Abstract
We demonstrate the presence of nonstructural protein 1 (NS1)-specific antibodies in a significant proportion of convalescent-phase human serum samples obtained from a cohort in an area where Japanese encephalitis virus (JEV) is endemic. Sera containing antibodies to NS1 but not those with antibodies to other JEV proteins, such as envelope, brought about complement-mediated lysis of JEV-infected BHK-21 cells. Target cells infected with a recombinant poxvirus expressing JEV NS1 on the cell surface confirmed the NS1 specificity of cytolytic antibodies. Mouse anti-NS1 cytolytic sera caused a complement-dependent reduction in virus output from infected human cells, demonstrating their important role in viral control. Antibodies elicited by JEV NS1 did not cross lyse West Nile virus- or dengue virus-infected cells despite immunoprecipitating the NS1 proteins of these related flaviviruses. Additionally, JEV NS1 failed to bind complement factor H, in contrast to NS1 of West Nile virus, suggesting that the NS1 proteins of different flaviviruses have distinctly different mechanisms for interacting with the host. Our results also point to an important role for JEV NS1-specific human immune responses in protection against JE and provide a strong case for inclusion of the NS1 protein in next generation of JEV vaccines.
The genus Flavivirus, many of whose more than 70 members are arthropod-borne human pathogens, such as dengue virus (DENV), West Nile virus (WNV), yellow fever virus (YFV), tick-borne encephalitis virus, and Japanese encephalitis virus (JEV), has assumed increasing public health importance in recent years. The single-strand, positive-sense RNA genomes of flaviviruses encode a single polyprotein, which is cotranslationally cleaved to produce three structural proteins (capsid [C], membrane [M], and envelope [E]) and seven nonstructural (NS) proteins (NS1, NS2a, NS2b, NS3, NS4a, NS4b, and NS5). NS1, a glycoprotein, is perhaps the most versatile among these, being involved both in vital processes such as viral RNA synthesis and in multiple interactions with the host, in ways that appear to benefit both pathogen and host. Following translocation into the lumen of the endoplasmic reticulum through a hydrophobic signal sequence that is encoded by the carboxyl terminus of E (17), NS1 undergoes glycosylation followed by rapid dimerization (44, 52). In DENV infection of cultured mammalian cells, extracellular NS1 was additionally detected as hexamers (19, 51). Despite the apparent absence of a canonical hydrophobic membrane anchor domain, the NS1s from JEV, Kunjin virus, DENV, and YFV have all been shown to be present on the surface of virus-infected cells (8, 23, 50). The mechanistic details of this membrane anchor still remain uncertain.
The ability of DENV NS1 to bind host complement (9, 49) pointed to a role for this protein in DENV pathogenesis. Serum NS1 levels in both DENV and WNV patients correlate directly with disease severity (1, 36). Promotion of immune complex formation (54), ability to elicit autoantibodies with reactivity to platelets and extracellular matrix (10), and damage inflicted on endothelial cells (34) are some of the mechanisms proposed to explain pathogenesis mediated by DENV NS1. Recent studies with WNV NS1 demonstrated its ability to bind human complement factor H, suggesting a role in reducing the host's ability to bring about complement-mediated control of early virus replication (11).
Critical differences between the functions of NS1s encoded by different pathogenic flaviviruses and their contributions to pathology are evident from the published reports, with DENV NS1 believed to be involved in complement activation and the consequent capillary leak syndrome of dengue hemorrhagic fever (6), while WNV NS1 appears relatively more benign and has more to do with modulation of the host innate immune response (11). We have not encountered reports of adverse impacts of JEV NS1 in infected individuals.
Paradoxically, several studies have pointed to a role for flavivirus NS1-specific immune responses in protection against flaviviruses. Passive immunization studies using monoclonal antibodies (MAbs) (24, 28, 29, 55) as well as immunization of mice using naked DNA constructs expressing NS1 (35, 40) revealed that antibodies directed to prM or E of DENV and NS1 of DENV and JEV are protective. Studies by different groups have shown that active immunization with purified NS1 or passive immunization with MAbs against YFV and DENV NS1 provides protection from lethal viral challenge in the absence of neutralizing antibodies (24, 45, 48). A panel of anti-WNV NS1 MAbs revealed multiple antibody-mediated mechanisms for protection, some mediated through complement and others via the Fc receptor (12). Those authors went on to show that anti-NS1 MAbs that facilitate phagocytosis and clearance of WNV-infected cells through Fc-γ receptors I and/or IV belonged to the IgG2a subclass and bound to cell surface-associated NS1 (13).
Earlier studies also pointed to the cytolytic potential of NS1 antibodies, a property that might contribute significantly to their protective ability. Passive immunization experiments using a panel of anti YFV NS1-specific MAbs showed a significant correlation between protection and in vitro complement-mediated cytolysis of YFV-infected mouse neuroblastoma cells (47). Additionally, immunization of mice with a DNA vaccine construct carrying JEV NS1 induced a strong antibody response exhibiting complement-mediated cytolysis of JEV-infected cells (35), but no neutralizing activity, and resulted in protection against subsequent challenge with virus. Cell-mediated immune responses directed to NS1 of JEV have also been reported to play a role in cytotoxic T-lymphocyte-mediated killing of JEV-infected murine target cells (41). Thus, NS1 appears to contribute to protection in the murine model by inducing both humoral and cell-mediated arms of the immune response.
It was therefore of interest to query whether NS1-specific antibodies in humans exposed to JEV exhibit cytolytic activity and to determine if these antibodies are capable of reducing virus production by infected cells. In this study we report for the first time the existence of detectable levels of anti-NS1 antibodies in a significant proportion of sera from humans infected with JEV and demonstrate their ability to induce antibody-dependent complement-mediated cytolysis of cells expressing JEV NS1 on the surface. These sera failed to cause lysis of cells infected with WNV or DENV, both of which cocirculate with JEV in the Indian subcontinent and have been reported in the region where we enrolled our volunteers, revealing stringent specificity and absence of flaviviral cross-reactivity for these cytolytic antibodies. Furthermore, we demonstrate the ability of NS1-specific antibodies elicited in mice to limit virus production in infected human SW-13 cell monolayers, which may explain, at least in part, the widely reported protective ability of flavivirus NS1. Significantly, we found no evidence for the ability of NS1 from JEV to bind human complement factor H, in contrast to the case for WNV NS1 (11). Taken together, these findings suggest that JEV NS1 may positively and significantly affect virus-specific protective immune responses.
MATERIALS AND METHODS
Cells and viruses.
CV-1, Vero, BHK-21, HeLa, HEK293T, SW-13, and human TK− cells were grown in minimal essential medium (MEM) supplemented with 10% fetal bovine serum (FBS). HepG2 cells were grown in Dulbecco's modified Eagle medium supplemented with 10% FBS. The Aedes albopictus cell line C6/36 was grown at 28°C in MEM supplemented with 10% tryptose phosphate broth and 5% FBS. The JEV P20778 strain (National Institute of Virology, Pune, India) was propagated in C6/36 cells. Virus titers were determined by plaque assay on Vero cells.
Serum samples.
Blood samples (2.0 ml) were obtained from volunteers at the district hospital, Vijayanagar Institute of Medical Sciences, Bellary, Karnataka, India, following informed consent, and serum was separated. Volunteers were convalescent JEV patients residing in areas where JE is endemic in the states of Karnataka and Andhra Pradesh, India, where they had resided for at least 6 years at a stretch (n = 73), and sera were obtained at between 6 and 22 months after discharge from hospitalization for encephalitis. Prior exposure to JEV was confirmed by IgM antibody capture enzyme-linked immunosorbent assay (ELISA) using virus-infected mouse brain antigens (7), and flaviviral infection due to WNV was ruled out by a plaque reduction neutralization test, where the reciprocal of the serum dilution giving 90% or greater reduction in plaque count for all the serum samples was higher for JEV (ranging from 20 to 80) than for WNV (ranging from <10 to 20). In addition, relative quantities of viral proteins immunoprecipitated from metabolically labeled lysates of cells infected with JEV, WNV, and DENV further confirmed JEV as the infecting flavivirus. Where possible, multiple bleeds from a single individual obtained several months apart were sampled. No data from acute-phase sera are reported in this study owing to difficulties related to bleeding patients in this state. All the procedures were conducted in conformity with the ethical guidelines of the Indian Council of Medical Research, and the study was approved by the institutional human ethics committee.
Immunization of mice.
BALB/c mice were inoculated intraperitoneally with 107 PFU of poxvirus twice at 6-week intervals. Mice were bled by intraocular puncture a week after the booster inoculation.
Construction of recombinant vaccinia virus carrying JEV NS1.
