Abstract
The intrinsic conformational preferences of the non-proteinogenic amino acids constructed by incorporating the arginine side chain in the β position of 1-aminocyclopentane-1-carboxylic acid (either in a cis or a trans orientation relative to the amino group) have been investigated using computational methods. These compounds may be considered as constrained analogues of arginine (denoted as c5Arg) in which the orientation of the side chain is fixed by the cyclopentane moiety. Specifically, the N-acetyl-N′-methylamide derivatives of cis and trans-c5Arg have been examined in the gas phase and in solution using B3LYP/6-311+G(d,p) calculations and Molecular Dynamics simulations. Results indicate that the conformational space available to these compounds is highly restricted, their conformational preferences being dictated by the ability of the guanidinium group in the side chain to establish hydrogen-bond interactions with the backbone. A comparison with the behavior previously described for the analogous phenylalanine derivatives is presented.
Introduction
Among non-proteinogenic amino acids the conformational propensities of which can be exploited in the design of peptides analogues with well-defined backbone conformations are 1-aminocycloalkane-1-carboxylic acids1 (known in the abbreviated form as Acnc, with n referring to the ring size). Within this series, the cyclopropane (Ac3c),1,2 cyclobutane (Ac4c),1,2c,3 cyclopentane (Ac5c)1,2a,3b,4 and cyclohexane (Ac6c)1,3b,5 members have been deeply investigated and shown to exhibit a restricted conformational space characterized by a high propensity to adopt ϕψ backbone angles typical of the 310-/α-helix (with some distortion in the case of Ac3c).
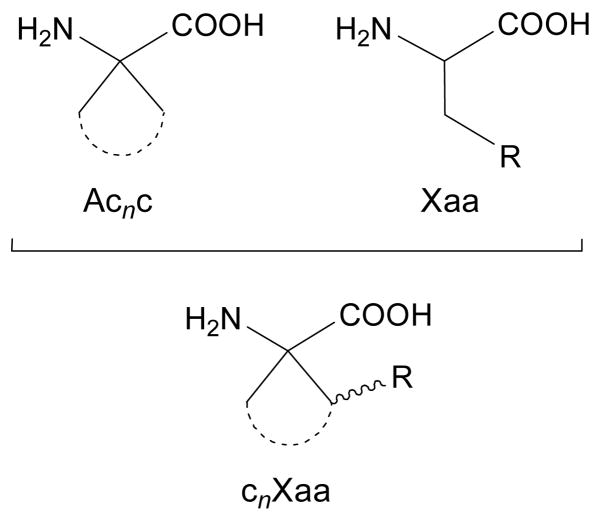
When considering a bioactive peptide, amino acids of the Acnc family have proven appropriate replacements for proteinogenic residues bearing aliphatic or aromatic side chains.6 However, the Acnc series may not be convenient to replace a proteinogenic amino acid containing a functionalized side chain which is directly involved in the peptide-receptor recognition process and is therefore essential for bioactivity. One may yet consider a new family of non-coded amino acids generated by attaching the functionalized side chain of a natural residue to the cycloalkane moiety in Acnc. This allows the combination of the necessary functionality with the particular conformational properties of the Acnc residues (Figure 1). Moreover, this may enable the specific orientation of the side chain functionality by selecting the appropriate cycloalkane size and stereochemistry.
Figure 1.
Structure of 1-aminocycloalkane-1-carboxylic acids (Acnc, n: cycle size) and a proteinogenic amino acid (represented, in general, as Xaa). Combination of the cyclic structure of Acnc with the side-chain functionality in Xaa gives rise to cnXaa residues.
Specifically, we have been working on the synthesis7 and structural study8 –both theoretical and experimentally– of the amino acids obtained by incorporating a phenyl substituent at one of the β carbons of Acnc (n = 3–6). The compounds thus obtained can be considered as phenylalanine (Phe) analogues and we denote them as cnPhe, where n indicates the size of the cycle, as in Acnc. Since the phenylalanine side chain in cnPhe is included in a cyclic structure, the Cα—Cβ bond can not rotate freely and, as a consequence, the orientation of the aromatic group is dictated by the size (n value) and stereochemistry of the cycloalkane ring. It should be noted that the additional phenyl substituent may exhibit a cis or a trans relative disposition with respect to the amino function. Accordingly, the different cnPhe stereoisomers can be regarded as a series of phenylalanine analogues with distinct well-defined side-chain orientations. Indeed, the different spatial arrangement attained by the aromatic substituent has proven useful in several applications related to the stabilization of particular peptide backbone conformations.8,9
Within a project aimed at imparting protection against proteolytic cleavage to a bioactive peptide with simultaneous stabilization of a folded conformation, we became interested in the replacement of an arginine residue (Arg) by a non-natural analogue. In particular, we have focused our attention on the arginine analogue bearing an Ac5c skeleton, that is, c5Arg according to the nomenclature described above (Figure 1). As it can be seen, in c5Arg the α carbon is separated from the guanidinium group by two carbon atoms, while this segment involves three carbon atoms in Arg. Therefore, from a rigorous point of view c5Arg is a substituted Ac5c-like derivative of nor-Arginine, where nor refers to a reduction of one carbon atom with respect to the side chain of conventional Arg. For an L configuration at the α carbon, the guanidilated side chain of arginine may exhibit a trans or a cis disposition relative to the amino moiety, respectively giving rise to trans- and cis-c5Arg (Figure 2). It should be considered that the charged side chain of c5Arg may interact with the backbone not only sterically but also electronically, and this may have a strong impact on the structural preferences of the peptide chain. In order to evaluate the behavior of the L enantiomer of both trans- and cis-c5Arg, we report a conformational study of the corresponding N-acetyl-N′-methylamide derivatives, hereafter denoted as Ac-t-L-c5Arg-NHMe and Ac-c-L-c5Arg-NHMe, respectively (Figure 2). Density Functional Theory (DFT) calculations at the B3LYP/6-311+G(d,p) level have been used to locate and characterize the minimum energy conformations. The influence of the solvent polarity on the conformational preferences has been examined using a Self Consistent Reaction Field (SCRF) method and molecular dynamics (MD) simulations with explicit solvent molecules.
Figure 2.
Structure of the compounds investigated, containing the trans (t) and cis (c) cyclopentane analogues of L-arginine. The backbone and side-chain dihedral angles are indicated.
Methods
The conformational properties of Ac-t-L-c5Arg-NHMe and Ac-c-L-c5Arg-NHMe have been investigated using the Gaussian-03 computer program.10 The structural search was performed considering that the compounds under study retain the restrictions imposed by the cyclopentane ring on the backbone in Ac5c.4a Accordingly, the five minimum energy conformations characterized for Ac-Ac5c-NHMe in ref. 4a were used to generate the starting structures for Ac-t-L-c5Arg-NHMe and Ac-c-L-c5Arg-NHMe. Although for Ac-Ac5c-NHMe such five minima were two-fold degenerate due to the symmetry of the molecule, i.e. {ϕ,ψ,χi} = {−ϕ,−ψ,−χi}, the chiral nature of the two c5Arg derivatives under study requires explicit consideration of both {ϕ,ψ,χi} and {−ϕ,−ψ,−χi} possibilities. The arrangement of the side group is defined by the flexible dihedral angles ξ1 and ξ2, which are expected to exhibit three different minima: trans (180°), gauche+ (60°) and gauche− (−60°). Consequently, 5 (minima of Ac-Ac5c-NHMe) × 2 (chiral nature of c5Arg) × 3 (minima of ξ1) × 3 (minima of ξ2) = 90 minima can be anticipated for the potential energy hypersurface (PEH) E = E(ϕ,ψ,χi,ξ1,ξ2) of each c5Arg-containing derivative. All these structures were used as starting points for subsequent full geometry optimizations.
