Utp10 and Utp20 in 40S ribosome maturation. RNA 131516-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elbashir, S. M., J. Harborth, K. Weber, and T. Tuschl. 2002. Analysis of gene function in somatic mammalian cells using small interfering RNAs. Methods 26199-213. [DOI] [PubMed] [Google Scholar]
- 5.Fath, S., P. Milkereit, A. V. Podtelejnikov, N. Bischler, P. Schultz, M. Bier, M. Mann, and H. Tschochner. 2000. Association of yeast RNA polymerase I with a nucleolar substructure active in rRNA synthesis and processing. J. Cell Biol. 149575-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fatica, A., and D. Tollervey. 2002. Making ribosomes. Curr. Opin. Cell Biol. 14313-318. [DOI] [PubMed] [Google Scholar]
- 7.Gallagher, J. E., D. A. Dunbar, S. Granneman, B. M. Mitchell, Y. Osheim, A. L. Beyer, and S. J. Baserga. 2004. RNA polymerase I transcription and pre-rRNA processing are linked by specific SSU processome components. Genes Dev. 182506-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerbi, S. A., and A. Borovjagin. 1997. U3 snoRNA may recycle through different compartments of the nucleolus. Chromosoma 105401-406. [DOI] [PubMed] [Google Scholar]
- 9.Gerczei, T., and C. C. Correll. 2004. Imp3p and Imp4p mediate formation of essential U3-precursor rRNA (pre-rRNA) duplexes, possibly to recruit the small subunit processome to the pre-rRNA. Proc. Natl. Acad. Sci. USA 10115301-15306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ginisty, H., F. Amalric, and P. Bouvet. 1998. Nucleolin functions in the first step of ribosomal RNA processing. EMBO J. 171476-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ginisty, H., G. Serin, L. Ghisolfi-Nieto, B. Roger, V. Libante, F. Amalric, and P. Bouvet. 2000. Interaction of nucleolin with an evolutionarily conserved pre-ribosomal RNA sequence is required for the assembly of the primary processing complex. J. Biol. Chem. 27518845-18850. [DOI] [PubMed] [Google Scholar]
- 12.Granneman, S., and S. J. Baserga. 2004. Ribosome biogenesis: of knobs and RNA processing. Exp. Cell Res. 29643-50. [DOI] [PubMed] [Google Scholar]
- 13.Granneman, S., J. E. Gallagher, J. Vogelzangs, W. Horstman, W. J. van Venrooij, S. J. Baserga, and G. J. Pruijn. 2003. The human Imp3 and Imp4 proteins form a ternary complex with hMpp10, which only interacts with the U3 snoRNA in 60-80S ribonucleoprotein complexes. Nucleic Acids Res. 311877-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Granneman, S., G. J. Pruijn, W. Horstman, W. J. van Venrooij, R. Lührmann, and N. J. Watkins. 2002. The hU3-55K protein requires 15.5K binding to the box B/C motif as well as flanking RNA elements for its association with the U3 small nucleolar RNA in vitro. J. Biol. Chem. 27748490-48500. [DOI] [PubMed] [Google Scholar]
- 15.Granneman, S., J. Vogelzangs, R. Lührmann, W. J. van Venrooij, G. J. Pruijn, and N. J. Watkins. 2004. Role of pre-rRNA base pairing and 80S complex formation in subnucleolar localization of the U3 snoRNP. Mol. Cell. Biol. 248600-8610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadjiolova, K. V., A. A. Hadjiolov, and J. P. Bachellerie. 1995. Actinomycin D stimulates the transcription of rRNA minigenes transfected into mouse cells. Implications for the in vivo hypersensitivity of rRNA gene transcription. Eur. J. Biochem. 228605-615. [DOI] [PubMed] [Google Scholar]
- 17.Henras, A. K., J. Soudet, M. Gerus, S. Lebaron, M. Caizergues-Ferrer, A. Mougin, and Y. Henry. 2008. The post-transcriptional steps of eukaryotic ribosome biogenesis. Cell. Mol. Life Sci. 652334-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez-Verdun, D. 2006. The nucleolus: a model for the organization of nuclear functions. Histochem. Cell Biol. 126135-148. [DOI] [PubMed] [Google Scholar]
- 19.Karbstein, K., and J. A. Doudna. 2006. GTP-dependent formation of a ribonucleoprotein subcomplex required for ribosome biogenesis. J. Mol. Biol. 356432-443. [DOI] [PubMed] [Google Scholar]
- 20.Karbstein, K., S. Jonas, and J. A. Doudna. 2005. An essential GTPase promotes assembly of preribosomal RNA processing complexes. Mol. Cell 20633-643. [DOI] [PubMed] [Google Scholar]
- 21.Kass, S., N. Craig, and B. Sollner-Webb. 1987. Primary processing of mammalian rRNA involves two adjacent cleavages and is not species specific. Mol. Cell. Biol. 72891-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kos, M., and D. Tollervey. 2005. The putative RNA helicase Dbp4p is required for release of the U14 snoRNA from preribosomes in Saccharomyces cerevisiae. Mol. Cell 2053-64. [DOI] [PubMed] [Google Scholar]
