Abstract
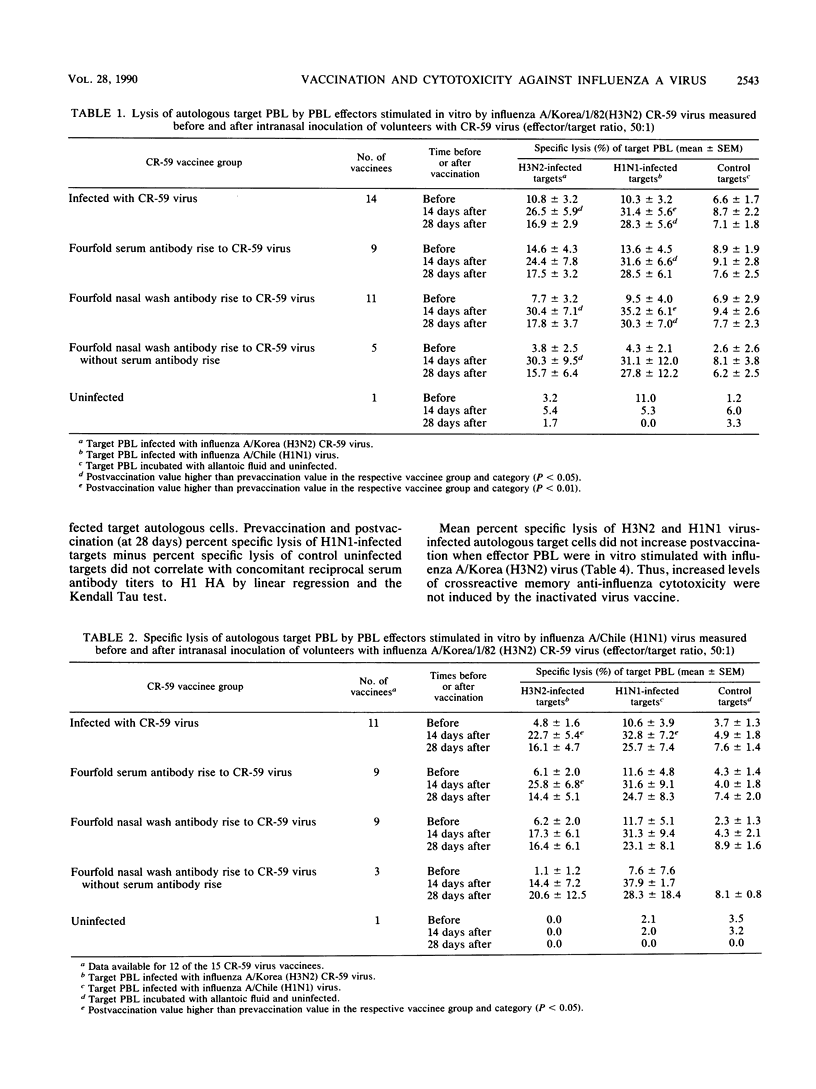
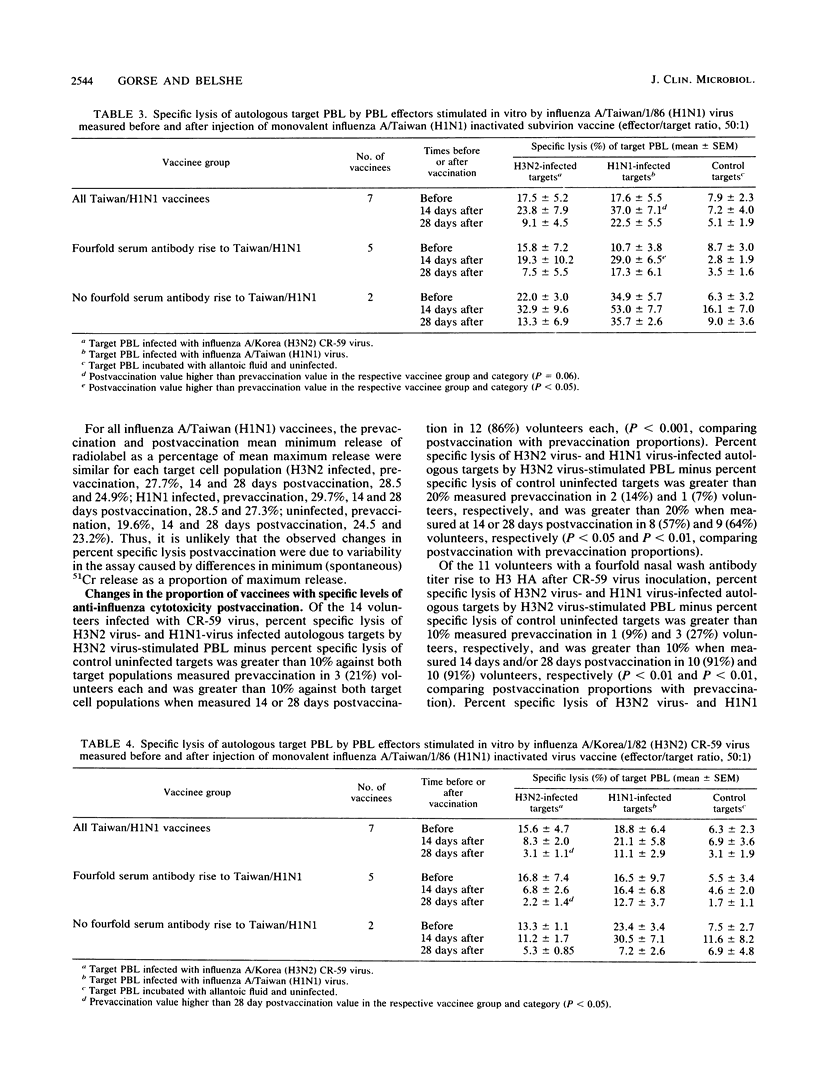
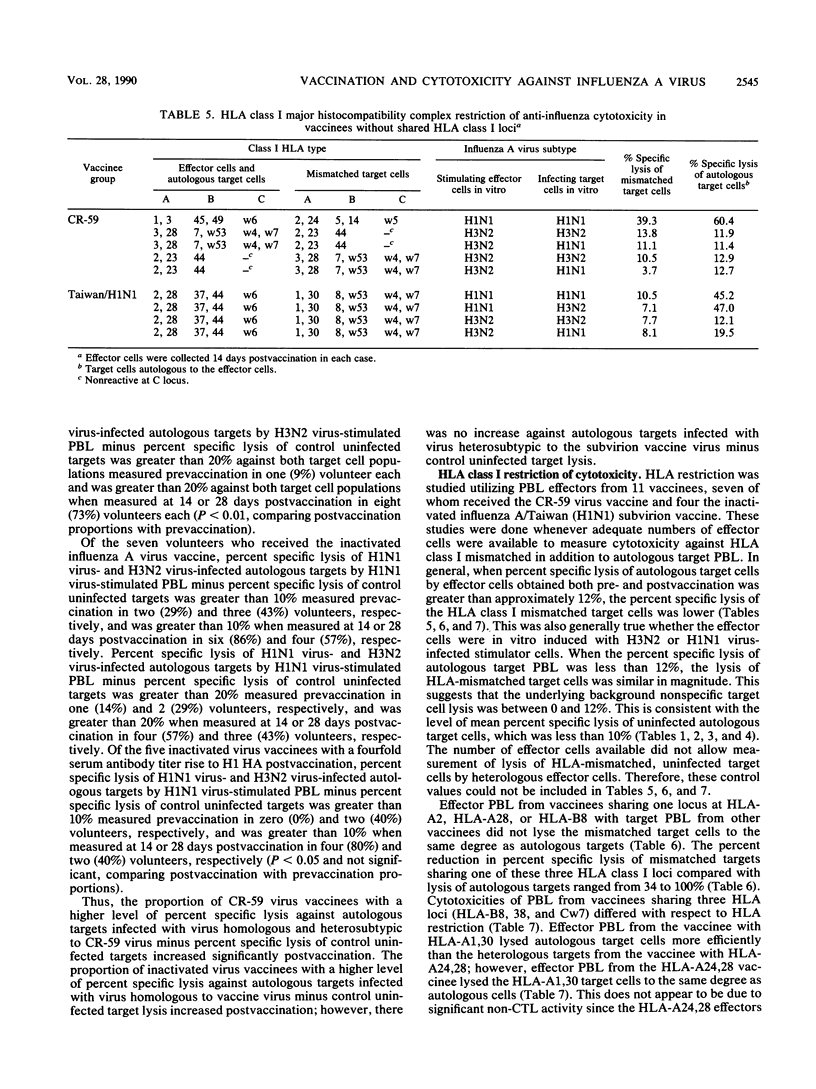
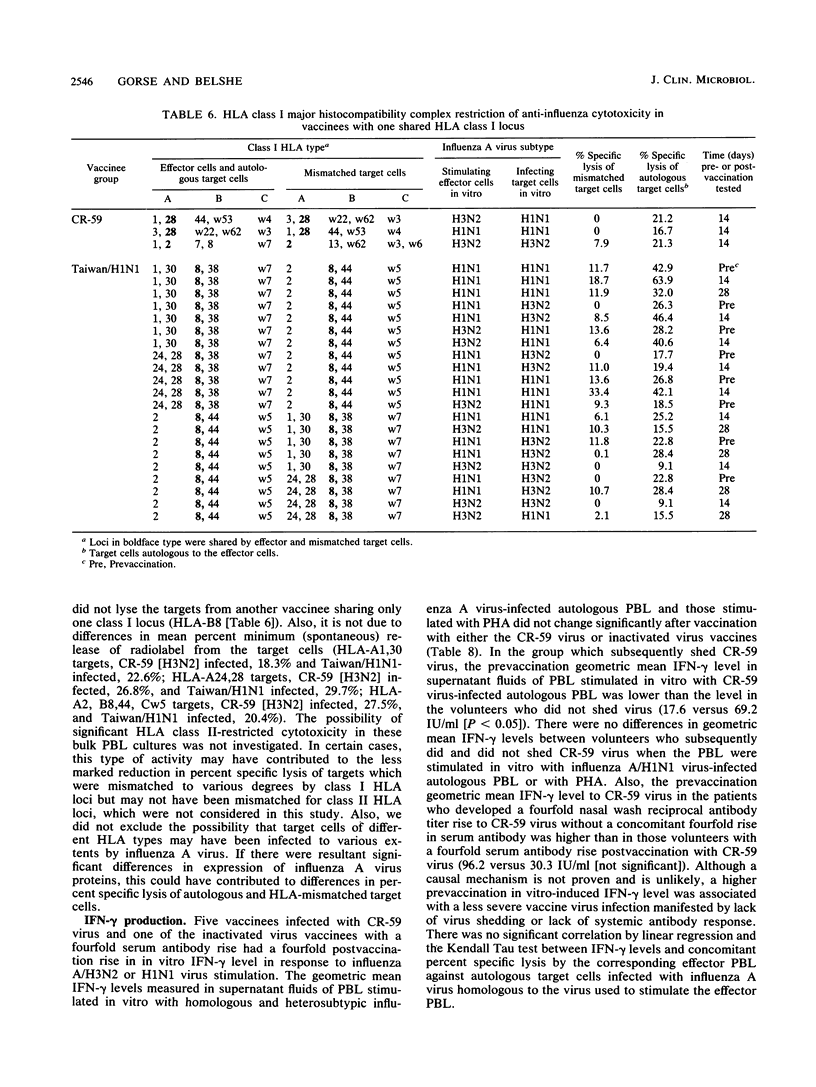
We studied anti-influenza cytotoxicity by bulk peripheral blood mononuclear leukocyte (PBL) cultures derived from older, chronically ill volunteers undergoing vaccination. Vaccinees received either cold-recombinant, live-attenuated influenza A/Korea/1/82 (H3N2) virus intranasally or inactivated monovalent influenza A/Taiwan/1/86 (H1N1) subvirion vaccine intramuscularly. PBL were collected pre- and postvaccination and in vitro stimulated by autologous PBL infected with influenza A virus homologous and heterosubtypic to the respective vaccine strain. Cytotoxicity was measured against influenza A virus-infected autologous and human leukocyte antigen (HLA)-mismatched PBL targets infected with influenza A virus homologous or heterosubtypic to the vaccine virus strain. Vaccinees infected with the live-attenuated virus developed significant rises in mean anti-influenza, HLA-restricted cytotoxicity that was cross-reactive against influenza A viruses homologous and heterosubtypic to the vaccine virus. The enhanced cross-reactive cytotoxicity was inducible postvaccination by in vitro stimulation with autologous PBL infected with the homologous influenza A (H3N2) virus and with influenza A (H1N1) virus. In contrast, after vaccination with inactivated monovalent subvirion vaccine, volunteers developed significant increases in mean anti-influenza, HLA-restricted cytotoxicity only against autologous PBL infected with homologous influenza A (H1N1) virus. Increased cytotoxicity occurred only after in vitro stimulation with autologous cells infected with homologous influenza A (H1N1) virus. Mean gamma