Abstract
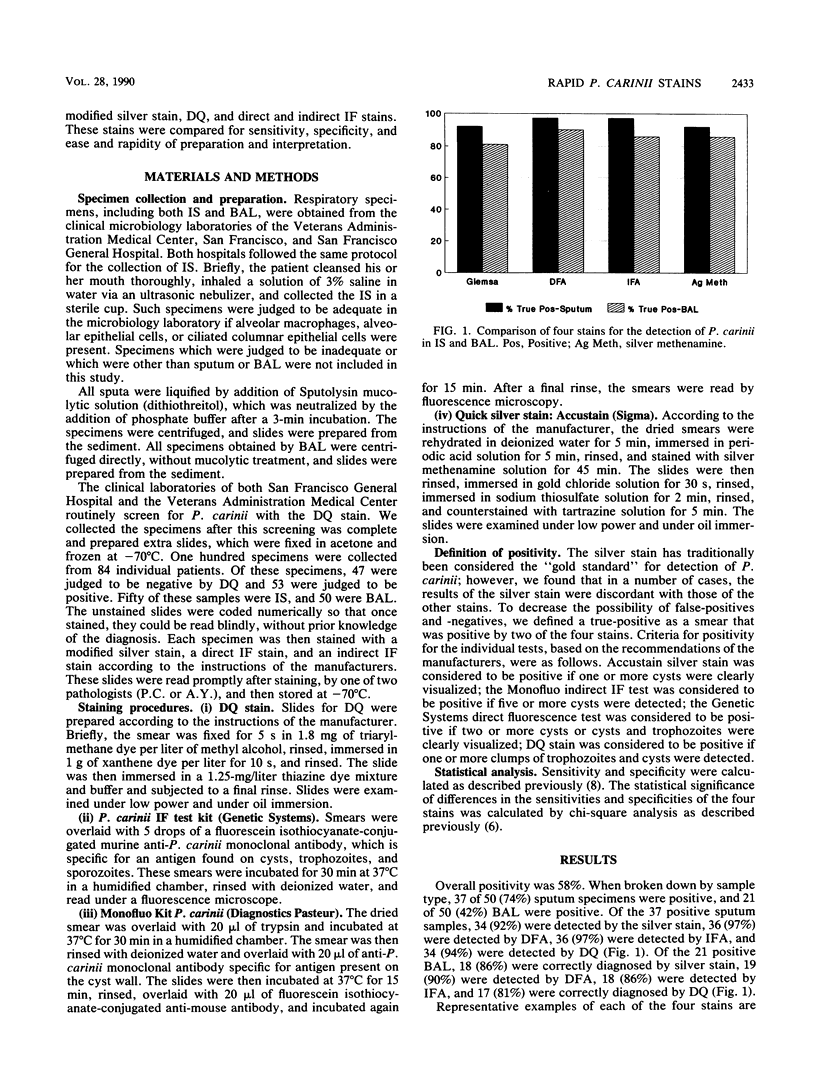
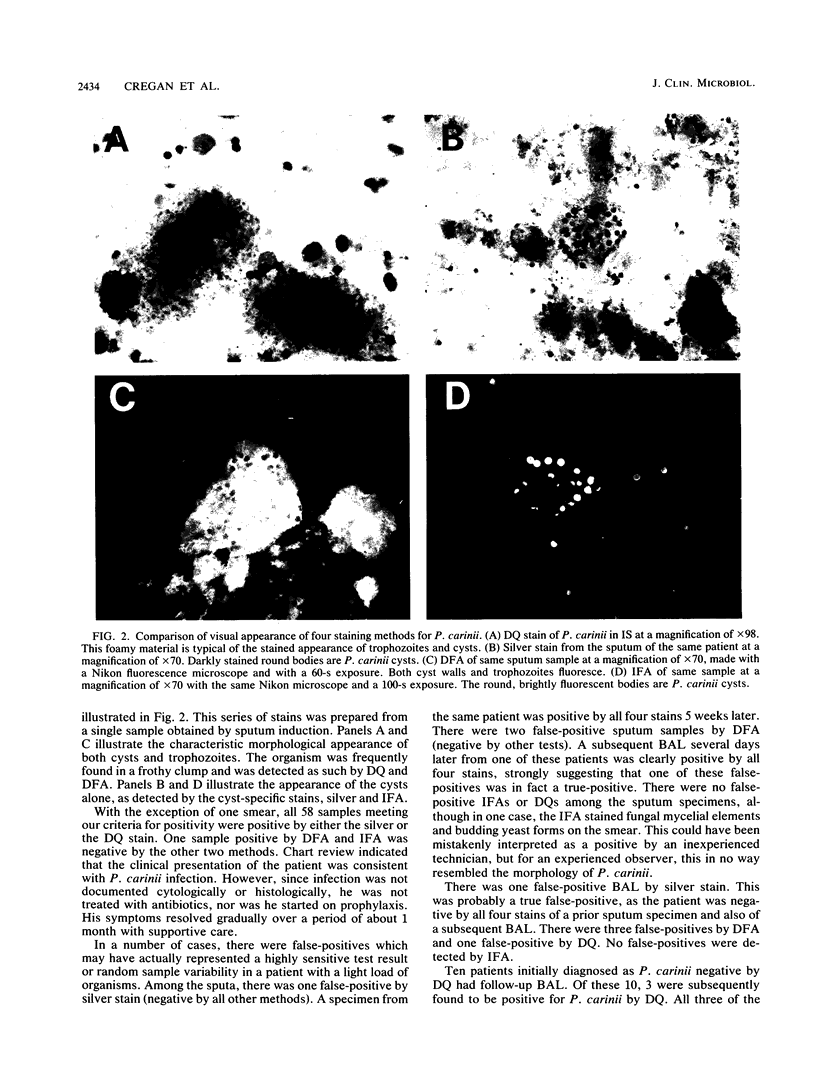
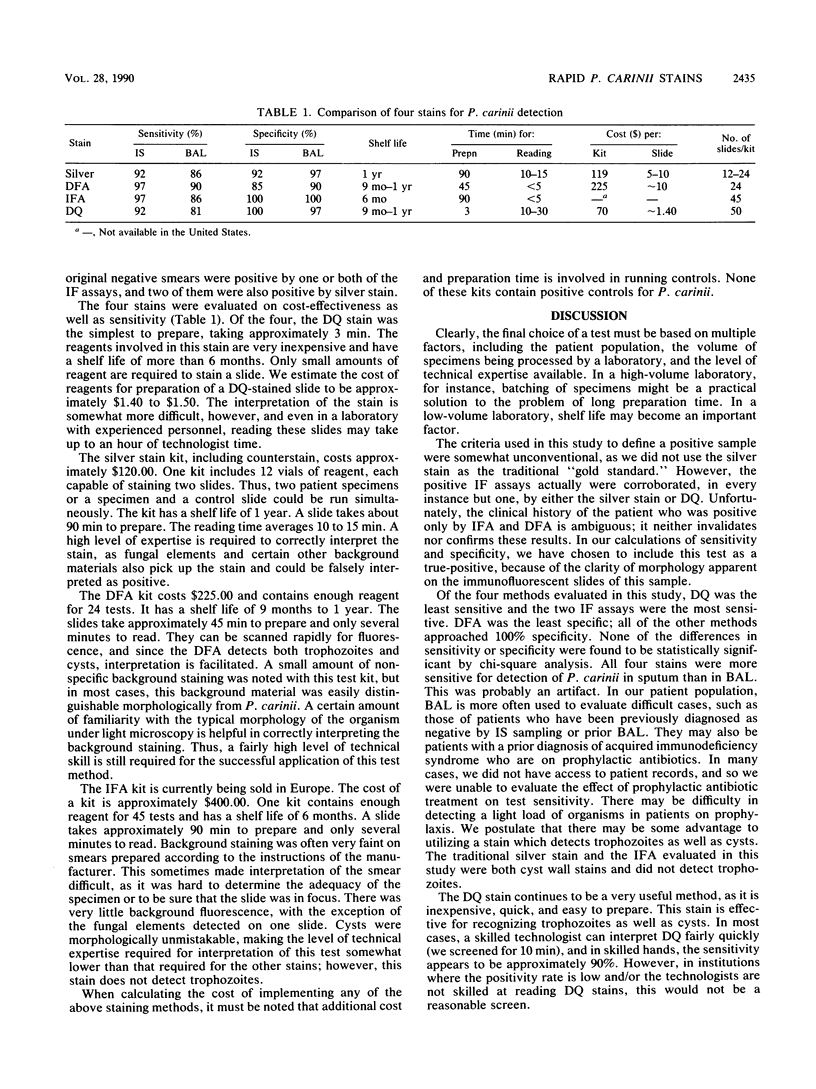
Four stains for the detection of Pneumocystis carinii in respiratory specimens were compared for sensitivity, specificity, preparation time, and ease of interpretation. One hundred specimens were collected. Of these, 50 were induced sputum specimens and 50 were bronchoalveolar lavage fluid. All specimens were stained with Diff-Quik (DQ) (a modified Giemsa stain), a quick silver stain, and direct and indirect immunofluorescence stains. A positive specimen was defined as any smear positive by two or more of the methods. Fifty-eight percent of specimens were positive. Seventy-four percent of the sputum specimens and 42% of the bronchoalveolar lavages were positive. The sensitivities for detection of P. carinii in sputum were 92% with silver stain, 97% with direct immunofluorescence assay (DFA), 97% with indirect immunofluorescence assay (IFA), and 92% with DQ. The sensitivities for detection in bronchoalveolar lavage were 86% with silver stain, 90% with DFA, 86% with IFA, and 81% with DQ. Preparation times varied from 90 min for the silver stain and IFA to 3 min for DQ. Costs of the tests varied from $1.50 per slide for DQ to $10.00 per slide for the silver stain and DFA. Reading times varied from 10 to 30 min for the silver stain and DQ to less than 5 min for the immunofluorescence assays. We conclude that all of these tests