Abstract
Several independent lines of evidence indicate that interferon-mediated innate responses are involved in controlling herpes simplex virus type 1 (HSV-1) infection and that the viral immediate-early regulatory protein ICP0 augments HSV-1 replication in interferon-treated cells. However, this is a complex situation in which the experimental outcome is determined by the choice of multiplicity of infection and cell type and by whether cultured cells or animal models are used. It is now known that neither STAT1 nor interferon regulatory factor 3 (IRF-3) play essential roles in the replication defect of ICP0-null mutant HSV-1 in cultured cells. This study set out to investigate the specific role of ICP0 in HSV-1 resistance to the interferon defense. We have used a cell line in which ICP0 expression can be induced at levels similar to those during the early stages of a normal infection to determine whether ICP0 by itself can interfere with interferon or IRF-3-dependent signaling and whether ICP0 enables the virus to circumvent the effects of interferon-stimulated genes (ISGs). We found that the presence of ICP0 was unable to compromise ISG induction by either interferon or double-stranded RNA. On the other hand, ICP0 preexpression reduced but did not eliminate the inhibitory effects of ISGs on HSV-1 infection, with the extent of the relief being highly dependent on multiplicity of infection. The results are discussed in terms of the relationships between ICP0 and intrinsic and innate antiviral resistance mechanisms.
The innate immune response mediated through the interferon (IFN) pathway is an important component of antiviral defense mediated by individual cells and whole organisms (10, 28). In turn, many viruses express proteins that counteract the effects of the IFN response (28). In the case of herpes simplex virus type 1 (HSV-1), highly defective HSV-1 mutants activate expression of IFN-stimulated genes (ISGs) through a mechanism that is independent of IFN itself but dependent on IFN regulatory factor 3 (IRF-3) (2, 3, 19, 23, 26). HSV-1 mutants that do not express the immediate-early (IE) regulatory protein ICP0 are more sensitive than the wild-type (wt) virus to IFN pretreatment of cultured cells (13, 20), and ICP0-null mutant HSV-1 is much more pathogenic in mice unable to respond to IFN (12, 15). Furthermore, a number of experimental systems have presented evidence suggesting that a specific function of ICP0 is to interfere with IFN and/or IRF-3-dependent IFN responses (3, 16-18, 21). However, we have reported recently that the replication defect of ICP0-null mutant HSV-1 is not complemented in cultured cells lacking either STAT1 or IRF-3 (9), which raises the question of whether the relative sensitivity of ICP0-null mutant HSV-1 to an IFN-induced antiviral state results from the absence of a specific effect of ICP0 on IFN pathways or is, rather, an indirect consequence of the disabled virus being intrinsically less able to replicate in cells expressing ISGs (9).
The investigation of these complex issues is difficult because sensitivity to IFN is highly dependent on multiplicity of infection (MOI) (9) and cell type (20). Therefore, we sought to develop a system in which the specific effects of ICP0 could be examined in the absence of HSV-1 infection and which avoids potential complications arising from the use of viral vectors or plasmid transfection technologies. In an accompanying paper, we describe the construction of a cell line that expresses ICP0 at physiological levels in an inducible manner (7). The cells allow 100% complementation of plaque formation by ICP0-null mutant HSV-1, and induction of ICP0 expression induces efficient reactivation of gene expression from quiescent HSV-1 genomes (7). We have used these cells to investigate whether, by itself, ICP0 is able to impede induction of ISGs in response to IFN (through the normal STAT1 signaling pathway) or to interfere with IRF-3-dependent activation of ISGs induced by double-stranded RNA, the archetypal pathogen-associated molecular pattern (PAMP). We found that preexpression of ICP0 had no deleterious effect on either pathway. On the other hand, preexpression of ICP0 decreased (but did not eliminate) the sensitivity of HSV-1 to an IFN-induced antiviral state. We discuss the relationship between ICP0 and intrinsic and innate cellular defenses to HSV-1 infection.
MATERIALS AND METHODS
Viruses and cells.
