Abstract
Hepatocyte growth factor (HGF), the ligand for the Met receptor tyrosine kinase, induces epithelial cell dispersal, invasion, and morphogenesis, events that require remodeling of the actin cytoskeleton. The scaffold protein Gab1 is essential for these biological responses downstream from Met. We have identified p21-activated kinase 4 (Pak4) as a novel Gab1-interacting protein. We show that in response to HGF, Gab1 and Pak4 associate and colocalize at the cell periphery within lamellipodia. The association between Pak4 and Gab1 is dependent on Gab1 phosphorylation but independent of Pak4 kinase activity. The interaction is mediated through a region in Gab1, which displays no homology to known Gab1 interaction motifs and through the guanine exchange factor-interacting domain of Pak4. In response to HGF, Gab1 and Pak4 synergize to enhance epithelial cell dispersal, migration, and invasion, whereas knockdown of Pak4 attenuates these responses. A Gab1 mutant unable to recruit Pak4 fails to promote epithelial cell dispersal and an invasive morphogenic program in response to HGF, demonstrating a physiological requirement for Gab1-Pak4 association. These data demonstrate a novel association between Gab1 and Pak4 and identify Pak4 as a key integrator of cell migration and invasive growth downstream from the Met receptor.
The dispersal of sheets of epithelia is a complex biological process that requires the coordinated function of numerous cellular proteins. One of the initial steps of cell motility involves the reorganization of the actin cytoskeleton and the initiation of a dynamic actin meshwork at the leading edge of the cell in ruffles or lamellipodia (11, 45). The actin cytoskeleton is tightly controlled via multiple signaling enzymes, which when deregulated can promote defects in the processes of cell invasion and motility and in oncogenic transformation.
The activation of the Met receptor tyrosine kinase (RTK), induced by the binding of its ligand, hepatocyte growth factor (HGF), modulates epithelial cell proliferation, survival, scatter of epithelial colonies, and invasion (35). Epithelial cells stimulated with HGF undergo a dramatic remodeling of their actin cytoskeleton, which is required for branching morphogenesis, cell migration, and invasion (37, 40). These biological processes downstream from the Met RTK are dependent on the scaffold protein Gab1, which is the major substrate/phosphoprotein recruited to Met (26, 29, 30, 34, 49).
Gab1 is a member of a family of adaptor proteins that act as a scaffold downstream from a broad range of growth factor, cytokine, and antigen receptors, linking them to downstream intracellular signaling pathways through the assembly of multiprotein complexes (15, 18, 24). Three mammalian Gab genes have been identified, the Gab1, Gab2, and Gab3 genes (15, 24). Gab1, but not Gab2 or Gab3, is a critical modulator of cell dispersal, invasion, and epithelial morphogenesis downstream from the Met receptor (26). Activation of the Met RTK by HGF promotes recruitment and tyrosine phosphorylation of Gab1, which in turn provides docking sites for the recruitment of multiple SH2 domain-containing signaling molecules, including the p85 subunit of PI(3)kinase (PI3K), phospholipase C-γ (PLC-γ), the adapter protein Crk, and the tyrosine phosphatase Shp2 (16, 23, 29, 31, 42, 43). Structure function studies have shown that the association of Gab1 with Shp2, Crk, and the PH domain of Gab1 is critical for Met-induced branching morphogenesis (21, 29, 31, 43). Hence, Gab1 subcellular localization, as well as its ability to form signaling complexes, is critical for Met-dependent biological responses.
The HGF/Met signaling axis activates Rho GTPases that play key roles in regulating the organization of the actin cytoskeleton in mammalian cells (37, 40, 51). Three members of this family, Cdc42, Rac, and Rho, induce the production of filopodia, lamellipodia, and stress fibers, respectively. This occurs in a variety of cell types, including epithelial cells and fibroblasts (17). These proteins are modulated downstream from HGF and are required for dispersal of epithelial cells in response to HGF (37, 40). Activation of Rac by HGF is mediated in part through the recruitment of Crk to Gab1. Overexpression of Crk enhances lamellipodia formation and cell spreading in response to HGF and consequently enhances cell motility and promotes epithelial dispersal (22, 23).
Members of the p21-activated kinase (Pak) family are major effectors of the RhoGTPases Cdc42 and Rac (19) and function to induce reorganization of the actin cytoskeleton (1, 2, 10). Pak proteins are Ser/Thr kinases that promote the reorganization of the actin cytoskeleton in response to upstream signals (44). The Pak family consists of six members, which are subdivided into two groups: Pak1 to -3 (group I) and Pak4 to -6 (group II) (19). This distinction is based on sequence similarities and also on the presence of an autoinhibitory region in group I, which is not present in group II Pak proteins. Pak1 and Pak4 are the best-studied members of each group and are widely expressed in a variety of tissue types (19). Both Pak1 and Pak4 have been shown to be involved in adhesion to the cell substratum and to link RhoGTPase signaling to the actin cytoskeleton (1, 9, 10, 50, 52, 53). HGF activates Pak1 (40) and stimulates the translocation of Pak4 to the leading edge of the epithelial cells in a PI3K-dependent manner (50). However, the mechanism or molecular function for this HGF-dependent response is not understood.
In this study, we have identified Pak4 as a novel Gab1-interacting protein downstream from the Met receptor. We define a new Gab1-Pak4 interaction domain and show that Pak4 is required for HGF-dependent epithelial cell dispersal, invasion, and morphogenesis.
MATERIALS AND METHODS
Antibodies.
Polyclonal anti-Erk1/2, anti-phospho-Erk1/2 (pTpY202/204), anti-phospho-Pak4(Ser474)/Pak5(Ser602)/Pak6(Ser560), anti-phospho-Gab1(Tyr627), and monoclonal anti-myc (9E10) antibodies were purchased from Cell Signaling Technology (Mississauga, Ontario, Canada). Monoclonal anti-Pak4 and anti-PY20 antibodies were purchased from BD Biosciences (San Diego, CA). Polyclonal anti-Gab1 was purchased from Upstate Biotechnology, Inc. (Lake Placid, NY). Rabbit monoclonal anti-Pak1 antibody was purchased from Epitomics (Burlingame, CA). Polyclonal anti-green fluorescent protein (anti-GFP), wheat germ agglutinin (WGA) Alexa 555, and Alexa 488-conjugated phalloidin were purchased from Molecular Probes (Burlington, Ontario, Canada). Monoclonal anti-HA.11 antibody was purchased from Covance (Berkeley, CA). Polyclonal antiactin, anticofilin, and anti-phospho-cofilin (Ser3) antibodies were purchased from Sigma-Aldrich (St. Louis, MO). Polyclonal anti-V5 antibody was purchased from GeneTex (San Antonio, TX). Anti-Met antibody (148) was generated by immunizing rabbits with the carboxy-terminal 16 amino acids (aa) of the human Met sequence, as previously described (39).
DNA constructs.
Pak4 cDNA was purchased from Origene (Rockville, MD). Myc-tagged Pak4 deletion constructs were generated by PCR and cloned into the NheI and NotI sites of pEBB (see Table S1 in the supplemental material). Gab1 deletion constructs containing an N-terminal hemagglutinin (HA) tag were generated by PCR amplification and cloning into BamHI and EcoRI sites of pcDNA1.1 (see Table S1 in the supplemental material). Pak4 and Gab1 amplifications were performed using high-fidelity Taq polymerase (Roche Diagnostics, Laval, Quebec, Canada), per the manufacturer's instructions. To generate Gab1ΔPak4, SmaI/XmaI restriction sites were generated at aa positions 116 and 234 of Gab1 pcDNA1.1 (see Table S1 in the supplemental material). Following restriction digestion with XmaI (New England Biolabs, Pickering, Ontario, Canada) and agarose gel purification (Qiagen, Mississauga, Ontario, Canada), the Gab1 pcDNA1.1 fragment was religated using T4 DNA ligase (New England Biolabs, Pickering, Ontario, Canada). The following constructs were described previously: pcDNA1.1 pcDNA1.1 HA-Gab1, pcDNA1.1 HA-Gab1ΔPH, GFP-Gab1, HA-Gab1ΔCrk, HA-Gab1ΔShp2, Gab1ΔPI3K, and HA-Gab1ΔGrb2 (13, 21, 27, 29, 30).
Cell culture and DNA transfections.