An NS1 gene with signal sequence (nucleotides 2388 to 3533 of the JEV genome) was generated by reverse transcription-PCR amplification of genomic RNA of JEV strain P20778 using the primers 5′-GGCCGGAATTCGCCGCCATGGGCGTCAACGCACGAGAC-3′ (forward) and 5′-CGCGCGTCGACTTACATATGAGCATCAACCTGTGATCTGAC-3′ (reverse) (start and stop codons are in bold, and restriction enzyme sites are underlined). The NS1 PCR product was digested with EcoRI and SalI and Klenow filled to blunt the ends. The EcoRI-SalI blunt NS1 fragment was cloned into SmaI-digested vaccinia virus insertion vector pGS20 under the transcriptional control of the vaccinia virus P7.5 early-late promoter. The JEV NS1 gene was flanked by the vaccinia virus thymidine kinase gene, which directed homologous recombination of the JEV NS1 sequence into the vaccinia virus genome following transfection of CV-1 cells infected with wild-type vaccinia virus WR strain at 1 h before transfection. The recombinant vaccinia virus containing the JEV NS1 gene was plaque purified four times on human TK− 293 cell monolayers in the presence of 25 μg/ml bromodeoxyuridine. The resulting virus was designated vNS1ss.
Immunoblot analysis.
Monolayers of the indicated cell lines were infected with JEV at a multiplicity of infection (MOI) of 5 for 48 h and with vNS1ss or control wild-type poxvirus (WR) at an MOI of 3 for 48 h. Lysates from approximately 1.0 × 105 cells were electrophoresed in each lane of a sodium dodecyl sulfate (SDS)-10% polyacrylamide gel, transferred to a nitrocellulose membrane, probed with rabbit antiserum specific for JEV NS1 (raised to bacterially expressed recombinant JEV NS1 [30]) followed by peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG), and developed with diaminobenzidene and H2O2. To detect NS1 dimers, samples were left unheated before electrophoresis. In some cases, samples were processed for electrophoresis without dithiothreitol (DTT).
Indirect immunofluorescence assay.
BHK-21 or SW-13 cells grown on coverslips were infected with vNS1ss or WR at an MOI of 5 for 12 h and with JEV at an MOI of 5 for 20 h following standardization to determine the optimal time point and MOI required to achieve infection of all the cells in the monolayer as well as maximum cell surface expression of NS1 with the least damage to cells from the virus. Longer periods of infection were found to result in progressively increasing cell death. The cells were fixed with 4% paraformaldehyde for 10 min at room temperature. The cells were then blocked with 1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) containing 1% normal goat serum and treated with human serum containing NS1 antibody (P3) at 4°C for 8 h. After being washed three times with PBS, the cells were further treated with fluorescein isothiocyanate (FITC)-conjugated goat-anti human IgG at room temperature for 1 h. Cells were examined using a Leica DMIRB inverted confocal laser scanning microscope with the pinhole set at 1.5. Expression of E protein was detected using MAb 1F10, specific to JEV E protein, which was generated and characterized in our laboratory.
Flow cytometry.
BHK-21 or SW-13 cells infected with JEV at an MOI of 5 for 8 h, 14 h, 20 h, and 24 h or with vNS1ss or WR at an MOI of 5 for 4 h, 8 h, 12 h, and 16 h were detached using 4 mM EDTA in PBS containing 10% FBS. After washing once with 1% BSA in PBS, cells were blocked with 1% BSA in PBS containing 1% normal goat serum for 30 min at 4°C. For analysis of cell surface expression of NS1, cells were treated with 20 μg/ml of MAb 9NS1 (a kind gift from Michael S. Diamond, Washington University School of Medicine, St. Louis, MO) raised against WNV NS1, which cross-reacts with JEV NS1, for 1 h at 4°C. Cells were washed twice with PBS, followed by treatment with FITC-conjugated goat anti-mouse IgG for 30 min at 4°C. The cells were washed twice with PBS before being fixed with 4% paraformaldehyde for 20 min at 4°C. Cell surface expression of NS1 was analyzed using a BD FACScan flow cytometer equipped with Cell Quest software. Propidium iodide was added to a final concentration of 200 μg/ml just before flow cytometry to aid in gating out dead cells. For analysis of total expression of NS1 and E in these cells at the indicated time points, the cells were fixed with 4% paraformaldehyde in PBS for 15 min and permeabilized with 0.1% saponin in PBS containing 1% BSA for 10 min before treatment with antibody. Expression of NS1 was detected using MAb 9NS1, while JEV E expression was detected using MAb1F10.
Radioimmunoprecipitation.
BHK-21 cells were infected with JEV, WNV, DENV-2, vNS1, or WR at an MOI of 5. At 14 h postinfection (p.i.) for JEV, WNV, and DENV-2 or 10 h p.i. for vNS1 and WR, the medium was removed and replaced with warm cysteine- and methionine-free RPMI 1640 medium (Invitrogen) containing 50 μCi of 35S protein labeling mix (BRIT, Mumbai) per ml and 1% FBS for 10 h at 37°C with 5% CO2. Cells were harvested, washed once with ice-cold PBS, and lysed with radioimmunoprecipitation assay lysis buffer (10 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.1% SDS, 1% Triton X-100, 1% deoxycholate) containing protease inhibitor cocktail. Lysate from 105 cells was used for each immunoprecipitation reaction with 10 μl human serum. Immunoprecipitated JEV and DENV proteins were eluted from the beads using Laemmli sample buffer (50 mM Tris [pH 6.8], 2% SDS, 10% glycerol, 0.1% bromophenol blue, and 100 mM DTT), separated by electrophoresis in 10% acrylamide NuPage bis-Tris gels (Invitrogen) using 3-(N-morpholino)propane sulfonic acid (MOPS) running buffer, transferred to a nitrocellulose membrane, and visualized in a Fuji BAS-1000 phosphorimager. This percentage of acrylamide and buffer system alone were found to successfully resolve an abundant nonspecific host cell protein from the NS1 protein of JEV and DENV. The identity of NS1 was further confirmed by immunoblotting of the same membrane with rabbit anti-NS1 antibody and peroxidase-conjugated goat anti-rabbit IgG. The immunoprecipitates of WNV lysates with human sera were eluted from the beads with Laemmli sample buffer without DTT (37) and electrophoresed in 10% polyacrylamide gels using the Laemmli buffer system, since the mobility of around 150 kDa for the immunoglobulin molecule ensured lack of interference from immunoglobulin heavy chains in the resolution of NS1 envelope and NS1′ from one another only under these conditions.
Antibody-dependent complement-mediated cytolytic assay.
The cytolytic potential of anti-NS1 antibody in human serum was measured by a previously described complement-mediated cytolytic assay with minor modifications (47). Preliminary experiments with a limited number of human sera were carried out with JEV-infected BHK-21 cells, while all subsequent analyses used the human adrenal gland-derived cell line SW-13, which was used previously to demonstrate inhibition of YFV proliferation by cytolytic mouse MAbs (46). Briefly, a confluent monolayer of cells grown in 96-well microtiter plates was infected with JEV or WNV at an MOI of 5 for 20 h, with DENV-2 at an MOI of 2 for 48 h, or with vNS1ss and WR at an MOI of 5 for 12 h. These time points were determined to be optimal based on analysis of NS1 expression on the surface of infected cells using flow cytometry and by measuring cytolysis of target cells at various time points p.i. Cells were labeled with Na251CrO4 for 90 min, followed by washing five times with MEM containing 2.5% FBS and 20 mM HEPES buffer, pH 7.4. Heat-inactivated sera (56°C for 30 min) obtained from JE patients or naïve controls diluted 1:10 in 50 μl of MEM containing 2.5% heat-inactivated FBS were added in triplicate to the monolayer and incubated at 37°C for 30 min, and then 50 μl of 1:10 diluted rabbit complement (for BHK-21 cells) or pooled human complement preserved serum (for SW-13 cells) was added and incubated for an additional 4 h. Released chromium in the harvested supernatant samples was counted in a Packard Cobra Auto-gamma (model D5002) counter. Wells with heat-inactivated rabbit or human complement and medium alone were used as negative controls. Percent specific lysis was calculated using the formula (experimental 51Cr release − spontaneous release)/(maximum release − spontaneous release) × 100, in which spontaneous release was the value obtained without antibody and complement and maximum lysis was the value obtained after lysis of the cell monolayer with 0.2% Triton X-100.
Effect of anti-NS1 antibody and complement on JEV proliferation.