The viruses used were wt HSV-1 strain 17+ and its derivative in1863 that contains the lacZ gene under the control of the human cytomegalovirus promoter/enhancer inserted into the tk gene (kindly provided by Chris Preston). HSV-1 mutant virus in1374 contains the tsK lesion in ICP4, a deletion of the ICP0 gene, and a mutation within VP16 that inactivates its ability to stimulate IE gene expression (27). All viruses were grown in BHK (baby hamster kidney) cells and titrated in U2OS cells, in which ICP0 is not required for efficient replication of HSV-1. Virus in1374 was propagated at the permissive temperature of 31°C and grown in the presence of 2.5 mM hexamethylbisacetamide (27). Human diploid fibroblasts (HFs) and U2OS cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. BHK cells were grown in Glasgow modified Eagle's medium supplemented with 10% newborn calf serum and 10% tryptose phosphate broth. HepaRG hepatocyte cells (11) were grown in William's medium E supplemented with 10% fetal bovine serum Gold (PAA Laboratories Ltd.), 2 mM glutamine, 5 μg/ml insulin, and 0.5 μM hydrocortisone. All cell growth media were supplemented with 100 units/ml penicillin and 100 μg/ml streptomycin. Lentivirus-transduced cells were maintained with continuous antibiotic selection, as appropriate.
Induction of ICP0 expression.
The construction and characterization of a cell system that allows efficient inducible expression of ICP0 are described in an accompanying paper (7). The control cells used to construct the inducible cell line are named HA-TetR cells, and the inducible cells themselves are named HA-cICP0 cells. HA-cICP0 cells were treated with medium containing tetracycline (catalog no. T7660; Sigma-Aldrich) at 0.1 μg/ml for 24 h. Tetracycline was maintained in the medium throughout the duration of an experiment after the initial induction in order to maintain expression of ICP0.
Virus plaque, yield, and reactivation assays.
For plaque assays, cells were seeded into 24-well dishes at 1 × 105 cells per well and then infected the following day with appropriate sequential threefold dilutions of in1863 or dl1403/CMVlacZ. After virus adsorption, the cells were overlaid with medium containing 1% human serum, and then the cells were stained for β-galactosidase-positive plaques 24 h later, as described previously (14). For virus yield assays, cells in 12-well dishes were infected with viruses at the multiplicities stated in the text; 24 h later the cells were scraped into the medium, and the mixture was sonicated and clarified prior to titration on U2OS cells.
IFN methods.
For IFN treatment experiments, medium containing human IFN-β (catalogue no. 407318; Calbiochem) at the stated concentrations was added to the cells 1 day after seeding; the cells were then incubated for a further 24 h before the next stage of experimentation (as detailed in the text). The cells were maintained with medium containing IFN-β at the same concentration throughout the course of the subsequent experiment. For induction of ISG expression by double-stranded RNA, cells were seeded into 24-well plates, and then the following day they were washed twice with serum-free medium before the addition of serum-free medium containing poly(I·C) (catalog no. P1530; Sigma-Aldrich) at 100 μg/ml. After incubation for 2 h, the cells were washed four times with serum-free medium, and then normal medium was replaced with or without tetracycline, as relevant. The cells were then incubated overnight before being harvested for Western blot analysis.
Infections and Western blot analysis.
Cells were seeded into 24-well dishes at 1 × 105 cells per well and then harvested the following day. Cell monolayers were washed twice with phosphate-buffered saline before being harvested in sodium dodecyl sulfate-polyacrylamide gel electrophoresis loading buffer. Proteins were resolved on 7.5% sodium dodecyl sulfate gels (12.5% gels for detection of ISG15) and then transferred to nitrocellulose membranes by Western blotting. The following antibodies were used: anti-ICP0 mouse monoclonal antibody (MAb) 11060 (5), anti-actin MAb AC-40 (Sigma-Aldrich), anti-ICP4 MAb 58S (29), anti-ISG15 rabbit serum H-150 (sc-50366; Santa Cruz Biotechnology, Inc.), and anti-phosphor-STAT1 (tyrosine 701) (58D6; Cell Signaling Technology).
RESULTS
ICP0 is unable to interfere with induction of ISGs by IFN or via an IRF-3-dependent mechanism.
