Abstract
The loss of p53 induces spontaneous tumors in mice, and p53 mutations are found in approximately 50% of human tumors. These tumors are generally caused by a number of events, including genomic instability, checkpoint defects, mitotic defects, deregulation of transcriptional targets, impaired apoptosis, and G1 deregulation or a combination of these effects. In order to determine the role of proteins involved in G1 control in tumorigenesis, we focused on Cdk2 and Cdk4, two cyclin-dependent kinases that in association with cyclin E and cyclin D promote the G1/S phase transition. We analyzed the consequence of loss of Cdk2 in p53-null animals by generating Cdk2−/− p53−/− mice. These mice are viable and developed spontaneous tumors, predominantly lymphoblastic lymphomas, similar to p53−/− mice. In contrast, the genotypes Cdk4−/− p53−/− were mostly lethal, with few exceptions, and Cdk2−/− Cdk4−/−p53−/− mice die during embryogenesis at embryonic day 13.5. To study the oncogenic potential, we generated mouse embryonic fibroblasts (MEFs) and found that p53−/−, Cdk2−/− p53−/−, Cdk4−/− p53−/−, and Cdk2−/− Cdk4−/− p53−/− MEFs grew at similar rates without entering senescence. Ras-transformed MEFs of these genotypes were able to form colonies in vitro and induce tumors in nude mice. Our results suggest that tumorigenicity mediated by p53 loss does not require either Cdk2 or Cdk4, which necessitates considering the use of broad-spectrum cell cycle inhibitors as a means of effective anti-Cdk cancer therapy.
p53 is a transcription factor or tumor suppressor, and loss of one or two alleles has been shown to induce tumors in mice (13, 22). Transcription of p21 is regulated by p53, and Cdk2 inhibition by p21 is believed to be essential for the establishment of G1 arrest upon DNA damage by various stresses and for cellular senescence (15, 43). p21 is not necessarily the sole effector of p53-mediated G1 arrest as exemplified by the involvement of additional targets such as Ptprv (14). The role of p21 binding to Cdk/cyclin D complexes might serve contradictory functions such as acting as assembly factor for Cdk/cyclin D complexes (9) besides inhibiting Cdk activity. p53 is also believed to inhibit Cdk4 activity through p21 and by repression of Cdk4 synthesis (16, 18).
Loss of G1 control in the cell cycle appears to be an important step contributing to tumorigenesis (for a review, see references 17 and 24). Overexpression of Cdk2, Cdk4, cyclin E, and cyclin D1 has been observed in various types of tumors (3, 6, 20, 25, 29, 36, 45). While it has been shown that cancer cells can still proliferate in the absence of Cdk2, Cdk4 activity was reported to be essential for ErbB-2-driven mammary carcinogenesis (46, 47).
Mouse models lacking one or more of the cell cycle proteins have been generated in order to study cell cycle control in vivo. Cdk2−/− mice are viable but sterile. Mouse embryonic fibroblasts (MEFs) generated from Cdk2−/− mice display minor proliferation defects (4, 31). Loss of Cdk4 expression causes insulin-deficient diabetes and delayed S phase entry in MEFs (32, 42). Concomitant loss of both Cdk2 and Cdk4 has more dramatic effects on the cell cycle. Embryos lacking both Cdk2 and Cdk4 die during embryogenesis due to heart defects. MEFs isolated from these embryos, display decreased proliferation rates, severely delayed S phase entry, and premature entry into senescence (5).
While Cdk/cyclin complexes regulate progression of the cell cycle from one phase to the next, the timely entry into the cell cycle and arrest depends on pathways that are mediated by two groups of inhibitors called the INK4 and CIP/KIP families of proteins. The INK4 family proteins include p15INK4b, p16INK4a, p18INK4c, and p19INK4d, which inhibit Cdk4/cyclin D and Cdk6/cyclin D complexes (for a review, see reference 23). The CIP/KIP family includes p21Cip1/Waf1, p27Kip1, and p57Kip2 (for a review, see reference 38). p27 binds and inhibits Cdk2/cyclin E and Cdk1/cyclin E complexes, and loss of this inhibition is one step for cells entering into the DNA synthesis (S) phase.
In an effort to study how Cdk2 and Cdk4 activities contribute to the tumorigenicity witnessed in p53−/− mice and cells, we generated Cdk2−/− p53−/− and Cdk4−/− p53−/− mice. Cdk2−/− p53−/− mice developed spontaneous tumors, including hemangiosarcoma, granulosa cell tumor, muscle sarcoma, and most predominantly thymic lymphoma similar to p53−/− mice. Only a few Cdk4−/− p53−/− mice were born and did not live long. However, Ras overexpression transformed Cdk4−/− p53−/− MEFs in vitro and they formed tumors when injected in nude mice. Previous work from our laboratory has demonstrated that Cdk2 and Cdk4 function cooperatively during the G1/S transition. In order to study if the loss of both would have any influence on p53−/− mouse tumorigenicity, we generated mice lacking Cdk2, Cdk4, and p53. Cdk2−/− Cdk4−/−p53−/− mice were embryonic lethal, and therefore we resorted to characterized MEFs. Our results indicate that Cdk2−/− Cdk4−/− cells bypass senescence and continue to grow in the absence of p53. We have also shown that either subsequent or concomitant loss of Cdk2 and Cdk4 has little or no effect on the transformation and tumorigenic potential of p53−/− cells. We demonstrated that growth properties of Cdk2−/− Cdk4−/− p53−/− cells were not mediated through decreased expression of p21. Our results collectively reveal a dominating control of the cell cycle by p53, which cannot be reversed by the loss of Cdk2 and Cdk4.
MATERIALS AND METHODS
Generation of Cdk2−/− p53−/−, Cdk4−/− p53−/−, Cdk2−/− Cdk4−/− p53−/−, and Cdk2−/− Cdk4−/− p21−/− mice.
Cdk2/4+/− mice were crossed with p53+/− mice (a kind gift from Tyler Jacks [22]) to generate Cdk2/4+/− p53+/− mice. Cdk2/4+/− p53+/− mice were later intercrossed to generate Cdk2/4−/− p53−/− mice. Cdk2+/− Cdk4+/− mice (5) were crossed with p53+/− mice. Cdk2+/− Cdk4+/− p53+/− mice were intercrossed to generate Cdk2−/− Cdk4−/− p53−/− animals. Cdk2+/− Cdk4+/− mice were crossed with p21+/− mice (obtained from Jackson Laboratory, allele from Tyler Jacks Cdkn1atm1Tyj [8]) to generate heterozygous Cdk2+/− Cdk4+/− p21+/− mice. Male and female triple-heterozygote animals were intercrossed to generate Cdk2−/− Cdk4−/− p21−/− mice. The mice used in this study are of mixed C57BL/6 × 129S1/SvlmJ background. Mice were routinely genotyped by isolating tail DNA using HOTshot lysis (41). A piece of mouse tail was incubated in 94°C for 30 min in 70 μl lysis buffer containing 25 mM NaOH and 0.2 mM EDTA followed by addition of 70 μl of neutralization buffer at room temperature. One microliter of the HOTshot was used for PCR analysis (41). Cdk2 PCR was performed with the following oligonucleotides: PKO0292 (forward; GTGACCCTGTGGTACCGAGCACCTG), PKO0344 (reverse; GGTTTTGCTCTTGGATGTGGGCATGG), and PKO0294 (Neo; CCCGTGATATTGCTGAAGAGCTTGGCGG). The sizes of the PCR products are 195 bp for the wild type (WT) and 500 bp for the null mutant. Cdk4 PCR was carried out with the following oligonucleotides: PKO0104 (forward; CGGAAGGCAGAGATTCGCTTAT), PKO0105 (reverse; CCAGCCTGAAGCTAAGAGTAGCTGT), and PKO0294 (Neo). The sizes of the PCR products are 195 bp for the WT and 315 bp for the null mutant. p53 PCR was made with the oligonucleotides PKO0468 (GTAGTGGATGGTGGTATACTCAGAGCCGGCCT), PKO0469 (GTGGCGGACCGCTATCAGGACATAGCGTTGGCT), and PKO0340 (ACAGCGTGGTGGTACCTTAT). The sizes of the PCR products are 375 bp for the WT and 525 bp for the null mutant. p21 PCR was done with the oligonucleotides PKO0662 (AAGCCTTGATTCTGATGTGGGC), PKO0663 (TGACGAAGTCAAAGTTCCACC), and PKO0664 (GCTATCAGGACATAGCGTTGGC). The sizes of the PCR products are 872 bp for the WT and 700 bp for the null mutant. All PCRs were performed for 30 cycles with annealing temperature for Cdk2 at 60°C, Cdk4 at 62°C, p53 at 60°C, and p21 at 60°C. Mice were group housed under standard conditions with food and water available ad libitum and were maintained on a 12-h light/dark cycle. Mice were fed a standard chow diet containing 6% crude fat and were treated humanely in compliance with the National Institutes of Health guidelines for animal care and use.
Histopathology and microscopy.
Histopathology was performed as described by Aleem et al. (2). Microscopy was performed using an Olympus Vanox AHBS3 microscope.
Isolation and culture of embryonic fibroblasts.
Fibroblasts were prepared from embryos at 12.5 or 13.5 days postcoitum as described previously (4). Embryos whose head and other red organs were removed were smashed into pieces using a razor blade in a 10-cm dish with 5 ml trypsin (Gibco; no. 25300-054). The smashed embryo was incubated in trypsin for 15 min at 37°C followed by dilution in 25 ml Dulbecco's modified minimum essential medium (DMEM) by pipetting up and down. The cells were centrifuged and seeded in 100-mm culture dishes (passage 0). MEFs were routinely cultured in a humidified 5% CO2 atmosphere at 37°C in DMEM (Invitrogen; no. 10569-010) supplemented with 10% (wt/vol) fetal calf serum (Gemini Bio-Products; no. 100-106) and 1% penicillin-streptomycin (Invitrogen; no. 15140-122).
