Abstract
No two roses smell exactly alike, but our brain accurately bundles these variations into a single percept `rose'. We found that ensembles of rat olfactory bulb neurons decorrelate complex mixtures that vary by as little as a single missing component, whereas olfactory (piriform) cortical neural ensembles perform pattern completion in response to an absent component, essentially filling in the missing information and allowing perceptual stability. This piriform cortical ensemble activity predicts olfactory perception.
The need for perceptual discrimination must be balanced with the need for perceptual stability. Without an ability to ignore some differences between input patterns, nearly all experiences would be unique, with each presentation of a similar stimulus being devoid of previously acquired associations and meaning. Computational modeling and experimental data suggest that some cortical circuits balance discrimination and stability through the network emergent functions of pattern separation and pattern completion1-5. Simply put, pattern separation allows partially overlapping input patterns to be decorrelated and processed as being distinct. Pattern completion is a memory-based phenomenon6 that allows degraded input patterns to be compared to existing templates and, if they are sufficiently close to those templates, `completed' and processed as a match. These processes have been examined in some detail in the hippocampal formation, where slight changes in the spatial distribution of visual cues can be completed to promote stability in hippocampal neuronal place fields and presumably stability in spatial maps and perception. With further change or degradation in the visual spatial patterns, hippocampal place fields shift, presumably along with spatial perception. In olfaction, the need for pattern separation and completion is particularly intense, as most natural odors derive from odorant mixtures, evoking complex spatiotemporal patterns of olfactory sensory neuron and olfactory bulb activity7. Given this complexity, it is rare for a given stimulus to always have the exact same components in the exact same proportions, yet it is possible for a noisy or degraded stimulus to reliably evoke a stable percept. On the other hand, if the component make-up changes sufficiently, discrimination ensues. Evidence for pattern completion would be consistent with the view of olfactory perception as an object-oriented sense, where sensation of a familiar complex mixture evokes perception of a unique odor object, distinct from its components8-11, and generally irreducible to its components12.
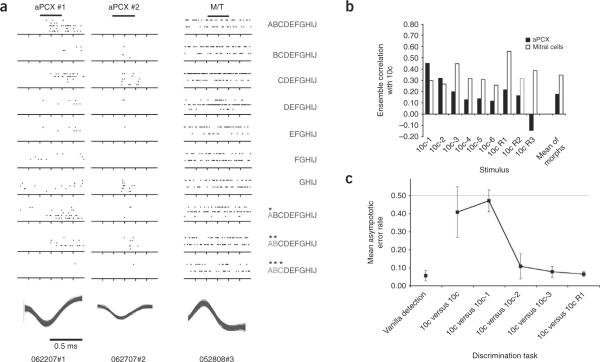
We isolated single units with stable odor-evoked activity over at least 45 min in the anterior piriform cortex (aPCX) and olfactory bulb (n = 59 aPCX neurons; 25 olfactory bulb mitral/tufted cells) and examined responses to odorant mixtures in accord with University of Oklahoma Institutional Animal Care and Use Committee approval and U.S. Public Health Service standards. The standard odorant mixture that we used consisted of ten approximately equal concentration (based on vapor pressure and dilution in mineral oil) monomolecular odorants (as described in the Supplementary Methods and Supplementary Table 1 online). All data presented here used the same initial mixture of ten odorants (10c, for example, ABCDEFGHIJ), from which components were removed (for example, 10c-1 (10 components with 1 removed), 10c-2 (10 components with 2 removed), etc.) or replaced (10cR1, 10cR2, etc). This core mixture and its subsets were repeatedly presented during testing, and, given the speed at which cortical units become familiarized to odor mixtures even under anesthesia13, it was assumed they were familiar to the rats. We examined aPCX single units responding to the odorant mixtures (two typical examples are shown in Fig. 1a). These units responded to several different mixture combinations, and a response to one mixture did not predict responsiveness to other mixtures, despite the linear morphing from one mixture to the next (see Supplementary Fig. 1 and Supplementary Results online).