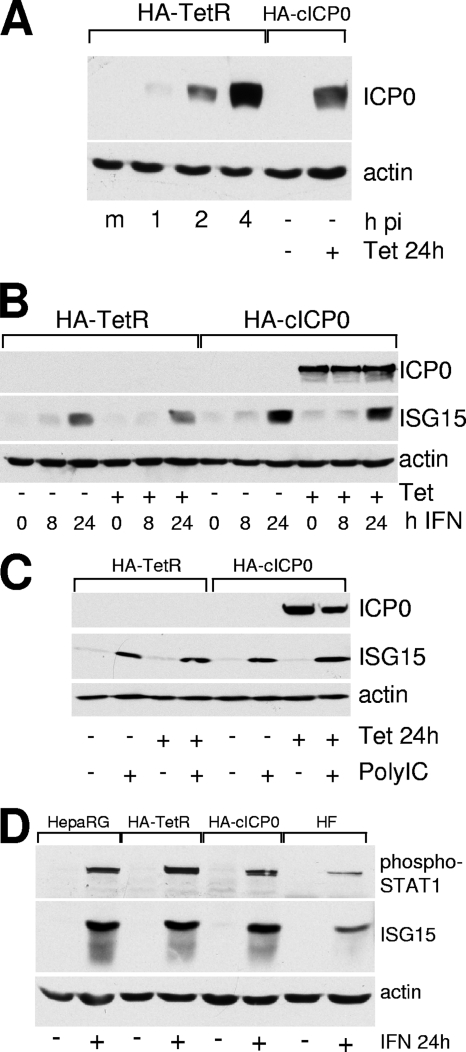
In the accompanying paper we describe the construction and characterization of a cell line, HA-cICP0, in which ICP0 expression can be induced in virtually all cells simply by adding tetracycline to the medium (7). The parental HA-TetR cell line is based on HepaRG hepatocyte cells (11), which are a good host for HSV-1 and in which replication of ICP0-null mutant virus is substantially compromised (6, 7). To assess the relative levels of ICP0 expressed during HSV-1 infection and after tetracycline induction, HA-TetR cells were infected with wt HSV-1 (MOI of 1), and samples harvested at 1, 2, and 4 h after infection were analyzed for ICP0 expression in comparison with samples of HA-cICP0 cells that were untreated or had been treated with tetracycline. Figure 1A illustrates that HA-cICP0 cells express ICP0 in amounts that are similar to those during the first 2 to 4 h of wt HSV-1 infection. Densitometric analysis indicated that the amounts of ICP0 at the 1-, 2-, and 4-h time points were induced 0.08-, 0.42-, and 1.36-fold relative to the level in HA-cICP0 cells.
FIG. 1.
(A) Comparison of the level of ICP0 expression during the first 4 h of infection of HA-TetR cells with the level expressed after induction of HA-cICP0 cells. HA-TetR cells were infected with wt HSV-1 at an MOI of 1 PFU/cell, and then samples were harvested at 1, 2, and 4 h after infection (m, mock-infected control). These samples were compared for ICP0 expression levels by Western blotting to extracts of HA-cICP0 cells with or without tetracycline treatment (0.1 μg/ml; 24 h). p.i., postinfection. (B) ICP0 does not impede induction of ISGs by IFN. HA-TetR and HA-cICP0 cells were untreated or treated with tetracycline (0.1 μg/ml) for 24 h; cells were subsequently treated with IFN (50 U/ml) for the times indicated in the continuous presence of tetracycline. Samples were harvested and analyzed by Western blotting for ICP0, ISG15, and actin. (C) ICP0 does not impede induction of ISGs by poly(I·C). This experiment was similar to that described in panel B, but poly(I·C) treatment was used to stimulate ISG expression through the IRF-3 pathway. (D) Comparison of induction of phosphorylated STAT1 and ISG15 after treatment of HepaRG, HA-TetR, HA-cICP0, and HFs with IFN-β (100 U/ml) for 24 h. Actin provides the loading control. +, with the indicated treatment; −, no treatment.
Having established a system in which virtually all cells of a population could be induced to express ICP0 that was fully functional in terms of complementation of ICP0-null mutant HSV-1 (7), we were in a position to test specific effects of the protein. An issue of current interest is whether ICP0 of itself can specifically interfere with the IFN response. Previous work had reported that ICP0-null mutant HSV-1 is hypersensitive to IFN pretreatment (13, 20, 21), that defective HSV-1 mutants induce ISG expression though an IRF-3-dependent pathway (3, 19, 23, 26), and that ICP0 expressed from viral vectors can impede this response (3, 17). We recently tested the hypothesis that these effects might be a major factor in the plaque-forming defect of ICP0-null mutant HSV-1 in HFs. Perhaps surprisingly, we found that neither STAT1 nor IRF-3 played any role in the ICP0-null mutant defect and that the previously reported apparent hypersensitivity of ICP0-null mutant HSV-1 was a complex issue that was critically dependent on MOI and choice of assay (9). At a low MOI (0.001), wt HSV-1 yield was as sensitive to the effects of IFN pretreatment as that of the mutant, but with increasing MOIs the wt eventually became resistant while the sensitivity of the mutant, although decreased by increasing the multiplicity, could not be reduced to zero (9). These and other observations in our previous paper left open the question whether ICP0 itself could impede the IFN response. HA-cICP0 cells provided an ideal opportunity to test this hypothesis.
