Abstract
Upon prolonged arrest in mitosis, cells undergo adaptation and exit mitosis without cell division. These tetraploid cells are either eliminated by apoptosis or arrested in the subsequent G1 phase in a spindle checkpoint- and p53-dependent manner. p53 has long been known to be activated by spindle poisons, such as nocodazole and Taxol, although the underlying mechanism remains elusive. Here we present evidence that stabilization and activation of p53 by spindle disruption requires the spindle checkpoint kinase TTK/hMps1. TTK/hMps1 phoshorylates the N-terminal domain of p53 at Thr18, and this phosphorylation disrupts the interaction with MDM2 and abrogates MDM2-mediated p53 ubiquitination. Phosphorylation at Thr18 enhances p53-dependent activation of not only p21 but also Lats2, two mediators of the postmitotic checkpoint. Furthermore, a phospho-mimicking substitution at Thr18 (T18D) is more competent than the phospho-deficient mutant (T18A) in rescuing the tetraploid checkpoint defect of p53-depleted cells. Our findings therefore provide a mechanism connecting the spindle checkpoint with p53 in the maintenance of genome stability.
The tumor suppressor protein p53 is frequently mutated in cancers (21). p53 is a transcription factor composed of an N-terminal activation domain, a central DNA binding core, and a C-terminal tetramerization domain followed by a regulatory basic region (33). In response to stress, p53 is stabilized and activated to modulate expression of target genes, such as the p21 gene, which is required for stress-induced cell cycle arrest, and the bax and puma genes, which participate in apoptosis (10, 26).
The stability and activity of p53 are regulated by posttranslational modifications including phosphorylation and acetylation (22, 30). p53 can be phosphorylated by multiple kinases, as in phosphorylation at Ser15 by ATM/ATR, at Thr18 by casein kinase I, and at Ser20 by CHK1/CHK2. The protein levels of p53 are controlled in part by MDM2. MDM2 binds to the N-terminal domain of p53 and promotes p53 ubiquitination and degradation by its E3 ubiquitin ligase activity (13). Phosphorylation within the N-terminal MDM2-binding domain of p53 at Thr18 and Ser20 is involved in the modulation of p53 stability, most likely by interfering with the interaction between p53 and MDM2 (14, 30).
During mitosis, the spindle assembly checkpoint inhibits anaphase onset until all sister chromatids are properly attached to the mitotic spindle and aligned at the metaphase plate (20). Numerous proteins are involved in this checkpoint, including Bub1, BubR1, Bub3, MAD1, MAD2, and TTK/hMps1. Upon treatment with spindle-damaging agents, such as nocodazole or Taxol, cells are arrested at preanaphase because of activation of the spindle checkpoint. Cells ultimately exit mitosis upon prolonged arrest and enter G1 without sister chromatid segregation and cytokinesis, a process known as mitotic slippage or adaptation (17, 25). In p53-competent cells, these tetraploid cells either arrest in G1 or undergo apoptosis by p53-dependent tetraploidy checkpoint activation. Cells lacking p53 continue to duplicate their DNA, resulting in polyploidization (12, 18). Thus, the activation of p53 prevents further DNA replication and polyploidy. Interestingly, an intact spindle assembly checkpoint is required for proper execution of the postmitotic checkpoint (32), although the underlying mechanistic details remain elusive.
p21, the p53-induced target, plays an important role in the p53-mediated G1 tetraploid checkpoint. Like the lack of functional p53, a deficiency of p21 leads to polyploidy after spindle disruption (12). Apart from p21, large tumor suppressor 2 (Lats2) has also been shown to be involved. p53 activates the expression of Lats2, which then binds and inactivates MDM2, therefore promoting the stabilization of p53 (3). Significantly, Lats2 is induced earlier than MDM2 or p21 after spindle disruption (3), suggesting different mechanisms are instigated for the expression of these genes. In support of this notion, distinct patterns of p53 phosphorylation were observed after DNA damage and after spindle damage (29). Although the ATM-CHK2 and ATR-CHK1 axes are known to mediate the DNA damage response upstream of p53, they do not appear to be activated by spindle disruption (32). Thus, the mechanism underlying p53 activation in response to spindle damage remains obscure. It has been suggested that an intact spindle assembly checkpoint is required for the G1 tetraploid checkpoint. A deficiency of the spindle checkpoint protein MAD1, MAD2, or BubR1 causes polyploidy after spindle damage (9, 32). It is tempting to speculate that the spindle checkpoint machinery may somehow regulate p53, although the connection remains to be elucidated.
TTK/hMps1 is a spindle checkpoint kinase essential for loading of the checkpoint complex on kinetochores (1, 16, 19, 31). The family of Mps1 proteins has been described for several species, such as Saccharomyces cerevisiae (Mps1p), the mouse (ESK/mMps1), Xenopus laevis (xMps1), and humans (TTK/PYT/hMps1) and is required for the spindle assembly checkpoint and centrosome duplication (36). In S. cerevisiae, Mps1p interacts with and phosphorylates Spc42p, an integral component of the spindle pole body, and assists the assembly of Spc42p in the spindle pole body (6). In addition, Mps1p phosphorylates Dam1, a kinetochore component, and may regulate coupling of kinetochores to microtubule plus ends (28). In humans, TTK/hMps1 has been shown to interact with and phosphorylate BLM at Ser144, and this phosphorylation appears to regulate chromosome stability (15). TTK/hMps1 may also be involved in a noncanonical Smad signaling pathway by phosphorylating R-Smads (Smad2 and Smad3) (37). Furthermore, TTK/hMps1 may participate in DNA damage checkpoints through the CHK2 signaling pathway (35).
In this study, we sought to address the link between the spindle assembly checkpoint and the p53-dependent postmitotic checkpoint, and we found that TTK/hMps1 phosphorylates p53 and may participate in the activation of p53 after spindle damage to prevent polyploidization.
MATERIALS AND METHODS
Cell culture conditions and treatments.
293T cells were maintained in Dulbecco's modified Eagle's medium (HyClone), LNCaP cells in RPMI-1640 medium (HyClone), and U2OS cells in McCoy's medium supplemented with 10% fetal bovine serum (Gibco), 100 U/ml of penicillin, and 100 μg/ml of streptomycin (Gibco). For ionizing radiation (IR) treatment, cells were exposed to 8 Gy of X-rays using the Torres 150D inspection system. Cells were treated with Taxol (100 nM), nocodazole (50 ng/ml), cycloheximide (20 μg/ml), or MG132 (25 μM) and then harvested as appropriate.
Generation of the stable Tet-on U2OS-shp53 line was achieved by sequential transfection of pcDNA6/TR (Invitrogen), which expresses the Tet repressor, and pBabe-shp53, which expresses a short hairpin RNA (shRNA) that targets p53 under the control of the Tet operator.