To measure the effect of anti-NS1 antibody and complement on virus production in vitro, monolayers of SW-13 cells in six-well tissue culture plates were infected with JEV at an MOI of 0.05 for 1 h. Residual extracellular virus was then removed by washing five times with MEM containing 2.5% FBS. The medium was then replaced with MEM-10% FBS containing heat-inactivated pooled sera from vNS1ss-immunized mice (1:10 dilution) and active or heat-inactivated pooled human complement preserved serum (1:10 dilution) in triplicate wells. Pooled sera of mice immunized with wild-type vaccinia virus WR with active or heat-inactivated complement served as controls. Cells were incubated for 72 h at 37°C with 5% CO2. At 24-h intervals, culture supernatants were collected for determination of infectious virus titer by plaque assay on Vero cell monolayers.
Interaction of JEV NS1 with human complement factor H.
BHK-21 cells infected with JEV or WNV at an MOI of 5 or mock-infected BHK-21 cells were grown in serum-free OptiMEM (Invitrogen) for 48 h. Clarified culture supernatant was then ammonium sulfate fractionated (20 to 60% saturation) to enrich NS1. The fraction containing NS1 was identified, and the amount of NS1 was quantified by Western blot analysis of serial dilutions of the relevant fractions using polyclonal anti-JEV NS1 rabbit serum cross-reactive to WNV NS1 and purified Escherichia coli-expressed JEV NS1 (30) as a standard. Approximately 7 μg of NS1 was mixed with 70 μl of normal human serum in GVB-Mg2+-EGTA buffer (1.019 g/liter sodium diethyl barbiturate, 150 mM sodium chloride, 0.1% gelatin, 10 mM EGTA, and 10 mM MgCl2) overnight at 4°C. This mixture was then immunoprecipitated for 5 h at 4°C using protein G-Sepharose beads coated with MAb 9NS1 (a kind gift from Michael S. Diamond, Washington University School of Medicine, St. Louis, MO) raised against WNV NS1, which cross-reacts with JEV NS1. After washing four times with GVB-Mg2+-EGTA buffer, proteins in the immunoprecipitate were separated by electrophoresis in SDS-7.5% polyacrylamide gels, transferred to nitrocellulose membranes, and immunoblotted using 1:1,000 diluted sheep-anti human fH (Biodesign, Saco, ME) followed by 1:2,000 diluted horseradish peroxidase (HRP)-conjugated rabbit anti-sheep IgG (KPL, Gaithersburg, MD). Matching quantities of culture supernatant from mock-infected BHK-21 cells were used as a negative control. The presence of sizeable quantities of both JEV NS1 and WNV NS1 trapped on the beads by MAb 9NS1 was confirmed by immunoblotting the membrane with rabbit anti-NS1 antibody and HRP-conjugated goat anti-rabbit IgG. Blots were developed with the ECL Plus Western blotting detection system (GE Healthcare, United Kingdom).
ELISA.
Microtiter plates (Costar, Corning Inc., NY) were coated with NS1-enriched fractions obtained by 40 to 60% saturated ammonium sulfate precipitation of culture supernatant from vNS1-infected CV-1 cells grown in serum-free OptiMEM (Invitrogen). Wells coated with a matching quantity of culture supernatant from WR-infected CV-1 cells served as negative controls. After overnight incubation at 4°C, the wells were blocked with 3% BSA in PBS for 2 h at room temperature, and then 100 μl of 1:100 diluted serum sample was added and incubated for 30 min at 37°C. After the wells were washed four times with PBS-0.05% Tween 20, 100 μl of 1:5,000 diluted goat anti-human IgG-HRP (Jackson ImmunoResearch Laboratories, Inc.) was added and incubated for 30 min at room temperature. Wells were washed four times with PBS-0.05% Tween 20 and developed with 3,3′,5,5′-tetramethylbenzidine substrate. Each serum sample was tested in triplicate wells, and the P/N ratio for each sample was calculated as mean absorbance in vNS1 supernatant-coated wells (P, positive)/mean absorbance in WR supernatant-coated wells (N, negative). The cutoff value of the P/N ratio for distinguishing serum samples with NS1 antibodies was set at 2.0, since the highest value of the P/N ratio in control individuals (n = 12) plus 1.96 times the standard deviation of the mean was 1.64.
Statistical analysis.
Values provided for antibody-mediated cytolysis represent means ± standard deviations from three independent experiments. To determine the effect of anti-NS1 antibody and complement on virus production, virus titrations were performed in triplicate in two independent experiments, and the P values were calculated by a two-tailed paired t test using GraphPad Prism 4 software (Graph-Pad Software Inc, San Diego, CA). NS1 binding to cell surfaces was performed in triplicate and the data analyzed by analysis of variance as reported for DENV (4).
RESULTS
Expression of JEV NS1 in cells infected with recombinant vaccinia virus vNS1ss.
Synthesis of JEV NS1 protein in cells infected with recombinant vaccinia virus vNS1ss was initially studied in four different mammalian cell lines, namely, 293T, BHK-21, Vero, and CV-1. While both NS1 and NS1′ were detected in JEV-infected BHK-21 cell lysate as expected (Fig. 1A, lane 10), NS1 protein with a size similar to that of authentic NS1 was observed in all the cell lines tested, with the amount of NS1 synthesized in BHK-21 cells being comparatively higher (Fig. 1A, lane 4). Flavivirus NS1 forms SDS-stable dimers which are highly sensitive to heat treatment and are believed to represent one of the functional forms of NS1 (18, 51, 52). Dimerization of NS1 is a prerequisite for the export of NS1 to the plasma membrane and its secretion into the extracellular fluid (44, 46). As expected, we detected three heat-sensitive forms of NS1, which represent homo- and heterodimers of NS1 and NS1′ (Fig. 1B, lane 4), in JE-infected cell lysates and two dimeric forms in the culture supernatant (Fig. 1B, lane 8), in keeping with the reported inefficient secretion of NS1′ homodimer (35). In vNS1ss-infected cell lysate (Fig. 1C, lanes 3 and 4) and culture supernatant (Fig. 1C, lanes 7 and 8), we detected, as expected, a single heat-sensitive dimer of NS1. These results indicate that similar to JEV-infected cells, NS1 expressed by vNS1ss forms dimers intracellularly which are secreted as dimers into the culture supernatant, further authenticating the recombinant poxvirus-expressed JEV NS1. It should, however, be pointed out that the relative intensities of the monomeric and dimeric forms of NS1 and NS1′ may not necessarily reflect their relative abundances in the samples, since the rabbit serum used, raised to bacterially expressed denatured monomeric form of NS1, may not recognize all the forms with equal efficiency. A nonspecific band of around 68 kDa was observed in all the lanes.
FIG. 1.
Expression of JEV NS1 in vNS1ss-infected cell lines. (A) Cell lysates of WR (−)- or vNS1ss (+)-infected 293T (lanes 1 and 2), BHK-21 (lanes 3 and 4), Vero (lanes 5 and 6), and CV-1 (lanes 7 and 8) cells were immunoblotted using rabbit anti-NS1 antibody and HRP-conjugated goat anti-rabbit IgG. Lysates of uninfected (lane 9) and JEV-infected (lane 10) BHK-21 cells were used as negative and positive controls, respectively. The positions of NS1 and NS1′ are indicated by arrows on the right. The positions and sizes of protein standards are given on the left. (B and C) Immunoblot analysis of cell-associated and secreted forms of NS1 in infected cultured cells. Whole-cell lysates (L) or culture supernatants (S) of mock-infected BHK-21 cells or BHK-21 cells infected with JEV (B) or WR or vNS1ss (C) were immunoblotted using rabbit anti-NS1 antibody followed by HRP-conjugated goat anti-rabbit IgG. Samples in the presence of DTT were either heated (+) or left unheated (−) prior to electrophoresis. The positions of the protein standards are indicated on the left. Positions of NS1 monomer and dimer are indicated on the right.
NS1 is detected on the surface of vNS1ss-infected cells.