All cell lines were maintained in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum (FBS) and 50 μg/ml gentamicin (Invitrogen, Carlsbad, CA). Madin-Darby canine kidney (MDCK) epithelial cells expressing GFP-Gab1 and/or mcherry-Pak4 were maintained in geneticin (400 μg/ml) (Invitrogen, Carlsbad, CA) and/or hygromycin (75 μg/ml) (Invitrogen, Carlsbad, CA). For transient expression of proteins, 1 × 106 HEK293 cells were seeded 24 h prior to performing transient transfections using Lipofectamine Plus (Invitrogen, Carlsbad, CA), according to the manufacturer's instructions. Media were replaced 3 h posttransfection, and cells lysed 24 to 48 h posttransfection in 1% NP-40 lysis buffer (50 mM Tris-HCl, pH 7.4; 150 mM NaCl; 2 mM EGTA, pH 8.0; 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM phenylmethylsulfonyl fluoride, 1 mM Na3VO4). The homogenates were centrifuged at 13,000 rpm for 15 min to remove debris.
Immunoprecipitation and immunoblotting.
HEK293 cell protein lysates (500 μg) were used for each immunoprecipitation. Antibodies were allowed to bind for 1 h at 4°C with gentle rocking, and 10 μl of protein A- or protein G-Sepharose beads were then added to collect immune complexes. Beads were washed three times in lysis buffer, resuspended in 20 μl of Laemmlli sample buffer, and boiled for 5 min. For lambda phosphatase treatment, prior to immunoprecipitation, lysates were incubated with 2,000 units of lambda phosphatase (New England Biolabs, Pickering, Ontario, Canada) for 30 min at 30°C. Samples were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose. Membranes were blocked with 3% bovine serum albumin and probed with appropriate antibodies, as described, and then with horseradish peroxidase-conjugated secondary antibodies. All immunoblots were visualized by enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ).
Confocal immunofluorescence microscopy.
HeLa, MDCK, or MDCK cells expressing CSF-Met N1358H (G17) (12) together with Gab1ΔPH (29) or the myristoylated Gab1ΔPH rescue protein (32) (2 × 104) were seeded on glass coverslips (Bellco Glass, Inc., Vineland, NJ) in 24-well plates (Nalgene NUNC, Rochester, NY) and were transfected with the indicated DNA using Lipofectamine Plus (Invitrogen, Carlsbad, CA) 16 h postplating, according to the manufacturer's instructions. Cells were serum starved for 2 h prior to HGF or colony-stimulating factor 1 (CSF-1) treatment. Coverslips were washed once with PBS and then fixed with 2% paraformaldehyde (PFA) (Fisher Scientific) in PBS for 20 min. Coverslips were then washed four times in PBS, and residual PFA was removed with three 5-min washes in 100 mM glycine in PBS. Cells were permeabilized with 0.3% Triton X-100/PBS and blocked for 30 min in blocking buffer (5% bovine serum albumin, 0.2% Triton X-100, 0.05% Tween 20, PBS). Coverslips were incubated with primary and secondary antibodies diluted in blocking buffer for 1 h and 40 min, respectively, at room temperature. Coverslips were mounted with Immumount (Thermo-Shandon, Pittsburgh, PA). Confocal images were taken using a Zeiss 510 Meta laser scanning confocal microscope (Carl Zeiss, Canada Ltd, Toronto, Ontario, Canada) with a 100× objective. Image analysis was carried out using the LSM 5 image browser (Empix Imaging, Mississauga, Ontario, Canada). Confocal live cell imaging was performed with a spinning disk confocal microscope from Quorum Technologies. Data from the spinning disk microscopy were analyzed using Volocity 4.1 software (Improvision, Coventry, England).
Scatter assay.
MDCK cells or stable cell lines expressing CSF-Met N1358H (G17) alone or expressing HA-Gab1 (WT3) (29) or HA-Gab1ΔPak4 were seeded (5 × 103/well) overnight in 24-well dishes (Nalgene NUNC, Rochester, NY), and 24 h later, HGF, 0.34 ng/ml or 1.35 ng/ml, defined as 0.25 units or 1.00 units, respectively (47), or CSF-1 (50 ng) (54) was added for 24 h. Phase-contrast images were captured with a Zeiss Axiovision 135 microscope with a 10× objective (Carl Zeiss Canada Ltd, Toronto, Ontario, Canada).
Collagen assay.
The ability of MDCK cells to form branching tubules was assayed, as previously described, with a few modifications (54). Briefly, 5 × 103 cells were suspended in 500 μl of Vitrogen 100 collagen solution (now commercially available as Pur-Col by Inamed Biomaterials, Fremont, CA), according to the manufacturer's protocols, and layered over 350 μl of the collagen solution in a 24-well plate. Cells were maintained in Leibowitz medium containing 5% FBS and allowed to form cysts for 6 days. Cysts were stimulated through the addition of HGF (20.25 ng/ml) or CSF-1 (50 ng/ml) to Leibowitz medium supplemented with 2% FBS. The medium was replaced every 5 days for the duration of the assay. Images were then acquired with a Zeiss Axiovision 135 microscope with a 10× objective (Carl Zeiss Canada Ltd, Toronto, Ontario, Canada).
Migration and invasion assays.
MDCK cells (5 × 104) were counted and seeded directly onto 6.5-mm Corning Costar transwell chambers for migration assays or transwells coated with 100 μg/cm2 Matrigel (BD Biosciences, San Jose, CA) for invasion assays. Complete media were added to both the top and bottom wells, and cells were incubated at 37°C overnight. For HGF stimulations, 34 ng/ml of HGF was added to the bottom wells. Following overnight incubation, cells on both sides of the transwells were fixed with 10% neutral buffered formalin for 20 min at room temperature. After washing with double-distilled water, cells were stained with 0.1% crystal violet in 20% methanol for 20 min at room temperature. Cells on the top layer were scraped off, and membranes were left to dry overnight. Images were captured using a Retiga 1300 digital camera (Qimaging, Burnaby, British Columbia, Canada) and a Zeiss Axioskop microscope (Carl Zeiss Canada Ltd., Toronto, Ontario, Canada). Image analysis and quantification were performed using the Scion Image-NIH equivalent program for Microsoft Windows (Scion Company, Frederick, MD).
Pak4 knockdown.
Small interfering RNA (siRNA)-mediated knockdown of Pak4 in MDCK cells was accomplished using the reverse transfection of adherent cells with siRNA in 6-well plates (Qiagen, Mississauga, ON), per the manufacturer's protocol. Briefly, 1 × 105 cells were seeded per well in complete medium. A total of 24 h later, 150 nM of Pak4 siRNA duplex (duplex 1, CCGGCTGGTGGCCGTCAAGAA; or duplex 4, CGAGAACGTGGTGGAGATGTA) was mixed with 20 μl HiPerfect transfection reagent (Qiagen, Mississauga, Ontario, Canada). AllStars negative control siRNA (no.1027281; Qiagen, Mississauga, Ontario, Canada) was used as the scrambled siRNA negative control. Cells were collected 96 h posttransfection and used to perform assays.
RESULTS
Pak4 is a novel Gab1 binding partner.
Recruitment of Gab1 is critical for Met-dependent biological responses in epithelial cells. To date, multiple proteins have been shown to associate with Gab1 downstream from Met, playing key roles in Met signaling. These interactions were identified based on predicted SH2 domain binding sites (18). However, no unbiased screens for Gab1-associated proteins have been performed. To identify novel Gab1-associated protein complexes following Met receptor activation, we generated a Gab1 TAP-tag fusion protein and used this in a proteomic screen to identify Gab1 binding partners. This technique has been successful in isolating and identifying protein complexes in yeast and mammalian cells (20, 38). Stable lines of HEK293 cells expressing a Gab1 TAP-tag fusion protein were generated, and Gab1 TAP-tag-associated protein complexes were isolated following stimulation with HGF for 5 min. Protein complexes were analyzed by mass spectrometry (data not shown). We were able to isolate, as a proof of principle, known Gab1 binding partners, Grb2, Shp2, and the p85 subunit of PI3K, by mass spectrometry and by Western blot analysis in a Gab1 TAP-tag, HGF-dependent protein complex (see Fig. S1 in the supplemental material; also data not shown). We were interested in pursuing novel Gab1 binding partners and identified Pak4, a Ser/Thr kinase.
To establish if Pak4 was a Met-dependent Gab1 binding protein, HEK293 cells were transiently transfected with HA-tagged Gab1 and myc-tagged Pak4, in the absence or presence of Met RTK. Transient overexpression of Met leads to its activation in the absence of ligand (39). Although weak binding of Gab1 and Pak4, as established by coimmunoprecipitation, is observed in the absence of Met, this association was significantly increased in the presence of active Met (Fig. 1A). Moreover, phosphorylation of serine 474 of Pak4, which is thought to play a positive role in regulating the activity and function of Pak4 (36), was significantly elevated in the presence of Met (Fig. 1A). To examine if the association of Pak4 was unique to Gab1 alone or capable of binding to all Gab family members downstream from the Met receptor, transient transfections of Pak4 with Gab1, Gab2, and Gab3 were performed. Interestingly, Pak4 was found to associate solely with Gab1 and not with Gab2 or Gab3, suggesting a specific interaction between Pak4 with Gab1 (see Fig. S2 in the supplemental material).