Plasmids and siRNA transfection.
Plasmids expressing hemagglutinin (HA)-tagged full-length p53 or ΔΝ96 (amino acids 1 to 96 deleted) or ΔC30 (amino acids 363 to 393 deleted) p53 were gifts from X. Chen (University of California, Davis). The p53 Thr18 phosphorylation site T18A (Thr changed to Ala) and T18D (Thr changed to Asp) mutants were generated by PCR-based site-directed mutagenesis and cloned into pcDNA3.1 (Invitrogen). To generate small interfering RNA (siRNA)-resistant p53-expressing constructs, conservative changes were made in the nucleotides targeted by p53 siRNA (5′-GACTCATCTGGTAATCTAC-3′; changes are underlined). Wild-type (WT) or kinase-defective (KD) (D647A) TTK was cloned into the BamHI and XhoI sites of the pXJ-HA vector (23) for mammalian expression. Plasmids were introduced into cells either by calcium phosphate (for 293T cells) or Lipofectamine 2000 (for other cells) transfection.
To construct the plasmid that expresses H2B-green fluorescent protein (GFP), H2B was amplified from LNCaP cDNA by PCR and was cloned into the HindIII and SalI sites of the pEGFP-N1 vector (Clontech).
For luciferase reporter assays, an oligonucleotide corresponding to p53RE-I in the Lats2 promoter (3) was cloned into the NheI and XhoI sites of the pGL3 promoter vector (Promega) to make the Lats2 reporter. The p21-min reporter carries a minimal p53 binding site derived from the p21 promoter. The p21 reporter, which contains a 2.4-kb p21 promoter cloned upstream of the luciferase cassette, was as described previously (7).
All siRNAs were synthesized and annealed by Qiagen. The sequences targeted by TTK, CHK2, BubR1, and p53 siRNA were 5′-TGAACAAAGTGAGAGACAT-3′ (TK1), 5′-AATGTGTGAATGACAACTACT-3′, 5′-CAATACTCTTCAGCAGCAG-3′, and 5′-GACTCCAGTGGTAATCTAC-3′, respectively. Transfection of siRNA was performed using calcium phosphate precipitation in 293T cells and Oligofectamine (Invitrogen) in other cell lines.
Cell lysis, immunoprecipitation, and immunoblotting.
Cells were lysed in TEGN buffer (10 mM Tris, pH 7.5, 1 mM EDTA, 420 mM NaCl, 10% glycerol, and 0.5% Nonidet P-40) containing 10 mM NaF, 10 mM β-glycerophosphate, 1 mM Na3VO4, and 1 mM dithiothreitol (DTT). For immunoprecipitation, the lysates were diluted with an equal volume of TEG buffer (10 mM Tris, pH 7.5, 1 mM EDTA, and 20% glycerol) and rocked with antibodies and 10 μl of 50% protein G beads (Pierce) for 2 h at 4°C. The immunoprecipitates were washed three times in 10S buffer (50 mM HEPES, pH 7.9, 250 mM NaCl, 0.2% NP-40, 0.1% Triton X-100, and 0.01% sodium dodecyl sulfate [SDS]), boiled in protein sample buffer (2 M β-mercaptoethanol, 12% SDS, 0.5 M Tris, pH 6.8, 0.5 mg/ml bromophenol blue, and 30% glycerol), and analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) followed by Western blotting. Antibodies used were the following: PAb1801, PAb421, TTK (sc540; Santa Cruz), CHK2 (K0087; MBL), BubR1 (K0169-3; MBL), phospho-p53 (Thr18) (no. 2529; Cell Signaling), phospho-p53 (Ser15) (P1001-27D; U.S. Biological), phospho-p53 (Ser20) (P1001-27G; U.S. Biological), MDM2 (sc965; Santa Cruz), p21 (OP79; Oncogene Science), actin (A2066; Sigma), HA (16B12; Covance), and His (sc8036; Santa Cruz).
Recombinant proteins.
His-tagged and glutathione S-transferase (GST) fusion proteins were expressed in Escherichia coli strain BL21(DE3) LysE and purified as described previously (24, 35).
GST pulldown assay.
Assays were performed as described previously (23) using the recombinant GST-TTK and His-p53 proteins.
In vitro kinase assay and immunoprecipitation/kinase assay.
For in vitro kinase assays, purified recombinant WT or KD GST-TTK fusion proteins were incubated with purified His-p53 in kinase buffer (20 mM Tris, pH 7.5, 10 mM MgCl2, 2 mM MnCl2, and 1 mM DTT) supplemented with 50 μM ATP and 3 μCi of [γ-32P]ATP at 30°C for 15 min. The reaction was stopped by the addition of a 0.5 volume of protein sample buffer, and the proteins were resolved by SDS-PAGE. The phosphorylation of p53 was visualized by autoradiography and Western blotting using specific anti-phospho-p53 antibodies.
For immunoprecipitation/kinase assays, 293T cells were lysed in CSK buffer [10 mM piperazine-N,N′bis(2-ethanesulfonic acid) (pH 7.0), 100 mM NaCl, 3 mM MgCl2, 300 mM sucrose, and 0.1% NP-40). TTK was immunoprecipitated from the cell lysates with anti-TTK (sc-540) and washed three times in CSK buffer, to which 0.7 M LiCl was added in the second wash. After two washes in kinase buffer, kinase reactions were carried out as described above using His-p53 as a substrate.
In vivo ubiquitination assays.
H1299 cells were cotransfected with TTK, p53, and His-tagged ubiquitin. Transfected cells were pretreated or not with MG132 and then harvested and sonicated in buffer A (6 M guanidine-HCl, 0.1 M Na2HPO4-NaH2PO4, 10 mM imidazole, pH 8.0). Cell lysates were rocked with 30 μl of 50% Ni beads (Qiagen) at room temperature for 1 h. The precipitated beads were washed once in buffer A, once in buffer TI (20 mM imidazole, 0.2% Triton X-100, 25 mM Tris-HCl, pH 6.8) mixed with buffer A (1:3), and three times in TI buffer. Proteins bound to beads were boiled in sample buffer and analyzed by SDS-PAGE followed by Western blotting.
Cell cycle analysis.
For flow cytometric analysis, cells were detached using trypsin and collected in culture medium by centrifugation. Cells were fixed in cold methanol and stored at −20°C for at least 2 h. Cells were rehydrated with cold phosphate-buffered saline and resuspended in phosphate-buffered saline containing 60 μg/ml propidium iodide (Sigma) and 50 μg/ml of RNase A. Samples were analyzed using a FACSCalibur flow cytometer (Becton Dickinson).