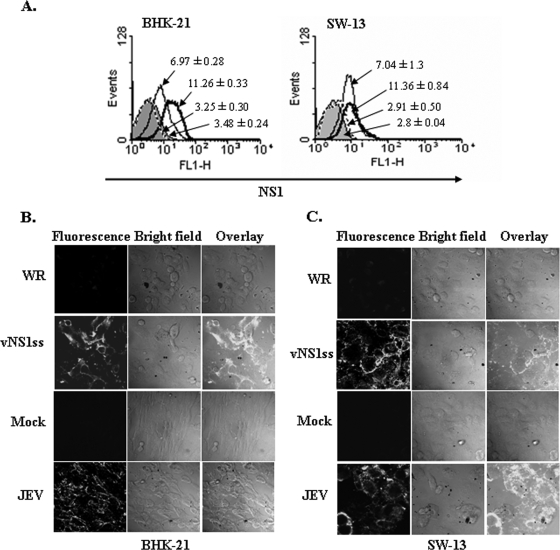
The JEV NS1 protein, like NS1 of several other flaviviruses, has been detected on the surface of cells infected with JEV or transfected with NS1 gene constructs (35, 44) despite lacking a stretch of hydrophobic amino acids that could serve as a potential membrane anchor domain (53). We confirmed expression of recombinant NS1 on the surface of vNS1ss-infected BHK-21 and SW-13 cells by flow cytometry using MAb 9NS1 followed by FITC-conjugated goat anti-mouse IgG. As seen in Fig. 2A, left panel, there was an approximately 3.5-fold increase in the mean fluorescence intensity in vNS1ss-infected BHK-21 cells compared to WR-infected cells at 12 h p.i. (11.26 ± 0.33 and 3.25 ± 0.30, respectively), indicating that NS1 is expressed on the surface of vNS1ss-infected cells, similar to the case for JEV-infected cells. Similar results were observed with vNS1ss- and WR-infected SW-13 cells (Fig. 2A, right panel; mean fluorescence intensities, 11.36 ± 0.84 and 2.91 ± 0.50, respectively). These results were further confirmed by indirect immunofluorescence assay of nonpermeabilized infected cells, where the surface staining was observed on all the vNS1ss- and JEV-infected BHK-21 (Fig. 2B) and SW-13 (Fig. 2C) cells but not on control WR-infected cells. Thus, unlike the case for DENV NS1 (42), surface expression of JEV NS1 appears to occur in the absence of fusion to the N-terminal segment of NS2a or any other viral proteins, consistent with the previous observation that the processing and maturation of JEV NS1 do not require NS2a or NS2b (18).
FIG. 2.
Determination of surface expression of NS1 in vNS1ss-infected cells. (A) BHK-21 or SW-13 cells were mock infected (shaded area) or infected with JEV (thin solid line) at an MOI of 5 for 20 h or with vNS1ss (bold solid line) or WR (dotted line) at an MOI of 5 for 12 h and stained for surface NS1 with MAb 9NS1 followed by FITC-conjugated goat anti-mouse IgG as described in Materials and Methods. Cells were acquired using a BD FACScan and analyzed with WinMIDI software. The data are representative of three independent experiments. (B and C) Surface localization of NS1 in vNS1ss-infected cells by indirect immunofluorescence. BHK-21 cells (B) and SW-13 cells (C) infected with vNS1ss or WR for 12 h were fixed with 4% paraformaldehyde. Unpermeabilized cells were stained with serum from patient P3 followed by FITC-conjugated goat anti-human IgG as described in Materials and Methods. BHK-21 and SW-13 cells infected with JEV for 20 h were used as a positive control. Stained cells were observed under a Leica DMIRB inverted laser scanning confocal microscope.
Presence of JEV NS1-reactive antibodies in human convalescent patient sera.
Humoral immune responses elicited after flaviviral infections in mice and humans are directed to multiple viral proteins (20, 38). Of the 73 serum samples from convalescent JEV patients tested by ELISA as well as by immunoprecipitation of metabolically labeled JEV-infected BHK-21 cell lysates, 45 samples recognized the NS1 protein, a representative set of which is shown in Fig. 3A. Immunoblotting of the immunoprecipitated samples with rabbit anti-JEV NS1 serum confirmed the identity of NS1 in the precipitates (Fig. 3B). The patient sera also contained antibodies to other JEV proteins, predominantly E and prM, as can be seen in Fig. 3A. In order to further confirm the specificity of the human antibodies for NS1, we immunoprecipitated metabolically labeled lysates of BHK-21 cells infected with vNS1ss or WR using these sera. As evident from Fig. 3C, all the serum samples which were positive in Fig. 3A and B for NS1 reactivity also immunoprecipitated the recombinant NS1 protein from vNS1-infected BHK-21 lysates. JEV NS1 cross-reactive MAb 2E11 raised to DENV NS1 and a JEV E-specific MAb, 1F10, served as positive and negative controls, respectively. In a few cases where we could obtain sera from the same individual more than once over a period of 16 months, we did not observe significant variation in their reactivity to the JEV proteins (data not shown). Thus, in individuals living in regions where JEV is endemic, serum antibodies to JEV proteins persist for at least 1 to 2 years after recovery from acute JE, perhaps owing to repeated exposure to virus.
FIG. 3.
JEV NS1 is recognized by JE patient sera. (A) Lysates of 35S-labeled JEV infected (+) or uninfected (−) BHK-21 cells immunoprecipitated with JE patient sera (P1 to P10), healthy JEV contact serum determined to contain antibodies to E but not to JEV NS1 (HC), and negative control normal human serum (C) were boiled in Laemmli sample buffer, electrophoresed in a 10% polyacrylamide NuPage (Invitrogen) bis-Tris gel with MOPS running buffer, transferred to a nitrocellulose membrane, and visualized by fluorography as described in Materials and Methods. Sizes of molecular mass standards and positions of prM, E, NS1, and NS1′ are indicated on the right and left, respectively. L, total lysate. (B) The immunoprecipitated proteins in panel A were immunoblotted using rabbit anti-JEV NS1 antibody followed by HRP-conjugated goat anti-rabbit IgG as described in Materials and Methods. (C) Human patient sera immunoprecipitate JEV NS1 from vNS1-infected BHK-21 cell lysates. Immunoprecipitates of 35S-labeled lysates of vNS1ss (upper panel)- or WR (lower panel)-infected BHK-21 cells with the human serum samples used for panel A along with MAb 2E11 and 1F10 were electrophoresed in an SDS-10% polyacrylamide gel and visualized by fluorography. L, lysate.
Antibodies to NS1 in JE patient sera induce complement-mediated cytolysis of cells expressing NS1 on the surface.
In earlier studies using MAbs to YFV NS1 in the mouse model, their protective capacity by passive immunization correlated with the in vitro complement-mediated cytolysis of YFV-infected cells (47). In our preliminary experiments, sera from patient 1 (P1), P2, and P5, all of which showed various levels of anti-NS1 antibodies as evident from Fig. 3A and B, brought about various levels of cytolysis of BHK21 cells infected with JEV for 20 h, while serum from P6, which had no detectable antibodies to JEV NS1 but had significant reactivity to E and prM (Fig. 3A), did not show any cytolytic activity (see Fig. 6B). Over the past 11 years that we have sampled human sera from regions of JEV endemicity, we have never encountered samples with NS1 antibodies alone in the absence of E-specific antibodies, although the reverse was often the case. Therefore, in order to exclude any role for antibodies to other JEV proteins in bringing about cytolysis, we used vNS1ss-infected target cells, which expressed JEV NS1 as the sole viral protein, for assessing the cytolytic potential of NS1-specific antibodies.
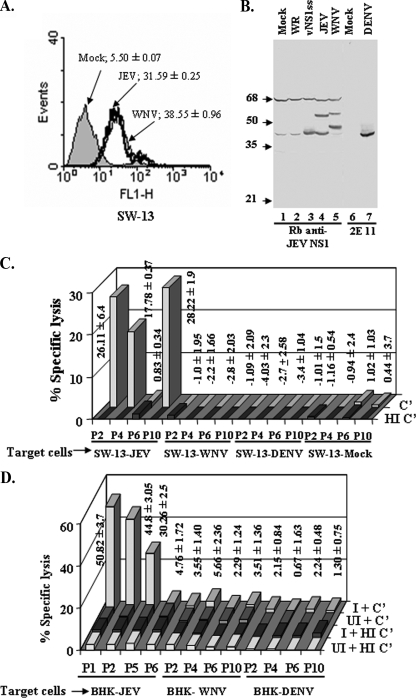
FIG. 6.
Antibodies to JEV NS1 fail to induce complement-mediated lysis of WNV- or DENV-infected cells. (A) Confirmation of surface expression of NS1 in JEV- and WNV-infected SW-13 cells. SW-13 cells were mock infected (shaded area) or infected with JEV (thick solid line) or WNV (thin solid line) at an MOI of 5 for 20 h and stained for surface NS1 with MAb 9NS1 followed by FITC-conjugated goat anti-mouse IgG as described in Materials and Methods. Cells were acquired using a BD FACScan and analyzed with WinMIDI software. The data are representative of three independent experiments. (B) Immunoblot analysis of expression of NS1 in SW-13 cells infected with vNS1ss or WR at an MOI of 5 for 12 h, with JEV or WNV at an MOI of 5 for 20 h, or with DENV-2 at an MOI of 2 for 48 h. Lysates from approximately 1.0 × 105 cells were electrophoresed in each lane of an SDS-10% polyacrylamide gel; transferred to a nitrocellulose membrane; probed with either rabbit antiserum specific for JEV NS1, which cross-reacts with WNV NS1, or MAb 2E11 specific for DENV NS1, followed by peroxidase-conjugated goat anti-rabbit IgG or goat anti-mouse IgG, respectively; and developed with diaminobenzidene and H2O2. (C and D) The sera obtained from JE convalescent patients were analyzed for their ability to lyse JEV-, WNV-, DENV-2-, or mock infected SW-13 cells (C) or BHK-21 cells (D) in the presence of active (C′) or heat-inactivated (HI C′) complement . Values represent means ± standard deviations of the percent specific lysis in the presence of active complement observed in three independent experiments.