Figure 1.
Odor discrimination and stability. (a) Representative responses of two different aPCX single units and a mitral/tufted (M/T) cell to morphed complex odor mixtures. Raster plots show responses to 2-5 repeats of each 2-s stimulus. Waveforms are overlays of at least 100 consecutive spikes. Letter series to the right of each raster plot indicates the mixture of components that was present. Starred letters indicate component replacement (removal of a component from the mixture and replacement with a different component). Note that as stimulus mixtures morphed gradually, unit responses did not, and were not predictable from the change in stimulus. (b) Cross-correlation analyses of mitral/tufted and aPCX pyramidal cell ensemble responses to the 10c mix versus the various morphs. (c) Behavioral performance in a two-alternative forced-choice odor-discrimination task matched predictions from the aPCX ensembles, with discrimination of a single missing component being significantly more difficult than discrimination of a replaced component. The marked improvement that we observed in behavioral discrimination of 10c-2 relative to the gradual change in ensemble decorrelation could reflect a threshold phenomenon in cortical to behavioral translation. Error bars represent s.e.m.
We incorporated all of the cells tested in a brain region with a given stimulus set into a virtual ensemble and calculated a correlation matrix comparing ensemble responses to different odorant mixtures (stimulus components were all approximately 100 p.p.m.; Fig. 1). Across all mixture morphs (10c-1, 10cR1, etc.), the aPCX ensembles showed significantly greater mean decorrelation from the full mixture than did mitral/tufted cells (t16 = 2.73, P < 0.02). However, there was a significant difference in the pattern of correlation shift in mitral/tufted cells and aPCX ensembles as the mixtures diverged from 10c (Fig. 1b). Although mitral/tufted cell ensembles showed similar levels of decorrelation across all mixture morphs, aPCX ensemble decorrelation increased as more components were removed or replaced. For example, with this odor set, repeated presentations of the 10c mixture produced a correlation in mitral/tufted cell ensemble response of 0.73 (correlating responses to repeated 10c stimuli). Removing one component (10c versus 10c-1) produced a significant decorrelation in mitral/tufted cell ensemble response to 0.27 (observed Z score, Zobs = 2.17, P < 0.05). In aPCX ensembles, repeating the 10c mixture resulted in a cross-correlation of 0.53, which showed no significant decorrelation (0.45) when comparing 10c versus 10c-1 (Zobs = 0.32, P > 0.05). Removing additional components, or replacing even a single component, created a significant mean decorrelation in aPCX ensemble response compared with that of mitral/tufted cells (t14 = 3.66, P < 0.01).
On the basis of our cortical ensemble data, we predicted that rats would have difficulty behaviorally discriminating the 10c mixture from a mixture with one component missing, whereas they would have no difficulty discriminating the 10c mixture from the same mixture with one component replaced, although both stimuli had 90% overlap with the 10c mix. As predicted (Fig. 1c), rats (n = 4; see Supplementary Methods and Supplementary Fig. 2 online for details) had difficulty discriminating 10c from 10c-1, but were proficient at discriminating 10c from 10cR1. Stimulus mixtures that overlapped 80% or less were more easily discriminated. ANOVA of error rates across discrimination stimuli showed a significant main effect of odor mixture (F5,15 = 9.13, P < 0.001).
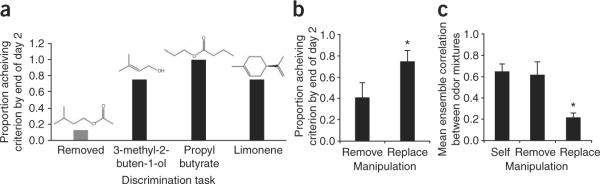
Similar behavioral and aPCX ensemble results were generally obtained regardless of the specific components that were removed or replaced. For example (Fig. 2a), rats had difficulty discriminating the loss of isoamyl acetate, yet easily discriminated its replacement with a variety of molecularly similar or dissimilar uncommon components. Across several single-component removal and replacement protocols (see Supplementary Table 2 online), replacement was significantly more easily behaviorally detected (t6 = 2.00, P < 0.05; Fig. 2b) and induced greater aPCX ensemble decorrelation (t4 = 5.33, P < 0.01; Fig. 2c) than single-component removal.
