Abstract
Trophoblast differentiation during placentation involves an epithelial-mesenchymal transition (EMT) with loss of E-cadherin and gain of trophoblast invasiveness. Mice harboring a point mutation that renders inactive the mitogen-activated protein kinase kinase kinase MEKK4 exhibit dysregulated placental development with increased trophoblast invasion. Isolated MEKK4 kinase-inactive trophoblast stem (TS) cells cultured under undifferentiating, self-renewing conditions in the presence of fibroblast growth factor 4 (FGF4) display increased expression of Slug, Twist, and matrix metalloproteinase 2 (MMP2), loss of E-cadherin, and hyperinvasion of extracellular matrix, each a hallmark of EMT. MEKK4 kinase-inactive TS cells show a preferential differentiation to Tpbpα- and Gcm1-positive trophoblasts, which are indicative of spongiotrophoblast and syncytiotrophoblast differentiation, respectively. FGF4-stimulated Jun N-terminal kinase (JNK) and p38 activity is markedly reduced in MEKK4 kinase-inactive TS cells. Chemical inhibition of JNK in wild-type TS cells induced a similar EMT response as loss of MEKK4 kinase activity, including inhibition of E-cadherin expression and increased expression of Slug, MMP2, Tpbpα, and Gcm1. Chromatin immunoprecipitation analyses revealed changes in AP-1 composition with increased Fra-2 and decreased Fra-1 and JunB binding to the regulatory regions of Gcm1 and MMP2 genes in MEKK4 kinase-inactive TS cells. Our results define MEKK4 as a signaling hub for FGF4 activation of JNK that is required for maintenance of TS cells in an undifferentiated state.
Trophoblasts are the first cell type to be specified at the blastocyst stage and differentiate to form the extraembryonic epithelial cells of the placenta (20). Differentiation of trophoblast stem (TS) cells is necessary for trophoblast invasion of the uterus for establishment of the placenta. Once implantation is complete, different trophoblast lineages assume specific functions in the placenta. TS cell acquisition of the ability to migrate and invade extracellular matrix required for placentation is a functional hallmark of epithelial-mesenchymal transition (EMT) (21). Once invasion is complete, the trophoblasts assume an epithelial phenotype with loss of motility and invasiveness characteristic of a mesenchymal-epithelial transition (MET) (9, 27). Other than the role of fibroblast growth factor 4 (FGF4) activation of ERK1/2 in controlling TS cell self-renewal and survival, little is known about the signaling pathways controlling TS cell maintenance, suppression of the commitment to an invasive phenotype, and differentiation (18, 22, 26).
We have found that MEKK4, a mitogen-activated protein kinase kinase kinase (MAP3K) that regulates Jun N-terminal kinase (JNK) and p38 activity, is expressed strongly in the developing embryo, TS cells, and cells derived from TS cells. Targeted mutation of the required active-site lysine renders MEKK4 kinase inactive in murine ES cells (3) and results in dramatic changes in embryonic development. Loss of MEKK4 kinase activity in developing mouse embryos results in lethality due to both neural tube closure defects and skeletal malformations (3). Herein we show that extraembryonic development is perturbed in MEKK4 kinase-inactive concepti, resulting in disruptions in placental development. These defects are highly penetrant in fetuses homozygous for kinase-inactive MEKK4 and are also observed with fetuses heterozygous for the mutant MEKK4 allele because of the dominant negative properties of the kinase-inactive MEKK4 protein.
Little is known about how growth factors control tissue stem cell maintenance and self-renewal and signal to control inhibition of differentiation. In this report we show that changes in the regulation of TS cell differentiation account for the disruption in placental development seen with mice harboring kinase-inactive MEKK4. We show that loss of MEKK4 kinase activity in TS cells inhibits FGF4 activation of JNK and p38, promoting the loss of E-cadherin expression, increased invasiveness, and expression of factors that promote selective TS cell differentiation toward spongiotrophoblast and syncytiotrophoblast lineages. Chemical inhibition of JNK mimics many of the changes observed in the kinase-inactive MEKK4 TS cells. Thus, MEKK4 signaling to JNK is required for FGF4 to suppress TS cell EMT, invasiveness, and differentiation. The results show that MEKK4 is a key hub kinase that regulates the fundamental decision of TS cells to undergo renewal for stem cell maintenance or to induce a program of increased motility, invasiveness, and differentiation for placental development. These findings show for the first time the requirement of FGF4-stimulated MEKK4 activation of JNK for maintenance of a tissue stem cell.
MATERIALS AND METHODS
Mouse strains and genotyping.
MEKK4WT/K1361R mice (3) were maintained on both mixed (C57BL/6 × 129/SvEv) and pure (129/SvEv) backgrounds. Placentas and TS cells isolated from both mouse backgrounds yielded identical results. PCR genotyping was used for mice, embryos, and TS cells as previously described (3). All animal and embryo work was performed according to university and federal guidelines for the use of animals.
Placental isolation and histology.
Embryos and placentas were isolated at embryonic day 7.5 (E7.5), E8.5, E9.5, and E13.5 from timed matings of either wild-type or heterozygote MEKK4WT/K1361R mice. Embryos were used for genotyping. Placentas were fixed at 4°C overnight in 4% paraformaldehyde-phosphate-buffered saline (PBS) and frozen as previously described (3). Ten-micrometer cryosections were used for analyses. Sections were stained with hematoxylin and eosin using standard techniques.
In situ hybridization.
In situ hybridizations were performed as described previously (3, 24). Slides were incubated overnight at 66°C with digoxigenin-labeled antisense probes for PlI, Tpbpα, and Gcml, which were generated from constructs provided as a kind gift by David Threadgill (UNC Chapel Hill). Probes were detected with alkaline phosphatase-conjugated antidigoxigenin antibodies (Boehringer Mannheim) and BM purple (Boehringer Mannheim).
Trophoblast stem cell isolation and primary cell culture.
For wild-type and heterozygote cells, TS cells were isolated using the method of Tanaka et al. from blastocysts derived from timed matings of MEKK4WT/K1361R mice (22). TS cells were cultured without feeders in 30% TS medium containing RPMI 1640, 20% heat-inactivated fetal bovine serum, 1% sodium pyruvate, 1% penicillin-streptomycin, 1% glutamine, and 100 μM β-mercaptoethanol and 70% conditioned medium isolated from mitomycin-treated primary mouse embryonic fibroblasts (MEFs) cultured in TS medium. Medium was supplemented with FGF4 (37.5 ng/ml) and heparin (1 mg/ml). MEFs were isolated from 129SvEv mice at E14.5 as previously described (3). For treatment with inhibitors, undifferentiated TS cells were treated for 2 days with either dimethyl sulfoxide (DMSO), 50 μM SP600125 (JNK inhibitor), 10 μM SB203580 (p38 inhibitor), or 10 μM U0126 (MEK inhibitor). Tissue culture plastic was from Fisher, medium reagents were from Gibco, and chemicals were from Sigma.
Immunofluorescence.
For immunostaining of placental cryosections, sections were washed in PBS and permeabilized for 5 minutes in 0.2% Triton in PBS. Slides were washed and blocked in 10% donkey serum and incubated overnight at 4°C in block containing rabbit anti-MEKK4 antibody (3), rat anti-PECAM antibody (BD Pharmingen), and mouse anticytokeratin antibody (Sigma). Slides were washed and incubated for 1 hour with fluorescently labeled secondary antibodies (Jackson Immunoresearch) and 4′,6-diamidino-2-phenylindole (DAPI; Molecular Probes). TS cells were cultured on coverglasses for 2 days, treated with DMSO or inhibitors for 2 days, and then fixed in 3% paraformaldehyde-PBS for 10 minutes. Coverslips were washed and permeabilized for 5 minutes in 0.2% Triton in PBS. After incubation for 1 hour in block, coverslips were incubated overnight at 4°C in block containing mouse anti-E-cadherin antibody (BD Transduction). Coverslips were washed and incubated for 1 hour at room temperature in block containing Cy3-donkey anti-mouse secondary and DAPI.
Western blotting of whole-cell and nuclear lysates.
TS cells were washed once with PBS and lysed on ice in buffer A containing 20 mM Tris (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton-X, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium vanadate, 1 mM sodium fluoride, 0.05 mM dithiothreitol, 1 μg/ml leupeptin, and 17 μg/ml aprotinin A. Ten micrograms of lysate was subjected to Western blotting with phospho-specific antibodies to JNK and p38 (Cell Signaling) or with antibodies recognizing total α-tubulin (Sigma), β-catenin (Sigma), E-cadherin (BD Transduction), or MEKK3 (BD Transduction). Bands were quantitated by densitometry using ImageJ.
For nuclear lysates, TS cells were washed once with PBS and scraped on ice in homogenization buffer containing 10 mM HEPES (pH 7.4), 10 mM KCl, 1.5 mM MgCl2, 0.1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium vanadate, 0.05 mM dithiothreitol, 1 μg/ml leupeptin, and 17 μg/ml aprotinin A. Cells were disrupted by five passages through a 25-gauge needle. Cells were spun and pellets were washed with homogenization buffer. Pellets were extracted with 30 μl of extraction buffer containing 10 mM HEPES (pH 7.4), 400 mM NaCl, 1.5 mM MgCl2, 0.1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM sodium vanadate, 0.05 mM dithiothreitol, 1 μg/ml leupeptin, and 17 μg/ml aprotinin A with shaking at 4°C for 15 minutes. Pellets were spun for 15 minutes at 4°C and 5 μg of the supernatant was analyzed by Western blotting for AP-1 transcription family members using antibodies from Santa Cruz Biotechnology for Fra-1, Fra-2, JunB, ATF-2, and c-Jun.
