Abstract
The transmission of traits across generations has typically been attributed to the inheritance by offspring of genomic information from parental generations. However, recent evidence suggests that epigenetic mechanisms are capable of mediating this type of transmission. In the case of maternal care, there is evidence for the behavioral transmission of postpartum behavior from mothers to female offspring. The neuroendocrine and molecular mediators of this transmission have been explored in rats and implicate estrogen-oxytocin interactions and the differential methylation of hypothalamic estrogen receptors. These maternal effects can influence multiple aspects of neurobiology and behavior of offspring and this particular mode of inheritance is dynamic in response to environmental variation. In this review, evidence for the generational transmission of maternal care and the mechanisms underlying this transmission will be discussed as will the implications of this inheritance system for offspring development and for the transmission of environmental information from parents to offspring.
Keywords: maternal, epigenetic, DNA methylation, estrogen receptor α, oxytocin, environment, cross-fostering
Maternal effects have been demonstrated across many species and serve as an important cue to offspring development. In mammals, the lengthy period of prenatal and postnatal mother-infant interaction provides an opportunity for mothers to influence offspring through a variety of mechanisms. During gestation, interactions between mother and fetus are critical for growth and development and variations in these interactions can have long-term consequences for offspring physiological and psychological health. These effects have best been demonstrated through study of prenatal stress [1] and maternal malnutrition [2; 3] in which changes to the mother’s neuroendocrine system and physiology produces a shift in fetal neurodevelopment. Likewise, the care received by an infant early in life can produce changes in the development of neural systems regulating response to novelty and social behavior [4]. Thus, the maternal environment experienced by a developing organism can play a critical role in shaping adult patterns of behavior. Moreover, there can be transmission of these effects to subsequent generations through alterations in the reproductive behavior of offspring. Thus maternal care can be transmitted from mothers to daughters and grand-daughters. The mechanisms mediating this transmission have been explored in rodents and involve epigenetic alterations to steroid receptor genes that produce long-term changes in gene expression and behavior. In this review, the evidence for the context-dependent epigenetic transmission of reproductive behavior and the consequences of these generational effects on offspring development will be discussed. In addition, the role of environment in modulating the epigenetic effects of maternal care will be explored.
Matrilineal Transmission of Maternal Care
In both humans and primates there is evidence for the matrilineal transmission of maternal behavior. In the case of child abuse, there is a striking trans-generational continuity in humans. It is currently estimated that up to 70% of abusive parents were themselves abused, whereas 20–30% of abused infants are likely to become abusers [5; 6]. Women reared in institutional settings without experiencing parental care display less sensitivity and are more confrontational towards their own children [7]. An inter-generational transmission of maternal care and overprotection as rated by the Parental Bonding Index (PBI), a self-report retrospective assessment of parental interactions [8], has also been shown between women and their daughters [9] and this transmission of parental style appears to be independent of socioeconomic status, maternal or daughter temperament or depression. A mother’s own attachment to her mother is a good predictor of her infant’s attachment, especially for secure and disorganized patterns of attachment [10; 11; 12; 13]. Sroufe and colleagues have also reported preliminary results from a prospective study suggesting evidence for the transmission of attachment classifications as measured in the Strange Situation Task [14] from mother to daughter and grand-daughter [15; 16]. This task explores changes in the behavior of an infant following brief removal and reintroduction of the mother during an observed session.
In primate studies, Dario Maestripieri and colleagues have demonstrated the influence of abusive parenting styles of rhesus macaques in modulating the subsequent maternal behavior of offspring, providing evidence that over 50% of offspring who had received abusive parenting during the first 6 months of life would then exhibit abusive parenting themselves as adults [17; 18; 19]. Infants cross-fostered from an abusive female to a non-abusive female were not found to abuse their own offspring suggesting the role of the postnatal environment in mediating these effects [20]. Such a transmission of abuse has long been suspected from observational studies of rhesus and pigtail macaques social groups where infant abuse is highly concentrated within certain matrilines and among closely related females [19; 21]. However, this generational transmission is not limited to abusive behaviors. Amongst captive vervet monkeys, the best predictor of the frequency of mother-infant contact is the level of contact a female had received from her mother during the first six months of life [22]. Matrilineal transmission of maternal rejection rates has also been observed amongst rhesus monkeys [23]. Moreover, the overall frequency of maternal behaviors has been found to differ in rhesus matrilines which may be passed intergenerationally [24].
