Abstract
Natural killer (NK) cells are associated with the innate immune response and are important in many viral infections. Recent studies indicate that NK cells can control human immunodeficiency virus type 1 (HIV-1) replication. We studied the effect of NK cells on HIV-1 replication in a subpopulation of HIV-1-infected individuals termed elite suppressors (ES) or elite controllers. These patients maintain a clinically undetectable viral load without treatment and thus provide a fascinating cohort in which to study the immunological response to HIV-1. Using an autologous system, we analyzed the effects of NK cells and CD8+ T cells on viral replication in CD4+ T lymphoblasts. Although we had postulated that NK cells of ES would be highly effective at controlling viral replication, we found that NK cells from some, but not all, ES were capable of inhibiting replication in the presence of interleukin-2, and the inhibition was less robust than that mediated by CD8+ T cells. Additionally, we examined whether particular alleles of the KIR receptors, specifically KIR3DS1 and KIR3DL1, or allele-ligand combinations correlated with the control of HIV-1 replication by NK cells and whether any specific KIR alleles were overrepresented in ES. Our ES cohort did not differ from the general population with respect to the frequency of individual KIR. However, of the eight ES studied, the four exhibiting the most NK cell-mediated control of viral replication also had the fewest activating KIR and were haplotype A. Thus, the strong NK cell-mediated inhibition of viral replication is not necessary for the immunological control of HIV-1 in all ES.
A small subset of untreated, human immunodeficiency virus type 1 (HIV-1)-infected individuals referred to as elite suppressors (ES) control viremia to levels that are undetectable by ultrasensitive commercial assays while maintaining high CD4+ T-cell counts (13, 37). While defective virus has been shown to account for the control of virus in some patients, examining multiple host factors in ES with replication-competent virus (9) already has provided critical information on the immune response to HIV-1 and may yield important insights into future therapies and vaccine development.
Research on ES suggests that CD8+ T cells play a crucial role in an effective response to HIV-1. CD8+ T cells from ES are capable of controlling viral replication in autologous CD4+ T cells significantly better than CD8+ T cells from progressors (36), and only the former proliferate (29) and secrete multiple cytokines (8) in response to HIV-1 antigens. Furthermore, certain class I HLA alleles, such as HLA-B*27 and HLA-B*57, which appear to be important in the cytotoxic T-lymphocyte (CTL) response, are overrepresented in ES (15, 19, 21, 30, 32). A second, less well studied cytotoxic cell also may play a role in the control of HIV-1. Natural killer (NK) cells are part of the innate immune system and are an important component of the host response to many viral infections. They act on target cells via cytokine release and cytolysis in response to the integration of signals from inhibitory and activating receptors.
The striking propensity of HIV-1 to evolve rapidly in response to immunologic or pharmacologic pressure suggests that the virus has the capability to evade the NK cell response, and indeed selection for evasive measures seems to have occurred. The virus-induced downregulation of HLA-A and -B molecules on infected cells provides some protection against the CTL response; at the same time, however, HLA-C molecules are not downregulated upon infection (12). NK cell interaction with HLA-C can inhibit NK cytotoxic effects, and thus the retention of HLA-C on infected cells can provide some protection against the NK cell response. Additionally, a variety of alterations in NK cell function have been observed during HIV-1 infection. NK cells of patients with chronic HIV-1 have altered phenotypes and effector capabilities: NK cells from viremic patients have an increased expression of inhibitory receptors, and there is an expansion of the defective CD56− NK cells compared to the levels in patients on highly active antiretroviral therapy or in ES (7, 27). These changes may be due to alterations in the cytokine environment during infection, which can affect the activation of the NK cells (39); they also may be due to direct interactions between HIV-1 gene products and the NK cells (20). Although the precise cause is unknown, the result is the development of defective NK cells that express an altered receptor and NK cell marker phenotype.
Studies specifically examining a role for NK cells in the response to HIV-1 have yielded conflicting results. During acute HIV-1 infection, the NK cell population is activated and expands, particularly the cytotoxic CD56dim population (2, 3). This activation declines in the chronic phase, and at least one study suggests that the drop in the viral load (VL) of patients during acute infection occurs before the CD8+ T-cell response is fully activated; this could be attributed to the effect of NK cells (2). At the same time, the study of exposed, uninfected individuals shows a correlation between resistance to acquiring HIV-1 infection and NK cell activation levels, cytokine release, and cytotoxicity to NK cell-sensitive cell lines (33, 38). Additionally, a recent whole-genome association study identified three single-nucleotide polymorphisms that appear to be important for the host control of HIV-1 (16). Two of these may have an impact on NK cell function, one that is associated with HLA-B*57 and a second that correlates with higher HLA-C mRNA expression. Taken together, such data suggest that NK cells are important for preventing HIV-1 infection and/or reducing the magnitude of viral replication in acute infection, thereby contributing to the ability of ES to control viremia.
In this study, we provide the first characterization of NK cells in patients who naturally control HIV-1 infection. Considering that the effectiveness of CD8+ T cells against viral replication is well documented, we directly compared the effect of NK cells to that of CD8+ T cells from ES on viral replication to put the effect of NK cells in perspective. We studied the NK cell response by measuring the change in p24 production when autologous effector cell populations were coincubated with infected CD4+ lymphoblasts with and without the addition of interleukin-2 (IL-2). Additionally, we examined the killer immunoglobulin-like receptors (KIR) and KIR ligand genotype of ES patients to determine whether any KIR are overrepresented in ES and whether KIR-ligand combinations correlated with the HIV-1 inhibitory activity of the NK cells from specific patients. Previous studies have identified correlations between the expression of certain KIR and progression to AIDS in chronic progressors (25, 26); however, a connection between KIR, KIR ligands, and the control of HIV-1 has yet to be identified in ES. The results of these studies significantly advance the understanding of the nature of NK cells and of their potential role in reducing HIV-1 replication.
