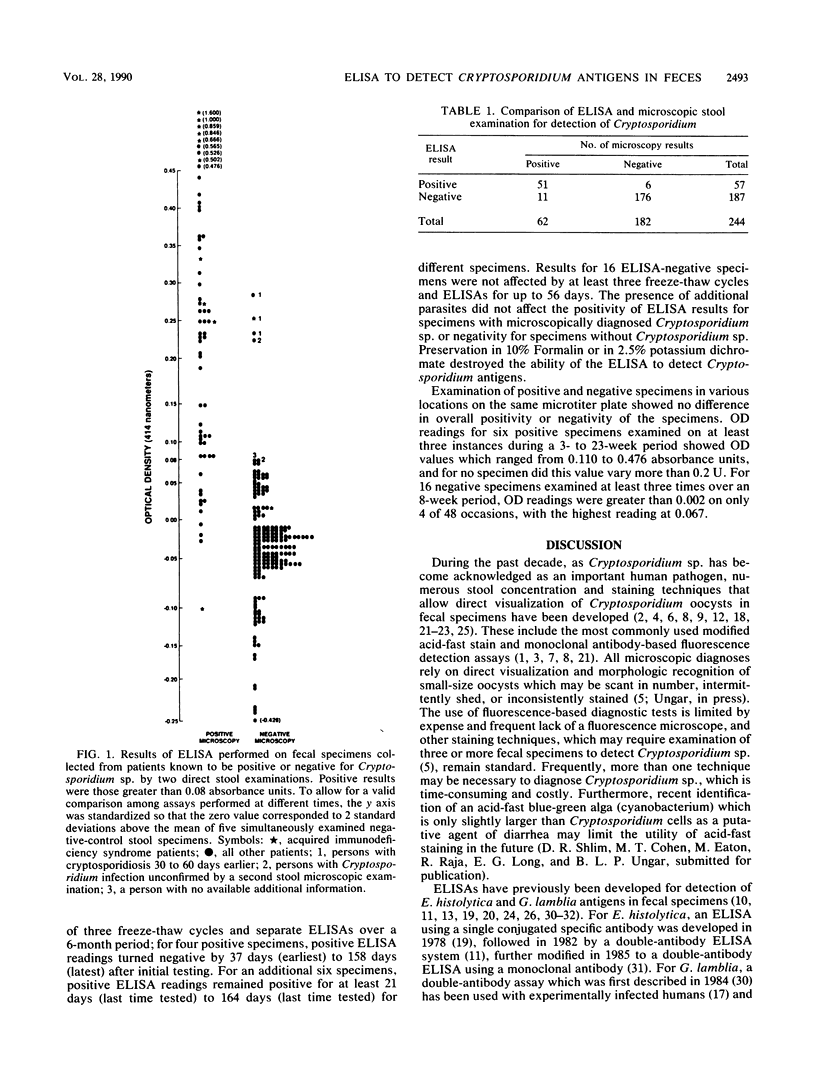
Abstract
Cryptosporidium sp. is a ubiquitous 4- to 6-micron protozoan parasite infecting the intestinal tract of humans. It causes mild to fulminant diarrhea in patients, especially immunocompromised persons, and it may be hard to detect by microscopic fecal examination. An indirect, double-antibody enzyme-linked immunosorbent assay (ELISA) was developed using specifically produced goat and rabbit antisera to detect Cryptosporidium antigens in human feces. Of 62 frozen stools from patients with cryptosporidiosis, as detected by at least two microscopic diagnostic techniques, 51 were positive by ELISA; all ELISA-negative specimens came from patients with fewer than five oocysts per 0.01 ml of concentrated fecal sample examined after modified acid-fast or fluorescent monoclonal antibody staining. A total of 182 specimens from persons without Cryptosporidium infection were negative by ELISA in 176 instances; 3 ELISA-positive specimens came from patients with cryptosporidiosis diagnosed earlier. The sensitivity of the assay was 82.3%, and specificity was 96.7%. The predictive value of a positive ELISA was 89.5%, and the predictive value of a negative ELISA was 94.2%. The ELISA was not affected by the presence of eight other intestinal parasites but was sometimes affected by repeated freezing and thawing of fecal specimens. All fecal specimens were heated to 100 degrees C for 2 min to reduce proteolytic enzyme activity, although the necessity of this step needs further evaluation. This first-generation ELISA is a simple, rapid, easily standardized test for Cryptosporidium antigens in stool samples which will be useful for diagnosis and for large-scale epidemiologic studies.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrowood M. J., Sterling C. R. Comparison of conventional staining methods and monoclonal antibody-based methods for Cryptosporidium oocyst detection. J Clin Microbiol. 1989 Jul;27(7):1490–1495. doi: 10.1128/jcm.27.7.1490-1495.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asahi H., Kumada M., Kato K., Koyama T. A simple staining method for cryptosporidian oocysts and sporozoites. Jpn J Med Sci Biol. 1988 Jun;41(3):117–121. doi: 10.7883/yoken1952.41.117. [DOI] [PubMed] [Google Scholar]
- Baron E. J., Schenone C., Tanenbaum B. Comparison of three methods for detection of Cryptosporidium oocysts in a low-prevalence population. J Clin Microbiol. 1989 Jan;27(1):223–224. doi: 10.1128/jcm.27.1.223-224.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronsdon M. A. Rapid dimethyl sulfoxide-modified acid-fast stain of Cryptosporidium oocysts in stool specimens. J Clin Microbiol. 1984 Jun;19(6):952–953. doi: 10.1128/jcm.19.6.952-953.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford F. G., Vermund S. H. Human cryptosporidiosis. Crit Rev Microbiol. 1988;16(2):113–159. doi: 10.3109/10408418809104469. [DOI] [PubMed] [Google Scholar]