The challenge of investigating the behavioral transmission of traits in humans and primates is the longitudinal nature of these studies. However, these questions can also be addressed in a rodent model, permitting use of a species in which the fecundity and life-span allow the study of multiple generations of offspring behavior in a short period of time. One experimental approach is to manipulate maternal care received by offspring and then characterize offspring mother-infant interactions. Reducing the normal exposure of female mouse pups to maternal interactions through early weaning is associated with lower levels of licking/grooming (LG) and nursing toward their own pups [25]. Female rat pups that are artificially separated from their mothers, either for short repeated periods [26] or who experience complete maternal deprivation [27], exhibit impaired maternal care; retrieving fewer pups during a Retrieval Test and exhibiting reduced pup licking and crouching behaviors.
An alternative approach to studying these transgenerational effects of maternal care in rodents is to observe the transmission of individual differences in behavior. This approach has been implemented in the study of the maternal effects of natural variations in maternal care in Long-Evans rats [4]. During the first week postpartum, lactating female rats engage in a high frequency of pup licking/grooming (LG). This behavior serves to stimulate pups, modify body and brain temperature, and allows the dam to reclaim salt and water to meet the physiological demands of lactation [28; 29; 30; 31]. The frequency with which dams engage in LG varies considerably between individuals yet shows a high level of stability within individuals [32]. Thus, females can be characterized as engaging in High, Mid, or Low levels of maternal LG. This characterization is achieved through extensive home cage observation during the first week postpartum. Observation of large cohorts (40–100) of lactating females suggests that LG is a normally distributed behavior [32]. The selection of females as High, Mid, or Low licking/grooming (LG) mothers is based on the mean and standard deviation of this measure for the maternal cohort. High licking/grooming mothers are defined as females whose mean LG frequency over days 1–6 postpartum is greater than 1 SD above the mean, Low LG mothers are defined as females whose mean frequency of LG is greater than 1 SD below the mean, and Mid LG mothers are defined as females whose mean frequency scores for LG is within 1 SD of the mean. Offspring of High, Mid, and Low LG dams exhibit levels of licking/grooming that are highly correlated to the behavior exhibited by their mothers [32; 33; 34]. Moreover, cross-fostering female offspring between High and Low LG dams confirms the role of postnatal care in mediating this transmission. Thus, females born to Low LG dams and then fostered to High LG dams will exhibit high levels of LG toward their own pups whereas females born to High LG dams and then fostered to Low LG dams will exhibit low levels of LG [32; 34].
Influence of Maternal Care on Offspring Neurobiology and Behavior
Taken together, these studies implicate a strong relationship between mother’s care and the maternal behavior of offspring. Data from cross-fostering studies conducted in primates and rodents suggests that this inheritance is not genetic, in the sense that it is not mediated by sequence variations in DNA, but rather is behavioral, relying on the quality of the postpartum mother-infant interaction. However, regardless of whether the etiology of this transmission is genetic or behavioral there must ultimately be a neurobiological change in offspring that has consequences for the behavioral patterns displayed in adulthood. The impact of natural variations in maternal care on gene expression and neuroendocrine function has been explored extensively in rodents. Initial studies focused on the consequences of maternal LG for the physiological and behavioral response to stress. Offspring reared by Low LG dams were found to have prolonged elevations in adrenocorticotropin (ACTH) and corticosterone following restraint stress, reduced hippocampal glucocorticoid receptor (GR) mRNA, and elevated hypothalamic corticotrophin releasing hormone (CRH) mRNA [35; 36]. These initial findings suggested that offspring of Low LG dams have elevated hypothalamic-pituitary-adrenal (HPA) activity as a consequence of decreased capacity to down-regulate the release of CRH and ACTH. The release of corticosterone following activation of the HPA axis has negative-feedback effects on the stress response through interaction with hippocampal glucocorticoid receptors [37]. Decreased levels of GR mRNA in the hippocampus result in a decreased capacity to achieve baseline levels of corticosterone following the cessation of a stressor. Interestingly, in these initial studies, a negative linear correlation was demonstrated between the levels of maternal LG received and adult plasma levels of corticosterone following restraint stress [36]. Behaviorally, these neuroendocrine changes result in decreased exploratory behavior and increased inhibition on tests such as the open-field and elevated plus maze [35].