Serum starvation and β-galactosidase staining.
A total of 2.5 million cells were plated in a 15-cm dish and grown to confluence for a period of 3 to 4 days in DMEM with 10% serum. After 3 days, the medium was removed and cells were washed with phosphate-buffered saline (PBS). Twenty milliliters of fresh DMEM containing 0.1% serum was added to start the serum starvation. After 72 h of serum starvation, the cells were split into several 10-cm dishes for different time points for the bromodeoxyuridine (BrdU) incorporation assay and in 15-cm dishes for protein analysis. β-Galactosidase stainings were performed as described previously (5).
Cycloheximide treatment.
One million cells plated in a 10-cm dish and grown for 24 h were treated with 50 μg/ml of cycloheximide for 0, 2, 4, or 6 h. The cells were trypsinized and processed for Western blot analysis with antibodies specific for Hsp90, Cdk2, and Cdk1.
FACS analysis.
Cells were harvested 1 h after addition of 31 μl of BrdU (10 mg/ml PBS) (Invitrogen; no. B23151) in a 10-cm plate containing 10 ml medium by trypsinization. Cells were washed with PBS once and fixed with 70% cold ethanol with rigorous vortexing. Cells were washed with PBS-1% bovine serum albumin (BSA) and treated with 1 ml 2N HCl- 0.5% Triton X-100 followed by 1 ml 1 M sodium tetra borate (pH 8.5). The cells were washed again with PBS-1% BSA. After centrifugation, the cell pellet was incubated with anti-BrdU Alexa 488-conjugated antibodies (Invitrogen; no. A21303) for 30 min. The cells were washed once with PBS-1% BSA and once with PBS. After centrifugation, the cells were resuspended in the following solution: 11.4 ml PBS, 600 μl propidium iodide (50 μg/ml), and 24 μl RNase (10 mg/ml). The cells were resuspended in about one million cells/ml and analyzed by fluorescence-activated cell sorting (FACS) (Guava EasyCyte; Guava Technologies).
Protein analysis.
Harvested MEFs were resuspended in the following buffer: 50 mM HEPES (pH 7), 150 mM NaCl, 2.5 mM EGTA, 1 mM EDTA, 10 mM β-glycerol phosphate, 0.1% Tween 20, 10% glycerol, 1 mM dithiothreitol, 2 mM NaF, and 1× protease inhibitors (10 μg/ml each of leupeptin, chymostatin, and pepstatin [Chemicon, Temecula, CA]). The cells were vortexed, frozen in liquid nitrogen, and thawed in ice three times. Lysates were centrifuged for 45 min at 18,000 × g at 4°C, and supernatants were frozen in liquid nitrogen. Protein concentrations were determined using the Bradford protein assay (Bio-Rad; no. 500-0006). Lysates were analyzed by immunoblotting, immunoprecipitation, and kinase assays. Affinity-purified antibodies against Cdk1, Cdk2, Cdk4, Cdk6, and cyclin B1 have been described previously (4). Antibodies against Emi1 were a kind gift from Peter Jackson. Other antibodies are commercially available: 0.2 μg/ml rabbit anti-cyclin A (Santa Cruz; H-432, no. Sc-751), 2 μg/ml rabbit anti-cyclin D1 (NeoMarkers; no. RB-010-P), 0.1 μg/ml rabbit anti-Cdk4 (Clontech; no. 3517-1), 0.5 μg/ml rabbit anti-p27 (Zymed; no. 71-9600), 0.1 μg/ml mouse anti-p53 (Cell Signaling; no. 2524), 0.2 μg/ml 1:500 mouse anti-p21 (Santa Cruz; no. Sc-6246), and 0.2 μg/ml goat anti-β-actin (Santa Cruz; I-19, no. Sc-1616). For immunoprecipitation, 10 μl of antibodies conjugated to agarose beads was used (Santa Cruz; no. Sc-xxx-AC) as well as Rb-agarose beads (Santa Cruz; IF8, no. Sc-102), or p13Suc1-agarose beads (Upstate Biotechnology; no. 14-132). Kinase assays were performed as described previously (4).
Colony formation assay and tumorigenesis.
MEFs were infected with pBABE-RasV12G (PKB889; plasmid generated by Scott Lowe and Bob Weinberg). Ten thousand cells were plated in a 10-cm plate and grown in DMEM with a high glucose concentration and 10% fetal calf serum for 10 days. The medium was changed after every 3 days. The colonies which appeared around 10 days were stained with Giemsa stain (Sigma; no. GS500). RasG12V-transformed cells were injected into the flanks of C3H/HeNCr nude mice.
RESULTS
Generation of Cdk2−/− p53−/− mice.
The loss of the tumor suppressor p53 has been shown to induce spontaneous tumors, from which mice succumb to death within 6 to 8 months of age (13, 22). Cdk2 is one of the primary regulators of the G1/S-phase transition, and mice lacking Cdk2 are viable, implying the involvement of additional cyclin-dependent kinases in cell cycle control (4, 31). In pursuit of investigating the role of the G1/S phase transition mediated by Cdk2 activity in spontaneous tumorigenesis caused by p53 loss or mutation, we generated mice lacking both Cdk2 and p53. We generated 1,149 mice by intercrossing Cdk2+/− p53+/− mice, yielding 41 double-homozygous Cdk2−/− p53−/− mice (Table 1). The percentage of Cdk2−/− p53−/− mice observed was 3.6%, slightly below the expected Mendelian ratio of 6.25%. Visual comparison of healthy WT, Cdk2−/−, p53−/−, and Cdk2−/− p53−/− mice indicated no significant difference in size or appearance until about 6 months of age.
TABLE 1.
Mice of various genotypes generated by Cdk2+/− p53−/− intercrossing
| Genotype | No. of micea | % of mice
|
|
|---|---|---|---|
| Observed | Expected | ||
| Cdk2+/+p53+/+ | 75 | 6.5 | 6.25 |
| Cdk2+/+p53+/− | 163 | 14.2 | 12.5 |
| Cdk2+/+p53−/− | 51 | 4.4 | 6.25 |
| Cdk2+/−p53+/+ | 165 | 14.4 | 12.5 |
| Cdk2+/−p53+/− | 328 | 28.5 | 25 |
| Cdk2+/−p53−/− | 91 | 7.9 | 12.5 |
| Cdk2−/−p53+/+ | 92 | 8.0 | 6.25 |
| Cdk2−/−p53+/− | 143 | 12.4 | 12.5 |
| Cdk2−/−p53−/− | 41* | 3.6 | 6.25 |
| Total | 1,149 | ||
*, significant difference from the expected Mendelian ratio.
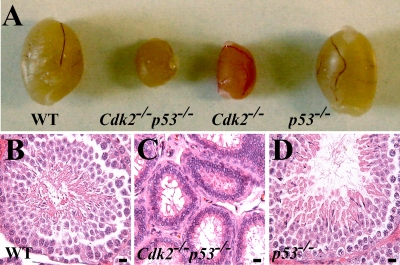
In addition to analyzing Cdk2−/− p53−/− mice for tumors (described below), we screened them for known phenotypes that had previously been observed in Cdk2−/− mice. The testes in Cdk2−/− p53−/− mice were smaller, as had been observed in Cdk2−/− mice, and the double-knockout mice never produced pups when mated with WT animals (Fig. 1; data not shown). Therefore, the loss of p53 did not rescue the sterility in Cdk2−/− mice, indicating that p53-mediated apoptosis is not the sole cause of the Cdk2−/− sterility (4, 31).
FIG. 1.
Cdk2−/− p53−/− and Cdk2−/− mice are sterile. (A) Representative photograph comparing testes from WT, Cdk2−/− p53−/−, Cdk2−/−, and p53−/− mice. The pictures indicate that testes of both Cdk2−/− and Cdk2−/− p53−/− mice are smaller than WT testis. (B to D) Histology of testis sections indicates that the seminiferous tubules of Cdk2−/− p53−/− mice are atrophic like the ones from Cdk2−/− mice. Bars, 40 μm.