MATERIALS AND METHODS
Patient information.
The ES used in this study have been described previously (4, 5). They have maintained VLs of <50 copies/ml for a median of 10 years, and their median CD4+ T-cell count is 898 cells/ml. Uninfected donors were HIV-1-seronegative laboratory volunteers. The protocol was approved by the institutional review board of Johns Hopkins University School of Medicine. Informed consent was obtained before phlebotomy.
NK and CD8+ T-cell assay and autologous virus assay.
PBMCs from donors and ES were separated into three populations. One was activated with 0.5 μg/ml phytohemagglutinin (PHA) and incubated for 3 days at 37°C in RPMI medium with 10% fetal calf serum and 100 μl/ml of recombinant IL-2 to make CD4+ lymphoblasts, which were used as target cells; the second and third populations were used for NK and CD8+ T-cell isolation. NK cells and CD8+ T cells were isolated using the MACS NK cell isolation kit and CD8+ T-cell isolation kit (Miltenyi Biotec) according to the manufacturer's instructions. These kits negatively depleted the unwanted populations in peripheral blood mononuclear cells (PBMCs), resulting in untouched CD8+ T cells and NK cells. Following isolation, cells from ES patients were sorted via fluorescence-activated cell sorting to remove CD3+ and CD8 bright cells from the NK cells and CD4+, CD56+, and CD16+ from the CD8+ T cells; this was done exclusively for ES patients. Antibodies used for fluorescence-activated cell sorting included CD56-fluorescein isothiocyanate (FITC), CD16-phycoerythrin (CD16-PE), CD3-FITC, and CD8-PE; all fluorescent monoclonal antibodies were from Becton Dickinson, aside from CD8-PE, which was from Invitrogen. NK and CD8+ T cells from both ES and uninfected donors then were stained with CD56, CD16, CD3, and CD8 to ensure that CD8+ T-cell populations had very low (<1%) NK cell contamination, and that NK cells had very low (<1%) CD8+ T-cell contamination. NK cells were, on average, 94% CD16+, CD56+, or double positive; CD8+ T cells were, on average, 90% double positive (CD8+ CD3+). There was no statistical difference between the purity of the CD8+ T or NK cells of the ES and those of the donors.
After 3 days of activation, lymphoblasts were depleted for CD8 and CD16 using antibodies from Invitrogen and sheep anti-mouse secondary antibody conjugated to magnetic beads (Dynal). The resulting blast cells were less than 5% CD56, CD16, or CD8 positive, with no difference between the cells of the ES and uninfected donors. Cells were infected via spinoculation (31) with the reference HIV-1 isolate Ba-L for 2 h at 1,200 × g at 25°C and then immediately washed and resuspended in RPMI medium with 10% human AB serum and 1% penicillin-streptomycin, either with or without 50 U/ml IL-2. These infected target cells then were aliquoted into 96-well plates at 105 cells/well. CD8+ T cells and NK cells were added to the infected lymphoblasts at 105 cells/well in triplicate, as were NK cells resuspended in medium with 50 U/ml IL-2. NK cells and CD8+ T cells were added to target cells derived from the same infection event, so there was no difference in the frequencies of infected cells for the CD8+ T-cell assay and for the NK cell assay for each patient. Reaction mixtures were incubated for 7 days at 37°C, and the supernatant was sampled at days 3, 5, and 7 postinfection, except for one ES patient for whom samples were taken at days 4, 5, and 7 postinfection. For half of the patients, samples also were taken 1 h postinfection to serve as a baseline control. The levels of p24 antigen in the supernatant were determined using the Alliance HIV-1-1 p24 antigen enzyme-linked immunosorbent assay kit as per the manufacturer's instructions (Perkin-Elmer).
For the experiments using autologous virus, identical procedures were followed, with the exception that the CD8+ T-cell effect was not assayed; instead, only NK cells were isolated, and their effects against both Ba-L virus and the patient's autologous virus were evaluated at the same time. Autologous virus was isolated as previously described (9).
NK and CD8+ T-cell secondary assay.
For the secondary assay, PBMCs from three ES patients were divided into three aliquots. One aliquot served as the target cells and was activated with PHA and then depleted of CD8+ cells and CD16+ cells as described above; the second was depleted of CD4+ T cells using a CD4+ selection kit (Miltenyi Biotech); the third was depleted of CD4+ cells using a CD4+ selection kit (Miltenyi Biotech) and of CD16+ cells using anti-CD16 antibody (Invitrogen) and sheep anti-mouse secondary antibody conjugated to magnetic beads (Dynal). The target cells then were infected as described above, and 105 target cells were incubated with 105 of the two effector cell populations in quadruplicate. Supernatant aliquots were taken at days 3, 5, and 7 postinfection, and the levels of p24 antigen in the supernatant were determined using the Alliance HIV-1 p24 antigen enzyme-linked immunosorbent assay kit per the manufacturer's instructions (Perkin-Elmer).
DNA isolation.
DNA isolation from whole PBMCs was conducted using the Gentra PureGene DNA isolation kit (Qiagen). PBMCs were lysed with the cell lysis solution and then frozen at −80°C. Upon thawing, DNA was isolated per the manufacturer's instructions and hydrated in 100 μl distilled H2O overnight at 4°C (24).
KIR and KIR ligand genotyping.
KIR genotyping utilized the Olerup SSP KIR genotyping kit (Qiagen); results were analyzed on a 1% agarose gel with ethidium bromide. Because this kit was unable to distinguish between KIR3DS1 and one of the KIR3DL1 alleles, all samples also were screened for KIR3DS1 using a second round of PCRs. KIR2DL1 and DRB1 also were analyzed using this second PCR method and served as controls by which to compare the two KIR typing methods (24).