In addition to these HPA effects, offspring of low LG dams have a decreased density of benzodiazepine receptors in the amygdala compared to the offspring of High LG dams [36; 38; 39; 40] and GABA subunit expression is altered by maternal LG with implications for benzodiazepine binding [41]. Offspring of Low LG dams also exhibit impaired performance on tests of spatial learning and memory, elevated hippocampal brain derived neurotrophic factor (BDNF) mRNA, and increased hippocampal choline acetyltransferase and synaptophysin [42]. Neuronal survival in the hippocampus is decreased and apoptosis increased amongst the offspring of Low LG dams associated with decreased levels of fibroblast growth factor [43; 44]. Dopaminergic release associated with stress responsivity in males is also altered as a function of LG [45; 46].
The Neurobiology of Maternal Licking/Grooming
Research on the neuroendocrine consequences of maternal LG for female offspring has focused primarily on systems related to the expression of maternal care itself. Activation of maternal care is thought to involve several nuclei including the bed nucleus of the stria terminalis (BNST), lateral septum (LS), and medial preoptic area (MPOA) [47; 48]. Evidence from neuroanatomical and hormonal manipulation of maternal care suggests that the MPOA in particular is essential for the expression of postpartum maternal interactions with pups [49; 50]. Investigation of the neuroendocrine correlates of individual differences in maternal LG likewise implicates the MPOA. Lactating females characterized as Low LG during the first week postpartum have decreased levels of oxytocin receptor (OTR) binding in the MPOA compared to High LG dams [51; 52]. Consequently, central infusion of a selective OTR antagonist results in a reduction in LG in High LG lactating dams with negligible effects in Low LG dams [51]. In addition to these hypothalamic systems, there is evidence for the role of mesolimbic dopamine activity in the expression of LG [45]. Prior to the onset of LG in High LG dams there is a steady increase in the release of dopamine (DA) in the nucleus accumbens (NA). The magnitude of this increase predicts the length of time a female will engage in pup LG and these elevated DA levels return to baseline once the dam stops engaging in LG behavior. Amongst Low LG dams, DA levels do not increase substantially prior to LG and thus bouts of this behavior are of a very short duration. It is hypothesized that connections between hypothalamic oxytocin neurons and mesolimbic dopamine neurons may mediate this response [45; 53] resulting in the stable individual differences observed between High and Low LG dams. However, the relationship between these systems has yet to be determined
Neuroendocrine Effects of Maternal LG on Female Offspring
In addressing the issue of the transmission of maternal care we must first understand the neuroendocrine systems in female offspring that are altered by maternal LG. As is the case with High and Low LG dams, the offspring of these females display altered levels of hypothalamic oxytocin receptor binding [51]. Thus, offspring of Low LG dams have reduced oxytocin receptor binding during the postpartum period. Moreover, offspring of Low LG dams that have been ovarietomized and given a high dose of estrogen do not have elevated oxytocin receptor binding in the MPOA [51]. Initially this finding was somewhat puzzling and contrary to what would be predicted based on previous research of estrogen-oxytocin interactions. The promoter region of the oxytocin receptor gene contains response elements for estrogen which serve to increase expression of the gene [54; 55]. Thus following heightened exposure to estrogen, such as at parturition, there should be an increase in the levels of OTR to facilitate the physiological and behavioral demands of newborn pups. The lack of estrogen sensitivity displayed by the offspring of Low LG dams is similar to that observed amongst mice lacking a functional copy of estrogen receptor alpha (ERα)[56]. The interaction between estrogen and estrogen receptors is essential in mediating the transcriptional effects of this ligand. The estrogen-ERα complex forms and activated transcription factor which can interact with estrogen response elements in gene promoter regions and alter levels of transcription [57; 58]. In the absence of ERα, the ability of estrogen to modify transcription is diminished resulting in low levels of oxytocin receptor binding in estrogen-treated ERα knockout mice [56]. Analysis of levels of ERα in the offspring of High and Low LG dams suggest that differences in estrogen sensitivity are mediated by this same mechanism. Expression of ERα in the MPOA of both lactating and non-lactating female offspring of Low LG dams is significantly reduced compared to that of the offspring of High LG dams [59]. Thus, hypothetically, the elevated levels of plasma estrogen that occur late in gestation would not increase levels of oxytocin receptors in the MPOA of Low LG female offspring with consequences for maternal LG.