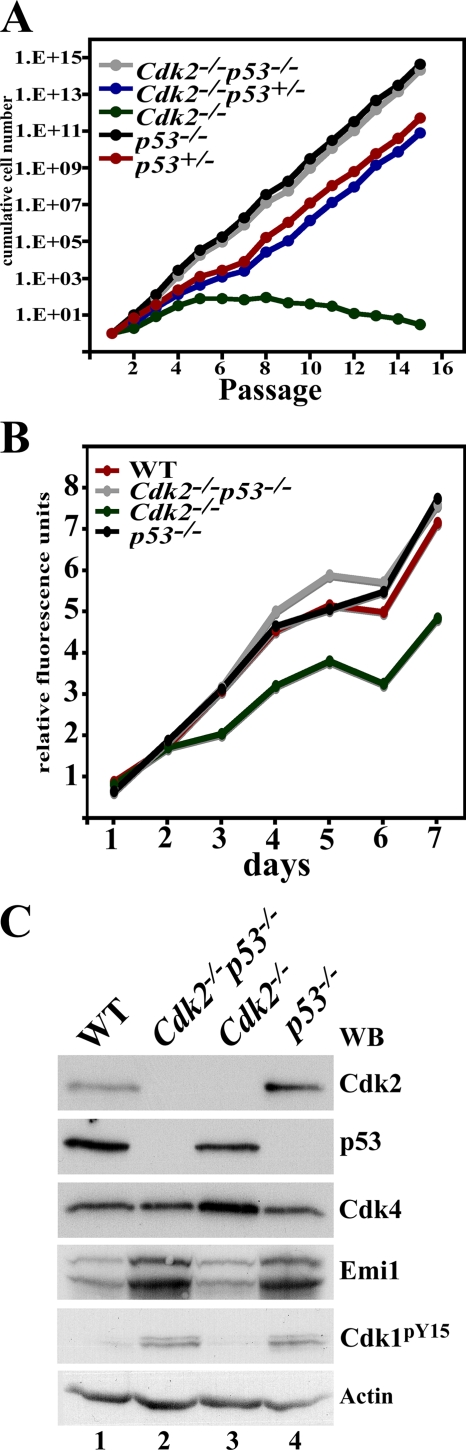
Cdk2−/− MEFs display minor growth defects (4), and therefore we performed 3T3 assays (40). Cdk2−/− MEFs stopped dividing after about 6 passages and did appear to enter senescence, whereas all other genotypes continued growing. Cdk2−/− p53−/− MEFs grew at dynamics similar to those of p53−/− MEFs. p53+/− and Cdk2−/− p53+/− MEFs seemed to switch to an increased rate at passage 7 (Fig. 2A). To analyze proliferation, rather than immortalization, we monitored the growth of MEFs over a period of 7 days (Fig. 2B). Cdk2−/− MEFs displayed a reduced proliferation potential, whereas p53−/− and Cdk2−/− p53−/− MEFs proliferated at a rate similar to that of WT MEFs. Western blot analysis of MEF extracts indicated that in p53−/− and Cdk2−/− p53−/− MEFs, levels of Emi1 and phosphorylation of Cdk1 at tyrosine 15 were elevated (Fig. 2C) compared to those in WT and Cdk2−/− extracts (see Discussion).
FIG. 2.
Loss of p53 improves growth of Cdk2−/− MEFs. (A) Analysis of spontaneous immortalization of MEFs using a 3T3 assay. The five genotypes plotted are Cdk2−/−, Cdk2−/− p53−/−, p53−/−, p53+/−, and Cdk2−/− p53+/−. The number of passages is plotted on the x axis, and cumulative growth is plotted on the y axis. (B) Proliferation assay performed on passage 6 for a period of 7 days for four genotypes: WT, Cdk2−/− p53−/−, Cdk2−/−, and p53−/−. Cdk2−/− MEFs display a decreased growth rate, which is rescued by loss of p53. (C) Western blot (WB) analysis of protein extracts from MEFs using antibodies against Cdk2, p53, Cdk4, Emi1, Cdk1pY15, and actin.
Cdk2−/− p53−/− mice develop spontaneous tumors.
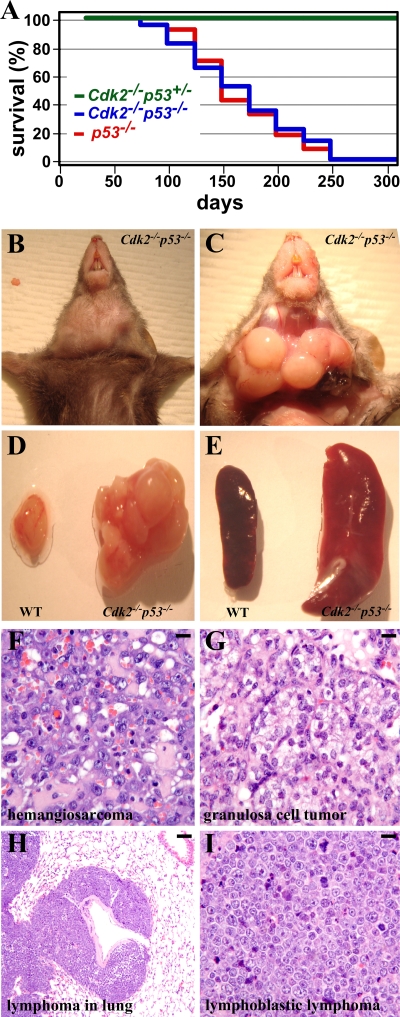
p53−/− mice have been reported to develop spontaneous tumors, predominantly thymic lymphomas (22). We followed a cohort of Cdk2−/− p53−/− mice to investigate the potential effects of the loss of Cdk2 in the development of spontaneous tumors in the absence of p53. All of the mice analyzed developed some form of tumor and died spontaneously or had to be sacrificed due to ill health before 250 days of life (Fig. 3A). Of the 21 mice analyzed in detail, 12 developed lymphoblastic lymphoma (Fig. 3B to I and Table 2). Some developed lymphoma in the lungs, one female developed a granulosa cell tumor, and one developed a hemangiosarcoma (Fig. 3F to I and Table 2). Most mice analyzed had enlarged spleen (Fig. 3E) and lymph nodes. Four mice were sick but had no obvious tumors (Table 2). Control p53−/− mice developed tumors and spontaneously died or had to be sacrificed before 250 days of age (data not shown; see also reference 22). Development of spontaneous tumors in Cdk2−/− p53−/− mice underscores the dispensability of Cdk2 activity in the formation of tumors.
FIG. 3.
Cdk2−/− p53−/− mice develop spontaneous tumors. (A) Survival curve generated after following 25 p53−/− mice and 30 Cdk2−/− p53−/− mice for a period of 275 days. (B and C) Representative thymic lymphoma found in one of the Cdk2−/− p53−/− mice. (D) Thymic lymphoma found in Cdk2−/− p53−/− mice compared with the normal thymus of a WT animal of the same age. (E) Comparison of the spleens of a WT animal and Cdk2−/− p53−/− mice. (F to I) Histological sections of tumors found in Cdk2−/− p53−/− mice (Table 2). Sections have been stained with hematoxylin and eosin. Magnifications: panels F, G, and I, ×40; panel H, ×10. Bars: 100 μm (H) and 300 μm (F, G, and I).
TABLE 2.
Pathological findings for Cdk2−/− p53−/− mice
| Animal | Sex | Day | Pathological finding(s) for Cdk2−/−p53−/− micea |
|---|---|---|---|
| KM123 | Male | 32 | Skin abscess |
| KM135 | Male | 238 | Lymphoblastic lymphoma, hematopoietic neoplasm, skin sarcoma |
| KM108 | Female | 175 | Lymphoblastic lymphoma |
| KM1139 | Male | 108 | Lymphoblastic lymphoma |
| KM1161 | Male | 113 | Sick, no obvious tumor |
| KM1184 | Male | 66 | Sick, no obvious tumor |
| KM1240 | Female | 62 | Lymphoblastic lymphoma |
| KM1269 | Male | 264 | Lymphoblastic lymphoma |
| KM1304 | Female | 137 | Lymphoblastic lymphoma |
| KM1571 | Female | 98 | Lymphoblastic lymphoma |
| KM1574 | Male | 99 | Lymphoblastic lymphoma, lymphoma in lungs, muscle sarcoma, hematopoietic neoplasm |
| KM1637 | Male | 154 | Lymphoma in lungs, thymic hyperplasia |
| KM1671 | Male | 237 | Lymphoblastic lymphoma, hemangiosarcoma |
| KM1677 | Female | 253 | Granulosa cell tumor |
| KM1697 | Male | 235 | Lymphoma, hematopoietic neoplasm |
| KM1703 | Male | 256 | Skin acanthosis |
| KM1756 | Male | 117 | Lymphoblastic lymphoma, hematopoietic neoplasm |
| KM1793 | Female | 199 | Lymphoblastic lymphoma |
| KM1823 | Male | 185 | Sick, but no obvious tumor |
| KM1843 | Female | 168 | Sick, but no obvious tumor |
| KM1869 | Male | 95 | Lymphoblastic lymphoma |
The following animals were found dead (with sex and day of death shown in parentheses): KM1152 (male, day 179), KM1171 (male, day 218), KM1172 (male, day 184), KM1317 (male, day 208), KM1419 (male, day 105), KM1429 (male, day 173), KM1430 (female, day 109), KM1470 (male, 173), and KM1480 (female, day 105).
Genetic interaction between Cdk4 and p53.
The loss of control of the G1/S phase transition is one of the hallmarks of tumorigenesis. Since Cdk2 plays a nonessential role in the G1/S phase transition, we aimed to unravel potential compensatory activity by other genes. Cdk4 was a strong candidate, considering that it may play a more significant role in tumorigenesis (46, 47) and might compensate for the loss of Cdk2 (5). In the process of generating double-mutant mice lacking both p53 and Cdk4, only 2 live Cdk4−/− p53−/− mice were born out of 218 animals produced by intercrossing Cdk4+/− p53+/− mice. The birth rate was exceedingly low (less than 1%), suggesting approximately 85% lethality compared to the expected Mendelian birth rate for double-knockout mice (Table 3). The live Cdk4−/− p53−/− mice belonged to the same litter: one of which was found dead at 69 days, and the other was observed to be ill and had to be sacrificed, although pathology revealed no obvious tumors in the sick Cdk4−/− p53−/− mouse (data not shown).
TABLE 3.
Mice generated by Cdk4+/− p53+/− crosses
| Genotype | No. of micea | % of mice
|
|
|---|---|---|---|
| Observed | Expected | ||
| Cdk4+/+p53+/+ | 21 | 9.6 | 6.25 |
| Cdk4+/+p53+/− | 44 | 20.2 | 12.5 |
| Cdk4+/+p53−/− | 9 | 4.1 | 6.25 |
| Cdk4+/−p53+/+ | 38 | 17.4 | 12.5 |
| Cdk4+/−p53+/− | 72 | 33.0 | 25 |
| Cdk4+/−p53−/− | 17 | 7.8 | 12.5 |
| Cdk4−/−p53+/+ | 4* | 1.8 | 6.25 |
| Cdk4−/−p53+/− | 11 | 5.0 | 12.5 |
| Cdk4−/−p53−/− | 2* | 0.9 | 6.25 |
| Total | 218 | ||
*, significant difference from the expected Mendelian ratio.