Quantitative real-time RT-PCR and chromatin immunoprecipitation (ChIP) analyses.
Total RNA was isolated from murine trophoblast stem cells using the RNeasy minikit (Qiagen). The High-Capacity cDNA reverse transcription (RT) kit (Applied Biosystems) was used to synthesize cDNA from 3 μg of total RNA, which was DNase treated only if the subsequent real-time assays were not designed to detect mRNA exclusively. Real-time RT-PCR was performed on diluted cDNA using the Applied Biosystems 7500 Fast real-time PCR system (standard program) and either inventoried TaqMan gene expression assays or Sybr green reagents with primer pairs designed using Primer Express software version 3.0 (Applied Biosystems). Primer pairs for detecting murine proliferins 1 to 4 (PLF-1 to -4) and placental lactogen 1α (PL-1α) with the Sybr green assay were as follows: PLF 5′ primer, TCAACCATGCTCCTGGATACTG; PLF 3′ primer, GGCAACATTCTTCCACAATAACG; PL-1α 5′ primer, TTATCTTGGCCGCAGATGTG; PL-1α 3′ primer, TCTGTCTGTTATCCAAGTTTTATCGAA. Murine β-actin served as the endogenous control, and undifferentiated wild-type MEKK4 was the calibrator for calculating differences in expression levels using the 2−ΔΔCT method. Final results represent the pooling of at least two experiments in which cDNA was assayed in duplicate or triplicate.
For ChIP analyses, undifferentiated wild-type and kinase-inactive TS cells were fixed for 10 minutes with 1% formaldehyde, sonicated, and immunoprecipitated with anti-Fra-1, -Fra-2, and -JunB antibodies. Cross-linking was reversed by incubation at 65°C overnight and DNA was purified using the MinElute PCR purification kit (Qiagen). PCRs for Gcm1 and matrix metalloproteinase 2 (MMP2) were carried out at 59°C for 35 cycles. Primers for the third intron of murine Gcm1 generated a 236-bp product: Gcm1 5′ primer, ATTCGTGAATAAGTAGGGAGGA; Gcm1 3′ primer, GCTGATTCTGAGTCTGAGGTGTC. Primer for −2723 to −2344 in the MMP2 promoter generated a 401-bp product. The MMP2 5′ primer was CTCACAGGACCCTCACCAGAC and the MMP2 3′ primer was GCCTTAGGAAGACATGGAACCC.
RESULTS
Expression of MEKK4 in placental trophoblasts.
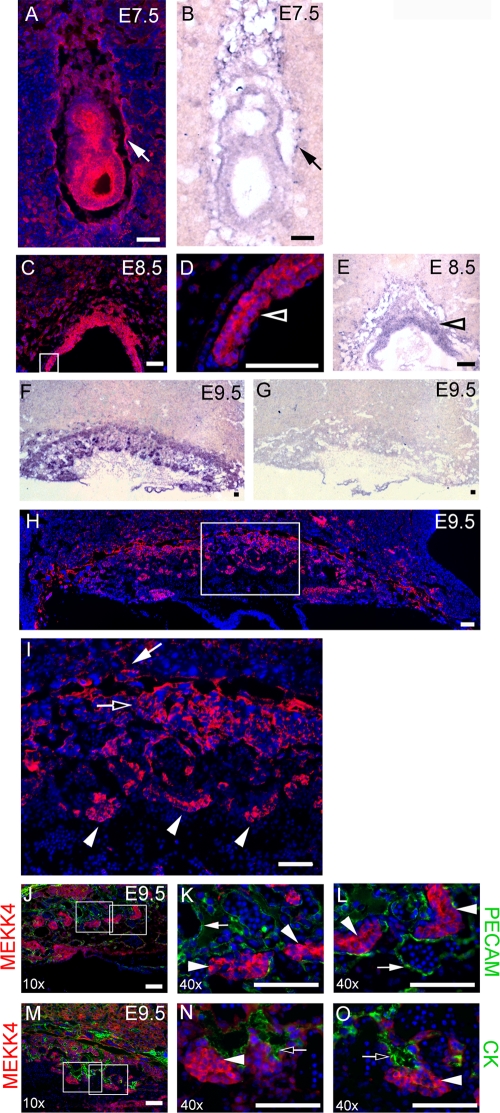
To understand the role of MEKK4 in trophoblasts, MEKK4 expression during placental development from E7.5 to E13.5 was examined by immunostaining with an anti-MEKK4 antibody and by in situ hybridization with an antisense probe for MEKK4. MEKK4 is expressed strongly in the developing embryo (Fig. 1A and B) (3, 7). In addition, MEKK4 was found in the giant cells surrounding the embryo and in the ectoplacental cone of the E7.5 placenta by both immunostaining (Fig. 1A) and in situ hybridization (Fig. 1B). At E8.5, robust staining for MEKK4 was observed in the chorion of the placenta (Fig. 1C, D, and E). In situ hybridization detected MEKK4 message in all three of the trophoblast cell layers of the E9.5 placenta that included the labyrinth, spongiotrophoblast, and giant cell layers (Fig. 1F), but staining was not observed with a control MEKK4 sense probe (Fig. 1G). Further, anti-MEKK4 immunostaining of E9.5 placentas revealed MEKK4 staining in all three trophoblast layers, with the strongest staining in the undifferentiated cuboidal trophoblasts (Fig. 1H and I). At E13.5, MEKK4 protein and message were only weakly detectable, indicating a loss of MEKK4 expression with placental differentiation and maturation (data not shown). Costaining of E9.5 placentas with anti-PECAM antibody to detect endothelial cells and anti-MEKK4 antibody showed that MEKK4 does not colocalize with PECAM-positive endothelial cells but is highly expressed in the undifferentiated trophoblasts (Fig. 1J, K, and L). All trophoblast cells express keratins, a family of intermediate filament proteins. Differentiation of trophoblasts induces the expression of a more extensive keratin scaffold network that is detectable with a pan-anticytokeratin antibody. Costaining with anticytokeratin antibody and anti-MEKK4 antibody demonstrated colocalization of MEKK4 and cytokeratin (Fig. 1M, N, and O). However, MEKK4 expression was reduced in the more cytokeratin-positive differentiated trophoblasts relative to the undifferentiated trophoblasts. These data indicate that MEKK4 is highly expressed specifically in the undifferentiated trophoblasts of the placenta, and with differentiation to mature trophoblast lineages, MEKK4 expression decreases significantly. This finding is indicative of MEKK4 playing a significant function in trophoblasts that is lost or significantly diminished with differentiation.
FIG. 1.
MEKK4 is expressed strongly in the trophoblast lineages of the developing placenta. MEKK4 was visualized in cryosections with anti-MEKK4 immunostaining (red) (A, C, D, H, and I) and by in situ hybridization with MEKK4 antisense probe (purple) (B, E, and F) or control MEKK4 sense probe (G). Nuclei were counterstained with DAPI (blue). (A) Anti-MEKK4 immunostaining shows that MEKK4 is expressed not only in the developing embryo but also in the invading primary giant cells lining the implantation site and the ectoplacental cone. (B) MEKK4 expression by in situ hybridization shows an identical expression pattern of MEKK4 message compared to the MEKK4 protein shown in panel A. (C and D) Strong MEKK4 expression in E8.5 chorion visualized by anti-MEKK4 immunostaining. The box indicates the area shown in the inset in panel D. (E) Strong expression of MEKK4 message in E8.5 chorion by in situ hybridization with antisense MEKK4 probe. (F) In situ hybridization with antisense MEKK4 probe in an E9.5 cryosection. (G) Control in situ hybridization with sense MEKK4 probe in an E9.5 cryosection. (H and I) Anti-MEKK4 immunostaining of E9.5 placenta (red). A white box indicates the area shown in the inset in panel I. For panels A to I, closed arrows indicate trophoblast giant cells, open arrows indicate spongiotrophoblasts, closed arrowheads indicate undifferentiated cuboidal trophoblasts, and open arrowheads indicate chorion. (J to O) Immunofluorescence microscopy of E9.5 placental cryosections stained with an anti-MEKK4 antibody (red) and counterstained with the nuclear stain DAPI (blue). (J to L) MEKK4 does not colocalize with PECAM-positive endothelial cells. Sections were also costained with anti-PECAM antibody (green). (M to O) MEKK4 colocalizes with cytokeratin (CK). Sections were also costained with anti-CK antibody. White boxes in panel J indicate the area that is enlarged in the insets in panels K and L. White boxes in panel M indicate areas enlarged in the insets in panels N and O. Filled arrowheads indicate MEKK4 in undifferentiated cuboidal trophoblasts. For panels J to O, filled arrows indicate PECAM-positive endothelial cells. Open arrows indicate sites with CK staining (green) having colocalization with MEKK4 (red). Bar, 100 μm.
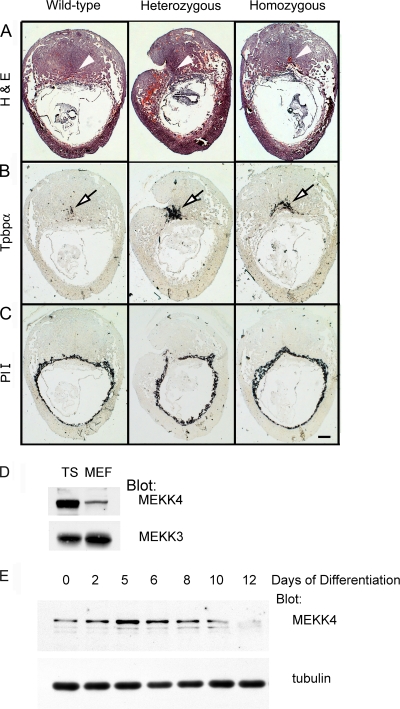
Placentas from both heterozygote and homozygote MEKK4K1361R E9.5 embryos display spatial malformations and dysregulated expression of Tbpbα.