All geometry optimizations were performed using the B3LYP functional11,12 combined with the 6-311+G(d,p) basis set.13 Frequency analyses were carried out to verify the nature of the minimum state of all the stationary points obtained and to calculate the zero-point vibrational energies (ZPVE) and both thermal and entropic corrections. These statistical terms were then used to compute the conformational Gibbs free energies in the gas phase at 298K (ΔGgp).
To obtain an estimation of the solvation effects on the relative stability of the different minima, single point calculations were conducted on the optimized structures using a SCRF model. Specifically, the Polarizable Continuum Model (PCM) developed by Tomasi and co-workers14 was used to describe water and chloroform as solvents. The PCM model represents the polarization of the liquid by a charge density appearing on the surface of the cavity created in the solvent. This cavity is built using a molecular shape algorithm. PCM calculations were performed in the framework of the B3LYP/6-311+G(d,p) level using the standard protocol and considering the dielectric constants of water (ε = 78.4) and chloroform (ε = 4.9) to obtain the free energies of solvation (ΔGsolv) of the minimum energy conformations. Within this context, it should be emphasized that previous studies indicated that solute geometry relaxations in solution and single point calculations on the optimized geometries in the gas phase give almost identical ΔGsolv values.15 The conformational free energies in solution (ΔGconf) at the B3LYP/6-311+G(d,p) level were estimated using the classical thermodynamics scheme: ΔGconf= ΔGgp + ΔGsolv.
MD simulations in water solution were performed using the NAMD program.16 The simulated peptides Ac-t-L-c5Arg-NHMe and Ac-c-L-c5Arg-NHMe were placed in the center of a cubic simulation box (a= 31.1 Å) filled with 338 explicit water molecules, which were represented using the TIP3 model,17 and a negatively charged chloride atom as counterion. Atom pair distance cutoffs were applied at 14 Å to compute van der Waals interactions. The electrostatic interactions were computed using the nontruncated electrostatic potential by means of Ewald Summations. The real space term was determined by the van der Waals cutoff (14 Å), while the reciprocal term was estimated by interpolation of the effective charge into a charges mesh with a grid thickness of 5 points per volume unit, i.e. the Particle-Mesh Ewald (PME) method.18 Bond lengths were constrained using the SHAKE algorithm19 and the numerical integration step was 2 fs.
Before the MD run series was started, 5 × 103 steps of energy minimization were performed to relax conformational and structural tensions. Different consecutive rounds of short MD runs were performed to equilibrate the density, temperature, and pressure: 0.50 ns of NVT-MD at 298 K (thermal relaxation) followed by 0.25 ns of isobaric relaxation (NPT-MD). Both temperature and pressure were controlled by the weak coupling method, the Berendsen thermo-barostat20 using a time constant for heat bath coupling, and a pressure relaxation time of 1 ps. The coordinates of the NPT-MD production runs, which were 10 ns long, were saved every 500 steps (1 ps intervals) for subsequent analysis.
Results and Discussion
Geometry optimization at the B3LYP/6-311+G(d,p) level led to the characterization of 28 and 23 different minimum energy structures for Ac-t-L-c5Arg-NHMe and Ac-c-L-c5Arg-NHMe, respectively. These minima are within relative energy (ΔE) intervals of 36.7 and 20.8 kcal/mol, respectively. Figure 3 represents the ϕψ backbone dihedral angles of these minima using a color scale (dark blue-to-white) to show the ΔE increase through intervals of 5 kcal/mol. As it can be seen, almost all regions of the Ramachandran map are visited because of the large number of minima characterized. However, the conformational space available to the compounds investigated is relatively restricted, especially that corresponding to the trans-c5Arg derivative. Thus, only 8 out of the 28 minima found for Ac-t-L-c5Arg-NHMe have ΔE values lower than 5 kcal/mol, and all 8 exhibit conformations in the αL region (left-handed α-helix; ϕ,ψ ≈ 60°,50°) of the Ramachandran map. Regarding cis-c5Arg, 7 (out of 23) minimum energy conformations have ΔE < 5 kcal/mol and correspond to three different backbone conformations, namely αL, (equatorial C7 or inverse γ-turn; ϕ,ψ ≈ −60°,60°) and C5 (fully extended; ϕ,ψ ≈ ±180°,±180°). Accordingly, the relative stability of the minimum energy conformations characterized for these c5Arg derivatives is strongly influenced by the cis/trans disposition of the charged substituent.
Figure 3.
Distribution on the Ramachandran map of the minimum energy conformations characterized at the B3LYP/6-311+G(d,p) level for the two c5Arg derivatives under study. The color and size of the symbols used to represent the backbone conformations depend on the relative energy (ΔE) values. Specifically, large and dark blue circles correspond to the more stable minima, while small and empty circles are the least stable ones, i.e. both the intensity of the color and the size of the circles decrease when the relative enery increases.
The next two sections present a detailed description of those minimum energy conformations characterized for the compounds under study that are more favored, not only in the gas phase but also in chloroform and water solutions. These minima are denoted using three labels. The first one refers to the backbone conformation type, defined by the ϕψ dihedral angles. The second label corresponds to the puckering of the cyclopentane ring, i.e. endo/exo-envelope (E) or twist (T) conformations (Scheme 1). Finally, the third label indicates the conformation of the guanidinium side chain, that is, the trans (t), gauche+ (g+) or gauche− (g−) arrangement of the dihedral angles ξ1 and ξ2.
Scheme 1.
Ac-t-L-c5Arg-NHMe
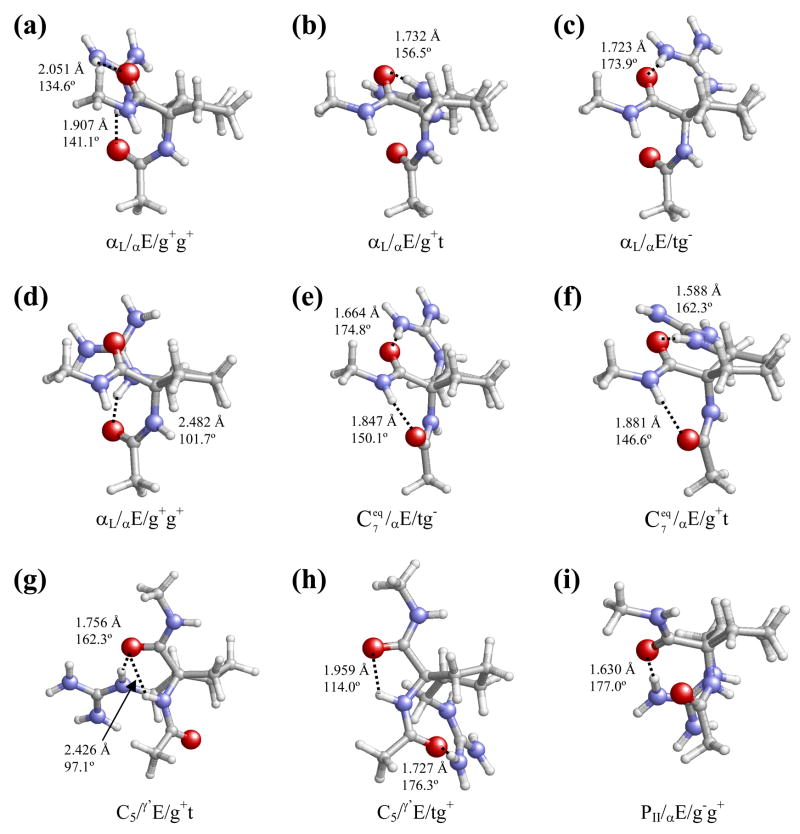
Table 1 lists the backbone and side-chain dihedral angles of the 13 minimum energy conformations calculated for the trans-c5Arg derivative with ΔE < 7 kcal/mol. The global minimum corresponds to an αL/γ′ E/g−t conformation (Figure 4a), in which the backbone adopts an α-helical structure and the cyclopentane ring accommodates a Cγ′-exo envelope (γ′E) arrangement. This geometry, combined with the gauche−/trans disposition of ξ1/ξ2, allows the formation of a strong hydrogen bond between the guanidinium NH and the carbonyl oxygen of the acetyl blocking group [d(H···O) = 1.706 Å, <N–H···O = 170.0°]. Modification of the envelope arrangement of the cyclopentane moiety from Cγ′-exo (γ′E) to Cγ′-endo (γ′E) gives rise to a new minimum (αL/γ′E/g−t, Figure 4b), that maintains all other conformational features present in the global minimum, including the side-chain···backbone interaction. This γ′E-to-γ′E transition is associated with an energy penalty of 1.6 kcal/mol.