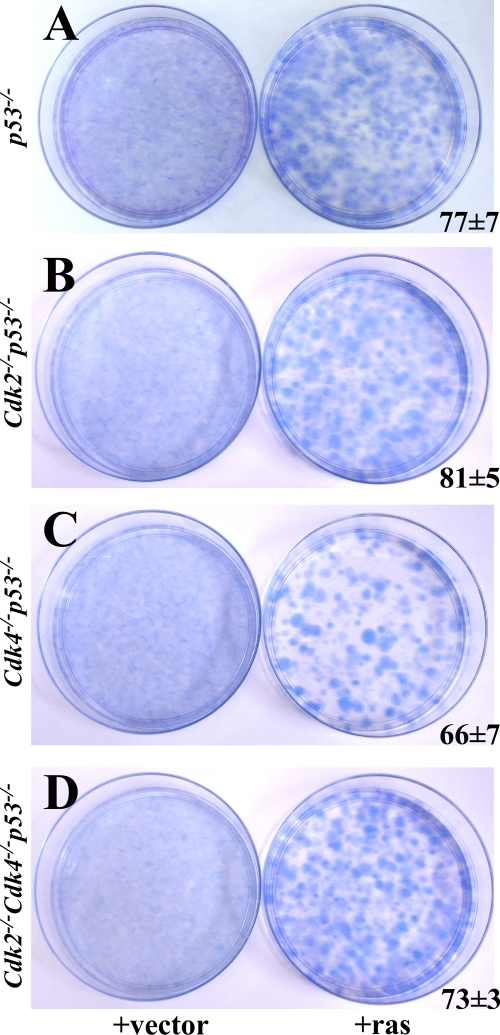
Generation of a statistically significant number of Cdk4−/− p53−/− mice to study in vivo tumorigenesis became untenable due to the low birth rate and short life span. To address the loss of Cdk4 in vitro, Cdk4−/− p53−/− MEFs were generated and infected with activated RasG12V to study tumorigenicity. Ras-transformed Cdk2−/− p53−/− and Cdk4−/− p53−/− MEFs formed colonies in vitro (Fig. 4B and C) and formed tumors when these cells were injected into nude mice (data not shown). These results indicate that the loss of either Cdk2 or Cdk4 does not substantially affect tumorigenesis mediated by the loss of p53.
FIG. 4.
Ras transforms MEFs lacking p53−/−. (A to D) p53−/−, Cdk2−/− p53−/−, Cdk4−/− p53−/−, and Cdk2−/− Cdk4−/− p53−/− MEFs were infected with empty vector (left) or activated RasG12V (right). Colonies were stained with Giemsa after 10 days of culture. The numbers of colonies are the averages from three 10-cm plates.
Generation of triple-mutant Cdk2−/− Cdk4−/− p53−/− MEFs.
Since the independent loss of either Cdk2 or Cdk4 did not influence tumorigenesis in mice or cells lacking p53, the simultaneous loss of both could result in a more pronounced phenotype. The generation of triple-knockout mice was inherently complicated due in part to the fact that Cdk2−/− Cdk4−/− embryos die around embryonic day 15 (E15) due to heart defects (5) and Cdk4−/− p53−/− mice could not be generated in large numbers (Table 3). Investigation of the effect of inhibition of Cdk4 in Cdk2−/− p53−/− MEFs appeared to be a straightforward alternative conveniently offsetting the aforementioned complications. Short hairpin RNA silencing of Cdk4 did not adversely affect the growth of Cdk2−/− p53−/− MEFs (data not shown). This finding was unanticipated in light of the fact that MEFs lacking both Cdk2 and Cdk4 displayed severely pronounced growth defects (5).
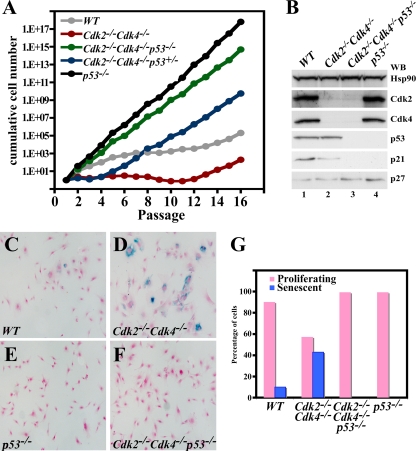
Emboldened by these new findings, we proceeded to generate Cdk2−/− Cdk4−/− p53−/− triple-mutant mice. Loss of p53 did not rescue the embryonic lethality of Cdk2−/− Cdk4−/−, and the triple-mutant embryos died around E13.5 (Table 4; data not shown). Cdk2−/− Cdk4−/− p53−/− MEFs were generated to determine their growth behavior and their ability to become immortal (Fig. 5A) using a 3T3 protocol (40). WT cells (Fig. 5A) stopped proliferating and entered senescence-related growth arrest after about 6 passages and remained in a stagnant state until about passage 12, when they became spontaneously immortal. p53−/− MEFs never slowed down in growth and could be passaged many times without a decrease in the growth rate. In contrast, Cdk2−/− Cdk4−/− MEFs grew much slower than WT cells and stopped dividing after approximately three passages, as we have described before (5). Interestingly, the loss of p53 was immensely effective in improving growth of the otherwise slow-growing Cdk2−/− Cdk4−/− MEFs, and loss of even one allele of p53 was sufficient for this improvement, although in a delayed fashion.
TABLE 4.
Generation of triple-knockout embryos
| Day of deatha | No. of embryos observed
|
|
|---|---|---|
| Total | Cdk2−/−Cdk4−/−p53−/− | |
| 12.5 | 81 | 5 |
| 13.5 | 39 | 2 |
| 14.5 | 50 | 0 |
| 16.5 | 26 | 0 |
Number of days after pregnancy.
FIG. 5.
Loss of p53 improves growth of Cdk2−/− Cdk4−/− cells. (A) 3T3 assay performed for 16 passages using WT, Cdk2−/− Cdk4−/−, Cdk2−/− Cdk4−/− p53−/−, Cdk2−/− Cdk4−/− p53+/−, and p53−/− MEFs. The x axis shows the number of passages, and the y axis indicates the cumulative cell number for each passage. (B) Western blot (WB) analysis. One hundred micrograms of protein lysate from passage 3 MEFs of WT (lane 1), Cdk2−/− Cdk4−/− (lane 2), Cdk2−/− Cdk4−/− p53−/− (lane 3), and p53−/− (lane 4) genotypes was separated on a 12.5% gel, and proteins were transferred to a polyvinylidene difluoride membrane. Antibodies against Hsp90, Cdk2, Cdk4, p53, p21, and p27 were used for detection. (C to F) β-Galactosidase staining. Shown are representative images from WT (C), Cdk2−/− Cdk4−/− (D), p53−/− (E), and Cdk2−/− Cdk4−/− p53−/− (F) MEFs stained with β-galactosidase as a marker for senescence. (G) Graph depicting the percentage of proliferating cells and senescent cells at passage 3 in WT, Cdk2−/− Cdk4−/−, Cdk2−/− Cdk4−/− p53−/−, and p53−/− MEFs, counting at least 200 cells for each genotype.
Loss of Cdk2 and Cdk4 does not prevent the cellular transformation in p53−/− MEFs.
Expression of activated Ras in MEFs with mutant p53 is known to transform cells and form tumors when injected into nude mice (19, 39, 49). Cellular transformation can be analyzed by performing colony formation assays. p53−/−, Cdk2−/− p53−/−, Cdk4−/− p53−/−, and Cdk2−/− Cdk4−/− p53−/− MEFs were infected with viruses expressing constitutively active RasG12V and plated for colony formation assays. MEFs of all genotypes analyzed formed colonies after 10 days of growth, whereas cells infected only with the empty vector failed (Fig. 4A to D).
Ras-transformed cells were injected into nude mice to study their potential to form tumors. All nude mice injected with Ras-transformed p53−/− (6/6), Cdk2−/− p53−/− (4/4), Cdk4−/− p53−/− (4/4), and Cdk2−/− Cdk4−/− p53−/− (6/6) MEFs developed tumors after approximately 12 days (data not shown). No significant difference in size or onset of the tumor formation was observed, although there was a moderate statistically insignificant reduction in size in tumors induced by the induction of Cdk2−/− Cdk4−/− p53−/− or Cdk4−/− p53−/− MEFs (Table 5). Our results suggest that either the subsequent or concomitant loss of Cdk2 and Cdk4 does not prevent tumor cell growth in a mutant p53 background in this particular system.
TABLE 5.
Tumor sizes measured after 21 days in four nude mice of different genotypes
| Animal | Tumor size (cm3) in mouse
|
|||
|---|---|---|---|---|
| p53−/− | Cdk2−/−p53−/− | Cdk4−/−p53−/− | Cdk2−/−Cdk4−/−p53−/− | |
| 1 | 24.6 | 16.2 | 17.4 | 14.1 |
| 2 | 15.3 | 19.4 | 14 | 13.6 |
| 3 | 10.1 | 17.9 | 14.7 | 9.9 |
| 4 | 22.6 | 36.3 | 12 | 9.2 |
| Avg | 18.2 ± 6.7 | 22.5 ± 9.3 | 14.5 ± 2.2 | 11.7 ± 2.5 |
Molecular connection between p53 and Cdks.
The paradoxical observation that concomitant loss of both Cdk2 and Cdk4 leads to growth defects (5), and its reversal in a p53-null background warranted an investigation of the underlying molecular mechanism. As a first step, the expression levels of cell cycle proteins in MEFs were analyzed by Western blots. Absence of Cdk2 and Cdk4 confirmed the genotyping of Cdk2−/− Cdk4−/− and Cdk2−/− Cdk4−/− p53−/− MEFs. The expression of p21 was hard to detect in cells lacking p53, as has been reported previously (28), whereas p27 expression was comparable in all genotypes (Fig. 5B).