interferon levels in supernatant fluids of influenza A virus-stimulated effector PBL did not increase postvaccination, despite increased levels of anti-influenza cytotoxicity displayed by the effector cells. We conclude that the live-attenuated influenza A virus infection induced a broader range of enhanced anti-influenza cytotoxicity than did the inactivated subvirion vaccine.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belshe R. B., Van Voris L. P., Mufson M. A. Parenteral administration of live respiratory syncytial virus vaccine: results of a field trial. J Infect Dis. 1982 Mar;145(3):311–319. doi: 10.1093/infdis/145.3.311. [DOI] [PubMed] [Google Scholar]
- Biddison W. E., Shaw S., Nelson D. L. Virus specificity of human influenza virus-immune cytotoxic T cells. J Immunol. 1979 Feb;122(2):660–664. [PubMed] [Google Scholar]
- Bourgault I., Gomez A., Gomard E., Picard F., Levy J. P., Gomrad E. A virus-specific CD4+ cell-mediated cytolytic activity revealed by CD8+ cell elimination regularly develops in uncloned human antiviral cell lines. J Immunol. 1989 Jan 1;142(1):252–256. [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Cox N. J., Maassab H. F., Kendal A. P. Comparative studies of wild-type and cold-mutant (temperature-sensitive) influenza viruses: nonrandom reassortment of genes during preparation of live virus vaccine candidates by recombination at 25 degrees between recent H3N2 and H1N1 epidemic strains and cold-adapted A/An Arbor/6/60. Virology. 1979 Aug;97(1):190–194. doi: 10.1016/0042-6822(79)90386-6. [DOI] [PubMed] [Google Scholar]
