Abstract
The Y family DNA polymerase Rev1 has been proposed to play a regulatory role in the replication of damaged templates. To elucidate the mechanism by which Rev1 promotes DNA damage bypass, we have analyzed the progression of replication on UV light-damaged DNA in mouse embryonic fibroblasts that contain a defined deletion in the N-terminal BRCT domain of Rev1 or that are deficient for Rev1. We provide evidence that Rev1 plays a coordinating role in two modes of DNA damage bypass, i.e., an early and a late pathway. The cells carrying the deletion in the BRCT domain are deficient for the early pathway, reflecting a role of the BRCT domain of Rev1 in mutagenic translesion synthesis. Rev1-deficient cells display a defect in both modes of DNA damage bypass. Despite the persistent defect in the late replicational bypass of fork-blocking (6-4)pyrimidine-pyrimidone photoproducts, overall replication is not strongly affected by Rev1 deficiency. This results in almost completely replicated templates that contain gaps encompassing the photoproducts. These gaps are inducers of DNA damage signaling leading to an irreversible G2 arrest. Our results corroborate a model in which Rev1-mediated DNA damage bypass at postreplicative gaps quenches irreversible DNA damage responses.
Unrepaired DNA damage usually leads to an arrest of replicative polymerases. Nonetheless, prokaryotic and eukaryotic cells display progression of replication on damaged templates, allowing replication to be completed and averting replication fork collapse (12). Direct bypass of the fork-blocking lesion, permitting replication to proceed with little delay, would be an attractive mechanism to release an arrested replicon. Several early studies using bacteria and mammalian cells, however, have indicated that replicating cells exposed to UV light initially synthesize smaller DNA strands than undamaged cells (reviewed in reference 32). At a later stage, these molecules are converted into DNA of high molecular weight, possibly via late, postreplicative, filling of the lesion-containing gap by so-called postreplication repair. The presence of single-stranded gaps in both sister chromatids behind a replication fork was recently visualized by electron microscopy on DNA of UV-exposed budding yeast Saccharomyces cerevisiae (34).
Damage avoidance and translesion synthesis are two pathways that allow cells to replicate damaged templates (3, 12). Damage avoidance (also called template switching-dependent synthesis) uses the undamaged sister chromatid as a template. This can be achieved by replication fork regression or by strand invasion with the sister chromatid and results in error-free bypass of DNA lesions (7, 65). Translesion synthesis, on the other hand, is characterized by insertion of a nucleotide opposite the lesion by specialized DNA polymerases of the Y family (48). The reduced stringency of the active site and a lack of proofreading activity of translesion synthesis polymerases imply that translesion synthesis is an inherently mutagenic process.
The Y family polymerase Rev1 is proposed to play a central role in translesion synthesis in lower and higher eukaryotic cells (13). Rev1 has a unique and highly distributive deoxycytidyl transferase activity, incorporating deoxycytidines opposite guanine, uracil, abasic sites, and a variety of damaged guanines in vitro (20, 21, 39, 43, 66). The in vivo function of the catalytic domain of Rev1 in translesion synthesis in mammalian cells has not been fully elucidated although we previously showed that it is required for the generation of G·C to C·G transversions during somatic hypermutation of immunoglobulin genes (27). This process most likely involves Rev1-mediated translesion synthesis on abasic sites. For various other DNA lesions, such as UV photoproducts, Rev1 plays a poorly defined noncatalytic role in mutagenic translesion synthesis in vivo that largely depends on its N-terminal BRCT domain (28, 33, 42). The C terminus of mammalian Rev1 is important for its intranuclear localization and interacts with other Y family translesion synthesis polymerases (Pols), including Pol η, Pol κ, and Pol ι (2, 16, 41, 45, 54), and Rev7, a subunit of the B family DNA Pol ζ (38, 41, 44). These interactions have led to a model describing Rev1 as a protein that promotes the switch between translesion synthesis Pols, inserting nucleotides opposite damaged nucleotides, and B family Pols that perform the extension step in translesion synthesis (13, 51).
Primer extension studies suggest that Rev1 may act in translesion synthesis at stalled replication forks. Thus, in vitro Rev1 can stimulate Rev3, the catalytic subunit of the B family DNA Pol ζ, to extend from nucleotides incorporated opposite various DNA adducts (1, 19, 61). Translesion synthesis in S. cerevisiae depends on monoubiquitination of the essential DNA sliding clamp PCNA, which is induced upon replication fork stalling (24). Recently, it was found that Rev1 physically interacts with PCNA (17, 49, 63). The nature of this interaction is controversial since one study indicated that it may require the C-terminal part of Rev1 (49), and another provided evidence that the N-terminal BRCT domain of Rev1 mediates PCNA binding (17). In addition, a ubiquitin-binding motif has been identified in the C terminus of Rev1, and this was suggested to mediate a functional interaction of Rev1 with ubiquitinated PCNA (18, 63). Monoubiquitination of PCNA and the stalling of the replicative polymerase regulate the exchange between replicative Pol δ and Pol η and, similarly, monoubiquitinated PCNA might also facilitate switching between Rev1 and the replicative polymerase (67).
It was proposed recently that Rev1 mediates translesion synthesis by direct extension of stalled replicons but in a ubiquitinated PCNA-independent fashion (10). However, Rev1 was also proposed to be essential for translesion synthesis at lesion-containing postreplicative gaps (26). To unravel the contribution of Rev1 in the regulation of DNA damage bypass in mammalian cells in vivo, we have analyzed progression of replication on damaged DNA in UV-exposed mouse embryonic fibroblasts (MEFs) that were either fully deficient for Rev1 (27) or contained a deletion of the BRCT domain of Rev1 (28). We found that Rev1 is essential for two temporally distinct modes of DNA damage bypass that may depend on postreplicative gap filling. The earliest mode of bypass involves translesion synthesis, mediated by the BRCT domain of Rev1. Rev1-deficient cells accumulate gaps opposite UV-induced (6-4)pyrimidine-pyrimidone photoproducts [(6-4)PP] while replicating their genome to near completeness. The resulting single-stranded DNA gaps are potent inducers of DNA damage signaling, resulting in a cell cycle arrest at G2/M.
MATERIALS AND METHODS
Isolation, immortalization, and culturing of MEFs.
Wild-type, RevB/B, and Rev1−/− MEFs of hybrid 129/Ola and C57BL/6 background were isolated from embryos of crosses of RevB/− or Rev1+/− mice (27, 28). RevB/B Xpc−/−, Rev1−/− Xpc−/−, and Xpc−/− MEFs were isolated from crosses of Rev1+/B Xpc−/− or Rev1+/− Xpc−/− mice that were derived from crosses between Xpc+/− mice (8) and RevB/B or Rev1+/− mice, respectively. Mouse embryonic fibroblasts (MEFs) were isolated by trypsinization of finely minced, 13.5-day-old embryos at 37°C for 15 min. MEFs were cultured in MEF medium (Dulbecco's modified Eagle's medium containing 4,500 mg/liter glucose, GlutaMax, and pyruvate [Invitrogen]) supplemented with 10% heat-inactivated fetal calf serum, antibiotics, and 0.4 μM 2-mercaptoethanol (Invitrogen) at 37°C and 5% CO2. The cells were immortalized by infection with a retroviral vector expressing a short hairpin RNA against mouse p53 (9).
UV exposure and survival assays.
UV-C exposure (Philips TUV lamp, predominantly 254 nm) was performed on exponentially growing cells washed once with phosphate-buffered saline (PBS). Fresh MEF medium was added to the cells after UV-C exposure.
Sensitivity of MEFs to UV-C light was determined by clonogenic survival. Log phase cells were irradiated with UV-C doses up to 10 J/m2, trypsinized, and counted. A fixed number of cells was seeded in p90 dishes and incubated for 10 days at 5% CO2 and 37°C. Clones were fixed, stained with methylene blue, and counted. The cloning efficiency of unexposed cells was set at 100%.
Labeling of replicating DNA and generation and visualization of DNA fibers.
Exponentially growing MEFs were pulse labeled with 25 μM iododeoxyuridine (IdU) at 37°C for 20 min, mock treated or exposed to either 20 J/m2 or 40 J/m2 UV-C light, and labeled with 250 μM bromodeoxyuridine (BrdU) for 20, 40, or 60 min. Isolation of DNA fibers and immunolabeling were carried out as described previously (25). Briefly, 2 μl of cells resuspended in PBS (106 cells/ml) was diluted 1:1 with unlabeled cells and spotted onto cleaned glass slides. Cells were lysed with 7 μl of 0.5% sodium dodecyl sulfate (SDS) in 200 mM Tris-HCl (pH 5.5)-50 mM EDTA (6 min at 20°C). Slides were tilted at 15° to horizontal, allowing a stream of DNA to run slowly down the slide. Next, slides were air dried and then fixed in methanol-acetic acid (3:1). Fixed DNA fiber spreads were hydrated and denatured (2.5 M HCl for 1 h). BrdU incorporation was detected using anti-BrdU (1:1,000; Ab-Direct Serotech) and AlexaFluor 488-labeled goat anti-rat antibody (1:1,000; Molecular Probes, Inc.) antibodies. Mouse anti-IdU/BrdU (1:1,000; Caltag Laboratories) and Cy3-labeled sheep anti-mouse antibody (1:1,000; Sigma Aldrich) were used to detect IdU incorporation. Finally, slides were thoroughly rinsed and mounted in mounting medium containing glycerol and 4′,6′-diamidino-2-phenylindole (DAPI). Spread and immunolabeled DNA fibers were imaged using an Olympus confocal laser scanning microscope. The lengths of DNA tracts corresponding to IdU and BrdU labeling were measured using Olympus software, and IdU/BrdU ratios were analyzed.