RESULTS
Downregulation of TTK/hMps1 by siRNA attenuates the p53 response induced by spindle disruption and DNA damage.
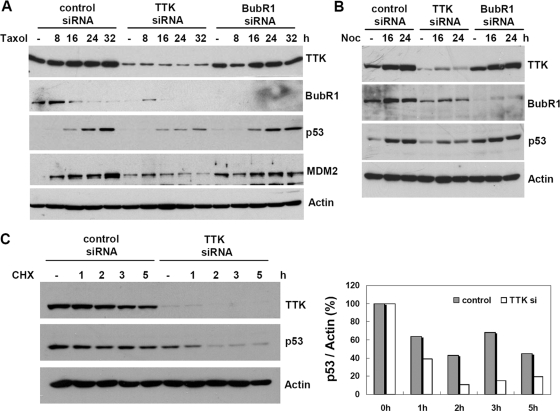
p53 is induced upon spindle disruption; however, the underlying mechanism remains elusive. Although ATM/ATR and CHK2/CHK1 are largely responsible for p53 responses after DNA damage, neither is required for p53 activation by spindle poisons, such as nocodazole and Taxol (32). Since spindle disruption activates the spindle checkpoint, we set out to investigate whether the spindle checkpoint kinase TTK was involved. To address this possibility, U2OS cells were transfected with TTK siRNA and, for comparison, siRNA targeting another spindle checkpoint kinase, BubR1. After treatment with Taxol, p53 induction was diminished significantly in the TTK knockdown cells (Fig. 1A). In contrast, downregulation of BubR1 had no apparent effect on the p53 response, demonstrating that specificity exists in addition to a dysfunctional spindle checkpoint. Similar results were obtained using nocodazole in the LNCaP cell line (Fig. 1B) or with another TTK-targeting siRNA (TK3) in U2OS cells (see Fig. S1A in the supplemental material). The diminished p53 response could be due to the markedly reduced p53 half-life in TTK-depleted, Taxol-treated cells (Fig. 1C). Notably, ionizing radiation-induced p53 responses including expression of the p53 downstream targets MDM2 and p21 were also decreased in TTK-ablated U2OS (see Fig. S1B in the supplemental material) and LNCaP (see Fig. S1C in the supplemental material) cells. These data suggest a larger role for TTK in p53 stress responses and point to the possibility that TTK may be a specific upstream activator in the response of p53 to spindle poisons.
FIG. 1.
TTK/hMps1 knockdown by siRNA mitigates p53 responses after Taxol or nocodazole treatment. U2OS cells (A) or LNCaP cells (B) were transfected with control, TTK, or BubR1 siRNA. The cells were treated with Taxol (100 nM) (A) or nocodazole (Noc) (50 ng/ml) (B), harvested at the indicated times, and analyzed by Western blotting for the indicated proteins. (C) The half-life of p53 was reduced in TTK/hMps1-downregulated cells treated with Taxol. Control or TTK siRNA-transfected U2OS cells were treated with Taxol for 20 h. Cycloheximide (CHX) (20 μg/ml) was then added, and cells were collected at the indicated times. The lysates were analyzed by Western blotting using the indicated antibodies. Levels of p53 were normalized to actin and are shown on the right.
TTK/hMps1 interacts directly with p53.
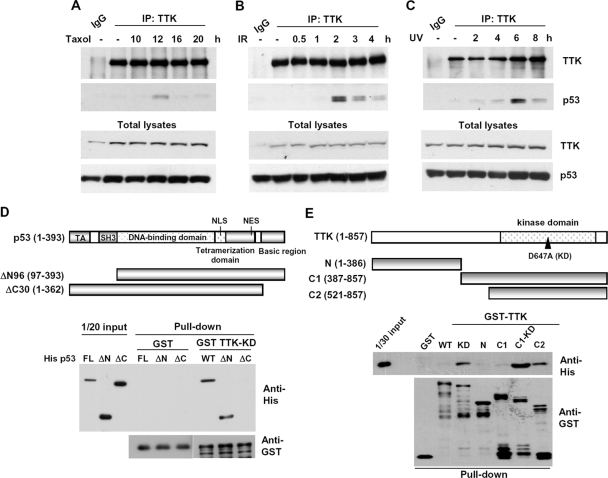
To determine whether TTK and p53 interact in vivo, endogenous TTK was immunoprecipitated from 293T cell lysates using an anti-TTK antibody. The results showed that the anti-TTK antibody brought down not only TTK but also p53 after spindle damage, which activates TTK (Fig. 2A). The interaction was similarly enhanced when cells were treated with IR or UV (Fig. 2B and C), suggesting that DNA damage may also modulate the interaction between these two proteins.
FIG. 2.
TTK/hMps1 interacts with p53 in vitro and in vivo after spindle disruption or DNA damage in 293T cells. 293T cells were treated with Taxol (100 nM) (A), IR (8 Gy) (B), or UV (30 J/m2) (C) and then collected at the indicated times. TTK was immunoprecipitated from these cells using an anti-TTK antibody, and p53 in the immune complex was detected by Western blotting using the anti-p53 antibody PAb1801. (D and E) p53 C terminus interacted directly with the kinase domain of TTK/hMps1 in vitro. Recombinant His-tagged p53 or the truncation mutants ΔN and ΔC (D) were mixed with GST-fused full-length or truncated TTK (E) in GST pulldown assays. Bound proteins were detected by Western blotting.
To determine whether the interaction was direct, in vitro GST pull-down assays were performed using bacterially produced GST-tagged TTK and His-p53. The results indicate that GST-TTK but not GST pulled down His-p53, suggesting that the two proteins could physically interact. To map the interacting domains in these two proteins, various truncation mutants were generated (Fig. 2D and E). The interacting domain in p53 was localized to the C-terminal 30 amino acids, since deletion of this region but not the N-terminal activation domain completely abolished the interaction (Fig. 2D). The p53-interacting domain in TTK resided in the C-terminal kinase domain; the N-terminal domain of TTK could not bring down p53 (Fig. 2E). In both contexts, full-length and C1, the KD mutants interacted better than did the WT (Fig. 2E), suggesting that autophosphorylation of TTK may negatively regulate the interaction.
TTK/hMps1 stabilizes p53 through phosphorylation of the p53 N-terminal domain.