During the study of embryos from MEKK4WT/K1361R intercrosses, delayed development of the kinase-inactive homozygote and heterozygote embryos was observed. Further, resorptions were common, with 24% of all embryos reabsorbed or in a state of resorption (16 of 66 embryos) at E9.5. Examination of placentas from embryos isolated at E9.5 from MEKK4WT/K1361R intercrosses revealed spatial malformations of both placentas and decidua of heterozygote and homozygote MEKK4 kinase-inactive placentas relative to wild-type littermates (Fig. 2A). To measure the presence of the different trophoblast cell types in the MEKK4 kinase-inactive placentas, in situ hybridizations for markers of the three layers of the placenta (giant cell, spongiotrophoblast, and labyrinth) were performed. Expression of the spongiotrophoblast marker Tpbpα was clearly elevated in the placentas from both heterozygote and homozygote kinase-inactive embryos, with levels being even higher in heterozygotes (Fig. 2B). This finding is likely due to the dominant negative properties of the kinase-inactive mutant and the defined ability of the kinase-inactive protein to dimerize with the wild-type protein and interfere with MEKK4 signaling to JNK and p38, resulting in severe neural tube and skeletal defects (3). The mRNA levels of the primary giant cell marker PlI were only slightly increased, particularly in the homozygous MEKK4 kinase-inactive animals (Fig. 2C), but not as dramatically as that observed for Tpbpα (Fig. 2Β). In situ hybridizations for the labyrinth syncytiotrophoblast marker Gcm1 yielded weak signals, making it difficult to assess changes in this marker (data not shown). The marked increase in Tpbpα expression observed in the developing placentas from MEKK4 kinase-inactive mice indicated an abnormal trophoblast differentiation response.
FIG. 2.
Altered morphology of kinase-inactive (K1361R) placentas with dysregulated expression of Tpbpα. Placental sections from littermate E9.5 embryos with wild-type (MEKK4WT; left), heterozygous (MEKK4WT/K1361R; center), or homozygous kinase-inactive (MEKK4K1361R; right) genotypes. (A) Hematoxylin and eosin (H&E) staining of E9.5 placentas. White arrowheads indicate the location of the placenta. (B) In situ hybridization with antisense probe to the spongiotrophoblast marker Tpbpα, showing dysregulated expression of Tpbpα. Open black arrows indicate sites of Tpbpα expression. (C) In situ hybridization with antisense probe to the primary giant cell marker placental lactogen I (PlI). Bar, 0.1 mm. MEKK4 is expressed strongly in trophoblast stem cells and expression is reduced with differentiation. (D) MEKK4 is expressed more strongly in stem cells than in fibroblasts. Thirty micrograms of TS cell or MEF lysate was probed with anti-MEKK4 or anti-ΜΕΚΚ3 antibodies. (E) MEKK4 expression is reduced by TS cell differentiation. TS cells were differentiated by removal of FGF4 and fibroblast-conditioned medium for the indicated times and probed with anti-MEKK4 or anti-α-tubulin antibodies.
MEKK4 function in TS cells.
TS cells were isolated from blastocyst outgrowths according to the method of Tanaka et al. (22) and used to define the function of MEKK4 in trophoblasts. Similar to the immunostaining and in situ hybridization results observed in the developing embryo and placenta (Fig. 1), immunoblotting demonstrated that MEKK4 is expressed at high levels in isolated TS cells relative to other cell types such as mouse embryo fibroblasts; in contrast, the MAP3K MEKK3 is expressed similarly in these two cell types (Fig. 2D). We also compared MEKK4 expression in vitro in wild-type renewing TS cells versus differentiating TS cells induced by the removal of FGF4 and fibroblast-conditioned medium. Similar to what occurred in the placenta, induction of trophoblast differentiation resulted in reduced MEKK4 expression levels (Fig. 2E). Differentiation of TS cells resulted in increased levels of a degradation fragment of MEKK4 that runs just below the full-length form of MEKK4. This change was followed by a reduction in the total levels of MEKK4 by 12 days of differentiation, a time that yields clearly defined spongiotrophoblasts, syncytiotrophoblasts, and giant cells in cultures (Fig. 2E).
TS cells were isolated from blastocyst outgrowths from 12 litters of MEKK4WT/K1361R intercrosses. Of the 61 isolated blastocysts, 16% failed to hatch and were discarded. Of the 51 outgrowths, 73% yielded TS cell colonies, with 45% of these clones generating enough cells to be successfully genotyped. At this stage of isolation, there was a clear loss of TS cells homozygous for the MEKK4K1361R mutation (Table 1). The homozygote MEKK4K1361R colonies failed to stabilize and terminally differentiated (Table 1). Interestingly, there was a significant reduction in the number of heterozygote MEKK4WT/K1361R outgrowths relative to wild-type outgrowths that formed stable TS cell colonies (Table 1).
TABLE 1.
Genotypes of trophoblast stem cells isolated from blastocyst outgrowths from MEKK4WT/K1361R intercrosses
| Stem cell type | Total no. of outgrowths | % with indicated genotype
|
||
|---|---|---|---|---|
| Wild type | Heterozygote | Homozygote | ||
| Genotyped | 23 | 39.1 | 52.2 | 8.7 |
| Stable clones | 12 | 58.3 | 41.7 | 0 |
Due to the potential loss of homozygote MEKK4K1361R cells due to failed hatching and attachment, we isolated TS cells from the extraembryonic ectoderm. TS cells can be isolated from the extraembryonic ectoderm of the E6.5 conceptus (22). However, MEKK4K1361R embryos, including the extraembryonic placenta, have delayed development requiring the isolation of homozygous TS cells at E7.5, a stage that is extremely difficult to isolate in wild-type TS cells (22). As predicted, no wild-type or heterozygous MEKK4WT/K1361R TS cells were isolated at E7.5, but TS cells homozygous for kinase-inactive MEKK4 were isolated, consistent with the MEKK4 kinase-inactive homozygote embryos being developmentally delayed. We were successful in obtaining stable homozygote MEKK4K1361R TS cells that could be cultured for over 6 months.
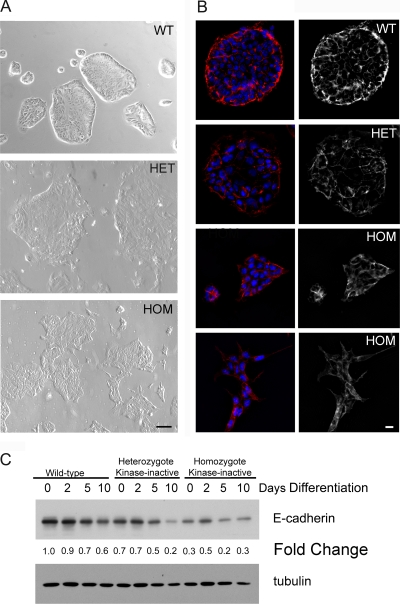
Even in the presence of FGF4 and conditioned medium used to maintain wild-type TS cells in an undifferentiated self-renewing state, both the heterozygous and homozygous MEKK4 kinase-inactive cells have a dramatically altered morphology and diminished E-cadherin expression (Fig. 3). Examination of the morphology of the different TS cell populations revealed that the wild-type TS cells grew in tight colonies of epithelial sheets with a characteristic cobblestone appearance (Fig. 3A). In contrast, heterozygous and homozygous MEKK4 kinase-inactive TS cells had a dramatically altered spindle-like morphology characteristic of a mesenchymal phenotype (Fig. 3A), with the homozygous MEKK4 kinase-inactive cells having a stronger mesenchymal cell-like morphology than the heterozygous cells. Figure 3B also shows that there was a substantial loss of E-cadherin expression in both the heterozygous and homozygous MEKK4 kinase-inactive TS cells under undifferentiating culture conditions in the presence of FGF4 and fibroblast-conditioned medium. The loss of E-cadherin expression was confirmed by immunoblotting (Fig. 3C). Thus, TS cells harboring kinase-inactive MEKK4 have the morphological characteristics of trophoblasts differentiating into a mesenchymal phenotype.
FIG. 3.
Reduced E-cadherin expression in TS cells homozygous for MEKK4K1361R. (A) Phase microscopy of wild-type and mutant TS cells cultured under undifferentiating conditions in the presence of FGF4 and conditioned medium is shown. Wild-type TS cells colonies exist as tight epithelial sheets. Heterozygote MEKK4WT/K1361R TS cell colonies are more irregularly shaped and slightly differentiated. Homozygote MEKK4K1361R TS cell colonies appear differentiated with irregularly shaped, polarized cells. Bar, 100 μm. (B) E-cadherin expression in wild-type and kinase-inactive TS cells cultured under undifferentiating conditions. Cells were immunostained with anti-E-cadherin antibodies (red) and nuclei were counterstained with DAPI (blue). Strong peripheral E-cadherin staining was observed in wild-type TS cells. Peripheral E-cadherin staining was slightly decreased in heterozygote kinase-inactive TS cells and further decreased in homozygote kinase-inactive TS cells. (C) Western blotting of whole-cell extracts from wild-type and kinase-inactive TS cells. Blots were probed with anti-E-cadherin and antitubulin antibodies. Changes were expressed relative to undifferentiated wild-type TS cells. WT, wild-type cells; HET, heterozygote kinase-inactive cells; HOM, homozygote kinase-inactive cells. Bar, 10 μm. Data shown are representative results from three independent experiments.