Alkaline DNA unwinding.
The progression of replicons, as measured by alkaline DNA unwinding, was essentially performed as described previously (29). Briefly, 105 MEFs were seeded per well of a 24-well plate and cultured overnight. Subsequently, MEFs were pulse labeled with [3H]thymidine (2 μCi/ml; 76 Ci/mmol) for 30 min, washed with PBS, immediately exposed to 10 J/m2 UV-C, and cultured in medium for up to 6 h. In some experiments the cells were incubated after UV irradiation in MEF medium containing [3H]thymidine (5 μCi/ml; 76 Ci/mmol). The cells were washed twice with 0.15 M NaCl and incubated in the dark on ice in 0.5 ml of ice-cold unwinding solution containing 0.15 M NaCl and 0.03 M NaOH for 30 min. Unwinding was terminated by forceful injection of 1 ml of 0.02 M NaH2PO4. The cell lysates were sonicated for 15 s using a Sonifier 250 apparatus (Branson); SDS was added up to 0.25%, and the plates were stored at −20°C. To separate single-stranded DNA (ssDNA) from double-stranded DNA, hydroxyl apatite columns were washed with 0.5 M K2HPO4 followed by 10 mM NaH2PO4 (pH 6.8). After each cell lysate was loaded, the columns were washed twice with 10 mM NaH2PO4 (pH 6.8). ssDNA was eluted with 0.13 M K2HPO4 (pH 6.8), and double-stranded DNA was eluted with 0.25 M K2HPO4 (pH 6.8). Radioactivity was quantified by liquid scintillation counting.
Alkaline sucrose gradient sedimentation.
Elongation of nascent DNA in UV-exposed MEFs and the average molecular mass of nascent DNA fragments were determined as described previously (59, 60). Briefly, cells were prelabeled in MEF medium containing [14C]thymidine for approximately 24 h to label parental DNA. After exposure to 5 or 10 J/m2 of UV-C, MEFs were incubated for up to 6 h in MEF medium containing [3H]thymidine to label nascent DNA. Cells were collected, and a small aliquot of a concentrated cell suspension was permeabilized by freeze-thawing twice. Subsequently, the cell lysate was treated with T4 endonuclease V, which cuts specifically at cyclobutane pyrimidine dimer (CPD) lesions (14). Following unwinding on top of a linear alkaline sucrose gradient, gradients were centrifuged in a Beckman SW55Ti rotor at 40,000 × g for 123 min (ω2t = 1.3 × 1011 rads2/s) at 20°C. The gradients were fractionated, and each fraction was assayed for radioactivity (60). Digestion of [14C]thymidine-labeled parental DNA with T4 endonuclease V results in a size distribution of DNA fragments that is indicative for the inter-CPD distance. This distribution remains constant in time since CPDs are poorly removed in mouse cells (14). When daughter-strand DNA becomes larger than the inter-CPD distance, the replication fork has bypassed the CPD.
Construction of gapped plasmids containing a site-specific UV lesion and translesion synthesis assay in mammalian cells.
The generation of gapped plasmids containing a site-specific (6-4)PP or CPD was described previously (22). The quantitative translesion synthesis assay using gapped plasmids containing a defined lesion was performed as described previously (22). Briefly, wild-type and Rev1−/− MEFs were transfected with a plasmid mixture containing the carrier plasmid pUC18, a normalizing gapped plasmid without a lesion (chloramphenicol resistant [Cmr]), and a gapped plasmid containing either a (6-4)PP or a CPD at a TT dimer in the single-strand region (kanamycin resistant [Kanr]). Twenty-four hours after transfection, plasmids were isolated using alkali. An indicator Escherichia coli strain was transformed with the mixture, and transformants were selected on agar plates containing kanamycin and chloramphenicol. Efficiency of gap filling was calculated by dividing the number of Kanr colonies by the number of Cmr colonies.
Cell cycle analysis.
For cell cycle analysis, wild-type, Xpc−/−, Rev1−/−, Rev1B/B Xpc−/−, and Rev1−/− Xpc−/− primary MEFs from passage two were seeded at a density of 106 cells per 90-mm dish and the next day were exposed to 0 or 2 J/m2 of UV-C. Immediately after UV-C exposure, replicating cells were subsequently pulse labeled in MEF medium with 10 μM BrdU for 30 min and chased in MEF medium with 5 μM thymidine. Cells were trypsinized and fixed in 70% ethanol at 6 to 48 h after UV-C exposure. BrdU incorporation in ethanol-fixed cells was determined using a monoclonal mouse anti-BrdU antibody (M0744) and a fluorescein isothiocyanate-conjugated rabbit anti-mouse immunoglobulin G1 antibody (553443; BD Pharmingen) as described previously (52). DNA was labeled using propidium iodide (33 μg/ml). DNA content and BrdU incorporation were determined in 10,000 cells using a FACSCalibur apparatus (Becton Dickinson) and analyzed using WINMDI software (The Scripps Research Institute, La Jolla, CA).
Immunostaining of (6-4)PP and CPD.
Immortalized Rev1−/− Xpc−/− and Xpc−/− MEF lines were seeded on coverslips at a density of 2.5 × 105 cells per well in a six-well plate. The next day, the coverslips with exponentially growing cells were exposed to 0 or 5 J/m2 of UV-C. Immediately after UV irradiation, medium containing 300 ng/ml of nocodazole was added. Sixteen hours after UV exposure, cells were fixed with 2% formalin containing 0.2% Triton X-100 for 15 min, washed three times with PBS, and stored up to 2 days in +4°C until used. For immunostaining the coverslips with irradiated cells were blocked with 3% bovine serum albumin in PBS-0.015% Tween 20 for 30 min at room temperature and incubated with anti-(6-4)PP or anti-CPD antibody (1:400; clone 64 M-2 or TDM2, respectively; MBL, Japan) for 2 h in PBS-0.015% Tween 20 containing 0.5% bovine serum albumin. To visualize the signal, goat anti-mouse AlexaFluor 488-conjugated secondary antibody (1:1,000, Molecular Probes, Leiden, The Netherlands) was used. Cells were counterstained with DAPI as a nuclear dye, the coverslips were mounted on the glass slides, and the samples were analyzed by fluorescent microscopy.
Western blot analysis.
One million MEFs were exposed to 5 J/m2 UV-C and cultured for up to 8 h before preparation of protein extracts and Western blot analysis. MEFs were lysed in 1× protein sample buffer, and proteins were separated by 10% SDS-polyacrylamide gel electrophoresis, followed by overnight transfer at 4°C to Hybond-P membranes (Amersham Biosciences) in transfer buffer consisting of 24.8 mM Tris base, 192 mM glycine, and 20% methanol at 1 mA/cm2. Membranes were incubated with a mouse monoclonal antibody against β-actin (Calbiochem) or with a rabbit polyclonal antiserum against Chk1 phosphorylated at Ser 317 (Bethyl). After incubation with secondary antibodies conjugated to peroxidase (Bio-Rad), proteins were visualized by enhanced chemiluminescence detection.
RESULTS
Differential UV sensitivities of Rev1B/B and Rev1−/− MEFs.
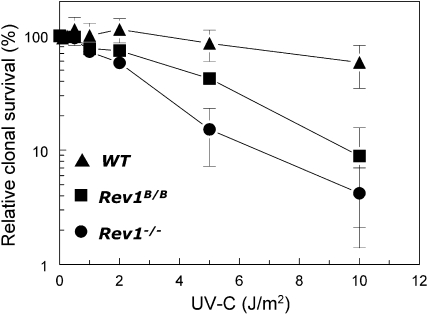
To elucidate the role of Rev1 in responses to UV-C light, we generated immortalized MEF lines homozygous for a targeted disruption of two exons encoding a part of the N-terminal BRCT domain of Rev1 (Rev1B/B MEFs) (28) that resulted in a shortened Rev1 protein or of the catalytic domain that produced an out-of-frame C terminus resulting in Rev1 deficiency (Rev1−/− MEFs) (27). An immortalized isogenic wild-type MEF line was generated as a control. These cell lines were exposed to a range of UV-C doses, and clonogenic survival was assayed to investigate their sensitivity to UV-C light (Fig. 1). Rev1−/− MEFs were more sensitive to UV-C light than wild-type MEFs, whereas Rev1B/B MEFs were only moderately sensitive. These results indicate that the Rev1 BRCT domain plays a role in protection against UV-induced lethality and that other domains of Rev1 may have roles additional to the BRCT domain.