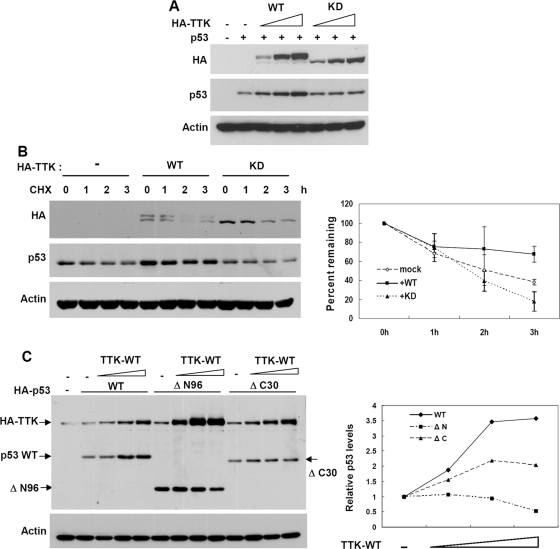
p53 is an unstable protein, and with stress it can be stabilized by phosphorylation. Our data that ablation of TTK by siRNA reduced the p53 response prompted us to investigate whether TTK could stabilize p53 by phosphorylating p53. To assess this possibility, HCT116 cells were first transfected with p53 together with increasing amounts of TTK-WT or -KD. As shown in Fig. 3A, p53 was increased by coexpressed TTK-WT, but no significant change was observed with coexpression with TTK-KD, indicating the kinase activity of TTK is essential for this effect. In agreement with this, the stability of p53 was higher when it was coexpressed with the WT than with TTK-KD (Fig. 3B). To further determine the responsive domain in p53, increasing amounts of TTK-WT were coexpressed with either full-length p53 or the truncation mutant ΔN96 or ΔC30 in p53-null H1299 cells. Deletion of the N-terminal domain (ΔN96) abrogated the stabilization by TTK compared with results for full-length p53, whereas deletion of the C terminus (ΔC30), a domain required for interaction, reduced the effect (Fig. 3C). Taken together, these results indicate that overexpression of TTK stabilizes p53 through the N-terminal transactivation domain and the effect is dependent on the kinase activity of TTK. It was noted that not only ΔN96 but also coexpressed TTK were consistently expressed at higher levels (Fig. 3C). Although the underlying cause is unclear, we speculate that this might be due to the lack of an MDM2-binding domain in the ΔN96 protein.
FIG. 3.
TTK/hMps1 stabilizes p53 through the N-terminal domain. (A) Overexpression of TTK-WT but not KD stabilized p53. HCT116 cells were transfected with p53 and TTK-WT or KD. The cell lysates were analyzed by Western blotting using the indicated antibodies. Actin was used as a loading control. (B) The half-life of p53 was enhanced by coexpressed WT-TTK. Cells were transfected and analyzed as for panel A except that cycloheximide (CHX) (20 μg/ml) was added before harvest for the indicated length of time. Levels of p53 were quantified and normalized to actin and are shown on the right. Averages for two independent experiments are displayed. (C) WT-TTK failed to stabilize the N-terminal-domain-deleted mutant of p53. p53-null H1299 cells were transfected with HA-TTK and either full-length HA-p53 or the truncation mutants, ΔN96 (amino acids 1 to 96 deleted) or ΔC30 (amino acids 363 to 393 deleted). Protein expression was detected by Western blotting using an anti-HA antibody. Levels of p53 were normalized to actin and are shown on the right as n-fold increases compared to results for the construct transfected alone.
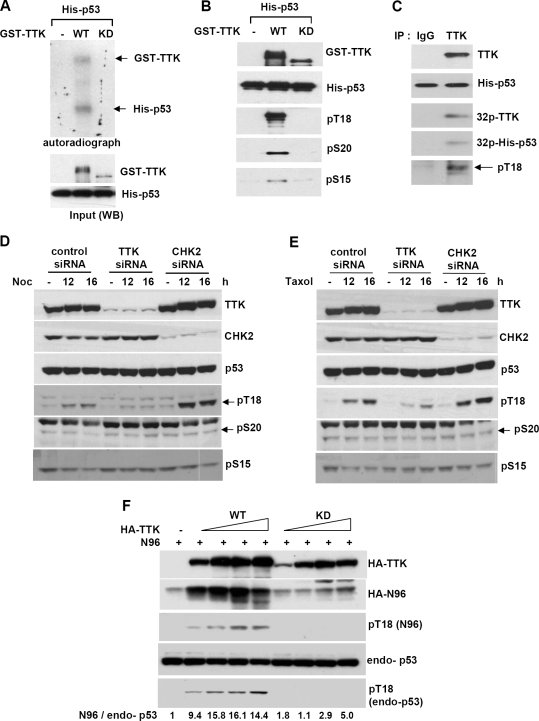
To determine whether TTK phosphorylates the N-terminal domain of p53, in vitro kinase assays were employed using bacterially produced GST-TTK, WT or KD, and His-p53. As shown in Fig. 4A, GST-TTK-WT but not GST-TTK-KD phosphorylated p53. The phosphorylation sites on p53, as determined by Western blotting using specific anti-phospho-p53 antibodies, included Thr18, Ser20, and (weakly) Ser15 (Fig. 4B). Similarly, TTK immunoprecipitated from 293T cells phosphorylated p53 at Thr18 (Fig. 4C) and weakly at Ser20 (data not shown); phosphorylation at Ser15 was not detected (data not shown).
FIG. 4.
TTK/hMps1 phosphorylates the N-terminal domain of p53. (A) TTK phosphorylated p53 in vitro. Kinase assays were performed by incubating purified His-tagged p53 with purified GST-TTK-WT or -KD in the presence of [γ-32P]ATP. The products were resolved by SDS-PAGE and visualized by autoradiography. (B) TTK phosphorylated p53 on Thr18 and Ser20 and weakly on Ser15 in vitro. Kinase assays were conducted as described for panel A, and phosphorylated p53 was detected by Western blotting using residue-specific phospho-p53 antibodies against Thr18, Ser20, or Ser15. (C) Immunoprecipitated endogenous TTK phosphorylated p53 on Thr18. IP/kinase assays were performed using TTK immunoprecipitated from 293T cells. Normal rabbit immunoglobulin G (IgG) was used as an IP control. Phosphorylation of the substrate (His-p53) was detected by autoradiography or Western blotting using an anti-phospho-Thr18-specific p53 antibody. (D and E) Downregulation of TTK but not CHK2 by siRNA diminished spindle damage-induced p53 Thr18 phosphorylation. 293T cells transfected with control, TTK, or CHK2 siRNA were treated with nocodazole (Noc) (50 ng/ml) (D) or Taxol (100 nM) (E). Lysates were collected at the indicated times and analyzed by Western blotting using the indicated antibodies. (F) Overexpression of TTK-WT but not TTK-KD markedly stabilized the p53 N-terminal domain (N96, amino acids 1 to 96) and increased Thr18 phosphorylation of both transfected and endogenous p53. 293T cells were transfected with HA-N96 and HA-TTK-WT or -KD. Cell lysates were analyzed by Western blotting using specific antibodies. Numbers indicate relative levels of p53N96 after normalization to endogenous p53 (endo-p53) that is at a constant level in 293T cells.
