Abstract
Objective
To evaluate the benefit of Metfomin added to Clomiphene Citrate in a primary ovulation induction protocol in PCOS patients
Design
Prospective randomised controlled study
Setting
Tygerberg Academic Hospital, Stellenbosch University and the Institute of Reproductive Medicine at Vincent Pallotti Hospital, Cape Town
Patients
107 patients presenting with PCOS
Study
Group A was pre-treated with metformin 850 mg twice a day for at least 6 weeks before clomiphene was added and the metformin was used throughout the study period. Group B received clomiphene without pre-treatment with metformin. In both groups clomiphene was given at a starting dose of 50 mg day 4–8 and increase with increments of 50 mg to a maximum of 150 mg if no response was achieved.
Results
The ovulation rate achieved in women in the M+C/C arm was 34/52 (65.4%) compared to 36/55 (65.5%) in the C/C arm. The treatment effect ((M+C/C) – C/C) is 0% with 95% confidence interval of −18.1% to 18%. The per protocol ovulation results were 34/42 (81%) in the M+C/C arm compared to 36/48 (75%) in the C/C arm. The ovulation rate difference was 6% with 95% confidence interval −11% to 22%. In a comparison of successful ovulating versus non-ovulating women from the trial the following were significant baseline determinants: lower median weight in the ovulating group (77 kg versus 86 kg, p = .021), lower median bmi (29.0 versus 32.9, p = .009), lower median DHEAS at baseline (4.6 compared to 7.0, p = .049), lower median 17OH-progesterone (2.2 versus 4.6, p = .027) and higher baseline median SHBG ( 37.8 compared to 28.5, p = .036).
Conclusion
Although identical ovulation rates were observed in both arms equivalence could not be concluded with respect to the specified criteria.
Keywords: Metformin, Clomiphene citrate, Ovulation induction, Weight loss
Introduction
Polycystic ovary syndrome is one of the most common endocrinopathies, affecting 5–10% of women of reproductive age [1]. Various criteria have been proposed for the diagnosis of PCOS which hampered research into this common disorder [2, 3]. Fortunately, in 2003 a joint consensus meeting between the American Society of Reproductive Medicine and the European Society of Human Reproduction and Embryology proposed a unifying definition [3]. Oligo-anovulation due to ovarian dysfunction continues to be the pivotal feature that makes this syndrome the major cause of anovulatory infertility in developed countries [4].
Clomiphene citrate (C/C) was the first agent used in experiments for ovulation induction in oligomenorrheic women [5]. For many years it was and may still be the first therapeutic option managing anovulatory infertility. The treatment with C/C in anovulatory PCOS women is associated with an ovulation rate of 60–85% and a pregnancy rate of 30–40% [6]. Reasons for this discrepancy may be due to the anti-oestrogenic effect of C/C acting at both endometrial and ovarian levels, in addition to the development of a hostile cervical mucus [7].
The addition of metformin to C/C in C/C-resistant women significantly improves the ovulation rate. A meta analysis in a Cochrane review reported a significant benefit for metformin compared to placebo for ovulation in anovulatory women with PCOS [8]. Another metanalysis showed a significant positive effect of metformin when added to C/C in the C/C-resistant PCOS patient [9]. In a recent review by Moll et al. [10], they confirmed the superiority of the combination of Metformin and C/C in the C/C resistant patient regarding ovulation and pregnancy rates. This review also concluded that there is no difference in effectivity between Metformin and C/C in therapy-naïve women.
The aim of this study was to evaluate the benefit of metformin if added to C/C in a primary ovulation induction protocol in comparison to C/C alone. Pregnancy outcome was not a primary aim of the study.