FIG. 1.
Pak4 associates with Gab1 following Met receptor activation. (A) Following transient transfection of HA-Gab1, myc-Pak4, and Met in HEK293 cells, total cell lysates (500 μg) were immunoprecipitated with anti-HA and anti-myc (9E10) sera. Immunoprecipitations (IP) and whole-cell lysates (WCL) (30 μg) were separated by SDS-PAGE, and immunoblot analysis was performed with indicated antibodies. (B) Transient transfection of HA-Gab1, myc-Pak4, myc-Pak1, and Met in HEK293 cells. Proteins were extracted, and total cell lysates (500 μg) were immunoprecipitated by anti-HA and anti-myc (9E10) sera and separated by SDS-PAGE, and immunoblot analysis was performed. Immunoblot analysis was performed on whole-cell lysate (30 μg), and proteins were probed with antisera against HA, myc, Met, and actin. (C) HeLa cells were plated at a density of 1 × 106 cells/10-cm dish and were stimulated for the indicated time points with 135 ng/ml of HGF. Proteins from total cell lysate (500 μg) were immunoprecipitated with anti-Gab1 sera, separated by SDS-PAGE, and immunobloted with antisera against Pak4. Proteins from whole-cell lysate (30 μg) were probed with antisera against phospho-Pak4(S474), phospho-ERK1/2 (pTpY202/204), and total ERK1/2. (D) HEK293 cells were transiently transfected with myc-Pak4, HA-Gab1, and Met. Prior to coimmunoprecipitation, cells were treated with 2,000 units of lambda phosphatase for 30 min at 30°C. Immunoprecipitation and whole-cell lysates (30 μg) were separated by SDS-PAGE and immunoblotted with antisera againt HA, myc (9E10), Met, phospho-MetY1234/35, phospho-Gab1Y627, and actin. (E) HEK293 cells were transiently trasnsfected with constitutively active HA-Pak4 (S445NS447E) and kinase-dead HA-Pak4(K350) together with GFP-Gab1 and Met. Proteins were extracted, and total cell lysates (500 μg) were immunoprecipitated by anti-HA and anti-GFP sera and separated by SDS-PAGE, and immunoblot analysis was performed. Immunoblot blot analysis was performed on whole-cell lysate (30 μg), and proteins were probed with antisera against HA, GFP, phospho-Pak4(Ser474), Met, and actin.
To evaluate the possibility that group I Pak family members could also bind to Gab1, we tested the ability of Pak1 to associate with Gab1. We have previously shown that Pak1 is activated, phosphorylated, and translocated to membrane ruffles at the edge of lamellipodia 15 min post-HGF stimulation (40). However, transient transfection of myc-Pak1 together with HA-Gab1 and Met showed no association between Gab1 and Pak1, whereas under these conditions, Pak4 coimmunoprecipitated with Gab1, delineating a specific Gab1-Pak4 complex (Fig. 1B). We next determined whether the association between Pak4 and Gab1 could occur with endogenous proteins. Following stimulation of HeLa cells with HGF, coimmunoprecipitation of endogenous Pak4 with Gab1 was detected as early as 5 min post-HGF-stimulation and maintained up to 30 min poststimulation (Fig. 1C). To examine the requirements for the Gab1-Pak4 association, we established if Gab1 phosphorylation and Pak4 activation were required. A requirement for Gab1 phosphorylation was examined by pretreating protein lysates prepared from HEK293 cells transiently transfected with Met, Gab1, and Pak4 with lambda phosphatase, which acts to nonspecifically remove tyrosine, serine, and threonine phosphate residues (55). Pretreatment with lambda phosphatase abrogates coimmunoprecipitation of Gab1 with Pak4, supporting a requirement for Gab1 and/or Pak4 phosphorylation for this interaction (Fig. 1D). To establish if Pak4 catalytic activity was necessary, constitutively active Pak4 (Pak4S445NS447E) (36) and kinase-dead Pak4 (Pak4K350M) (1) were transiently transfected with Met and Gab1 in HEK293 cells (Fig. 1E). The Pak4K350M protein associated with Gab1 at levels similar to those of the constitutively active Pak4, demonstrating that Pak4 activation was not necessary for the association between Gab1 and Pak4 (Fig. 1E).
HGF stimulation for 2 hours leads to the translocation of Pak4 to the cell periphery in MDCK cells (50). To establish if Pak4 rapidly translocated to the cell cortex in response to HGF, we generated MDCK cells that stably expressed GFP-Pak4. In response to HGF, Pak4 rapidly translocated to the cell periphery and localized within newly forming peripheral ruffles as early as 3 min post-HGF stimulation and subsequently to newly forming lamellipodia at 7 min (Fig. 2A). To establish that Pak4 is recruited to lamellipodia, MDCK cells expressing GFP-Pak4 were stimulated with HGF and stained with WGA Alexa 555, a marker for the plasma membrane. Localization of GFP-Pak4 and WGA (555), as examined by confocal microscopy on fixed cells through multiple z-stacks (Fig. 2B), revealed that Pak4 is not localized to the plasma membrane in the absence of HGF stimulation (no overlap with WGA staining) but is recruited following stimulation with HGF to the limits of the cell colony and is further enhanced in lamellipodia (Fig. 2B and data not shown). Moreover, following transient transfection of HA-Gab1 and myc-Pak4 in HeLa cells, confocal images of fixed cells further demonstrated that these two proteins colocalize at the cell periphery within lamellipodia at 5 min post-HGF stimulation (Fig. 2C), supporting the ability of these two proteins to form a complex following HGF stimulation. To determine if recruitment of Pak4 to the cell cortex is dependent on Gab1, MDCK cells stably expressing HA-Gab1ΔPH were stimulated and localization of Pak4 was examined. Membrane recruitment of Gab1 via its PH domain is required for the biological activity of Gab1 (29), and this function can be rescued through the addition of the myristoylation (Myr) signal from c-src (Myr-Gab1ΔPH) (32). In MDCK cells expressing Gab1ΔPH, neither Gab1ΔPH nor Pak4 translocated to the cell cortex. However, in MDCK cells expressing the Myr-Gab1ΔPH protein, which localizes to the cell membrane in the absence of HGF stimulation, Pak4 was recruited to the membrane in response to HGF but not in its absence (Fig. 2D). Therefore, Pak4 and Gab1 colocalize in a complex within lamellipodia, following Met receptor stimulation.
FIG. 2.
Pak4 colocalizes with Gab1 at the leading edge in lamellipodia in HGF-stimulated cells. (A) MDCK cells stably expressing GFP-Pak4 (2 × 104) were plated on glass-bottom dishes and the next day stimulated with HGF. Spinning disk time-lapse microscopy of living cells with single time frames taken at the indicated times post-HGF stimulation with a 63× objective. Images shown represent a 0.2-μm-thickness slice of z-stack. (B) MDCK cells stably expressing GFP-Pak4 were plated on coverslips and the next day stimulated with HGF. Following stimulation, cells were stained with plasma membrane-specific protein WGA Alexa Fluor 555. Confocal images were taken using a Zeiss 510 Meta laser scanning confocal microscope. Confocal images were taken with a 100× objective. Image representative of 0.2-μm z-stack. Bar represents 10 μm. (C) HeLa cells were plated on coverslips and were transiently transfected with HA-Gab1 and myc-Pak4. Following overnight incubation, cells were serum starved for 2 h and stimulated with 135 ng/ml HGF at 37°C for the indicated time points. Coverslips were fixed in 3% PFA and stained with anti-HA (left) and anti-myc sera (middle). Confocal images were taken with a 100× objective. Bar represents 10 μm. (D) Stable MDCK cells expressing chimeric receptor CSF-MetΔGrb2 and overexpressing HA-Gab1ΔPH or myr-HA-Gab1ΔPH were plated on coverslips and transiently transfected with mCherry-Pak4. Following overnight incubation, cells were serum starved for 2 h, stimulated with 50 ng of CSF-1, and then analyzed for the ability of Gab1 to be properly recruited to the plasma membrane. Coverslips were fixed in 3% PFA and stained with anti-HA. Confocal images were taken with a 100× objective. Bar represents 10 μm.
Association of Gab1 with Pak4 requires the GID of Pak4.