Table 1.
Dihedral anglesa and relative energies in the gas phase (ΔE) for the minimum energy conformations with ΔE < 7.0 kcal/mol characterized for Ac-t-L-c5Arg-NHMe at the B3LYP/6-311+G(d,p) level.
| # | Conformer | Backbone dihedral angles | Cyclopentane dihedral angles | Side group | ΔEb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ω0 | ϕ | ψ | ω | χ1 | χ2 | χ3 | χ4 | χ5 | ξ1, ξ2 | ||||
| 1 | αL/γ′E/g−t | 174.8 | 71.9 | 19.8 | 174.5 | −0.1 | 25.3 | −40.9 | 41.2 | −25.2 | −63.3, 166.9 | 0.0c | |
| 2 | αL/γ′E/g−t | 174.6 | 71.2 | 19.6 | 173.6 | −3.9 | −21.2 | 38.6 | −41.3 | 27.8 | −59.3, 164.3 | 1.6 | |
| 3 | αL/γ′E/tg+ | 177.5 | 79.0 | 14.3 | 177.2 | −7.4 | 31.2 | −42.8 | 38.3 | −18.9 | −162.8, 96.8 | 2.5 | |
| 4 | αL/γ′E/g−g− | 176.2 | 59.1 | 45.5 | 177.9 | 4.6 | 21.0 | −38.8 | 42.0 | −28.6 | −62.7, −94.1 | 2.8 | |
| 5 | αL/αE/g+t | 172.2 | 56.9 | 42.2 | 178.8 | −39.0 | 22.3 | 4.1 | −29.2 | 41.7 | 68.4, 174.4 | 3.0 | |
| 6 | αL/γ′E/g+t | 173.0 | 74.0 | 21.1 | 176.6 | −8.7 | 32.0 | −42.9 | 37.8 | −17.7 | 75.1, 148.8 | 3.2 | |
| 7 | αL/γ′E/tg+ | 177.8 | 79.0 | 16.3 | 176.5 | −11.1 | −13.3 | 33.0 | −40.4 | 31.7 | −161.8, 98.1 | 3.8 | |
| 8 | αL/γ′E/g−g− | 174.7 | 80.5 | 17.5 | 174.3 | 10.4 | 15.4 | −35.5 | 42.4 | −32.6 | −50.3, −87.0 | 4.4 | |
| 9 |
|
−177.8 | −73.8 | 74.8 | −175.5 | 45.0 | −34.8 | 11.1 | 17.0 | −38.2 | −155.2, 85.4 | 5.2 | |
| 10 | βL/αE/g−t | 164.7 | 150.9 | 119.9 | 179.0 | −44.9 | 36.8 | −13.5 | 15.2 | 37.0 | −68.5, 125.5 | 5.6 | |
| 11 | αL/αE/tg+ | 174.9 | 60.0 | 48.4 | −179.4 | 45.3 | −38.2 | 16.6 | 11.9 | −35.4 | −143.9, 82.9 | 6.2 | |
| 12 | PII/γ′E/g−t | −170.5 | 71.0 | 155.3 | −176.0 | −5.1 | 17.8 | 34.0 | −37.7 | 26.4 | −65.0, 159.9 | 6.5 | |
| 13 |
|
−170.5 | −75.2 | 57.3 | 179.2 | −41.0 | 24.8 | 15.6 | −27.4 | 41.9 | 147.0, −98.3 | 6.7 | |
Figure 4.
Lower minimum energy conformations of Ac-t-L-c5Arg-NHMe obtained from B3LYP/6-311+G(d,p) calculations. The 13 structures depicted correspond to the minima listed in Table 1, i.e. minimum energy conformations with ΔE < 7 kcal/mol.
The conformation adopted by both the peptide backbone and the cyclopentane ring in the lowest energy conformer is maintained in the third (αL/γ′E/tg+, Figure 4c), fourth (αL/γ′E/g−g−, Figure 4d), sixth (αL/γ′E/g+t, Figure 4f) and eighth (αL/γ′E/g−g−, Figure 4h) minima, which present ΔE values ranging from 2.5 to 4.4 kcal/mol. Thus, minima # 1, 3, 4, 6 and 8 differ mainly in the arrangement of the guanidinium substituent. The diverse orientations exhibited by this group translate into different hydrogen-bonding schemes involving the donor sites in the side chain (NH/NH2) and the backbone carbonyl groups. Notably enough, two of such side-chain···backbone interactions exist simultaneously in minimum # 4 (Figure 4d), although none of them present an optimal geometry. Moreover, to allow their formation, the ψ angle deviates by around 25° from the value characterizing the rest of αL/γ′E conformers (Table 1).
On the other hand, changing the cyclopentane envelope arrangement in minimum # 3 from Cγ′-exo to Cγ′-endo results in a new minimum (# 7, αL/γ′E/tg+, Figure 4g), which is 1.3 kcal/mol higher in energy. This destabilization is similar to that produced on going from the first to the second minima, which implies the same conformational change.
Finally, two other minimum energy conformations with an αL backbone structure and ΔE < 7 kcal/mol were located for Ac-t-L-c5Arg-NHMe, namely conformers # 5 (αL/αE/g+t, Figure 4e) and # 11 (αL/αE/tg+, Figure 4k). They present ΔE values of 3.0 and 6.2 kcal/mol, respectively, and are the only minima in Table 1 exhibiting an αL backbone conformation in which the flap of the cyclopentane envelope is occupied by the α instead of the γ′ carbon. The fact that no minima with an atom other than Cγ′ at the envelope flap is located below ΔE = 3 kcal/mol is a significant difference with respect to the unsubstituted derivative Ac-Ac5c-NHMe, for which the global minimum was found to exhibit an αE cyclopentane arrangement.4a
The most stable conformer with a backbone disposition other than αL is # 9, which is unfavored by 5.2 kcal/mol with respect to the global minimum. In this structure ( , Figure 4i), the terminal backbone CO and NH sites form an intramolecular hydrogen bond [d(H···O)= 1.948 Å, <N–H···O= 141.6°] defining a seven-membered cycle (C7 or γ-turn conformation) and the cyclopentane ring adopts a Cα-exo envelope arrangement. Furthermore, the side chain orientation allows the formation of an additional hydrogen bond involving the c5Arg CO and the guanidinium NH2 [d(H···O)= 1.671 Å, <N–H···O= 175.3]. The other minimum with a C7 backbone structure ( , Figure 4m) exhibits similar backbone···backbone and side-chain···backbone interactions and differs from the former in the endo position occupied by Cα within the cyclopentane envelope.
Two additional types of peptide backbone conformation, corresponding to extended (β-pleated sheet) or semi-extended (polyproline II) structures were detected among the minima characterized for Ac-t-L-c5Arg-NHMe with ΔE < 7 kcal/mol, namely minima # 10 (βL/αE/g−t, Figure 4j) and # 12 (PII/γ′E/g−t, Figure 4l). They share a common disposition for the guanidinium side group (gauche-/trans arrangement of ξ1/ξ2), that, combined with the different backbone and cyclopentane conformations, leads to the formation of two and one side-chain···backbone interactions, respectively, for minima # 10 and 12.
