Abstract
BACKGROUND:
There is evidence that combination therapy (CT) in the form of long-acting beta2-agonists (LABAs) and inhaled corticosteroids can improve lung function for patients with chronic obstructive pulmonary disease (COPD).
OBJECTIVE:
To determine the cost-effectiveness of using CT in none, all or a selected group of COPD patients.
METHODS:
A Markov model was designed to compare four treatment strategies: no use of CT regardless of COPD severity (patients receive LABA only); use of CT in patients with stage 3 disease only (forced expiratory volume in 1 s [FEV1] less than 35% of predicted); use of CT in patients with stages 2 and 3 disease only (FEV1 less than 50% of predicted); and use of CT in all patients regardless of severity of COPD. Estimates of mortality, exacerbation and disease progression rates, quality-adjusted life years (QALYs) and costs were derived from the literature. Three-year and lifetime time horizons were used. The analysis was conducted from a health systems perspective.
RESULTS:
CT was associated with a cost of $39,000 per QALY if given to patients with stage 3 disease, $47,500 per QALY if given to patients with stages 2 and 3 disease, and $450,333 per QALY if given to all COPD patients. Results were robust to various assumptions tested in a Monte Carlo simulation.
CONCLUSION:
Providing CT for COPD patients in stage 2 or 3 disease is cost-effective. The message to family physicians and specialists is that as FEV1 worsens and reaches 50% of predicted values, CT is recommended.
Keywords: COPD, Cost-effectiveness analysis, Economics, Lung diseases, Pharmaceuticals
Abstract
HISTORIQUE :
Selon certaines données probantes, la polythérapie sous forme de bêta2-agonistes à action prolongée (BAAP) et de corticoïdes par aérosol peut améliorer la fonction pulmonaire des patients atteints d’une maladie pulmonaire obstructive chronique (MPOC).
OBJECTIF :
Déterminer le rapport coût-efficacité de l’utilisation de la polythérapie chez aucun patient atteint de MPOC, tous les patients atteints de MPOC ou certains de ces patients.
MÉTHODOLOGIE :
Les auteurs ont conçu un modèle de Markov pour comparer quatre stratégies de traitement : ne pas recourir à la polythérapie, quelle que soit la gravité de la maladie (les patients ne reçoivent que des BAAP), recourir à la polythérapie seulement chez les patients atteints d’une MPOC de phase 3 (volume expiratoire maximal par seconde [VEMS] inférieur à 35 % de celui prévu), utilisation de la polythérapie seulement en présence d’une maladie de phase 2 ou 3 (VEMS inférieur à 50 % de celui prévu) et recourir à la polythérapie chez tous les patients quelle que soit la gravité de la MPOC. Les auteurs ont dérivé des publications les estimations des taux de mortalité, d’exacerbation et d’évolution du diagnostic, les années de vie pondérée par la qualité (AVPQ) et les coûts. Ils ont utilisé un horizon prévisionnel de trois ans et d’une vie entière. Ils ont effectué l’analyse selon la perspective du système de santé.
RÉSULTATS :
La polythérapie s’associe à un coût de 39 000 $ par AVPQ si elle est administrée aux patients atteints de la maladie de phase trois, de 47 500 $ par AVPQ si elle est administrée aux patients atteints de la maladie de phase 2 ou 3, et de 450 333 $ par AVPQ si elle est administrée à tous les patients atteints d’une MPOC. Les résultats résistaient à diverses hypothèses vérifiées dans une simulation de Monte Carlo.
CONCLUSION :
L’administration d’une polythérapie aux patients atteints d’une MPOC de phase 2 ou 3 est rentable. Le message à transmettre aux médecins de famille et aux spécialistes, c’est que lorsque le VEMS s’aggrave et atteint 50 %, la polythérapie est recommandée.
Chronic obstructive pulmonary disease (COPD) is a respiratory disorder that is characterized by progressive, partially reversible airway obstruction, systemic manifestations, and increasing frequency and severity of exacerbations. COPD is a major cause of morbidity and mortality throughout the world (1). COPD affects more than 16 million people in the United States, generating over US$14 billion in direct costs and US$9 billion in indirect costs annually (2). Moreover, significant increases in the prevalence and mortality of the disease are predicted in the coming decades (3,4), resulting in increasing demands on health care.
There is evidence that combination therapy (CT) in the form of long-acting beta2-agonists (LABAs) (5) and inhaled corticosteroids (ICSs) (6) can improve clinical outcomes for patients with COPD (7–10). Recent guidelines for the management of COPD recommend LABA plus ICS for symptomatic patients with a forced expiratory volume in 1 s (FEV1) of less than 50% of predicted, particularly if there have been three or more exacerbations per year (1,11).
However, these guidelines have not assessed the cost implications of this recommendation. CT may cost almost double that of LABA monotherapy. Meaningful information regarding the cost-effectiveness of using CT is necessary for decision-makers to rationally guide their management recommendations.
METHODS
Model design
Economic models are used when complete observational data are not available to assess the economic outcomes of alternative strategies. In Markov models, a series of health states are defined; subjects remain in these states for a given cycle period (eg, three months), and subjects move between these states, or remain in them, incurring costs and health outcomes, from one cycle to the next. The probabilities of moving between states will depend on the treatment strategy, and it is the differences between these probabilities that generate different outcomes (costs and health outcomes). A Markov model was designed to estimate the cost-effectiveness of using CT in patients with varying severities of COPD modelled over the natural history of COPD. In this model, a cohort with COPD was followed over three years divided into three-month cycles to allow maximum flexibility for movement of patients between disease severity categories. A lifetime time horizon was also used to determine the long-term outcomes of CT. All modelling assumptions and their sources are shown in Table 1.