Trophoblasts expressing MEKK4K1361R are highly invasive through the extracellular matrix.
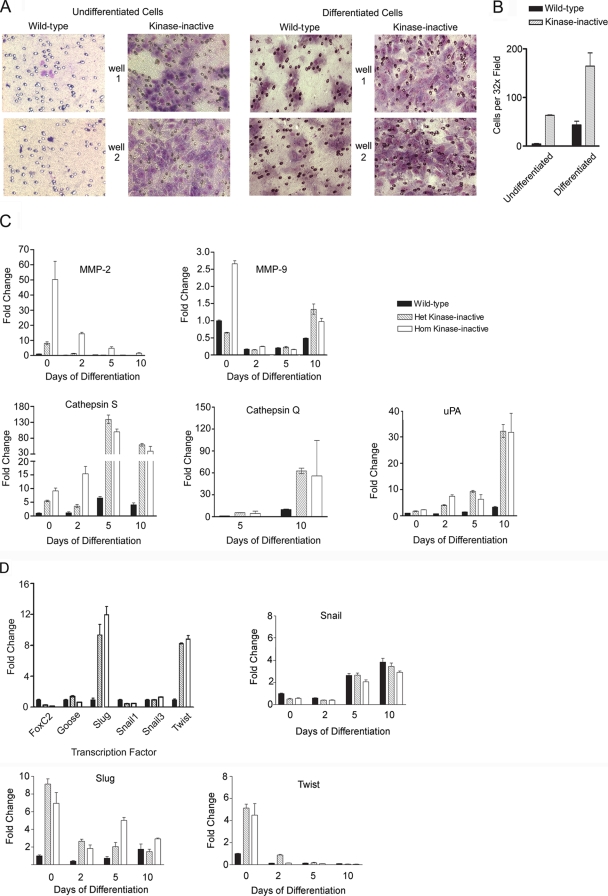
Figure 4 shows that TS cells expressing MEKK4K1361R are highly invasive through growth factor-reduced Matrigel. Undifferentiated wild-type TS cells are epithelial in nature and noninvasive. In contrast, TS cells expressing even one MEKK4 kinase-inactive allele are highly invasive (Fig. 4A). TS cells homozygous for MEKK4K1361R are similarly highly invasive, as the heterozygous MEKK4WT/K1361R TS cells (data not shown). Withdrawal of FGF4 and fibroblast-conditioned medium induces wild-type TS cell differentiation and invasion (Fig. 4A). Figure 4B quantitates the invasiveness of undifferentiated and 2-day-differentiated wild-type and heterozygous MEKK4 kinase-inactive trophoblasts. It is clear that the expression of MEKK4K1361R markedly increases trophoblast invasiveness in the presence or absence of FGF4 and fibroblast-conditioned medium. Wild-type and heterozygous MEKK4K1361R-expressing trophoblasts that invaded and migrated through the extracellular matrix-coated filters under differentiating conditions were collected and used to monitor specific trophoblast marker expression in the invading cells. The abundance of the trophoblast markers in the invading cells was compared to that of 2-day differentiated trophoblasts. Invading trophoblasts had elevated Tpbpα expression compared to 2-day differentiated trophoblasts (data not shown), suggesting that the spongiotrophoblast marker Tpbpα is strongly expressed in invasive trophoblasts. Interestingly, elevated Tpbpα was observed in the hyperinvasive trophoblasts in the placentas of MEKK4WT/K1361R and MEKK4K1361R embryos (Fig. 2B).
FIG. 4.
Increased invasiveness of MEKK4 kinase-inactive TS cells. (A) Invasion of undifferentiated or differentiated wild-type and heterozygote kinase-inactive TS cells through growth factor-reduced Matrigel. Chambers were Wright-stained and 10× images from two transwell chambers are shown for each condition. (B) Quantitation of the TS cell invasion shown in panel A. Data shown are the means ± ranges of two independent experiments performed in duplicate. Five 32× fields were counted for each transwell chamber. (C) Increased expression of proteases in kinase-inactive TS cells. Message levels were measured by quantitative RT-PCR of TS cells differentiated for the indicated number of days. Data are the means ± ranges of three independent experiments performed in duplicate and normalized to undifferentiated wild-type levels. (D) Increased expression of EMT-inducing transcription factors Slug and Twist in MEKK4 kinase-inactive TS cells. TS cells were cultured under undifferentiating conditions in the presence of FGF4 and conditioned medium. Message levels were measured by quantitative RT-PCR. Data normalized to wild-type undifferentiated TS cell levels are the means ± ranges of two independent experiments performed in duplicate. Het, heterozygous; Hom, homozygous.
Galardin is a specific inhibitor of MMPs (16). Incubation of 2-day differentiated wild-type and MEKK4K1361R TS cells with galardin inhibited invasiveness 29 and 14%, respectively, indicating that MMP activity was associated with invasiveness (data not shown). To further test this hypothesis, real-time PCR analysis was performed for specific proteases known to be expressed by trophoblasts (14). Figure 4C shows that MMP2 and cathepsin S are elevated in both the heterozygous and homozygous MEKK4 kinase-inactive TS cells cultured under undifferentiating conditions. MMP9 is elevated in homozygous but not heterozygous MEKK4 kinase-inactive trophoblasts. Differentiation of TS cells by removal of FGF4 and conditioned medium induced the expression of other proteases, like urokinase plasminogen activator and cathepsin Q, with message induction being significantly greater in kinase-inactive TS cells than in wild-type cells (Fig. 4C). These findings in combination with the loss of the E-cadherin, spindle-like morphology characteristic of motile mesenchymal cells and the increased invasiveness observed with inhibition of MEKK4 kinase activity demonstrate that MEKK4 regulates several cellular hallmarks that define EMT.
Increased expression of the transcriptional regulators of EMT Slug and Twist in MEKK4K1361R TS cells.
Several transcription factors that repress E-cadherin expression, including Snail and bHLH families, are thought to function in development and EMT. Snail family members include Snail1, Snail2 (Slug), and Snail3. These zinc finger transcription factors share a common organization with similar DNA binding domains in their C termini and are thought to act mainly as transcriptional repressors. Snail and Slug directly repress E-cadherin expression by binding to E-box elements in the E-cadherin promoter (5, 6). Snail and Slug are also capable of repressing the expression of several polarity genes that contribute to epithelial traits. Twist is a bHLH transcription factor also capable of repressing E-cadherin expression, but the mechanism is not well defined. Twist has been shown to induce EMT in specific epithelial cell types (25). Figure 4D shows that both Slug and Twist, but not other EMT-inducing transcription factors, are induced in MEKK4K1361R TS cells. Detailed analysis of the expression of Snail, Slug, and Twist with trophoblast differentiation revealed the induction of Snail by FGF4 withdrawal (Fig. 4D). This induction of Snail is consistent with the EMT that occurs with the differentiation of epithelial trophoblast stem cells into invasive mesenchymal trophoblasts. Significantly, Slug and Twist, which are overexpressed in kinase-inactive TS cells, are not induced by trophoblast differentiation (Fig. 4D). Instead, the overexpression of Slug and Twist by the mutant TS cells reflects the aberrant expression of these transcription factors. The expression of these transcription factors indicates the mechanism for loss of E-cadherin and the induction of EMT in MEKK4K1361R TS cells and is consistent with the highly invasive characteristics of the kinase-inactive trophoblast stem cells.
MEKK4K1361R TS cells have inhibited JNK and p38 activities and diminished responsiveness to FGF4.
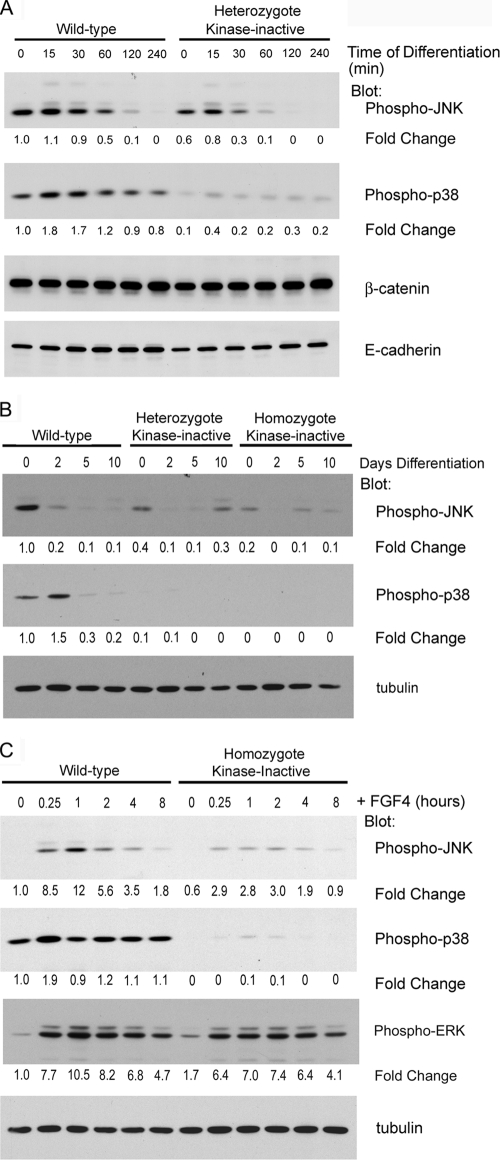
MEKK4 regulates the JNK and p38 MAPK pathways (1, 2). In the presence of FGF4 and conditioned medium, levels of activated JNK and p38 are significantly inhibited in MEKK4WT/K1361R TS cells relative to wild-type TS cells (Fig. 5A). Whereas JNK activity is inhibited approximately 40% in cycling TS cells expressing MEKK4K1361R, p38 activity is almost completely inhibited compared to wild-type TS cells. Removal of FGF4 and conditioned medium resulted in a rapid decrease within minutes in phosphorylated JNK in both wild-type and MEKK4WT/K1361R TS cell populations (Fig. 5A), indicating that FGF4 and conditioned medium sustain activation of JNK in TS cells. In contrast, there was a measurable transient increase in p38 activity, which may be related to the stress of growth factor withdrawal. Temporally, the loss of JNK activity was significantly more rapid in MEKK4WT/K1361R TS cells, but by 240 min JNK activity was undetectable in both TS cell populations. Thus, expression of kinase-inactive MEKK4 inhibits both JNK and p38 in self-renewing TS cells, and the kinetics of loss of JNK activity upon removal of FGF4 and fibroblast-conditioned medium are faster in MEKK4WT/K1361R than in wild-type TS cells. The results are consistent with MEKK4 being a primary hub-like MAP3K in the control of JNK and p38 in TS cells.