Human cells are protected from inappropriate complement attack by membrane-bound complement inhibitory proteins, mainly CD59, decay-accelerating factor, and membrane cofactor protein, that either prevent complement activation or block the formation of membrane attack complex (5, 21, 43, 56). In order to confirm the relevance of anti-NS1-dependent complement-mediated lysis, we carried out this assay for representative human serum samples using three different human cell lines, namely, HeLa, HEK293T, and SW-13, along with human complement. All three human cell lines showed similar levels of complement-mediated cytolysis with no significant difference between them (data not shown), and all further experiments were carried out using SW-13 cells.
NS1 antibody-dependent cytolytic activity was detected in all the serum samples that had anti-NS1 antibody measured by radioimmunoprecipitation and ELISA when vNS1-infected SW13 or BHK-21 cells were used as targets (Fig. 4A and B). No cytolysis was detected when serum samples that lack NS1 antibody (P6, P7, and HC) (Fig. 4A and B) were tested, indicating that the ability to activate complement-mediated lysis was a property unique to antibodies directed to the NS1 protein of JEV. The values for specific lysis of SW-13 cells (13 to 29%) (Fig. 4A) in the presence of human complement were, however, less than those for lysis of BHK-21 cells using rabbit complement (28 to 65%) (Fig. 4B), as would be expected from the high levels of complement-inhibitory proteins on human cell surfaces, with the exception of serum from P10 (Fig. 4B). Two naïve sera tested with BHK-21 cells gave values of 1.34 ± 0.78 and 1.14 ± 0.8 for cytolysis, and one naïve serum tested with SW-13 cells gave a value of −0.3 ± 0.77. We obtained the best levels of cytolysis in vNS1ss-infected BHK-21 and SW-13 cells at 12 h p.i. and in JEV-infected BHK-21 and SW-13 cells at 20 h p.i., time points at which near-maximum levels of NS1 expression on the surface were observed in both cell lines by flow cytometry (Fig. 2A). At 24 h p.i. and 16 h p.i., respectively, for JEV and vNS1, time points at which the surface expression of NS1 detected by flow cytometry was marginally higher, specific lysis was in fact reduced (data not shown), primarily due to higher background leaching of chromium from the cells, presumably owing to virus-induced damage to cell membranes.
FIG. 4.
Complement-mediated cytolytic potential of anti-NS1 antibodies in human serum samples. The sera obtained from JE convalescent patients and one healthy control (HC) were analyzed for their ability to lyse vNS1ss-infected SW-13 cells (A) or BHK-21 cells (B) in the presence of active complement (C′) or heat-inactivated complement (HI C′). WR-infected cells served as negative control target cells. Human complement was used for SW-13 cells and rabbit complement for BHK-21 cells. Values represent means ± standard deviations of the percent specific lysis of vNS1ss-infected target cells in the presence of active complement observed in three independent experiments.
JEV NS1-specific cytolytic antibodies do not cross lyse WNV- or DENV-infected cells.
WNV and DENV are both known to cocirculate with JEV in the districts from where our human volunteers were recruited. NS1 proteins of both these viruses have been reported to contribute to pathology (1, 2, 3, 11, 15, 32-34, 36). It was therefore of interest to query whether antibodies elicited by JEV NS1 would cross recognize the NS1 proteins of these flaviviruses. Several sera from humans living in areas where JEV is endemic cross precipitated WNV NS1 in addition to WNV E (Fig. 5A). WNV NS1 protein in these immunoprecipitates was confirmed by Western blotting with rabbit serum specific to JEV NS1, which cross-reacts with WNV NS1 (Fig. 5B). Sera from P8, P9, and P10 also recognized DENV NS1 (Fig. 5C), as confirmed by Western blotting with MAb 2E11 (Fig. 5D). The repertoire as well as the quantity of proteins immunoprecipitated from WNV or DENV lysates by the serum samples was, however, less than that of JEV. This coupled with absence of recognition of DENV E protein (Fig. 5C) further confirmed JEV as the infecting agent in these individuals.
FIG. 5.
Antibodies to JEV NS1 in human sera recognize NS1 of WNV and DENV. (A) Lysates of 35S-labeled WNV-infected (+) or uninfected (−) BHK-21 cells immunoprecipitated with negative control serum (C) or the JE patient sera indicated above the lanes, eluted with Laemmli sample buffer without DTT, electrophoresed in an SDS-10% polyacrylamide gel, transferred to a nitrocellulose membrane, and visualized by fluorography. (B) The immunoprecipitated proteins in panel A were immunoblotted using WNV cross-reactive rabbit anti-JEV NS1 antibody followed by HRP-conjugated goat anti-rabbit IgG as described in Materials and Methods. Blots were developed with the ECL Plus Western blotting detection system (GE Healthcare, United Kingdom). (C) Lysates of 35S-labeled DENV-2-infected (+) or uninfected (−) BHK-21 cells immunoprecipitated with control serum (C) or the JE patient sera indicated above the lanes were electrophoresed in a 10% polyacrylamide NuPage (Invitrogen) bis-Tris gel with MOPS running buffer, transferred to a nitrocellulose membrane, and visualized by fluorography. (D) The immunoprecipitated proteins in panel C were immunoblotted using MAb 2E11 followed by HRP-conjugated goat anti-mouse IgM as described in Materials and Methods. L, lysate. The asterisk in panels C and D denotes the position of DENV NS1.
We wished to determine if the cross recognition of the NS1 proteins of WNV and DENV by these human sera would result in cross lysis of WNV- and DENV-infected cells, since cell surface expression of NS1 is a common feature reported for multiple flaviviruses, including WNV and DENV (8, 13, 23, 25, 42, 50). Indeed, we were also able to demonstrate surface expression of NS1 on JEV- and WNV-infected SW-13 cells using MAb 9NS1 (Fig. 6A); for DENV, we were able to detect weak surface NS1 using a pool of human sera P8 and P10 (data not shown). Robust infection of target cells with all viruses used was evident, however, when verified by Western blotting of infected cell lysates (Fig. 6 B). We did not detect any cytolytic activity whatsoever toward SW-13 and BHK-21 cells (Fig. 6C and D) infected with WNV or DENV for 20 h and 48 h, respectively, for any of the four sera tested (P2, P4, P6, and P10), revealing the exquisite specificity of cytolytic anti-JEV NS1 antibodies.
JEV NS1-specific antibodies inhibit virus proliferation in cultured cells.
We next addressed whether these NS1-specific cytolytic antibodies can ultimately limit virus spread and contribute to control of viral load. Monolayers of JEV-infected SW-13 cells, when treated with sera pooled from vNS1ss-immunized mice determined to contain significant levels of NS1-specific antibodies (Fig. 7A), failed to support normal levels of virus proliferation in the presence of active complement, as seen from the titer of virus released into the culture medium at various time points (Fig. 7B) (P = 0.003 for 48 h p.i.). From a 6-fold reduction evident at 24 h, as much as a 22-fold reduction in virus produced at 48 h p.i. was recorded. No significant reduction in virus titer was observed in the presence of active complement when pooled sera from WR-immunized mice were used or in the presence of sera from vNS1-immunized mice along with heat-inactivated complement, convincingly demonstrating the ability of NS1-specific antibodies to limit virus spread by lysis of JEV-infected cells.
FIG. 7.
Propagation of JEV in SW-13 cell monolayers in the presence of anti-NS1 antibody and complement. (A) Pooled sera from vNS1ss-immunized mice or control serum from WR-immunized mice was immunoprecipitated using radiolabeled JEV-infected BHK-21 cell lysates to determine the presence of anti-NS1 antibody. Samples were electrophoresed in an SDS-10% polyacrylamide gel, transferred to a nitrocellulose membrane, and visualized by fluorography. The presence of NS1 in the immunoprecipitate was confirmed by immunoblotting using rabbit anti-NS1 antibody. (B) Pooled sera from vNS1ss or WR-immunized mice were used in the presence of active (C′) or heat-inactivated (HI C′) human complement as described in Materials and Methods. Virus in the culture medium at the indicated time points was measured by plaque assay. Results are representative of two independent experiments performed in triplicates. Error bars represent standard deviations. *, P = 0.003.