To identify which region of Pak4 is required for association with Gab1, Pak4 deletion constructs were created and a structure function analysis was performed. Pak4 possesses an N-terminal p21-binding (CRIB) domain and a C-terminal kinase domain (Fig. 3A). Deletion mutants generated to remove the CRIB domain still retained the ability to bind Gab1 (Fig. 3B and data not shown). Deletion of the kinase domain and the newly identified guanine exchange factor H1 (GEF-H1)-interacting domain (GID) (7) abrogated Gab1 binding. However, a Pak4 mutant that possessed a GID and a kinase domain retained binding to Gab1, whereas a similar Pak4 mutant lacking the GID failed to bind (Fig. 3B). Taken together, our data localized the region in Pak4 required for binding to Gab1 to a previously described region in Pak4 which is responsible for associating with GEF-H1 (Fig. 3B) (7).
FIG. 3.
Association of Pak4 with Gab1 occurs through GID of Pak4. (A) Schematic representation of Pak4 deletion constructs. Numbers in parentheses represent amino acids in Pak4 sequence. (B) HEK293 cells were transiently transfected with myc-Pak4 deletion constructs together with HA-Gab1 and Met. Proteins from total cell lysate (500 μg) were immunoprecipitated (IP) with anti-myc antisera, separated by SDS-PAGE, and immunoblotted with antisera for HA and myc (9E10). Proteins from whole-cell lysates (30 μg) were immunoblotted with antisera against HA, Met, and myc (9E10).
Association of Pak4 with Gab1 is independent of known Gab1-binding sites.
In response to HGF, Gab1 associates with multiple proteins in a phosphotyrosine-dependent manner through specific interactions with the SH2 domains of Shp2, Crk, PLC-γ, and the p85 subunit of PI3K. To determine the mechanism through which Pak4 is recruited to Gab1, Gab1 mutants deficient in their ability to recruit known binding proteins were examined for their ability to associate with Pak4 (Fig. 4A). Mutant Gab1 proteins unable to recruit Grb2, PI3K, Crk/PLC-γ, or Shp2 still retain the ability to recruit Pak4 (Fig. 4B), providing evidence that the association of Pak4 and Gab1 is mediated by an unidentified domain within Gab1. To delineate the region of Gab1 responsible for mediating the association with Pak4, Gab1 deletion constructs were generated (Fig. 5A). Deletion constructs revealed that Pak4 recruitment to Gab1 is dependent on a region of Gab1 localized between the PH domain and the first Crk phosphotyrosine binding site (aa 116 to 234) (Fig. 5A and 5B). This region in Gab1 possesses no known binding sites for other proteins (Fig. 5A). A Gab1 mutant, Gab1 (1-234), which contains only the PH domain and aa 116 to 234, was still capable of associating with Pak4 (Fig. 5B). Similarly, decreased association is observed between Pak4 and a Gab1 mutant lacking the PH domain, which fails to localize to the plasma membrane (Fig. 5B).
FIG. 4.
Association of Pak4 and Gab1 is not mediated through known Gab1-binding sites. (A) Schematic representation of Gab1 mutant constructs. (B) HEK293 cells were transiently transfected with HA-tagged Gab1 mutants lacking the binding sites for Grb2, the p85 subunit of PI3K, Crk/PLC-γ, and Shp2 in combination with myc-Pak4 and/or Met. Gab1 protein lysates (500 μg) were immunoprecipitated (IP) with sera against anti-HA, resolved by SDS-PAGE, and transferred to a nitrocellulose membrane. The membrane was immunoblotted with antisera against myc (9E10), phosphotyrosine (PY20), HA, and actin. WCL, whole-cell lysate.
FIG. 5.
Pak4 association to Gab1 is mediated through an unidentified domain of Gab1. A) Schematic representation of Gab1 deletion constructs. Numbers in parentheses represent amino acids in Gab1 sequence. IP, immunoprecipitated MBM, Met binding motif. (B) HA-tagged Gab1 deletion constructs were transiently transfected in combination with myc-Pak4 and Met. Proteins from total cell lysates (500 μg) were immunoprecipitated (IP) with anti-myc (9E10) sera, separated by SDS-PAGE, and immunoblotted with antisera against HA and myc (9E10). Proteins from whole-cell lysates (WCL) (30 μg) were immunoblotted with antisera against HA, myc (9E10), and actin. Asterisks delineate the mature Gab1 construct. Numbers at left are molecular weight markers (in thousands). (C) Schematic model of Gab1ΔPak4. (D) Gab1 deletion construct lacking aa 116 to 234 (Gab1ΔPak4) was examined for its ability to associate with Pak4 downstream from Met. HA-Gab1 and HA-Gab1ΔPak4 were transiently transfected in combination with myc-Pak4 and Met. Proteins from total cell lysates (500 μg) were immunoprecipitated with anti-myc (9E10) sera, separated by SDS-PAGE, and immunoblotted with antisera against HA and myc (9E10). Proteins from whole-cell lysates (30 μg) were immunoblotted with antisera against HA, myc (9E10), phospho-Gab1Y627, Met, and actin. (E) Gab1ΔPak4 was examined for its ability to associate with known Gab1-binding partners. HA-Gab1 and HA-Gab1ΔPak4 were transiently transfected in combination with Met. Proteins from total cell lysates (500 μg) were immunoprecipitated with anti-HA sera, separated by SDS-PAGE, and immunoblotted with antisera against phosphotyrosine (PY20), HA, Shp2, and Crk. Proteins from whole-cell lysates (30 μg) were immunoblotted with antisera against Crk, Shp2, and actin. (F) A competition assay was performed with increasing concentrations of the HA-tagged Pak4-ASM of Gab1 (HA-Pak4-ASM) to compete for binding of Pak4 with Gab1. HEK293 cells were transiently transfected with GFP-Gab1, myc-Pak4, Met, and the HA-Pak4-ASM. Proteins from total cell lysates (500 μg) were immunoprecipitated with anti-myc (9E10) sera, separated by SDS-PAGE, and immunoblotted with antisera against HA and GFP. Proteins from whole-cell lysates (30 μg) were immunoblotted with antisera against HA, myc (9E10), GFP and actin. (G) Bar graphs representing the data shown in panel F. The proportions of Gab1 associating to Pak4 and the Pak4-ASM binding to Pak4 and the proportion of phospho-Pak4 compared to total Pak4 following increasing expression of the Pak4-ASM of Gab1.
To test if aa 116 to 234 are essential for Pak4 association with Gab1, we generated a Gab1 mutant that lacks this domain (Fig. 5C). Following transient cotransfection of HEK293 cells, the Gab1Δ116-234 (Gab1ΔPak4) mutant failed to coimmunoprecipitate with Pak4 (Fig. 5D), demonstrating that aa 116 to 234 are essential for Pak4 recruitment. Importantly, the Gab1ΔPak4 mutant was robustly phosphorylated downstream from Met (Fig. 5D) and recruited other known signaling proteins, including Shp2 and Crk, to levels similar to that of the wild-type (WT) Gab1 protein (Fig. 5E). To establish whether the Gab1 aa 116 to 234, which are required for Pak4 recruitment, reflect a Pak4-association motif (ASM) of Gab1, we examined if increasing overexpression of the Pak4-ASM (aa 116 to 234) of Gab1 (HA-Pak4-ASM) (Fig. 5F and G) would compete for Pak4 association with Gab1. With increasing titration, we observed both a loss of association between Pak4 and full-length Gab1 and a decrease in phosphorylation on serine 474 of Pak4 (Fig. 5F and G). Notably the Pak4-ASM of Gab1 coimmunoprecipitated with full-length Pak4, demonstrating that it was both sufficient and necessary for Pak4 interaction (Fig. 5F and G).
Pak4 loss decreases cofilin phosphorylation.
To examine the requirement of Pak4 in Met-dependent signaling, the consequence of loss of Pak4 on known downstream effectors of Pak4 in response to HGF was examined. We employed a siRNA strategy against Pak4 to knockdown endogenous protein levels. Efficient knockdown of Pak4 was achieved with MDCK cells (Fig. 6A) compared to mock and scrambled siRNA (Fig. 6A). Pak4 is a known regulator of LIM kinase (LIMK) and consequently cofilin activity (9). Inactivation of cofilin results in part from phosphorylation on serine 3 by LIMK, which prevents association of cofilin with actin. We therefore examined the level of phosphorylation of cofilin in MDCK cells treated with Pak4 siRNA or scrambled siRNA and stimulated with HGF. In response to HGF, no significant change in the level of cofilin phosphorylation was observed at early time points (Fig. 6B and C). However, at later time points following HGF stimulation (45 to 60 min), the level of cofilin phosphorylation in cells transfected with scrambled compared to Pak4 siRNA was twofold higher (Fig. 6C). This change was consistent in three independent experiments. As a control, the phosphorylation status of Erk was examined to look at the efficiency of stimulation. No significant difference was observed in Erk activation in scrambled compared to Pak4 siRNA-treated cells. This demonstrates a requirement for Pak4 in the regulation of enzymes involved in the remodeling of the actin cytoskeleton downstream from Met. Furthermore, we observed marked differences in the actin cytoskeleton of MDCK cells depleted of Pak4 upon HGF stimulation. Phalloidin staining of Pak4 siRNA-treated MDCK cells reveals a decrease in lamellipodia formation and the actin cytoskeletal network on cells at the edge of the colony (Fig. 6D). In contrast, in scrambled siRNA-treated MDCK cells, polymerized actin was visualized by phalloidin staining at the cell cortex (Fig. 6D).
