Abstract
Geminiviruses replicate single-stranded DNA genomes through double-stranded intermediates that associate with cellular histone proteins. Unlike RNA viruses, they are subject to RNA-directed methylation pathways that target viral chromatin and likely lead to transcriptional gene silencing (TGS). Here we present evidence that the related geminivirus proteins AL2 and L2 are able to suppress this aspect of host defense. AL2 and L2 interact with and inactivate adenosine kinase (ADK), which is required for efficient production of S-adenosyl methionine, an essential methyltransferase cofactor. We demonstrate that the viral proteins can reverse TGS of a green fluorescent protein (GFP) transgene in Nicotiana benthamiana when overexpressed from a Potato virus X vector and that reversal of TGS by geminiviruses requires L2 function. We also show that AL2 and L2 cause ectopic expression of endogenous Arabidopsis thaliana loci silenced by methylation in a manner that correlates with ADK inhibition. However, at one exceptional locus, ADK inhibition was insufficient and TGS reversal required the transcriptional activation domain of AL2. Using restriction-sensitive PCR and bisulfite sequencing, we showed that AL2-mediated TGS suppression is accompanied by reduced cytosine methylation. Finally, using a methylation-sensitive single-nucleotide extension assay, we showed that transgenic expression of AL2 or L2 causes global reduction in cytosine methylation. Our results provide further evidence that viral chromatin methylation is an important host defense and allow us to propose that as a countermeasure, geminivirus proteins reverse TGS by nonspecifically inhibiting cellular transmethylation reactions. To our knowledge, this is the first report that viral proteins can inhibit TGS.
Covalent modification of eukaryotic DNA and associated histones is a fundamental epigenetic mechanism that governs the expression of selected genes and genetic elements and distinguishes heterochromatic and euchromatic regions of the genome. For example, cytosine methylation is associated with the promoters of transcriptionally silenced genes and with repressed heterochromatic sequences, including centromeric repeats, transposons, and retroelements (9, 25). How sequences are targeted for methylation is not entirely understood, but in organisms such as fission yeast and plants, small interfering RNAs produced by mechanisms related to RNA interference guide methylation to homologous sequences in a process termed RNA-directed DNA methylation (2, 7, 16, 27). Promoter methylation is typical of transcriptional gene silencing (TGS), although coding region methylation (body methylation) may also be present in both repressed and active genes (9, 24, 54, 55). Body methylation sometimes also occurs as a consequence of posttranscriptional gene silencing (PTGS), although its role in this process is not clear (18).
In mammals, 5-methylcytosine is limited to CG dinucleotides, whereas in plants, methylation can additionally occur at CNG (N is A, T, G, or C) and CHH (asymmetric sites, where H is A, T, or C) sequences. Methylation protects the integrity of the genome by suppressing transposon activity and may also prevent rearrangement in repeat-rich regions (2, 7, 16, 27). It also is critically important for gene regulation during development. Null mutations in mouse methyltransferase genes result in embryonic lethality (13), while in Arabidopsis thaliana mutations in methyltransferases involved in CG maintenance, methylation (met1) or non-CG methylation (ddc triple mutants drm1/2 and cmt3) leads to severe developmental abnormalities (8, 28, 41). Alterations in DNA methylation can have detrimental outcomes, including cancer, in adult organisms as well (20, 33).
The geminiviruses are a diverse family of single-stranded DNA pathogens that infect a wide variety of plant species and are responsible for significant crop losses (14, 38). These viruses have small (2.5 to 3.0 kb), circular, monopartite or bipartite genomes that specify four to seven proteins. Geminiviruses do not encode polymerases and are therefore dependent on host machinery for replication and transcription, which occur on viral chromatin templates that consist of double-stranded (ds) DNA replicative intermediates associated with cellular histones (32). Thus, they are attractive models for fundamental host processes, including the epigenetic regulation of replication and gene expression.
It is well established that plants employ PTGS as an adaptive defense against RNA viruses and that, as a countermeasure, viruses encode an array of silencing suppressor proteins (10, 23, 39, 49). DNA virus transcripts are also vulnerable to cytoplasmic PTGS, and several proteins produced by geminiviruses and their satellites are known to act as silencing suppressors (4, 47). In addition, there is considerable evidence that plants methylate geminivirus chromatin as an epigenetic defense. The results of early studies indicated that in vitro methylation of geminivirus DNA greatly impaired replication and transcription in protoplasts (6, 11). More recently, we have shown that methylation-deficient Arabidopsis mutants are hypersusceptible to geminiviruses and that RNA-directed DNA methylation pathway components (e.g., ARGONAUTE 4) are necessary for host recovery from infection. Geminivirus DNA and associated histones are methylated in infected plants, and viral DNA methylation is reduced in mutants that display enhanced disease. In contrast, the small amount of viral DNA present in tissue that has recovered from infection is hypermethylated (35).
Given that methylation and, likely, TGS act as defenses against DNA viruses, we hypothesized that geminiviruses produce proteins capable of interfering with these processes. In this study, we examine the role of AL2 and L2 proteins in this regard. The 15-kDa AL2, also known as AC2, C2, or TrAP (transcriptional activator protein), is encoded by members of the genus Begomovirus, including Tomato golden mosaic virus (TGMV) and Cabbage leaf curl virus (CaLCuV). AL2 is a transcription factor required for the expression of late viral genes (43). In contrast, the related L2 protein found in the Curtovirus genus, including Beet curly top virus (BCTV), is not a transcription factor (17). AL2 can suppress PTGS by a mechanism that depends on its ability to activate transcription (transcription-dependent suppression) (46). In addition, both AL2 and L2 can inactivate adenosine kinase (ADK) (52), which is required for the efficient production of S-adenosyl methionine (SAM), an essential methyltransferase cofactor (31). We have demonstrated that AL2 and L2 can inhibit PTGS by a mechanism that involves ADK inactivation (transcription-independent suppression) (51, 53). Here we present evidence that these proteins use a similar mechanism to reverse TGS and that the expression of AL2 and L2 in transgenic plants causes genome-wide reductions in cytosine methylation.
MATERIALS AND METHODS
Primers.
Primer sequences used for cloning, genotyping, and reverse transcription (RT)-PCR are listed in Table S1 in the supplemental material.
Silenced GFP-transgenic N. benthamiana.
N. benthamiana line 16C containing an active 35S-green fluorescent protein (GFP) transgene was inoculated with Tobacco rattle virus (TRV) vector expressing a portion of the 35S promoter sequence to induce TGS (TRV::35S), a method previously shown to cause heritable TGS of the transgene (19, 40). After 3 to 4 weeks, flowers were observed under UV light, and silenced (red) flowers were tagged and seeds harvested. The resulting plants were allowed to self-fertilize to generate line 16-TGS, and heritable silencing was confirmed in T2-generation seedlings under UV light and by nuclear run-on analysis as previously described (43). Line 16C and TRV::35S were kindly provided by David Baulcombe.
Recombinant PVX and TRV vectors.