Materials and methods
Patients
This study was approved by the Ethical Committee of Stellenbosch University at Tygerberg Academic Hospital (2003/013). Informed consent was obtained from each patient involved. A total number of 107 patients diagnosed with PCOS were enrolled for ovulation induction in a treatment period of 24 months, June 2004 to June 2006. The inclusion criteria required that all couples needed to present with a history of infertility for at least 18 months. The diagnosis of PCOS was based on the recent Rotterdam consensus statement. All patients had a complete infertility and PCOS work up consisting of height, weight and body mass index (BMI); hysterosalpingogram(HSG); basal hormonal tests (FSH, LH, TSH, Prolactin, 17-OH Progesterone, DHEAS, SHBG, Testosterone, fasting insulin, fasting glucose and fasting lipid profile; Abbott architect biochemical assay was used); semen analysis on the husband; and where indicated, a diagnostic hysteroscopy and laparoscopy were performed. Patients with known tubal factors, azoospermia or severe oligoteratozoospermia were excluded from this study. The Tygerberg strict criteria was used to evaluate the sperm morphology and the rest of the semen parameters were evaluated according to the WHO manual 1999 [11].
All obese patients (BMI [kg/m2] >25) were instructed to lose at least 5% of their weight and to participate in exercise for at least 40 min per day, 3 days per week. The mean BMI was >28. They were motivated regarding short-term positive impact of weight loss regarding ovulation induction and long term benefits on development of Diabetes Mellitus, ischaemic heart disease and lipid abnormalities. Specific data on weight loss prior to randomization or during the study is, however, not available.
Study
This was a prospective randomised controlled trial of 107 consecutive PCOS patients. The sample size was limited to the number of patients recruited during the 24 months of active recruitment.
The randomization was computer generated and patients were randomised into two groups. A nursing sister (blinded to the clinical data of the patients) had access to the randomisation list and was responsible for allocating consecutive patients to a group. Group A received pre-treatment with metformin 850 mg twice a day for at least 6 weeks before C/C was added and the metformin was used throughout the study period. Group B received C/C alone without pre-treatment with placebo. In both groups C/C was given at a starting dose of 50 mg day 4–8 and was increased with increments of 50 mg to a maximum of 150 mg if no response was achieved. Four cycles of ovulation induction were attempted. The primary outcome of the study was successful ovulation. We regarded pregnancy as a positive response and patients loss to follow-up as a negative result for intention to treat analysis.
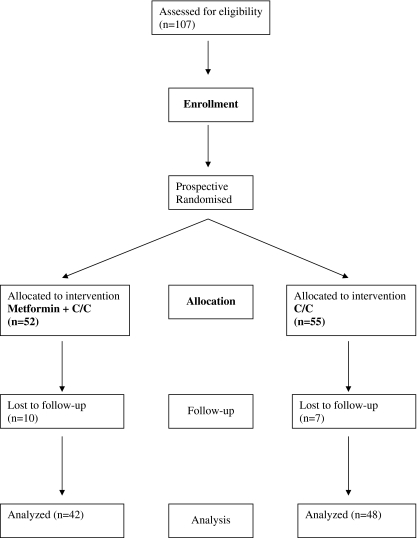
The patients were followed up with transvaginal ultrasound to record follicular growth and endometrial response. Day 21 progesterone was drawn to confirm ovulation (Fig. 1).
Fig. 1.
Study flowchart
Statistical analysis
An intention to treat analysis was performed for the primary outcome of ovulation success.
A test for equivalence was done by means of a 2-sided 95% confidence interval for the difference between the ovulation rates. The equivalence limits specified were −.1 to .1. A per protocol analysis for the primary outcome was also done using the same criteria.
A secondary analysis of the baseline patient factors associated with ovulation was also performed. The Mann-Whitney test was used to compare the ovulation and non-ovulation groups with respect to characteristics such as 17OH Progesterone, Testosterone, SHBG and fasting insulin. The median difference and 95% confidence interval were calculated for the significant factors.
For the tables of baseline characteristics the median as well as the first and third quartiles were used as descriptive statistics. Stata 9 was used for the statistical computations.