TABLE 1.
Model inputs (three-month cycle)
| Inputs | Stage 1 | Stage 2 | Stage 3 | References |
|---|---|---|---|---|
| Baseline distribution of patients, % | 93 | 4 | 3 | 12 |
| Progression to next disease stage, % | 2.97 (2.87–3.07) | 9.94 (9.84–10.04) | n/a | 25 |
| Exacerbations per year (LABA), % | 0.17 (0.153–0.187) | 0.59 (0.531–0.649) | 0.83 (0.747–0.913) | 14 |
| Mild | 93.7 | 26.0 | 0 | 16,23,36,37 |
| Moderate | 3.8 | 61.9 | 69.8 | 16,23,36,37 |
| Severe | 2.5 | 12.0 | 30.2 | 16,23,36,37 |
| Relative risk (CT versus LABA) | 0.86 (0.77–0.97) | 0.86 (0.77–0.97) | 0.86 (0.77–0.97) | 17–20 |
| All-cause mortality rates (events/100 person-years) | 3.92 (3.72–4.12) | 6.16 (5.85–6.47) | 9.24 (8.78–9.71) | 38 |
| QALY | 0.897 (0.807–0.987) | 0.750 (0.675–0.825) | 0.549 (0.494–0.604) | 24,39 |
| Reduction in QALY due to: | ||||
| Mild exacerbation | −0.17 (0.15–0.19 | −0.17 (0.15–0.19) | −0.17 (0.15–0.19) | 40 |
| Moderate exacerbation | −0.47 (0.42–0.52) | −0.47 (0.42–0.52) | −0.47 (0.42–0.52) | 41 |
| Severe exacerbation | −0.47 (0.42–0.52) | −0.47 (0.42–0.52) | −0.47 (0.42–0.52) | 41 |
| Cost of COPD per year, $ | 687 (618–756) | 658 (752–724) | 1,752 (1,576–1,927) | 25 |
| Cost of mild exacerbation, $ | 60 (54–66) | 60 (54–66) | 60 (54–66) | 25 |
| Cost of moderate exacerbation, $ | 270 (243–297) | 270 (243–297) | 270 (243–297) | 25 |
| Cost of severe exacerbation, $ | 4,827 (4,344–5,309) | 4,827 (4,344–5,309) | 4,827 (4,344–5,309) | 25 |
| Average daily cost of LABA, $ | 1.61 (1.47–1.75) | 1.61 (1.47–1.75) | 1.61 (1.47–1.75) | 42 |
| Average daily cost of CT, $ | 2.77 (2.60–4.18) | 2.77 (2.60–4.18) | 2.77 (2.60–4.18) | 42 |
Values for sensitivity analysis are in parenthesis. Doses: Oxeze (AstraZeneca Canada): 12 μg formoterol per dose twice daily; Serevent (GlaxoSmithKline Canada): 50 μg salmeterol twice daily; Advair (GlaxoSmithKline Canada) (usual dose): 50 μg salmeterol/250 μg fluticasone propionate per dose twice daily; Advair (GlaxoSmithKline Canada) (high dose): 50 μg salmeterol/500 μg fluticasone propionate per dose twice daily; Symbicort (AstraZeneca Canada): 200 μg budesonide/6 μg formoterol per dose twice daily. COPD Chronic obstructive pulmonary disease; CT Combination therapy; LABA Long-acting beta2-agonist; n/a Not available; QALY Quality-adjusted life years
Natural history of disease
The cohort of COPD patients in the model was representative of those who had COPD in a national (United States) population-based sample survey (12). The sample in the survey consisted of 79% men and 21% women, 87% white, mean (± SD) age of 61±7.7 years, and 97% who were current or former smokers (12). Only age was used in the model, because key variables were not available by sex or smoking status. To model the natural history of COPD, all patients were divided into three mutually exclusive disease severity strata based on the criteria of the American Thoracic Society (13):
Stage 1 disease was defined as FEV1 50.0% of predicted or greater;
Stage 2 disease was defined as FEV1 of 35.0% to 49.9% of predicted; and
Stage 3 disease was defined as FEV1 less than 35.0% of predicted.
Based on the Third National Health and Nutrition Examination Survey, it was estimated that initially, at baseline, 93% of the total pool of patients had stage 1 disease, 4% had stage 2 disease and 3% had stage 3 disease (12).
The model assumed that FEV1 declined over time. Based on results from the Lung Health Study Research Group (14), it was assumed that the mean rate of FEV1 reduction for each severity group was 47 mL per patient per year (11.75 mL over a three-month period). Using this assumption, the estimated probability for each person in stage 1 moving into stage 2 was 0.74% over a three-month period while the estimated probability for a person in stage 2 moving into stage 3 was 2.48%. Furthermore, it was assumed that the risk of mortality and the frequency and severity of exacerbations increased with declining FEV1 (ie, exacerbation and mortality rates varied by stage of disease) (15).