FIG. 1.
UV hypersensitivity of Rev1-mutant MEF lines. The clonogenic survival of unexposed cells was set at 100%. Data represent the average of three independent experiments. WT, wild type.
The Rev1 BRCT domain is essential for an early pathway for bypassing UV-damaged DNA.
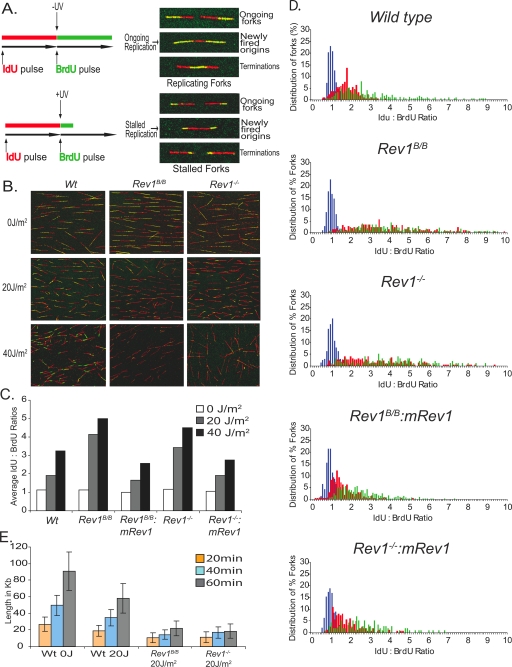
DNA fiber labeling is a sensitive assay to determine the progression of replicons on single DNA molecules in vivo, shortly after exposure to UV-C (Fig. 2A). To test whether the different UV sensitivities of wild-type, Rev1B/B, and Rev1−/− MEF lines were caused by a difference in bypassing damaged DNA, early after exposure we performed DNA fiber labeling on these cell lines. Thus, cells were incubated with IdU for 20 min to label replicating DNA, exposed to 0 to 40 J/m2 UV-C, and subsequently incubated with BrdU for 20 to 60 min. Combed DNA was stained by specific antibodies for IdU and BrdU and visualized by fluorescent microscopy, and finally tract lengths and replication rates during IdU and BrdU labeling were determined (Fig. 2B to D). Under normal replication conditions, at an undamaged template, the ratio of IdU to BrdU is expected to be approximately 1 (Fig. 2C). Mock-treated wild-type, Rev1B/B, and Rev1−/− MEFs indeed showed a ratio of approximately 1 and displayed similar replication rates (see Fig. S1 in the supplemental material). This indicates that Rev1 is dispensable for replication fork progression on undamaged DNA. Following exposure to 20 J/m2 or 40 J/m2 UV-C, however, Rev1B/B and Rev1−/− MEFs showed much stronger, and very similar, increases in the ratio of IdU to BrdU than wild-type MEFs (Fig. 2C and D). This suggests a defect in an early DNA damage bypass mode in both Rev1-mutant cell lines. A time course experiment revealed that, following UV-C exposure, average tract lengths of nascent DNA fibers in Rev1B/B and Rev1−/− MEFs slightly increased in time although to a much smaller extent than in wild-type MEFs (Fig. 2E). Stable expression of a mouse Rev1 cDNA in Rev1B/B and Rev1−/− MEFs corrected the defect in fork progression (Fig. 2C and D), demonstrating that the phenotype is attributable to the Rev1 mutations. Since Rev1B/B and Rev1−/− MEFs display similar phenotypes, we conclude that early progression of forks stalled at UV damage requires a functional Rev1 BRCT domain.
FIG. 2.
UV-exposed Rev1B/B and Rev1−/− MEFs display similar reductions in tract lengths of DNA fibers. (A) Schematic representation of replication labeling and representative replication forks during ongoing and stalled replication. (B) Representative set of DNA fibers of wild-type (Wt), Rev1B/B, and Rev1−/− MEFs exposed to 0, 20, or 40 J/m2 UV-C. (C) Average ratio of IdU to BrdU in wild-type, Rev1B/B, and Rev1−/− MEFs and Rev1 mutant MEFs complemented with mouse Rev1. (D) Distribution of percentages of replication forks at corresponding IdU/BrdU ratios. Blue bars, 0 J/m2 UV-C; red bars, 20 J/m2 UV-C; green bars, 40 J/m2 UV-C. (E) Length of replicating forks during BrdU labeling in unexposed wild-type cells and wild-type, Rev1B/B, and Rev1−/− MEFs at 20, 40, and 60 min after exposure to 20 J/m2 UV-C.
Rev1 but not its BRCT domain is essential for a late pathway of DNA damage bypass.
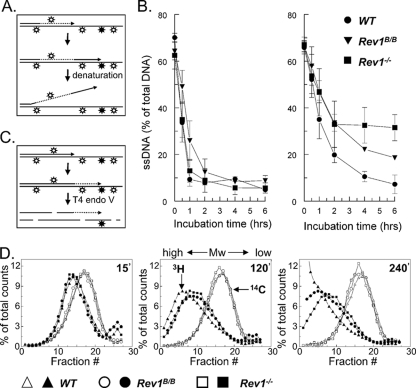
The early progression of forks stalled at UV photoproducts was defective to a similar extent in Rev1B/B and Rev1−/− MEFs, as judged from the fiber analyses. Therefore, the difference in UV-C sensitivity between these cell lines must be explained by another function of Rev1, possibly also in DNA damage bypass. We asked whether Rev1B/B and Rev1−/− MEFs differ in the progression of arrested replicons at later times after UV exposure. To this aim we measured DNA damage bypass in MEFs up to 6 h after exposure by using an alkaline DNA unwinding assay (29). In this assay lack of progression of arrested replicons in cells pulse-labeled with [3H]thymidine just before UV treatment is evidenced by the persistence of [3H]thymidine at locally unwound DNA ends (Fig. 3A). No difference in fork progression was found between untreated cell lines (Fig. 3B, left panel), underscoring that a deficiency in Rev1 does not significantly affect the rate of replication on undamaged DNA. Following treatment with 10 J/m2 UV-C, a dose that is virtually nontoxic to wild-type MEFs (Fig. 1), these cells show only a slight delay in the progression of replicons. During the first 2 h after exposure, in Rev1−/− and Rev1B/B MEFs the progression of replicons is perturbed to the same extent, which is in agreement with the defect in early DNA damage bypass observed by DNA fiber labeling. At the UV-C dose used, part of the forks will not encounter a blocking lesion before the replication fork is 30 to 50 kbp beyond the [3H]thymidine label. This explains the residual progression of replicons in Rev1−/− and Rev1B/B MEFs at early time points after treatment. Starting 2 h after exposure, Rev1−/− MEFs display a strong and persistent arrest (Fig. 3B, right panel). This indicates that UV damage is not further bypassed in these cells. In contrast, replicons in Rev1B/B MEFs continue to progress with kinetics similar to the wild-type MEFs (Fig. 3B, right panel).
FIG. 3.
Delayed progression of replicons in Rev1 mutant cells. (A) Scheme of the alkaline DNA unwinding assay. Nascent DNA is pulse labeled with [3H]thymidine (dotted line) just before induction of UV damage (open and closed signs) (top). Cells were cultured in medium without label (middle). Stalling of a fork at a UV lesion results in a DNA end containing [3H]thymidine that is locally denatured using alkaline, followed by analysis using hydroxyl apatite (bottom). (B) Level of [3H]thymidine in ssDNA after alkaline unwinding. Results for mock-treated cells are shown in the left panel. Right panel shows results for cells exposed to 10 J/m2 UV-C. (C) Scheme of alkaline sucrose gradient sedimentation using T4 endonuclease V. Template DNA was uniformly labeled with [14C]thymidine (solid line) followed by exposure to 10 J/m2 UV-C inducing CPD (open symbols) and (6-4)PP (closed symbols) (top). Elongating daughter strands were labeled with [3H]-thymidine (dotted line; middle). At different time points, cells were lysed, and [14C]thymidine containing DNA was cleaved by T4 endonuclease V at a CPD, followed by size fractionation using alkaline sucrose gradients (bottom). The [14C]thymidine-labeled inter-CPD size distribution serves as an internal standard since CPDs are not removed in mouse cells. (D) Alkaline sucrose gradient profiles of wild-type, Rev1B/B, and Rev1−/− MEF lines at 15, 120, and 240 min after exposure to 10 J/m2 UV-C. WT, wild type; Mw, molecular weight.