KIR ligands were determined using two complementary methods. HLA-A and HLA-B typing for ES patients was conducted as previously described (9), and from this patients were determined to carry either Bw4T80, Bw4I80, or Bw6 using the HLA sequence database from the Anthony Nolan Trust (34). Where allele information was insufficient to determine the presence of the Bw6 and/or the sequence of the Bw4 domain, HLA-A, HLA-B, and HLA-C KIR ligands were determined using the Olerup SSP KIR HLA ligand genotyping kit (Qiagen).
RESULTS
The effectiveness of NK cells against HIV-1 infection currently is unclear, although at least one study indicates that NK cells are important in the acute phase of infection (2). To examine their potential role in reducing viral replication, we studied the effect of autologous NK cells and CD8+ T cells on infected CD4+ lymphoblasts from ES. Considering evidence that the KIR receptors may affect HIV-1 disease progression (18, 25, 26) and the ability to suppress HIV replication in vitro (1), we also characterized the KIR and major KIR ligands of our elite patients.
Effect of NK cells and CD8+ T cells on p24 production.
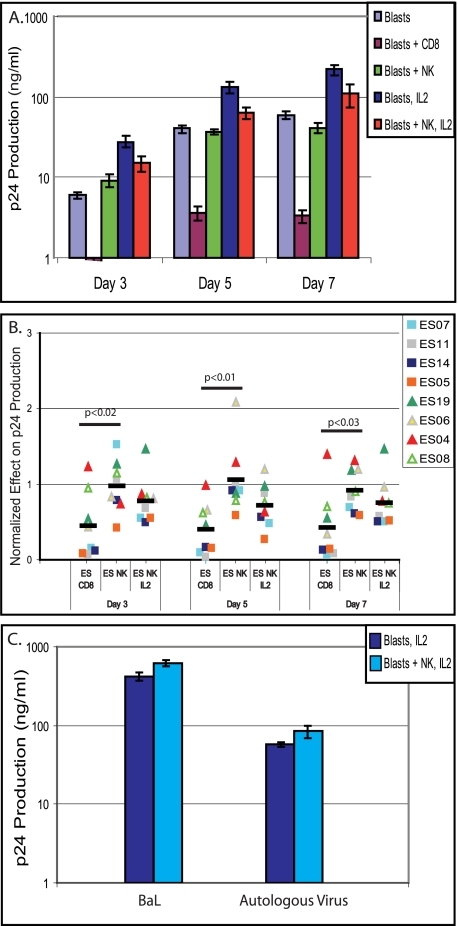
To evaluate the ability of NK cells to control HIV-1 infection, we infected autologous CD4+ CD8− CD16− lymphoblasts from ES and uninfected patients with the reference HIV-1 isolate Ba-L, and then we incubated these cells with autologous, unstimulated CD8+ T cells, NK cells, or NK cells in the presence of IL-2 using 1:1 effector/target ratios. We found no significant difference in the number of NK cells or CD8+ T cells isolated from uninfected donors and those of ES (data not shown). For controls, we included both infected cells alone and infected cells with IL-2 added. When p24 levels were measured immediately after infection, they were below or at the limit of detection, confirming that the values measured subsequently reflected new viral replication (data not shown). We then evaluated p24 production in all ES and uninfected patients in triplicate at days 3, 5, and 7 postinfection. Figure 1A presents results from a representative patient (ES07). This patient showed a >1 log reduction in p24 production when CD8+ T cells were added to the lymphoblasts and no significant reduction when NK cells were added. Additionally, this patient had the expected increase in viral replication when IL-2 was added to the media and exhibited a quarter to a third of a log of NK cell-induced reduction in viral replication when in the presence of exogenous IL-2. To compare the effects of the NK and CD8+ T cells on p24 production across the patient cohorts, the data from each patient were normalized to those for the control lymphoblast-only samples for each patient; data for samples from experiments with NK cells in the presence of exogenous IL-2 were normalized to those for the lymphoblast with IL-2 controls (Fig. 1B).
FIG. 1.
Effect of NK cells and CD8+ T cells on viral replication in infected CD4+ T lymphoblasts, as measured by p24 production. (A) Representative results for one patient showing the p24 production of different cell combinations, in triplicate, at three different time points. (B) p24 production of CD4+ T lymphoblasts infected with the laboratory HIV-1 strain Ba-L in the presence of NK cells or CD8+ T cells, normalized to the production by lymphoblasts alone; data for NK cells with IL-2 were normalized to data for lymphoblasts with IL-2. Symbols represent individual ES patients, as noted in the key. (C) Representative results for one patient (ES04) of two who were assayed by infecting them with their respective autologous virus. The p24 production of control blasts and blasts with NK cells added, in triplicate, at day 7 postinfection is shown.