For analytical purposes, COPD exacerbations were sub-classified into three mutually exclusive categories (13):
Mild, defined as worsening of symptoms requiring outpatient physician services and institution of medications and/or antimicrobials (ie, exacerbation therapy).
Moderate, defined as clinical episodes requiring emergency department services or urgent visits to a physician’s office (including institution of exacerbation therapy).
Severe, defined as requiring inpatient hospital care (including institution of exacerbation therapy).
The total expected number of exacerbations per person (all severities) annually per health state was 0.17 for stage 1, 0.59 for stage 2 and 0.83 for stage 3 (14). In stage 1, 93.7% of exacerbations were assumed to be mild while in stage 2, 74% of clinically apparent exacerbations were assumed to be moderate or severe. For patients in stage 3, it was assumed that 30% of exacerbations would require hospitalization (16).
Modelling strategy
The effects of the two therapies (CT and LABA monotherapy) were evaluated using four different strategies in a Markov model:
Strategy 1 (base case): All patients were treated with LABA monotherapy.
Strategy 2: In addition to the base case, ICS is given to patients with stage 3 disease only.
Strategy 3: In addition to the base case, ICS is given to patients with stage 2 and stage 3 disease only.
Strategy 4: In addition to the base case, ICS is given to all patients.
For each strategy, all patients were followed for three years in three-month periods. For each three-month period, probability of death and exacerbation were input for each cohort within each disease category. To estimate exacerbation rates for strategies using CT, a single RR reduction of 13.6% (see Efficacy below) was applied based on four randomized controlled trials (17–20).
From one three-month cycle to the next, a small proportion of patients moved into a higher disease severity category based on the expected declines in FEV1. Patients who died during the three-month period were censored from further analysis. Survivors of each three-month cycle continued through another cycle, wherein a similar set of probabilities (survival, exacerbation and disease progression) was applied. A total of 12 cycles translated into 36 months of follow-up time (40 cycles for lifetime model). All analyses were conducted using TreeAge Pro Suite (TreeAge Software Inc, USA).
Health outcomes
The health outcomes considered were health-related quality of life and mortality. A quality-adjusted life year (QALY) value based on the EQ-5D was obtained for the duration of each of the three stages (Table 1). The EQ-5D provides a descriptive health status score ranging from −0.59 to 1.00 (21). A score of 0.0 represents death and a score of 1.0 represents perfect health. The minimally important clinical difference for the EQ-5D is 0.074 (21). Each time a person had an exacerbation, his or her QALY value (ie, health status) was reduced by a specified amount for that three-month cycle.
The baseline mortality rates for ICSs (22) were used, because these were the only ones available by stage of disease. All-cause yearly mortality rates for COPD were estimated to be 3.92% for stage 1, 6.16% for stage 2 and 9.24% for stage 3 disease (23). All-cause mortality was chosen because COPD patients may die from complications of COPD as well as other causes, and the distinction is not always clear.
Efficacy
The key variable that distinguishes treatment regimens is efficacy. A systematic review of the COPD literature on clinical trials for treatment with CT and LABA monotherapy was conducted. The inclusion criteria were that the study was a randomized controlled trial, patients only had COPD, the intervention arms included LABA and CT, and the number of exacerbations and mortality rates were indicated. Based on these inclusion criteria, four studies were identified and their data abstracted (17–20). Table 2 shows the key indicators for each trial. Efficacy for treatment regimens was measured as the difference in risk of exacerbations between regimens. The weighted average of the four trials was used as the RR. A single value of RR was used for all stages of COPD. Based on the four randomized controlled trials on CT (17–20), there was no evidence to indicate that mortality would differ by intervention and it was assumed in the base case that there was no mortality reduction in CT versus LABA monotherapy.
TABLE 2.
Selected findings from abstracted clinical trials*
| Study | Comparators | Mean FEV1 (baseline % predicted) | Change from baseline | Relative risk (95% CI) |
|---|---|---|---|---|
| Calverley et al (17) | Salmeterol, n=372 | 44.3 | 73 mL | 0.93 |
| Salmeterol + fluticasone, n=358 | 44.8 | 133 mL | (0.801–1.08) | |
| Calverley et al (18) | Salmeterol, n=1521 | 43.6 | –21 mL | 0.88 |
| Salmeterol + fluticasone, n=1533 | 44.3 | 29 mL | (0.81–0.95) | |
| Calverley et al (19) | Formoterol, n=255 | 36.0 | 5% | 0.745 |
| Formoterol + budesonide, n=254 | 36.0 | 14% | (0.587–0.945) | |
| Szafranski et al (20) | Formoterol, n=201 | 36.0 | 14% | 0.771 |
| Formoterol + budesonide, n=208 | 36.0 | 15% | (0.599–0.992) |
Trials were identified via systematic review. FEV1 Forced expiratory volume in 1 s
Costs
Cost of care consisted of routine maintenance costs, incremental costs resulting from exacerbations and costs of treatment regimens. Routine maintenance services for persons with COPD were based on those found in Oostenbrink et al (24). The amount of services for routine maintenance care varied according to severity level. Unit costs for each of the services are shown in Table 3. The second component was the number of exacerbations by level of severity, based on data from Sin et al (25) (Table 1). Services and costs increase by level of severity. A mild exacerbation requires a physician visit and medications, a moderate one assumes additional emergency services and a severe one assumes additional hospitalization, including intensive care unit care. An increased risk of pneumonia has been observed in patients prescribed ICSs both in the Towards a Revolution in COPD Health (TORCH) study (18) and in a retrospective analysis of a Quebec administrative database (26). However, this observation was based on either a post hoc analysis or a retrospective analysis, respectively, and requires further confirmation. Thus, costs associated with pneumonia were excluded from the analysis.