To explain the difference in progression of replicons between both Rev1 mutants late after UV treatment, we hypothesized that although in Rev1B/B MEFs an early pathway for DNA damage bypass is defective, a late pathway for DNA damage bypass may be normal. This hypothesis was tested using a sensitive alkaline sucrose gradient-based assay that measures maturation of nascent DNA beyond an internal standard comprised of template fragments of intra-CPDs (Fig. 3C) (60). When exposed to UV-C, Rev1−/− and Rev1B/B MEFs generated nascent strand molecules that are larger than the average inter-CPD distance, indicating that Rev1 is dispensable for replicative bypass of most CPDs in vivo (Fig. 3D). In agreement with the alkaline DNA unwinding assays, no apparent differences in fork progression were observed between Rev1−/− and Rev1B/B MEF lines during the first 2 h following UV exposure. At 4 h after irradiation, however, the percentage of mature DNA molecules is considerably lower in the Rev1−/− MEF line than in the Rev1B/B MEFs (Fig. 3D). The apparent residual progression of replicons in the Rev1−/− cells probably is caused by nucleotide excision repair (NER) of stalling lesions before arrival of the fork (see below). This result further substantiates the notion that only the Rev1−/− MEF line is deficient in a late pathway for DNA damage bypass, as was suggested by the alkaline DNA unwinding assay.
Bypass of episomal (6-4)PP and CPD is affected in Rev1 mutants.
To define the involvement of Rev1 in translesion synthesis, we used a quantitative translesion synthesis assay, based on a transfected plasmid containing a site-specific (6-4)PP or CPD in a single-strand gap (4, 5). The results show a more than threefold reduction in translesion synthesis events at (6-4)PP in Rev1−/− MEFs compared to the wild type (Table 1). Analysis of mutational spectra revealed that in wild-type MEFs, most translesion synthesis events at (6-4)PP are mutagenic and depend on Rev1, in particular, a transversion of G·C to T·A at the guanine immediately 5′ to the lesion (Table 2). In this assay also translesion synthesis of a CPD-TT is affected to some extent in Rev1−/− MEFs (Tables 1 and 2). Rev1B/B MEFs displayed a milder defect in translesion synthesis of both damages than Rev1−/− MEFs. Although it is unknown whether this assay completely mimics damage bypass at the genome, it reveals a clear contribution of Rev1 in mutagenic translesion synthesis of (6-4)PP photoproducts.
TABLE 1.
Rev1-dependent gap filling on DNA templates containing a defined UV lesiona
| Cell line | Template with (6-4)PP-TT
|
Template with CPD-TT
|
||||||
|---|---|---|---|---|---|---|---|---|
| No. of Kanr colonies | No. of Cmr colonies | Gap repair (%)b | TLS (%)c | No. of Kanr colonies | No. of Cmr colonies | Gap repair (%)b | TLS (%)c | |
| Wild type | 922 ± 205 | 1,280 ± 244 | 72 ± 3 | 72 ± 3 | 403 ± 60 | 416 ± 85 | 98 ± 7 | 94 ± 6 |
| Rev1B/B | 561 ± 123 | 1,122 ± 198 | 50 ± 4 | ND | 280 ± 57 | 413 ± 55 | 67 ± 7 | ND |
| Rev1−/− | 304 ± 30 | 1,160 ± 163 | 26 ± 2 | 22 ± 2 | 169 ± 11 | 368 ± 63 | 48 ± 14 | 41 ± 12 |
Values are means ± standard deviations.
Ratio of Kanr colonies to Cmr colonies.
TLS, translesion synthesis; ND, not determined. Only plasmids containing no deletions are included (Table 2).
TABLE 2.
Translesion synthesis events in wild-type and Rev1-deficient MEFs
| Nucleotide(s) inserted opposite lesiona | No. (%) of colonies with events per genotype at UV lesion:
|
|||
|---|---|---|---|---|
| (6-4)PP-TT
|
CPD-TT
|
|||
| Wild type | Rev1−/− | Wild type | Rev1−/− | |
| C-AA-C | 8 (17) | 24 (55) | 41 (87) | 33 (85) |
| A-AA-C | 1 (2) | 2 (4) | ||
| G-AA-C | 1 (2) | |||
| C-TA-C | 1 (2) | |||
| C-AC-C | 1 (2) | |||
| C-AG-C | 2 (4) | |||
| C-AT-C | 2 (4) | 2 (5) | ||
| C-AA-A | 20 (43) | 4 (9) | ||
| C-AA-G | 1 (2) | |||
| C-AA-T | 2 (5) | |||
| Δ-AA-C | 1 (2) | |||
| C-AΔ-C | 2 (4) | 1 (2) | ||
| C-GT-C | 1 (2) | |||
| C-AC-A | 1 (2) | |||
| C-AT-A | 1 (2) | |||
| C-GA-A | 4 (9) | 2 (5) | ||
| C-TA-A | 3 (6) | 1 (2) | ||
| A-AA-A | 1 (2) | |||
| Overall totalb | 47 (100) | 44 (100) | 47 (100) | 39 (100) |
| Total mutagenic | 39 (83) | 14 (32) | 4 (9) | |
Inserted nucleotides are in boldface. Δ indicates one nucleotide deletion.
Numbers of colonies with deletions/insertions were as follows: for (6-4)PP-TT in Rev1−/− cells, 6 (14%); for CPD-TT in wild-type and Rev1−/− cells, 2 (4%) and 6 (15%), respectively.
Rev1 is required for efficient progression through S and G2 phases.
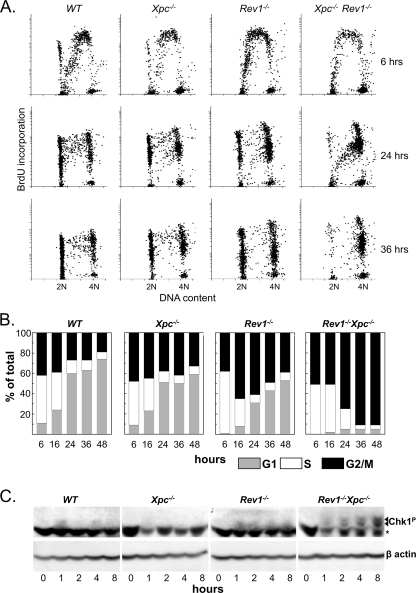
Based on these data, we hypothesized that Rev1 may be required for filling of postreplicative gaps opposite (6-4)PP by translesion synthesis. This predicts that in the absence of Rev1, most of the genome should still be replicated, despite the persistence of nonbypassed (6-4)PP. We wanted to investigate this by analyzing S-phase progression of primary MEFs using flow cytometry. To maximize the sensitivity of these assays we introduced complete deficiency for the NER protein Xpc, rendering the cells virtually unable to remove (6-4)PP. Compared to Xpc−/− MEFs, UV-C-exposed Rev1−/− Xpc−/− MEFs progress much more slowly through the S phase and ultimately arrest indefinitely at the G2 phase, at a ploidy that is indistinguishable from 4N (Fig. 4A and B). Similar results were obtained using immortalized MEF lines irradiated with 5 J/m2 UV-C (data not shown). Thus, the genome appears replicated to completion in Rev1−/− Xpc−/− MEFs, despite persistently blocked replication forks at most (6-4)PP (see also below). Compared with Rev1−/− Xpc−/− MEFs, Rev1−/− MEFs display a more rapid progression through S and late S/G2 phases. This suggests that NER removes most (6-4)PP prior to the arrival of replication forks, thereby preventing persistent fork arrests in Rev1−/− MEFs (Fig. 4A and B). Virtually no defect in cell cycle progression was seen in the UV-C-treated Xpc−/− MEFs, indicating that, owing to Rev1-mediated bypass of the large number of unrepaired (6-4)PP, cell cycle responses are not triggered to any major extent. UV-C-exposed Rev1B/BXpc−/− MEFs showed cell cycle progression similar to Xpc−/− cells (see Fig. S2 in the supplemental material), in support of the notion that the BRCT domain of Rev1 is not essential for late DNA damage bypass pathways of photoproducts. In mock-treated MEFs, cell cycle progression was similar for all cell populations tested (data not shown). These data support our previous results indicating that efficient bypass of (6-4)PP by a late, postreplicative, DNA damage bypass pathway requires Rev1 and suggest that, despite the defect in the Rev1-deficient mutant, virtually the entire genome is replicated.
FIG. 4.
Reduced S and G2/M phase progression in Rev1-deficient MEFs and induction of DNA damage signaling. (A) Cell cycle profiles of primary MEFs pulse labeled with BrdU immediately after exposure to 2 J/m2 of UV-C, followed by chase, to determine S-phase progression. (B) Quantification of UV-C exposed cells in G1, S, and G2/M. (C) Western blots showing induction of Chk1 phosphorylation by UV in mutant cell lines (arrows). The asterisk indicates a cross-reacting band. β-Actin served as a loading control. WT, wild type.
Rev1 is essential for postreplicative gap filling.