HA-TetR and HA-cICP0 cells were treated with tetracycline or left untreated, and then 24 h later the cells were challenged with IFN-β at 50 U/ml. Samples were prepared for Western blot analysis at 8 and 24 h after IFN treatment. The results clearly show that ISG induction, as detected by expression of ISG15, was unaffected by the presence of ICP0 (Fig. 1B). Therefore, ICP0 does not interfere with any step of the pathway leading from IFN engagement with its receptor at the cell surface through to synthesis of a representative ISG. We next tested whether ICP0 can interfere with an IRF-3-dependent response. IRF-3 is a crucial factor in the signal transduction pathway by which engagement of various PAMPs leads to stimulation of IFN-β gene transcription and subsequent induction of ISGs (28). PAMPs can include defective HSV-1 mutants (as noted above) and double-stranded RNA, one of the strongest ISG inducers. Since poly(I·C) can be used as a surrogate for authentic double-stranded RNA, we treated HA-TetR and HA-cICP0 cells with poly(I·C) after treatment with tetracycline, using controls analogous those of the above experiment. Figure 1C shows that poly(I·C) stimulated ISG15 expression in HA-TetR and HA-cICP0 cells, and the extent of the induction was unaffected by prior induction of ICP0 expression. Therefore, it is clear that in this cell type and by this assay, ICP0 does not interfere with IRF-3-dependent signal transduction. Although this conclusion might seem at variance with some previously published work (3, 16, 17), we note that this is a simple direct test without complicating features, and more recent analysis using other systems in another laboratory has yielded results that are consistent with those shown in Fig. 1B (Karen Mossman, personal communication).
Since many previous studies investigating the relationship between IFN, HSV-1 infection, and ICP0 have been performed in HFs, we compared HepaRG and derivative cells with HFs in terms of production of phosphorylated STAT1 and ISG15 induction. Figure 1D illustrates that neither of these two key indicators of an intact IFN response is defective in HepaRG lines compared to HFs. However, in order to test whether preexpression of ICP0 could counteract the induction of ISGs that occurs in response to defective HSV-1 infection in HFs and Vero cells (3, 19, 23), we also tested whether infection with defective HSV-1 mutant in1374 or UV-inactivated HSV-1 mutant in1312 (which carries the same lesions as in1374 in VP16, ICP0, and ICP4) induced ISG15 in HepaRG-derived cells. Although in1374 induced ISG15 expression in HFs (9), no such response was detectable in HepaRG-derived cells (data not shown). Therefore, we could not test whether preexpression of ICP0 interferes with this response using the tools described here. However, if it did so, it would have to be at a stage upstream of IRF-3 activation since ISG induction by defective HSV-1 is dependent on IRF-3 (2, 9), and the results shown in Fig. 1C demonstrate that events downstream of IRF-3 are not impeded by preexpressed ICP0.
ICP0 can partially overcome a preexisting IFN-induced antiviral state.
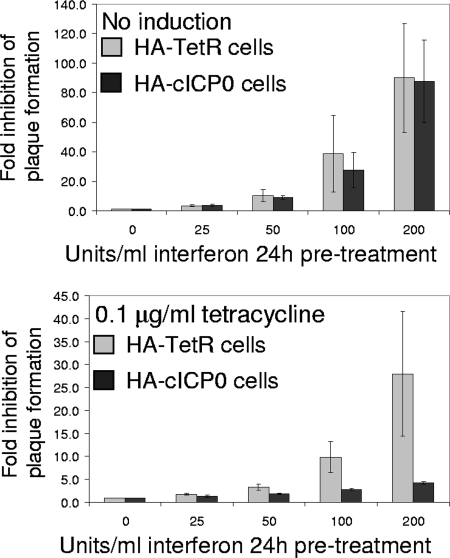
The above results demonstrate that ICP0 cannot interfere with major components of the IFN response, but considerable previous work implies that ICP0 renders the virus less sensitive to an IFN-induced antiviral state, at least at higher MOIs (9, 13, 19, 20). Therefore, we used the HA-cICP0 cell system to investigate whether such cells became more permissive for HSV-1 infection after IFN treatment if ICP0 was preexpressed. We conducted two series of experiments, first using simple plaque assays and, second, by measuring virus yields after infection at a range of multiplicities.