Cellular senescence in general is believed to limit cellular proliferation and protects cells from transformation (for a review, see reference 10). Senescence in MEFs is defined as a replicative arrest upon continuous passage in vitro. These cells are larger than proliferating cells, display a flat morphology, are metabolically active, and express the marker β-galactosidase (12). MEFs in passage 3 were stained for the expression of β-galactosidase to measure the percentage of senescent cells (Fig. 5C to F). As reported, Cdk2−/− Cdk4−/− MEFs had an increased population of senescent cells compared to WT or p53−/− MEFs (5). In contrast, senescence was not detected in Cdk2−/− Cdk4−/− p53−/− MEF cultures (compare panel F to panel D). Quantification of the number of cells after β-galactosidase staining indicated that 40% of Cdk2−/− Cdk4−/− MEFs were senescent compared to less than 10% in WT cells at passage 3 (Fig. 5G). Our results indicate that the loss of p53 prevents premature entry of Cdk2−/− Cdk4−/− MEFs into senescence.
Loss of p53 does not rescue the S phase entry defect in Cdk2−/− Cdk4−/− MEFs.
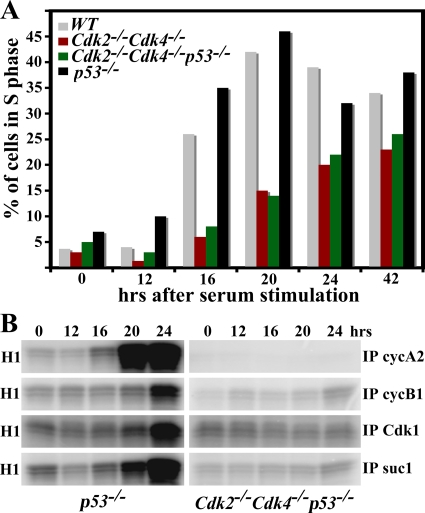
Cdk2/cyclin E, Cdk1/cyclin E, Cdk4/cyclin D, and Cdk6/cyclin D complexes promote entry into S phase. Cdk2−/− Cdk4−/− MEFs enter S phase delayed compared to WT cells (5). Bypass of senescence in Cdk2−/− Cdk4−/p53−/− MEFs prompted us to investigate the kinetics of S-phase entry. MEFs were grown to confluence, serum starved, stimulated with serum, and harvested at different time points for S phase entry measurement by both BrdU incorporation and protein analysis. Both WT and p53−/− MEFs peaked with 40 to 45% of cells in S phase after 20 h (Fig. 6A). Cdk2−/− Cdk4−/− MEFs entered S phase delayed, and less than 25% of these cells were found in S phase after 42 h. Strikingly, Cdk2−/− Cdk4−/− p53−/− MEFs entered S phase with the same kinetics as Cdk2−/− Cdk4−/− MEFs. These results indicate that while the loss of p53 bypasses senescence and improved growth, it did not rescue the S phase entry defect observed in Cdk2−/− Cdk4−/− MEFs.
FIG. 6.
Cdk2−/− Cdk4−/− p53−/− MEFs display a defect in S phase entry. (A) Histogram of FACS analysis of cells in S phase. MEFs were collected after release from serum starvation at different time points (x axis) after 1 h of pulse-labeling with BrdU and were stained with propidium iodide followed by FACS analysis. The y axis represents the percentage of cells in S phase in the different MEF genotypes: WT, Cdk2−/− Cdk4−/−, Cdk2−/− Cdk4−/− p53−/−, and p53−/−. (B) Kinase assay. Two hundred fifty micrograms of protein lysate prepared from Cdk2−/− Cdk4−/− p53−/− and p53−/− MEFs 0, 12, 16, 20, and 24 h after serum stimulation was used. Cyclin A2 (cycA2), cyclin B1 (cycB1), and Cdk1 antibodies coupled to protein A-agarose beads or suc1 beads were used for immunoprecipitation (IP) followed by an in vitro kinase assay using the substrate histone H1.
The cell cycle is regulated by the temporal activation of Cdk/cyclin complexes. Thus measurement of their kinase activities would serve as an indication of the phase of the cell cycle. Cdks or cyclins were immunoprecipitated, and their ability to phosphorylate histone H1 in vitro was measured to determine the kinase activity. The activity of cyclin A2, an S phase marker, peaked at about 20 h in p53−/− MEFs (Fig. 6B, left top panel), which entered S phase with kinetics similar to those of WT cells (Fig. 6A). The kinase activity associated with G2-phase markers cyclin B and Cdk1 was markedly increased in p53−/− MEFs about 24 h after serum stimulation. Similarly, Cdk1/2 activity was determined by using suc1 beads, which bind to Cdk1 and Cdk2 and are markers for both S and G2 phase (7). Cdk1/2 activity was beginning to increase at 20 h and peaked at 24 h. In contrast, we were unable to detect any kinase activity of any of the proteins checked in Cdk2−/− Cdk4−/− p53−/− MEFs during the first 24 h (Fig. 6B, right panels). However, cyclin A2, cyclin B1, Cdk1, and suc1 immunoprecipitates had detectable kinase in triple-knockout MEFs after 42 h, when cells start loosing their synchrony (data not shown, see also Fig. 7B). These results are consistent with BrdU incorporation assays and confirm the fact that Cdk2−/− Cdk4−/− p53−/−MEFs entered S phase at a much lower rate than p53−/− MEFs.
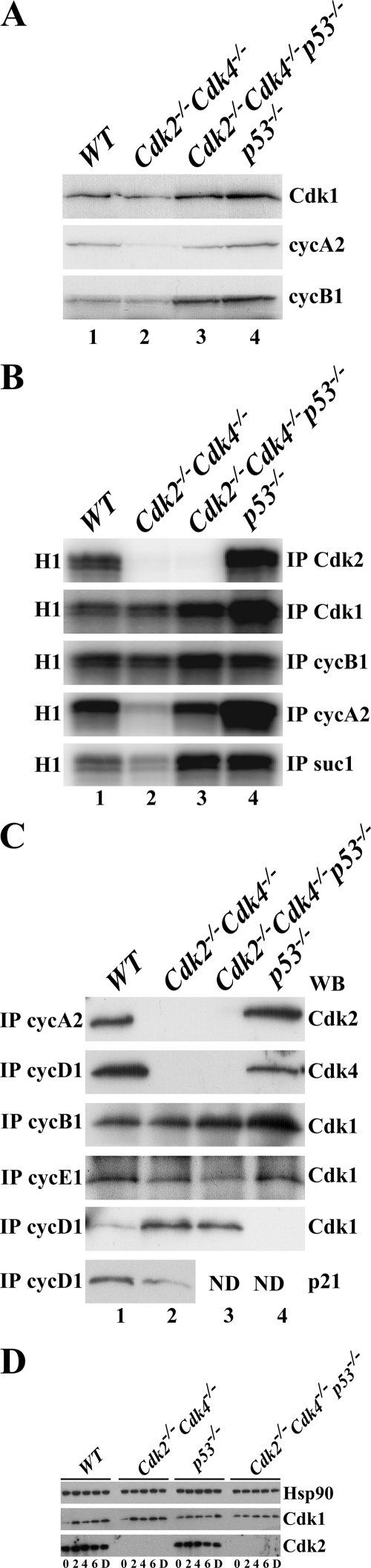
FIG. 7.
Cdk1 binds to all cyclins. (A) Western blot analysis. One hundred micrograms of protein extract from WT, Cdk2−/− Cdk4−/−, Cdk2−/− Cdk4−/− p53−/−, and p53−/− MEFs was separated on a 12.5% Tris-HCl gel, and proteins were transferred to a polyvinylidene difluoride membrane. Antibodies against Cdk1, cyclin A2 (cycA2), and cyclin B1 (cycB1) were used to probe the membranes. (B) Kinase assays. Two hundred fifty micrograms of protein lysate from WT, Cdk2−/− Cdk4−/−, Cdk2−/− Cdk4−/− p53−/−, and p53−/− MEFs was used for immunoprecipitation (IP) using protein A-agarose beads coupled to Cdk2, Cdk1, cyclin B1, and cyclin A2 antibodies or suc1 beads. The immunoprecipitated complexes were used to perform an in vitro kinase assay using histone H1 as a substrate and radiolabeled ATP. (C) Immunoprecipitation followed by Western blot (WB) analysis. Protein A-agarose beads coupled to cyclin A2, cyclin D1, cyclin B1, and cyclin E antibodies were used to immunoprecipitate 250 μg protein lysate. The immunoprecipitated proteins were probed with anti- bodies against Cdk2, Cdk4, Cdk1, and p21. ND, not determined. (D) Cycloheximide treatment. Cdk2−/−Cdk4−/− and Cdk2−/−Cdk4−/−p53−/− MEFs at passage 4 were treated with cycloheximide for 3 or 6 h. Western blot analysis was performed on these cell extracts using antibodies specific for HSP90, Cdk1, and Cdk2.
Cdk2−/− Cdk4−/− p53−/− MEFs display increased Cdk1 activity.
Understanding the molecular mechanism underlying the immortalization and transformation potential of Cdk2−/− Cdk4−/−p53−/+ MEFs is essential for exploitation of this pathway for the potential development of drugs for cancer treatment. Western blots were performed to reveal the expression of Cdk1, cyclin A2, and cyclin B1 (Fig. 7A). While the protein expression level of cyclin A2 in MEFs lacking p53 was similar to that in WT cells, it appeared increased compared to the level in Cdk2−/− Cdk4−/− MEFs. In contrast, Cdk1 and cyclin B1 levels were markedly higher in both Cdk2−/− Cdk4−/− p53−/− and p53−/− MEFs than those in WT or Cdk2−/− Cdk4−/− MEFs (Fig. 7A, compare lanes 3 and 4 to lane 1). Therefore, the loss of p53 results in increased expression of Cdk1 and cyclin B1.