Results
One-hundred and seven women were randomised during the 24 months of active recruitment; 52 women were assigned to M+C/C and 55 women to C/C (Fig 1). The loss the follow-up in both arms were similar: 10 women (16%) in M+C/C compared to 7 women (11%) in C/C, p = .603. Since the duration of the treatment is different with M+C/C being much longer, one would expect this arm to have a higher dropout. (Reasons for absconding mostly unknown.) There were two women diagnosed with pregnancy before follow-up, one in each arm. These two women received C/C 50 mg and did not attend their first follow-up. They were regarded as having had a successful ovulation at 50 mg. The baseline characteristics in the two arms of the study were similar and are given in Table 1.
Table 1.
Baseline characteristics of the treatment groups
| variable | N(=52) | M+C/C | Q1–Q3a | N(=55) | C/C | Q1–Q3a |
|---|---|---|---|---|---|---|
| median | median | |||||
| height | 44 | 1.61 | 1.57–1.66 | 44 | 1.61 | 1.53–1.66 |
| weight | 50 | 78.90 | 68.70–91.00 | 51 | 76.60 | 62.00–93.00 |
| BMI | 44 | 30.48 | 26.55–34.05 | 44 | 30.71 | 24.05–34.48 |
| FSH | 50 | 4.15 | 3.10–5.10 | 53 | 4.20 | 2.80–5.30 |
| LH | 50 | 9.95 | 5.80–11.90 | 53 | 8.90 | 6.10–13.60 |
| DHEAS | 51 | 5.20 | 3.90–7.40 | 49 | 4.80 | 3.40–6.40 |
| 17-OH progesterone | 49 | 3.90 | 1.90–7.20 | 52 | 2.55 | 0.80–5.40 |
| testosterone | 50 | 2.35 | 1.90–3.10 | 53 | 2.00 | 1.80–2.90 |
| SHBG | 48 | 30.25 | 21.45–45.25 | 50 | 29.55 | 20.40–47.30 |
| fasting insulin | 52 | 17.20 | 8.75–26.95 | 51 | 13.60 | 6.60–25.00 |
| fasting glucose | 52 | 5.00 | 4.60–5.35 | 53 | 5.10 | 4.60–5.40 |
| tsh | 48 | 1.62 | 1.05–2.27 | 53 | 1.55 | 1.09–1.96 |
| prolactin | 49 | 9.70 | 7.20–15.70 | 52 | 8.70 | 7.50–12.85 |
| hdl | 45 | 1.10 | 0.90–1.30 | 48 | 1.24 | 1.10–1.50 |
| ldl | 42 | 3.30 | 2.50–3.80 | 48 | 3.40 | 2.95–4.26 |
| triglyceride | 45 | 1.10 | 0.75–1.78 | 49 | 0.86 | 0.67–1.20 |
| cholesterol | 46 | 5.10 | 4.10–5.61 | 51 | 5.20 | 4.68–6.20 |
| morphology | 43 | 4.00 | 2.00–7.00 | 52 | 7.00 | 5.00–9.00 |
| count | % | count | % | |||
| obese | 44 | 23 | 52 | 44 | 24 | 55 |
aQ1–Q3: Range from 1st to 3rd quartile
The ovulation rate achieved in women in the M+C/C arm was 34/42 (81%) compared to 36/48 (75%) in the C/C arm. (Table 2) The treatment effect ((M+C/C) – C/C) is 6% with 90% confidence interval of −9% to 20%. Since this interval, does not fit within the equivalence interval we cannot conclude equivalence. Using the confidence interval we cannot conclude superiority of metformin and C/C versus C/C alone since the interval spans 0%, the reference value of no difference between the arms. In this analysis, the patients who were lost to follow-up were excluded.
Table 2.