To probe for physiological relevance, in particular, in the response to spindle disruption, TTK was first downregulated by siRNA in 293T cells and the phosphorylation of p53 was examined. In control cells, phosphorylation at p53 Thr18 but not Ser15 and Ser20 was induced by the spindle poisons nocodazole (Fig. 4D) and Taxol (Fig. 4E), whereas in TTK knockdown cells, such phosphorylation was significantly diminished. Similar results were seen using another TTK siRNA, TK3 (see Fig. S2A in the supplemental material). Notably, CHK2 depletion did not affect the phosphorylation, indicating that unlike the effects observed upon DNA damage (35), the effect of TTK depletion could not be mediated through CHK2 in response to spindle damage. In line with the above observations, Thr18 phosphorylation on a transfected p53 N-terminal fragment (N96; amino acids 1 to 96) and endogenous p53 was enhanced with concomitant increases in the N96 protein by overexpressed WT but not KD TTK (Fig. 4F). It was noted that TTK ablation also dampened IR- and UV-induced p53 Thr18 phosphorylation (see Fig. S2B and S2C in the supplemental material). Collectively, our results suggest that TTK/hMps1 is the principal p53 Thr18 kinase in vivo and Thr18 is the major N-terminal site that harbors phosphorylation after spindle damage.
Overexpression of WT but not TTK/hMps1-KD protects p53 from MDM2-mediated degradation.
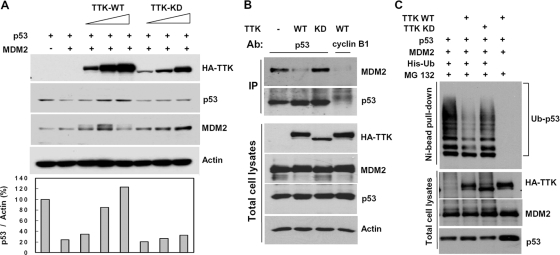
MDM2 promotes p53 ubiquitination and degradation through its E3 ubiquitin ligase activity. In H1299 cells, coexpression of p53 with MDM2 significantly reduced p53 levels (Fig. 5A). Increased TTK-WT rescued MDM2-induced reduction of p53 (Fig. 5A), suggesting that TTK may protect p53 from MDM2-mediated degradation. To determine whether TTK pageed the interaction between p53 and MDM2, immunoprecipitation was performed using lysates of H1299 cells expressing TTK (WT or KD), p53, and MDM2. Overexpression of TTK-WT but not TTK-KD significantly decreased the amount of MDM2 bound to p53 (Fig. 5B). Consistently, overexpression of TTK-WT reduced the amount of polyubiquitinated p53 (Fig. 5C). Together, these data indicate that TTK may stabilize p53 by inhibiting the feedback control of p53 by its E3 ligase, MDM2.
FIG. 5.
Overexpression of WT TTK/hMps1 but not TTK-KD protects p53 from MDM2-mediated degradation. (A) MDM2-induced degradation of p53 was rescued by coexpression of TTK-WT but not TTK-KD. H1299 cells were transfected with p53 and MDM2 together with HA-TTK-WT or -KD. The lysates were analyzed by Western blotting. Levels of p53 were quantified and normalized to actin levels and are shown in the lower panel. (B) The p53-MDM2 interaction was disrupted by coexpression of the WT but not TTK-KD. H1299 cells were transfected as for panel A and treated with MG132 (25 μM) for 6 to 8 h before harvesting. p53 was immunoprecipitated using anti-p53 antibody PAb421, and MDM2 in the immune complex was detected by Western blotting. An anti-cyclin B1 antibody was used as an IP control. (C) p53 ubiquitination was abrogated by coexpressed TTK-WT but not TTK-KD. H1299 cells were transfected as for panel A except that His-Ub was included in the transfection. Transfected cells were treated with MG132 (25 nM) for 10 h before harvesting and processed for Ni bead pull-down assay. The complexes pulled down were analyzed by Western blotting using the anti-p53 antibody DO-1.
Downregulation of TTK causes a postmitotic checkpoint defect and polyploidy.
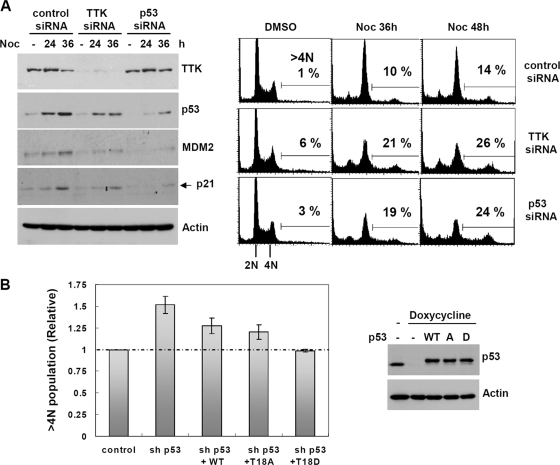
Upon spindle damage, cells respond by arresting in mitosis. In the case of prolonged arrest, cells ultimately exit mitosis and enter G1 without sister chromatid segregation and cytokinesis. In cells with normal p53 function, these tetraploid cells then arrest at the G1 postmitotic checkpoint or undergo apoptosis (12, 17). Since TTK phosphorylated and stabilized p53 upon spindle damage, we set out to determine whether the TTK-mediated p53 response was involved in the postmitotic checkpoint. As shown in Fig. 6A (right panel), TTK downregulation and p53 ablation increased ploidy (>4N population) upon prolonged incubation of U2OS cells with nocodazole. These results indicate that although a residual amount of TTK was able to transiently retain the cells in the M phase upon spindle disruption (data not shown), it was not sufficient for cells to arrest in the postmitotic G1 phase upon mitotic slippage. In agreement with this, the expression of the p53 downstream targets p21 and MDM2 were reduced in TTK knockdown cells (Fig. 6A, left panels). These results suggest that TTK may contribute to the p53-dependent postmitotic checkpoint, perhaps by phosphorylating p53.
FIG. 6.
TTK/hMps1 ablation ameliorates the postmitotic checkpoint. (A) Control, TTK, or p53 siRNA-transfected U2OS cells were treated with nocodazole (Noc) (50 ng/ml) for the indicated times and processed for flow cytometry analysis (right panel). Protein levels were determined by Western blotting and are shown on the left. DMSO, dimethyl sulfoxide. (B) Tetraploidy was more efficiently suppressed by the phospho-mimicking T18D mutant than by the WT or the phospho-deficient T18A mutant. Tet-on U2OS-shp53 cells were transfected with HA-tagged, siRNA-resistant WT, T18A, or T18D p53 together with H2B-GFP in the absence or presence of doxycycline (1 μg/ml) to induce the expression of p53-targeting shRNA. Twenty-four hours after transfection, cells were treated with Noc for 48 h and processed for flow cytometry as for panel A, except that GFP-positive cells were gated and analyzed. Expression of p53 was examined by Western blotting and is shown on the right.