In order to determine if the increased expression of Cdk1 and/or cyclin B1 translates into increased activity, these proteins were immunoprecipitated and in vitro kinase assays were performed (Fig. 7B). The loss of p53 led to increased Cdk2 activity in p53−/− MEFs, whereas both Cdk2−/− Cdk4−/− and Cdk2−/− Cdk4−/− p53−/− MEFs displayed no Cdk2 activity, as expected. In addition, Cdk1 activity was elevated in Cdk2−/− Cdk4−/− p53−/− MEFs and cyclin A2-associated activity was restored to a level similar to that of WT cells in Cdk2−/− Cdk4−/− p53−/− MEFs. Both cyclin B1 and suc1 immunoprecipitates showed elevated activity in the absence of p53. These results suggest that the loss of p53 increases the activity of cyclin A2, cyclin B1, and Cdk1 complexes regardless of the presence of Cdk2 and Cdk4 (Fig. 7B, compare lane 2 to lane 3). The increase in Cdk1 activity is likely to be related to the observed growth behavior of Cdk2−/− Cdk4−/− p53−/− MEFs.
Cdk1 binds to all cyclins.
Immunoprecipitations were performed to determine the composition of Cdk/cyclin complexes in MEFs of all four genotypes. The results indicated that cyclin A2 bound to Cdk2 and cyclin D1 to Cdk4 in both WT and p53−/− MEFs and not in Cdk2−/− Cdk4−/− and Cdk2−/− Cdk4−/− p53−/− MEFs, as expected (Fig. 7C, top two panels). Cdk1 bound to cyclin B1 and cyclin E1 was detected in all genotypes (as has been shown previously [2]); however, there was increased binding of cyclin B1 in p53−/− MEFs, most likely due to the abundant presence of cyclin B1 (Fig. 7C, third panel from top, lane 4). Detection of binding of Cdk1 to cyclin D1 in both Cdk2−/− Cdk4−/− and Cdk2−/− Cdk4−/− p53−/− MEFs (Fig. 7C, second panel from bottom) and binding of p21 to cyclin D1 in both WT and Cdk2−/− Cdk4−/− MEFs was unexpected (Fig. 7C, bottom panel). These findings indicate that Cdk1 can form complexes with cyclin D1 besides cyclins E, A, and B. However, the activity and functional relevance of Cdk1/cyclin D1 complexes in vivo will need to be determined.
Growth properties of Cdk2−/− Cdk4−/− MEFs by p53 loss is p21 independent.
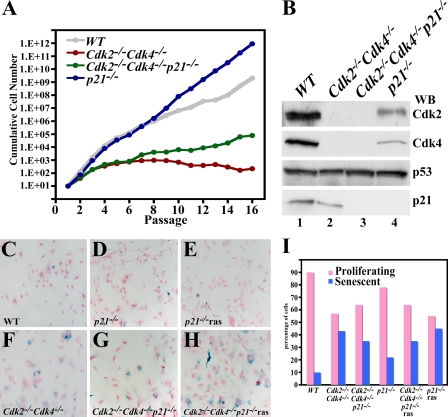
p21 is a p53-inducible gene (15), its expression levels are very low in the absence of p53, and p21 can bind directly to Cdk1 and inhibit its activity in the absence of Cdk2 (35). Loss of p21 binding and inhibition of Cdk1 could account for increased Cdk1 activity in the absence of p53. To test this hypothesis, Cdk2−/− Cdk4−/− p21−/− triple-mutant mice were generated. Loss of p21 did not affect the embryonic lethality observed in Cdk2−/− Cdk4−/− embryos (data not shown). Therefore, Cdk2−/− Cdk4−/− p21−/− MEFs were generated and subsequent 3T3 analysis indicated that both WT and p21−/− MEFs grew at a similar rate (Fig. 8A). While Cdk2−/− Cdk4−/− MEFs continued to grow slowly until passage 16, Cdk2−/− Cdk4−/− p21−/− MEFs grew slightly better than Cdk2−/− Cdk4−/− MEFs but not as efficiently as WT, p21−/−, or p53−/− MEFs. Western blot analysis confirmed on the protein level the genotypes of all the cell types used (Fig. 8B).
FIG. 8.
Loss of p21 does not immortalize Cdk2−/− Cdk4−/− MEFs. (A) 3T3 assay of WT, Cdk2−/− Cdk4−/−, Cdk2−/− Cdk4−/− p21−/−, and p21−/− MEFs. The y axis depicts the cumulative cell number for each passage. (B) Western blot (WB) analysis. One hundred micrograms of protein lysate from passage 3 WT (lanes 1), Cdk2−/− Cdk4−/− (lanes 2), Cdk2−/− Cdk4−/− p21−/− (lanes 3), and p21−/− (lanes 4) MEFs were separated on a 12.5% Tris-HCl gel. Proteins were transferred to a polyvinylidene difluoride membrane and probed with antibodies against Cdk2, Cdk4, p53, and p21. (C to H) Representative pictures depicting senescent cells in WT, Cdk2−/− Cdk4−/−, Cdk2−/− Cdk4−/− p21−/−, p21−/−, Cdk2−/− Cdk4−/− p21−/− (expressing Ras), and p21−/− (expressing Ras) MEFs after β-galactosidase and neutral red staining. (I) Histogram generated after random counting of at least 200 cells for each genotype. The senescent cells are indicated with a blue bar, and nonsenescent cells are indicated with pink bars.
Determination of the percentage of senescent cells by β-galactosidase staining indicated that both WT and p21−/− MEFs displayed very few senescent cells compared to the substantial percentage of senescent cells in both Cdk2−/− Cdk4−/− and Cdk2−/− Cdk4−/− p21−/− MEFs (Fig. 8C, D, F, G, and I). Expression of constitutively active Ras did not transform these cells, as evidenced by their entry into senescence (Fig. 8E and H) and the inability to form colonies (data not shown). Our results indicate that p21 is unlikely be the sole target through which p53 controls the cell cycle, as loss of p21, unlike p53, does not lead to the rescue of senescence and growth properties in Cdk2−/− Cdk4−/− MEFs. Therefore, we envision a novel mechanism by which p53 controls the G1 phase of the cell cycle in a way that requires neither its main transcriptional target, p21, nor the two G1 cyclin-dependent kinases, Cdk2 and Cdk4.
DISCUSSION
The loss of p53 function results in tumors in mice and also in humans. However, in mice it takes 6 to 8 months for these tumors to form. Induction of tumors is a long process that might include downstream effectors in addition to the primary changes. In the present study, we aimed to investigate the contribution of deregulating the G1/S phase transition in p53-null tumors. To address this, we generated a series of mouse mutants, including Cdk2−/− p53−/−, Cdk4−/− p53−/−, Cdk2−/− Cdk4−/− p53−/−, and Cdk2−/− Cdk4−/− p21−/− mice. Of these, only Cdk2−/− p53−/− mice were viable and could be followed for up to approximately 8 months to study tumor formation. The onset and spectrum of the tumors observed in Cdk2−/− p53−/− mice were comparable to those of p53−/− mice, which implies that the loss of Cdk2 by itself is not a major determinant of tumorigenesis. Since other mutant mice we generated were not viable, we decided to investigate the oncogenic potential of Cdk4−/− p53−/−, Cdk2−/− Cdk4−/− p53−/−, and Cdk2−/− Cdk4−/− p21−/− MEFs and the induction of tumors when injected in nude mice. Ras transformation of MEFs constitutes the first step in this process, and we proved that Cdk2−/− Cdk4−/− p21−/− MEFs were unable to undergo this transformation. Ras-transformed Cdk2−/− p53−/−, Cdk4−/− p53−/−, and Cdk2−/− Cdk4−/− p53−/− MEFs did form tumors in nude mice, which indicated that the loss of Cdk2, Cdk4, or both together was not able to inhibit tumor growth in this particular model. To gain more insight into this somewhat surprising observation, we investigated the underlying molecular mechanisms. We found that the loss of p53 affects the cell cycle in a profound way. For example, the loss of p53 increased the protein levels of Cdk1, cyclin B, and cyclin A2, which resulted in a substantial increase in the activity of several Cdk/cyclin complexes. Additionally, the loss of p53 prevented premature entry into senescence of Cdk2−/− Cdk4−/− MEFs but had no effect on their delayed S phase entry. Presumably, the combination of these changes resulted in the immortalization of all the MEFs analyzed when combined with p53−/−.
The effect of p53 on cell cycle regulation can be caused by several potential mechanisms, including (i) induction of p21Cip1/Waf1; (ii) direct transcriptional repression of Cdk1, cyclin B1, and cyclin A2; (iii) inhibition of protein degradation by induction of Emi1; (iv) modulation of the Rb/E2F pathway; or (v) indirect effects.
The induction of p21Cip1/Waf1 by p53 and the subsequent inhibition of Cdk2 and possibly other Cdks is well known (15). Our experiments with Cdk2−/− Cdk4−/− p21−/− MEFs (Fig. 8) suggested that p21 is unlikely to be the only determinant of the effects of loss of p53 that we have observed. Nevertheless, the likelihood of loss or low levels of p21 (due to the loss of p53) contributing to the increased activity of Cdk/cyclin complexes cannot be ruled out.