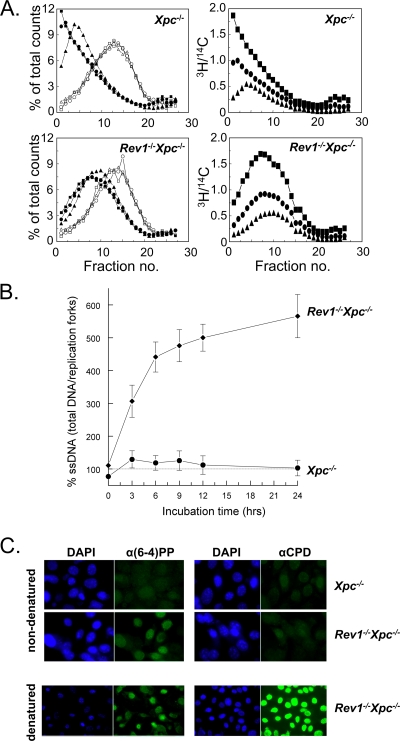
The progression of UV-exposed Rev1−/− Xpc−/− MEFs through the S phase predicts that small gaps should persist opposite (6-4)PP and that replication continues between (6-4)PP in the template in mammalian cells. To analyze this, we performed an alkaline sucrose sedimentation assay using Rev1−/− Xpc−/− and Xpc−/− MEF lines that do not remove (6-4)PP (Fig. 5A, left panel). In the Rev1−/− Xpc−/− MEF line, the length of nascent DNA fragments hardly increases beyond 4 h after UV treatment, whereas Xpc−/− MEFs display normal daughter strand maturation (Fig. 5A, left panel). This demonstrates a strong dependence on Rev1 for the bypass of photoproducts. The molecular mass of the stagnant fragments in the Rev1−/− Xpc−/− MEF line is less than threefold larger than that of the inter-CPD molecules (data not shown). Since the ratio between UV-C-induced (6-4)PP and CPD is approximately 1 to 3 (14), this underscores our previous results that forks arrest at most of the (6-4)PP, but not at the CPD, in Rev1-deficient cells. Importantly, although in Rev1−/− Xpc−/− MEFs the size of the replicated fragments hardly increases after 4 h, these cells continue to incorporate [3H]thymidine for at least 6 h at nearly wild-type rates (Fig. 5A, right panels) in inter-(6-4)PP daughter fragments. The rate of incorporation is completely independent of the size of the intra-(6-4)PP daughter fragments. These data suggest that most of the genome is replicated, which is in agreement with the observed progression of Rev1−/− Xpc−/− MEFs to 4N ploidy (Fig. 4A and B). This result also implies that replication of damaged templates in mammalian cells continues beyond nonbypassed (6-4)PP, both in the lagging and the leading strands.
FIG. 5.
Identification of strand discontinuities in Rev1-deficient MEFs. (A) At left are alkaline sucrose gradient profiles of Xpc−/− and Rev1−/− Xpc−/− MEF lines at 2 h (triangles), 4 h (circles), and 6 h (squares) after exposure to 5 J/m2 UV-C. Open symbols, [14C]thymidine-labeled DNA; closed symbols, [3H]thymidine-labeled DNA. Panels at right show a continuation of replication during time depicted as the ratio of [3H]thymidine counts per fraction and the total amount of [14C]thymidine counts in the gradient. (B) Alkaline DNA unwinding of DNA that was pulse labeled by [3H]thymidine before and continuously labeled for up to 24 h after exposure to 10 J/m2 UV-C. For each time point, the percentage of ssDNA from total DNA of mock-treated cells was set at 100%. (C) Immunostaining of (6-4)PP and CPD in Xpc−/− and Rev1−/− Xpc−/− MEFs exposed to 5 J/m2 UV-C using antibodies specifically recognizing UV lesions in ssDNA. α, anti.
We predicted that the generation of gaps at persistent fork arrests will result in the enhanced formation of substrates for local DNA strand unwinding using alkaline, as described above. To provide further evidence that strand discontinuities persist in Rev1−/− Xpc−/− MEFs, we performed an alkaline DNA unwinding experiment in which nascent DNA was continuously labeled with [3H]thymidine both prior to and after UV treatment. UV-exposed Xpc−/− MEFs barely displayed an increase in the formation of labeled ssDNA after alkaline treatment compared to unexposed cells (Fig. 5B). This result indicates that this cell line does not accumulate persistent strand interruptions during replication of damaged DNA. However, a strong time-dependent increase in the number of strand discontinuities after alkaline denaturation was found for the Rev1−/− Xpc−/− MEF line (Fig. 5B). This result supports the model that Rev1 is essential for replicative bypass of UV damages via a postreplicative gap-filling process. We exclude the possibility that the increase in the formation of strand discontinuities is predominantly caused by induction of double-strand DNA breaks as a consequence of apoptosis since the cells showed no significant apoptotic response to UV (see Fig. S3 in the supplemental material).
Based on these data, we anticipated the persistence of ssDNA gaps containing replication fork-blocking lesions in Rev1−/− Xpc−/− cells. To investigate this, UV-exposed Xpc−/− and Rev1−/− Xpc−/− MEFs were accumulated at G2/M using nocodazole, and cells were subsequently stained for UV lesions using antibodies that are specific for CPD or (6-4)PP embedded in (unreplicated) ssDNA. Fluorescence microscopy revealed that unreplicated (6-4)PP were readily detectable in nondenatured DNA of Rev1−/− Xpc−/− MEFs arrested after S phase but not in Xpc−/− mutant MEFs (Fig. 5C). This result confirms that Rev1 is required for gap filling opposite genomic (6-4)PP. Although CPDs were easily detected in denatured DNA of both cell lines, no significant staining was found for CPD in both cell lines (Fig. 5C). This again is consistent with the notion that most genomic CPDs are not a substrate for Rev1-mediated bypass.
Photoproducts embedded in ssDNA gaps induce DNA damage signaling.
We have demonstrated above that Rev1 deficiency results in patches of single-stranded template DNA containing unreplicated (6-4)PP. This implies the presence of free 5′ primer-template junctions at the other end of the single-strand gap relative to the fork-blocking lesions. In S. cerevisiae, free 5′ primer-template junctions bind the Rad9-Rad1-Hus1 (9-1-1) clamp that is involved in triggering an ATR-mediated DNA damage signal (36). We previously predicted that a postreplicative gap opposite a fork-blocking lesion would provide such a free primer-template junction and therefore might be instrumental in DNA damage signaling (26). To investigate this, we monitored phosphorylation of the DNA damage response transducer Chk1 (11) in Rev1−/− Xpc−/− MEFs after irradiation with UV-C (5 J/m2). Indeed, a significant phosphorylated (at Ser317) Chk1 signal was detected only in the Rev1−/− Xpc−/− MEFs that accumulate most postreplicative gaps but not in wild-type, Xpc−/−, or Rev1−/− MEFs (Fig. 4C). This result indicates that the gaps that oppose fork-blocking damages are indeed inducers of the DNA damage response and is consistent with the irreversible arrest of UV light-exposed primary Rev1−/− Xpc−/− MEFs cells at G2.
DISCUSSION
In the present study we have investigated the role of Rev1 in the bypass of UV lesions in mammalian cells. We found that in UV-exposed Rev1-deficient MEFs, progression of replicons is severely compromised, resulting in the generation of DNA molecules that are not converted into high molecular weight. Nonetheless, Rev1-deficient MEFs progress through S phase while accumulating strand discontinuities that contain the replication fork-blocking lesions. The Rev1 BRCT domain is specifically required for efficient progression of stalled forks early after UV exposure. Our results suggest that mammalian Rev1 acts not only in this early translesion synthesis pathway, involving its BRCT domain, but also in a slower postreplication repair pathway, which occurs independently of the BRCT domain of Rev1.
The DNA fiber assay is a powerful method to study the progression of replicons on individual DNA molecules early after UV treatment. Although this assay does not discriminate between the direct extension of stalled forks and early translesion synthesis by filling of postreplicative gaps, the assay indicated that both Rev1 mutants are deficient in the extension of stalled forks within 1 h after UV treatment. This conclusion is supported by the results of the alkaline DNA unwinding assays that show an identical delay in damage bypass in both mutants in the first 2 h after UV treatment (Fig. 3B). This result contrasts with recent fiber assay data showing that the Rev1 BRCT domain does not play a significant role in early DNA damage bypass in chicken DT40 cells (10). The authors of this work used Rev1-disrupted cells that were complemented by a transfected Rev1 BRCT mutant gene driven by an exogenous promoter. This ectopic Rev1 construct may result in unregulated expression of the protein, which could mask the phenotype of the BRCT mutation. Our Rev1B/B mouse cells express the Rev1 mutant protein from the endogenous Rev1 promoter at a level that is similar to that of wild-type Rev1 (28). Expression from the endogenous promoter also ensures a possible cell cycle phase-dependent expression of Rev1 in mammalian cells, as was found for S. cerevisiae (62).