TABLE 3.
Unit costs
| Item | Cost, $ | Units | Source |
|---|---|---|---|
| Routine items | |||
| Outpatient visit – internist | 35.68 | Visits | AHCIP – code 0303A INMD |
| Outpatient visit – general practitioner | 28.97 | Visits | AHCIP Code 0303A |
| Spirometry | 99.56 | Procedures | AHCIP – codes 0338C ($39.68), 0338A ($9.40), 0338N ($25.74), 0338H ($24.74). |
| Beta-adrenergics | 4.72 | Days | AHC DBP – General salbutomol, DIN 00002232987, $0.59/puff, 8 per day |
| Theophylline | 0.50 | Days | AHC DBP – 400 mg per day, 0ral, $0.5036 |
| Inhaled steroids | 1.26 | Days | AHC DBP – Flovent, 500 mg per day, 250 mg aerosol $0.6302 |
| Exacerbation items | |||
| Intensive care unit days | 2,916.00 | Days | AHW MIS – 3 x daily ward rate |
| Ward days | 972.00 | Days | AHW MIS |
| Emergency room visits | 461.42 | Visits | ACCS – code 863 ($418); AHCIP – code 0303A E/M ($43.42) |
| Antibiotics | 6.28 | Days | AHC DBP – Clarythromycin, 1000 mg, 500 mg oral ($3.1442) |
| Systemic steroids | 0.13 | Days | AHC DBP – Prednisone 30 mg per day 5 mg $0.022 |
ACCS Ambulatory Case Classification System, in Health Costing in Alberta, 2004 (44); AHC DBP – Alberta Health Care Drug Benefit Plan (42); AHCIP Alberta Health Care Insurance Plan, Schedule of Medical Benefits, 2005 (43); AHW MIS Alberta Health and Wellness Management Information System (44); DIN Drug identification number
Costs were updated to 2006 levels using the Alberta general Consumer Price Index, obtained from Statistics Canada. Finally, the third component, drug costs for alternative treatment regimens, were based on recommended doses and 2005 drug prices as listed in the Alberta Health and Wellness drug plan formulary. All costs are in Canadian dollars. The costs were alternately discounted at an annual rate of 5% and 3%.
Cost-effectiveness
The incremental cost-effectiveness ratio (ICER) is the ratio between the differences in costs and in QALYs in each successive strategy. Each strategy was ordered according to its cost (27). The differences in costs and QALYs were then calculated between each successive strategy, generating an ICER for each successive strategy. Each ICER was compared with a minimally acceptable value (ie, representing society’s willingness to pay for an additional unit of improvement) to conclude whether a particular strategy was cost-effective. A cost per QALY threshold of $50,000 is commonly used as a threshold for determining cost-effectiveness (28). Therefore, strategies of CT were considered to be cost-effective if their ICER was below a decision threshold of $50,000 per QALY gained.
Sensitivity analysis
To determine the robustness of the data, a multivariate probabilistic analysis was conducted of all key variables to different (but clinically plausible) ranges of assumptions (shown in parentheses in Table 1). Using Monte Carlo simulation, the variables were randomly sampled, assuming a triangular distribution, and 100,000 sample sets were produced. Based on the 100,000 sample sets, an ‘acceptability curve’, which depicts the proportion of simulations for strategies using CT that had ICERs (compared with LABA monotherapy) below a range of cost per QALY thresholds, was generated (29). Therefore, the curve represents the probability that CT is cost-effective (compared with LABA monotherapy) at particular cost per QALY thresholds; hence, the term ‘acceptability curve’.
Furthermore, the entrance criterion in TORCH (18) was FEV1 less than 60% predicted, while the entrance criterion in the other three trials (17,19,20) was FEV1 less than 50% predicted. Therefore, a separate sensitivity analysis was conducted, excluding TORCH (18) from the estimate of RR reduction. The weighted RR reduction from the three trials (17,19,20) not including TORCH (18) was 25% (14% when including TORCH; Table 1).
All four randomized trials included in the present analysis (17–20) found no evidence to indicate that mortality differed between LABA monotherapy and CT. Only one of the four COPD trials (18) was powered to see a mortality effect from treatment. CT was shown to have a lower mortality rate than the other study groups, although the finding was not statistically significant. In the TORCH trial, proportion of deaths (all causes) over three years was 13.4% (1–[1316/1521]) in the LABA study group and 12.7% (1–[1339/1533]) in the CT study group. The relative risk reduction in all-cause mortality between CT and LABA monotherapy was therefore 5% (13.4–[12.7/13.4]) over three years. In a separate sensitivity analysis, a reduction in the all-cause probability of death for CT based on the result from TORCH (18) was included.