CD8+ T cells of ES patients generally exhibited superior control of HIV replication compared to that of NK cells, although the addition of IL-2 greatly enhanced NK cell function. Previously, it has been shown that unstimulated CD8+ T cells from ES are able to control viral replication, resulting in a reduction in p24 production by infected, autologous CD4+ lymphoblasts (36); by comparing the effect of these cells in ES to that of NK cells, we were able to put the effectiveness of NK cells into context. The addition of CD8+ T cells from ES to cultures of infected, autologous CD4+ lymphoblasts sometimes reduced p24 production to almost undetectable levels (Fig. 1B), thus displaying significantly greater inhibition than that caused by unstimulated NK cells; however, in some of our ES patients the inhibition caused by NK cells with IL-2 approached that caused by unstimulated CD8+ T cells (Fig. 1B). We observed that individuals with the strongest CD8+ T-cell effect frequently also had the strongest NK cell effect when exogenous IL-2 was added. We used Student's t test to determine whether there were differences between the p24 levels of individuals with strong NK effect and weak NK effect. There was no significant variation between the control samples' p24 levels for those patients with strong NK effect compared to those with little NK effect at any of the three time points for the four patients with the strongest NK effect (data not shown). Additionally, in one experiment we tested the NK effect in a transwell assay (data not shown) and found no inhibition of viral replication, suggesting that the mechanism of inhibition by NK cells is cytolytic in nature. In evaluating the efficacy of our assay, we also conducted it on eight uninfected donors (data not shown). Donors showed little to no inhibition of viral replication by CD8+ T cells, as anticipated, and showed a variable effect by the NK cells. The effect of NK cells with IL-2 was larger than that in the absence of IL-2 and generally mirrored the effect shown by ES. Additionally, for two ES, we evaluated the effect of NK cells on CD4+ lymphoblasts infected with autologous virus (9). For both patients, there was no significant difference (P > 0.1) between the NK effect on the viral replication of the patients' autologous virus and their effect on the Ba-L strain virus (Fig. 1C).
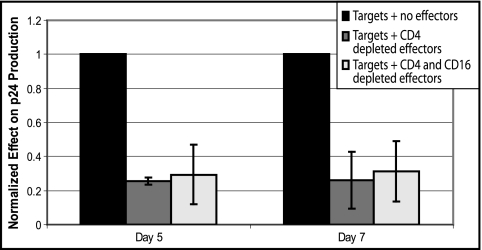
Our initial assay utilized isolated populations of CD8+ T cells and NK cells. This approach does not account for the possibility that a delicate balance of dendritic cells or other accessory immune cells alters the effectiveness of NK cells, so a secondary assay was developed. Instead of adding a purified population of effector cells to autologous infected CD4+ lymphoblasts, we depleted PBMCs of specific cellular subsets and used the remaining cells as effectors. All effectors were depleted of CD4+ T cells, as CD4+ T cells could serve as secondary target cells; a subset of the CD4-depleted effectors also were depleted of CD16+ cells to model an effector cell population lacking NK cells. We then compared the ability of these depleted cell populations to inhibit viral replication. The CD4− effector cells resulted in a significant decrease in p24 production (∼70 to 80%); the CD4− CD16− cells yielded a similar though slightly greater decrease in p24 production, but there was not a statistically significant difference between the effect of the two effector populations (Fig. 2). The assay confirmed that effector cells containing CD8+ T cells with or without NK cells were effective at decreasing viral replication in ES and also indicated that depleting the effector cells of accessory immune cells did not decrease the NK cell activity.
FIG. 2.
Results of alternate assay. Cell populations were depleted of CD16+ cells and CD4+ cells, or depleted of CD4+ cells alone, and used as effector cells against infected, autologous CD4+ T lymphoblast target cells. Data represent results from three ES patients. Error bars are standard deviations; P values are based on Student's t tests.
Characterization of ES NK cells: KIR and KIR ligand genotyping.
Epidemiological studies have identified correlations between the KIR receptor phenotype or genotype and a slower progression to AIDS (18, 25, 26). As the ES in our cohort have maintained both undetectable VLs and high CD4+ T-cell counts for a median of 10 years, we characterized KIR in our cohort to determine whether receptors implicated in slower progression also are overrepresented in our cohort of ES. The combination of KIR3DS1 and its putative ligand, HLA-B molecules with an I80 mutation in the Bw4 domain, has been shown to correlate with slower progression to AIDS and increased NK cell activity against HIV-1 replication (1, 25). While other studies have presented both supporting and conflicting data about this KIR-ligand combination (6, 10, 18, 22), the role of this receptor in patients who naturally control HIV infection has not been assessed. We hypothesized that NK cells from individuals possessing the KIR3DS1 and ligand combination exhibit a higher inhibition of viral replication.
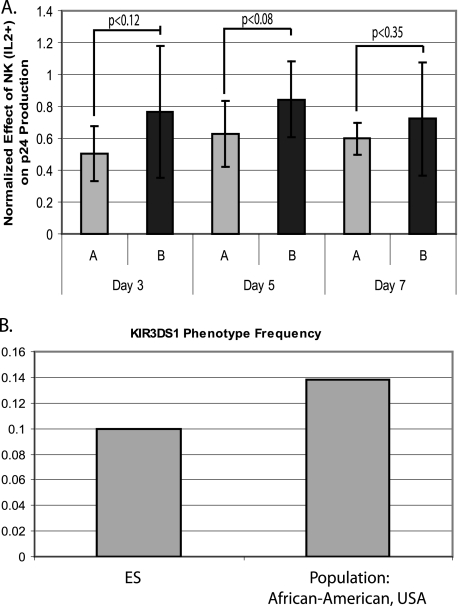
We determined the KIR haplotype of the ES studied in our functional assays as well as that of 12 additional ES from whom we were able to obtain DNA. All of our ES patients were positive for the KIR3DL1 receptor, but only 2 of the 20 had the activating allele of this receptor, KIR3DS1. This frequency was nearly identical to published phenotype frequencies of African-American cohorts, 0.138 (14, 28), which is notable, as all of our ES are African-American (Fig. 3B). Only one of the two KIR3DS1+ individuals (ES08) possessed an HLA-B Bw4I80 ligand, and that was in the form of HLA-B*570301 (Table 1). ES08 was studied in the functional assay but showed little NK cell-induced control of viral replication (Fig. 1B).
FIG. 3.