JEV NS1 does not bind to human complement factor H.
We next investigated the ability of JEV NS1 to bind the human complement factor H, a property demonstrated for WNV NS1 (11). Despite sizeable quantities of JEV NS1 immunoprecipitated by MAb 9NS1 (Fig. 8A, lane 3), coimmunoprecipitation of human factor H was not observed with JEV NS1 (Fig. 8B, lane 3). However, WNV NS1, used as a positive control, immunoprecipitated significant quantities of human factor H (Fig. 8B, lane 2). The inability of JEV NS1 to bind and neutralize complement factor H suggests that JEV, unlike WNV, does not utilize NS1 to modulate or downregulate host innate immunity.
FIG. 8.
JEV NS1 does not bind to human complement factor H. The culture supernatant containing NS1 (∼7 μg) and 70 μl of normal human serum (NHS) were mixed in GVB-Mg2+-EGTA buffer overnight and immunoprecipitated as described in Materials and Methods using protein G beads coated with MAb 9NS1 raised to WNV NS1, which cross-reacts with JEV NS1. Electrophoresed immunoprecipitated proteins were blotted with rabbit anti-NS1 antibody followed by HRP-conjugated goat anti-rabbit IgG (A) or sheep anti-human fH antibody followed by HRP-conjugated rabbit anti-sheep IgG (B).
DISCUSSION
The multifunctional NS1 glycoprotein of flaviviruses is unusual, as this NS protein is secreted into the extracellular fluid as well as expressed on the surface of infected cells (35, 44, 53). We employed a recombinant vaccinia virus expression system to express high levels of JEV NS1 on the cell surface. Immunoblot, indirect immunofluorescence, and flow cytometry analyses showed that NS1 expressed by vNS1ss formed dimers intracellularly, was secreted as dimers into the culture supernatant, and was expressed on the surface of infected cells, similar to NS1 in JEV-infected cells. In this aspect, the JEV NS1 differed from that of DENV, which could be expressed on surfaces of cells only if contiguous with the N terminus of the NS2a protein (42).
In the present study, we demonstrated for the first time that antibodies directed to NS1 in convalescent JE patients induced antibody-dependent complement-mediated cytolysis of cells expressing JEV NS1 on the surface. The patient sera containing anti-E antibody but lacking anti-NS1 antibody failed to bring about complement-mediated cytolysis. Anti-NS1 antibodies from mice immunized with vNS1ss also effected a 22-fold reduction in virus produced by infected human SW-13 cell monolayers. This reduction in virus titer was distinct from virus reduction brought about by E-specific neutralizing antibodies, as it was complement dependent; additionally, these mouse antisera were completely devoid of JEV-neutralizing activity in a classical plaque reduction neutralization test (V. D. Krishna et al., unpublished data). Of particular interest was the complete absence of cytolytic activity when the human sera were tested using WNV- or DENV-infected cells. At present, we do not know the identity of the epitope(s) recognized by these cytolytic antibodies. While in YFV also only anti-NS1 and not anti-E antibodies sensitized infected cells to complement-mediated lysis (46), one study using MAbs to E of WNV indicated that E-specific antibodies can induce complement-mediated lysis (39).
Several human serum samples from individuals living in areas of endemicity that we tested revealed the ability to recognize cell surface NS1 as judged by flow cytometry (Fig. 3 and data not shown). Mouse MAbs that promoted phagocytosis and clearance of WNV-infected cells also possessed this ability to recognize cell surface NS1 and belonged predominantly to the mouse IgG2a subclass (13). Those authors therefore recommended the use of NS1 in vaccines and therapeutics against WNV. We are currently investigating the isotype of human NS1-specific antibodies responsible for cytolysis of JEV-infected cells.
High levels of NS1 accumulate in serum during WNV and DENV infection and correlated with development of severe disease in DENV patients (1, 3, 31, 36, 54). The roles of secreted and surface NS1 in flavivirus pathogenesis are not very clear, although in DENV infection it was observed that NS1-induced autoantibodies cross-reacted with platelets and extracellular matrix proteins (10, 15). DENV NS1 also bound endothelial cells, leading perhaps to apoptosis and vascular leakage (3, 32-34), mediated through NS1 antibodies. Furthermore, preincubation of Huh7 cells with the secreted form of NS1 increased DENV production after infection with the homologous DENV-1 strain, demonstrating that the accumulation of NS1 in the cell enhances subsequent DENV infection (2). The recently demonstrated ability of soluble DENV NS1 to bind uninfected cell surfaces via heparan sulfate and chondroitin sulfate E (4) also contributes to risks of antibody-mediated destruction of uninfected cells and resultant pathology. However, we could not detect binding of JEV NS1 to human cell surfaces in limited investigations using human sera that had JEV NS1 reactivity (data not shown). Thus, while there is widespread recognition of the contribution of NS1 to pathogenesis in DENV infections, we have not encountered similar reports on a correlation between levels of antibodies to JEV NS1 and severity of disease. We also failed to observe binding of JEV NS1 to human complement factor H, in contrast to the case for NS1 of WNV (11). These multiple reports highlight the striking divergence between the flaviviruses in their ability to deploy the NS1 protein for mediating pathological manifestations. Keeping in mind the coendemicity of multiple flaviviruses in affected regions of the globe, the non-cross-reactive nature of the cytolytic anti-JEV NS1 antibodies toward DENV or WNV that we observed may serve to ensure the absence of adverse effects in the event of sequential infection with closely related flaviviruses.
Conversely, passive immunization with MAbs to NS1 or active immunization with NS1 demonstrated that YFV, DENV, and tick-borne encephalitis virus NS1 can induce protective immunity (14, 16, 22, 24, 26, 27, 45, 47, 48). Multiple studies demonstrated conclusively that MAbs directed to NS1 were protective, primarily due to their ability to bind cell surface NS1 (12, 13). A strong correlation between complement-fixing activity of the antibodies and protective ability was also demonstrated (35, 47). This paradoxical property of flavivirus NS1 of contributing to severe pathogenesis on the one hand and to protective immunity on the other constitutes challenges for vaccine design. Of note, no report documenting any role in viral pathogenesis for NS1 of JEV exists, throwing open a window of opportunity for its use in prophylactic approaches that can induce flavivirus cross-reactive immunity.
In our cohort, the rate of recovery from encephalitis and progressive improvement in neurological sequelae over a period of 6 to 22 months after discharge from hospitalization was similar in individuals who tested positive or negative for serum anti-NS1 antibodies (Krishna et al., unpublished data). In addition, we have encountered healthy individuals with no previous history of clinical encephalitis who have levels of circulating NS1-specific serum antibodies comparable in titer to those found in convalescent patients.
A survey of the roles of NS1 of multiple flaviviruses thus reveals a predominant protective role for humoral immune responses directed to NS1. Even for DENV, where the virus appears to have developed unique strategies to subvert NS1-specific antibodies and bring about severe pathology as seen in dengue hemorrhagic fever and dengue shock syndrome, reports nevertheless document the ability of NS1-specific antibodies to protect against lethal viral challenge in the mouse model (16, 24). Furthermore, cell-mediated immune responses directed to the NS1 protein also correlated with protective immunity, both in humans, as evident from our earlier studies of a population in an area of JEV endemicity (30), and in our ongoing investigations in the mouse model (Krishna et al., unpublished data). By immunization with NS1-containing experimental vaccines followed by challenge using JEV, WNV, or DENV, (Krishna et al., unpublished data), we are currently investigating in animal models the question of whether inclusion of NS1 can enhance the protective efficacy of currently used E-based JEV vaccines.
Acknowledgments
We thank Prida Malasit, Siriraj Hospital, Mahidol University, Bangkok, Thailand; Michael S. Diamond, Washington University School of Medicine, St. Louis, MO; and Deepak Gadkari, National Institute of Virology, Pune, India, for the generous gifts of MAbs 2E11 and 9NS1 and polyclonal rabbit anti-JEV NS1 serum, respectively. We thank M. Veerashankar and Magandi Haridattatreya of the Vijayanagar Institute of Medical Sciences, Bellary, for help in collection of serum samples.
The infrastructure facilities provided to the Department of Microbiology and Cell Biology by the Indian Council of Medical Research under a CARM grant and the Department of Science and Technology under FIST are gratefully acknowledged.
Footnotes
Published ahead of print on 4 March 2009.