The binary Potato virus X (PVX) vector pgR106 (26) and the TRV binary vectors pTV00 (TRV RNA 2) and pBINTRA6 (TRV RNA 1) (36) were gifts of David Baulcombe. Recombinant PVX was constructed using AL2, AL21-114, AL2-C33A, and L2 sequences from previously described constructs (51, 53). All genes were first cloned into pUC19 and then resequenced and added to the pgR106 vector as AscI-SalI or a ClaI-SalI fragments by standard cloning procedures. For TRV vectors, MET1, SAHH, and ADK sequences from cloned N. benthamiana cDNAs were used (51). (The GenBank sequence accession number for SAHH is FJ222442, for MET1 is FJ222441, and for ADK is AY741533). Portions of these genes (∼500 nucleotides [nt] of SAHH, ∼600 nt of ADK, and ∼750 nt of MET1) were cloned into pTV00 as ClaI-SalI fragments. The resulting PVX and TRV vectors were transformed into Agrobacterium tumefaciens strain GV3101, and glycerol stocks stored at −80°C prior to use. Details of PVX and TRV vector construction and cloning of N. benthamiana cDNAs are provided in the supplemental material.
Infection of 16-TGS N. benthamiana plants with recombinant RNA virus vectors and geminiviruses.
Two to 3 weeks postgermination, 16-TGS plants were observed under UV light and any showing GFP expression were eliminated. Infiltration of plants with A. tumefaciens GV3101 harboring the PVX vectors was carried out as previously described (51). Successful infection was indicated by mosaic PVX symptoms after 7 days. A similar procedure was used for pTV00-based vectors, except that a culture containing pBINTRA6 was added at a 2:1 ratio prior to infiltration. Because TRV normally produces asymptomatic infections, virus-induced gene silencing (VIGS) was confirmed in parallel using pTV09, which targets the sulfur allele of magnesium chelatase and causes photobleaching in infected plants (22). After 2 to 6 weeks, infected plants were photographed under UV light by using a Nikon CoolPix 990 handheld digital camera equipped with UV and yellow filters, and tissue samples taken for RNA analysis.
The 16-TGS plants were also agroinoculated with CaLCuV, BCTV, and BCTV L2− mutant virus (17) as previously described (35, 44). Photographs were taken at 3 weeks postinoculation using a Nikon D40 digital camera equipped with UV and yellow filters, and tissue samples taken for RNA analysis.
Transgenic Arabidopsis studies.
A dexamethasone (dex) induction system was chosen to make AL2, AL21-114, L2, and ADK dsRNA (dsADK) transgenic lines (1, 29). The TGMV AL2, AL21-114, and BCTV L2 genes (51) were cloned into pTA7001, containing a dex-inducible promoter, as SpeI-XhoI fragments. The dsADK construct was generated by insertion of an inverted repeat sequence corresponding to ∼600 nt of Arabidopsis ADK2 cDNA (51, 52). The TA7001 AL2, AL21-114, L2, and dsADK constructs were transformed into the Agrobacterium strain GV3101 and then into the Colombia ecotype of Arabidopsis thaliana by using the floral dip procedure (3). T1 seeds were selected on Murashige and Skoog medium (Invitrogen) containing B vitamins and 30 μg/ml hygromycin. Plants with well-developed cotyledons and roots were transplanted to soil and genotyped with primers specific for the transgene and dex promoter cassette by using an Extract-N-Amp PCR kit (Sigma). T2 seeds were harvested from positive lines and selected again on medium containing hygromycin, and the resulting plants used for experiments.
Transgenic plants were grown until bolting (3 to 5 weeks). Dex stock solution (20 mM in 100% ethanol) was diluted 1:1,000 into sterile water containing 0.05% Silwet L-77 (Vac-In-Stuff; Lehle Seeds). The diluted dex (or water) was sprayed onto plants with a Paasche VLSTPRO double-action airbrush attached to a Husky (Home Depot, Inc.) 4-gallon air compressor set to 40 lb/in2. After being sprayed, plants were laid on damp paper towels under a dome for 24 h and then returned to the growth chamber for 24 to 48 h. Plants were resprayed every 48 to 72 h for 1 to 2 weeks, and then leaves were harvested and extracts prepared for RNA analysis (see below). Thus, the effects of transgenes were monitored between 9 and 17 days after dex treatment in different experiments. ADK activity present in dsADK transgenic lines was measured as previously described (52).
RNA extraction and analysis.
RNA extraction, Northern blotting, and RT-PCR procedures have been described previously (51). Briefly, 1 ml Trizol reagent (Invitrogen) was used to extract RNA from 0.1 g of plant tissue. Northern blot assays were performed with 5 to 10 μg of RNA. Antisense riboprobes were synthesized with [α-32P]UTP and a Maxiscript T7 kit, and DNA probes were synthesized with [α-32P]dCTP and a Strip-EZ DNA kit (Applied Biosystems). RT-PCR was carried out using a SuperScript III one-step RT-PCR kit (Invitrogen) with 1 μg of RNA.
Methylation-sensitive PCR.
Appropriate enzymes were selected based on target AtSN1 and Athila sequences by using REBASE (New England Biolabs), a database containing methylation sensitivity data for restriction endonucleases. An amount of 100 ng of genomic DNA from dex- or water-treated transgenic Arabidopsis plants was incubated with 2 units of methylation-sensitive restriction enzyme in 20-μl reaction mixtures for at least 14 h. The enzymes were heat inactivated, and then 20 ng of the cleaved DNA was loaded into PCR mixtures containing primers for the target loci. Primers specific for the Arabidopsis 18S ribosomal DNA (rDNA) sequence served as controls.
Bisulfite sequencing.
Bisulfite sequencing of AtSN1 was carried out as previously described (12, 35), using PCR primers specific for AtSN1 (56).
Methylation-sensitive extension assay.
The extension assay was performed essentially as described previously (5, 34). Arabidopsis genomic DNA (1 μg) was digested for 16 h with a 10-fold excess of MspI. An additional 1-μg DNA aliquot from the mock samples was incubated without restriction enzyme and served as a background control. Single-nucleotide-extension reactions were carried out using 500 ng DNA, 1× PCR buffer (Invitrogen), 1.0 mM MgCl2, 0.25 units of Taq DNA polymerase (New England Biolabs, Beverly, MA), 0.5 μl [32P]dCTP (800 Ci/mmol; Perkin-Elmer) in 25-μl reaction mixtures for 1 h. A 10-μl amount was loaded onto each of two DE-81 filters (Whatman) and washed as described (5, 34). Radioactivity was determined by scintillation counting.
RESULTS
AL2 and L2 reverse TGS of a GFP transgene in a transcription-independent manner.
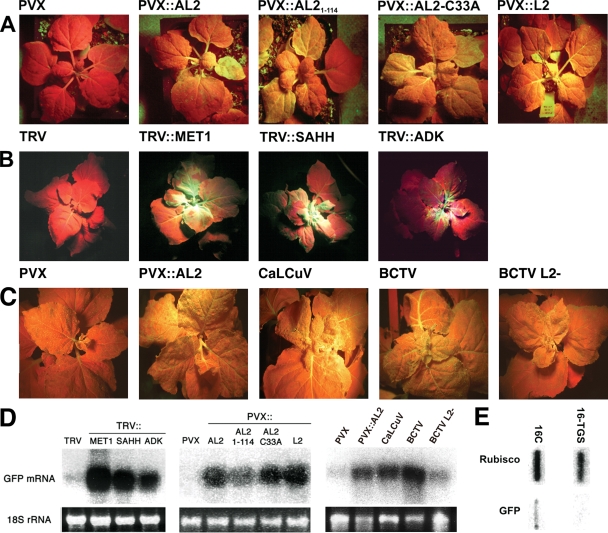
To investigate the effects of AL2 and L2 on TGS, we created a Nicotiana benthamiana line containing a transcriptionally silenced GFP transgene. N. benthamiana line 16C plants, which contain an active GFP transgene driven by the 35S promoter, were inoculated with a TRV vector expressing a portion of the 35S promoter sequence to induce TGS (19, 40). Plants were observed under UV light, and seed was collected from red flowers, where GFP expression was silenced. The resulting silenced progeny, which do not express GFP as judged by red fluorescence in UV light at the time of germination, were self-fertilized to establish the silenced (16-TGS) line. Nuclear run-on analysis confirmed that the heritable silencing in 16-TGS plants was transcriptional in nature (Fig. 1E).