To determine if the effect of TTK downregulation on the postmitotic checkpoint was indeed mediated through p53 Thr18 phosphorylation and stabilization, we performed replacement assays in which endogenous p53 was first depleted using stably expressed shRNA and then substituted by transient transfection with either HA-tagged WT p53, the phospho-deficient T18A mutant (with Thr18 changed to Ala). or the phospho-mimicking T18D mutant (with Thr18 changed to Asp). Consistent with its regulation, the phospho-mimicking T18D mutant was more capable than the WT or the T18A mutant in suppressing the polyploidy (>4N population) induced by prolonged nocodazole treatment (Fig. 6B).
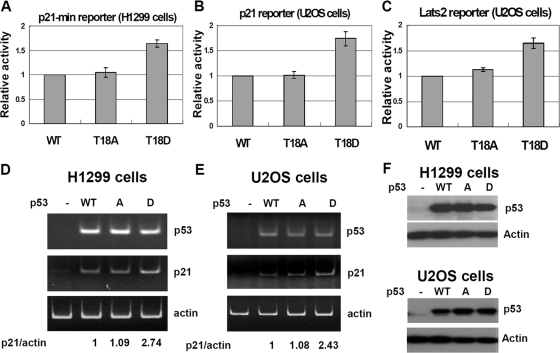
Since p21 and Lats2 have been implicated in the postmitotic checkpoint downstream of p53 (3, 12), the promoter activity of these two genes in response to p53 was examined. In the luciferase reporter assays, the phospho-mimicking T18D mutant transactivated these promoters about 50% better than did the WT or the phospho-deficient T18A mutant (Fig. 7A to C). Expression of endogenous p21 RNA, as assayed by RT-PCR, was also higher in T18D-transfected cells than in WT or T18A-transfected cells (Fig. 7D and E). Since Thr18 phosphorylation is specifically induced by spindle damage in a TTK-dependent manner, these results further highlight the involvement of TTK and the significance of Thr18 phosphorylation in expression of these genes in the event of postmitotic arrest.
FIG. 7.
Phospho-mimicking mutation at Thr18 increases p53 activity. (A) H1299 cells were transfected with WT p53 or the T18A or T18D mutant and a luciferase reporter driven by a p53 response element derived from the p21 promoter. Luciferase activity was determined 16 to 24 h later. (B and C) The Tet-on U2OS-shp53 cells that inducibly express p53 shRNA were grown in the presence of doxycycline and transfected with HA-tagged, RNA interference-resistant WT p53 or a mutant and a luciferase reporter driven by either the p21 promoter (B) or a p53 response element derived from the Lats2 promoter (C). The luciferase activity was then assayed as for panel A. Results from three independent, duplicated experiments are presented. (D and E) Expression of the endogenous p21 gene was elevated by transfection with the p53 T18D mutant. RNA was isolated from transfected H1299 (D) or Tet-on U2OS (E) cells and processed for RT-PCR. (F) Western blots showing relative expression levels of WT and mutant p53 in transfected H1299 (upper panel) or Tet-on U2OS (lower panel) cells.
DISCUSSION
In this report, we have attempted to address the molecular basis of p53 activation in the spindle damage-induced postmitotic tetraploidy checkpoint. We demonstrate that this activation requires the spindle checkpoint kinase TTK/hMps1. TTK/hMps1, upon its activation by spindle poisons, phosphorylates p53 at Thr18, thus disrupting the interaction between p53 and MDM2 and leading to p53 stabilization. Our study not only has unraveled a novel mechanism of p53 activation, especially in a circumstance where ATM-CHK2 and ATR-CHK1 signaling are not induced, but also provides an explanation for the long-known requirement of the spindle assembly checkpoint for p53-dependent postmitotic arrest (2, 12, 32).
The importance of the p53-dependent tetraploid checkpoint has been well elucidated. Tetraploidy, whether from chromosome nondisjunction or adaptation from prolonged mitotic arrest as presented in this study, is linked to marked increases in chromosome instability and tumorigenesis (5, 17, 34). When p53 is compromised, for example by mutation, these tetraploid cells proceed to the next division, form multipolar spindles, and inevitably become aneuploids (27). The link with tumor development is further strengthened by a study that showed that p53−/− tetraploid cells are more tumorigenic than p53−/− diploid cells (8). How p53 is activated in this process has not been completely clear. In this report, we have presented evidence that the spindle checkpoint kinase TTK/hMps1 is involved. TTK directly phosphorylated a critical site (Thr18) involved in the interaction with MDM2, the p53 E3 ligase. Furthermore, downregulation of TTK not only abrogated the spindle damage-induced p53 response but also disrupted the p53-dependent G1 tetraploid checkpoint. Finally, the tetraploid checkpoint was reestablished in p53-ablated cells by overexpression of the phospho-mimicking p53T18D mutant and less so by that of the phospho-deficient T18A mutant, further strengthening the physiological relevance of the phosphorylation.
TTK/hMps1, however, may not be the only spindle checkpoint kinase involved. Recently BubR1 was implicated in the activation of p53 by spindle damage (9). BubR1 bound the N terminus of p53 and, following immunoprecipitation, phosphorylated p53 at Ser15 and Ser46 (9). Downregulation of BubR1 in HCT116 cells by shRNA significantly lowered the levels of p53 induced by nocodazole, which is in contrast to our findings that depletion of BubR1 in either U2OS or LNCaP cells did not affect the induction of p53 by either Taxol or nocodazole (Fig. 1). Whether the difference was due to distinct cellular contexts remains to be determined. Nevertheless, we did observe that without MAD2, an important mediator of the spindle checkpoint downstream of TTK, TTK-mediated p53 Thr18 phosphorylation was diminished in 293T cells (see Fig. S3A in the supplemental material), although the intrinsic kinase activity of TTK, as assessed by immoprecipitation (IP)/kinase assay, was not affected (data not shown). Concordantly, an increase in tetraploidy was observed in MAD2 knockdown, nocodazole-treated HCT116 cells, although to a lesser degree than in the TTK-depleted cells (see Fig. S3B in the supplemental material). This is consistent with a previous report that showed that reduction of MAD2 as well as MAD1 in HCT116 cells promoted tetraploidy upon mitotic slippage (32). These results suggest that additional spindle checkpoint components are somehow involved in Thr18 phosphorylation. It is possible that phosphorylation of p53 by TTK, as happens upon spindle damage, requires the participation of the spindle checkpoint machinery, either serving as a platform or for proper localization of TTK and p53. Alternatively, we cannot exclude the possibility that another MAD1- and MAD2-dependent spindle checkpoint kinase may also be involved in p53 Thr18 phosphorylation.