FIG. 5.
FGF4 stimulation of JNK phosphorylation is reduced in kinase-inactive TS cells. Western blot analysis of wild-type, heterozygote, and homozygote kinase-inactive TS cell extracts is shown. Blots were probed with antibodies against phospho-JNK, phospho-p38, α-tubulin, β-catenin, and E-cadherin. Changes are relative to wild-type TS cells (time zero). (A) Removal of FGF4 from TS cells results in a loss of JNK phosphorylation. FGF4 and conditioned medium was removed for the indicated times. Phospho-JNK levels decreased after an hour of differentiation. Phospho-JNK and phospho-p38 levels were significantly reduced in kinase-inactive TS cells. (B) Differentiation results in a loss of phospho-JNK protein and a slight enhancement in phospho-p38 protein levels. Kinase-inactive TS cells exhibited a 60 to 80% reduction in phospho-JNK levels and a nearly complete loss of phospho-p38. (C) FGF4-stimulated JNK phosphorylation is diminished in kinase-inactive TS cells. TS cell were cultured for 24 h in the absence of FGF4 in TS medium containing TGF-β. FGF4 (10 ng/ml) was added for the indicated times. Data shown are representative results from three independent experiments.
Examination of changes in JNK and p38 activities during a 10-day period following removal of FGF4 and conditioned medium revealed a loss in JNK activity in both wild-type and kinase-inactive TS cells (Fig. 5B). Similar to the findings in Fig. 5A, both undifferentiated heterozygous MEKK4WT/K1361R and homozygous MEKK4K1361R TS cells had significantly inhibited JNK and p38 activities compared to wild-type TS cells. After 2 days of differentiation, JNK was reduced 80% in wild-type TS cells but completely lost in kinase-inactive TS cells. JNK activity remained very low in all differentiated cell populations (Fig. 5B). However, extended differentiation times showed a recovery of JNK phosphorylation after 12 days of FGF4 withdrawal, suggesting an additional role for JNK in the late stages of differentiation (see Fig. S1 in the supplemental material). At 12 days of differentiation, MEKK4 levels were reduced, suggesting another MAP3K may regulate JNK at later stages of differentiation (Fig. 2E). Overall, the findings indicate that the rapid loss of JNK activity is a normal TS cell response to FGF4 and conditioned medium withdrawal and the induction of differentiation. MEKK4 harboring the K1361R mutation, as shown previously (2, 3), functions as an inhibitory mutant. Figure 5 shows that expression of MEKK4K1361R inhibits JNK and p38 activities in undifferentiated TS cells. Diminished JNK and/or p38 activity in MEKK4K1361R TS cells appears to release the repression of differentiation that would normally occur only with FGF4 withdrawal.
Addition of FGF4 to wild-type TS cells results in a robust activation of JNK (12-fold) and a modest activation of p38 (Fig. 5C). FGF4-induced activation of JNK was reduced by 80% in homozygous MEKK4K1361R TS cells. Similarly, FGF4-induced activation of p38 was completely lost in kinase-inactive TS cells (Fig. 5C). However, kinase-inactive TS cells retained a robust FGF4-induced activation of ERK that was similar to wild-type TS cells (Fig. 5C). Together, these data suggest that MEKK4 is the primary MAP3K for FGF4 activation of JNK and p38 in TS cells.
Chemical inhibition of JNK results in the induction of Slug and loss of E-cadherin in undifferentiated wild-type TS cells.
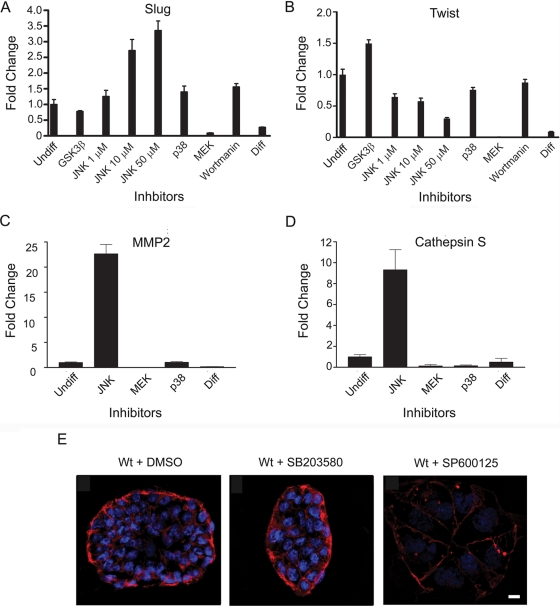
Small-molecule inhibitors for JNK, p38, GSK3β, MEK1/2, and phosphatidylinositol 3-kinase were used to screen for pathways controlling the expression of Slug (Fig. 6A) and Twist (Fig. 6B) in wild-type TS cells cultured under undifferentiating conditions. Of the inhibitors tested, only the JNK inhibitor induced the expression of Slug. Induction of Slug expression was dose dependent for the JNK inhibitor, consistent with a specific small-molecule inhibition of JNK activity inducing Slug expression. In contrast, JNK inhibitor had no effect on Twist expression, providing discrimination between the regulation of Slug and Twist by kinase-inactive MEKK4. Twist expression was increased with the GSK3β inhibitor, but not by inhibition of p38, MEK1/2, or phosphatidylinositol 3-kinase. Interestingly, MEKK4 binds GSK3β (1), and it is possible that the GSK3β activity associated with its interaction with MEKK4 is involved in the control of Twist expression. Inhibition of JNK in wild-type TS cells resulted in increased MMP2 and cathepsin S message levels but had negligible effects on MMP9 and MMP14 (Fig. 6C and D and data not shown). These increases in MMP2 and cathepsin S were similar to those observed in kinase-inactive TS cells (compare Fig. 4C and 6C and D). Importantly, JNK inhibition in wild-type TS cells induces a loss of E-cadherin expression and the onset of a mesenchymal phenotype (Fig. 6E), consistent with the induction of Slug. Thus, JNK inhibition of wild-type TS cells is sufficient to induce several hallmarks of EMT that strongly resemble kinase-inactive TS cells.
FIG. 6.
Chemical inhibition of JNK induces the expression of Slug, MMP2, and cathepsin S and a loss of E-cadherin expression. (A, B, C, and D) Wild-type TS cells were cultured under undifferentiating conditions in the presence of FGF4 and conditioned medium and treated for 2 days with either DMSO, 30 μM GSK3β inhibitor, JNK inhibitor, 10 μM p38 inhibitor, 20 μM MEK inhibitor, 0.1 μM wortmannin, or differentiated (Diff) for 2 days in the absence of FGF4 and conditioned medium. Message levels were measured by quantitative RT-PCR. Data were normalized to wild-type undifferentiated DMSO-treated TS cell levels. Data are the means ± ranges of two independent experiments performed in duplicate. (E) Wild-type TS cells cultured under undifferentiating conditions were treated for 2 days with either DMSO, 10 μM SB203580 (p38 inhibitor), or 50 μM SP600125 (JNK inhibitor). Cells were immunostained with anti-E-cadherin antibody (red) and nuclei were counterstained with DAPI (blue). Strong peripheral E-cadherin staining in wild-type TS cells treated with DMSO or p38 inhibitor was lost with JNK inhibitor treatment. Data shown are representative images from three independent experiments. Wt, wild type. Bar, 10 μm.
We discovered that chemical inhibition of p38 in TS cells results in JNK activation (data not shown). This is due apparently to a negative feedback regulation that is undefined in mechanism. The fact that chemical inhibition of p38 results in JNK activation prevents the ability to use p38 inhibitors in wild-type TS cells to study p38 function, because JNK activity represses the EMT response. The combination of JNK and p38 inhibitors was toxic to TS cells.
JNK suppresses expression of Tpbpα and Gcm1 in TS cells.