FIG. 1.
Overexpression of AL2 and L2, as well as geminivirus infection, reverses TGS of a GFP transgene. (A) Transgenic N. benthamiana plants containing a transcriptionally silenced 35S-GFP transgene (line 16-TGS) were inoculated with PVX vector or PVX expressing the indicated viral protein. Plants were photographed under UV light 2 weeks postinoculation. Uniform dark red color resulting from chlorophyll autofluorescence in the absence of GFP is evident in the PVX-inoculated plant. Release of TGS is indicated by the presence of GFP (yellow-green color) in veins and mesophyll of upper leaves following inoculation with PVX::AL2, AL21-114, AL2-C33A, and L2. Results shown are representative of at least three independent trials with 4 to 8 plants per treatment. (B) As described for panel A except that 16-TGS plants were inoculated with VIGS TRV vector or TRV expressing sequences derived from the indicated endogenous genes in order to reduce their expression. Plants were photographed under UV light 4 weeks postinoculation. (C) Line 16-TGS plants were inoculated with PVX (negative control), PVX::AL2 (positive control), CaLCuV, BCTV, or BCTV L2-2 mutant (L2−) virus. Plants were photographed under UV light three weeks postinoculation. Results shown are representative of two independent trials with 16 plants per treatment. (D) Northern blot of RNA from leaves of 16-TGS plants inoculated as indicated. The 32P-labeled probe was specific for GFP mRNA. The 18S rRNA loading controls were visualized by staining the gel with ethidium bromide. (E) Nuclear run-on analysis indicating GFP transcription in line 16C and transcriptional silencing in derivative line 16-TGS. Rubisco served as a control.
Reversal assays were carried out by using PVX vectors to express TGMV AL2 and BCTV L2 proteins (50). Silenced 16-TGS plants were inoculated with PVX (empty vector control), PVX::AL2, PVX::AL21-114, PVX::AL2-C33A, and PVX::L2. AL21-114 lacks the C-terminal 15 amino acids that constitute the transcription activation domain, and the AL2-C33A mutant protein has reduced transcription activation activity (about one-third that of the wild type) (15, 53). PVX infection had no impact on GFP silencing. However, the geminivirus proteins intensified PVX symptoms (not shown), and GFP expression was evident by UV inspection in all 16-TGS plants inoculated with all PVX::AL2/L2 recombinant viruses. Yellow-green GFP fluorescence was especially apparent in leaf petioles and upper leaves where PVX symptoms were most pronounced (Fig. 1A). The presence of GFP mRNA was confirmed by Northern blot analysis (Fig. 1D).
TRV vectors were employed to knock down the expression of selected cellular genes by VIGS (36). The 16-TGS plants were inoculated with TRV (empty vector control), TRV::MET1 (positive control), TRV::SAHH, and TRV::ADK. The 500- to 750-nt targeting sequences used in these experiments were obtained from cloned N. benthamiana cDNAs (51 and our unpublished data). MET1 is a CG-specific methyltransferase required for TGS (19). SAHH refers to S-adenosyl homocysteine hydrolase, an enzyme essential to the methyl cycle that generates SAM and which also is required for TGS (37). The ADK construct targeted the methyl cycle-associated enzyme that is inhibited by AL2 and L2 (a diagram of the methyl cycle is presented in Fig. S1 in the supplemental material) (31, 52). As illustrated in Fig. 1B and D, all TRV treatments except the empty vector were able to restore GFP expression. In addition, the targeting sequences also enhanced TRV symptoms, which normally are very mild or absent (not shown). However, unlike the results with the viral proteins, which reversed silencing to roughly equivalent extents, clear differences in effectiveness were noted. MET1 targeting resulted in the most-robust GFP expression, followed in order by SAHH and ADK. This is in keeping with the known functions of these enzymes: MET1 activity is required for TGS maintenance, and SAHH is required to regenerate SAM, while ADK is an important but accessory activity that increases methyl cycle activity. We concluded that AL2 and L2 can reverse TGS of a GFP transgene by a mechanism that does not depend on transcriptional activation and which correlates with methyl cycle inhibition.
Geminiviruses reverse GFP transgene TGS in an L2-dependent manner.
The experiment described above showed that overexpression of AL2 or L2 from a PVX vector causes expression of the silenced GFP transgene in N. benthamiana line 16-TGS. To determine whether TGS reversal also occurs in the context of a natural geminivirus infection, 16-TGS plants were inoculated with CaLCuV or BCTV. In addition, we further examined the role of L2 by including a BCTV L2− mutant virus. The BCTV mutant L2-2, which contains a stop codon that allows the translation of only the first 72 (of 173) amino acids of L2, was selected for this study because it replicates to nearly wild-type levels and generates symptoms similar to those of wild-type virus (17). CaLCuV AL2− mutants could not be used because in begomoviruses, AL2 function is required to activate the expression of the BR1 movement protein and so AL2 mutants are not systemically infectious (43). PVX was used as a negative control, and PVX::AL2 served as a positive control.
Infection of 16-TGS plants by CaLCuV and wild-type BCTV caused reversal of GFP silencing, as indicated by the presence of yellow-green fluorescence in symptomatic tissue (Fig. 1C) and by Northern blot analysis of GFP mRNA levels (Fig. 1D). Further, the location of GFP expression reflected virus tissue tropism. Begomoviruses show a preference for phloem tissue, although many can also invade mesophyll cells in N. benthamiana, and GFP expression was apparent in both phloem and mesophyll of CaLCuV-infected plants. In contrast, curtoviruses, such as BCTV, are more tightly limited to phloem, and GFP expression was mostly confined to this tissue (Fig. 1C). Reversal of TGS was dependent on L2 function, as the BCTV L2− mutant virus did not cause GFP expression in regions that were clearly symptomatic (Fig. 1C and D). We concluded that CaLCuV and BCTV can reverse TGS in a biologically relevant context and that reversal depends on L2 (and, by extension, AL2) function.
AL2 and L2 cause ectopic expression of TGS-silenced Arabidopsis loci by transcription-dependent and -independent mechanisms.
To confirm and further evaluate TGS reversal activity, we constructed transgenic Arabidopsis lines expressing AL2, AL21-114, L2, or GFP (negative control) from a dex-inducible promoter. An inducible-expression system was chosen because previous studies showed that the expression of full-length AL2 is not compatible with development (44). Transgenes were confirmed by genomic PCR, and expression levels were verified by Northern blot analysis or semiquantitative RT-PCR (sqRT-PCR) (data not shown). At least two independent transgenic lines expressing AL2, AL21-114, or L2 mRNA following dex treatment but not after water (mock) treatment were selected for this study. In each experiment, extracts from individual plants were retested for transgene expression by Northern blotting or sqRT-PCR. Those showing high levels of transgene mRNAs following dex treatment were again used for sqRT-PCR with primers to amplify transcripts from selected endogenous loci known to be silenced by methylation or from control genes (45, 55).