HA-TetR and HA-cICP0 cells were pretreated with IFN-β at concentrations varying from 0 to 200 U/ml, and then plaque assays were conducted with wt HSV-1 (in1863). Figure 2 (upper panel) shows that plaque formation was inhibited by nearly 2 orders of magnitude in both cell types at the highest IFN concentration used. In a parallel experiment, both cell types were first incubated in the presence of tetracycline for 24 h to induce ICP0 expression in HA-cICP0 cells before IFN treatment. Preexpression of ICP0 reduced the IFN sensitivity of wt HSV-1 by about 20-fold, but note that plaque numbers were still reduced on average by fourfold in ICP0-expressing cells (Fig. 2, lower panel). Therefore, it appears that ICP0 reduces but does not eliminate HSV-1 sensitivity to an induced IFN response, as detected simply by plaque formation.
FIG. 2.
Effect of ICP0 preexpression on sensitivity to IFN pretreatment of HSV-1 plaque formation. HA-TetR and HA-cICP0 cells were treated with tetracycline (0.1 μg/ml) for 24 h (lower panel) or left untreated (upper panel) before subsequent treatment for 24 h with increasing concentrations of IFN-β, as indicated. Plaque assays using in1863 were then conducted on replicate wells under each condition, and the numbers of plaques were counted. The results are expressed as relative inhibition of plaque numbers compared to samples not treated with IFN. The data show means and standard deviations of plaque reduction factors from four or five determinations at each concentration of IFN.
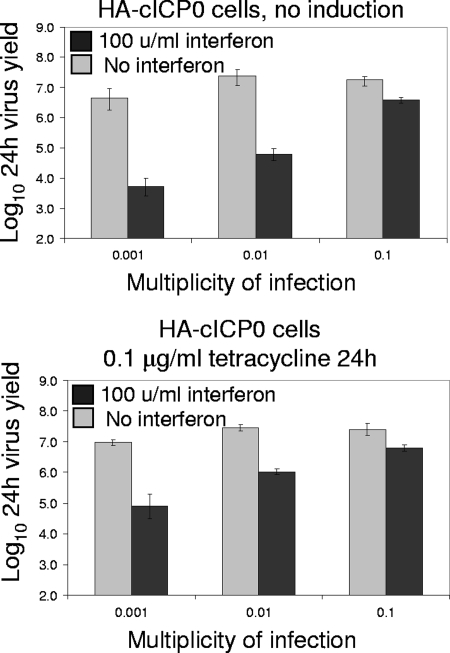
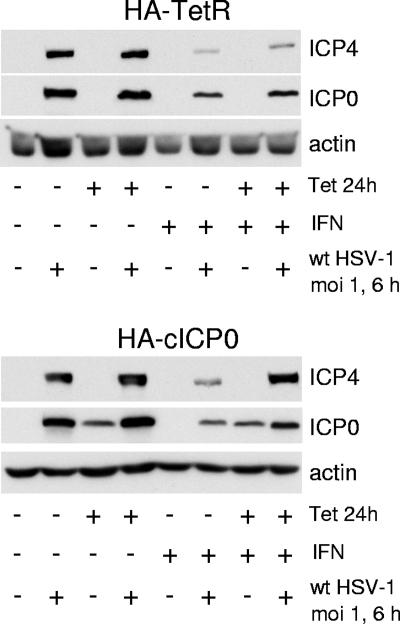
However, since plaque sizes were significantly reduced after IFN pretreatment, we also measured virus yields 24 h after infection at various multiplicities of HA-cICP0 cells with or without preinduction of ICP0 expression. As observed previously (9), sensitivity of wt HSV-1 to IFN pretreatment in this assay is low at a relatively high MOI (MOI of 0.1), but virus yields can be reduced by up to 3 orders of magnitude if input multiplicity is reduced to 0.001 (Fig. 3, upper panel). Although preexpression of ICP0 increased wt HSV-1 yields at a low MOI, virus replication was still inhibited by around 100-fold in these experiments (Fig. 3, lower panel). The apparent differences between the results shown in Fig. 2 and 3 can be explained by reduced plaque size and probably reduced yields of virus per infected cell in all cell types in the presence of IFN. It is clear that preexpression of ICP0 is unable to eliminate the effects of an IFN-induced antiviral state during at a low MOI. At higher MOIs, however, ICP0 preexpression relieves IFN-induced repression to a significant extent. This conclusion was further supported by Western blot analysis of cells infected with wt HSV-1 at an MOI of 1 for 6 h, following a protocol of ICP0 induction and IFN pretreatment (Fig. 4). This experiment reveals a marked increase in ICP4 expression in IFN-treated cells if ICP0 is induced prior to infection (Fig. 4, lower panel, far right lane).
FIG. 3.