- 23.Krogan, N. J., W. T. Peng, G. Cagney, M. D. Robinson, R. Haw, G. Zhong, X. Guo, X. Zhang, V. Canadien, D. P. Richards, B. K. Beattie, A. Lalev, W. Zhang, A. P. Davierwala, S. Mnaimneh, A. Starostine, A. P. Tikuisis, J. Grigull, N. Datta, J. E. Bray, T. R. Hughes, A. Emili, and J. F. Greenblatt. 2004. High-definition macromolecular composition of yeast RNA-processing complexes. Mol. Cell 13225-239. [DOI] [PubMed] [Google Scholar]
- 24.Lazdins, I. B., M. Delannoy, and B. Sollner-Webb. 1997. Analysis of nucleolar transcription and processing domains and pre-rRNA movements by in situ hybridization. Chromosoma 105481-495. [DOI] [PubMed] [Google Scholar]
- 25.Leary, D. J., M. P. Terns, and S. Huang. 2004. Components of U3 snoRNA-containing complexes shuttle between nuclei and the cytoplasm and differentially localize in nucleoli: implications for assembly and function. Mol. Biol. Cell 15281-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lemm, I., C. Girard, A. N. Kuhn, N. J. Watkins, M. Schneider, R. Bordonne, and R. Luhrmann. 2006. Ongoing U snRNP biogenesis is required for the integrity of Cajal bodies. Mol. Biol. Cell 173221-3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liang, W. Q., J. A. Clark, and M. J. Fournier. 1997. The rRNA-processing function of the yeast U14 small nucleolar RNA can be rescued by a conserved RNA helicase-like protein. Mol. Cell. Biol. 174124-4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olson, M. O., and M. Dundr. 2005. The moving parts of the nucleolus. Histochem. Cell Biol. 123203-216. [DOI] [PubMed] [Google Scholar]
- 29.Perez-Fernandez, J., A. Roman, J. De Las Rivas, X. R. Bustelo, and M. Dosil. 2007. The 90S preribosome is a multimodular structure that is assembled through a hierarchical mechanism. Mol. Cell. Biol. 275414-5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prieto, J. L., and B. McStay. 2007. Recruitment of factors linking transcription and processing of pre-rRNA to NOR chromatin is UBF-dependent and occurs independent of transcription in human cells. Genes Dev. 212041-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raska, I., P. J. Shaw, and D. Cmarko. 2006. Structure and function of the nucleolus in the spotlight. Curr. Opin. Cell Biol. 18325-334. [DOI] [PubMed] [Google Scholar]
- 32.Roger, B., A. Moisand, F. Amalric, and P. Bouvet. 2003. Nucleolin provides a link between RNA polymerase I transcription and pre-ribosome assembly. Chromosoma 111399-407. [DOI] [PubMed] [Google Scholar]
- 33.Ruggero, D., and P. P. Pandolfi. 2003. Does the ribosome translate cancer? Nat. Rev. Cancer 3179-192. [DOI] [PubMed] [Google Scholar]
- 34.Saez-Vasquez, J., D. Caparros-Ruiz, F. Barneche, and M. Echeverria. 2004. A plant snoRNP complex containing snoRNAs, fibrillarin, and nucleolin-like proteins is competent for both rRNA gene binding and pre-rRNA processing in vitro. Mol. Cell. Biol. 247284-7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sweet, T., W. Yen, K. Khalili, and S. Amini. 2008. Evidence for involvement of NFBP in processing of ribosomal RNA. J. Cell. Physiol. 214381-388. [DOI] [PubMed] [Google Scholar]
- 36.Warner, J. R. 1999. The economics of ribosome biosynthesis in yeast. Trends Biochem. Sci. 24437-440. [DOI] [PubMed] [Google Scholar]
- 37.Watkins, N. J., I. Lemm, D. Ingelfinger, C. Schneider, M. Hossbach, H. Urlaub, and R. Lührmann. 2004. Assembly and maturation of the U3 snoRNP in the nucleoplasm in a large dynamic multiprotein complex. Mol. Cell 16789-798. [DOI] [PubMed] [Google Scholar]
- 38.Watkins, N. J., V. Segault, B. Charpentier, S. Nottrott, P. Fabrizio, A. Bachi, M. Wilm, M. Rosbash, C. Branlant, and R. Lührmann. 2000. A common core RNP structure shared between the small nucleolar box C/D RNPs and the spliceosomal U4 snRNP. Cell 103457-466. [DOI] [PubMed] [Google Scholar]
- 39.Wegierski, T., E. Billy, F. Nasr, and W. Filipowicz. 2001. Bms1p, a G-domain-containing protein, associates with Rcl1p and is required for 18S rRNA biogenesis in yeast. RNA 71254-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wehner, K. A., J. E. Gallagher, and S. J. Baserga. 2002. Components of an interdependent unit within the SSU processome regulate and mediate its activity. Mol. Cell. Biol. 227258-7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wormsley, S., D. A. Samarsky, M. J. Fournier, and S. J. Baserga. 2001. An unexpected, conserved element of the U3 snoRNA is required for Mpp10p association. RNA 7904-919. [DOI] [PMC free article] [PubMed] [Google Scholar]