Little or no expression of test loci was evident in plants treated with water or with a 0.05% Silwet L77 solution which is used to deliver dex (Fig. 2 and data not shown). However, following dex treatment of AL2 plants, ectopic expression was observed from all silenced loci tested, including a putative F-box family protein (At2g17690), the retrotransposons AtSN1 (short interspersed element) and Athila (long terminal repeat element), and DNA transposon CACTA-like (At2g04770) (Fig. 2). All but one of these (CACTA-like) were also overexpressed in dex-treated AL21-114 and L2 plants. Plants expressing GFP (negative control) showed no increase in the transcription of any of the sequences tested (not shown).
FIG. 2.
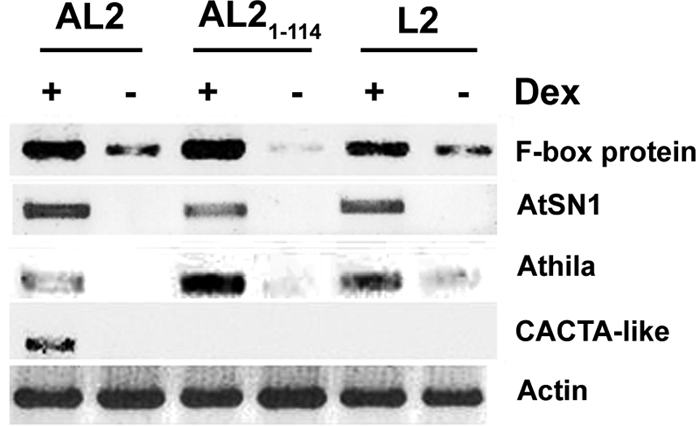
AL2, AL21-114, and L2 cause overexpression of methylated, TGS-silenced loci. Arabidopsis plants containing the indicated transgenes under the control of a dex-inducible promoter were treated with dex (+) or water (−), and expression from the endogenous silenced loci was evaluated by sqRT-PCR. Actin served as a control. The data are representative of results obtained in at least two experiments using two independent lines for each transgene.
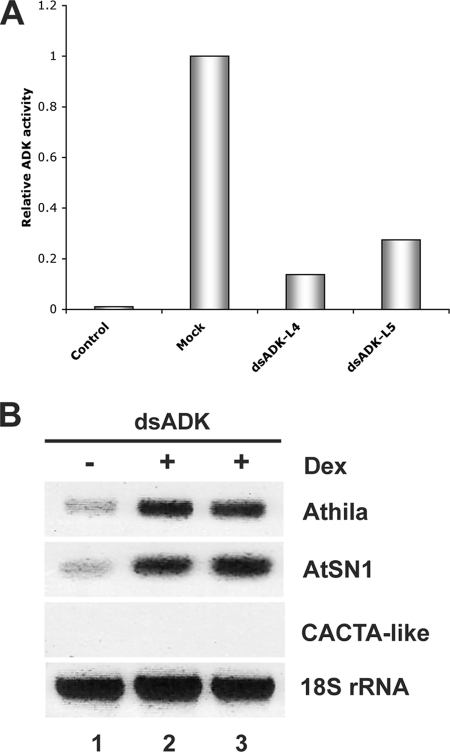
It is not yet clear why the expression of CACTA-like was enhanced only by AL2. However, it is consistent with the idea that this protein can suppress silencing by transcription-dependent and transcription-independent means, whereas AL21-114 and L2 are limited to the latter mechanism. Because the transcription-independent mechanism involves inhibition of cellular ADK activity, we next examined the consequences of dex-induced expression of an inverted repeat transgene corresponding to Arabidopsis ADK2 (dsADK). Due to the high level of similarity between the two Arabidopsis ADK genes (88% identity at the nucleotide level), we expected dsRNA produced from the dsADK transgene to target both mRNAs by RNA interference. Plants from two independent transgenic lines with reduced ADK activity following dex induction were selected (Fig. 3A), and the expression of silenced loci was examined by sqRT-PCR. Remarkably, overexpression of Athila and AtSN1 but not CACTA-like was evident in extracts from dex-induced dsADK-treated plants (Fig. 3B). We concluded that transgenic expression of AL2, AL21-114, and L2 can reverse TGS of endogenous loci silenced by methylation and that, consistent with the transcription-independent suppression mechanism, dsADK treatment phenocopies AL21-114 and L2. However, TGS suppression of the CACTA-like sequence appears to require the transcription-dependent mechanism of AL2.
FIG. 3.
dsADK causes ectopic expression of TGS-silenced loci. (A) Arabidopsis plants containing a dex-inducible transgene (dsADK) designed to express ADKds RNA were treated with dex or water, and ADK activity was evaluated. The histogram shows relative ADK activity in extracts of plants from two independent lines (dsADK-L4 and dsADK-L5) following dex or water (mock) treatment. Mock extracts were obtained from a dsADK-L4 plant. (B) Expression of the indicated endogenous loci in extracts from dsADK plants treated with dex (+) or water (−) was assessed by sqRT-PCR. 18S rRNA served as control. Lane 1, mock; lane 2, dsADK-L4; lane 3, dsADK-L5.
Transgenic expression of AL2 reduces cytosine methylation at previously silenced loci.
To determine whether the ectopic expression observed following AL2 induction was associated with reduced cytosine methylation, we first analyzed Athila and AtSN1 genomic sequences using methylation-sensitive PCR. This method involves digestion of genomic DNA with restriction endonucleases that are unable to cleave methylated target sequences, followed by PCR with locus-specific primers that flank a target site. Destruction of template due to reduced methylation is expected to result in a smaller amount of PCR product.
We tested several endonucleases which are blocked by methylation of the underlined cytosine residues, including AlwNI (CAGNNNCTG), BmrI (ACTGGG), BspCNI (CTCAG), EcoO109I (A/GGGNCCC/T), and HpyCH4V (TGCA). We observed that 2 of 6 sites tested in Athila and 3 of 12 sites in AtSN1 showed obvious decreases in PCR product in extracts prepared from AL2 transgenic plants treated with dex compared to the amount in extracts from water-treated plants (Fig. 4). These results strongly suggested that the presence of AL2 resulted in partial demethylation of cytosine residues at some of the sites examined.
FIG. 4.
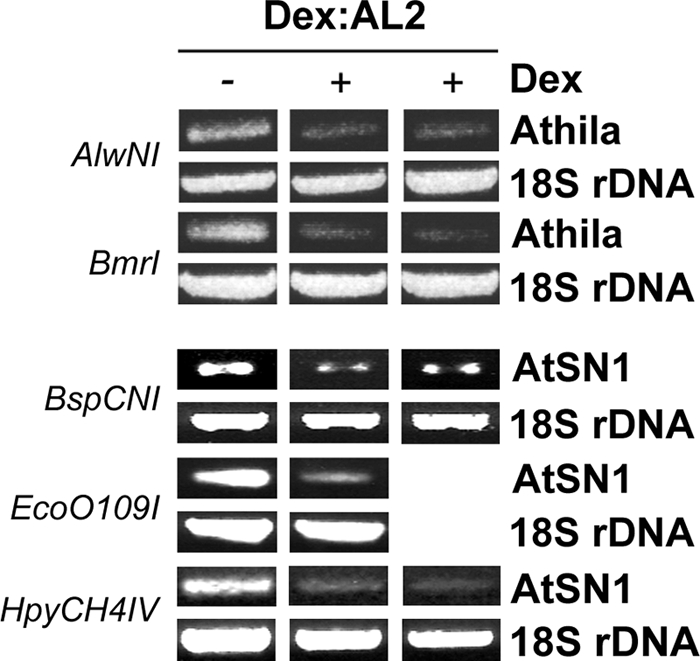
Restriction-sensitive PCR shows reduced cytosine methylation in the Athila and AtSN1 loci following expression of AL2. Arabidopsis plants containing a dex-inducible AL2 transgene (one or two independent lines) were treated with dex (+) or water (−). DNA extracts were incubated with the indicated methylation-sensitive restriction enzymes prior to genomic PCR with primers flanking the restriction site. Destruction of template due to reduced methylation is expected to result in a smaller amount of PCR product. 18S rDNA served as control.