Effect of ICP0 preexpression on sensitivity to IFN pretreatment of HSV-1 replication in 24-h virus yield experiments. HA-cICP0 cells were treated with tetracycline (0.1 μg/ml) for 24 h (lower panel) or left untreated (upper panel) before subsequent treatment for 24 h with or without IFN-β (100 units/ml). The cells were then infected with in1863 at the indicated multiplicities. Total progeny virus was harvested at 24 h after infection and titrated on U2OS cells. The data show the means of two independent determinations, with the ranges of the individual results indicated by the error bars.
FIG. 4.
Effect of ICP0 preexpression on IFN-induced repression of HSV-1 IE gene expression. HA-TetR (upper panel) and HA-cICP0 cells (lower panel) were treated with tetracycline (0.1 μg/ml) for 24 h or left untreated before subsequent treatment for 24 h with IFN-β (100 U/ml), as indicated. Selected wells were then infected with wt HSV-1 at an MOI of 1 PFU/cell for 6 h, and then whole-cell extracts were prepared and analyzed by Western blotting, probing for ICP4, ICP0, and actin as indicated. +, with the indicated treatment; −, no treatment.
Taken together, the results shown in Fig. 1 to 4 demonstrate that (i) ICP0 is unable to impede induction of ISGs by two major pathways and (ii) that it is unable to eliminate completely the inhibitory effects of ISGs themselves although it does result in a decrease in their effectiveness, especially in higher-multiplicity infections.
DISCUSSION
This study is concerned with the relationship between ICP0 and IFN-mediated antiviral defense. This is a pertinent question since IFN pathways are important for controlling HSV-1 infection in mice, and ICP0-null mutant viruses are particularly sensitive to this defense (12, 15). Although the extent of the effect differs with cell type and multiplicity, several studies have reported that ICP0-null mutant HSV-1 is less able to replicate than wt virus in the face of an antiviral defense induced by IFN pretreatment (1, 9, 13, 19, 20). Defective HSV-1 mutants induce an IFN response via an IRF-3-dependent pathway (2, 3, 16-19, 21, 23, 26), and ICP0 has been implicated in counteracting ISG induction in a variety of different assays (3, 16, 18, 21). However, complete depletion of IRF-3 function had no effect on the plaque-forming efficiency of ICP0-null mutant HSV-1 (9), reopening the question of whether ICP0 plays a specific role in impeding IRF-3-dependent induction of ISGs. The experiments presented here clearly demonstrate that ICP0 expressed in the nucleus in amounts similar to those at early times of infection is unable to inhibit either IFN or IRF-3-dependent induction of a typical ISG. Nonetheless, preexpression of ICP0 significantly improved HSV-1 plaque formation in IFN-pretreated cells and partially protected virus replication efficiency from an IFN-induced antiviral state.
An important consideration for analysis of the results presented here is that the level of ICP0 expressed in induced HA-cICP0 cells, although sufficient to complement plaque formation by ICP0-null mutant HSV-1 and to derepress quiescent HSV-1 genomes at maximum efficiencies (7), is restricted to that pertaining during the first few hours of infection (Fig. 1 and 4). Therefore, it is clear that amounts of ICP0 sufficient to be fully effective in its core biological functions (lytic replication and derepression of quiescent genomes) do not affect IFN- or IRF-3-dependent signaling events. However, ICP0 accumulates to much higher levels at later times of a normal infection and also in most other expression systems, such as plasmid transfection, ICP4-defective HSV-1 mutant infections, or adenoviral vectors engineered for high-level expression. Accordingly, this study would not detect effects that are dependent on very high levels of ICP0 expression. Therefore, we cannot exclude the possibility that interference in IFN pathways might occur during later stages of HSV-1 infection in an ICP0-dependent manner. However, if this were to occur, it would be unlikely to have significant biological impact on the infected cell itself because at this stage the infection would have progressed to a point analogous to one at a high MOI and would, therefore, be relatively resistant to inhibition by the IFN response.
Regardless of whether high levels of ICP0 are able to achieve effects undetected in this study, the results shown in Fig. 3 and 4 demonstrate that ICP0 expression at the levels in induced HA-cICP0 cells are able to diminish the inhibitory effects of ISGs on HSV-1 replication efficiency. We suggest two working hypotheses that are consistent with these observations. The first is that ICP0 improves HSV-1 replication in IFN-treated cells nonspecifically by increasing the robustness of viral gene expression to an extent that overcomes, at least partially, the inhibitory effect of ISGs. The second possibility is that ICP0 directly targets the inhibitory effects of one or more ISGs in addition to its effects on cellular intrinsic resistance pathways. Indeed, it is possible that the intrinsic and innate resistance mechanisms have elements in common. We note that the cellular promyelocytic leukemia (PML) and Sp100 proteins, prominent components of nuclear substructures known as PML nuclear bodies, or ND10, are both expressed from IFN-responsive genes and that both have been implicated in an intrinsic cellular antiviral defense (4, 6, 8, 22). Furthermore, it has been suggested that PML is required for the IFN-induced anti-HSV-1 defense (1) although the quantitative impact of this effect does not explain the full extent of IFN-mediated inhibition (9), nor is PML solely responsible for the intrinsic (IFN independent) antiviral defense (6, 8, 9).