KIR haplotype of ES. (A) Comparison of the inhibition of viral replication by ES (n = 8) and uninfected donors (n = 8) who had haplotype A and B KIR on days 3, 5, and 7 postinfection. Inhibition was measured by normalizing the p24 produced by cells with NK cells and IL-2 added to p24 produced by infected, autologous lymphoblasts alone. Error bars are standard deviations; P values are based on Student's t tests. (B) Frequency of the KIR3DS1 allele in our cohort (ES; n = 20) compared to data from an African-American cohort.
TABLE 1.
Allele and ligand composition of KIR3DS1 and KIR3DL1 from ES individuals
| ES no.a | KIRb
|
HLA-B allelec | KIR haplotype | |
|---|---|---|---|---|
| 3DL1 | 3DS1 | |||
| ES01 | + | + | B27 | |
| B14 | B | |||
| ES02 | + | - | B5703 | |
| B5101 | B | |||
| ES03 | + | - | B5702 | |
| B5101 | B | |||
| ES04 | + | - | B4402/4419N | |
| B0801 | B | |||
| ES05 | + | - | B5703 | |
| B5802 | A | |||
| ES06 | + | - | B5703 | |
| B1503 | B | |||
| ES07 | + | - | B5703 | |
| B8101/8102 | A | |||
| ES08 | + | + | B5703 | |
| B4402/4419N | B | |||
| ES09 | + | - | B5703 | |
| B2703 | B | |||
| ES11 | + | - | B1516 | |
| B3508 | A | |||
| ES12 | + | - | B5703 | |
| B5301 | B | |||
| ES14 | + | - | B5201 | |
| B7801 | A | |||
| ES15 | + | - | B5703 | |
| B0702/0761 | B | |||
| ES18 | + | - | B5703 | |
| B42 | A | |||
| ES19 | + | - | B5703 | |
| B0702/0761 | B | |||
| ES23 | + | - | B5703 | |
| B1510 | B | |||
| ES27 | + | - | B5703 | |
| B1503 | B | |||
| ES31 | + | - | B5801/5811 | |
| B2705/2713 | A | |||
| ES32 | + | - | B5701 | |
| B3501 | A | |||
| ES33 | + | - | B1503 | |
| B1510/9503 | B | |||
Entries in boldface type indicate individuals who were tested in the assay and are represented in Fig. 1B.
+, individual possesses the given KIR3DS1 or KIR3DL1 allele; −, individual does not possesses the given KIR3DS1 or KIR3DL1 allele.
HLA-B Bw4I80 alleles are indicated in boldface type. Where sufficient allele information was not available, Bw4I80 status was determined with help from a KIR ligand-typing kit, as noted in Materials and Methods. A slash (/) separating HLA-B alleles indicates that the HLA typing was not able to distinguish between these alleles.
There were no obvious differences in the frequency of other individual KIR receptors between our ES cohort and published population statistics from African-Americans (data not shown) (14, 28). Interestingly, however, among the eight ES on whom we conducted our functional assay, the four individuals who were haplotype A, and therefore had only one activating receptor, exhibited the strongest NK cell effect on viral replication (Table 1). This difference in effectiveness did not reach significance (P < 0.1 for days 3, 5, and 7). To further investigate this, we determined the KIR haplotypes of the eight seronegative donors on whom we had conducted the NK and CD8+ T-cell assays and incorporated these data into that of the ES on whom we had conducted the NK and CD8+ T-cell assays. The trend of the increased NK cell control of replication correlating with the possession of haplotype A KIR persisted, although it decreased slightly (P = 0.12 for day 3 and P = 0.08 for day 5) (Fig. 3A).
DISCUSSION
ES control viremia and retain high CD4+ T-cell counts through mechanisms that currently are unclear. We hypothesized that an effective early response to infection would repress viral replication quickly, resulting in a smaller viral reservoir and providing time for the CTL response to develop fully and subsequently control HIV-1 infection. NK cells are important in the early response to many viral infections, and we thus hypothesized that they are important in HIV-1 infection as well. Using an autologous system that examined viral replication at three different time points, we determined that the NK cells of some individuals significantly reduce viral replication in vitro: three of our eight subjects showed approximately 50% reduction in viral replication within the 7-day time course. An NK cell effect, however, was not a universal attribute of ES and did not correlate with genotypes of KIR3DL1 and KIR3DS1 or their ligands. As it is widely accepted that CD8+ T cells play an important role in controlling viremia in ES, we compared the ability of purified populations of CD8+ T cells and NK cells to inhibit viral replication in vitro. While there was a great deal of variation within the cohort, CD8+ T cells invariably reduced viral replication to a greater extent than did NK cells, although in the presence of exogenous IL-2 the NK cell effect rivaled that of the CD8+ T cells in some patients. Individuals with stronger NK responses in the presence of exogenous IL-2 also tended to have stronger CD8+ T-cell responses, as seen in Fig. 1B. This could not be explained by differences in the baseline level of infection as measured by p24 production, as there was no statistical difference between the p24 levels of the control samples in the stronger controls compared to that of the weaker controllers. Additionally, NK cells similarly were effective against viral replication of autologous virus and against Ba-L strain virus in two ES.
Several important studies have provided evidence both for and against a role for NK cells in HIV-1 infection. In the presence of exogenous IL-2, NK cells from healthy donors have been found to be effective at lysing endogenously infected CD4+ T cells from infected patients, specifically via the NKG2D receptor; in one study of uninfected donors, NK cells reduced viral replication in an ex vivo, autologous cell assay (1, 17). In contrast, in infected rhesus macaques with progressive disease, the depletion of NK cells with anti-CD16 antibodies had no effect on VL or CD4+ T-cell levels, suggesting that NK cells did not have a measurable effect on these parameters (11). However, perhaps the most compelling evidence for NK function during HIV-1 infection has been the large population studies identifying correlations between slow progression to AIDS and certain KIR receptors, specifically KIR3DS1 and KIR3DL1 (18, 25, 26). Considering that ES are essentially the ultimate slow progressors, they serve as the logical model group with which to follow up on these population studies and to determine the role that NK cells, and KIR in particular, serve in controlling HIV-1 infection.