RESULTS
Cost-effectiveness
Results are expressed on a per-person basis over the entire group (all stages of COPD) and are expressed in Canadian dollars. The cost statistic includes the cost of drug therapy plus the cost of maintenance (routine care) and treatment of exacerbations. The per-person costs and total number of QALYs are also shown in Table 4. If CT is given to patients with stage 3 disease, the incremental cost is $39 per patient over three years ($152 over a lifetime). All values are discounted at 3%. If CT is given to patients with stages 2 or 3 disease, the incremental cost is $95 per patient over three years ($354 over a lifetime). If CT is given to all patients with COPD, the incremental cost is $1,351 per patient over three years ($3,140 over a lifetime).
Table 4.
Cost-effectiveness of various treatment strategies for three-year and lifetime models
| Treatment scenario | Cost per patient, $ | QALYs | ICER*, $ per QALY |
|---|---|---|---|
| Three-year model | |||
| LABA monotherapy for all stages | 3,719 | 2.405 | – |
| CT for stage 3 only | 3,758 | 2.406 | 39,000 |
| CT for stages 2 and 3 | 3,853 | 2.408 | 47,500 |
| CT for all stages | 5,204 | 2.411 | 450,333 |
| Lifetime model | |||
| LABA monotherapy for all stages | 9,636 | 6.763 | – |
| CT for stage 3 only | 9,788 | 6.769 | 25,333 |
| CT for stages 2 and 3 | 10,142 | 6.776 | 50,571 |
| CT for all stages | 13,282 | 6.783 | 448,571 |
The incremental cost-effectiveness ratio (ICER) is the ratio between the differences in costs and quality-adjusted life years (QALYs) in each successive strategy. Determining whether a strategy was cost-effective required comparing each ICER to a minimally acceptable value (ie, represents society’s willingness to pay for an additional unit of improvement). A cost per QALY threshold of $50,000 is commonly used as a threshold for determining cost-effectiveness (28). Therefore, strategies of combination therapy (CT) were considered to be cost-effective if their ICER was below a decision threshold of $50,000 per QALY gained. LABA Long-acting beta2-agonists
The base-case strategy (LABA monotherapy) was associated with 2.405 QALYs per patient over three years (6.763 QALYs over a lifetime). If CT was given to patients with stage 3 disease, there was 2.406 QALYs per patient over three years (6.769 QALYs over a lifetime). If CT was given to patients with stage 2 or 3 disease, there were 2.408 QALYs per patient over three years (6.776 QALYs over a lifetime). If CT is given to all patients with COPD, there were 2.411 QALYs per patient over three years (6.783 QALYs over a lifetime).
The ICER is presented in successive order. Beginning with the lowest cost strategy (LABA monotherapy for all cases), if the strategy is changed and and CT is provided for COPD stage 3 cases, the ICER would be $39,000 per QALY ($25,333 over a lifetime). The ICER for a strategy of providing CT for stages 2 and 3 is $47,500 per QALY ($50,571 over a lifetime), and $450,333 per QALY for all stages ($448,571 over a lifetime).
Sensitivity analysis
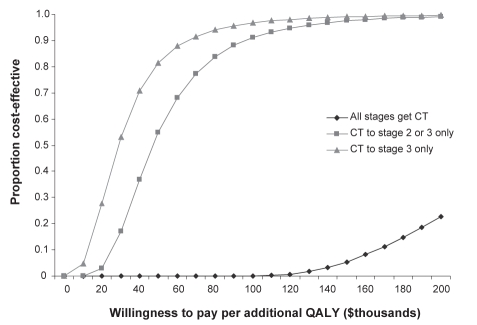
Using a threshold level of $50,000 as a benchmark (30) for determining cost-effectiveness of CT, 80% of the sample sets generated from the Monte Carlo simulation were cost-effective when CT was provided to stage 3 disease and 55% when CT was provided to stages 2 and 3 disease (Figure 1). None of the sample sets were cost-effective when providing CT to all stages. The lifetime model produced similar results.
Figure 1).
Acceptability curve for a three-year model, constructed from 100,000 sample sets generated from a Monte Carlo simulation. Using long-acting beta2-agonist (LABA) monotherapy as the comparator, the curve depicts the proportion of incremental cost-effectiveness ratio from strategies using combination therapy (CT) that were below a range of cost per quality-adjusted life year (QALY) thresholds. Therefore, the curve represents the probability that CT is cost-effective (compared with LABA monotherapy) at particular cost per QALY thresholds
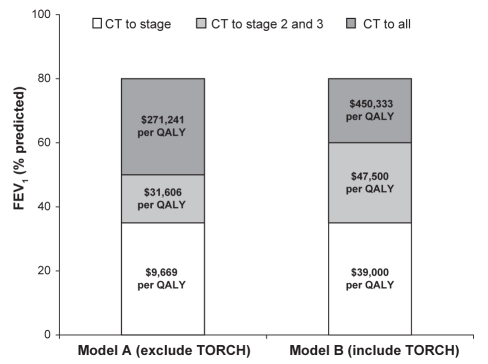
When excluding TORCH (18) in the estimate of RR reduction, beginning with the lowest cost strategy (LABA mono-therapy for all cases): if CT is provided for COPD stage 3 cases, the ICER would be $9,669 per QALY ($7,828 over a lifetime). The ICER for a strategy of providing CT for stages 2 and 3 is $31,606 per QALY ($26,356 over a lifetime), and $271,241 per QALY for all stages ($235,827 over a lifetime). Figure 2 shows the effects on the economic model of including clinical outcomes from the TORCH trial (18) compared with using only the clinical outcomes derived from three earlier trials (17,19,20). Because the CT and LABA groups in TORCH were much larger (n=1533 and n=1521, respectively) than in the other three studies combined (n=820 and n=828, respectively), the economic outcomes are heavily weighted by TORCH findings alone. As well, the FEV1 per cent predicted inclusion criterion for TORCH was higher than the three earlier studies (less than 60% versus less than 50%, respectively).