Table 2 shows the conformational Gibbs free energies in the gas phase at 298K (ΔGgp) for the minima listed in Table 1. It is worth noting that the addition of the ZPVE, thermal and entropic contributions to the ΔE values does not produce significant changes in the relative stability order outlined above. Thus, assuming a Boltzmann distribution, αL/γ′E/g−t is the only conformation with a significant population at room temperature in the gas phase, since the ΔGgp values of all other minima are above 1.5 kcal/mol (Table 2). Furthermore, the backbone adopts an αL conformation in the seven structures with lower ΔGgp values, indicating that the preference for this helical fold is not altered by the addition of the statistical corrections. Not surprisingly, ΔGgp values above 7 kcal/mol were obtained for all the minima with ΔE > 7 kcal/mol and, therefore, the contribution of these structures to the conformational preferences of Ac-t-L-c5Arg-NHMe are completely negligible.
Table 2.
Relative conformational Gibbs free energiesa at 298K for selectedb minimum energy conformations of Ac-t-L-c5Arg-NHMe in the gas phase (ΔGgp), chloroform ( ) and aqueous solution ( ) characterized at the B3LYP/6-311+G(d,p) level.
| # | Conformer | ΔGgp | ||||
|---|---|---|---|---|---|---|
| 1 | αL/γ′E/g−t | 0.0c | 2.0 | 3.2 | ||
| 2 | αL/γ′E/g−t | 1.6 | 3.1 | 4.8 | ||
| 3 | αL/γ′E/tg+ | 2.8 | 0.0 | 0.0 | ||
| 4 | αL/γ′E/g−g− | 4.1 | 4.0 | 5.2 | ||
| 5 | αL/αE/g+t | 2.4 | 3.6 | 4.1 | ||
| 6 | αL/γ′E/g+t | 3.8 | 3.9 | 4.7 | ||
| 7 | αL/γ′E/tg+ | 4.2 | 1.2 | 1.3 | ||
| 8 | αL/γ′E/g−g− | 6.2 | 5.9 | 7.4 | ||
| 9 |
|
6.4 | 1.5 | 1.3 | ||
| 10 | βL/αE/g−t | 6.9 | 6.7 | 8.4 | ||
| 11 | αL/αE/tg+ | 6.4 | 3.6 | 3.1 | ||
| 12 | PII/γ′E/g−t | 5.7 | 7.7 | 8.6 | ||
| 13 |
|
8.2 | 3.8 | 4.2 |
In kcal/mol.
Minimum energy conformations with ΔE < 7.0 kcal/mol (see Table 1).
G= −856.436015 a.u
The conformational free energies estimated in chloroform ( ) and water ( ) solutions at the same temperature are also included in Table 2. As it can be seen, αL/γ′E/tg+ (Figure 4c) becomes the most favored conformation in both solvents, with αL/γ′E/tg+ (Figure 4g) being destabilized by only 1.2 and 1.3 kcal/mol in chloroform and water, respectively. These results indicate that the solvent affects the arrangement of the side chain and, to some extent, the puckering of the cyclopentane ring but not the backbone, which in solution retains the preference for the α-helical conformation previously detected in the gas phase.
At this point, it is interesting to establish a comparison between the results obtained in this work for Ac-t-L-c5Arg-NHMe and those recently reported for the analogous phenylalanine derivative, Ac-t-L-c5Phe-NHMe.8a Thus, for the trans-c5Phe-containing compound, four different peptide backbone conformations were found to be energetically accessible in the gas phase. They correspond to , C5, and αR structures, the three latter being destabilized with respect to the former by 0.6, 1.0, and 1.5 kcal/mol, respectively. In comparison, only the αL backbone conformation is accessible to Ac-t-L-c5Arg-NHMe, with no other being located within ΔE < 5 kcal/mol (Table 1). The cyclopentane ring puckering propensities are also significantly different for the two compounds. Thus, αE, γE and αE arrangements were characterized in the accessible minima of the trans-c5Phe derivative,8a whereas for Ac-t-L-c5Arg-NHMe only the γ′E disposition is detected. Regarding the behavior in solution, the environment was found to alter the conformational preferences of the trans-c5Phe derivative from a quantitative point of view but not qualitatively, that is, both compounds retain the main conformational trends observed in the gas phase.
This comparison provides evidence for the different roles played by the guanidinium and phenyl side groups in directing the conformational preferences of Ac5c and indicates that the presence of a charged guanidinium group in the neighborhood of the carbonyl terminus (trans-c5Arg) imposes more severe conformational constraints than those induced when an aromatic group is incorporated in the same position (trans-c5Phe). This distinct behavior should be attributed to the different types of interactions that each side group may establish with the backbone. Thus, the phenyl substituent may affect the conformational propensities of the rest of the molecule by steric reasons or through the establishment of weak attractive interactions of the N–H···π type21 with the NH groups in the peptide backbone. In comparison, the guanidinium side chain mainly interacts with the backbone through the formation of hydrogen bonds. The latter interactions have a more marked directional character, involve the CO instead of the NH backbone groups and are associated with a much higher energy. As a consequence, the guanidinium side chain specifically oriented by the cyclopentane ring towards the carbonyl terminus has a greater impact on the conformational properties of the peptide backbone than a phenyl substituent, and induces conformations different to those encountered for peptides incorporating the unsubstituted Ac5c4a or the phenylalanine counterpart, trans-c5Phe.8a
Ac-C-L-c5Arg-NHMe
The relevant structural parameters of the minimum energy conformations with ΔE < 7 kcal/mol characterized for Ac-c-L-c5Arg-NHMe are listed in Table 3. As it can be seen, only 9 minima satisfy this energetic criterion. It should be noted that, at variance with the compound described in the previous section, in this c5Arg derivative the charged guanidinium group exhibits a cis relative orientation with respect to the amino substituent (Figure 2). This should reflect in different interactions between the side chain and the rest of the molecule (both the cyclopentane ring and the peptide backbone) and therefore lead to different conformational propensities.
Table 3.
Dihedral anglesa and relative energies in the gas phase (ΔE) for the minimum energy conformations with ΔE < 7.0 kcal/mol characterized for Ac-c-L-c5Arg-NHMe at the B3LYP/6-311+G(d,p) level.
| # | Conformer | Backbone dihedral angles | Cyclopentane dihedral angles | Side group | ΔEb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ω0 | ϕ | ψ | ω | χ1 | χ2 | χ3 | χ4 | χ5 | ξ1, ξ2 | ||||
| 1 | αL/αE/g+g+ | −178.5 | 48.0 | 48.6 | 180.0 | 40.4 | −26.7 | 2.0 | 23.8 | −39.6 | 63.0, 84.6 | 0.0c | |
| 2 | αL/αE/g+t | −178.5 | 48.6 | 51.6 | 179.4 | 43.4 | −32.1 | 7.7 | 19.9 | −38.9 | 69.5, −163.2 | 0.5 | |
| 3 | αL/αE/tg− | 180.0 | 49.8 | 45.0 | −178.7 | 44.6 | −35.2 | 11.5 | 16.5 | −37.3 | 151.6, −86.3 | 1.4 | |
| 4 | αL/αE/g+g+ | −178.3 | 49.6 | 42.5 | 178.1 | 41.3 | −28.6 | 4.0 | 22.2 | −39.0 | 54.2, 84.6 | 1.5 | |
| 5 |
|
−172.0 | −72.9 | 60.3 | 179.2 | 42.3 | −29.9 | 5.4 | 21.0 | −38.8 | 152.8, −84.2 | 2.3 | |
| 6 |
|
−173.0 | −72.8 | 67.1 | −179.6 | 42.6 | −28.7 | 2.9 | 24.3 | −41.1 | 70.3, 161.9 | 3.1 | |
| 7 | C5/γ′E/g+t | 169.4 | 179.4 | 131.4 | 176.9 | 11.7 | −33.6 | 42.7 | −35.7 | 14.6 | 61.9, 166.4 | 4.9 | |
| 8 | C5/γ′E/tg+ | 169.5 | −179.9 | 172.2 | −177.3 | 13.9 | −34.6 | 42.1 | −33.5 | 11.9 | −156.0, 99.8 | 6.1 | |
| 9 | PII/αE/g−g+ | −172.0 | −57.8 | 129.1 | −177.5 | 43.8 | −29.4 | 3.3 | 24.5 | −41.8 | −66.6, 111.4 | 6.9 | |
The lowest energy minimum characterized for Ac-c-L-c5Arg-NHMe (αL/αE/g+g+, Figure 5a) corresponds to an αL backbone conformation with the cyclopentane ring arranged as a Cα-exo envelope and the two side-chain dihedral angles in gauche+. This spatial organization orientates both backbone carbonyl oxygens (those in the acetyl group and the c5Arg residue) towards the guanidinium side chain, thus allowing the existence of two side-chain···backbone hydrogen bonds. The second (αL/αE/g+t, Figure 5b), third (αL/αE/tg−, Figure 5c) and fourth (αL/αE/g+g+, Figure 5d) minima only differ from the global one in the orientation of the guanidinium side group. These conformational transitions bring about significant changes in the hydrogen bonding scheme and an energy destabilization ranging from 0.5 to 1.5 kcal/mol.