The most likely explanation for the observed lack of consistent NK cell-mediated viral inhibition in ES is that the factors that allow an individual to control HIV-1 infection are multifaceted and unique to each patient. While CTLs and certain HLA molecules like B*57 have been shown to contribute to the control of HIV-1 replication in ES, there are ES who do not possess a strong CTL response or these specific HLA genes (15, 32). Likewise, NK cells may prove to be important in some individuals, but our data suggest that they are not a necessary factor for the long-term immunological control of HIV-1, at least with respect to the direct inhibition of viral replication. NK cells may be critical to controlling HIV-1 infection through a different mechanism than that examined here. Plasmacytoid dendritic cells (pDC) produce alpha interferon (IFN-α) and IFN-β, which are critical to the chronic immune activation that primes progression to AIDS in the rhesus macaque model (23). Perhaps the importance of NK cells and their specific receptors is due not to their ability to directly impact viral replication but rather to their ability to modulate critical upstream elements of the immune response and improve the conditions for long-term defense against the virus.
A striking epidemiological study indicates that KIR3DS1 is important in delaying progression to AIDS, but discordant data from several studies makes it clear that the significance of KIR3DS1 is not yet fully elucidated (6, 10, 18, 22). While one study showed that the possession of the KIR3DS1 receptor correlated with slow progression to AIDS only in conjunction with its presumptive ligand, HLA-B Bw4I80, other studies have found no synergistic relationship with the ligand, or, in fact, no impact of the ligand at all (6, 10, 25). Considering that the HLA-B Bw4I80 group includes HLA molecules that are known to be important in the CTL response to HIV-1 such as B*57, it is difficult to dissect the role of the ligand in the CTL response versus its role in the NK response. The aforementioned studies provided a critical look at the impact of KIR on HIV-1 progression. However, no previous study has examined the presence of this receptor allele in ES, and these patients are distinguished from previous study participants in that they appear never to progress to AIDS. While our patient sample was relatively small (20 ES), we did not find that KIR3DS1 was overrepresented in our ES cohort compared to population statistics for African-Americans, nor were the patients in our cohort who exhibited an NK response against infected cells predominately KIR3DS1 positive. It is possible that there are fundamental differences between the mechanisms that allow ES to control viremia and those that delay the onset of AIDS in viremic patients; additionally, receptors other than KIR, such as the NKG2D receptor that was examined by Fogli and colleagues, may play a role in ES (17).
While there was no relationship between the possession of KIR3DS1 and the control of viremia, the four ES who had the KIR haplotype A had stronger NK cell-mediated suppression of viral replication than those who had haplotype B. KIR haplotypes A and B are defined by the presence of specific KIR receptors in the genome (24, 35). While haplotype B individuals carry the gene for any or all of six KIR receptors, five of which are activating (2DL5, 2DS1, 2DS2, 2DS3, 2DS5, or 3DS1), individuals lacking these receptors are considered haplotype A. Gaudieri and colleagues found that haplotype B individuals exhibited faster CD4 decline and faster progression to AIDS (18), suggesting that this balance of inhibitory and activating receptors warrants more investigation. The trend toward the increased control of viral replication with the possession of KIR haplotype A persisted in the eight uninfected donors on whom we conducted the assay, though the significance of the trend did decrease when these data were added. It would be of interest to further investigate this correlation in a larger cohort.
To our knowledge, this study presents the first comparison between the magnitude of the NK effect and CD8+ T-cell effect in ES, and it examines the presence of critical KIR and their ligands in these unique patients. As happens in autologous systems, there was a great deal of variation within the cohort, and each patient's response could have been affected by any variety of factors occurring in their immune system when the samples were taken. However, it seems clear that the direct NK cell-mediated suppression of HIV-1 replication is not a universal attribute of ES. Additionally, it is clear that in ES who do exhibit NK cell-mediated suppression, the NK cells are less effective than CD8+ T cells. Most likely the factors permitting an individual to control HIV-1 infection are multifaceted and unique to each individual, involving a role for HIV-specific CTLs and, at times, a role for NK cells.
Acknowledgments
We thank Jason Dinoso for consultation and help with this project. We also thank Mary Carrington and Maureen Martin for their help and advice with respect to the KIR3DS1 typing.
This work was supported by NIH grants R56 AI73185-01A1 (J.N.B.) and the HHMI (R.F.S.).
Footnotes
Published ahead of print on 11 February 2009.