The plasmid-based translesion synthesis assays indicated that a (6-4)PP and, to a lesser extent, a CPD is substrate for Rev1-mediated translesion synthesis in mouse cells (Tables 1 and 2). These results support and extend previous studies showing that in S. cerevisiae and chicken DT40 cells, Rev1 is essential for (6-4)PP bypass on plasmids (15, 53). Although Rev1 can uniquely incorporate cytosines during replication, these nucleotides are virtually not incorporated at UV photoproducts in yeast and mammals (Table 2) (15, 28). For these reasons we infer that mammalian Rev1 has a regulatory or accessory, rather than a catalytic, role in translesion synthesis. The partial dependence of translesion synthesis, similar to yeast, opposite a CPD on mouse Rev1, as found in the plasmid-based assay, may provide an explanation for the hypermutability of CPDs in Pol η-deficient cells, which is the default translesion synthesis polymerase to replicate CPDs (22). In contrast to the plasmid-based assay, the alkaline sucrose sedimentation gradients indicated that the Rev1 mutant cells are proficient for the bypass of genomic CPDs. In support of this result, we were unable to demonstrate CPDs embedded in ssDNA using immunocytochemistry although (6-4)PP were readily detected (Fig. 5C). Thus, we hypothesize that in wild-type cells, Rev1-mediated mutagenic translesion synthesis of CPD is a minor pathway that acts as a backup for Pol η.
Our work indicates that both early translesion synthesis of genomic (6-4)PP as well as a late postreplicative pathway for DNA damage bypass, starting at 2 h after UV treatment, are severely compromised in Rev1-deficient cells whereas cells with deletions of Rev1 and BRCT are defective only in early DNA damage bypass (Table 1; Fig. 2 and 3). These results demonstrate divergent roles of the Rev1 BRCT domain and the rest of Rev1 in DNA damage bypass. In addition, the nearly complete defect in genomic (6-4)PP bypass in Rev1−/− MEFs indicates that in Rev1-deficient cells damage avoidance, using the undamaged sister chromatid as a template, may be a quantitatively minor process. Although we cannot formally exclude the possibility that in mammalian cells damage avoidance also requires Rev1, these data indicate that translesion synthesis is the major pathway of replicational bypass of (6-4)PP in MEFs. The contrasting finding that Rev1-deficient DT40 cells have wild-type levels of postreplication repair (10) may be explained by the observation that these cells display significant, Rev1-independent damage avoidance (53). This may obscure the defect of Rev1 in postreplicative translesion synthesis in these cells. Also in S. cerevisiae damage avoidance is a major mechanism for the replicative bypass of DNA damage (6, 65). This predominance of damage avoidance in yeast may explain the observation that in S. cerevisiae versus mammalian cells at least 100-fold fewer mutations per UV-induced DNA lesion are introduced (see Table S1 in the supplemental material). In S. cerevisiae, damage avoidance is mediated by a complex of Mms2-Ubc13-Rad5 that polyubiquitinates PCNA (55). The mammalian homologues of genes encoding the damage avoidance proteins Mms2 and Rad5 suppress DNA damage-induced chromosomal instability and mutagenesis to some extent (40, 56, 57). This suggests that mammals do have the capacity to perform damage avoidance.
Based on electron microscopy and two-dimensional gel electrophoretic studies of damaged DNA, it was proposed that in S. cerevisiae replication of both the leading and the lagging strands can reinitiate downstream of a fork-blocking lesion. The resulting gaps of up to a few thousand base pairs may be filled at a later stage by postreplicative translesion synthesis (34;reviewed in reference 32). In the Rev1−/− Xpc−/− MEF line exposed to 5 J/m2 UV-C, replication forks stall at (6-4)PP lesions that are separated by, on the average, 60 kbp (58). Nevertheless, efficient replication at intra-(6-4)PP fragments continues after UV treatment (Fig. 5A), and this replication does not depend on the size of these fragments. This process results in daughter DNA strands containing persistent discontinuities (Fig. 5B and C) while replication proceeds to a 4N genome complement (Fig. 4A). These results suggest that efficient replication continues directly beyond the lesion, both at the lagging and at the leading strands. This finding indicates also that in mammalian cells leading strand replication can reprime downstream of replication-blocking lesions, resulting in duplication of virtually the entire genome before arrest at G2. An alternative explanation for the efficient continuation of replication in UV-damaged Rev1−/− Xpc−/− MEFs is the activation of cryptic origins of replication at intra-(6-4)PP fragments. However, as most small intra-(6-4)PP fragments will lack a cryptic origin (31), firing of cryptic origins may not explain the efficient incorporation at small intra-(6-4)PP fragments in Rev1−/− Xpc−/− MEFs.
We recently proposed that Rev1 is recruited at 5′ primer-template junctions at postreplicative gaps (26), based on the finding that the Rev1-binding protein Rev7 interacts with the 9-1-1 clamp (46, 50) in S. cerevisiae. The 9-1-1 clamp is recruited to stalled forks independently of PCNA (46) at 5′ primer-template junctions in vitro (35, 36). As in S. cerevisiae cells, these junctions in mammalian cells might be generated in vivo by repriming of the replication fork downstream of the blocking lesion, resulting in the formation of ssDNA gaps encompassing the template damages. In vitro, Rev1 has the ability to translocate on ssDNA to a 3′ primer-template junction (37) where the stalled fork resides. This translocation reaction could allow Rev1 to perform its postulated noncatalytic function in switching between translesion and extending polymerases (13, 26). In support of epistatic roles for 9-1-1 and Rev1 in DNA damage bypass by postreplicative translesion synthesis, 9-1-1-mutated S. cerevisiae displays a similar hypomutable phenotype as Rev1 mutants (30, 33, 47, 63). In contrast to yeast, however, the direct interaction of translesion synthesis proteins with the 9-1-1 clamp in mammalian cells remains to be investigated.
We previously predicted (26) that the presence of lesion-containing single-stranded molecules with a free 5′ primer-template junction, induced by repriming of replication, provides a strong signal for the activation of ATR- and 9-1-1-mediated DNA damage signaling (36). Indeed, in the Rev1−/− Xpc−/− MEFs that accumulate gaps opposite (6-4)PP, phosphorylation of Chk1 was induced by UV-C, but no significant signaling was observed in Xpc−/− cells (Fig. 4C). Thus, Rev1-mediated postreplicative gap filling of unrepaired (6-4)PP suppresses DNA damage signaling. Conversely, an excess of unreplicated template damage during S phase induces an irreversible G2/M arrest (Fig. 4A and B), presumably triggered by ATR and 9-1-1. Along the same line, it was recently reported that a defect in damage avoidance at postreplicative gaps in S. cerevisiae triggers DNA damage responses in this organism when cells are exposed to chronic low-dose UV irradiation (23). Interestingly, substrates for ATR-mediated phosphorylation may interact with the BRCT domain of Rev1 (64), suggesting that ATR activation may regulate early DNA damage bypass via the BRCT domain of Rev1.
This study provides evidence for Rev1-mediated DNA damage bypass that comes in two modes that might be triggered by binding of Rev1 at 9-1-1 at a 5′ primer-template junction. First, Rev1 is involved in a relatively early DNA damage bypass pathway that might involve an interaction with monoubiquitinated PCNA, possibly mediated by interaction with the N-terminal BRCT domain of Rev1 (17, 49, 63). Depending on the type of lesion, Rev1 may incorporate deoxycytidine or could recruit another DNA Pol to perform translesion synthesis at the stalled replication fork. This mode of lesion bypass is mutagenic since Rev1B/B cells have lost UV-induced transversions at TT bipyrimidines (28). Second, Rev1, but not its BRCT domain, is required for postreplication repair by gap filling, which dominates at a later stage. This late role of Rev1 may represent a second translesion synthesis pathway although we cannot exclude the possibility that it denotes involvement of Rev1 in damage avoidance rather than in a secondary translesion synthesis pathway.
Although the precise molecular function of Rev1 in DNA damage bypass remains elusive, our data demonstrate catalytic and noncatalytic functions not only in multiple modes of DNA damage bypass but also in quenching DNA damage signaling. For this reason, we infer a central coordinating role for Rev1 in regulating the cellular outcome of exposure to genotoxic agents in mammalian cells.
Supplementary Material
Acknowledgments
We thank A. R. Filon, J. W. A. Verspuy, L. Carlée, and A. A. van Zeeland for valuable help.
This work was financially supported by grants from the Dutch Cancer Society (UL 2001-2517) and the European Union (IP FP6-512113) to N.D.W.; from the Flight Attendant Medical Research Institute, Florida, and the Israel Science Foundation (1136/08) to Z.L.; and from the Hungarian National Office for Research and Technology (KFKT-1-2006-0010) and Howard Hughes Medical Institute (55005612) to L.H.