Figure 5.
Lower minimum energy conformations of Ac-c-L-c5Arg-NHMe obtained from B3LYP/6-311+G(d,p) calculations. The 9 structures depicted correspond to the minima listed in Table 3, i.e. minimum energy conformations with ΔE < 7 kcal/mol.
The next minimum ( , Figure 5e) adopts a different backbone conformation. Specifically, the backbone acetyl CO and methylamide NH groups form a seven-membered hydrogen-bonded ring typical of the C7 or γ-turn conformation. Additionally, this structure is stabilized by a strong side-chain···backbone interaction involving one guanidinium NH2 and the c5Arg CO group. The arrangement of the cyclopentane ring is identical to that observed for the four preceding minima. Conformer # 6 ( , Figure 5f) differs from # 5 in the orientation of the side group only, and this is associated with a change in the side-chain···backbone hydrogen-bonding pattern and an energy cost of 0.8 kcal/mol.
The next two structures, minima # 7 (C5/γ′E/g+t, Figure 5g) and # 8 (C5/γ′E/tg+, Figure 5h), correspond to a C5 peptide backbone conformation, characterized by the presence of a hydrogen bond linking the c5Arg NH and CO sites and closing a five-membered cycle. In the case of minimum # 7, the geometry of this pseudocycle is severely distorted –as evidenced by the small ψ angle– to allow the involvement of the same CO group in a strong hydrogen bond with the guanidinium NH site. The different orientation of the guanidinium side chain in minimum # 8 leads to an interaction with the acetyl CO group (instead of the c5Arg CO), and the C5 conformation accommodated by the peptide backbone is completely regular. It is also noteworthy that # 7 is the most stable minimum of the cis-c5Arg derivative in which the flap of the cyclopentane envelope is not occupied by the α carbon. This means a significant difference with reference to the behavior described above for the trans-c5Arg derivative.
Finally, the last conformer in Table 3 (PII/αE/g−g+, Figure 5i) is unfavored by 6.9 kcal/mol with respect to the global minimum and corresponds to a polyproline II conformation stabilized by a single side-chain···backbone interaction.
Inspection of Table 4 indicates that the lowest ΔGgp value corresponds to the αL/αE/g+t conformation (Figure 5b), while the αL/αE/g+g+ minimum (Figure 5d) is destabilized by 1.8 kcal/mol. Accordingly, if a Boltzmann distribution is assumed, only the αL backbone conformation and the Cα-exo envelope arrangement of the cyclopentane ring are populated in the gas phase at 298 K. For all the minima with ΔE > 7.0 kcal/mol, ΔGgp values above 5.1 kcal/mol were obtained and, therefore, the contribution of these structures to describe the conformational preferences of cis-c5Arg can be considered as negligible.
Table 4.
Relative conformational Gibbs free energiesa at 298K for selectedb minimum energy conformations of Ac-c-L-c5Arg-NHMe in the gas phase (ΔGgp), chloroform ( ) and aqueous solution ( ) characterized at the B3LYP/6-311+G(d,p) level.
| # | Conformer | ΔGgp | ||||
|---|---|---|---|---|---|---|
| 1 | αL/αE/g+g+ | 2.0 | 2.4 | 4.6 | ||
| 2 | αL/αE/g+t | 0.0c | 3.1 | 4.9 | ||
| 3 | αL/αE/tg− | 2.1 | 0.0 | 0.0 | ||
| 4 | αL/αE/g+g+ | 1.8 | 3.4 | 5.4 | ||
| 5 |
|
3.4 | 1.0 | 3.3 | ||
| 6 |
|
2.9 | 4.5 | 7.5 | ||
| 7 | C5/γ′E/g+t | 8.2 | 5.6 | 6.9 | ||
| 8 | C5/γ′E/tg+ | 5.5 | 3.9 | 6.1 | ||
| 9 | PII/αE/g−g+ | 7.7 | 3.8 | 4.3 |
In kcal/mol.
Minimum energy conformations with ΔE < 7.0 kcal/mol (see Table 3).
G=−856.429193 a.u.
The values of and are also listed in Table 4. As it can be seen, the only conformations with are αL/αE/tg− (Figure 5c), which is the global minimum in chloroform solution, and (Figure 5e), that is destabilized by 1.0 kcal/mol. Accordingly, both the αL and backbone conformations are expected to exhibit significant populations in this organic solvent, while the disposition of the cyclopentane ring and the guanidinium side chain seems to be restricted to the Cα-exo envelope and tg− arrangements, respectively. On the other hand, the lowest energy minimum in aqueous solution is αL/αE/tg− (Figure 5c), all the other structures being destabilized by more than 3.3 kcal/mol. It is worth noting that the αL/αE/g+t conformation (Figure 5b), which presented the lowest ΔGgp value, is unfavored by 3.1 and 4.9 kcal/mol in chloroform and water, respectively.
Again, significant differences are detected when the results obtained in this work for Ac-c-L-c5Arg-NHMe are compared to those previously described for Ac-c-L-c5Phe-NHMe.8a Notably, the conformational space available to the latter compound was found to be more restricted than that of the trans derivative as a consequence of the high proximity between the amino terminus and the bulky, rigidly held aromatic substituent. Thus, only equatorial C7 conformers were found to be accessible at room temperature for Ac-c-L-c5Phe-NHMe. Indeed, for this compound, minima with an αL backbone arrangement were destabilized by more than 5 kcal/mol, whereas this is the only backbone structure energetically accessible to cis-c5Arg (Table 3). Also the cyclopentane arrangement was found to be substantially different. Thus, the βE disposition, which places the β carbon bearing the bulky phenyl ring out of the plane defined by the other cyclopentane atoms, proved the most favorable one for the cis-c5Phe derivative,8a whereas cis-c5Arg largely prefers the αE arrangement. In chloroform and aqueous solution, remained the only structure energetically accessible to Ac-c-L-c5Phe-NHMe. Accordingly, the conformational preferences of cis-c5Phe and cis-c5Arg are substantially different since they depend, to a large extent, on the need to relieve steric hindrance in the former case and on the ability of the side chain to form hydrogen bonds with the backbone in the latter.
Classical Molecular Dynamics simulations in aqueous solution
In the absence of experimental data, classical MD simulations with explicit solvent molecules are valuable for describing the favored low-energy conformations of peptides. In order to explore the conformational energy surfaces of Ac-t-L-c5Arg-NHMe and Ac-c-L-c5Arg-NHMe in aqueous solution using this methodology, a specific force-field parametrization to represent the stretching, bending, torsional, van der Waals and electrostic interactions of these constrained peptides was required. In a previous study we showed that no special electronic effect is present in Ac5c4a and, therefore, the stretching, bending, torsional and van der Waals parameters for Ac5c and its derivatives can be directly transferred from the Amber force-field.22 Accordingly, electrostatic charges have been the only force-field parameters specifically developed for trans- and cis-c5Arg.