Figure 2.
Piriform cortical ensembles predict behavioral discrimination. (a) Rats could not detect removal of isoamyl acetate from a 10c mixture; however, rats could discriminate mixtures in which isoamyl acetate was replaced with any of three different molecules. (b) Over all mixture morphs, mixes with single replaced components were significantly easier for rats to discriminate from the 10c standard than mixes with a single removed component. (c) Similarly, cortical ensembles decorrelated mixes with single replaced components from the standard 10c mix significantly greater than mixes with a single removed component. Error bars represent s.e.m. Asterisks indicate a significant difference (P < 0.05) between Replace and Remove manipulations.
In summary, these results demonstrate that, despite the fact that individual piriform cortical cells can and do respond differentially to similar, overlapping mixtures (for example, 10c and 10c-1; Fig. 1a), the cortical ensemble disregards this difference and functions consistent with pattern completion. The rat's perceptual performance matches that of the cortical ensemble. This emergent property of the ensemble allows minor variation in the stimulus to be disregarded, a function that is critical for perceptual stability. The enhanced pattern completion observed in aPCX relative to its afferent olfactory bulb input is similar to that observed across subregions of the hippocampal formation, such as dentate gyrus (pattern separation) and area CA3 (pattern completion)2,14. Together, these results help explain both the stability of olfactory percepts of complex, varying mixtures and sensitivity to tainted food odors15.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by grant R01-DC03906 to D.A.W. and grant R01-DC008982 to R.L.R and D.A.W. from the US National Institute on Deafness and other Communication Disorders.
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
References
- 1.Hasselmo ME. Trends Cogn. Sci. 1999;3:351–359. doi: 10.1016/s1364-6613(99)01365-0. [DOI] [PubMed] [Google Scholar]
- 2.Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- 3.Marr D. Phil. Trans. R. Soc. Lond. B. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 4.O'Reilly RC, McClelland JL. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- 5.Treves A, Rolls ET. Hippocampus. 1992;2:189–199. doi: 10.1002/hipo.450020209. [DOI] [PubMed] [Google Scholar]
- 6.Hebb DO. The Organization of Behavior: a Neuropsychological Theory. New York; John Wiley: 1949. [Google Scholar]
- 7.Lin DY, Shea SD, Katz LC. Neuron. 2006;50:937–949. doi: 10.1016/j.neuron.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 8.Polak EH. J. Theor. Biol. 1973;40:469–484. doi: 10.1016/0022-5193(73)90005-2. [DOI] [PubMed] [Google Scholar]
- 9.Wilson DA, Stevenson RJ. Learning to Smell: Olfactory Perception From Neurobiology to Behavior. Baltimore, Maryland; Johns Hopkins University Press: 2006. [Google Scholar]
- 10.Hopfield JJ. Proc. Natl. Acad. Sci. USA. 1991;88:6462–6466. doi: 10.1073/pnas.88.15.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haberly LB. Chem. Senses. 2001;26:551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- 12.Laing DG, Francis GW. Physiol. Behav. 1989;46:809–814. doi: 10.1016/0031-9384(89)90041-3. [DOI] [PubMed] [Google Scholar]
- 13.Wilson DAJ. Neurophysiol. 2003;90:65–72. doi: 10.1152/jn.00133.2003. [DOI] [PubMed] [Google Scholar]
- 14.Bakker A, Kirwan CB, Miller M, Stark CEL. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva Ferreira AC, Hogg T, Guedes de Pinho P. J. Agric. Food Chem. 2003;51:1377–1381. doi: 10.1021/jf025847o. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.