The increased expression of Cdk1, cyclin B, and cyclin A2 in the absence of p53 might suggest that p53 as a transcription factor represses the expression of these genes directly. In fact, there are several reports which have attempted to establish a direct connection between Cdk1 and p53. p53-mediated transcriptional repression of Cdk1 has been shown to occur through the CCAAT-binding NF-Y transcription factor (21, 48). It is also believed that p53 can bind directly to the Cdk1 promoter and inhibit its activity (1). We therefore measured the increase of Cdk1 mRNA by reverse transcription-PCR in the absence of p53 but did not detect a notable difference (data not shown). When p53−/− MEFs were treated with cycloheximide to inhibit translation, we still detected increased levels of Cdk1 (Fig. 7D). Based on our results, we conclude that it is unlikely that p53 represses Cdk1 and cyclin transcription directly, which opens the possibility of secondary effects. Protein degradation could also be affected since we have observed an increase in Emi1 levels (Fig. 2C), which could lead to inhibition of the ubiquitin ligase APC (anaphase-promoting complex/cyclosome) (30, 33) and therefore increases in cyclin A2 or cyclin B1 protein levels.
It is known that Cdk1, cyclin A2, and probably cyclin B are targets of E2F transcription factors. Any increase in Cdk activity would lead to hyperphosphorylation of Rb, which then would dissociate from E2F, subsequently resulting in elevated transcription of E2F targets like Cdk1, etc. This in effect would increase the Cdk1 protein levels and thus its activity. Since this is a feedback loop, any minor positive change can result in upward spiraling. Because of the nature of a feedback loop, it is almost impossible to pinpoint a particular change that results in increased Rb phosphorylation. In other words, the loss of p53 might initially induce only a small increase in Cdk1 levels but the signal can be amplified in the feedback loop, resulting in even higher levels of Cdk1. One example where this feedback loop was disrupted was in Cdk2−/− Cdk4−/− MEFs (5). The loss of Cdk2 and Cdk4 leads to hypophosphorylation of Rb and therefore to a repression of Cdk1 transcription. As a result, Cdk2−/− Cdk4−/− MEFs display a decreased proliferation rate, a delayed entry into S phase, premature entry into senescence, and impaired spontaneous immortalization. Interestingly, our results indicate that loss of p53 rescues all Cdk2−/− Cdk4−/− MEF phenotypes with the exception of S phase entry. The most likely explanation for this rescue is the restoration of Cdk1 expression indicating its central role in cell cycle control. Recently, we have reported that the loss of Rb rescues the known defects of Cdk2−/− Cdk4−/− MEFs (27). Like the loss of p53, the loss of Rb restores Cdk1 kinase activity and prevents premature entry into senescence. The major difference is that the loss of Rb rescues the S phase entry defect, whereas p53 does not (Fig. 6). This would indicate that Rb is more potent in promoting S phase compared to p53, albeit both increase Cdk1 activity. Another major difference is that Rb affects the expression of Cdk1 directly since Cdk1 is an E2F target gene, whereas in the case of p53 several possibilities can be envisioned.
Recently, it has been reported that p53 represses the expression of c-Myc through the induction of the microRNA miR-145 (26, 34). Since the expression of cyclin E, cyclin A, cyclin B, Cdk1, and many other cell cycle regulators is controlled by c-Myc, it is possible that our observations are related to control of c-Myc. Future studies will have to focus on this aspect of p53 control.
We have demonstrated that MEFs lacking both Cdk2 and Cdk4 still retain their transformation potential and become tumorigenic in the absence of p53. Senescence is thought to function as a cancer-preventing mechanism, and it can be induced to treat cancer (for a review, see reference 11). In the absence of p53, examined cells did not undergo senescence. Cdk1 and Cdk6, which so far have not been thought to be as essential for S phase entry as Cdk2, still retain the potential to drive the S phase entry powerful enough to make cells tumorigenic. This is a significant observation in terms of cancer treatment. Our observation, indeed, questions the rationale of using cell cycle inhibitors against both Cdk2 and Cdk4 in the treatment of cancers caused by p53 mutations, although it might still be an effective strategy in cancers harboring WT p53. The fact that the cells used for the tumor assay had to be Ras transformed and the probability that Ras might cooperate in an unknown way with the loss of Cdk2 and Cdk4 deserves attention. Loss of Cdk4 is believed to prevent tumorigenicity in cells lacking Arf or p53 (50). The difference in behavior of Cdk4−/− p53−/− MEFs in our experiments might just be due to the difference of p53-knockout cells in our analysis compared to the use of a dominant-negative construct to inhibit p53 activity in the previous study (50). The fact that loss of Cdk4 activity prevents immortalization of Arf mutant cells but not p53 mutant cells indicates that Arf and p53 participate in independent pathways by which they control the G1 phase. In fact, p53-independent functions of Arf have been reported in previous studies (44; for a review, see reference 37). Cdk4 kinase activity has also been shown to be essential for Erb-2-driven breast cancer (47). The difficulty associated with the generation of large numbers of Cdk4−/− p53−/− mice and their inability to survive beyond a few months prevented us from investigating tumor formation in vivo. In vitro, Ras-transformed Cdk4−/−p53−/− MEFs were able to form tumors in nude mice.
Although it is already known that the p53 pathway is connected to the cell cycle, we conclude that this connection is closer and more complex than previously thought and extends beyond the inhibitor p21 but encompasses the expression and activity of Cdk1. In terms of p53-null tumors, our results suggest that these cells can proliferate independently of Cdk2 and Cdk4. Our findings improve the understanding of p53-mediated cell cycle control by inspiring us to shift our attention to Cdk1 and consequently pushing Cdk1 chemical inhibitors closer to the front stage. Nevertheless, the relevance of Cdk2/Cdk4 inhibitors for tumors with WT p53 cannot be ruled out from our studies.
Acknowledgments
We thank Matt McCollum and Angie Smith for animal care and Tyler Jacks for mice. We are indebted to Hiroaki Kiyokawa, Xianghong Zou, Stefano Campaner, and Bruno Amati for discussion of unpublished results. We are thankful to Kasim Diril, Shuhui Lim, Yili Yang, Shuhei Kotoshiba, Xinde Zheng, and Weimin Li for comments on the manuscript and all the members of the Kaldis lab for support. We are also thankful to Miriam Anver (SAIC) for help with analyzing the tumors and Peter Jackson (Genentech) for the Emi1 antibodies.
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Published ahead of print on 23 March 2009.
REFERENCES
- 1.Ababneh, M., C. Gotz, and M. Montenarh. 2001. Downregulation of the Cdc2/cyclin B protein kinase activity by binding of p53 to p34Cdc2. Biochem. Biophys. Res. Commun. 283507-512. [DOI] [PubMed] [Google Scholar]
- 2.Aleem, E., H. Kiyokawa, and P. Kaldis. 2005. Cdc2-cyclin E complexes regulate the G1/S phase transition. Nat. Cell Biol. 7831-836. [DOI] [PubMed] [Google Scholar]
- 3.An, H. X., M. W. Beckmann, G. Reifenberger, H. G. Bender, and D. Niederacher. 1999. Gene amplification and overexpression of CDK4 in sporadic breast carcinomas is associated with high tumor cell proliferation. Am. J. Pathol. 154113-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berthet, C., E. Aleem, V. Coppola, L. Tessarollo, and P. Kaldis. 2003. Cdk2 knockout mice are viable. Curr. Biol. 131775-1785. [DOI] [PubMed] [Google Scholar]
- 5.Berthet, C., K. D. Klarmann, M. B. Hilton, H. C. Suh, J. R. Keller, H. Kiyokawa, and P. Kaldis. 2006. Combined loss of Cdk2 and Cdk4 results in embryonic lethality and Rb hypophosphorylation. Dev. Cell 10563-573. [DOI] [PubMed] [Google Scholar]
- 6.Bianchi, A. B., S. M. Fischer, A. I. Robles, E. M. Rinchik, and C. J. Conti. 1993. Overexpression of cyclin D1 in mouse skin carcinogenesis. Oncogene 81127-1133. [PubMed] [Google Scholar]
- 7.Brizuela, L., G. Draetta, and D. Beach. 1987. p13suc1 acts in the fisson yeast cell division cycle as a component of the p34cdc2 protein kinase. EMBO J. 63507-3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brugarolas, J., C. Chandrasekaran, J. I. Gordon, D. Beach, T. Jacks, and G. J. Hannon. 1995. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 377552-557. [DOI] [PubMed] [Google Scholar]
- 9.Cheng, M., P. Olivier, J. A. Diehl, M. Fero, M. F. Roussel, J. M. Roberts, and C. J. Sherr. 1999. The p21Cip1 and p27Kip1 CDK ‘inhibitors’ are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 181571-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collado, M., M. A. Blasco, and M. Serrano. 2007. Cellular senescence in cancer and aging. Cell 130223-233. [DOI] [PubMed] [Google Scholar]