- Fayer R., Ungar B. L. Cryptosporidium spp. and cryptosporidiosis. Microbiol Rev. 1986 Dec;50(4):458–483. doi: 10.1128/mr.50.4.458-483.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia L. S., Brewer T. C., Bruckner D. A. Fluorescence detection of Cryptosporidium oocysts in human fecal specimens by using monoclonal antibodies. J Clin Microbiol. 1987 Jan;25(1):119–121. doi: 10.1128/jcm.25.1.119-121.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia L. S., Brewer T. C., Bruckner D. A. Incidence of Cryptosporidium in all patients submitting stool specimens for ova and parasite examination: monoclonal antibody IFA method. Diagn Microbiol Infect Dis. 1988 Sep;11(1):25–27. doi: 10.1016/0732-8893(88)90070-3. [DOI] [PubMed] [Google Scholar]
- Garcia L. S., Bruckner D. A., Brewer T. C., Shimizu R. Y. Techniques for the recovery and identification of Cryptosporidium oocysts from stool specimens. J Clin Microbiol. 1983 Jul;18(1):185–190. doi: 10.1128/jcm.18.1.185-190.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy M. S. Preliminary observations using a multi-layer ELISA method for the detection of Entamoeba histolytica trophozoite antigens in stool samples. Trans R Soc Trop Med Hyg. 1982;76(3):396–400. doi: 10.1016/0035-9203(82)90199-7. [DOI] [PubMed] [Google Scholar]
- Henriksen S. A., Pohlenz J. F. Staining of cryptosporidia by a modified Ziehl-Neelsen technique. Acta Vet Scand. 1981;22(3-4):594–596. doi: 10.1186/BF03548684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knisley C. V., Engelkirk P. G., Pickering L. K., West M. S., Janoff E. N. Rapid detection of giardia antigen in stool with the use of enzyme immunoassays. Am J Clin Pathol. 1989 Jun;91(6):704–708. doi: 10.1093/ajcp/91.6.704. [DOI] [PubMed] [Google Scholar]
- Lumb R., Lanser J. A., O'Donoghue P. J. Electrophoretic and immunoblot analysis of Cryptosporidium oocysts. Immunol Cell Biol. 1988 Oct-Dec;66(Pt 5-6):369–376. doi: 10.1038/icb.1988.48. [DOI] [PubMed] [Google Scholar]
- Ma P., Soave R. Three-step stool examination for cryptosporidiosis in 10 homosexual men with protracted watery diarrhea. J Infect Dis. 1983 May;147(5):824–828. doi: 10.1093/infdis/147.5.824. [DOI] [PubMed] [Google Scholar]
- Mead J. R., Arrowood M. J., Sterling C. R. Antigens of Cryptosporidium sporozoites recognized by immune sera of infected animals and humans. J Parasitol. 1988 Feb;74(1):135–143. [PubMed] [Google Scholar]
- Nash T. E., Herrington D. A., Levine M. M. Usefulness of an enzyme-linked immunosorbent assay for detection of Giardia antigen in feces. J Clin Microbiol. 1987 Jul;25(7):1169–1171. doi: 10.1128/jcm.25.7.1169-1171.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root D. M., Cole F. X., Williamson J. A. The development and standaridization of an ELISA method for the detection of Entamoeba histolytica antigens in fecal samples. Arch Invest Med (Mex) 1978;9 (Suppl 1):203–210. [PubMed] [Google Scholar]