Per protocol ovulation outcome by dosage
| Dose | M+C/C (%) | C/C (%) | Difference (%) | CI(lower to upper) | p-value |
|---|---|---|---|---|---|
| 50 mg | 20/27 (74) | 18/23 (78) | 4 | −26 to 20 | .776 |
| 100 mg | 11/12 (92) | 11/14 (79) | 13 | −17 to 40 | .566 |
| 150 mg | 3/3 (100) | 7/11 (64) | 36 | −24 to 65 | .332 |
| All | 34/42 (81) | 36/48 (75) | 6 | −11 to 22 | .592 |
The estimated treatment effect by C/C dosage show an increased effect by dose. However the sample size within each dose is small and a test for a dose by treatment effect is not significant, p = .414. The 95% confidence intervals for the estimated treatment effect is also given for completeness (Table 2).
The descriptive characteristics of the factors considered as possible determinants for ovulation are mentioned in the Materials and Methods section (Table 3). These factors were weight and body mass index (BMI), hysterosalpingogram(HSG), basal hormonal tests (FSH, LH, TSH, Prolactin, 17-OH Progesterone, DHEAS, SHBG, Testosterone, fasting insulin, fasting glucose and fasting lipid profile.), and semen analysis. The Mann Whitney test was used to do a non-parametric comparison of ovulating versus non-ovulating women, for each of the factors, to assess if any of these factors were associated with ovulation outcome. From this analysis, weight (p = .021), DHEAS (p = .05), 17OH-progesterone (p = .027), SHBG (p = .036) and BMI (p = .009) were significant factors. Marginal risk factors for ovulation outcome were height (p = .097) and fasting glucose (p = .085).
Table 3.
Baseline characteristics of ovulation groups
| variable | N(=20) | No ovulation | N(=70) | Ovulation | Mann-Whitney | ||
|---|---|---|---|---|---|---|---|
| median | Q1–Q3a | median | Q1–Q3a | p-value | |||
| height | 17 | 1.58 | 1.52–1.61 | 60 | 1.62 | 1.55–1.67 | 0.097 |
| weight | 19 | 86 | 73.5–90 | 66 | 73 | 62–86.4 | 0.021 |
| BMI | 17 | 32.9 | 29.8–37.3 | 60 | 29.4 | 23.2–33.7 | 0.009 |
| FSH | 20 | 4.3 | 3.45–5.5 | 67 | 4 | 2.8–5.1 | 0.274 |
| LH | 20 | 9.3 | 6.7–11.9 | 66 | 8.55 | 5.7–12.1 | 0.602 |
| DHEAS | 17 | 7 | 4.7–7.8 | 66 | 4.7 | 3.4–6.3 | 0.049 |
| 17-OH progesterone | 20 | 4.6 | 2.8–5.9 | 64 | 2.2 | 0.8–5.2 | 0.027 |
| testosterone | 19 | 2.5 | 1.9–3 | 68 | 2.29 | 1.8–3.1 | 0.531 |
| SHBG | 17 | 28.3 | 15.8–32.5 | 65 | 37.8 | 23–48.3 | 0.036 |
| fasting insulin | 19 | 17.2 | 8.8–25.8 | 68 | 13.65 | 5.8–19.8 | 0.164 |
| fasting glucose | 20 | 5.1 | 5–5.6 | 68 | 4.95 | 4.6–5.3 | 0.085 |
| tsh | 20 | 1.64 | 0.89–2.30 | 64 | 1.52 | 1.08–2.03 | 0.793 |
| prolactin | 19 | 9.6 | 7.7–15.9 | 65 | 9.6 | 7–14 | 0.785 |
| hdl | 18 | 1.3 | 1–1.5 | 59 | 1.2 | 1–1.5 | 0.484 |
| ldl | 19 | 3.2 | 2.9–3.8 | 55 | 3.4 | 2.8–4.2 | 0.326 |
| triglyceride | 18 | 0.9 | 0.7–1.2 | 60 | 0.94 | 0.67–1.3 | 0.995 |
| cholesterol | 19 | 4.87 | 4.5–5.6 | 62 | 5.15 | 4.6–6 | 0.632 |
| morphology | 19 | 7 | 4–9 | 65 | 5 | 3–8 | 0.267 |
aQ1–Q3: Range from 1st to 3rd quartile
To further evaluate the factors affecting ovulation, a logistic regression model was used where the factors found above were evaluated with an adjustment for a treatment effect. The variable SHBG is a significant factor after adjustment for treatment with odds ratio (OR) 1.04; 95% CI :1.0 to 1.07; p = .049. It is positively associated with ovulation. The variables 17OH-progresterone (OR = .82; 95%CI: .67 to .99; p = .043), BMI (OR = .90; 95%CI: .82 to .98; p = .0.018) and weight (OR = .97; 95%CI: .94 to 1.0; p = .049) were also significant factors after adjustment for treatment. These factors were negatively associated with ovulation. In this study all women with a BMI below 27 kg/m2 achieved ovulation irrespective of treatment received. The variables DHEAS and fasting glucose were no longer significant factors after adjustment for treatment.