- Ennis F. A., Meager A. Immune interferon produced to high levels by antigenic stimulation of human lymphocytes with influenza virus. J Exp Med. 1981 Nov 1;154(5):1279–1289. doi: 10.1084/jem.154.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis F. A., Rook A. H., Qi Y. H., Schild G. C., Riley D., Pratt R., Potter C. W. HLA restricted virus-specific cytotoxic T-lymphocyte responses to live and inactivated influenza vaccines. Lancet. 1981 Oct 24;2(8252):887–891. doi: 10.1016/s0140-6736(81)91389-1. [DOI] [PubMed] [Google Scholar]
- Ennis F. A., Yi-Hua Q., Schild G. C. Antibody and cytotoxic T lymphocyte responses of humans to live and inactivated influenza vaccines. J Gen Virol. 1982 Feb;58(Pt 2):273–281. doi: 10.1099/0022-1317-58-2-273. [DOI] [PubMed] [Google Scholar]
- Fleischer B., Becht H., Rott R. Recognition of viral antigens by human influenza A virus-specific T lymphocyte clones. J Immunol. 1985 Oct;135(4):2800–2804. [PubMed] [Google Scholar]
- Gorse G. J., Belshe R. B., Munn N. J. Local and systemic antibody responses in high-risk adults given live-attenuated and inactivated influenza A virus vaccines. J Clin Microbiol. 1988 May;26(5):911–918. doi: 10.1128/jcm.26.5.911-918.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorse G. J., Belshe R. B., Munn N. J. Safety of and serum antibody response to cold-recombinant influenza A and inactivated trivalent influenza virus vaccines in older adults with chronic diseases. J Clin Microbiol. 1986 Sep;24(3):336–342. doi: 10.1128/jcm.24.3.336-342.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorse G. J., Kopp W. C. Modulation by immunosuppressive agents of peripheral blood mononuclear cell responses to influenza A virus. J Lab Clin Med. 1987 Nov;110(5):592–601. [PubMed] [Google Scholar]