- Rosoff J. D., Sanders C. A., Sonnad S. S., De Lay P. R., Hadley W. K., Vincenzi F. F., Yajko D. M., O'Hanley P. D. Stool diagnosis of giardiasis using a commercially available enzyme immunoassay to detect Giardia-specific antigen 65 (GSA 65). J Clin Microbiol. 1989 Sep;27(9):1997–2002. doi: 10.1128/jcm.27.9.1997-2002.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusnak J., Hadfield T. L., Rhodes M. M., Gaines J. K. Detection of Cryptosporidium oocysts in human fecal specimens by an indirect immunofluorescence assay with monoclonal antibodies. J Clin Microbiol. 1989 May;27(5):1135–1136. doi: 10.1128/jcm.27.5.1135-1136.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soave R., Armstrong D. Cryptosporidium and cryptosporidiosis. Rev Infect Dis. 1986 Nov-Dec;8(6):1012–1023. doi: 10.1093/clinids/8.6.1012. [DOI] [PubMed] [Google Scholar]
- Stibbs H. H. Monoclonal antibody-based enzyme immunoassay for Giardia lamblia antigen in human stool. J Clin Microbiol. 1989 Nov;27(11):2582–2588. doi: 10.1128/jcm.27.11.2582-2588.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stibbs H. H., Ongerth J. E. Immunofluorescence detection of Cryptosporidium oocysts in fecal smears. J Clin Microbiol. 1986 Oct;24(4):517–521. doi: 10.1128/jcm.24.4.517-521.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stibbs H. H., Samadpour M., Manning J. F. Enzyme immunoassay for detection of Giardia lamblia cyst antigens in formalin-fixed and unfixed human stool. J Clin Microbiol. 1988 Sep;26(9):1665–1669. doi: 10.1128/jcm.26.9.1665-1669.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilley M., Upton S. J., Blagburn B. L., Anderson B. C. Identification of outer oocyst wall proteins of three Cryptosporidium (Apicomplexa: Cryptosporidiidae) species by 125I surface labeling. Infect Immun. 1990 Jan;58(1):252–253. doi: 10.1128/iai.58.1.252-253.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungar B. L., Nash T. E. Quantification of specific antibody response to Cryptosporidium antigens by laser densitometry. Infect Immun. 1986 Jul;53(1):124–128. doi: 10.1128/iai.53.1.124-128.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungar B. L., Soave R., Fayer R., Nash T. E. Enzyme immunoassay detection of immunoglobulin M and G antibodies to Cryptosporidium in immunocompetent and immunocompromised persons. J Infect Dis. 1986 Mar;153(3):570–578. doi: 10.1093/infdis/153.3.570. [DOI] [PubMed] [Google Scholar]
- Ungar B. L., Yolken R. H., Nash T. E., Quinn T. C. Enzyme-linked immunosorbent assay for the detection of Giardia lamblia in fecal specimens. J Infect Dis. 1984 Jan;149(1):90–97. doi: 10.1093/infdis/149.1.90. [DOI] [PubMed] [Google Scholar]
- Ungar B. L., Yolken R. H., Quinn T. C. Use of a monoclonal antibody in an enzyme immunoassay for the detection of Entamoeba histolytica in fecal specimens. Am J Trop Med Hyg. 1985 May;34(3):465–472. doi: 10.4269/ajtmh.1985.34.465. [DOI] [PubMed] [Google Scholar]
- Wienecka J., Olding-Stenkvist E., Schröder H., Huldt G. Detection of giardia antigen in stool samples by a semi-quantitative enzyme immunoassay (EIA) test. Scand J Infect Dis. 1989;21(4):443–448. doi: 10.3109/00365548909167450. [DOI] [PubMed] [Google Scholar]