Discussion
In the treatment of women with PCOS who wanted to become pregnant, our study could not establish equivalence or find any benefit of adding metformin to C/C compared to the standard treatment with C/C alone in women receiving these options as primary induction choice. We found no significant differences in outcome of ovulation induction in the two different groups studied. We also observed no difference in the discontinuation rate between the two groups.
In addition to the results of our study, three prospective randomised controlled trials were recently published [12–14]. In the first study by Moll et al. [12], they prospectively randomised 228 women. The primary outcome of this study was the ovulation rate. The ovulation rate in the metformin and C/C group was 64% compared with 72% in the placebo and C/C group which was not statistically significant. There was no difference in the pregnancy rates or the abortion rates of the two groups and the mean BMI was 28 in both groups.
In the second study by Legro et al. [13], 626 PCOS patients were randomised. The primary outcome of their study was live birth rates. They concluded that C/C (22.5%) is superior to metformin (7.2%) but similar to the combination group (26.8%) in achieving live birth rates. They did not observe any difference in the abortion rates between the three groups and observed a significantly better live birth rate if the BMI is less than 30 regardless of the treatment options used. The mean BMI in all three groups was ±35.
In the third study by Neveu et al. [14], they prospectively randomised 154 patients with PCOS. Pregnancy rates were equivalent in the three groups. They also observed a better ovulatory response in the women with a lower BMI in the C/C group and patients with a BMI of 27–35 responded better to metformin for ovulation induction. The mean BMI of the study was 31. This study had a better ovulation rate in the metformin and the combination group (P = 0.005), but no difference in pregnancy rates between the three groups (P = 0.332). These three studies concluded that it is not beneficial to add metformin to C/C in primary ovulation induction protocols.
In one of the first studies to address this topic, Nestler et al. [15] conducted a multicenter study. In this study they studied 61 obese PCOS women. The BMI of all these women was >28. They concluded that spontaneous ovulation induced by C/C may be increased in obese women with PCOS by decreasing serum insulin concentrations with metformin. This was not a prospective randomised control trial and it was also a very small study.
The lack of pre-treatment placebo in the C/C arm can be considered as a limitation of our study. The treatment comparison of this trial is however a comparison of the treatments as applied in usual care and should be interpreted as such. In our study, we prospectively randomised 107 patients and 17 (16.3%) patients were lost to follow up. In the study by Moll et al. [12], they lost 63 (27.6%) patients to follow up. In their study, more patients were lost in the metformin group, which might have been due to the side effects. In contrast, in our study a similar number of women were lost to follow up in the two groups studied; most of them absconded without any given reason. The sample size of the study is one of the major shortcomings of our study. To conclude equivalence from this trial we have to use a 72% 2-side confidence interval for the ovulation rate difference.