- Greenberg S. B., Six H. R., Drake S., Couch R. B. Cell cytotoxicity due to specific influenza antibody production in vitro after recent influenza antigen stimulation. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4622–4626. doi: 10.1073/pnas.76.9.4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan D. R., Griffith R., Braciale V. L., Braciale T. J. Influenza virus-specific human cytotoxic T cell clones: heterogeneity in antigenic specificity and restriction by class II MHC products. Cell Immunol. 1984 Oct 1;88(1):193–206. doi: 10.1016/0008-8749(84)90064-9. [DOI] [PubMed] [Google Scholar]
- Kuwano K., Scott M., Young J. F., Ennis F. A. HA2 subunit of influenza A H1 and H2 subtype viruses induces a protective cross-reactive cytotoxic T lymphocyte response. J Immunol. 1988 Feb 15;140(4):1264–1268. [PubMed] [Google Scholar]
- Lamb J. R., Eckels D. D., Lake P., Johnson A. H., Hartzman R. J., Woody J. N. Antigen-specific human T lymphocyte clones: induction, antigen specificity, and MHC restriction of influenza virus-immune clones. J Immunol. 1982 Jan;128(1):233–238. [PubMed] [Google Scholar]
- Lukacher A. E., Braciale V. L., Braciale T. J. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J Exp Med. 1984 Sep 1;160(3):814–826. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak N. K., Zhang Y. H., Ada G. L., Tannock G. A. Humoral and cellular responses of mice to infection with a cold-adapted influenza A virus variant. Infect Immun. 1982 Oct;38(1):218–225. doi: 10.1128/iai.38.1.218-225.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael A. J., Askonas B. A. Influenza virus-specific cytotoxic T cells in man; induction and properties of the cytotoxic cell. Eur J Immunol. 1978 Oct;8(10):705–711. doi: 10.1002/eji.1830081007. [DOI] [PubMed] [Google Scholar]