REFERENCES
- 1.Alcon, S., A. Talarmin, M. Debruyne, A. Falconar, V. Deubel, and M. Flamand. 2002. Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J. Clin. Microbiol. 40376-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcon-LePoder, S., M. T. Drouet, P. Roux, M. P. Frenkiel, M. Arborio, A. M. Durand-Schneider, M. Maurice, I. Le Blanc, J. Gruenberg, and M. Flamand. 2005. The secreted form of dengue virus nonstructural protein NS1 is endocytosed by hepatocytes and accumulates in late endosomes: implications for viral infectivity. J. Virol. 7911403-11411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avirutnan, P., N. Punyadee, S. Noisakran, C. Komoltri, S. Thiemmeca, K. Auethavornanan, A. Jairungsri, R. Kanlaya, N. Tangthawornchaikul, C. Puttikhunt, S. N. Pattanakitsakul, P. T. Yenchitsomanus, J. Mongkolsapaya, W. Kasinrerk, N. Sittisombut, M. Husmann, M. Blettner, S. Vasanawathana, S. Bhakdi, and P. Malasit. 2006. Vascular leakage in severe dengue virus infections: a potential role for the nonstructural viral protein NS1 and complement. J. Infect. Dis. 1931078-1088. [DOI] [PubMed] [Google Scholar]
- 4.Avirutnan, P., L. Zhang, N. Punyadee, A. Manuyakorn, C. Puttikhunt, W. Kasinrerk, P. Malasit, J. P. Atkinson, and M. S. Diamond. 2007. Secreted NS1 of dengue virus attaches to the surface of cells via interactions with heparan sulfate and chondroitin sulfate E. PLoS Pathog. 3e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barilla-LaBarca, M. L., M. K. Liszewski, J. D. Lambris, D. Hourcade, and J. P. Atkinson. 2002. Role of membrane cofactor protein (CD46) in regulation of C4b and C3b deposited on cells. J. Immunol. 1686298-6304. [DOI] [PubMed] [Google Scholar]
- 6.Bokisch, V. A., F. H. Top, Jr., P. K. Russell, F. J. Dixon, and H. J. Muller-Eberhard. 1973. The potential pathogenic role of complement in dengue hemorrhagic shock syndrome. N. Engl. J. Med. 289996-1000. [DOI] [PubMed] [Google Scholar]
- 7.Burke, D. S., A. Nisalak, M. A. Ussery, T. Laorakpongse, and S. Chantavibul. 1985. Kinetics of IgM and IgG responses to Japanese encephalitis virus in human serum and cerebrospinal fluid. J. Infect. Dis. 1511093-1099. [DOI] [PubMed] [Google Scholar]
- 8.Cardiff, R. D., and J. K. Lund. 1976. Distribution of dengue-2 antigens by electron immunocytochemistry. Infect. Immun. 131699-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cardiff, R. D., T. G. McCloud, W. E. Brandt, and P. K. Russell. 1970. Molecular size and charge relationships of the soluble complement-fixing antigens of dengue viruses. Virology 41569-572. [DOI] [PubMed] [Google Scholar]
- 10.Chang, H. H., H. F. Shyu, Y. M. Wang, D. S. Sun, R. H. Shyu, S. S. Tang, and Y. S. Huang. 2002. Facilitation of cell adhesion by immobilized dengue viral nonstructural protein 1 (NS1): arginine-glycine-aspartic acid structural mimicry within the dengue viral NS1 antigen. J. Infect. Dis. 186743-751. [DOI] [PubMed] [Google Scholar]
- 11.Chung, K. M., M. K. Liszewski, G. Nybakken, A. E. Davis, R. R. Townsend, D. H. Fremont, J. P. Atkinson, and M. S. Diamond. 2006. West Nile virus nonstructural protein NS1 inhibits complement activation by binding the regulatory protein factor H. Proc. Natl. Acad. Sci. USA 10319111-19116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung, K. M., G. E. Nybakken, B. S. Thompson, M. J. Engle, A. Marri, D. H. Fremont, and M. S. Diamond. 2006. Antibodies against West Nile virus nonstructural protein NS1 prevent lethal infection through Fc gamma receptor-dependent and -independent mechanisms. J. Virol. 801340-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung, K. M., B. S. Thompson, D. H. Fremont, and M. S. Diamond. 2007. Antibody recognition of cell surface-associated NS1 triggers Fc-gamma receptor-mediated phagocytosis and clearance of West Nile virus-infected cells. J. Virol. 819551-9555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Despres, P., J. Dietrich, M. Girard, and M. Bouloy. 1991. Recombinant baculoviruses expressing yellow fever virus E and NS1 proteins elicit protective immunity in mice. J. Gen. Virol. 722811-2816. [DOI] [PubMed] [Google Scholar]
- 15.Falconar, A. K. 1997. The dengue virus nonstructural-1 protein (NS1) generates antibodies to common epitopes on human blood clotting, integrin/adhesin proteins and binds to human endothelial cells: potential implications in haemorrhagic fever pathogenesis. Arch. Virol. 142897-916. [DOI] [PubMed] [Google Scholar]
- 16.Falgout, B., M. Bray, J. J. Schlesinger, and C. J. Lai. 1990. Immunization of mice with recombinant vaccinia virus expressing authentic dengue virus nonstructural protein NS1 protects against lethal dengue virus encephalitis. J. Virol. 644356-4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falgout, B., R. Chanock, and C. J. Lai. 1989. Proper processing of dengue virus nonstructural glycoprotein NS1 requires the N-terminal hydrophobic signal sequence and the downstream nonstructural protein NS2a. J. Virol. 631852-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan, W. F., and P. W. Mason. 1990. Membrane association and secretion of the Japanese encephalitis virus NS1 protein from cells expressing NS1 cDNA. Virology 177470-476. [DOI] [PubMed] [Google Scholar]
- 19.Flamand, M., F. Megret, M. Mathieu, J. Lepault, F. A. Rey, and V. Deubel. 1999. Dengue virus type 1 nonstructural glycoprotein NS1 is secreted from mammalian cells as a soluble hexamer in a glycosylation-dependent fashion. J. Virol. 736104-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia, G., D. W. Vaughn, and R. M. Del Angel. 1997. Recognition of synthetic oligopeptides from nonstructural proteins NS1 and NS3 of dengue-4 virus by sera from dengue virus-infected children. Am. J. Trop. Med. Hyg. 56466-470. [DOI] [PubMed] [Google Scholar]
- 21.Gorter, A., and S. Meri. 1999. Immune evasion of tumor cells using membrane-bound complement regulatory proteins. Immunol. Today 20576-582. [DOI] [PubMed] [Google Scholar]
- 22.Gould, E. A., A. Buckley, A. D. Barrett, and N. Cammack. 1986. Neutralizing (54K) and non-neutralizing (54K and 48K) monoclonal antibodies against structural and non-structural yellow fever virus proteins confer immunity in mice. J. Gen. Virol. 67591-595. [DOI] [PubMed] [Google Scholar]
- 23.Gould, E. A., A. Buckley, N. Cammack, A. D. Barrett, J. C. Clegg, R. Ishak, and M. G. Varma. 1985. Examination of the immunological relationships between flaviviruses using yellow fever virus monoclonal antibodies. J. Gen. Virol. 661369-1382. [DOI] [PubMed] [Google Scholar]
- 24.Henchal, E. A., L. S. Henchal, and J. J. Schlesinger. 1988. Synergistic interactions of anti-NS1 monoclonal antibodies protect passively immunized mice from lethal challenge with dengue 2 virus. J. Gen. Virol. 692101-2107. [DOI] [PubMed] [Google Scholar]
- 25.Jacobs, M. G., P. J. Robinson, C. Bletchly, J. M. Mackenzie, and P. R. Young. 2000. Dengue virus nonstructural protein 1 is expressed in a glycosyl-phosphatidylinositol-linked form that is capable of signal transduction. FASEB J. 141603-1610. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs, S. C., J. R. Stephenson, and G. W. Wilkinson. 1992. High-level expression of the tick-borne encephalitis virus NS1 protein by using an adenovirus-based vector: protection elicited in a murine model. J. Virol. 662086-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobs, S. C., J. R. Stephenson, and G. W. Wilkinson. 1994. Protection elicited by a replication-defective adenovirus vector expressing the tick-borne encephalitis virus non-structural glycoprotein NS1. J. Gen. Virol. 752399-2402. [DOI] [PubMed] [Google Scholar]
- 28.Kaufman, B. M., P. L. Summers, D. R. Dubois, W. H. Cohen, M. K. Gentry, R. L. Timchak, D. S. Burke, and K. H. Eckels. 1989. Monoclonal antibodies for dengue virus prM glycoprotein protect mice against lethal dengue infection. Am. J. Trop. Med. Hyg. 41576-580. [DOI] [PubMed] [Google Scholar]