The first model proposed above implies that the primary effect of ICP0 is to eliminate intrinsic cellular resistance to HSV-1 infection mediated through preexisting proteins that act to repress viral gene expression and that this repression is separable from the inhibitory effects of ISGs. In other words, in IFN-treated cells the intrinsic and innate antiviral effects are additive. In this model, ICP0-defective viruses appear more sensitive than the wt to the effects of IFN and ISGs because they are unable to impede the underlying intrinsic resistance and are thus subject to the additive effects of both defenses in IFN-treated cells. In contrast, the wt virus would be inhibited by only the innate response in such cells. In the second model, the intrinsic and innate defenses are still at least partially separable, but the mechanism by which ICP0 counters the intrinsic defense also renders less efficient the inhibitory effect of one or more ISGs themselves. Finally, it is important to make a distinction between the cultured cell systems and analyses presented here and the situation in mouse models, in which it is clear that IFN pathways play a central role in controlling HSV-1 infections, particularly of ICP0-null mutant virus (12, 15, 24, 25). The cultured cell systems allow dissection of molecular mechanisms while the animal models present a more holistic analysis in which the effects of intrinsic, innate, and acquired immunity combine to control the infection.
Acknowledgments
This work was supported by the Medical Research Council and in part by EC FP6 SME-STREP project TargetHerpes (LSHG-CT-2006-037517).
Chris Preston kindly supplied virus in1863. We thank Rick Randall (University of St. Andrews) for advice on IFN-related experiments. We thank members of the Everett laboratory for their input throughout the course of this project.
Footnotes
Published ahead of print on 4 March 2009.
REFERENCES
- 1.Chee, A. V., P. Lopez, P. P. Pandolfi, and B. Roizman. 2003. Promyelocytic leukemia protein mediates interferon-based anti-herpes simplex virus 1 effects. J. Virol. 777101-7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins, S. E., R. S. Noyce, and K. L. Mossman. 2004. Innate cellular response to virus particle entry requires IRF3 but not virus replication. J. Virol. 781706-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eidson, K. M., W. E. Hobbs, B. J. Manning, P. Carlson, and N. A. DeLuca. 2002. Expression of herpes simplex virus ICP0 inhibits the induction of interferon-stimulated genes by viral infection. J. Virol. 762180-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Everett, R. D., and M. K. Chelbi-Alix. 2007. PML and PML nuclear bodies: implications in antiviral defence. Biochimie 89819-830. [DOI] [PubMed] [Google Scholar]
- 5.Everett, R. D., A. Cross, and A. Orr. 1993. A truncated form of herpes simplex virus type 1 immediate-early protein Vmw110 is expressed in a cell type dependent manner. Virology 197751-756. [DOI] [PubMed] [Google Scholar]
- 6.Everett, R. D., C. Parada, P. Gripon, H. Sirma, and A. Orr. 2008. Replication of ICP0-null mutant herpes simplex virus type 1 is restricted by both PML and Sp100. J. Virol. 822661-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everett, R. D., M. L. Parsy, and A. Orr. 2009. Analysis of the functions of herpes simplex virus type 1 regulatory protein ICP0 that are critical for lytic infection and derepression of quiescent viral genomes. J. Virol. 834963-4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Everett, R. D., S. Rechter, P. Papior, N. Tavalai, T. Stamminger, and A. Orr. 2006. PML contributes to a cellular mechanism of repression of herpes simplex virus type 1 infection that is inactivated by ICP0. J. Virol. 807995-8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Everett, R. D., D. F. Young, R. E. Randall, and A. Orr. 2008. STAT-1- and IRF-3-dependent pathways are not essential for repression of ICP0-null mutant herpes simplex virus type 1 in human fibroblasts. J. Virol. 828871-8881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodbourn, S., L. Didcock, and R. E. Randall. 2000. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 812341-2364. [DOI] [PubMed] [Google Scholar]