Atomic charges for the five minimum energy conformations listed in Table 1 and 3 were calculated by fitting the HF/6-31G(d) quantum mechanical and the Coulombic molecular electrostatic potentials (MEPs) to a large set of points placed outside the nuclear region. It should be noted that the electrostatic parameters derived at this level of theory are fully compatible with the current Amber force-field.22 Electrostatic potential (ESP) fitting atomic centered charges for trans- and cis-c5Arg were derived by weighting the charges calculated for the corresponding minimum energy conformations according to Boltzmann populations.23 The weights were given by the standard Boltzmann formula using the ΔGgp values given in Tables 2 and 4. As the charges obtained for trans- and cis-c5Arg were similar, i.e. the absolute value of the largest was lower than 0.08 e.u., we decided to simplify the force-field by providing a unique set of electrostatic parameters for the two amino acids (Figure 6).
Figure 6.
Electrostatic parameters determined for c5Arg residues.
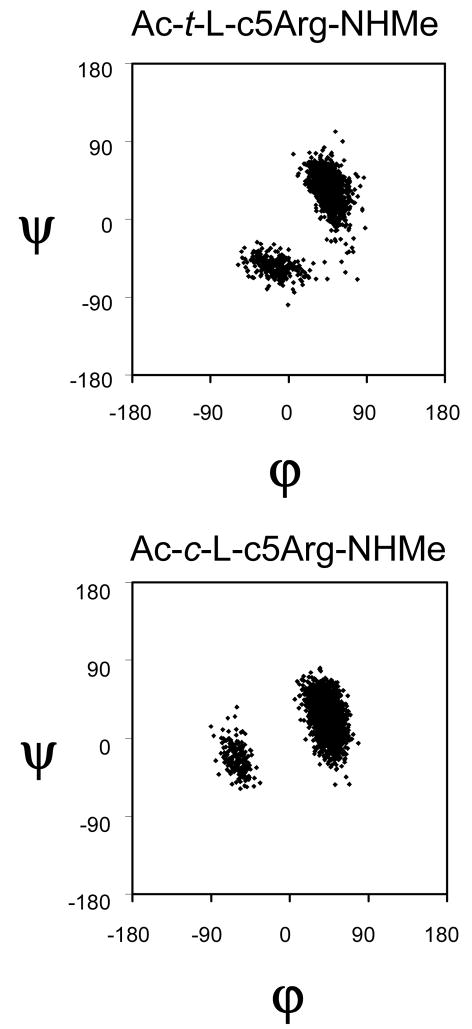
MD simulations of Ac-t-L-c5Arg-NHMe and Ac-c-L-c5Arg-NHMe were performed at 350 K. The lowest energy conformation was used as starting point of a trajectory that was 10 ns long for each compound. Figure 7 represents the accumulated Ramachandran plot for the trans- and cis-c5Arg dipeptides. In both cases the most populated conformation in aqueous solution corresponds to the αL, which is visited much more frequently than the conformation during the trajectory. This fact is in excellent agreement with the results displayed in Table 2 and 4, which indicate that the αL conformation is the lowest energy minimum. These evidences clearly confirm that the conformational space of both trans- and cis-c5Arg is severely restricted by the constrains imposed not only by the cyclopentane ring but also by the charged guanidinium group, which establish hydrogen-bond interactions with the peptide backbone.
Figure 7.
Accumulated Ramachandran plot for Ac-t-L-c5Arg-NHMe and Ac-c-L-c5Arg-NHMe derived from a MD trajectory 10 ns long in aqueous solution.
Influence of the guanidinium side group in the conformational properties
In order to evaluate quantitatively the consequences arising from the incorporation of the guanidinium side group in Ac5c to generate cis- and trans-c5Arg, the isodesmic reaction displayed in Scheme 1 has been considered. For different backbone conformations and cyclopentane ring arrangements, the energy (Esg) and free energy (Gsg) contribution associated with the side group in Scheme 1 were estimated according to equations (1) and (2), respectively.
| (1) |
| (2) |
In these equations, Esg and Gsg provide an estimation of the energy and free energy contribution, respectively, associated to the incorporation of the guanidinium side group for a given backbone conformation and cyclopentane ring puckering of Ac5c. Table 5 shows the values calculated considering selected minimum energy conformations of both Ac-t-L-c5Arg-NHMe (Table 1) and Ac-c-L-c5Arg-NHMe (Table 3), namely the most stable ones among those for which Ac-Ac5c-NHMe was found 4a to exhibit a minimum with similar backbone conformation and cyclopentane puckering. As no αL minimum with an αE cyclopentane arrangement was characterized for the cis-c5Arg derivative, the αL/αE/g+g+ conformation (the global minimum in Table 3) was considered in this case. On the other hand, it should be noted that the minimum energy conformations previously obtained for Ac-Ac5c-NHMe through B3LYP/6-311G(d,p) calculations4a have been re-optimized at the B3LYP/6-311+G(d,p) level.
Table 5.
Energy (Esg) and free energy (Gsg) contributions associated with the guanidinium side group for selected backbone conformations of Ac-t-L-c5Arg-NHMe and Ac-c-L-c5Arg-NHMe.
| Compound | Conf. L-c5Arg | Conf. Ac5ca | Esg | Gsg | ||
|---|---|---|---|---|---|---|
| Ac-t-L-c5Arg-NHMe | αL/αE/g+t | αL/αE | −17.1 | −13.8 | ||
|
|
|
−12.5 | −8.4 | |||
|
|
|
−11.1 | −7.3 | |||
| Ac-c-L-c5Arg-NHMe | αL/αE/g+g+ | αL/αE | −19.5 | −13.7 | ||
|
|
|
−14.7 | −10.9 | |||
| C5/γ′E/g+t | C5/γ′E | −13.2 | −9.8 |
From ref. 4a [minima re-optimized at the B3LYP/6-311+G(d,p) level]
The negative values obtained for both Esg and Gsg (Table 5) reveal significant favorable interactions for all the conformations of Ac-t-L-c5Arg-NHMe and Ac-c-L-c5Arg-NHMe considered. Specifically, the attractive interactions between the charged side group and the polar backbone amide groups produce a significant stabilization for Ceq and backbone conformations. The strength of this effect is fully the αL, and C5 consistent with the relative energies and free energies obtained for such conformations, the most and least attractive interaction being obtained for the αL and C5 structures, respectively. Overall, these results indicate that the remarkable preference of c5Arg towards the αL helical conformation is due to the formation of strong side-chain···backbone interactions, which are more attractive than those established for other backbone conformations. As expected, Gsg is higher than Esg in all cases, which should be attributed to the unfavorable entropic contribution associated with the disappearance of the strong side chain···backbone interactions.
Conclusions
The conformational preferences of Ac-t-L-c5Arg-NHMe and Ac-c-L-c5Arg-NHMe have been explored using quantum mechanical calculations at the B3LYP/6-311+G(d,p) level. Results indicate that the cis and, particularly, the trans stereoisomers of c5Arg prefer an α-helical conformation. Thus, all the minima found for Ac-t-L-c5Arg-NHMe and Ac-c-L-c5Arg-NHMe with ΔE ≤ 4.4 and 1.5 kcal/mol, respectively, exhibit this peptide backbone structure. Furthermore, the preference for the α-helical conformation is retained in solution. Also the cyclopentane ring puckering is significantly affected by the presence and orientation of the guanidinium side chain, and thus, cis- and trans-c5Arg show a marked preference for the Cγ′-exo and Cα-exo envelope arrangements, respectively, in all environmental conditions considered.