Figure 2).
Model A represents the economic model describing combination therapy (CT) versus long-acting beta2-agonist (LABA) monotherapy based on three trials using a forced expiratory volume in 1 s (FEV1) of less than 50% predicted as the cut-off for stage 2 chronic obstructive pulmonary disease (COPD) (17,19,20). Model B uses the identical economic model but clinical outcomes from the Towards a Revolution in COPD Health (TORCH) trial (18) are included with the previous three studies. The FEV1 cut-off for stage 2 COPD was less than 60% predicted and therefore patients with milder COPD are included in this analysis. Note that that model is weighted according to patient numbers in each study and therefore model B is heavily weighted toward clinical outcomes of TORCH. QALY Quality-adjusted life year
When including a RR reduction in all-cause mortality in those receiving CT, beginning with the lowest cost strategy (LABA monotherapy for all cases): if CT is provided for COPD stage 3 cases, the ICER would be $28,638 per QALY ($23,033 over a lifetime). The ICER for a strategy of providing CT for stages 2 and 3 is $62,811 per QALY ($49,915 over a lifetime), and $414,831 per QALY for all stages ($263,527 over a lifetime).
DISCUSSION
We developed an economic model of four alternate strategies to measure the cost-effectiveness of providing LABA monotherapy versus CT (LABA plus ICS), based on the provision of combined therapies (instead of LABA alone) for persons with mild, moderate and severe COPD. The ICERs for the alternative strategies are as follows: for providing CT only to persons with severe COPD, the ICER is $39,000 per QALY; for providing CT to persons with moderate and severe COPD, the ICER is $47,500; and if everyone was provided CT, the ICER is $450,333.
Using a decision threshold of $50,000 per QALY (30), this would imply that CT be given to persons in stages 2 (moderate) and 3 (severe) COPD, but not stage 1 (mild COPD). The cut-off point would have to be very high indeed to recommend CT for everyone. We tested the robustness of these findings to various assumptions using Monte Carlo simulation and found the estimates to be stable.
Our results can be compared with those of Löfdahl et al (31), who conducted an economic analysis based on the results of one of the four clinical trials included in our efficacy analysis (20). Löfdahl et al (31) estimated that the cost per person for CT (including therapy for exacerbations and drug costs) was lower for CT than for LABA monotherapy. These results held for the entire group (all of whom had a predicted value of FEV1 of less than 50%) as well as for those with a predicted FEV1 of less than 30%, and are consistent with our results. However, their results are limited to the observed time in the trial of 12 months and to more severe cases. As well, they do not address health outcomes nor does their analysis provide guidance as to whether CT should be recommended to all or a selected group of COPD patients. Modelling efforts allowed us to make predictions for a wider group (including all severity levels) and over a longer time span.
Our study also had several strengths that increase the validity of our findings. First, the natural history of COPD has been established in large observational studies. Efficacy was based on the best available evidence. Second, our model was developed based on the standards and recommendations of expert panels and committees (32). Third, we conducted both a probabilistic sensitivity analysis and a separate sensitivity analysis excluding TORCH (18) and including mortality effects. Even including the milder population recruited by TORCH (18) still showed a benefit of CT compared with LABA (Figure 2). It is also clear that treating patients with very early disease (FEV1 greater than 60% predicted) does not meet cost-effectiveness criteria. Moreover, including a mortality reduction for CT only slightly improved the cost-effectiveness results. Therefore, our conclusions remain consistent at a decision threshold of $50,000 per QALY.
However, there are limitations to our analysis. Only four good-quality trials have prospectively examined the effects of CT on exacerbation frequency in COPD (17–20). None of the trials directly examined the effects in the mildest COPD population. All four trials, however, showed consistent results using two different formulations of combination inhalers (formoterol/budesonide or salmeterol/fluticasone). Furthermore, within the population of patients studied with placebo, combination inhaler or LABA monotherapy, there was no clear, statistically significant effect on mortality. Our economic model included data from over 4700 subjects from four independent and relatively current trials (17–20) and, as such, should yield representative and contemporary outcomes.
Only one of the four COPD trials (18) was powered to see a mortality effect from treatment. All four randomized trials included in our analysis (17–20) found no evidence to indicate that mortality differed between LABA monotherapy and CT. Still, a recent study examining the effects of a LABA in asthma found increased mortality in the LABA treatment group, particularly in a subset of patients who did not receive concomitant treatment with ICS (33). The relevance of this finding to COPD is uncertain because in asthma, the use of ICS is protective against asthma-related mortality (34) and concomitant use of LABA with ICS appears to be both safe and efficacious (35). If a mortality effect is associated with LABA, it is uncertain whether it is due to a class effect or is unique to the agent that was studied (salmeterol). However, because there is a theoretical overlap in the pathophysiology of adult asthma with COPD and previous studies have suggested a risk with the excessive use of beta2-agonists, it may be prudent to discourage the isolated use of LABAs as maintenance monotherapy in COPD unless other safety data become available. This argument becomes particularly cogent with the clear benefits shown in our analysis of CT compared with LABA monotherapy.