Although Thr18 but not Ser15 or Ser20 was found to be the site that gains phosphorylation upon spindle damage (Fig. 4D and E), our study does not exclude the possibility that TTK may also act on another part of the p53 protein or even on MDM2. During the course of our study, we at times observed that MDM2 levels were increased by coexpression of TTK, and overexpression of WT TTK caused the appearance of a slower-migrating form of MDM2 (Fig. 5A), suggesting a functional interplay between TTK and MDM2. An interesting but unproven theory is that MDM2 may be stabilized by binding to TTK and its E3 ligase activity toward substrates may be modified by TTK-mediated phosphorylation. The fact that replacement of Thr18 with Ala did not completely abolish the stabilization of p53 by TTK (data not shown) further supports these possibilities.
Another issue arising in our study is the relative contribution of the p53 N-terminal and C-terminal domains in TTK-mediated stabilization. Mechanistically, TTK may phosphorylate the p53 N terminus (at Thr18) by binding to the last 30 amino acids of p53, thereby stabilizing p53. Direct physical interaction may not be sufficient in stabilizing p53, since p53 coexpressed with KD TTK was not more stable than p53 expressed alone (Fig. 3B). Instead, protein-protein interaction through the p53 C terminus may facilitate N-terminal phosphorylation by TTK in vivo. This argument is supported by our observation that ΔC30 was less stabilized by TTK than was full-length p53 (Fig. 3C), and in this case, the kinase activity of TTK is still required (see Fig. S4A in the supplemental material). Our other data also suggest that the p53 C-terminal domain is not absolutely required for p53 phosphorylation by TTK, since His-ΔC30 was as good a substrate as the full-length protein in in vitro kinase assays when both proteins were abundantly present in pure forms (see Fig. S4B in the supplemental material). These results, taken together, support a model that interaction facilitates phosphorylation. On the other hand, phosphorylation of p53 or even TTK itself may in turn modulate the interaction between the two proteins, since Taxol- or DNA damage-induced interaction appeared to be transient (Fig. 2A, B, and C) and GST-TTK-KD appeared to bind p53 more efficiently than GST-TTK-WT (Fig. 2E). The mechanistic details await further investigation.
The relationship between TTK and p53 may extend beyond phosphorylation. Bhonde et al. (4) have reported that DNA-damage-activated p53 suppressed the expression of TTK at the mRNA level. Indeed, we have observed an inverse correlation at the protein level between TTK and p53 in response to nocodazole treatment (Fig. 6). We believe that this may serve as a feedback mechanism to downregulate TTK when the checkpoint is satisfied or the mission is completed, thereby keeping the activity of TTK in check.
The role of TTK/hMps1 in genome stability is not limited to phosphorylation of p53. For example, yeast Mps1 phosphorylates the kinetochore protein Dam1, and such an event is required for efficient coupling of kinetochores to microtubule plus ends (28). TTK/hMps1 also phosphorylates the Bloom syndrome protein, BLM, which is important for accurate chromosome segregation (15). In addition, Mps1 phosphorylation of mortalin participates in centrosome duplication (11). Furthermore, TTK/hMps1 phosphorylates CHK2 at Thr68 and is involved in proper G2/M arrest after DNA damage (35). These, together with the results reported here, point to the multifaceted functions of Mps1. By phosphorylation of targets involved in either checkpoints or assembly of mitotic spindles, Mps1 may exert its profound impact on the maintenance of genome stability. Further investigation is warranted to decipher its role in tumorigenesis.
Supplementary Material
Acknowledgments
We thank Yen-Hsiu Yeh for her expert technical assistance.
This work was supported by funding from Academia Sinica to S.-Y. Shieh.
Footnotes
Published ahead of print on 30 March 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Abrieu, A., L. Magnaghi-Jaulin, J. A. Kahana, M. Peter, A. Castro, S. Vigneron, T. Lorca, D. W. Cleveland, and J. C. Labbe. 2001. Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell 10683-93. [DOI] [PubMed] [Google Scholar]
- 2.Andreassen, P. R., O. D. Lohez, F. B. Lacroix, and R. L. Margolis. 2001. Tetraploid state induces p53-dependent arrest of nontransformed mammalian cells in G1. Mol. Biol. Cell 121315-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aylon, Y., D. Michael, A. Shmueli, N. Yabuta, H. Nojima, and M. Oren. 2006. A positive feedback loop between the p53 and Lats2 tumor suppressors prevents tetraploidization. Genes Dev. 202687-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhonde, M. R., M.-L. Hanski, J. Budczies, M. Cao, B. Gillissen, D. Moorthy, F. Simonetta, H. Scherubl, M. Truss, C. Hagemeier, H.-W. Mewes, P. T. Daniel, M. Zeitz, and C. Hanski. 2006. DNA damage-induced expression of p53 suppresses mitotic checkpoint kinase hMps1—the lack of this suppression in p53mut cells contributes to apoptosis. J. Biol. Chem. 2818675-8685. [DOI] [PubMed] [Google Scholar]
- 5.Blagosklonny, M. V. 2006. Prolonged mitosis versus tetraploid checkpoint—how p53 measures the duration of mitosis. Cell Cycle 5971-975. [DOI] [PubMed] [Google Scholar]
- 6.Castillo, A. R., J. B. Meehl, G. Morgan, A. Schutz-Geschwender, and M. Winey. 2002. The yeast protein kinase Mps1p is required for assembly of the integral spindle pole body component Spc42p. J. Cell Biol. 156453-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Como, C. J., C. Gaiddon, and C. Prives. 1999. p73 function is inhibited by tumor-derived p53 mutants in mammalian cells. Mol. Cell. Biol. 191438-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujiwara, T., M. Bandi, M. Nitta, E. V. Ivanova, R. T. Bronson, and D. Pellman. 2005. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature 4371043-1047. [DOI] [PubMed] [Google Scholar]