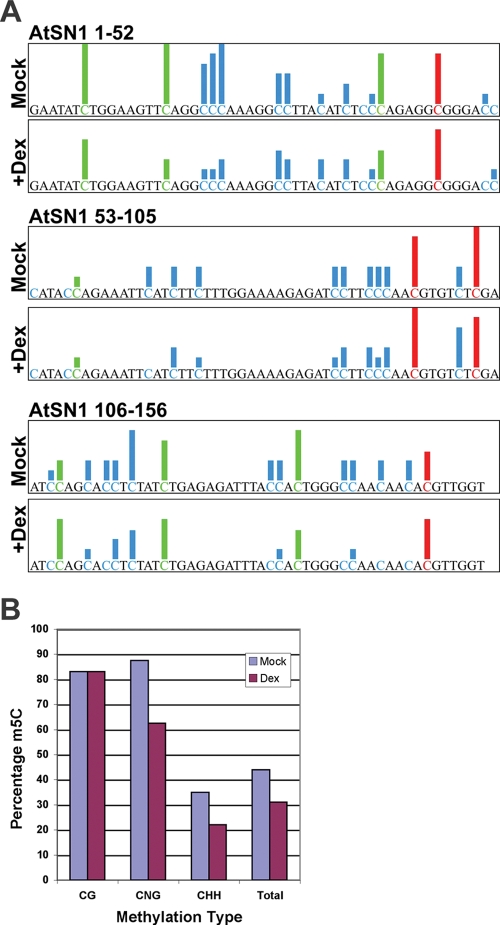
To investigate methylation status at higher resolution, bisulfite sequencing of a 156-bp fragment of AtSN1 was performed. Within this sequence, there are 4 CG, 7 CNG, and 33 CHH sites. DNA obtained from dex- or water-treated AL2 plants was treated with bisulfite reagent to convert unmethylated cytosines to uracil, followed by PCR to amplify the AtSN1 locus. PCR products were cloned, and the sequences of six clones were compiled for each treatment. We found that the presence of AL2 resulted in a 14% decrease in the total number of cytosine residues methylated, with most reductions occurring at non-CG sites (∼28% CNG and 13% CHH) (Fig. 5). Primary sequence data are presented in Fig. S2 in the supplemental material.
FIG. 5.
Bisulfite sequencing reveals reduced cytosine methylation in the AtSN1 locus following expression of AL2. DNA was obtained from dex-inducible AL2 transgenic Arabidopsis plants after they were sprayed with dex or water (mock). Following bisulfite treatment, the AtSN1 locus was amplified by PCR and cloned. Six clones each were sequenced from dex- or water-treated plants. (A) Summary of bisulfite sequencing data. Red represents CG sites, green CNG sites, and blue CHH sites. Height of bars is proportional to the number of clones with a methylated cytosine, ranging from 0 to 6. The 156-nt sequence is divided into three parts, with data from water treatment shown above data from dex treatment. (B) The histogram represents the proportions of cytosine residues methylated in different sequence contexts. m5C, 5-methylcytosine.
Transgenic expression of AL2 or L2 causes genome-wide reductions in cytosine methylation.
To gain a more-global view of the effects of AL2 and L2 on the methylation status of genomic DNA, we adopted a methylation-sensitive extension assay (5, 34). These experiments took advantage of the fact that methylation of the external cytosine in the target site of MspI (C↓CGG) prevents cleavage. (Methylation of the internal cytosine has no effect on MspI activity.) Following incubation of genomic DNA with MspI, a single-nucleotide extension assay was performed using [32P]dCTP and Taq DNA polymerase, which lacks a 3′ → 5′ exonuclease activity. Assuming complete extension, the extent of nucleotide incorporation depends on the number of MspI sites cleaved, which in turn depends on the degree of cytosine methylation, in this case at CNG sites within the MspI recognition sequence.
We first confirmed that treatment of wild-type plants with dex, which is delivered in a solution containing 0.05% Silwet L77 to facilitate cuticle penetration, had no significant impact on methylation compared to that of water treatment (see Fig. S3B in the supplemental material). We also determined that extension reactions were ∼90% complete by 15 min, reached maximum levels at 45 min, and remained at this plateau for at least 120 min (see Fig. S3A in the supplemental material). As an additional control, we also demonstrated that pUC plasmid DNA added to excess genomic DNA extract was cleaved to completion by MspI under our experimental conditions (not shown).
Methylation extension assays were then performed for 60 min using individual DNA extracts from three dex-treated plants from each of two independent AL2 or L2 transgenic lines. Extracts from water-treated AL2 or L2 plants were used as controls. The results of this analysis indicated that the expression of AL2 resulted in a 1.5- to 2.3-fold increase and the expression of L2 in a 2.1- to 2.6-fold increase in the incorporation of labeled cytosine (Fig. 6). Student's t test confirmed that the differences were significant at the 95% to 99% confidence interval. Thus, the expression of AL2 and L2 resulted in increased MspI cleavage, which reflects a corresponding global decrease in methylation levels at the CNG sites queried.
FIG. 6.