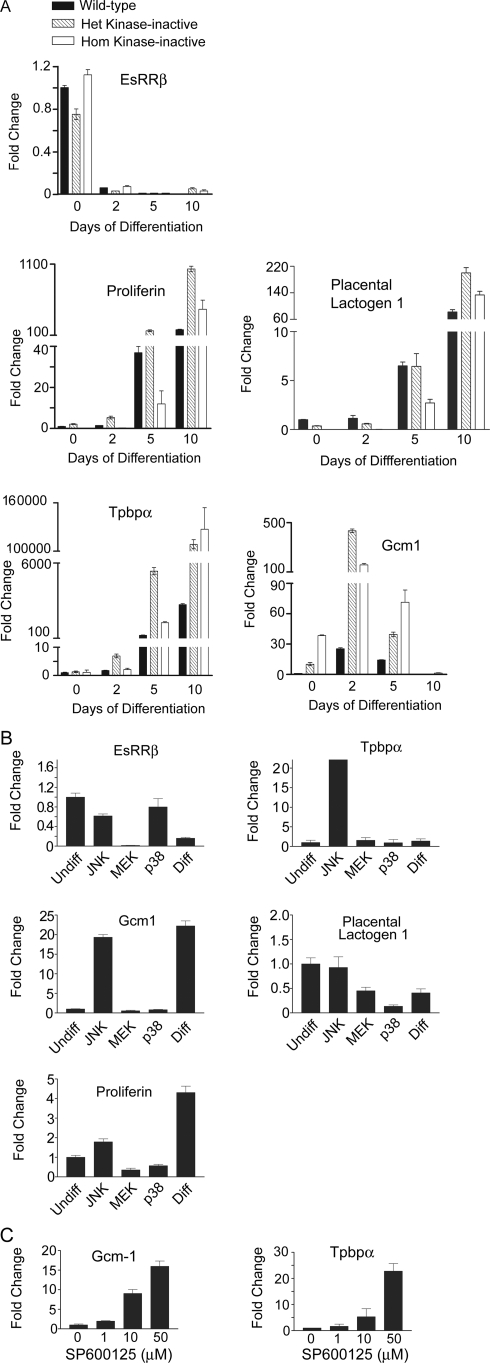
Quantitative RT-PCR using probes for markers of the different trophoblast lineages was used to further define the properties of wild-type, heterozygote, and homozygote MEKK4K1361R TS cell cultures (Fig. 7A). EsRRβ, a trophoblast stem cell marker, was highly abundant in both wild-type and kinase-inactive MEKK4 TS cells (Fig. 7A). Additional TS cell markers, like Cdx2 and Eomes, were also highly expressed in both wild-type and kinase-inactive TS cells (data not shown). Differentiation of TS cells by removal of FGF4 and conditioned medium resulted in a dramatic loss of EsRRβ, indicating a loss of stem cell potential (Fig. 7A). Giant cells are the default differentiation pathway and proliferin and PlI are giant cell markers (20). Proliferin and PlI were expressed at low levels in undifferentiated wild-type, heterozygous MEKK4WT/K1361R, and homozygous MEKK4K1361R TS cells (Fig. 7A). At 5 and 10 days of differentiation, a significant increase in the message levels for the two giant cell markers was observed in wild-type, MEKK4WT/K1361R, and MEKK4K1361R TS cells. At 10 days of differentiation, proliferin and PlI were only slightly elevated in kinase-inactive TS cells. Tpbpα, a marker of the spongiotrophoblasts, was induced sevenfold in the MEKK4WT/K1361R trophoblasts after 2 days of differentiation but remained at very low mRNA levels in wild-type trophoblasts. Further differentiation of trophoblasts for 5 to 10 days resulted in increased Tpbpα message. Strikingly, Tpbpα message was consistently higher in the MEKK4WT/K1361R and MEKK4K1361R trophoblasts than in wild-type cells at all stages of differentiation (Fig. 7A). This increase in Tpbpα levels in mutant TS cells is similar to those increases observed in kinase-inactive mutant placentas (compare Fig. 2B and 7A). Gcm1, a syncytiotrophoblast marker, is expressed at low levels in undifferentiated wild-type TS cells. Gcm1 message was dramatically upregulated in undifferentiated kinase-inactive TS cells, with a 10-fold and 38-fold increase in basal expression of Gcm1 in MEKK4WT/K1361R and MEKK4K1361R TS cells, respectively, relative to wild-type TS cells (Fig. 7A). Differentiation of wild-type TS cells for 2 days resulted in a 25-fold increase in Gcm1 levels; with further differentiation, Gcm1 levels returned to the low levels observed in undifferentiated cells (Fig. 7A). Differentiation of kinase-inactive TS cells resulted in a 435-fold and 158-fold elevation in Gcm1 message in MEKK4WT/K1361R and MEKK4K1361R TS cells, respectively, in direct contrast to the 25-fold change observed in wild-type TS cells (Fig. 7A). Further differentiation for 5 to 10 days resulted in the loss of Gcm1 expression, indicating that the pathways controlling the return of Gcm1 mRNA levels function similarly in both the wild-type and kinase-inactive trophoblasts. The morphological changes and altered expression of trophoblast markers indicated that the heterozygous and homozygous MEKK4 kinase-inactive TS cells have a diminished capacity to sustain maintenance of the undifferentiated TS cell phenotype and to repress differentiation toward the spongiotrophoblast and syncytiotrophoblast lineages.
FIG. 7.
JNK inhibition deregulates Tpbpα and Gcm1 expression in kinase-inactive TS cells. (A) Message levels were measured by quantitative RT-PCR of TS cells differentiated for the indicated number of days. Data are the means ± ranges of between two and five independent experiments performed in duplicate. Data were normalized to wild-type undifferentiated TS cell levels. (B) Inhibition of JNK with SP600125 in undifferentiated wild-type TS cells induces the deregulation of Tpbpα and Gcm1. Wild-type TS cells were treated for 2 days under undifferentiating conditions with DMSO (Undiff), 50 μM SP600125 (JNK inhibitor), 10 μM SB203580 (p38 inhibitor), 10 μM U0126 (MEK inhibitor), or under differentiating conditions with DMSO (Diff). Message levels were measured by quantitative RT-PCR. Data are the means ± ranges of between two and four independent experiments performed in duplicate. Data were normalized to wild-type undifferentiated levels. (C) Dose-dependent effects of JNK inhibitor SP600125 on Gcm1 and Tpbpα message levels. Wild-type TS cells were treated for two days with the indicated concentration of SP600125. mRNA levels were measured as for panel A.
MEKK4WT/K1361R and MEKK4K1361R TS cells have significantly inhibited JNK and p38 activities (Fig. 5). Therefore, undifferentiated wild-type TS cells were treated for 2 days with inhibitors for JNK, p38, or MEK1/2 as controls and expression of trophoblast cell type markers was analyzed by quantitative RT-PCR (Fig. 7B). Inhibition of JNK produced a modest decrease in the stem cell marker EsRRβ and had no or little effect on the giant cell markers PlI and proliferin (Fig. 7B). In striking contrast, dramatic increases in the message levels of both spongiotrophoblast (Tpbpα) and syncytiotrophoblast (Gcm1) markers were observed with JNK inhibition but not with inhibition of p38 or MEK1/2 (Fig. 7B). The induction of Gcm1 and Tpbpα was dose dependent for SP600125, indicating that JNK activity directly correlates with inhibition of Tpbpα and Gcm1 expression (Fig. 7C). In contrast, p38 or MEK1/2 inhibition did not enhance the expression of any trophoblast cell type markers but actually decreased expression of the giant cell markers (PlI and proliferin) (Fig. 7B). Activity of p38 is markedly inhibited in MEKK4WT/K1361R and MEKK4K1361R TS cells, but unlike inhibition of JNK, inhibition of p38 is not critical for regulation of Tpbpα and Gcm1 expression. Interestingly, inhibition of the ERK1/2 pathway with the MEK1/2 inhibitor U0126 caused a complete loss of the stem cell marker EsRRβ in the TS cells (Fig. 7B), suggesting an important function of ERK1/2 in EsRRβ expression. Cumulatively, these data indicate that JNK signaling selectively inhibits expression of Tpbpα and Gcm1. Further, it is the reduced JNK activity in TS cells expressing the kinase-inactive MEKK4 mutant protein, and not the loss of p38 activity, which is responsible for the enhanced Tpbpα and Gcm1 expression in kinase-inactive TS cells.
MEKK4 stimulation of JNK regulates AP-1 composition in TS cells.
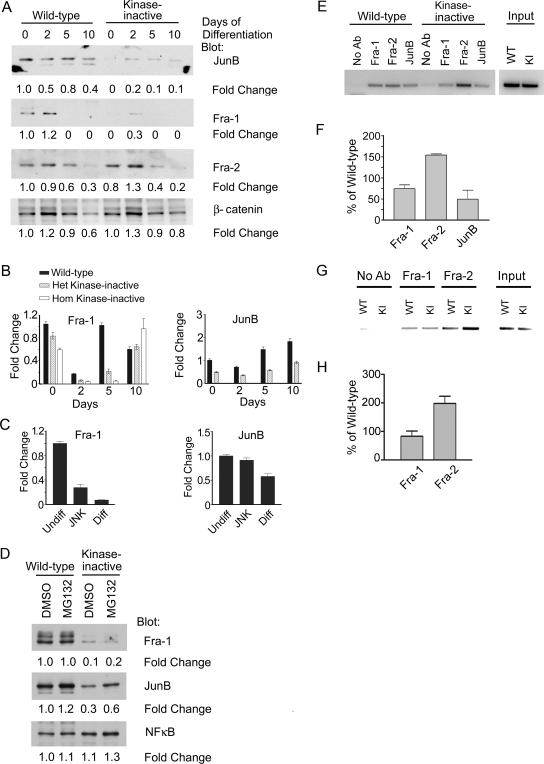
Loss of specific MAP3K signaling dramatically alters the expression of specific AP-1 complex proteins, resulting in changes in promoter-bound AP-1 composition and gene expression (8, 12, 13). We defined the expression of specific Jun and Fos proteins in wild-type and MEKK4WT/K1361R TS cells during trophoblast differentiation. The expression levels of ATF-2, c-Jun, and c-Fos were similar in wild-type and MEKK4 kinase-inactive TS cells (data not shown). In contrast, the expression levels of Fra-1, Fra-2, and JunB were altered (Fig. 8A). Expression levels of Fra-1 and JunB were inhibited and expression of Fra-2 was increased in MEKK4 kinase-inactive TS cells relative to wild-type TS cells.