- 11.Gripon, P., S. Rumin, S. Urban, J. Le Seyec, D. Glaise, I. Cannie, C. Guyomard, J. Lucas, C. Trepo, and C. Guguen-Guillouzo. 2002. Infection of a human hepatoma cell line by hepatitis B virus. Proc. Natl. Acad. Sci. USA 9915655-15660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halford, W. P., C. Weisend, J. Grace, M. Soboleski, D. J. Carr, J. W. Balliet, Y. Imai, T. P. Margolis, and B. M. Gebhardt. 2006. ICP0 antagonizes Stat 1-dependent repression of herpes simplex virus: implications for the regulation of viral latency. Virol. J. 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harle, P., B. Sainz, Jr., D. J. Carr, and W. P. Halford. 2002. The immediate-early protein, ICP0, is essential for the resistance of herpes simplex virus to interferon-alpha/beta. Virology 293295-304. [DOI] [PubMed] [Google Scholar]
- 14.Jamieson, D. R., L. H. Robinson, J. I. Daksis, M. J. Nicholl, and C. M. Preston. 1995. Quiescent viral genomes in human fibroblasts after infection with herpes simplex virus type 1 Vmw65 mutants. J. Gen. Virol. 761417-1431. [DOI] [PubMed] [Google Scholar]
- 15.Leib, D. A., T. E. Harrison, K. M. Laslo, M. A. Machalek, N. J. Moorman, and H. W. Virgin. 1999. Interferons regulate the phenotype of wild-type and mutant herpes simplex viruses in vivo. J. Exp. Med. 189663-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin, R., R. S. Noyce, S. E. Collins, R. D. Everett, and K. L. Mossman. 2004. The herpes simplex virus ICP0 RING finger domain inhibits IRF3- and IRF7-mediated activation of interferon-stimulated genes. J. Virol. 781675-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melroe, G. T., N. A. DeLuca, and D. M. Knipe. 2004. Herpes simplex virus 1 has multiple mechanisms for blocking virus-induced interferon production. J. Virol. 788411-8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melroe, G. T., L. Silva, P. A. Schaffer, and D. M. Knipe. 2007. Recruitment of activated IRF-3 and CBP/p300 to herpes simplex virus ICP0 nuclear foci: potential role in blocking IFN-beta induction. Virology 360305-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mossman, K. L., P. F. Macgregor, J. J. Rozmus, A. B. Goryachev, A. M. Edwards, and J. R. Smiley. 2001. Herpes simplex virus triggers and then disarms a host antiviral response. J. Virol. 75750-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mossman, K. L., H. A. Saffran, and J. R. Smiley. 2000. Herpes simplex virus ICP0 mutants are hypersensitive to interferon. J. Virol. 742052-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mossman, K. L., and J. R. Smiley. 2002. Herpes simplex virus ICP0 and ICP34.5 counteract distinct interferon-induced barriers to virus replication. J. Virol. 761995-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Negorev, D. G., O. V. Vladimirova, A. Ivanov, F. Rauscher, 3rd, and G. G. Maul. 2006. Differential role of Sp100 isoforms in interferon-mediated repression of herpes simplex virus type 1 immediate-early protein expression. J. Virol. 808019-8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicholl, M. J., L. H. Robinson, and C. M. Preston. 2000. Activation of cellular interferon-responsive genes after infection of human cells with herpes simplex virus type 1. J. Gen. Virol. 812215-2218. [DOI] [PubMed] [Google Scholar]
- 24.Pasieka, T. J., B. Lu, S. D. Crosby, K. M. Wylie, L. A. Morrison, D. E. Alexander, V. D. Menachery, and D. A. Leib. 2008. Herpes simplex virus virion host shutoff attenuates establishment of the antiviral state. J. Virol. 825527-5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasieka, T. J., B. Lu, and D. A. Leib. 2008. Enhanced pathogenesis of an attenuated herpes simplex virus for mice lacking Stat1. J. Virol. 826052-6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preston, C. M., A. N. Harman, and M. J. Nicholl. 2001. Activation of interferon response factor-3 in human cells infected with herpes simplex virus type 1 or human cytomegalovirus. J. Virol. 758909-8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Preston, C. M., and M. J. Nicholl. 2005. Human cytomegalovirus tegument protein pp71 directs long-term gene expression from quiescent herpes simplex virus genomes. J. Virol. 79525-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Randall, R. E., and S. Goodbourn. 2008. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 891-47. [DOI] [PubMed] [Google Scholar]
- 29.Showalter, S. D., M. Zweig, and B. Hampar. 1981. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect. Immun. 34684-692. [DOI] [PMC free article] [PubMed] [Google Scholar]