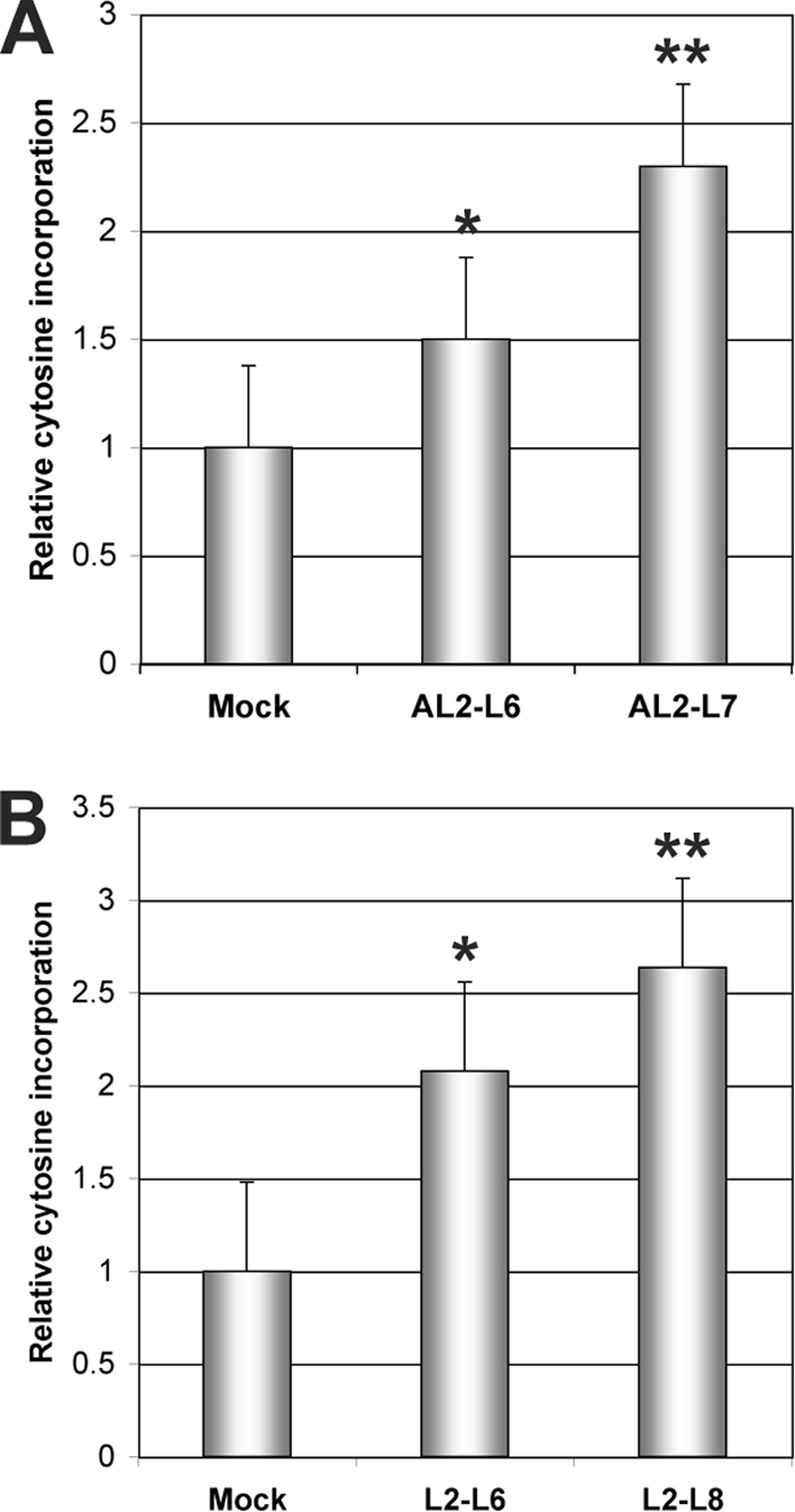
Transgenic expression of AL2 and L2 reduces genome-wide cytosine methylation. The histograms illustrate relative incorporation of dCTP observed in methylation-sensitive extension assays. DNA was obtained from dex- or water (mock)-treated Arabidopsis plants containing dex-inducible AL2 or L2 transgenes, digested to completion with MspI, and incubated with [32P]dCTP and Taq polymerase to allow single-nucleotide extension (see text). Increased incorporation reflects enhanced MspI cleavage due to reduced methylation. Assays were performed with DNA from three individual plants from each of two independent AL2 transgenic lines (AL2-L6 and AL2-L7) (A) or three individual plants from each of two independent L2 transgenic lines (L2-L6 and L2-L8) (B). Mock treatment assays contained DNA from three or four plants from the same transgenic lines. Asterisks indicate significant differences between dex- and mock-treated samples at the 95% (*) or 99% (**) confidence level, as determined by Student's t test.
DISCUSSION
Previous work has demonstrated that plants employ RNA-directed methylation of geminivirus DNA and associated histone proteins as an antiviral defense mechanism (35). Methylation likely leads to epigenetic transcriptional silencing of geminivirus chromatin. Here, using complementary approaches that exploit the advantages of N. benthamiana and Arabidopsis experimental systems, we present evidence that geminivirus AL2 and L2 proteins are able to counter this defense. Both proteins were shown to reverse TGS of a GFP transgene following expression from a PVX vector in N. benthamiana line 16-TGS. In addition, geminivirus infection of 16-TGS plants caused L2-dependent TGS reversal, suggesting that TGS suppression by geminiviruses is biologically relevant. Following dex-induced expression from AL2 and L2 transgenes in Arabidopsis, both proteins could reverse TGS of selected loci known to be silenced by methylation. To our knowledge, this is the first report that viral proteins can reverse TGS. However, given the similarities between small RNA-directed pathways in plants, the functional redundancy between pathway components, and the variety of viral silencing suppressors that inhibit various pathway enzymes or bind dsRNA, we anticipate that other DNA and RNA virus proteins will also prove capable of inhibiting TGS.
Earlier studies suggested that AL2 can suppress PTGS by at least two distinct mechanisms. A transcription-dependent mechanism is believed to involve the activation of host genes (e.g., Werner exonuclease-like 1 [WEL1]) that function as endogenous negative regulators of RNA-silencing pathways (46, 48). A second, transcription-independent mechanism is shared with L2 and with mutant AL2 proteins that are defective for transcription activation (e.g., AL21-114 and AL2-C33A). In this case, PTGS suppression results from interaction with and inhibition of ADK, which is required for optimal transmethylation activity (51-53). The studies presented here both support and extend the scope of the transcription-independent mechanism by showing that AL2, AL21-114, AL2-C33A, and L2, as well as VIGS targeting of ADK and the essential methyl cycle enzyme SAHH, can reverse TGS directed against a GFP transgene in N. benthamiana. That AL2, AL21-114, L2, and dsADK transgenes cause ectopic expression of Arabidopsis AtSN1, Athila, and F-box loci that are transcriptionally silenced by methylation provides powerful additional support. An interesting exception was the CACTA-like transposon, which is apparently refractory to the transcription-independent mechanism. The reasons for this are presently unclear. However, it is known that the AtSN1, Athila, and F-box loci are ectopically expressed in mutant backgrounds that primarily impact non-CG methylation (e.g., ddc) (45, 55). In contrast, CACTA-like expression is observed in a met1 mutant background, suggesting that CG methylation is important for silencing at this locus (55). Perhaps CG methylation patterns are more robustly maintained than non-CG methylation and, thus, less likely to be degraded by methyl cycle inhibition. Studies to determine how AL2 and L2 affect the expression of loci silenced primarily by CG or non-CG methylation and their effects on methylation in different sequence contexts are in progress. In this regard, it has been noted that a number of transposons are reactivated by mutations that cause demethylation and that for some (including CACTA), methylation in different contexts may function in an additive manner to bolster silencing (21, 30, 42). It will be interesting to see whether transgenic expression of AL2 and L2 causes differential reactivation of transposon classes and, if so, whether this also occurs in infected cells. Our work raises the possibility that host genome instability caused by transposon reactivation is a component of geminivirus pathogenesis.
Using restriction-sensitive PCR and bisulfite sequencing, we demonstrated that increased transcription of Athila and AtSN1 following dex-induced expression of an AL2 transgene is accompanied by reduced methylation of these loci. However, because methylation inhibition via ADK inactivation is not expected to be locus specific, we predicted that AL2 and L2 could affect global methylation patterns. Using a methylation-sensitive extension assay capable of assessing the CNG methylation status of MspI sites genome wide, we were able to confirm that reduced methylation caused by the viral proteins is indeed nonspecific in nature. Thus, AL2 and L2 could prove to be useful tools for attenuating global cytosine methylation, as well as other types of cellular transmethylation events that require SAM as a cofactor, such as histone methylation.