REFERENCES
- 1.Alter, G., M. P. Martin, N. Teigen, W. H. Carr, T. J. Suscovich, A. Schneidewind, H. Streeck, M. Waring, A. Meier, C. Brander, J. D. Lifson, T. M. Allen, M. Carrington, and M. Altfeld. 2007. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J. Exp. Med. 2043027-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alter, G., N. Teigen, R. Ahern, H. Streeck, A. Meier, E. S. Rosenberg, and M. Altfeld. 2007. Evolution of innate and adaptive effector cell functions during acute HIV-1 infection. J. Infect. Dis. 1951452-1460. [DOI] [PubMed] [Google Scholar]
- 3.Alter, G., N. Teigen, B. T. Davis, M. M. Addo, T. J. Suscovich, M. T. Waring, H. Streeck, M. N. Johnston, K. D. Staller, M. T. Zaman, X. G. Yu, M. Lichterfeld, N. Basgoz, E. S. Rosenberg, and M. Altfeld. 2005. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood 1063366-3369. [DOI] [PubMed] [Google Scholar]
- 4.Bailey, J. R., K. G. Lassen, H. C. Yang, T. C. Quinn, S. C. Ray, J. N. Blankson, and R. F. Siliciano. 2006. Neutralizing antibodies do not mediate suppression of human immunodeficiency virus type 1 in elite suppressors or selection of plasma virus variants in patients on highly active antiretroviral therapy. J. Virol. 804758-4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey, J. R., T. M. Williams, R. F. Siliciano, and J. N. Blankson. 2006. Maintenance of viral suppression in HIV-1-infected HLA-B*57+ elite suppressors despite CTL escape mutations. J. Exp. Med. 2031357-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbour, J. D., U. Sriram, S. J. Caillier, J. A. Levy, F. M. Hecht, and J. R. Oksenberg. 2007. Synergy or independence? Deciphering the interaction of HLA class I and NK cell KIR alleles in early HIV-1 disease progression. PLoS Pathog. 3e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker, E., J. Martinson, C. Brooks, A. Landay, and S. Deeks. 2007. Dysfunctional natural killer cells, in vivo, are governed by HIV viremia regardless of whether the infected individual is on antiretroviral therapy. AIDS 212363-2365. [DOI] [PubMed] [Google Scholar]
- 8.Betts, M. R., M. C. Nason, S. M. West, S. C. De Rosa, S. A. Migueles, J. Abraham, M. M. Lederman, J. M. Benito, P. A. Goepfert, M. Connors, M. Roederer, and R. A. Koup. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 1074781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blankson, J. N., J. R. Bailey, S. Thayil, H. C. Yang, K. Lassen, J. Lai, S. K. Gandhi, J. D. Siliciano, T. M. Williams, and R. F. Siliciano. 2007. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J. Virol. 812508-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulet, S., S. Sharafi, N. Simic, J. Bruneau, J. P. Routy, C. M. Tsoukas, and N. F. Bernard. 2008. Increased proportion of KIR3DS1 homozygotes in HIV-exposed uninfected individuals. AIDS 22595-599. [DOI] [PubMed] [Google Scholar]
- 11.Choi, E. I., K. A. Reimann, and N. L. Letvin. 2008. In vivo natural killer cell depletion during primary simian immunodeficiency virus infection in rhesus monkeys. J. Virol. 826758-6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen, G. B., R. T. Gandhi, D. M. Davis, O. Mandelboim, B. K. Chen, J. L. Strominger, and D. Baltimore. 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10661-671. [DOI] [PubMed] [Google Scholar]
- 13.Deeks, S. G., and B. D. Walker. 2007. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity 27406-416. [DOI] [PubMed] [Google Scholar]
- 14.Du, Z., D. W. Gjertson, E. F. Reed, and R. Rajalingam. 2007. Receptor-ligand analyses define minimal killer cell Ig-like receptor (KIR) in humans. Immunogenetics 591-15. [DOI] [PubMed] [Google Scholar]
- 15.Emu, B., E. Sinclair, H. Hatano, A. Ferre, B. Shacklett, J. N. Martin, J. M. McCune, and S. G. Deeks. 2008. HLA class I-restricted T-cell responses may contribute to the control of human immunodeficiency virus infection, but such responses are not always necessary for long-term virus control. J. Virol. 825398-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fellay, J., K. V. Shianna, D. Ge, S. Colombo, B. Ledergerber, M. Weale, K. Zhang, C. Gumbs, A. Castagna, A. Cossarizza, A. Cozzi-Lepri, A. De Luca, P. Easterbrook, P. Francioli, S. Mallal, J. Martinez-Picado, J. M. Miro, N. Obel, J. P. Smith, J. Wyniger, P. Descombes, S. E. Antonarakis, N. L. Letvin, A. J. McMichael, B. F. Haynes, A. Telenti, and D. B. Goldstein. 2007. A whole-genome association study of major determinants for host control of HIV-1. Science 317944-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fogli, M., D. Mavilio, E. Brunetta, S. Varchetta, K. Ata, G. Roby, C. Kovacs, D. Follmann, D. Pende, J. Ward, E. Barker, E. Marcenaro, A. Moretta, and A. S. Fauci. 2008. Lysis of endogenously infected CD4+ T cell blasts by rIL-2 activated autologous natural killer cells from HIV-infected viremic individuals. PLoS Pathog. 4e1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaudieri, S., D. DeSantis, E. McKinnon, C. Moore, D. Nolan, C. S. Witt, S. A. Mallal, and F. T. Christiansen. 2005. Killer immunoglobulin-like receptors and HLA act both independently and synergistically to modify HIV disease progression. Genes Immun. 6683-690. [DOI] [PubMed] [Google Scholar]
- 19.Han, Y., J. Lai, P. Barditch-Crovo, J. E. Gallant, T. M. Williams, R. F. Siliciano, and J. N. Blankson. 2008. The role of protective HCP5 and HLA-C associated polymorphisms in the control of HIV-1 replication in a subset of elite suppressors. AIDS 22541-544. [DOI] [PubMed] [Google Scholar]