FIG. 6.
Pak4 knockdown decreases HGF-induced cofilin phosphorylation and actin dynamics. (A) MDCK cells (1 × 105) were plated in 6-well dishes and the following day were transfected with 150 nM of Pak4 siRNA. Knockdown was examined 96 h later. Cells were lysed, and proteins from whole-cell lysates (20 μg) were immunoblotted with antisera against Pak4, Pak1, and actin. DUP, duplex. (B) MDCK cells treated with scrambled or Pak4 siRNA were stimulated with HGF for the indicated time points. Whole-cell lysates (20 μg) were immunoblotted with antisera against Pak4, Pak1, phospho-cofilin, cofilin, phospho-Erk, total Erk, and actin. (C) Densitometric analysis of phospho-cofilin blot shown in panel B were measured by NIH Image and compared with the total cofilin levels. Bars represent standard errors of the three separate experiments. (D) MDCK cells stained with phalloidin. Confocal images were taken with a 100× objective. Bar represents 10 μm.
Pak4/Gab1 enhances the breakdown of cell-cell contacts and epithelial cell scatter.
To address the biological relevance of the association of Gab1 with Pak4, we created stable MDCK cells lines overexpressing Gab1 and/or Pak4 (Fig. 7A). MDCK cells form tight colonies when grown in culture (Fig. 7) (14, 48). Stimulation of these colonies with HGF promotes the breakdown of cell-cell contacts, remodeling of the actin cytoskeleton, and dispersal of cells 12 to 16 h poststimulation, resulting in an event that resembles an epithelial mesenchymal transition (41). To address the impact of Pak4 on the ability of cells to undergo cell scatter, multiple stable cell lines overexpressing Gab1 (HA-Gab1 [7D6] and GFP-Gab1 [B1-3]), Pak4 (GFP-Pak4 CL9, CL11, and mCherry-Pak4 CL12), and both Gab1 and Pak4 (GFP-Gab1 and mCherry-Pak4 CL13, CL14, CL15, and CL16) were used to examine HGF-induced cell scatter. MDCK cells or cells expressing GFP alone (A2), GFP-Gab1 (B1-3), GFP-Pak4 (CL9), or both GFP-Gab1 and mcherry-Pak4 (CL14 and CL15) form tight colonies (Fig. 7B; see also Fig. S3 in the supplemental material). Interestingly, cells overexpressing both GFP-Gab1 and mcherry-Pak4 (CL13, CL14, CL15, and CL16) scattered in response to suboptimal levels of HGF (0.34 ng/ml), whereas cells overexpressing Gab1 or Pak4 alone failed to scatter in response to low levels of HGF (Fig. 7B and data not shown). As expected, all MDCK cells and those that expressed Gab1 or Pak4 alone were capable of scatter in response to higher levels of HGF (1.35 ng/ml) (see Fig. S3 in the supplemental material). Together, these data support that Gab1 and Pak4 synergize to mediate the scatter of MDCK cells downstream from the Met receptor.
FIG. 7.
Overexpression of Gab1 and Pak4 induces scatter of MDCK cells. (A) Proteins from whole-cell lysate (30 μg) from MDCK cells and cells overexpressing GFP-Gab1 and/or mcherry-Pak4 were subjected to SDS-PAGE, transferred to a nitrocellulose membrane, and immunoblotted with anti-GFP and anti-Pak4. The sizes of the molecular mass markers are indicated on the left. (B) MDCK cells stably expressing GFP alone (A2), GFP-Gab1, GFP-Pak4, and GFP- Pak4, and GFP-Gab1 and mcherry-Pak4 were plated (5 × 103) in 24-well plates. The following day, 0.34 ng/ml HGF was added to each well for 24 h. Phase-contrast images were taken to observe the extent of cell scatter.
Pak4 mediates Met-dependent cell migration and invasion.
Scatter of epithelial colonies reflects the breakdown of cell-cell junctions as well as cellular migration. To establish if Pak4 and Gab1 synergize to promote cell migration and invasion, we tested the ability of stable cell lines expressing Pak4 and Gab1 to enhance migration and invasion of single cells using Boyden chamber transwell assays. Overexpression of Gab1 or Pak4 alone resulted in no increase in migration or invasion in the absence of HGF (Fig. 8A and B). Similarly, overexpression of Gab1 and Pak4 had no significant effect on the ability of MDCK cells to migrate in the presence of HGF. However, when Pak4 and Gab1 were overexpressed together, we observed an increase in basal cell migration, but not in cell invasion, in the absence of HGF with four clones tested (Fig. 8A and B). We observed a significant increase in HGF-induced migration (5- to 10-fold) and invasion (3- to 6-fold) in cells expressing both proteins compared to cells overexpressing either Pak4 or Gab1 alone. Hence, this provides further evidence of synergy between Gab1 and Pak4 that plays a role in mediating the migratory and invasive response downstream from the Met receptor.
FIG. 8.
Overexpression of Gab1 and Pak4 induced migration and invasion of MDCK cells. MDCK cells overexpressing Gab1 and/or Pak4 were analyzed for their migration and invasion capacity in the presence of HGF (34 ng/ml). Cells were seeded (5 × 104 cells) onto (A) modified Boyden chambers or (B) Matrigel-coated Boyden chambers and assayed for their migration and invasion capacity, respectively. Using a Zeiss Axioskop microscope, bottom layers of the transwell were imaged in five separate fields for each condition, using a 10× objective in phase-contrast microscopy. Image analysis of these assays was carried out using Scion Image. Bars represent standard errors of the three experiments.
Pak4 is required for scatter and invasion downstream of Met.
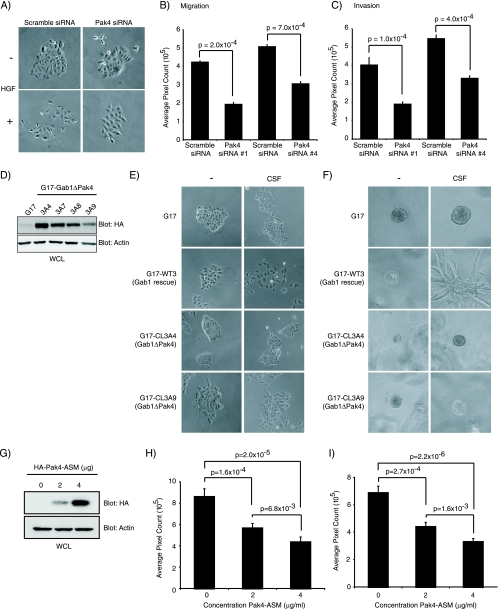
The above data demonstrate that Pak4-Gab1 overexpression enhances epithelial cell migration and invasion. To assess a requirement for Pak4 on these processes, the consequence of Pak4 knockdown was examined. MDCK cells in which Pak4 protein levels were reduced by two different siRNA (Fig. 6A) failed to scatter following HGF stimulation, compared to control MDCK or cells transfected with scrambled siRNA (Fig. 9 and data not shown). Moreover, following Pak4 knockdown, MDCK and HeLa cells showed decreased cell migration and invasion in single-cell Boyden chamber assays (Fig. 9B and C; see also Fig. S4 in the supplemental material). Overall decreases of 46% in migration and 44% in invasion were observed with MDCK cells (Fig. 9B and C, respectively) and of 45% in migration and 62% in invasion in HeLa cells (see Fig. S4 in the supplemental material).
FIG. 9.