In summary, we conclude that geminivirus AL2 and L2 proteins can suppress TGS by interfering with methylation, which is accomplished by inhibition of ADK. TGS suppression activities would be expected to occur in DNA viruses that are subject to genome methylation, and their existence reinforces the importance of this process as an epigenetic defense in plants. It is possible that mammalian DNA viruses are confronted with similar defenses, and certainly there are many (e.g., herpesviruses) that have latency pathways characterized by hypermethylation of the viral genome. Whether mammalian viruses produce proteins capable of directly suppressing methylation and/or reversing TGS remains to be seen.
Supplementary Material
Acknowledgments
We thank members of the Bisaro laboratory and the Plant Biotechnology Center for helpful discussions.
This work was supported by grants from the NSF (MCB-0743261) and the National Research Initiative of the USDA (2004-35301-14508) to D.M.B. The support of the Higher Education Commission of Pakistan (to S.A.) is gratefully acknowledged.
Footnotes
Published ahead of print on 11 March 2009.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Aoyama, T., and N.-H. Chua. 1997. A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J. 11605-612. [DOI] [PubMed] [Google Scholar]
- 2.Bender, J. 2004. DNA methylation and epigenetics. Annu. Rev. Plant Biol. 5541-68. [DOI] [PubMed] [Google Scholar]
- 3.Bent, A. 2006. Arabidopsis thaliana floral dip transformation method. Methods Mol. Biol. 34387-103. [DOI] [PubMed] [Google Scholar]
- 4.Bisaro, D. M. 2006. Silencing suppression by geminivirus proteins. Virology 344158-168. [DOI] [PubMed] [Google Scholar]
- 5.Boyko, A., P. Kathiria, F. J. Zemp, Y. Yao, I. Pogribny, and I. Kovalchuk. 2007. Transgenerational changes in the genome stability and methylation in pathogen-infected plants (virus-induced plant genome instability). Nucleic Acids Res. 351714-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brough, C. L., W. E. Gardiner, N. Inamdar, X. Y. Zhang, M. Ehrlich, and D. M. Bisaro. 1992. DNA methylation inhibits propagation of tomato golden mosaic virus DNA in transfected protoplasts. Plant Mol. Biol. 18703-712. [DOI] [PubMed] [Google Scholar]
- 7.Chan, S. W.-L., I. R. Henderson, and S. E. Jacobsen. 2005. Gardening the genome: DNA methylation in Arabidopsis thaliana. Nat. Rev. 6351-360. [DOI] [PubMed] [Google Scholar]
- 8.Chan, S. W.-L., I. R. Henderson, X. Zhang, G. Shah, J. S.-C. Chien, and S. E. Jacobsen. 2006. RNAi, DRD1, and histone methylation actively target developmentally important non-CG DNA methylation in Arabidopsis. PLoS Genet. 20791-0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cokus, S. J., S. Feng, X. Zhang, Z. Chen, B. Merriman, C. D. Haudenschild, S. Pradhan, S. F. Nelson, M. Pelligrini, and S. E. Jacobsen. 2008. Shotgun bisulphite sequencing of the Arabidopsis genome reveals DNA methylation patterning. Nature 452215-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding, S.-W., and O. Voinnet. 2007. Antiviral immunity directed by small RNAs. Cell 130413-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ermak, G., U. Paszkowski, M. Wohlmuth, O. M. Scheid, and J. Paszkowski. 1993. Cytosine methylation inhibits replication of African cassava mosaic virus by two distinct mechanisms. Nucleic Acids Res. 213445-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frommer, M., L. E. McDonald, D. S. Millar, C. M. Collis, F. Watt, G. W. Grigg, P. W. Malloy, and C. L. Paul. 1992. A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc. Natl. Acad. Sci. USA 891827-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goll, M. G., and T. H. Bestor. 2005. Eukaryotic cytosine methyltransferases. Annu. Rev. Biochem. 74481-514. [DOI] [PubMed] [Google Scholar]
- 14.Hanley-Bowdoin, L., S. Settlage, and D. Robertson. 2004. Reprogramming plant gene expression: a prerequisite to geminivirus replication. Mol. Plant Pathol. 5149-156. [DOI] [PubMed] [Google Scholar]
- 15.Hartitz, M. D., G. Sunter, and D. M. Bisaro. 1999. The geminivirus transactivator (TrAP) is a single-stranded DNA and zinc-binding phosphoprotein with an acidic activation domain. Virology 2631-14. [DOI] [PubMed] [Google Scholar]
- 16.Henderson, I. R., and S. E. Jacobsen. 2007. Epigenetic inheritance in plants. Nature 447418-424. [DOI] [PubMed] [Google Scholar]
- 17.Hormuzdi, S. G., and D. M. Bisaro. 1995. Genetic analysis of beet curly top virus: examination of the roles of L2 and L3 genes in viral pathogenesis. Virology 2061044-1054. [DOI] [PubMed] [Google Scholar]
- 18.Jones, L., A. J. Hamilton, O. Voinnet, C. L. Thomas, A. J. Maule, and D. C. Baulcombe. 1999. RNA-DNA interactions and DNA methylation in post-transcriptional gene silencing. Plant Cell 112291-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones, L., F. Ratcliff, and D. C. Baulcombe. 2001. RNA-directed transcriptional gene silencing in plants can be inherited independently of the RNA trigger and requires Met1 for maintenance. Curr. Biol. 11747-757. [DOI] [PubMed] [Google Scholar]
- 20.Jones, P. A., and S. B. Baylin. 2002. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 3415-428. [DOI] [PubMed] [Google Scholar]
- 21.Kato, M., A. Miura, J. Bender, S. E. Jacobsen, and T. Kakutani. 2003. Role of CG and non-CG methylation in immobilization of transposons in Arabidopsis. Curr. Biol. 13421-426. [DOI] [PubMed] [Google Scholar]
- 22.Kjemtrup, S., K. S. Sampson, C. G. Peele, L. V. Nguyen, M. Conkling, W. F. Thompson, and D. Robertson. 1998. Gene silencing from plant DNA carried by a geminivirus. Plant J. 1491-100. [DOI] [PubMed] [Google Scholar]
- 23.Li, F., and S.-W. Ding. 2006. Virus counterdefense: diverse strategies for evading the RNA-silencing immunity. Annu. Rev. Microbiol. 60503-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lippman, Z., A.-V. Gendrel, M. Black, M. W. Vaughn, N. Dedhia, W. R. McCrombie, K. Lavine, V. Mittal, B. May, K. D. Kasschau, J. C. Carrington, R. W. Doerge, V. Colot, and R. Martienssen. 2004. Role of transposable elements in heterochromatin and epigenetic control. Nature 430471-476. [DOI] [PubMed] [Google Scholar]
- 25.Lister, R., R. C. O'Malley, J. Tonti-Filippini, B. D. Gregory, C. C. Berry, A. H. Millar, and J. R. Ecker. 2008. Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell 133523-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu, R., I. Malcuit, P. Moffett, M. T. Ruiz, J. Peart, A.-J. Wu, J. P. Rathgen, A. Bendahmane, L. Day, and D. C. Baulcombe. 2003. High throughput virus-induced gene silencing implicates heat shock protein 90 in plant disease resistance. EMBO J. 225690-5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martienssen, R. A., M. Zaratiegui, and D. B. Goto. 2005. RNA interference and heterochromatin in the fission yeast Schizosaccharomyces pombe. Trends Genet. 21450-456. [DOI] [PubMed] [Google Scholar]