- 29.Kimura-Kuroda, J., and K. Yasui. 1988. Protection of mice against Japanese encephalitis virus by passive administration with monoclonal antibodies. J. Immunol. 1413606-3610. [PubMed] [Google Scholar]
- 30.Kumar, P., V. D. Krishna, P. Sulochana, G. Nirmala, M. Haridattatreya, and V. Satchidanandam. 2004. Cell-mediated immune responses in healthy children with a history of subclinical infection with Japanese encephalitis virus: analysis of CD4+ and CD8+ T cell target specificities by intracellular delivery of viral proteins using the human immunodeficiency virus Tat protein transduction domain. J. Gen. Virol. 85471-482. [DOI] [PubMed] [Google Scholar]
- 31.Libraty, D. H., P. R. Young, D. Pickering, T. P. Endy, S. Kalayanarooj, S. Green, D. W. Vaughn, A. Nisalak, F. A. Ennis, and A. L. Rothman. 2002. High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J. Infect. Dis. 1861165-1168. [DOI] [PubMed] [Google Scholar]
- 32.Lin, C. F., S. C. Chiu, Y. L. Hsiao, S. W. Wan, H. Y. Lei, A. L. Shiau, H. S. Liu, T. M. Yeh, S. H. Chen, C. C. Liu, and Y. S. Lin. 2005. Expression of cytokine, chemokine, and adhesion molecules during endothelial cell activation induced by antibodies against dengue virus nonstructural protein 1. J. Immunol. 174395-403. [DOI] [PubMed] [Google Scholar]
- 33.Lin, C. F., H. Y. Lei, A. L. Shiau, C. C. Liu, H. S. Liu, T. M. Yeh, S. H. Chen, and Y. S. Lin. 2003. Antibodies from dengue patient sera cross-react with endothelial cells and induce damage. J. Med. Virol. 6982-90. [DOI] [PubMed] [Google Scholar]
- 34.Lin, C. F., H. Y. Lei, A. L. Shiau, H. S. Liu, T. M. Yeh, S. H. Chen, C. C. Liu, S. C. Chiu, and Y. S. Lin. 2002. Endothelial cell apoptosis induced by antibodies against dengue virus nonstructural protein 1 via production of nitric oxide. J. Immunol. 169657-664. [DOI] [PubMed] [Google Scholar]
- 35.Lin, Y. L., L. K. Chen, C. L. Liao, C. T. Yeh, S. H. Ma, J. L. Chen, Y. L. Huang, S. S. Chen, and H. Y. Chiang. 1998. DNA immunization with Japanese encephalitis virus nonstructural protein NS1 elicits protective immunity in mice. J. Virol. 72191-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macdonald, J., J. Tonry, R. A. Hall, B. Williams, G. Palacios, M. S. Ashok, O. Jabado, D. Clark, R. B. Tesh, T. Briese, and W. I. Lipkin. 2005. NS1 protein secretion during the acute phase of West Nile virus infection. J. Virol. 7913924-13933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mason, P. W., S. Pincus, M. J. Fournier, T. L. Mason, R. E. Shope, and E. Paoletti. 1991. Japanese encephalitis virus-vaccinia recombinants produce particulate forms of the structural membrane proteins and induce high levels of protection against lethal JEV infection. Virology 180294-305. [DOI] [PubMed] [Google Scholar]
- 38.Matveeva, V. A., R. V. Popova, E. A. Kvetkova, L. O. Chernicina, V. I. Zlobin, N. M. Puchovskaya, and O. V. Morozova. 1995. Antibodies against tick-borne encephalitis virus (TBEV) non-structural and structural proteins in human sera and spinal fluid. Immunol. Lett. 461-4. [DOI] [PubMed] [Google Scholar]
- 39.Mehlhop, E., K. Whitby, T. Oliphant, A. Marri, M. Engle, and M. S. Diamond. 2005. Complement activation is required for induction of a protective antibody response against West Nile virus infection. J. Virol. 797466-7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mellado-Sanchez, G., J. Garcia-Cordero, R. Luria-Perez, L. Lazaro-Olan, L. Santos-Argumedo, B. Gutierrez-Castaneda, I. Estrada-Garcia, and L. Cedillo-Barron. 2005. DNA priming E and NS1 constructs—homologous proteins boosting immunization strategy to improve immune response against dengue in mice. Viral Immunol. 18709-721. [DOI] [PubMed] [Google Scholar]
- 41.Murali-Krishna, K., B. Ramireddy, V. Ravi, and R. Manjunath. 1995. Recognition of nonstructural protein peptides by cytotoxic T lymphocytes raised against Japanese encephalitis virus. Microbiol. Immunol. 391021-1024. [DOI] [PubMed] [Google Scholar]
- 42.Noisakran, S., T. Dechtawewat, P. Rinkaewkan, C. Puttikhunt, A. Kanjanahaluethai, W. Kasinrerk, N. Sittisombut, and P. Malasit. 2007. Characterization of dengue virus NS1 stably expressed in 293T cell lines. J. Virol. Methods 14267-80. [DOI] [PubMed] [Google Scholar]
- 43.Oglesby, T. J., C. J. Allen, M. K. Liszewski, D. J. White, and J. P. Atkinson. 1992. Membrane cofactor protein (CD46) protects cells from complement-mediated attack by an intrinsic mechanism. J. Exp. Med. 1751547-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pryor, M. J., and P. J. Wright. 1993. The effects of site-directed mutagenesis on the dimerization and secretion of the NS1 protein specified by dengue virus. Virology 194769-780. [DOI] [PubMed] [Google Scholar]
- 45.Schlesinger, J. J., M. W. Brandriss, C. B. Cropp, and T. P. Monath. 1986. Protection against yellow fever in monkeys by immunization with yellow fever virus nonstructural protein NS1. J. Virol. 601153-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlesinger, J. J., M. W. Brandriss, J. R. Putnak, and E. E. Walsh. 1990. Cell surface expression of yellow fever virus non-structural glycoprotein NS1: consequences of interaction with antibody. J. Gen. Virol. 71593-599. [DOI] [PubMed] [Google Scholar]
- 47.Schlesinger, J. J., M. W. Brandriss, and E. E. Walsh. 1985. Protection against 17D yellow fever encephalitis in mice by passive transfer of monoclonal antibodies to the nonstructural glycoprotein gp48 and by active immunization with gp48. J. Immunol. 1352805-2809. [PubMed] [Google Scholar]
- 48.Schlesinger, J. J., M. Foltzer, and S. Chapman. 1993. The Fc portion of antibody to yellow fever virus NS1 is a determinant of protection against YF encephalitis in mice. Virology 192132-141. [DOI] [PubMed] [Google Scholar]
- 49.Smith, T. J., W. E. Brandt, J. L. Swanson, J. M. McCown, and E. L. Buescher. 1970. Physical and biological properties of dengue-2 virus and associated antigens. J. Virol. 5524-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Westaway, E. G., and M. R. Goodman. 1987. Variation in distribution of the three flavivirus-specified glycoproteins detected by immunofluorescence in infected Vero cells. Arch. Virol. 94215-228. [DOI] [PubMed] [Google Scholar]
- 51.Winkler, G., S. E. Maxwell, C. Ruemmler, and V. Stollar. 1989. Newly synthesized dengue-2 virus nonstructural protein NS1 is a soluble protein but becomes partially hydrophobic and membrane-associated after dimerization. Virology 171302-305. [DOI] [PubMed] [Google Scholar]
- 52.Winkler, G., V. B. Randolph, G. R. Cleaves, T. E. Ryan, and V. Stollar. 1988. Evidence that the mature form of the flavivirus nonstructural protein NS1 is a dimer. Virology 162187-196. [DOI] [PubMed] [Google Scholar]
- 53.Wright, P. J., M. R. Cauchi, and M. L. Ng. 1989. Definition of the carboxy termini of the three glycoproteins specified by dengue virus type 2. Virology 17161-67. [DOI] [PubMed] [Google Scholar]
- 54.Young, P. R., P. A. Hilditch, C. Bletchly, and W. Halloran. 2000. An antigen capture enzyme-linked immunosorbent assay reveals high levels of the dengue virus protein NS1 in the sera of infected patients. J. Clin. Microbiol. 381053-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang, M. J., M. J. Wang, S. Z. Jiang, and W. Y. Ma. 1989. Passive protection of mice, goats, and monkeys against Japanese encephalitis with monoclonal antibodies. J. Med. Virol. 29133-138. [DOI] [PubMed] [Google Scholar]
- 56.Zhao, J., S. A. Rollins, S. E. Maher, A. L. Bothwell, and P. J. Sims. 1991. Amplified gene expression in CD59-transfected Chinese hamster ovary cells confers protection against the membrane attack complex of human complement. J. Biol. Chem. 26613418-13422. [PubMed] [Google Scholar]