- 11.Dimri, G. P. 2005. What has senescence got to do with cancer? Cancer Cell 7505-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimri, G. P., X. Lee, G. Basile, M. Acosta, G. Scott, C. Roskelley, E. E. Medrano, M. Linskens, I. Rubelj, O. Pereira-Smith, M. Peacocke, and J. Campisi. 1995. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 929363-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donehower, L. A., M. Harvey, B. L. Slagle, M. J. McArthur, C. A. Montgomery, Jr., J. S. Butel, and A. Bradley. 1992. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature 356215-221. [DOI] [PubMed] [Google Scholar]
- 14.Doumont, G., A. Martoriati, C. Beekman, S. Bogaerts, P. J. Mee, F. Bureau, E. Colombo, M. Alcalay, E. Bellefroid, F. Marchesi, E. Scanziani, P. G. Pelicci, and J. C. Marine. 2005. G1 checkpoint failure and increased tumor susceptibility in mice lacking the novel p53 target Ptprv. EMBO J. 243093-3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Deiry, W. S., T. Tokino, V. E. Velculescu, D. B. Levy, R. Parsons, J. M. Trent, D. Lin, E. Mercer, K. W. Kinzler, and B. Vogelstein. 1993. WAF1, a potent mediator of p53 tumor suppression. Cell 75817-825. [DOI] [PubMed] [Google Scholar]
- 16.Ewen, M. E., C. J. Oliver, H. K. Sluss, S. J. Miller, and D. S. Peeper. 1995. p53-dependent repression of CDK4 translation in TGF-beta-induced G1 cell-cycle arrest. Genes Dev. 9204-217. [DOI] [PubMed] [Google Scholar]
- 17.Hartwell, L. H., and M. B. Kastan. 1994. Cell cycle control and cancer. Science 2661821-1828. [DOI] [PubMed] [Google Scholar]
- 18.He, G., Z. H. Siddik, Z. Huang, R. Wang, J. Koomen, R. Kobayashi, A. R. Khokhar, and J. Kuang. 2005. Induction of p21 by p53 following DNA damage inhibits both Cdk4 and Cdk2 activities. Oncogene 242929-2943. [DOI] [PubMed] [Google Scholar]
- 19.Hinds, P., C. Finlay, and A. J. Levine. 1989. Mutation is required to activate the p53 gene for cooperation with the ras oncogene and transformation. J. Virol. 63739-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iida, H., M. Towatari, M. Tanimoto, Y. Morishita, Y. Kodera, and H. Saito. 1997. Overexpression of cyclin E in acute myelogenous leukemia. Blood 903707-3713. [PubMed] [Google Scholar]
- 21.Imbriano, C., A. Gurtner, F. Cocchiarella, S. Di Agostino, V. Basile, M. Gostissa, M. Dobbelstein, G. Del Sal, G. Piaggio, and R. Mantovani. 2005. Direct p53 transcriptional repression: in vivo analysis of CCAAT-containing G2/M promoters. Mol. Cell. Biol. 253737-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacks, T., L. Remington, B. O. Williams, E. M. Schmitt, S. Halachmi, R. T. Bronson, and R. A. Weinberg. 1994. Tumor spectrum analysis in p53-mutant mice. Curr. Biol. 41-7. [DOI] [PubMed] [Google Scholar]
- 23.Kim, W. Y., and N. E. Sharpless. 2006. The regulation of INK4/ARF in cancer and aging. Cell 127265-275. [DOI] [PubMed] [Google Scholar]
- 24.Kohn, K. W., J. Jackman, and P. M. O'Connor. 1994. Cell cycle control and cancer chemotherapy. J. Cell. Biochem. 54440-452. [DOI] [PubMed] [Google Scholar]
- 25.Lantsov, D., S. Meirmanov, M. Nakashima, H. Kondo, V. Saenko, Y. Naruke, H. Namba, M. Ito, A. Abrosimov, E. Lushnikov, I. Sekine, and S. Yamashita. 2005. Cyclin D1 overexpression in thyroid papillary microcarcinoma: its association with tumour size and aberrant beta-catenin expression. Histopathology 47248-256. [DOI] [PubMed] [Google Scholar]
- 26.Levy, N., E. Yonish-Rouach, M. Oren, and A. Kimchi. 1993. Complementation by wild-type p53 of interleukin-6 effects on M1 cells: induction of cell cycle exit and cooperativity with c-myc suppression. Mol. Cell. Biol. 137942-7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, W., S. Kotoshiba, C. Berthet, M. B. Hilton, and P. Kaldis. 2009. Rb/Cdk2/Cdk4 triple mutant mice elicit an alternative mechanism for regulation of the G1/S transition. Proc. Natl. Acad. Sci. USA 106486-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, Y., C. W. Jenkins, M. A. Nichols, and Y. Xiong. 1994. Cell cycle expression and p53 regulation of the cyclin-dependent kinase inhibitor p21. Oncogene 92261-2268. [PubMed] [Google Scholar]
- 29.Marone, M., G. Scambia, C. Giannitelli, G. Ferrandina, V. Masciullo, A. Bellacosa, P. Benedetti-Panici, and S. Mancuso. 1998. Analysis of cyclin E and CDK2 in ovarian cancer: gene amplification and RNA overexpression. Int. J. Cancer 7534-39. [DOI] [PubMed] [Google Scholar]
- 30.Miller, J. J., M. K. Summers, D. V. Hansen, M. V. Nachury, N. L. Lehman, A. Loktev, and P. K. Jackson. 2006. Emi1 stably binds and inhibits the anaphase-promoting complex/cyclosome as a pseudosubstrate inhibitor. Genes Dev. 202410-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ortega, S., I. Prieto, J. Odajima, A. Martin, P. Dubus, R. Sotillo, J. L. Barbero, M. Malumbres, and M. Barbacid. 2003. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat. Genet. 3525-31. [DOI] [PubMed] [Google Scholar]
- 32.Rane, S. G., P. Dubus, R. V. Mettus, E. J. Galbreath, G. Boden, E. P. Reddy, and M. Barbacid. 1999. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in β-islet cell hyperplasia. Nat. Genet. 2244-52. [DOI] [PubMed] [Google Scholar]
- 33.Reimann, J. D., E. Freed, J. Y. Hsu, E. R. Kramer, J. M. Peters, and P. K. Jackson. 2001. Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell 105645-655. [DOI] [PubMed] [Google Scholar]
- 34.Sachdeva, M., S. Zhu, F. Wu, H. Wu, V. Walia, S. Kumar, R. Elble, K. Watabe, and Y. Y. Mo. 2009. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc. Natl. Acad. Sci. USA 1063207-3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Satyanarayana, A., M. B. Hilton, and P. Kaldis. 2008. p21 inhibits Cdk1 in the absence of Cdk2 to maintain the G1/S phase DNA damage checkpoint. Mol. Biol. Cell 1965-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scuderi, R., K. A. Palucka, K. Pokrovskaja, M. Bjorkholm, K. G. Wiman, and P. Pisa. 1996. Cyclin E overexpression in relapsed adult acute lymphoblastic leukemias of B-cell lineage. Blood 873360-3367. [PubMed] [Google Scholar]
- 37.Sherr, C. J., and R. A. DePinho. 2000. Cellular senescence: mitotic clock or culture shock? Cell 102407-410. [DOI] [PubMed] [Google Scholar]
- 38.Sherr, C. J., and J. M. Roberts. 1999. Cdk inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 131501-1512. [DOI] [PubMed] [Google Scholar]
- 39.Taylor, W. R., S. E. Egan, M. Mowat, A. H. Greenberg, and J. A. Wright. 1992. Evidence for synergistic interactions between ras, myc and a mutant form of p53 in cellular transformation and tumor dissemination. Oncogene 71383-1390. [PubMed] [Google Scholar]
- 40.Todaro, G. J., and H. Green. 1963. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J. Cell Biol. 17299-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Truett, G. E., P. Heeger, R. L. Mynatt, A. A. Truett, J. A. Walker, and M. L. Warman. 2000. Preparation of PCR-quality mouse genomic DNA with hot sodium hydroxide and tris (HotSHOT). BioTechniques 2952-54. [DOI] [PubMed] [Google Scholar]
- 42.Tsutsui, T., B. Hesabi, D. S. Moons, P. P. Pandolfi, K. S. Hansel, A. Koff, and H. Kiyokawa. 1999. Targeted disruption of CDK4 delays cell cycle entry with enhanced p27Kip1 activity. Mol. Cell. Biol. 197011-7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waldman, T., K. W. Kinzler, and B. Vogelstein. 1995. p21 is necessary for the p53-mediated G1 arrest in human cancer cells. Cancer Res. 555187-5190. [PubMed] [Google Scholar]
- 44.Weber, J. D., J. R. Jeffers, J. E. Rehg, D. H. Randle, G. Lozano, M. F. Roussel, C. J. Sherr, and G. P. Zambetti. 2000. p53-independent functions of the p19ARF tumor suppressor. Genes Dev. 142358-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wunder, J. S., K. Eppert, S. R. Burrow, N. Gokgoz, R. S. Bell, and I. L. Andrulis. 1999. Co-amplification and overexpression of CDK4, SAS and MDM2 occurs frequently in human parosteal osteosarcomas. Oncogene 18783-788. [DOI] [PubMed] [Google Scholar]
- 46.Yu, Q., Y. Geng, and P. Sicinski. 2001. Specific protection against breast cancers by cyclin D1 ablation. Nature 4111017-1021. [DOI] [PubMed] [Google Scholar]
- 47.Yu, Q., E. Sicinska, Y. Geng, M. Ahnstrom, A. Zagozdzon, Y. Kong, H. Gardner, H. Kiyokawa, L. N. Harris, O. Stal, and P. Sicinski. 2006. Requirement for CDK4 kinase function in breast cancer. Cancer Cell 923-32. [DOI] [PubMed] [Google Scholar]
- 48.Yun, J., H. D. Chae, H. E. Choy, J. Chung, H. S. Yoo, M. H. Han, and D. Y. Shin. 1999. p53 negatively regulates cdc2 transcription via the CCAAT-binding NF-Y transcription factor. J. Biol. Chem. 27429677-29682. [DOI] [PubMed] [Google Scholar]
- 49.Zambetti, G. P., D. Olson, M. Labow, and A. J. Levine. 1992. A mutant p53 protein is required for maintenance of the transformed phenotype in cells transformed with p53 plus ras cDNAs. Proc. Natl. Acad. Sci. USA 893952-3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zou, X., D. Ray, A. Aziyu, K. Christov, A. D. Boiko, A. V. Gudkov, and H. Kiyokawa. 2002. Cdk4 disruption renders primary mouse cells resistant to oncogenic transformation, leading to Arf/p53-independent senescence. Genes Dev. 162923-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]