are viable options for the clinical laboratory, and the choice will be influenced by factors such as clinical volume, ability to batch specimens, and expertise of technological support. A reasonable option may be to use the quick and inexpensive DQ as a screening test and to confirm negative smears with a more sensitive assay.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedrossian C. W., Mason M. R., Gupta P. K. Rapid cytologic diagnosis of Pneumocystis: a comparison of effective techniques. Semin Diagn Pathol. 1989 Aug;6(3):245–261. [PubMed] [Google Scholar]
- Bigby T. D., Margolskee D., Curtis J. L., Michael P. F., Sheppard D., Hadley W. K., Hopewell P. C. The usefulness of induced sputum in the diagnosis of Pneumocystis carinii pneumonia in patients with the acquired immunodeficiency syndrome. Am Rev Respir Dis. 1986 Apr;133(4):515–518. doi: 10.1164/arrd.1986.133.4.515. [DOI] [PubMed] [Google Scholar]
- Blumenfeld W., Kovacs J. A. Use of a monoclonal antibody to detect Pneumocystis carinii in induced sputum and bronchoalveolar lavage fluid by immunoperoxidase staining. Arch Pathol Lab Med. 1988 Dec;112(12):1233–1236. [PubMed] [Google Scholar]
- Domingo J., Waksal H. W. Wright's stain in rapid diagnosis of Pneumocystis carinii. Am J Clin Pathol. 1984 Apr;81(4):511–514. doi: 10.1093/ajcp/81.4.511. [DOI] [PubMed] [Google Scholar]
- GROCOTT R. G. A stain for fungi in tissue sections and smears using Gomori's methenamine-silver nitrate technic. Am J Clin Pathol. 1955 Aug;25(8):975–979. doi: 10.1093/ajcp/25.8_ts.0975. [DOI] [PubMed] [Google Scholar]