Guidelines (1,11) recommend that ICS be considered for patients with both an FEV1 less than 50% predicted and with three or more exacerbations per year. Because these patients would already be receiving a long-acting bronchodilator, CT therapy would meet these recommendations. Our current analysis suggests that it would be cost-effective to start combination inhaler therapy for all TORCH stage 2 (FEV1 less than 60% predicted) or stage 3 (FEV1 less than 35% predicted) patients irrespective of the past frequency of exacerbations. This is a significant difference in approach. It would, in effect, promote the use of combination inhaler therapy more widely in this large COPD population. The message to family physicians and specialists would therefore be simple: as the FEV1 worsens and reaches 50% of predicted values, CT is recommended.
Footnotes
DISCLOSURES: Drs Mayers and Marciniuk received honoraria from GlaxoSmithKline Inc. Dr Jacobs received a workshop grant from GlaxoSmithKline Inc.
REFERENCES
- 1.Global Initiative for Chronic Obstructive Pulmonary Disease Global Initiative for Chronic Obstructive lung disease global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2006) 2006.
- 2.Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest. 2000;117:5S–9S. doi: 10.1378/chest.117.2_suppl.5s. [DOI] [PubMed] [Google Scholar]
- 3.Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global burden of disease study. Lancet. 1997;349:1498–504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- 4.Michaud CM, Murray CJ. Burden of disease-implications for future research. JAMA. 2001;285:535–9. doi: 10.1001/jama.285.5.535. [DOI] [PubMed] [Google Scholar]
- 5.Shukla VK, Husereau DR, Boucher M, et al. Technology Report no 27. Ottawa: Canadian Coordinating Office for Health Technology Assessment; 2002. Long-acting β2-agonists for maintenance therapy of stable chronic obstructive pulmonary disease: A systematic review. [Google Scholar]
- 6.World Health Organization and National Heart, Lung and Blood Institute . Bethesda: Global Initiative for Chronic Obstructive Lung Disease; 2003. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Executive Summary: updated 2003. [Google Scholar]
- 7.Wouters EF, Postma DS, Fokkens B, et al. Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: A randomised controlled trial. Thorax. 2005;60:480–7. doi: 10.1136/thx.2004.034280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dal Negro RW, Pomari C, Tognella S, Micheletto C. Salmeterol & fluticasone 50 microg/250 microg bid in combination provides a better long-term control than salmeterol 50 microg bid alone and placebo in COPD patients already treated with theophylline. Pulm Pharmacol Ther. 2003;16:241–6. doi: 10.1016/s1094-5539(03)00065-8. [DOI] [PubMed] [Google Scholar]
- 9.Mahler DA, Wire P, Horstman D, et al. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:1084–91. doi: 10.1164/rccm.2112055. [DOI] [PubMed] [Google Scholar]
- 10.Adcock IM, Maneechotesuwan K, Usmani O. Molecular interactions between glucocorticoids and long-acting β2-agonists. J All Clin Immunol. 2002;110:261–8. doi: 10.1067/mai.2002.129705. [DOI] [PubMed] [Google Scholar]
- 11.O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease – 2003. Can Respir J. 2003;10(Suppl A):11A–65A. doi: 10.1155/2003/567598. [DOI] [PubMed] [Google Scholar]
- 12.Sin DD, Stafinski T, Ng YC, et al. The impact of chronic obstructive pulmonary disease on work loss in the United States. Crit Care Med. 2002;165:704–7. doi: 10.1164/ajrccm.165.5.2104055. [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic Society Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;152:S77–S121. [PubMed] [Google Scholar]
- 14.The Lung Health Study Research Group Effect of inhaled triamicinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med. 2000;343:1902–9. doi: 10.1056/NEJM200012283432601. [DOI] [PubMed] [Google Scholar]
- 15.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–12. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 16.Niewoehner DE, Erbland ML, Deupree RH, et al. Effect of systematic glucocorticoids on exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1999;340:1941–7. doi: 10.1056/NEJM199906243402502. [DOI] [PubMed] [Google Scholar]
- 17.Calverley P, Pauwels R, Vestbo J, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: A randomised controlled trial. Lancet. 2003;361:449–56. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]
- 18.Calverley PM, Anderson J, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–89. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 19.Calverley PM, Boonsawat W, Cseke Z, et al. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J. 2003;22:912–9. doi: 10.1183/09031936.03.00027003. [DOI] [PubMed] [Google Scholar]
- 20.Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21:74–81. doi: 10.1183/09031936.03.00031402. [DOI] [PubMed] [Google Scholar]
- 21.Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res. 2005;14:1523–32. doi: 10.1007/s11136-004-7713-0. [DOI] [PubMed] [Google Scholar]