Pak4 knockdown affects HGF-induced cell scatter, migration, and invasion. (A) MDCK cells (5 × 104) were treated with Pak4 siRNA for 96 h in a 24-well plate, and a scatter assay was performed. The following day, 1.35 ng/ml of HGF were added to each well and incubated for 24 h. Cells were then photographed with a Zeiss Axioskop microscope. (B) A migration assay was performed using a modified Boyden chamber seeded with MDCK cells transfected with scramble or two different Pak4 siRNA. Results represent data performed in triplicate. (C) Invasion assay was performed using a modified Boyden chamber coated with Matrigel and MDCK cells transfected with scramble or two different Pak4 siRNA. Results represent data performed in triplicate. Bars represent standard errors. (D) Stable G17 cell lines expressing Gab1ΔPak4 were constructed. Blots represent relative levels of Gab1ΔPak4 expressed in the four different Gab1ΔPak4 constructs used. WCL, whole-cell lysate. (E) Stable MDCK cells (5 × 104) expressing CSF-MetΔGrb2 and Gab1ΔPak4 were plated in a 24-well plate, and a scatter assay was performed. The following day, 50 ng/ml of CSF-1 were added to each well and incubated for 24 h. Cells were then photographed with a Zeiss AxiosKop microscope. (F) Stable MDCK cells lines were seeded in collagen, allowed to form cysts for 5 days, and were stimulated with CSF-1 (50 ng/ml). Representative images are shown. Pictures were acquired at a magnification of ×10. (G) HeLa cells transiently transfected with 0, 2, and 4 μg of HA-Pak4-ASM. (H) A migration assay was performed using modified Boyden chambers seeded with HeLa cells transfected with 0, 2, and 4 μg of HA-Pak4-ASM. Results represent data performed in triplicate. (I) Invasion assay was performed using a modified Boyden chamber coated with Matrigel and MDCK cells transfected with 0, 2, and 4 μg of HA-Pak4-ASM. Results represent data performed in triplicate. Error bars represent standard errors of the means.
Gab1-Pak4 association is critical for HGF-induced cell scatter, invasion, and tubulogenesis.
To test the requirement for recruitment of Pak4 to a Gab1 complex, we utilized MDCK cells expressing a chimeric CSF-Met receptor mutant in which their ability to undergo a morphogenic program in response to CSF-1 is dependent on overexpression of Gab1 (12, 29). This has allowed structure function analyses of the requirement of Gab1-associated proteins for cell scatter and invasive tubulogenesis (21, 26, 29). This MDCK (G17) model was used to test the requirement for Pak4-Gab1 interaction by overexpressing the Gab1ΔPak4 mutant. Multiple clones of MDCK (G17) cells expressing Gab1ΔPak4 (CL3A4, CL3A7, CL3A8, and CL3A9) (Fig. 9D) failed to scatter or undergo a tubulogenic response to CSF-1 (Fig. 9D, E, and F; see also Fig. S5 and S6 in the supplemental material). Since we demonstrated that increasing concentrations of the Pak4-ASM of Gab1 can compete for association between Pak4 and Gab1 (Fig. 5F and G) to further test the requirement for Gab1-Pak4, migration/invasion assays were performed on HeLa cells in which the Pak4-ASM of Gab1 was overexpressed. Consistent with Pak4 knockdown causing a decrease in the migratory and invasive capacity of HeLa cells (see Fig. S4 in the supplemental material), the overexpression of the Pak4-ASM of Gab1 (Fig. 9G) resulted in a twofold decrease in the migratory (Fig. 9H) and invasive (Fig. 9I) capacity of HeLa cells in response to HGF. Hence, these data are consistent with a requirement of the Pak4-Gab1 association for an HGF-dependent scatter, invasive, and tubulogenic response.
DISCUSSION
Our results have provided insight into the molecular mechanism through which a Gab1-Pak4 complex modulates Met-dependent signals for cell migration and invasion. Pak4 was previously shown to translocate to membrane ruffles and lamellipodia following the stimulation of MDCK cells with HGF (50). We have now demonstrated that Pak4 is a downstream target of the Met receptor (Fig. 1). We provided a mechanism through which Pak4 recruitment to Gab1 promotes Pak4 localization to the cell periphery and newly forming lamellipodia and is essential for HGF-dependent, Gab1-mediated biological responses, including epithelial cell scatter, invasion, and tubulogenesis (Fig. 7, 8, and 9; see also Fig. S4, S5, and S6 in the supplemental material). Pak4 and Gab1 interact, as demonstrated by coimmunoprecipitation of proteins from both transiently transfected cells and endogenous proteins (Fig. 1). Notably, Pak4 associated with Gab1 in response to HGF stimulation and Met activation, but not with other Gab family members, including Gab2 and Gab3 (see Fig. S2 in the supplemental material). This is consistent with our data that a Gab1-Pak4 association is essential for HGF-induced cell migration, invasion, and tubulogenesis, which are biological responses dependent on Gab1 downstream from the Met receptor (16, 21, 26, 29, 31) and not on Gab2 (26) or Gab3 (our unpublished data).
We have shown that Pak4 recruitment to Gab1 is dependent on Met activity (Fig. 1). From structure function analysis, we have determined that recruitment of Pak4 to Gab1 is dependent on a domain in Gab1 not previously identified as a protein binding domain. From two-dimensional modeling studies, the Pak4-ASM of Gab1 (aa 116 to 234) displays no similarity to known protein binding motifs. When overexpressed, the Gab1-Pak4-ASM is sufficient to compete with Met-dependent recruitment of Pak4 to Gab1 (Fig. 5), and this inhibited migration and invasion following HGF stimulation, when increasing concentrations of Pak4-ASM were expressed in HeLa (Fig. 9G to I). Hence, the Gab1-Pak4-ASM is both necessary and sufficient to interact with Pak4.
Interestingly, treatment of protein lysates with lambda phosphatase, which is active on serine, threonine, and tyrosine residues, results in a decrease in the association of Gab1 and Pak4 (Fig. 1D), supporting a potential role for tyrosine phosphorylation of Gab1 for this interaction. The Gab1-Pak4-ASM contains tyrosine, serine, and threonine residues. Hence phosphorylation of the Gab1-Pak4-ASM may generate a phosphotyrosine or phosphoserine/threonine-dependent recruitment site for Pak4 or may modulate the structure of Gab1, enhancing the interaction with Pak4. The Pak4 domain required for binding to Gab1 is a domain previously identified to bind to GEF-H1 (GID). Interaction of the Pak4 GID with GEF-H1 occurs in an apparently phosphorylation-independent manner, demonstrating that phosphorylation is not essential for Pak4-GID binding (7). A comparison of the primary and secondary structures of Gab1-Pak4-ASM and GEF-H1 does not reveal similarities in their Pak4 interacting motifs, indicating that the Pak4 GID may reflect a multiprotein interacting domain. Since the GID of Pak4 interacts with both proteins, this would also indicate that Gab1 and GEF-H1 may compete for Pak4 binding and/or recruit Pak4 to distinct subcellular localizations.
The recruitment of Pak4 to Gab1 provides a mechanism to modulate subcellular localization of Pak4. The Gab1 scaffold protein is recruited to membrane ruffles and lamellipodia in response to HGF, and this is dependent on an intact Gab1 PH domain that binds to phosphoinositide 3,4,5 triphosphate (PIP3) phospholipids, in a PI3K-dependent manner (29). In support of a role for Gab1 in Pak4 localization, Pak4 fails to be recruited to the cell cortex in cells that overexpress a Gab1 mutant lacking its PH domain, that is, still competent for Pak4 interaction (Fig. 5B), and this recruitment is rescued by targeting the Gab1ΔPH domain mutant to the plasma membrane through the addition of the c-src myristoylation signal (Fig. 2D). Consistent with a requirement for a Pak4-Gab1 complex for Met-induced epithelial cell invasion and migration (Fig. 7 to 9), a Gab1ΔPak4 mutant that is unable to associate with Pak4 fails to promote an invasive morphogenic program in response to HGF (Fig. 9F; see also Fig. S6 in the supplemental material), even though recruitment of other signaling proteins, such as Shp2 and Crk, is unaltered (Fig. 5E), identifying Pak4 recruitment to Gab1 as critical for Gab1-mediated biological responses. Hence, Gab1 may act as a scaffold protein to localize Pak4 to subcellular compartments promoting association of Pak4 upstream regulators, as well as downstream effectors. This is consistent with our previous data in which upstream regulators of Pak4, activated Rac and Cdc42 (28), relocalize to membrane protrusions in MDCK cells in response to HGF (37, 40). Moreover, Pak4 activity is associated with enhanced activation of integrins and cell spreading (6, 25, 36), and Gab1 may act to accumulate Pak4 in integrin-rich protrusions (Fig. 2).
We show that a Gab1-Pak4 complex is critical for the breakdown of cell-cell contacts and for inducing cytoskeletal changes required for migration and invasion of epithelial cells in response to HGF (Fig. 7 to 9). The requirement for Pak4 for full activation of LIMK, which regulates filopodia and lamellipodia outgrowth (9), provides a mechanism through which Pak4 could modulate HGF-dependent cell migration and invasion. Consistent with a role for Pak4 in HGF-induced migration of a prostate cancer cell line being dependent on cofilin phosphorylation (3), we observed maximum stimulation of cofilin phosphorylation by 30 to 45 min post-HGF stimulation. A decrease in HGF-induced cofilin phosphorylation following Pak4 knockdown was seen (Fig. 6B and C), and we observed a decrease of the actin network at the leading edge of cells following knockdown of Pak4 (Fig. 6D). Phosphorylation of cofilin is thought to suppress its activity due to its inability to bind F-actin (8). However, recent data suggest that the phosphorylation status of cofilin is necessary for determining the direction of cellular protrusions (33, 46). In support of this, we observed a decrease in actin remodeling in MDCK cells following knockdown of Pak4 (Fig. 6D).