FIG. 8.
JNK inhibition alters AP-1 composition in TS cells. (A) Jun B and Fra-1 protein levels are significantly reduced in kinase-inactive TS cells. Results from Western blot analysis of nuclear lysates from wild-type and heterozygote kinase-inactive TS cells differentiated for the indicated times are shown. Blots were probed with antibodies against JunB, Fra-1, Fra-2, and β-catenin. Changes are relative to undifferentiated wild-type TS cells (time zero). (B) Fra-1 and JunB mRNA levels are significantly reduced in kinase-inactive TS cells. Fra-1 and JunB mRNA levels were measured by quantitative RT-PCR of TS cells differentiated for the indicated number of days. Data are the means ± ranges of two experiments. Data were normalized to wild-type undifferentiated levels. (C) Inhibition of JNK with SP600125 in undifferentiated TS cells induces the loss of Fra-1 message. Wild-type TS cells were treated for 2 days under undifferentiating conditions with DMSO (Undiff), 50 μM SP600125 (JNK inhibitor), or under differentiating conditions with DMSO (Diff). Fra-1 and JunB mRNA levels were measured by quantitative RT-PCR. Data are the means ± ranges of between two and four independent experiments. Data were normalized to wild-type undifferentiated levels. (D) Treatment with the proteasome inhibitor MG132 does not restore Fra-1 levels in kinase-inactive TS cells. Undifferentiated TS cells were treated with DMSO or MG132 for 5 hours and analyzed as described for panel A. (E) Alteration in AP-1 family member binding to the third intron of Gcm1 in undifferentiated MEKK4WT/K1361R cells. ChIP analysis was performed using antibodies to Fra-1, Fra-2, and JunB using samples obtained from undifferentiated MEKK4WT and MEKK4WT/K1361R TS cells. PCR was performed to a region containing an AP-1 consensus sequence (TGA G TCA) in the third intron of Gcm1. KI, kinase inactive. (F) Quantitation of ChIP analysis of Gcm1. Data shown are the means ± ranges of between two and three experiments. Bands were quantitated by densitometry using ImageJ and data were normalized to wild-type levels. (G) Alteration of AP-1 family member binding to the MMP2 promoter in undifferentiated MEKK4K1361R cells. ChIP analysis was performed with antibodies to Fra-1 and Fra-2 using samples obtained from undifferentiated MEKK4WT and MEKK4K1361R TS cells. PCR was performed to a region in the MMP2 promoter (−2723 to −2344) containing an AP-1 consensus sequence (TGA C TCA). (H) Quantitation of ChIP analysis results with the MMP2 promoter. Data shown are the means ± standard errors of the means of three experiments.
Quantitative RT-PCR analysis demonstrated that Fra-1 and JunB mRNA expression levels were reduced in MEKK4WT/K1361R TS cells (Fig. 8B). Inhibition of JNK in wild-type TS cells resulted in a strong inhibition of Fra-1 mRNA expression, indicating that JNK regulates the mRNA levels for Fra-1 (Fig. 8C). Unlike Fra-1, JNK inhibition had a weak effect on JunB mRNA levels. Binding of inactive JNK to JunB has been shown to promote the ubiquitination and degradation of JunB (12), and JNK has also been shown to activate the E3 ubiquitin ligase Itch, resulting in degradation of JunB and c-Jun in T cells (13). Proteasome inhibition using MG132 modestly increased JunB protein in both MEKK4WT/K1361R and wild-type TS cells (Fig. 8D), suggesting that altered proteasome-mediated degradation is not the major mechanism for regulating JunB protein levels in TS cells. Changes in JunB expression appear to be regulated by MEKK4 largely independently of JNK activity in TS cells.
To define the consequence of changes in Fra-1, Fra-2, and JunB expression, the binding of AP-1 components to the Gcm1 promoter was analyzed by ChIP (Fig. 8E and F). A consensus AP-1 site (TGA G TCA) is present in the third intron of the mouse Gcm1 gene that is absolutely conserved in the third intron of the human Gcm1 gene. ChIP revealed the binding of Fra-1, Fra-2, and JunB to the third intron of Gcm1 in wild-type undifferentiated TS cells (Fig. 8E). Reduced binding of Fra-1 and JunB and increased Fra-2 binding to the Gcm1 intron 3 in MEKK4WT/K1361R TS cells were observed (Fig. 8E and F). ChIP analysis of a consensus AP-1 site (TGA C TCA) in the MMP2 promoter revealed similar findings. A twofold increase in Fra-2 binding to the MMP2 promoter in MEKK4K1361R TS cells relative to wild-type TS cells was observed (Fig. 8G and H). Thus, loss of MEKK4 kinase activity changes the AP-1 repertoire binding to intron 3 of Gcm1 and the promoter of MMP2. These changes in AP-1 composition probably contribute to the dysregulated expression of these proteases in MEKK4 kinase-inactive TS cells. Cumulatively, the results define FGF4-stimulated MEKK4 regulation of JNK activity as the mechanism for repression of TS cell commitment to EMT and differentiation.
DISCUSSION
It is well known that FGF4 and TGFβ/activin along with other undefined factors maintain TS cells in a self-renewing state that includes repression of differentiation (18, 26). Even though FGF4 activation of ERK1/2 has defined antiapoptotic functions in TS cell renewal (26), signals promoting repression of differentiation, including trophoblast EMT, have not been characterized. MEKK4 is highly expressed in TS cells and MEKK4 expression is significantly reduced during differentiation, positioning MEKK4 as an important MAP3K in controlling TS cell function. MEKK4 is a 180-kDa serine/threonine protein kinase that interacts with several proteins known to regulate EMT, including GSK3β (1, 9), Cdc42 and Rac (11), axin, the negative regulator of β-catenin (17), and NIK, the NCK-interacting kinase (A. N. Abell and G. L. Johnson, unpublished data). MEKK4 signals to activate both JNK and p38 (1, 3). Our results clearly show that MEKK4 kinase activity is required for FGF4-stimulated JNK and p38 activities in TS cells. Kinase-inactive MEKK4 TS cells selectively differentiate to spongiotrophoblasts and syncytiotrophoblasts even in the presence of FGF4 and fibroblast-conditioned medium, indicating that MEKK4 signaling is essential for repression of differentiation. A major phenotype of the differentiation response observed with expression of kinase-inactive MEKK4 was EMT characterized by increased Slug, Twist, MMP2, and cathepsin S, loss of E-cadherin expression, and increased invasiveness.
The p38 MAPK has been shown to regulate EMT during gastrulation and is downstream of a signaling pathway involving NIK (28), which is a MEKK4 binding partner. A p38-interacting protein (p38IP) required for p38 activation in vivo is required for downregulation of E-cadherin during gastrulation. The p38IP was also found to be involved in neural tube closure, with p38IP mutants having exencephaly and spina bifida (28). Interestingly, MEKK4 kinase-inactive and knockout mice have defects in p38 activation and display both exencephaly and spina bifida (3). The p38 MAPK is also implicated in tumor cell EMT, particularly in response to transforming growth factor β (TGF-β) and in some cases tumor necrosis factor alpha.
JNK does not appear to be involved in EMT during gastrulation but is implicated as a positive regulator of TGF-β-induced EMT in several cell types (15, 19). Genetically, it was recently shown that TGF-β-stimulated EMT of primary murine tracheal epithelial cells was markedly blunted in a JNK1−/− background but not a JNK2−/− background (4). The role of JNK in positively regulating EMT in different cell types seems to involve AP-1 and the regulation of cadherins and specific Smads (4). Little is known about the role of JNK in controlling the EMT response in tissue stem cells.
Our results show that JNK is strongly activated by FGF4. TS cell differentiation induced by withdrawal of FGF4 results in a loss of JNK activity. Inhibition of JNK in TS cells by kinase-inactive MEKK4 or by chemical inhibition promotes the selective differentiation of cells to the spongiotrophoblast and syncytiotrophoblast lineages. These data define a clear role for FGF4 activation of JNK in repression of EMT, a JNK function that is clearly different from the positive regulation of TGF-β-initiated EMT responses in cultures of primary tissue epithelial cells and many transformed cell lines. Primary factors in fibroblast-conditioned medium for maintenance of TS cell renewal include TGF-β/activin (10), which in combination with FGF4 maintain TS cells in an undifferentiated, self-renewing state. This is an unusual function for TGF-β in primary epithelial cells, where TGF-β often inhibits proliferation of primary epithelial cells via a G1 cell cycle arrest program (23). Transformation can alter this control and bypass the G1 cell cycle arrest in response to TGF-β (23). Constitutive FGF4 signaling in TS cells has been shown to suppress the growth inhibitory effects of TGF-β/activin, and removal of FGF4 initiates a trophoblast differentiation program involving TGF-β (10). Mechanistically, loss of MEKK4 kinase activity removes a critical signaling response that is needed for FGF4 to control the response of other cytokines, such as TGF-β/activin. Our findings are consistent with the work of Erlebacher et al., who proposed that FGF4 signaling in TS cells inhibited specific TGF-β responses (10). Our findings demonstrate that FGF4 inhibition of TS cell differentiation requires MEKK4 activation of JNK. The increased expression of Gcm1 and MMP2 in MEKK4 kinase-inactive TS cells, whose expression is also induced by JNK inhibition, is consistent with a transcriptional repressor function for MEKK4-JNK signaling in FGF4 control of TS cell differentiation.