Footnotes
Published ahead of print on 30 March 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Acharya, M. K., R. E. Johnson, S. Prakash, and L. Prakash. 2006. Complex formation with Rev1 enhances the proficiency of Saccharomyces cerevisiae DNA polymerase zeta for mismatch extension and for extension opposite from DNA lesions. Mol. Cell. Biol. 269555-9563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akagi, J. I., C. Masutani, Y. Kataoka, T. Kan, E. Ohashi, T. Mori, H. Ohmori, and F. Hanaoka. 2009. Interaction with DNA polymerase eta is required for nuclear accumulation of REV1 and suppression of spontaneous mutations in human cells. DNA Repair 8585-599. [DOI] [PubMed] [Google Scholar]
- 3.Andersen, P. L., F. Xu, and W. Xiao. 2008. Eukaryotic DNA damage tolerance and translesion synthesis through covalent modifications of PCNA. Cell Res. 18162-173. [DOI] [PubMed] [Google Scholar]
- 4.Avkin, S., M. Goldsmith, S. Velasco-Miguel, N. E. Geacintov, E. C. Friedberg, and Z. Livneh. 2004. Quantitative analysis of translesion DNA synthesis across a benzo[a]pyrene-guanine adduct in mammalian cells: the role of DNA polymerase kappa. J. Biol. Chem. 27953298-53305. [DOI] [PubMed] [Google Scholar]
- 5.Avkin, S., Z. Sevilya, L. Toube, N. E. Geacintov, S. G. Chaney, M. Oren, and Z. Livneh. 2006. p53 and p21 regulate error-prone DNA repair to yield a lower mutation load. Mol. Cell 22407-413. [DOI] [PubMed] [Google Scholar]
- 6.Baynton, K., A. Bresson-Roy, and R. P. Fuchs. 1998. Analysis of damage tolerance pathways in Saccharomyces cerevisiae: a requirement for Rev3 DNA polymerase in translesion synthesis. Mol. Cell. Biol. 18960-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blastyák, A., L. Pintér, I. Unk, L. Prakash, S. Prakash, and L. Haracska. 2007. Yeast Rad5 protein required for postreplication repair has a DNA helicase activity specific for replication fork regression. Mol. Cell 28167-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheo, D. L., H. J. Ruven, L. B. Meira, R. E. Hammer, D. K. Burns, N. J. Tappe, A. A. van Zeeland, L. H. Mullenders, and E. C. Friedberg. 1997. Characterization of defective nucleotide excision repair in XPC mutant mice. Mutat. Res. 3741-9. [DOI] [PubMed] [Google Scholar]
- 9.Dirac, A. M., and R. Bernards. 2003. Reversal of senescence in mouse fibroblasts through lentiviral suppression of p53. J. Biol. Chem. 27811731-11734. [DOI] [PubMed] [Google Scholar]
- 10.Edmunds, C. E., L. J. Simpson, and J. E. Sale. 2008. PCNA ubiquitination and REV1 define temporally distinct mechanisms for controlling translesion synthesis in the avian cell line DT40. Mol. Cell 30519-529. [DOI] [PubMed] [Google Scholar]
- 11.Enders, G. H. 2008. Expanded roles for Chk1 in genome maintenance. J. Biol. Chem. 28317749-17752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedberg, E. C. 2005. Suffering in silence: the tolerance of DNA damage. Nat. Rev. Mol. Cell Biol. 6943-953. [DOI] [PubMed] [Google Scholar]
- 13.Friedberg, E. C., A. R. Lehmann, and R. P. P. Fuchs. 2005. Trading places: how do DNA polymerases switch during translesion DNA synthesis? Mol. Cell 18499-505. [DOI] [PubMed] [Google Scholar]
- 14.Friedberg, E. C., G. C. Walker, W. Siede, R. D. Wood, R. A. Schultz, and T. E. Ellenberger. 2005. DNA repair and mutagenesis, 2nd ed. ASM Press, Washington, DC.
- 15.Gibbs, P. E. M., J. McDonald, R. Woodgate, and C. W. Lawrence. 2005. The relative roles in vivo of Saccharomyces cerevisiae Pol η, Pol ζ, Rev1 protein and Pol32 in the bypass and mutation induction of an abasic site, T-T (6-4) photoadduct and T-T cis-syn cyclobutane dimer. Genetics 169575-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo, C., P. L. Fischhaber, M. J. Luk-Paszyc, Y. Masuda, J. Zhou, K. Kamiya, C. Kisker, and E. C. Friedberg. 2003. Mouse Rev1 protein interacts with multiple DNA polymerases involved in translesion DNA synthesis. EMBO J. 226621-6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo, C., E. Sonoda, T. S. Tang, J. L. Parker, A. B. Bielen, S. Takeda, H. D. Ulrich, and E. C. Friedberg. 2006. REV1 protein interacts with PCNA: significance of the REV1 BRCT domain in vitro and in vivo. Mol. Cell 23265-271. [DOI] [PubMed] [Google Scholar]
- 18.Guo, C., T. S. Tang, M. Bienko, J. L. Parker, A. B. Bielen, E. Sonoda, S. Takeda, H. D. Ulrich, I. Dikic, and E. C. Friedberg. 2006. Ubiquitin-binding motifs in REV1 protein are required for its role in the tolerance of DNA damage. Mol. Cell. Biol. 268892-8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo, D., Z. Xie, H. Shen, B. Zhao, and Z. Wang. 2004. Translesion synthesis of acetylaminofluorene-dG adducts by DNA polymerase zeta is stimulated by yeast Rev1 protein. Nucleic Acids Res. 321122-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haracska, L., S. Prakash, and L. Prakash. 2002. Yeast Rev1 protein is a G template-specific DNA polymerase. J. Biol. Chem. 27715546-15551. [DOI] [PubMed] [Google Scholar]
- 21.Haracska, L., I. Unk, R. E. Johnson, E. Johansson, P. M. Burgers, S. Prakash, and L. Prakash. 2001. Roles of yeast DNA polymerases delta and zeta and of Rev1 in the bypass of abasic sites. Genes Dev. 15945-954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hendel, A., O. Ziv, Q. Gueranger, N. Geacintov, and Z. Livneh. 2008. Reduced efficiency and increased mutagenicity of translesion DNA synthesis across a TT cyclobutane pyrimidine dimer, but not a TT 6-4 photoproduct, in human cells lacking DNA polymerase eta. DNA Repair 71636-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hishida, T., Y. Kubota, A. M. Carr, and H. Iwasaki. 2009. RAD6-RAD18-RAD5-pathway-dependent tolerance to chronic low-dose ultraviolet light. Nature 457612-615. [DOI] [PubMed] [Google Scholar]
- 24.Hoege, C., B. Pfander, G. L. Moldovan, G. Pyrowolakis, and S. Jentsch. 2002. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature 419135-141. [DOI] [PubMed] [Google Scholar]
- 25.Jackson, D. A., and A. Pombo. 1998. Replicon clusters are stable units of chromosome structure: evidence that nuclear organization contributes to the efficient activation and propagation of S phase in human cells. J. Cell Biol. 1401285-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jansen, J. G., M. I. Fousteri, and N. de Wind. 2007. Send in the clamps: control of DNA translesion synthesis in eukaryotes. Mol. Cell 28522-529. [DOI] [PubMed] [Google Scholar]
- 27.Jansen, J. G., P. Langerak, A. Tsaalbi-Shtylik, P. C. van den Berk, H. Jacobs, and N. de Wind. 2006. Strand-biased defect in C/G transversions in hypermutating immunoglobulin genes in Rev1-deficient mice. J. Exp. Med. 203319-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jansen, J. G., A. Tsaalbi-Shtylik, P. Langerak, F. Calléja, C. M. Meijers, H. Jacobs, and N. de Wind. 2005. The BRCT domain of mammalian Rev1 is involved in regulating DNA translesion synthesis. Nucleic Acids Res. 33356-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansson, F., A. Lagerqvist, K. Erixon, and D. Jenssen. 2004. A method to monitor replication fork progression in mammalian cells: nucleotide excision repair enhances and homologous recombination delays elongation along damaged DNA. Nucleic Acids Res. 32e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larimer, F. W., J. R. Perry, and A. A. Hardigree. 1989. The REV1 gene of Saccharomyces cerevisiae: isolation, sequence, and functional analysis. J. Bacteriol. 171230-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lebofsky, R., R. Heilig, M. Sonnleitner, J. Weissenbach, and A. Bensimon. 2006. DNA replication origin interference increases the spacing between initiation events in human cells. Mol. Biol. Cell 175337-5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehmann, A. R., and R. P. P. Fuchs. 2006. Gaps and forks in DNA replication: rediscovering old models. DNA Repair 51495-1498. [DOI] [PubMed] [Google Scholar]
- 33.Lemontt, J. F. 1971. Mutants of yeast defective in mutation induced by ultraviolet light. Genetics 6821-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopes, M., M. Foiani, and J. M. Sogo. 2006. Multiple mechanisms control chromosome integrity after replication fork uncoupling and restart at irreparable UV lesions. Mol. Cell 2115-27. [DOI] [PubMed] [Google Scholar]