The structural preferences exhibited by the c5Arg derivatives are in high contrast with those previously observed for the analogous phenylalanine derivatives, Ac-t-L-c5Phe-NHMe and Ac-c-L-c5Phe-NHMe, which have been shown to prefer the arrangement. The unique conformational properties observed for c5Arg should be attributed to the ability of the side-chain guanidinium group to establish hydrogen-bond interactions with the peptide backbone, which are particularly attractive when the backbone adopts a helical conformation. The present work provides evidence for the ability of the side chain to influence the peptide backbone conformation and, specifically, illustrates how the latter may be affected by the side-chain nature and orientation.
Supplementary Material
Scheme 2.
Acknowledgments
Gratitude is expressed to the Centre de Supercomputació de Catalunya (CESCA). Financial support from the Ministerio de Educación y Ciencia - FEDER (project CTQ2007-62245) and Gobierno de Aragón (research group E40) is gratefully acknowledged. This project has been funded in whole or in part with Federal funds from the National Cancer Institute, National Institutes of Health, under contract number N01-CO-12400. The content of this publication does not necessarily reflect the view of the policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organization imply endorsement by the U.S. Government. This research was supported [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Coordinates and energy of the minimum energy conformations characterized for Ac-t-L-c5Arg-NHMe and Ac-c-L-c5Arg-NHMe. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Toniolo C, Formaggio F, Kaptein B, Broxterman QB. Synlett. 2006:1295. [Google Scholar]; (b) Venkatraman J, Shankaramma SC, Balaram P. Chem Rev. 2001;101:3131. doi: 10.1021/cr000053z. [DOI] [PubMed] [Google Scholar]; (c) Toniolo C, Crisma M, Formaggio F, Peggion C. Biopolymers (Pept Sci) 2001;60:396. doi: 10.1002/1097-0282(2001)60:6<396::AID-BIP10184>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]; (d) Benedetti E. Biopolymers (Pept Sci) 1996;40:3. doi: 10.1002/(sici)1097-0282(1996)40:1<3::aid-bip2>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]; (e) Toniolo C, Benedetti E. Macromolecules. 1991;24:4004. [Google Scholar]
- 2.(a) Ballano G, Zanuy D, Jiménez AI, Cativiela C, Nussinov R, Alemán C. J Phys Chem B. 2008;112:13101. doi: 10.1021/jp8032116. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gómez-Catalán J, Alemán C, Pérez JJ. Theor Chem Acc. 2000;103:380. [Google Scholar]; (c) Alemán C. J Phys Chem B. 1997;101:5046. [Google Scholar]; (d) Valle G, Crisma M, Toniolo C, Holt EM, Tamura M, Bland J, Stammer CH. Int J Pept Protein Res. 1989;34:56. doi: 10.1111/j.1399-3011.1989.tb01009.x. [DOI] [PubMed] [Google Scholar]; (e) Benedetti E, Di Blasio B, Pavone V, Pedone C, Santini A, Crisma M, Valle G, Toniolo C. Biopolymers. 1989;28:175. [Google Scholar]; (f) Crisma M, Bonora GM, Toniolo C, Barone V, Benedetti E, Di Blasio B, Pavone V, Pedone C, Santini A, Fraternali F, Bavoso A, Lelj F. Int J Biol Macromol. 1989;11:345. doi: 10.1016/0141-8130(89)90006-8. [DOI] [PubMed] [Google Scholar]; (g) Benedetti E, Di Blasio B, Pavone V, Pedone C, Santini A, Barone V, Fraternalli F, Lelj F, Bavoso A, Crisma M, Toniolo C. Int J Biol Macromol. 1989;11:353. doi: 10.1016/0141-8130(89)90007-x. [DOI] [PubMed] [Google Scholar]; (h) Barone V, Fraternali F, Cristinziano PL, Lelj F, Rosa A. Biopolymers. 1988;27:1673. [Google Scholar]
- 3.(a) Casanovas J, Zanuy D, Nussinov R, Alemán C. Chem Phys Lett. 2006;429:558. doi: 10.1021/jp062804s. [DOI] [PubMed] [Google Scholar]; (b) Rao SN, Chan MF, Balaji VN. Bull Chem Soc Jpn. 1997;70:293. [Google Scholar]; (c) Gatos M, Formaggio F, Crisma M, Toniolo C, Bonora GM, Benedetti E, Di Blasio B, Iacovino R, Santini A, Saviano M, Kamphuis J. J Pept Sci. 1997;3:110. doi: 10.1002/(SICI)1099-1387(199703)3:2%3C110::AID-PSC88%3E3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]; (d) Balaji VN, Ramnarayan K, Chan MF, Rao SN. Peptide Res. 1995;8:178. [PubMed] [Google Scholar]
- 4.(a) Alemán C, Zanuy D, Casanovas J, Cativiela C, Nussinov R. J Phys Chem B. 2006;110:21264. doi: 10.1021/jp062804s. [DOI] [PubMed] [Google Scholar]; (b) Aschi M, Lucente G, Mazza F, Mollica A, Morera E, Nalli M, Paglialunga Paradisi M. Org Biomol Chem. 2003;1:1980. doi: 10.1039/b212247b. [DOI] [PubMed] [Google Scholar]; (c) Valle G, Crisma M, Toniolo C. Can J Chem. 1988;66:2575. [Google Scholar]; (d) Crisma M, Bonora GM, Toniolo C, Benedetti E, Bavoso A, Di Blasio B, Pavone V, Pedone C. Int J Biol Macromol. 1988;10:300. doi: 10.1016/0141-8130(89)90006-8. [DOI] [PubMed] [Google Scholar]; (e) Santini A, Barone V, Bavoso A, Benedetti E, Di Blasio B, Fraternali F, Lelj F, Pavone V, Pedone C, Crisma M, Bonora GM, Toniolo C. Int J Biol Macromol. 1988;10:292. doi: 10.1016/0141-8130(89)90007-x. [DOI] [PubMed] [Google Scholar]
- 5.(a) Harini VV, Aravinda S, Rai R, Shamala N, Balaram P. Chem Eur J. 2005;11:3609. doi: 10.1002/chem.200401124. [DOI] [PubMed] [Google Scholar]; (b) Crisma M, Bonora GM, Toniolo C, Bavoso A, Benedetti E, Di Blasio B, Pavone V, Pedone C. Macromolecules. 1988;21:2071. [Google Scholar]; (c) Pavone V, Benedetti E, Barone V, Di Blasio B, Lelj F, Pedone C, Santini A, Crisma M, Bonora GM, Toniolo C. Macromolecules. 1988;21:2064. [Google Scholar]; (d) Paul PKC, Sukumar M, Bardi R, Piazzesi AM, Valle G, Toniolo C, Balaram P. J Am Chem Soc. 1986;108:6363. [Google Scholar]