Pak1 also regulates LIMK phosphorylation, and HGF can regulate Pak1 localization through an undetermined mechanism (40). Since Pak1 was unable to associate with Gab1 downstream from the Met receptor (Fig. 1B), Pak4 is a specific Gab1 effector downstream of Met. Hence, even though Pak1 and Pak4 both localize to the leading edge of migrating cells in response to HGF, they are likely to be regulated independently and may be present within different subcellular compartments or complexes to regulate actin dynamics downstream from Met. Interestingly, sequence alignment of group I and group II Paks shows that only the family of group II Paks possesses a GID, providing support for the specificity that we observe (7), whereas group 1 Pak proteins, such as Pak1, interact with GEFs of the Cool/Pix family (4).
In summary, our data provide direct evidence for a novel signaling complex downstream from HGF, involving a Gab1-Pak4 complex, and identify a role for this complex in mediating the migratory and invasive morphogenic responses of sheets of epithelial cells downstream from the Met receptor. These findings have particular significance for human cancer. Many RTKs that are deregulated in human cancer (ErbB2, Met, and EGFR) signal through Gab1. Oncomine analysis of Gab1 revealed overexpression in multiple human cancers. Group I and group II Pak family members are elevated in multiple human cancers. A screen of human cancer cell lines revealed Pak4 to be overexpressed in 78% of these cancer lines and implicated Pak4 in ras transformation and anchorage-independent growth (6, 25). Therefore, the importance of Gab1 in the invasive growth of epithelial cells downstream from the Met receptor, plus the observation that deregulation of Met is associated with many human cancers (5) and that Gab1 and Pak4 expression is elevated in human cancer, highlights the importance in identifying the molecular mechanisms through which these proteins act together to enhance tumorigenesis, invasion, and the metastasis.
Supplementary Material
Acknowledgments
We thank members of the Park lab for critical reading of the manuscript. We are grateful to Genentech Inc. for HGF. Gab3 was kindly provided by Larry R. Rohrschneider, Gab2 by Benjamin Neel, and Pak4S445NS447E and Pak4K350M by Audrey Minden.
G.P. was supported by a studentship from McGill University Health Centre and Faculty of Medicine, McGill University. This research was supported by an operating grant to M.P. from the National Cancer Institute of Canada with funding from the Canadian Cancer Society. M.P. holds the Diane and Sal Guerrera Chair in Cancer Genetics at McGill University.
Footnotes
Published ahead of print on 16 March 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Abo, A., J. Qu, M. S. Cammarano, C. Dan, A. Fritsch, V. Baud, B. Belisle, and A. Minden. 1998. PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. EMBO J. 176527-6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adam, L., R. Vadlamudi, S. B. Kondapaka, J. Chernoff, J. Mendelsohn, and R. Kumar. 1998. Heregulin regulates cytoskeletal reorganization and cell migration through the p21-activated kinase-1 via phosphatidylinositol-3 kinase. J. Biol. Chem. 27328238-28246. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed, T., K. Shea, J. R. Masters, G. E. Jones, and C. M. Wells. 2008. A PAK4-LIMK1 pathway drives prostate cancer cell migration downstream of HGF. Cell. Signal. 201320-1328. [DOI] [PubMed] [Google Scholar]
- 4.Bagrodia, S., and R. A. Cerione. 1999. Pak to the future. Trends Cell Biol. 9350-355. [DOI] [PubMed] [Google Scholar]
- 5.Birchmeier, C., W. Birchmeier, E. Gherardi, and G. F. Vande Woude. 2003. Met, metastasis, motility and more. Nat. Rev. Mol. Cell Biol. 4915-925. [DOI] [PubMed] [Google Scholar]
- 6.Callow, M. G., F. Clairvoyant, S. Zhu, B. Schryver, D. B. Whyte, J. R. Bischoff, B. Jallal, and T. Smeal. 2002. Requirement for PAK4 in the anchorage-independent growth of human cancer cell lines. J. Biol. Chem. 277550-558. [DOI] [PubMed] [Google Scholar]
- 7.Callow, M. G., S. Zozulya, M. L. Gishizky, B. Jallal, and T. Smeal. 2005. PAK4 mediates morphological changes through the regulation of GEF-H1. J. Cell Sci. 1181861-1872. [DOI] [PubMed] [Google Scholar]
- 8.Condeelis, J. 2001. How is actin polymerization nucleated in vivo? Trends Cell Biol. 11288-293. [DOI] [PubMed] [Google Scholar]
- 9.Dan, C., A. Kelly, O. Bernard, and A. Minden. 2001. Cytoskeletal changes regulated by the PAK4 serine/threonine kinase are mediated by LIM kinase 1 and cofilin. J. Biol. Chem. 27632115-32121. [DOI] [PubMed] [Google Scholar]
- 10.Edwards, D. C., L. C. Sanders, G. M. Bokoch, and G. N. Gill. 1999. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat. Cell Biol. 1253-259. [DOI] [PubMed] [Google Scholar]
- 11.Faix, J., and K. Rottner. 2006. The making of filopodia. Curr. Opin. Cell Biol. 1818-25. [DOI] [PubMed] [Google Scholar]
- 12.Fournier, T. M., D. Kamikura, K. Teng, and M. Park. 1996. Branching tubulogenesis but not scatter of madin-darby canine kidney cells requires a functional Grb2 binding site in the Met receptor tyrosine kinase. J. Biol. Chem. 27122211-22217. [DOI] [PubMed] [Google Scholar]
- 13.Frigault, M. M., M. A. Naujokas, and M. Park. 2008. Gab2 requires membrane targeting and the Met binding motif to promote lamellipodia, cell scatter, and epithelial morphogenesis downstream from the Met receptor. J. Cell Physiol. 214694-705. [DOI] [PubMed] [Google Scholar]
- 14.Gherardi, E., J. Gray, M. Stoker, M. Perryman, and R. Furlong. 1989. Purification of scatter factor, a fibroblast-derived basic protein that modulates epithelial interactions and movement. Proc. Natl. Acad. Sci. USA 865844-5848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu, H., and B. G. Neel. 2003. The “Gab” in signal transduction. Trends Cell Biol. 13122-130. [DOI] [PubMed] [Google Scholar]
- 16.Gual, P., S. Giordano, T. A. Williams, S. Rocchi, E. Van Obberghen, and P. M. Comoglio. 2000. Sustained recruitment of phospholipase C-gamma to Gab1 is required for HGF-induced branching tubulogenesis. Oncogene 191509-1518. [DOI] [PubMed] [Google Scholar]
- 17.Hall, A. 2005. Rho GTPases and the control of cell behaviour. Biochem. Soc. Trans. 33891-895. [DOI] [PubMed] [Google Scholar]
- 18.Holgado-Madruga, M., D. R. Emlet, D. K. Moscatello, A. K. Godwin, and A. J. Wong. 1996. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature 379560-564. [DOI] [PubMed] [Google Scholar]
- 19.Jaffer, Z. M., and J. Chernoff. 2002. p21-activated kinases: three more join the Pak. Int. J. Biochem. Cell Biol. 34713-717. [DOI] [PubMed] [Google Scholar]
- 20.Knuesel, M., Y. Wan, Z. Xiao, E. Holinger, N. Lowe, W. Wang, and X. Liu. 2003. Identification of novel protein-protein interactions using a versatile mammalian tandem affinity purification expression system. Mol. Cell. Proteomics 21225-1233. [DOI] [PubMed] [Google Scholar]
- 21.Lamorte, L., S. Rodrigues, M. Naujokas, and M. Park. 2002. Crk synergizes with epidermal growth factor for epithelial invasion and morphogenesis and is required for the Met morphogenic program. J. Biol. Chem. 27737904-37911. [DOI] [PubMed] [Google Scholar]
- 22.Lamorte, L., S. Rodrigues, V. Sangwan, C. E. Turner, and M. Park. 2003. Crk associates with a multimolecular Paxillin/GIT2/beta-PIX complex and promotes Rac-dependent relocalization of Paxillin to focal contacts. Mol. Biol. Cell 142818-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamorte, L., I. Royal, M. Naujokas, and M. Park. 2002. Crk adapter proteins promote an epithelial-mesenchymal-like transition and are required for HGF-mediated cell spreading and breakdown of epithelial adherens junctions. Mol. Biol. Cell 131449-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu, Y., and L. R. Rohrschneider. 2002. The gift of Gab. FEBS Lett. 5151-7. [DOI] [PubMed] [Google Scholar]