- McMichael A. J., Gotch F. M., Noble G. R., Beare P. A. Cytotoxic T-cell immunity to influenza. N Engl J Med. 1983 Jul 7;309(1):13–17. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- Mock D. J., Domurat F., Roberts N. J., Jr, Walsh E. E., Licht M. R., Keng P. Macrophages are required for influenza virus infection of human lymphocytes. J Clin Invest. 1987 Feb;79(2):620–624. doi: 10.1172/JCI112856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Nelson D. L., Wright P. F., Tierney E. L., Phelan M. A., Chanock R. M. Secretory and systemic immunological response in children infected with live attenuated influenza A virus vaccines. Infect Immun. 1982 Jun;36(3):1102–1108. doi: 10.1128/iai.36.3.1102-1108.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Phelan M. A., Nelson D. L., Yarchoan R., Tierney E. L., Alling D. W., Chanock R. M. Hemagglutinin-specific enzyme-linked immunosorbent assay for antibodies to influenza A and B viruses. J Clin Microbiol. 1981 Mar;13(3):554–560. doi: 10.1128/jcm.13.3.554-560.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelan M. A., Mayner R. E., Bucher D. J., Ennis F. A. Purification of influenza virus glycoproteins for the preparation and standardization of immunological potency testing reagents. J Biol Stand. 1980;8(3):233–242. doi: 10.1016/s0092-1157(80)80039-4. [DOI] [PubMed] [Google Scholar]
- Reichman R. C., Pons V. G., Murphy B. R., Caplan E. A., Dolin R. Cell-mediated cytotoxicity following influenza infection and vaccination in humans. J Med Virol. 1979;4(1):1–14. doi: 10.1002/jmv.1890040102. [DOI] [PubMed] [Google Scholar]
- Roberts N. J., Jr, Horan P. K. Expression of viral antigens after infection of human lymphocytes, monocytes, and macrophages with influenza virus. J Infect Dis. 1985 Feb;151(2):308–313. doi: 10.1093/infdis/151.2.308. [DOI] [PubMed] [Google Scholar]
- Rouse B. T., Norley S., Martin S. Antiviral cytotoxic T lymphocyte induction and vaccination. Rev Infect Dis. 1988 Jan-Feb;10(1):16–33. doi: 10.1093/clinids/10.1.16. [DOI] [PubMed] [Google Scholar]