- Gal A. A., Koss M. N., Strigle S., Angritt P. Pneumocystis carinii infection in the acquired immune deficiency syndrome. Semin Diagn Pathol. 1989 Aug;6(3):287–299. [PubMed] [Google Scholar]
- Kim H. K., Hughes W. T. Comparison of methods for identification of Pneumocystis carinii in pulmonary aspirates. Am J Clin Pathol. 1973 Oct;60(4):462–466. doi: 10.1093/ajcp/60.4.462. [DOI] [PubMed] [Google Scholar]
- Kovacs J. A., Ng V. L., Masur H., Leoung G., Hadley W. K., Evans G., Lane H. C., Ognibene F. P., Shelhamer J., Parrillo J. E. Diagnosis of Pneumocystis carinii pneumonia: improved detection in sputum with use of monoclonal antibodies. N Engl J Med. 1988 Mar 10;318(10):589–593. doi: 10.1056/NEJM198803103181001. [DOI] [PubMed] [Google Scholar]
- Linder J., Radio S. J. Immunohistochemistry of Pneumocystis carinii. Semin Diagn Pathol. 1989 Aug;6(3):238–244. [PubMed] [Google Scholar]
- Musto L., Flanigan M., Elbadawi A. Ten-minute silver stain for Pneumocystis carinii and fungi in tissue sections. Arch Pathol Lab Med. 1982 Jun;106(6):292–294. [PubMed] [Google Scholar]
- Ng V. L., Gartner I., Weymouth L. A., Goodman C. D., Hopewell P. C., Hadley W. K. The use of mucolysed induced sputum for the identification of pulmonary pathogens associated with human immunodeficiency virus infection. Arch Pathol Lab Med. 1989 May;113(5):488–493. [PubMed] [Google Scholar]