In the secondary analysis of our study, we found that BMI was a significant baseline determinant and observed that all patients with a BMI < 27 ovulated. With a BMI > 27 there was no difference in ovulation rates between C/C alone or metformin and C/C. Legro et al. [13] observed a significantly higher rate of live births in women with a BMI less than 30 when compared to those with a BMI more than 30. However, in the study by Neveu et al. [14], they observed a better outcome when metformin was added in the more obese group with BMI 27–35. This improved outcome on metformin in the more obese patients was also observed in the study by Nestler et al. [15]. The role of BMI in our study is a very important finding and supports the current recommendation in literature to optimize the BMI first, lose weight if needed, before commencing any ovulation induction regimen [16]. Lifestyle changes (diet and exercise) are also encouraged to reduce the risk of type 2 diabetes and cardiovascular disease [17].
Other important factors observed in the current study were SHBG (sex hormone binding globulin) and 17hydroxy progesterone (17OH Progesterone). The variable SHBG was a significant factor and positively associated with ovulation. The physiological effect of SHBG is a lowering of the free androgen index. This may lead to an improved ovulation outcome. In a study by Ghazeeri et al. [18], rosiglitazone was administered to 25 obese, C/C-resistant, PCOS women who desired pregnancy. They observed a significant improvement in ovulation rates when rosiglitazone was added to C/C. One of the important findings was a significant rise in SHBG in the group of women treated with rosiglitazone. Our study confirms this finding of improved ovulation rates with a higher SHBG level. Several other investigators have similarly observed an increase in SHBG and a decrease in testosterone and androgenicity with improved conception rates in patients with weight loss [19, 20]. In a recent Cochrane review it was concluded that metformin significantly reduced androgen levels [8]. This subgroup of women with PCOS and high androgen levels may have an improved outcome when metformin is added for ovulation induction. However, more data is required before it can be concluded that this subgroup is a definite indication for the use of metformin. The variable 17OH-progesterone was also a significant factor and was negatively associated with ovulation. Fasting glucose and insulin had no positive or negative association with ovulation.
Based on the results of this trial, we conclude that the addition of metformin is not indicated in patients with primary anovulation. The sample size (n = 107) was the biggest limitation of our study. However, two other prospective randomised control trials had similar outcomes to our study [12, 13] regarding ovulation outcome. It was also concluded by these authors that metformin should not be added to patients in primary ovulation induction protocols [12–14]. In a recent meta-analysis, it was found that the addition of metformin is beneficial when added to C/C in the C/C-resistant PCOS women [9]. However, it is of utmost importance that all obese PCOS women should first be placed on an active exercise and weight loss programme before any treatment is offered.
Footnotes
Capsule
Clomiphene with Metformin achieved the same ovulation rate (65%) compared to Clomiphene alone. However the 2-sided 95% confidence interval for ovulation rate difference was −18.1% to 18%.
References
- 1.Frank S. Polycystic ovary syndrome. N Engl J Med. 1995;333:853–61. doi:10.1056/NEJM199509283331307. [DOI] [PubMed]
- 2.Zawadzki JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: toward a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriman GR, editors. polycystic ovary syndrome. Blackwell: Boston; 1992. p. 337–84.