- Snyder M. H., Betts R. F., DeBorde D., Tierney E. L., Clements M. L., Herrington D., Sears S. D., Dolin R., Maassab H. F., Murphy B. R. Four viral genes independently contribute to attenuation of live influenza A/Ann Arbor/6/60 (H2N2) cold-adapted reassortant virus vaccines. J Virol. 1988 Feb;62(2):488–495. doi: 10.1128/jvi.62.2.488-495.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Meide P. H., Dubbeld M., Schellekens H. Monoclonal antibodies to human immune interferon and their use in a sensitive solid-phase ELISA. J Immunol Methods. 1985 May 23;79(2):293–305. doi: 10.1016/0022-1759(85)90109-7. [DOI] [PubMed] [Google Scholar]
- Webster R. G., Askonas B. A. Cross-protection and cross-reactive cytotoxic T cells induced by influenza virus vaccines in mice. Eur J Immunol. 1980 May;10(5):396–401. doi: 10.1002/eji.1830100515. [DOI] [PubMed] [Google Scholar]
- Williams W. W., Hickson M. A., Kane M. A., Kendal A. P., Spika J. S., Hinman A. R. Immunization policies and vaccine coverage among adults. The risk for missed opportunities. Ann Intern Med. 1988 Apr;108(4):616–625. doi: 10.7326/0003-4819-108-4-616. [DOI] [PubMed] [Google Scholar]
- Yamada Y. K., Meager A., Yamada A., Ennis F. A. Human interferon alpha and gamma production by lymphocytes during the generation of influenza virus-specific cytotoxic T lymphocytes. J Gen Virol. 1986 Nov;67(Pt 11):2325–2334. doi: 10.1099/0022-1317-67-11-2325. [DOI] [PubMed] [Google Scholar]
- Yap K. L., Ada G. L. The recovery of mice from influenza virus infection: adoptive transfer of immunity with immune T lymphocytes. Scand J Immunol. 1978;7(5):389–397. doi: 10.1111/j.1365-3083.1978.tb00469.x. [DOI] [PubMed] [Google Scholar]