- 20.Iannello, A., O. Debbeche, S. Samarani, and A. Ahmad. 2008. Antiviral NK cell responses in HIV infection. II. Viral strategies for evasion and lessons for immunotherapy and vaccination. J. Leukoc. Biol. 8427-49. [DOI] [PubMed] [Google Scholar]
- 21.Lambotte, O., F. Boufassa, Y. Madec, A. Nguyen, C. Goujard, L. Meyer, C. Rouzioux, A. Venet, J. F. Delfraissy, et al. 2005. HIV controllers: a homogeneous group of HIV-1-infected patients with spontaneous control of viral replication. Clin. Infect. Dis. 411053-1056. [DOI] [PubMed] [Google Scholar]
- 22.Long, B. R., L. C. Ndhlovu, J. R. Oksenberg, L. L. Lanier, F. M. Hecht, D. F. Nixon, and J. D. Barbour. 2008. Conferral of enhanced natural killer cell function by KIR3DS1 in early human immunodeficiency virus type 1 infection. J. Virol. 824785-4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mandl, J. N., A. P. Barry, T. H. Vanderford, N. Kozyr, R. Chavan, S. Klucking, F. J. Barrat, R. L. Coffman, S. I. Staprans, and M. B. Feinberg. 2008. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat. Med. 141077-1087. [DOI] [PubMed] [Google Scholar]
- 24.Martin, M. P., and M. Carrington. 2008. KIR locus polymorphisms: genotyping and disease association analysis. Methods Mol. Biol. 41549-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin, M. P., X. Gao, J. H. Lee, G. W. Nelson, R. Detels, J. J. Goedert, S. Buchbinder, K. Hoots, D. Vlahov, J. Trowsdale, M. Wilson, S. J. O'Brien, and M. Carrington. 2002. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat. Genet. 31429-434. [DOI] [PubMed] [Google Scholar]
- 26.Martin, M. P., Y. Qi, X. Gao, E. Yamada, J. N. Martin, F. Pereyra, S. Colombo, E. E. Brown, W. L. Shupert, J. Phair, J. J. Goedert, S. Buchbinder, G. D. Kirk, A. Telenti, M. Connors, S. J. O'Brien, B. D. Walker, P. Parham, S. G. Deeks, D. W. McVicar, and M. Carrington. 2007. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat. Genet. 39733-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mavilio, D., J. Benjamin, M. Daucher, G. Lombardo, S. Kottilil, M. A. Planta, E. Marcenaro, C. Bottino, L. Moretta, A. Moretta, and A. S. Fauci. 2003. Natural killer cells in HIV-1 infection: dichotomous effects of viremia on inhibitory and activating receptors and their functional correlates. Proc. Natl. Acad. Sci. USA 10015011-15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Middleton, D., L. Menchaca, H. Rood, and R. Komerofsky. 2003. New allele frequency database: http://www.allelefrequencies.net. Tissue Antigens 61403-407. [DOI] [PubMed] [Google Scholar]
- 29.Migueles, S. A., A. C. Laborico, W. L. Shupert, M. S. Sabbaghian, R. Rabin, C. W. Hallahan, D. Van Baarle, S. Kostense, F. Miedema, M. McLaughlin, L. Ehler, J. Metcalf, S. Liu, and M. Connors. 2002. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat. Immunol. 31061-1068. [DOI] [PubMed] [Google Scholar]
- 30.Migueles, S. A., M. S. Sabbaghian, W. L. Shupert, M. P. Bettinotti, F. M. Marincola, L. Martino, C. W. Hallahan, S. M. Selig, D. Schwartz, J. Sullivan, and M. Connors. 2000. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. USA 972709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Doherty, U., W. J. Swiggard, and M. H. Malim. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 7410074-10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pereyra, F., M. M. Addo, D. E. Kaufmann, Y. Liu, T. Miura, A. Rathod, B. Baker, A. Trocha, R. Rosenberg, E. Mackey, P. Ueda, Z. Lu, D. Cohen, T. Wrin, C. J. Petropoulos, E. S. Rosenberg, and B. D. Walker. 2008. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J. Infect. Dis. 197563-571. [DOI] [PubMed] [Google Scholar]
- 33.Ravet, S., D. Scott-Algara, E. Bonnet, H. K. Tran, T. Tran, N. Nguyen, L. X. Truong, I. Theodorou, F. Barre-Sinoussi, G. Pancino, and P. Paul. 2007. Distinctive NK-cell receptor repertoires sustain high-level constitutive NK-cell activation in HIV-exposed uninfected individuals. Blood 1094296-4305. [DOI] [PubMed] [Google Scholar]
- 34.Robinson, J., M. J. Waller, P. Parham, N. de Groot, R. Bontrop, L. J. Kennedy, P. Stoehr, and S. G. Marsh. 2003. IMGT/HLA and IMGT/MHC: sequence databases for the study of the major histocompatibility complex. Nucleic Acids Res. 31311-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robinson, J., M. J. Waller, P. Stoehr, and S. G. Marsh. 2005. IPD—the Immuno Polymorphism Database. Nucleic Acids Res. 33D523-D526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sáez-Cirión, A., C. Lacabaratz, O. Lambotte, P. Versmisse, A. Urrutia, F. Boufassa, F. Barre-Sinoussi, J. F. Delfraissy, M. Sinet, G. Pancino, A. Venet, et al. 2007. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc. Natl. Acad. Sci. USA 1046776-6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sáez-Cirión, A., G. Pancino, M. Sinet, A. Venet, O. Lambotte, et al. 2007. HIV controllers: how do they tame the virus? Trends Immunol. 28532-540. [DOI] [PubMed] [Google Scholar]
- 38.Scott-Algara, D., L. X. Truong, P. Versmisse, A. David, T. T. Luong, N. V. Nguyen, I. Theodorou, F. Barre-Sinoussi, and G. Pancino. 2003. Cutting edge: increased NK cell activity in HIV-1-exposed but uninfected Vietnamese intravascular drug users. J. Immunol. 1715663-5667. [DOI] [PubMed] [Google Scholar]
- 39.Ward, J., and E. Barker. 2008. Role of natural killer cells in HIV pathogenesis. Curr. HIV/AIDS Rep. 544-50. [DOI] [PubMed] [Google Scholar]