- 35.Majka, J., S. K. Binz, M. S. Wold, and P. M. Burgers. 2006. Replication protein A directs loading of the DNA damage checkpoint clamp to 5′-DNA junctions. J. Biol. Chem. 28127855-27861. [DOI] [PubMed] [Google Scholar]
- 36.Majka, J., A. Niedziela-Majka, and P. M. Burgers. 2006. The checkpoint clamp activates Mec1 kinase during initiation of the DNA damage checkpoint. Mol. Cell 24891-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masuda, Y., and K. Kamiya. 2006. Role of single-stranded DNA in targeting REV1 to primer termini. J. Biol. Chem. 28124314-24321. [DOI] [PubMed] [Google Scholar]
- 38.Masuda, Y., M. Ohmae, K. Masuda, and K. Kamiya. 2003. Structure and enzymatic properties of a stable complex of the human REV1 and REV7 proteins. J. Biol. Chem. 27812356-12360. [DOI] [PubMed] [Google Scholar]
- 39.Masuda, Y., M. Takahashi, S. Fukuda, M. Sumii, and K. Kamiya. 2002. Mechanisms of dCMP transferase reactions catalyzed by mouse Rev1 protein. J. Biol. Chem. 2773040-3046. [DOI] [PubMed] [Google Scholar]
- 40.Motegi, A., H. J. Liaw, K. Y. Lee, H. P. Roest, A. Maas, X. Wu, H. Moinova, S. D. Markowitz, H. Ding, J. H. Hoeijmakers, and K. Myung. 2008. Polyubiquitination of proliferating cell nuclear antigen by HLTF and SHPRH prevents genomic instability from stalled replication forks. Proc. Natl. Acad. Sci. USA 10512411-12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murakumo, Y., T. Roth, H. Ishii, D. Rasio, S. Numata, C. M. Croce, and R. Fishel. 2000. A human REV7 homolog that interacts with the polymerase zeta catalytic subunit hREV3 and the spindle assembly checkpoint protein hMAD2. J. Biol. Chem. 2754391-4397. [DOI] [PubMed] [Google Scholar]
- 42.Nelson, J. R., P. E. M. Gibbs, A. M. Nowicka, D. C. Hinkle, and C. W. Lawrence. 2000. Evidence for a second function for Saccharomyces cerevisiae Rev1p. Mol. Microbiol. 37549-554. [DOI] [PubMed] [Google Scholar]
- 43.Nelson, J. R., C. W. Lawrence, and D. Hinkle. 1996. Deoxycytidyl transferase activity of yeast REV1 protein. Nature 382729-731. [DOI] [PubMed] [Google Scholar]
- 44.Nelson, J. R., C. W. Lawrence, and D. C. Hinkle. 1996. Thymine-thymine dimer bypass by yeast DNA polymerase zeta. Science 2721646-1649. [DOI] [PubMed] [Google Scholar]
- 45.Ohashi, E., Y. Murakumo, N. Kanjo, J. I. Akagi, C. Masutani, F. Hanaoka, and H. Ohmori. 2004. Interaction of hREV1 with three human Y-family DNA polymerases. Genes Cells 9523-531. [DOI] [PubMed] [Google Scholar]
- 46.Parrilla-Castellar, E. R., S. J. Arlander, and L. M. Karnitz. 2004. Dial 9-1-1 for DNA damage: the Rad9-Hus1-Rad1 (9-1-1) clamp complex. DNA Repair 31009-1014. [DOI] [PubMed] [Google Scholar]
- 47.Paulovich, A. G., C. D. Armour, and L. H. Hartwell. 1998. The Saccharomyces cerevisiae RAD9, RAD17, RAD24 and MEC3 genes are required for tolerating irreparable, ultraviolet-induced DNA damage. Genetics 15075-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prakash, S., R. E. Johnson, and L. Prakash. 2005. Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function. Annu. Rev. Biochem. 74317-353. [DOI] [PubMed] [Google Scholar]
- 49.Ross, A. L., L. J. Simpson, and J. E. Sale. 2005. Vertebrate DNA damage tolerance requires the C terminus but not BRCT or transferase domains of REV1. Nucleic Acids Res. 331280-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sabbioneda, J., B. K. Minesinger, M. Giannattasio, P. Plevani, M. Muzi-Falconi, and S. Jinks-Robertson. 2005. The 9-1-1 checkpoint clamp physically interacts with Polζ and is partially required for spontaneous Polζ-dependent mutagenesis in Saccharomyces cerevisiae. J. Biol. Chem. 28038657-38665. [DOI] [PubMed] [Google Scholar]
- 51.Shachar, S., O. Ziv, S. Avkin, S. Adar, J. Wittschieben, T. Reissner, S. Chaney, E. C. Friedberg, Z. Wang, T. Carell, N. Geacintov, and Z. Livneh. 2009. Two-polymerase mechanisms dictate error-free and error-prone translesion DNA synthesis in mammals. EMBO J. 28383-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stout, G. J., M. Oosten, F. Z. Acherrat, J. Wit, W. P. Vermeij, L. H. Mullenders, F. R. de Gruijl, and C. M. Backendorf. 2005. Selective DNA damage responses in murine Xpa−/−, Xpc−/−, and Csb−/− keratinocyte cultures. DNA Repair 41337-1344. [DOI] [PubMed] [Google Scholar]
- 53.Szüts, D., A. P. Marcus, M. Himoto, S. Iwai, and J. E. Sale. 2008. REV1 restrains DNA polymerase zeta to ensure frame fidelity during translesion synthesis of UV photoproducts in vivo. Nucleic Acids Res. 366767-6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tissier, A., P. L. Kannouche, M. P. Reck, A. R. Lehmann, R. P. P. Fuchs, and A. M. Cordonnier. 2004. Co-localization in replication foci and interaction of human Y-family members, DNA polymerase Polη and REVl protein. DNA Repair 31503-1514. [DOI] [PubMed] [Google Scholar]
- 55.Ulrich, H. D. 2004. How to activate a damage-tolerant polymerase: consequences of PCNA modifications by ubiquitin and SUMO. Cell Cycle 315-18. [PubMed] [Google Scholar]
- 56.Unk, I., I. Hajdú, K. Fátyol, J. Hurwitz, J. H. Yoon, L. Prakash, S. Prakash, and L. Haracska. 2008. Human HLTF functions as a ubiquitin ligase for proliferating cell nuclear antigen polyubiquitination. Proc. Natl. Acad. Sci. USA 1053768-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Unk, I., I. Hajdú, K. Fátyol, B. Szakál, A. Blastyák, V. Bermudez, J. Hurwitz, L. Prakash, S. Prakash, and L. Haracska. 2006. Human SHPRH is a ubiquitin ligase for Mms2-Ubc13-dependent polyubiquitylation of proliferating cell nuclear antigen. Proc. Natl. Acad. Sci. USA 10318107-18112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Hoffen, A., J. Venema, R. Meschini, A. A. van Zeeland, and L. H. Mullenders. 1995. Transcription-coupled repair removes both cyclobutane pyrimidine dimers and 6-4 photoproducts with equal efficiency and in a sequential way from transcribed DNA in xeroderma pigmentosum group C fibroblasts. EMBO J. 14360-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Zeeland, A. A., C. A. Smith, and P. C. Hanawalt. 1981. Sensitive determination of pyrimidine dimers in DNA of UV-irradiated mammalian cells. Introduction of T4 endonuclease V into frozen and thawed cells. Mutat. Res. 82173-189. [DOI] [PubMed] [Google Scholar]
- 60.Van Zeeland, A. A., and A. R. Filon. 1982. Post-replication repair: elongation of daughter strand DNA in UV-irradiated mammalian cells in culture. Prog. Mutat. Res. 4375-384. [Google Scholar]
- 61.Washington, M. T., I. G. Minko, R. E. Johnson, L. Haracska, T. M. Harris, R. S. Lloyd, S. Prakash, and L. Prakash. 2004. Efficient and error-free replication past a minor-groove N2-guanine adduct by the sequential action of yeast Rev1 and DNA polymerase ζ. Mol. Cell. Biol. 246900-6906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Waters, L. S., and G. C. Walker. 2006. The critical mutagenic translesion DNA polymerase Rev1 is highly expressed during G2/M phase rather than S phase. Proc. Natl. Acad. Sci. USA 1038971-8976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wood, A., P. Garg, and P. M. Burgers. 2007. A ubiquitin-binding motif in the translesion DNA polymerase Rev1 mediates its essential functional interaction with ubiquitinated proliferating cell nuclear antigen in response to DNA damage. J. Biol. Chem. 28220256-20263. [DOI] [PubMed] [Google Scholar]
- 64.Yu, X., C. C. Chini, M. He, G. Mer, and J. Chen. 2003. The BRCT domain is a phospho-protein binding domain. Science 302639-642. [DOI] [PubMed] [Google Scholar]
- 65.Zhang, H., and C. W. Lawrence. 2005. The error-free component of the RAD6/RAD18 DNA damage tolerance pathway of budding yeast employs sister-strand recombination. Proc. Natl. Acad. Sci. USA 10215954-15959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang, Y., X. Wu, O. Rechkoblit, N. E. Geacintov, J. S. Taylor, and Z. Wang. 2002. Response of human REV1 to different DNA damage: preferential dCMP insertion opposite the lesion. Nucleic Acids Res. 301630-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhuang, Z., R. E. Johnson, L. Haracska, L. Prakash, S. Prakash, and S. J. Benkovic. 2008. Regulation of polymerase exchange between Polη and Polδ by monoubiquitination of PCNA and the movement of DNA polymerase holoenzyme. Proc. Natl. Acad. Sci. USA 1055361-5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.