- 9.Ha, G.-H., K.-H. Baek, H.-S. Kim, S.-J. Jeong, C.-M. Kim, F. McKeon, and C.-W. Lee. 2007. p53 activation in response to mitotic spindle damage requires signaling via BubR1-mediated phosphorylation. Cancer Res. 677155-7164. [DOI] [PubMed] [Google Scholar]
- 10.Harms, K., S. Nozell, and X. Chen. 2004. The common and distinct target genes of the p53 family transcription factors. Cell. Mol. Life Sci. 61822-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanai, M., Z. Ma, H. Izumi, S.-H. Kim, C. P. Mattison, M. Winey, and K. Fukasawa. 2007. Physical and functional interaction between mortalin and Mps1 kinase. Genes Cells 12797-810. [DOI] [PubMed] [Google Scholar]
- 12.Lanni, J. S., and T. Jacks. 1998. Characterization of the p53-dependent postmitotic checkpoint following spindle disruption. Mol. Cell. Biol. 181055-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavin, M. F., and N. Gueven. 2006. The complexity of p53 stabilization and activation. Cell Death Differ. 13941-950. [DOI] [PubMed] [Google Scholar]
- 14.Lee, H. J., D. Srinivasan, D. Coomber, D. P. Lane, and C. S. Verma. 2007. Modulation of the p53-MDM2 interaction by phosphorylation of Thr18: a computational study. Cell Cycle 62604-2611. [DOI] [PubMed] [Google Scholar]
- 15.Leng, M., D. W. Chan, H. Luo, C. Zhu, J. Qin, and Y. Wang. 2006. Mps1-dependent mitotic BLM phosphorylation is important for chromosome stability. Proc. Natl. Acad. Sci. USA 10311485-11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindberg, R. A., W. H. Fischer, and T. Hunter. 1993. Characterization of a human protein threonine kinase isolated by screening an expression library with antibodies to phosphotyrosine. Oncogene 8351-359. [PubMed] [Google Scholar]
- 17.Margolis, R. L., O. D. Lohez, and P. R. Andreassen. 2003. G1 tetraploidy checkpoint and the suppression of tumorigenesis. J. Cell. Biochem. 88673-683. [DOI] [PubMed] [Google Scholar]
- 18.Meek, D. W. 2000. The role of p53 in the response to mitotic spindle damage. Pathol. Biol. (Paris) 48246-254. [PubMed] [Google Scholar]
- 19.Mills, G. B., R. Schmandt, M. McGill, A. Amendola, M. Hill, K. Jacobs, C. May, A. M. Rodricks, S. Campbell, and D. Hogg. 1992. Expression of TTK, a novel human protein kinase, is associated with cell proliferation. J. Biol. Chem. 26716000-16006. [PubMed] [Google Scholar]
- 20.Musacchio, A., and E. D. Salmon. 2007. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 8379-393. [DOI] [PubMed] [Google Scholar]
- 21.Olivier, M., R. Eeles, M. Hollstein, M. A. Khan, C. C. Harris, and P. Hainaut. 2002. The IARC TP53 database: new online mutation analysis and recommendations to users. Hum. Mutat. 19607-614. [DOI] [PubMed] [Google Scholar]
- 22.Olsson, A., C. Manzl, A. Strasser, and A. Villunger. 2007. How important are post-translational modifications in p53 for selectivity in target-gene transcription and tumour suppression? Cell Death Differ. 141561-1575. [DOI] [PubMed] [Google Scholar]
- 23.Ou, Y.-H., P.-H. Chung, F.-F. Hsu, T.-P. Sun, W.-Y. Chang, and S.-Y. Shieh. 2007. The candidate tumor suppressor BTG3 is a transcriptional target of p53 that inhibits E2F1. EMBO J. 263968-3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ou, Y.-H., P.-H. Chung, T.-P. Sun, and S.-Y. Shieh. 2005. p53 C-terminal phosphorylation by CHK1 and CHK2 participates in the regulation of DNA damage-induced C-terminal acetylation. Mol. Biol. Cell 161684-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rieder, C. L., and H. Maiato. 2004. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev. Cell 7637-651. [DOI] [PubMed] [Google Scholar]
- 26.Riley, T., E. Sontag, P. Chen, and A. Levine. 2008. Transcriptional control of human p53-regulated genes. Nat. Rev. Mol. Cell Biol. 9402-412. [DOI] [PubMed] [Google Scholar]
- 27.Shi, Q., and R. W. King. 2005. Chromosome nondisjunction yields tetraploid rather than aneuploid cells in human cell lines. Nature 4371038-1042. [DOI] [PubMed] [Google Scholar]
- 28.Shimogawa, M. M., B. Graczyk, M. K. Gardner, S. E. Francis, E. A. White, M. Ess, J. N. Molk, C. Ruse, S. Niessen, J. R. Yates III, E. G. D. Muller, K. Bloom, D. J. Odde, and T. N. Davis. 2006. Mps1 phosphorylation of Dam1 couples kinetochores to microtubule plus ends at metaphase. Curr. Biol. 161489-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart, Z. A., L. J. Tang, and J. A. Pietenpol. 2001. Increased p53 phosphorylation after microtubule disruption is mediated in a microtubule inhibitor- and cell-specific manner. Oncogene 20113-124. [DOI] [PubMed] [Google Scholar]
- 30.Toledo, F., and G. M. Wahl. 2006. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat. Rev. Cancer 6909-923. [DOI] [PubMed] [Google Scholar]
- 31.Vigneron, S., S. Prieto, C. Bernis, J. C. Labbe, A. Castro, and T. Lorca. 2004. Kinetochore localization of spindle checkpoint proteins: who controls whom? Mol. Biol. Cell 154584-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vogel, C., A. Kienitz, I. Hofmann, R. Muller, and H. Bastians. 2004. Crosstalk of the mitotic spindle assembly checkpoint with p53 to prevent polyploidy. Oncogene 236845-6853. [DOI] [PubMed] [Google Scholar]
- 33.Vousden, K. H., and D. P. Lane. 2007. p53 in health and disease. Nat. Rev. Mol. Cell Biol. 8275-283. [DOI] [PubMed] [Google Scholar]
- 34.Weaver, B. A. A., and D. W. Cleveland. 2005. Decoding the links between mitosis, cancer, and chemotherapy: the mitotic checkpoint, adaptation and cell death. Cancer Cell 87-12. [DOI] [PubMed] [Google Scholar]
- 35.Wei, J.-H., Y.-F. Chou, Y.-H. Ou, Y.-H. Yeh, S.-W. Tyan, T.-P. Sun, C.-Y. Shen, and S.-Y. Shieh. 2005. TTK/hMps1 participates in the regulation of DNA damage checkpoint response by phosphorylating CHK2 on threonine 68. J. Biol. Chem. 2807748-7757. [DOI] [PubMed] [Google Scholar]
- 36.Winey, M., and B. J. Huneycutt. 2002. Centrosomes and checkpoints: the MPS1 family of kinases. Oncogene 216161-6169. [DOI] [PubMed] [Google Scholar]
- 37.Zhu, S., W. Wang, D. C. Clarke, and X. Liu. 2007. Activation of Mps1 promotes transforming growth factor-beta-independent Smad signaling. J. Biol. Chem. 28218327-18338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.