- 3.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. [DOI] [PubMed]
- 4.Hamilton-Fairley D, Taylor A. Anovulation. BMJ. 2003;327:546–9. [DOI] [PMC free article] [PubMed]
- 5.Holtkamp DE, Greslin JG, Root CA, Lerner LJ. Gonadotropin inhibiting and anti-fecundity effects of chloramiphene. Proc Soc Exp Biol Med. 1960;105:197–201. [DOI] [PubMed]
- 6.Hughes E, Collins J, Vanderkerckhove P, Lilford R. Clomiphene citrate for ovulation induction in women with oligo-amenorrhoea. Cochrane Database Syst Rev. 2000;CD000056. [DOI] [PubMed]
- 7.Kousta E, White DM, Franks S. Modern use of clomiphene citrate in induction of ovulation. Hum Reprod Update. 1997;3:359–65. doi:10.1093/humupd/3.4.359. [DOI] [PubMed]
- 8.Lord JM, Flight IHK, Norman RJ. Metformin in polycystic ovary syndrome: systematic review and meta-analysis. BMJ. 2003;327:951–6. doi:10.1136/bmj.327.7421.951. [DOI] [PMC free article] [PubMed]
- 9.Siebert IT, Kruger TF, Steyn DW, Nosarka S. Is the addition of metformin efficacious in the treatment of clomiphene citrate-resistant patients with polycystic ovary syndrome? A structured literature review. Fertil Steril. 2006;86:1432–7. doi:10.1016/j.fertnstert.2006.06.014. [DOI] [PubMed]
- 10.Moll E, Van der Veen F, Van Wely M. The role of metformin in polycystic ovary syndrome: a systematic review. Hum Reprod Update. 2007;13(6):527–37. doi:10.1093/humupd/dmm026. [DOI] [PubMed]
- 11.World Health Organization. World Health Organization Laboratory Manual for Examination of Human Semen. Cambridge: Cambridge University Press; 1999.
- 12.Moll M, Bossuyt PMM, Korevaar JC, Lambalk CB, Van der Veen F. Effect of clomifene citrate plus metformin and clomifene citrate plus placebo on induction of ovulation in women with newly diagnosed polycystic ovary syndrome: randomised double-blind clinical trial. BMJ. 2006;332:1485–9. doi:10.1136/bmj.38867.631551.55. [DOI] [PMC free article] [PubMed]
- 13.Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, et al. Clomiphene, Metformin, or both for Infertility in the Polycystic Ovary Syndrome. N Engl J Med. 2007;356:551–66. doi:10.1056/NEJMoa063971. [DOI] [PubMed]
- 14.Neveu N, Granger L, St-Michel P, Lavoie HB. Comparison of clomiphene citrate, metformin, or the combination of both for first-line ovulation induction and achievement of pregnancy in 154 women with polycystic ovary syndrome. Fertil Steril. 2007;87:113–20. doi:10.1016/j.fertnstert.2006.05.069. [DOI] [PubMed]
- 15.Nestler JE, Jakubowicz DJ, Evans WS, Pasquali R. Effects of metformin on spontaneous and clomiphene-induced ovulation in the Polycystic Ovary Syndrome. N Engl J Med. 1998;338:1876–80. doi:10.1056/NEJM199806253382603. [DOI] [PubMed]
- 16.Norman RJ, Davies MJ, Lord J, Moran LJ. The role of lifestyle medication in polycystic ovary syndrome. Trends Endocrinol Metab. 2002;13:251–7. doi:10.1016/S1043-2760(02) 00612-4. [DOI] [PubMed]
- 17.Revised 2003 consensus on diagnostic criteria and longterm health risks related to polycystic ovary syndrome (PCOS). The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Hum Reprod. 2004;19(1):41–7. doi:10.1093/humrep/deh098. [DOI] [PubMed]
- 18.Ghazeeri G, Kutteh WH, Bryer-Ash M, Haas D. Effect of rosiglitazone on spontaneous and clomiphene citrate induced ovulation in women with polycystic ovary syndrome. Fertil Steril. 2003;79:562–6. doi:10.1016/S0015-0282(02) 04843-4. [DOI] [PubMed]
- 19.Clark AM, Thornley B, Tomlinson L, Galletley C, Norman RJ. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod. 1998;13:1502–5. doi:10.1093/humrep/13.6.1502. [DOI] [PubMed]
- 20.Kiddy DS, Hamilton-Fairley D, Bush A, et al. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf). 1992;36:105–11. doi:10.1111/j.1365-2265.1992.tb02909.x. [DOI] [PubMed]