- 6.(a) Prasad S, Mathur A, Jaggi M, Singh AT, Mukherjee R. J Pept Sci. 2007;13:544. doi: 10.1002/psc.886. [DOI] [PubMed] [Google Scholar]; (b) Labudda-Dawidowska O, Wierzba TH, Prahl A, Kowalczyk W, Gawinski L, Plackova M, Slaninová J, Lammek B. J Med Chem. 2005;48:8055. doi: 10.1021/jm0580353. [DOI] [PubMed] [Google Scholar]; (c) Kowalczyk W, Prahl A, Dawidowska O, Derdowska I, Sobolewski D, Hartrodt B, Neubert K, Slaninová J, Lammek B. J Pept Sci. 2005;11:584. doi: 10.1002/psc.656. [DOI] [PubMed] [Google Scholar]; (d) Kowalczyk W, Prahl A, Derdowska I, Dawidowska O, Slaninová J, Lammek B. J Med Chem. 2004;47:6020. doi: 10.1021/jm040813o. [DOI] [PubMed] [Google Scholar]; (e) García-Echeverría C, Gay B, Rahuel J, Furet P. Bioorg Med Chem Lett. 1999;9:2915. doi: 10.1016/s0960-894x(99)00501-6. [DOI] [PubMed] [Google Scholar]; (f) Breveglieri A, Guerrini R, Salvadori S, Bianchi C, Bryant SD, Attila M, Lazarus LH. J Med Chem. 1996;39:773. doi: 10.1021/jm950490j. [DOI] [PubMed] [Google Scholar]; (g) Sukumar M, Raj PA, Balaram P, Becker EL. Biochem Biophys Res Commun. 1985;128:339. doi: 10.1016/0006-291x(85)91684-5. [DOI] [PubMed] [Google Scholar]
- 7.(a) Lasa M, Cativiela C. Synlett. 2006:2517. [Google Scholar]; (b) Lasa M, López P, Cativiela C. Tetrahedron: Asymmetry. 2005;16:4022. [Google Scholar]; (c) Cativiela C, Lasa P, López P. Tetrahedron: Asymmetry. 2005;16:2613. [Google Scholar]; (d) Cativiela C, López M, Lasa M. Eur J Org Chem. 2004:3898. [Google Scholar]; (e) Alías M, Cativiela C, Jiménez AI, López P, Oliveros L, Marraud M. Chirality. 2001;13:48. doi: 10.1002/1520-636X(2001)13:1<48::AID-CHIR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]; (f) Cativiela C, Díaz-de-Villegas MD, Jiménez AI, López P, Marraud M, Oliveros L. Chirality. 1999;11:583. doi: 10.1002/(SICI)1520-636X(1999)11:7<583::AID-CHIR11>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 8.(a) Casanovas J, Jiménez AI, Cativiela C, Nussinov R, Alemán C. J Org Chem. 2008;73:644. doi: 10.1021/jo702107s. [DOI] [PubMed] [Google Scholar]; (b) Lasa M, Jiménez AI, Zurbano MM, Cativiela C. Tetrahedron Lett. 2005;46:8377. [Google Scholar]; (c) Alemán C, Jiménez AI, Cativiela C, Pérez JJ, Casanovas J. J Phys Chem B. 2002;106:11849. [Google Scholar]; (d) Gomez-Catalan J, Jiménez AI, Cativiela C, Perez JJ. J Pept Res. 2001;57:435. doi: 10.1034/j.1399-3011.2001.00840.x. [DOI] [PubMed] [Google Scholar]; (e) Jiménez AI, Cativiela C, Gómez-Catalán J, Pérez JJ, Aubry A, París M, Marraud M. J Am Chem Soc. 2000;122:5811. [Google Scholar]; (f) Jiménez AI, Cativiela C, París M, Peregrina JM, Avenoza A, Aubry A, Marraud M. Tetrahedron Lett. 1998;39:7841. [Google Scholar]; (g) Jiménez AI, Cativiela C, Aubry A, Marraud M. J Am Chem Soc. 1998;120:9452. [Google Scholar]; (h) Jiménez AI, Vanderesse R, Marraud M, Aubry A, Cativiela C. Tetrahedron Lett. 1997;38:7559. [Google Scholar]
- 9.(a) Crisma M, Toniolo C, Royo S, Jiménez AI, Cativiela C. Org Lett. 2006;8:6091. doi: 10.1021/ol062600u. [DOI] [PubMed] [Google Scholar]; (b) Crisma M, De Borggraeve WM, Peggion C, Formaggio F, Royo S, Jiménez AI, Cativiela C, Toniolo C. Chem Eur J. 2006;12:251. doi: 10.1002/chem.200500865. [DOI] [PubMed] [Google Scholar]; (c) Jiménez AI, Ballano G, Cativiela C. Angew Chem Int Ed. 2005;44:396. doi: 10.1002/anie.200461230. [DOI] [PubMed] [Google Scholar]; (d) Royo S, De Borggraeve WM, Peggion C, Formaggio F, Crisma M, Jiménez AI, Cativiela C, Toniolo C. J Am Chem Soc. 2005;127:2036. doi: 10.1021/ja043116u. [DOI] [PubMed] [Google Scholar]
- 10.Gaussian 03, Revision B.02, Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Montgomery, J. A.; Vreven, Jr., T.; Kudin, K. N.; Burant, J. C.; Millam, J. M.; Iyengar, S. S.; Tomasi, J.; Barone, V.; Mennucci, B.; Cossi, M.; Scalmani, G.; Rega, N.; Petersson, G. A.; Nakatsuji, H.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Klene, M.; Li, X.; Knox, J. E.; Hratchian, H. P.; Cross, J. B.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Ayala, P. Y.; Morokuma, K.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Zakrzewski, V. G.; Dapprich, S.; Daniels, A. D.; C. Strain, M.; Farkas, O.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Ortiz, J. V.; Cui, Q.; Baboul, A. G.; Clifford, S.; Cioslowski, J.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Challacombe, M.; Gill, P. M. W.; Johnson, B.; Chen, W.; Wong, M. W.; Gonzalez, C.; Pople, J. A. Gaussian, Inc., Pittsburgh PA, 2003.
- 11.Becke AD. J Chem Phys. 1993;98:1372. [Google Scholar]
- 12.Lee C, Yang W, Parr RG. Phys Rev B. 1993;37:785. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]
- 13.McLean AD, Chandler GS. J Chem Phys. 1980;72:5639. [Google Scholar]
- 14.(a) Miertus S, Scrocco E, Tomasi J. Chem Phys. 1981;55:117. [Google Scholar]; (b) Miertus S, Tomasi J. Chem Phys. 1982;65:239. [Google Scholar]; (c) Tomasi J, Persico M. Chem Phys. 1994;94:2027. [Google Scholar]; (b) Tomasi J, Mennucci B, Cammi R. Chem Rev. 2005;105:2999. doi: 10.1021/cr9904009. [DOI] [PubMed] [Google Scholar]
- 15.Hawkins GD, Cramer CJ, Truhlar DG. J Chem Phys B. 1998;102:3257. [Google Scholar]; (b) Jang YH, Goddard WA, III, Noyes KT, Sowers LC, Hwang S, Chung DS. J Phys Chem B. 2003;107:344. [Google Scholar]; (c) Iribarren JI, Casanovas J, Zanuy D, Alemán C. Chem Phys. 2004;302:77. [Google Scholar]
- 16.Kale L, Skeel R, Bhandarkar M, Brunner R, Gursoy A, Krawetz N, Phillips J, Shinozaki A, Varadarajan K, Schulten K. J Comput Phys. 1999;151:283. [Google Scholar]
- 17.Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML. J Chem Phys. 1982;79:926. [Google Scholar]
- 18.Darden T, York D, Pedersen L. J Chem Phys. 1993;98:10089. [Google Scholar]
- 19.Ryckaert JP, Ciccotti G, Berendsen HJC. Comput Phys. 1990;94:1683. [Google Scholar]
- 20.Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR. J Chem Phys. 1984;81:3684. [Google Scholar]
- 21.(a) Steiner T, Koellner G. J Mol Biol. 2001;305:535. doi: 10.1006/jmbi.2000.4301. [DOI] [PubMed] [Google Scholar]; (b) Worth GA, Wade RC. J Phys Chem. 1995;99:17473. [Google Scholar]; (c) Mitchell JBO, Nandi CL, McDonald IK, Thornton JM. J Mol Biol. 1994;239:315. doi: 10.1006/jmbi.1994.1370. [DOI] [PubMed] [Google Scholar]; (d) Mitchell JBO, Nandi CL, Ali S, McDonald IK, Thornton JM. Nature. 1993;366:413. [Google Scholar]
- 22.Cornell WD, Cieplak P, Bayly CI, Gould IR, Merz KM, Ferguson DM, Spellmeyer DC, Fox T, Caldwell JW, Kollman PA. J Am Chem Soc. 1995;117:5179. [Google Scholar]
- 23.Alemán C, Casanovas J Chem Soc Perkin Trans 2. 1994:563. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.