The function of MEKK4 control of JNK activity in TS cells in vivo is also consistent with our findings that MEKK4 kinase-inactive trophoblasts have an abnormal differentiation response during placentation. Normally, a gradient of FGF4 is generated by the developing embryo that controls trophoblast differentiation. However, in MEKK4 kinase-inactive trophoblasts the TS cells do not signal properly and have a dysregulated differentiation response. MEKK4 expression is highest in the developing embryo and particularly in TS cells, and expression is dramatically diminished during differentiation. High MEKK4 expression in renewing TS cells and loss of expression during differentiation is consistent with the necessary function of MEKK4 as a critical hub kinase in the maintenance of TS cell renewal.
Finally, our results have significant implications in understanding the integrated signaling responses for control of tissue stem cell maintenance, repression of differentiation, and changes in signaling for commitment of stem cells to differentiate. Kinases such as MEKK4 function as hubs that respond to different stimuli for the selective activation of pathways within a signaling network. MEKK4 coordinates the activity of the JNK and p38 modules in TS cells required for stem cell maintenance. It is likely that kinases other than MEKK4 will have similar functions in different tissue stem cells, and such hub kinases will control signal networks that integrate the complex cytokine profiles present in different stem cell niches. Identifying the kinases with hub-like functions such as MEKK4 will allow a rational chemical biology approach for selectively controlling renewal and differentiation of tissue stem cells from different tissues.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grant GM30324 (G.L.J.).
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print on 16 March 2009.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Abell, A. N., D. A. Granger, and G. L. Johnson. 2007. MEKK4 stimulation of p38 and JNK activity is negatively regulated by GSK3β. J. Biol. Chem. 28230476-30484. [DOI] [PubMed] [Google Scholar]
- 2.Abell, A. N., and G. L. Johnson. 2005. MEKK4 is an effector of the embryonic TRAF4 for JNK activation. J. Biol. Chem. 28035793-35796. [DOI] [PubMed] [Google Scholar]
- 3.Abell, A. N., J. A. Rivera-Perez, B. D. Cuevas, M. T. Uhlik, S. Sather, N. L. Johnson, S. K. Minton, J. M. Lauder, A. M. Winter-Vann, K. Nakamura, T. Magnuson, R. R. Vaillancourt, L. E. Heasley, and G. L. Johnson. 2005. Ablation of MEKK4 kinase activity causes neurulation and skeletal patterning defects in the mouse embryo. Mol. Cell. Biol. 258948-8959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alcorn, J. F., A. S. Guala, J. van der Velden, B. McElhinney, C. G. Irvin, R. J. Davis, and Y. M. Janssen-Heininger. 2008. Jun N-terminal kinase 1 regulates epithelial-to-mesenchymal transition induced by TGF-β1. J. Cell Sci. 1211036-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batlle, E., E. Sancho, C. Franci, D. Dominguez, M. Monfar, J. Baulida, and A. Garcia De Herreros. 2000. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat. Cell Biol. 284-89. [DOI] [PubMed] [Google Scholar]
- 6.Bolos, V., H. Peinado, M. A. Perez-Moreno, M. F. Fraga, M. Esteller, and A. Cano. 2003. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J. Cell Sci. 116499-511. [DOI] [PubMed] [Google Scholar]
- 7.Chi, H., M. R. Sarkisian, P. Rakic, and R. A. Flavell. 2005. Loss of mitogen-activated protein kinase kinase kinase 4 (MEKK4) results in enhanced apoptosis and defective neural tube development. Proc. Natl. Acad. Sci. USA 1023846-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuevas, B. D., M. T. Uhlik, T. P. Garrington, and G. L. Johnson. 2005. MEKK1 regulates the AP-1 dimer repertoire via control of JunB transcription and Fra-2 protein stability. Oncogene 24801-809. [DOI] [PubMed] [Google Scholar]
- 9.Doble, B. W., and J. R. Woodgett. 2007. Role of glycogen synthase kinase-3 in cell fate and epithelial-mesenchymal transitions. Cells Tissues Organs 18573-84. [DOI] [PubMed] [Google Scholar]
- 10.Erlebacher, A., K. A. Price, and L. H. Glimcher. 2004. Maintenance of mouse trophoblast stem cell proliferation by TGF-beta/activin. Dev. Biol. 275158-169. [DOI] [PubMed] [Google Scholar]
- 11.Fanger, G. R., N. L. Johnson, and G. L. Johnson. 1997. MEK kinases are regulated by EGF and selectively interact with Rac/Cdc42. EMBO J. 164961-4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fuchs, S. Y., B. Xie, V. Adler, V. A. Fried, R. J. Davis, and Z. Ronai. 1997. c-Jun NH2-terminal kinases target the ubiquitination of their associated transcription factors. J. Biol. Chem. 27232163-32168. [DOI] [PubMed] [Google Scholar]
- 13.Gao, M., T. Labuda, Y. Xia, E. Gallagher, D. Fang, Y. C. Liu, and M. Karin. 2004. Jun turnover is controlled through JNK-dependent phosphorylation of the E3 ligase Itch. Science 306271-275. [DOI] [PubMed] [Google Scholar]
- 14.Harvey, M. B., K. J. Leco, M. Y. Arcellana-Panlilio, X. Zhang, D. R. Edwards, and G. A. Schultz. 1995. Proteinase expression in early mouse embryos is regulated by leukaemia inhibitory factor and epidermal growth factor. Development 1211005-1014. [DOI] [PubMed] [Google Scholar]
- 15.Kuan, C. Y., D. D. Yang, D. R. Samanta Roy, R. J. Davis, P. Rakic, and R. A. Flavell. 1999. The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron 22667-676. [DOI] [PubMed] [Google Scholar]
- 16.Levy, D. E., F. Lapierre, W. Liang, W. Ye, C. W. Lange, X. Li, D. Grobelny, M. Casabonne, D. Tyrrell, K. Holme, A. Nadzan, and R. E. Galardy. 1998. Matrix metalloproteinase inhibitors: a structure-activity study. J. Med. Chem. 41199-223. [DOI] [PubMed] [Google Scholar]
- 17.Luo, W., W. W. Ng, L. H. Jin, Z. Ye, J. Han, and S. C. Lin. 2003. Axin utilizes distinct regions for competitive MEKK1 and MEKK4 binding and JNK activation. J. Biol. Chem. 27837451-37458. [DOI] [PubMed] [Google Scholar]
- 18.Ralston, A., and J. Rossant. 2006. How signaling promotes stem cell survival: trophoblast stem cells and Shp2. Dev. Cell 10275-276. [DOI] [PubMed] [Google Scholar]
- 19.Santibanez, J. F. 2006. JNK mediates TGF-β1-induced epithelial mesenchymal transdifferentiation of mouse transformed keratinocytes. FEBS Lett. 5805385-5391. [DOI] [PubMed] [Google Scholar]
- 20.Simmons, D. G., and J. C. Cross. 2005. Determinants of trophoblast lineage and cell subtype specification in the mouse placenta. Dev. Biol. 28412-24. [DOI] [PubMed] [Google Scholar]
- 21.Sutherland, A. 2003. Mechanisms of implantation in the mouse: differentiation and functional importance of trophoblast giant cell behavior. Dev. Biol. 258241-251. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka, S., T. Kunath, A. K. Hadjantonakis, A. Nagy, and J. Rossant. 1998. Promotion of trophoblast stem cell proliferation by FGF4. Science 2822072-2075. [DOI] [PubMed] [Google Scholar]
- 23.Ten Dijke, P., M. J. Goumans, F. Itoh, and S. Itoh. 2002. Regulation of cell proliferation by Smad proteins. J. Cell. Physiol. 1911-16. [DOI] [PubMed] [Google Scholar]
- 24.Uhlik, M. T., A. N. Abell, N. L. Johnson, W. Sun, B. D. Cuevas, K. E. Lobel-Rice, E. A. Horne, M. L. Dell'Acqua, and G. L. Johnson. 2003. Rac-MEKK3-MKK3 scaffolding for p38 MAPK activation during hyperosmotic shock. Nat. Cell Biol. 51104-1110. [DOI] [PubMed] [Google Scholar]
- 25.Yang, J., S. A. Mani, J. L. Donaher, S. Ramaswamy, R. A. Itzykson, C. Come, P. Savagner, I. Gitelman, A. Richardson, and R. A. Weinberg. 2004. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117927-939. [DOI] [PubMed] [Google Scholar]
- 26.Yang, W., L. D. Klaman, B. Chen, T. Araki, H. Harada, S. M. Thomas, E. L. George, and B. G. Neel. 2006. An Shp2/SFK/Ras/Erk signaling pathway controls trophoblast stem cell survival. Dev. Cell 10317-327. [DOI] [PubMed] [Google Scholar]
- 27.Zavadil, J., and E. P. Bottinger. 2005. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene 245764-5774. [DOI] [PubMed] [Google Scholar]
- 28.Zohn, I. E., Y. Li, E. Y. Skolnik, K. V. Anderson, J. Han, and L. Niswander. 2006. p38 and a p38-interacting protein are critical for downregulation of E-cadherin during mouse gastrulation. Cell 125957-969. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.