- 22.Sin DD, McAlister FA, Man SF, Anthonisen NR. Contemporary management of chronic obstructive pulmonary disease: Scientific review. JAMA. 2003;290:2301–12. doi: 10.1001/jama.290.17.2301. [DOI] [PubMed] [Google Scholar]
- 23.Paggiaro PL, Dahle R, Bakran I, et al. Multicentre randomised placebo-controlled trial of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease. International COPD study group. Lancet. 1998;351:773–80. doi: 10.1016/s0140-6736(97)03471-5. [DOI] [PubMed] [Google Scholar]
- 24.Oostenbrink JB, Rutten-van Molken MP, Monz BU, Fitzgerald JM. Probabilistic Markov model to assess the cost-effectiveness of bronchodilator therapy in COPD patients in different countries. Value Health. 2005;8:32–46. doi: 10.1111/j.1524-4733.2005.03086.x. [DOI] [PubMed] [Google Scholar]
- 25.Sin DD, Golmohammadi K, Jacobs P. Cost-effectiveness of inhaled corticosteroids for chronic obstructive pulmonary disease according to disease severity. Am J Med. 2004;116:325–31. doi: 10.1016/j.amjmed.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 26.Ernst P, Gonzalez AV, Brassard P, Suissa S. Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am J Respir Crit Care Med. 2007;176:162–6. doi: 10.1164/rccm.200611-1630OC. [DOI] [PubMed] [Google Scholar]
- 27.Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. 3rd edn. New York: Oxford University Press; 2005. [Google Scholar]
- 28.Laupacis A, Feeny D, Detsky AS, Tugwell PX. How attractive does a new technology have to be to warrant adoption and utilization? Tentative guidelines for using clinical and economic evaluations. CMAJ. 1992;146:473–81. [PMC free article] [PubMed] [Google Scholar]
- 29.Briggs AH. Handling uncertainty in economic evaluation. In: Drummond MF, McGuire A, editors. Economic Evaluation of Health Care: Merging Theory with Practice. New York: Oxford University Press; 2001. [Google Scholar]
- 30.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: In search of a standard. Med Decis Making. 2000;20:332–42. doi: 10.1177/0272989X0002000310. [DOI] [PubMed] [Google Scholar]
- 31.Löfdahl C, Ericsson A, Svensson K, Andreasson E. Cost effectiveness of budesonide/formoterol in a single inhaler for COPD compared with each monocomponent used alone. Pharmacoeconomics. 2005;23:365–75. doi: 10.2165/00019053-200523040-00006. [DOI] [PubMed] [Google Scholar]
- 32.Canadian Coordinating Office for Health Technology Assessment (CCOHTA) Guidelines for economic evaluation of pharmaceuticals: Canada. 2nd edn. Ottawa: Canadian Coordinating Office for Health Technology Assessment; 1997. [DOI] [PubMed] [Google Scholar]
- 33.Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM. The salmeterol multicenter asthma research trial: A comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15–26. doi: 10.1378/chest.129.1.15. [DOI] [PubMed] [Google Scholar]
- 34.Suissa S, Ernst P, Benayoun S, et al. Low-dose inhaled corticosteroids and the prevention of death from asthma. N Engl J Med. 2000;343:332–6. doi: 10.1056/NEJM200008033430504. [DOI] [PubMed] [Google Scholar]
- 35.Ernst P, McIvor A, Ducharme FM, et al. Canadian Asthma Guideline Group. Safety and effectiveness of long-acting inhaled beta-agonist bronchodilators when taken with inhaled corticosteroids. Ann Intern Med. 2008;145:692–4. doi: 10.7326/0003-4819-145-9-200611070-00012. [DOI] [PubMed] [Google Scholar]
- 36.Vestbo J, Sorenson T, Lange P, et al. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: A randomised controlled trial. Lancet. 1999;353:1819–23. doi: 10.1016/s0140-6736(98)10019-3. [DOI] [PubMed] [Google Scholar]
- 37.Burge PS, Calverley PM, Jones PW, et al. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: The ISOLDE trial. BMJ. 2000;320:1297–303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anthonisen NR, Wright EC, Hodgkin JE. Prognosis in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1986;133:14–20. doi: 10.1164/arrd.1986.133.1.14. [DOI] [PubMed] [Google Scholar]
- 39.Borg S, Ericsson A, Wedzicha J, et al. A computer simulation model of the natural history and economic impact of chronic obstructive pulmonary disease. Value Health. 2004;7:153–67. doi: 10.1111/j.1524-4733.2004.72318.x. [DOI] [PubMed] [Google Scholar]
- 40.Paterson C, Langan CE, McKaig GA, et al. Assessing patient outcomes in acute exacerbations of chronic bronchitis: The measure your medical outcome profile (MYMOP), medical outcomes study 6-item general health survey (MOS-6A) and EuroQol (EQ-5D) Qual Life Res. 2000;9:521–7. doi: 10.1023/a:1008930521566. [DOI] [PubMed] [Google Scholar]
- 41.Spencer S, Calverley PM, Burge PS, et al. Inhaled salmeterol in obstructive lung disease. Health status deterioration in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:122–8. doi: 10.1164/ajrccm.163.1.2005009. [DOI] [PubMed] [Google Scholar]
- 42.Alberta Health and Wellness Alberta Health Care Drug Benefit Plan<http://www.ab.bluecross.ca/dbl/pdfs/ahwdbl_april_print.pdf> (Version current at June 20, 2005).
- 43.Alberta Health and Wellness . Alberta Health Care Insurance Plan. Schedule of Medical Benefits. Edmonton: Alberta Health and Wellness; 2005. [Google Scholar]
- 44.Alberta Health and Wellness . Health Costing in Alberta. Annual Report. Edmonton: Alberta Health and Wellness; 2005. [Google Scholar]