- 28.Mathieu, O., J. Reinders, M. Caikovski, C. Smathajitt, and J. Paszkowski. 2007. Transgenerational stability of the Arabidopsis epigenome is coordinated by CG methylation. Cell 130851-862. [DOI] [PubMed] [Google Scholar]
- 29.McNellis, T. W., M. B. Mudgett, K. Li, T. Aoyama, D. Horvath, N.-H. Chua, and B. J. Staskawicz. 1998. Glucocorticoid-inducible expression of bacterial avirulence gene in transgenic Arabidopsis induced hypersensitive cell death. Plant J. 14247-257. [DOI] [PubMed] [Google Scholar]
- 30.Miura, A., S. Yonebayashi, K. Wantanabe, T. Toyama, H. Shimada, and T. Kakutani. 2001. Mobilization of transposons by mutation abolishing full DNA methylation in Arabidopsis. Nature 411212-214. [DOI] [PubMed] [Google Scholar]
- 31.Moffatt, B. A., Y. Y. Stevens, M. S. Allen, J. D. Snider, L. A. Periera, M. I. Todorova, P. S. Summers, E. A. Weretilnyk, L. Martin-Caffrey, and C. Wagner. 2002. Adenosine kinase deficiency is associated with developmental abnormalities and reduced transmethylation. Plant Physiol. 128812-821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pilartz, M., and H. Jeske. 2003. Mapping of abutilon mosaic geminivirus minichromosomes. J. Virol. 7710808-10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plass, C. 2002. Cancer epigenomics. Hum. Mol. Genet. 112479-2488. [DOI] [PubMed] [Google Scholar]
- 34.Pogribny, I., P. Yi, and S. J. James. 1999. A sensitive new method for rapid detection of abnormal methylation patterns in global DNA within CpG islands. Biochem. Biophys. Res. Commun. 262624-628. [DOI] [PubMed] [Google Scholar]
- 35.Raja, P., B. C. Sanville, R. C. Buchmann, and D. M. Bisaro. 2008. Viral genome methylation as an epigenetic defense against geminiviruses. J. Virol. 828997-9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ratcliff, F., A. M. Martin-Hernandez, and D. C. Baulcombe. 2001. Tobacco rattle virus as a vector for analysis of gene function by silencing. Plant J. 25237-245. [DOI] [PubMed] [Google Scholar]
- 37.Rocha, P. S. C. F., M. Sheikh, R. Melchiorre, M. Fagard, S. Boutet, R. Loach, B. A. Moffatt, C. Wagner, H. Vaucheret, and I. Furner. 2005. An Arabidopsis HOMOLOGY-DEPENDENT GENE SILENCING1 gene codes for an S-adenosyl-l-homocysteine hydrolase required for DNA methylation-dependent gene silencing. Plant Cell 17404-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rojas, M. R., C. Hagen, W. J. Lucas, and R. L. Gilbertson. 2005. Exploiting chinks in the plant's armor: evolution and emergence of geminiviruses. Annu. Rev. Phytopathol. 43361-394. [DOI] [PubMed] [Google Scholar]
- 39.Roth, B. M., G. J. Pruss, and V. B. Vance. 2004. Plant viral suppressors of RNA silencing. Virus Res. 10297-108. [DOI] [PubMed] [Google Scholar]
- 40.Ruiz, M. T., O. Voinnet, and D. C. Baulcombe. 1998. Initiation and maintenance of virus-induced gene silencing. Plant Cell 10937-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saze, H., O. Mittelsten, and J. Paszkowski. 2003. Maintenance of CG methylation is essential for epigenetic inheritance during plant gametogenesis. Nat. Genet. 3465-69. [DOI] [PubMed] [Google Scholar]
- 42.Singer, T., C. Yordan, and R. A. Martienssen. 2001. Robertson's mutator transposons in A. thaliana are regulated by the chromatin-remodeling gene Decrease in DNA Methylation (DDM1). Genes Dev. 15591-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sunter, G., and D. M. Bisaro. 1992. Transactivation of geminivirus AR1 and BR1 gene expression by the viral AL2 gene product occurs at the level of transcription. Plant Cell 41321-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sunter, G., J. Sunter, and D. M. Bisaro. 2001. Plants expressing tomato golden mosaic virus AL2 or beet curly top virus L2 transgenes show enhanced susceptibility to infection by DNA and RNA viruses. Virology 28559-70. [DOI] [PubMed] [Google Scholar]
- 45.Tran, R. K., D. Zilberman, C. de Bustos, R. F. Ditt, J. G. Henikoff, A. M. Lindroth, J. Delrow, T. Boyle, S. Kwong, T. D. Bryson, S. E. Jacobsen, and S. Henikoff. 2005. Chromatin and siRNA pathways cooperate to maintain DNA methylation of small transposable elements in Arabidopsis. Genome Biol. 6R90.1-R90.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trinks, D., R. Rajeswaran, P. V. Shivaprasad, R. Akbergenov, E. J. Oakeley, K. Veluthambi, T. Hohn, and M. Pooggin. 2005. Suppression of RNA silencing by a geminivirus nuclear protein, AC2, correlates with transactivation of host genes. J. Virol. 792517-2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vanitharani, R., P. Chellappan, and C. M. Fauquet. 2005. Geminiviruses and RNA silencing. Trends Plant Sci. 10144-151. [DOI] [PubMed] [Google Scholar]
- 48.van Wezel, R., H. Liu, Z. Wu, J. Stanley, and Y. Hong. 2003. Contribution of the zinc finger to zinc and DNA binding by a suppressor of posttrancriptional gene silencing. J. Virol. 77696-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Voinnet, O. 2005. Induction and suppression of RNA silencing: insights from viral infections. Nat. Rev. Genet. 6206-220. [DOI] [PubMed] [Google Scholar]
- 50.Voinnet, O., Y. M. Pinto, and D. C. Baulcombe. 1999. Suppression of gene silencing: a general strategy used by diverse DNA and RNA viruses of plants. Proc. Natl. Acad. Sci. USA 9614147-14152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang, H., K. J. Buckley, X. Yang, R. C. Buchmann, and D. M. Bisaro. 2005. Adenosine kinase inhibition and suppression of RNA silencing by geminivirus AL2 and L2 proteins. J. Virol. 797410-7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang, H., L. Hao, C.-Y. Shung, G. Sunter, and D. M. Bisaro. 2003. Adenosine kinase is inactivated by geminivirus AL2 and L2 proteins. Plant Cell 153020-3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang, X., S. Baliji, R. C. Buchmann, H. Wang, J. A. Lindbo, G. Sunter, and D. M. Bisaro. 2007. Functional modulation of the geminivirus AL2 transcription factor and silencing suppressor by self-interaction. J. Virol. 8111972-11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang, X. 2008. The epigenetic landscape of plants. Science 320489-492. [DOI] [PubMed] [Google Scholar]
- 55.Zhang, X., J. Yazaki, A. Sundaresan, S. Cokus, S. W.-L. Chan, H. Chen, I. R. Henderson, P. Shinn, M. Pellegrini, and S. E. Jacobsen. 2006. Genome-wide high-resolution mapping and functional analysis of DNA methylation in Arabidopsis. Cell 1261189-1201. [DOI] [PubMed] [Google Scholar]
- 56.Zilberman, D., X. Cao, and S. E. Jacobsen. 2003. Argonaute4 control of locus-specific siRNA accumulation and DNA and histone methylation. Science 299716-719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.