- 25.Liu, Y., H. Xiao, Y. Tian, T. Nekrasova, X. Hao, H. J. Lee, N. Suh, C. S. Yang, and A. Minden. 2008. The pak4 protein kinase plays a key role in cell survival and tumorigenesis in athymic mice. Mol. Cancer Res. 61215-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lock, L. S., C. R. Maroun, M. A. Naujokas, and M. Park. 2002. Distinct recruitment and function of Gab1 and Gab2 in Met receptor-mediated epithelial morphogenesis. Mol. Biol. Cell 132132-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lock, L. S., I. Royal, M. A. Naujokas, and M. Park. 2000. Identification of an atypical Grb2 carboxyl-terminal SH3 domain binding site in Gab docking proteins reveals Grb2-dependent and -independent recruitment of Gab1 to receptor tyrosine kinases. J. Biol. Chem. 27531536-31545. [DOI] [PubMed] [Google Scholar]
- 28.Manser, E., T. Leung, H. Salihuddin, Z. S. Zhao, and L. Lim. 1994. A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 36740-46. [DOI] [PubMed] [Google Scholar]
- 29.Maroun, C. R., M. Holgado-Madruga, I. Royal, M. A. Naujokas, T. M. Fournier, A. J. Wong, and M. Park. 1999. The Gab1 PH domain is required for localization of Gab1 at sites of cell-cell contact and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol. Cell. Biol. 191784-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maroun, C. R., D. K. Moscatello, M. A. Naujokas, M. Holgado-Madruga, A. J. Wong, and M. Park. 1999. A conserved inositol phospholipid binding site within the pleckstrin homology domain of the Gab1 docking protein is required for epithelial morphogenesis. J. Biol. Chem. 27431719-31726. [DOI] [PubMed] [Google Scholar]
- 31.Maroun, C. R., M. A. Naujokas, M. Holgado-Madruga, A. J. Wong, and M. Park. 2000. The tyrosine phosphatase SHP-2 is required for sustained activation of extracellular signal-regulated kinase and epithelial morphogenesis downstream from the Met receptor tyrosine kinase. Mol. Cell. Biol. 208513-8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maroun, C. R., M. A. Naujokas, and M. Park. 2003. Membrane targeting of Grb2-associated binder-1 (Gab1) scaffolding protein through Src myristoylation sequence substitutes for Gab1 pleckstrin homology domain and switches an epidermal growth factor response to an invasive morphogenic program. Mol. Biol. Cell 141691-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mouneimne, G., V. DesMarais, M. Sidani, E. Scemes, W. Wang, X. Song, R. Eddy, and J. Condeelis. 2006. Spatial and temporal control of cofilin activity is required for directional sensing during chemotaxis. Curr. Biol. 162193-2205. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen, L., M. Holgado-Madruga, C. Maroun, E. D. Fixman, D. Kamikura, T. Fournier, A. Charest, M. L. Tremblay, A. J. Wong, and M. Park. 1997. Association of the multisubstrate docking protein Gab1 with the hepatocyte growth factor receptor requires a functional Grb2 binding site involving tyrosine 1356. J. Biol. Chem. 27220811-20819. [DOI] [PubMed] [Google Scholar]
- 35.Peschard, P., and M. Park. 2007. From Tpr-Met to Met, tumorigenesis and tubes. Oncogene 261276-1285. [DOI] [PubMed] [Google Scholar]
- 36.Qu, J., M. S. Cammarano, Q. Shi, K. C. Ha, P. de Lanerolle, and A. Minden. 2001. Activated PAK4 regulates cell adhesion and anchorage-independent growth. Mol. Cell. Biol. 213523-3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ridley, A. J., P. M. Comoglio, and A. Hall. 1995. Regulation of scatter factor/hepatocyte growth factor responses by Ras, Rac, and Rho in MDCK cells. Mol. Cell. Biol. 151110-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rigaut, G., A. Shevchenko, B. Rutz, M. Wilm, M. Mann, and B. Seraphin. 1999. A generic protein purification method for protein complex characterization and proteome exploration. Nat. Biotechnol. 171030-1032. [DOI] [PubMed] [Google Scholar]
- 39.Rodrigues, G. A., M. A. Naujokas, and M. Park. 1991. Alternative splicing generates isoforms of the met receptor tyrosine kinase which undergo differential processing. Mol. Cell. Biol. 112962-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Royal, I., N. Lamarche-Vane, L. Lamorte, K. Kaibuchi, and M. Park. 2000. Activation of cdc42, rac, PAK, and rho-kinase in response to hepatocyte growth factor differentially regulates epithelial cell colony spreading and dissociation. Mol. Biol. Cell 111709-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Royal, I., and M. Park. 1995. Hepatocyte growth factor-induced scatter of Madin-Darby canine kidney cells requires phosphatidylinositol 3-kinase. J. Biol. Chem. 27027780-27787. [DOI] [PubMed] [Google Scholar]
- 42.Sakkab, D., M. Lewitzky, G. Posern, U. Schaeper, M. Sachs, W. Birchmeier, and S. M. Feller. 2000. Signaling of hepatocyte growth factor/scatter factor (HGF) to the small GTPase Rap1 via the large docking protein Gab1 and the adapter protein CRKL. J. Biol. Chem. 27510772-10778. [DOI] [PubMed] [Google Scholar]
- 43.Schaeper, U., N. H. Gehring, K. P. Fuchs, M. Sachs, B. Kempkes, and W. Birchmeier. 2000. Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J. Cell Biol. 1491419-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sells, M. A., U. G. Knaus, S. Bagrodia, D. M. Ambrose, G. M. Bokoch, and J. Chernoff. 1997. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr. Biol. 7202-210. [DOI] [PubMed] [Google Scholar]
- 45.Small, J. V., and G. P. Resch. 2005. The comings and goings of actin: coupling protrusion and retraction in cell motility. Curr. Opin. Cell Biol. 17517-523. [DOI] [PubMed] [Google Scholar]
- 46.Song, X., X. Chen, H. Yamaguchi, G. Mouneimne, J. S. Condeelis, and R. J. Eddy. 2006. Initiation of cofilin activity in response to EGF is uncoupled from cofilin phosphorylation and dephosphorylation in carcinoma cells. J. Cell Sci. 1192871-2881. [DOI] [PubMed] [Google Scholar]
- 47.Stoker, M., E. Gherardi, M. Perryman, and J. Gray. 1987. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature 327239-242. [DOI] [PubMed] [Google Scholar]
- 48.Weidner, K. M., J. Behrens, J. Vandekerckhove, and W. Birchmeier. 1990. Scatter factor: molecular characteristics and effect on the invasiveness of epithelial cells. J. Cell Biol. 1112097-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weidner, K. M., S. Di Cesare, M. Sachs, V. Brinkmann, J. Behrens, and W. Birchmeier. 1996. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature 384173-176. [DOI] [PubMed] [Google Scholar]
- 50.Wells, C. M., A. Abo, and A. J. Ridley. 2002. PAK4 is activated via PI3K in HGF-stimulated epithelial cells. J. Cell Sci. 1153947-3956. [DOI] [PubMed] [Google Scholar]
- 51.Wells, C. M., T. Ahmed, J. R. Masters, and G. E. Jones. 2005. Rho family GTPases are activated during HGF-stimulated prostate cancer-cell scattering. Cell Motil. Cytoskelet. 62180-194. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, H., Z. Li, E. K. Viklund, and S. Stromblad. 2002. P21-activated kinase 4 interacts with integrin alpha v beta 5 and regulates alpha v beta 5-mediated cell migration. J. Cell Biol. 1581287-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou, H., and R. H. Kramer. 2005. Integrin engagement differentially modulates epithelial cell motility by RhoA/ROCK and PAK1. J. Biol. Chem. 28010624-10635. [DOI] [PubMed] [Google Scholar]
- 54.Zhu, H., M. A. Naujokas, and M. Park. 1994. Receptor chimeras indicate that the met tyrosine kinase mediates the motility and morphogenic responses of hepatocyte growth/scatter factor. Cell Growth Differ. 5359-366. [PubMed] [Google Scholar]
- 55.Zhuo, S., J. C. Clemens, D. J. Hakes, D. Barford, and J. E. Dixon. 1993. Expression, purification, crystallization, and biochemical characterization of a recombinant protein phosphatase. J. Biol. Chem. 26817754-17761. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.