Mechanisms Mediating Long-Term Changes in Gene Expression
The experience of maternal LG in infancy clearly has enduring effects on neurobiology and behavior. Having described these effects, the question now becomes: “How are these long-term effects achieved?” Though infancy and adulthood are separated by a relatively short period of time in rodents compared to humans or primates, this is still a lengthy interval during which time offspring have been weaned from the mother and housed with peers. Determining the mechanisms capable of maintaining stable effects on gene expression requires and understanding of the molecular mechanisms that regulate gene expression. In the cell nucleus, DNA is wrapped around a complex of histone proteins and it is clusters of these DNA/histone complexes that form chromatin [60]. However, in order to be expressed, DNA must come into contact with RNA polymerase and transcription factors. Thus, gene expression can only occur when DNA is in an active state where it is unwrapped from the histone proteins and the nucleic acids sequences are exposed [60; 61]. Our knowledge of these processes is advancing rapidly and hence “epigenetic”, which can have many meanings, has come to refer to the changes in chromatin and DNA structure which alter gene expression and hence phenotype that do not involve changes to the sequence of DNA. The molecular mechanisms involved in the epigenetics of the genome are numerous and complex however one particular mechanism produces stable changes in gene expression and thus may be essential to understanding the maternal effects previously described in rodents. Within the DNA sequence, there are specific sites where a methyl group can attach to cytosine through an enzymatic reaction resulting in 5-methylcytosine [60; 62]. The sites where this can occur reside primarily within the regulatory regions of a gene, in the promoter area upstream from the transcription start site. At a functional level, methylation prevents access of transcription factors and RNA polymerase to DNA resulting in silencing of the gene. In addition to the gene silencing that occurs in the presence of DNA methylation, these methyl groups attract other protein complexes which promote histone deacetylation, further inhibiting the likelihood of gene expression [63]. The bond between the cytosine and methyl group is very strong, resulting in a stable yet potentially reversible change in gene expression. DNA methylation patterns are maintained after cell division and thus passed from parent to daughter cells and it is through this form of epigenetic modification that cellular differentiation occurs [64]. Though several examples of environmentally-induced changes in DNA methylation have been demonstrated [65; 66; 67], the question is whether the changes in gene expression, particularly expression of ERα, that have been associated with postnatal mother-infant interactions are associated with these epigenetic modifications.
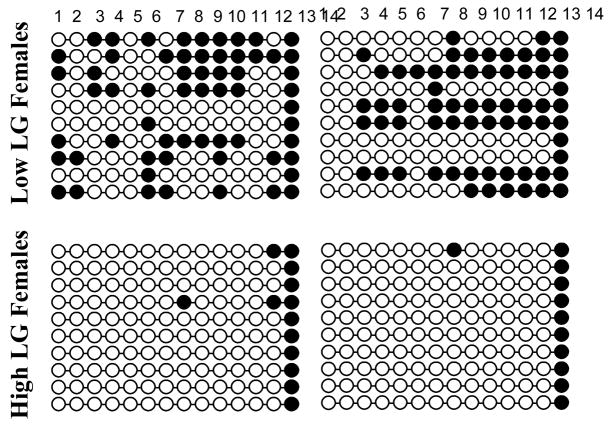
Differential expression of ERα within the MPOA of the offspring of Low compared to High LG dams emerges during the first week postpartum and is maintained into adulthood. Moreover, analysis of levels of ERα amongst offspring cross-fostered between High and Low LG dams confirms that this change in gene expression is mediated by postnatal maternal care [68]. Thus, offspring born to Low LG dams then cross-fostered at birth to High LG dams have elevated levels of ERα in the MPOA whereas offspring born to High LG dams that are cross-fostered at birth to Low LG dams have decreased levels of ERα. Analysis of the 1b region of ERα promoter, which shares a 70% homology with the human ER promoter B [69; 70], indicates that there are 14 potential sites at which methylation can occur. Using bisulfate mapping, a technique which indicates the location of 5-methylcytosine within a sequence of DNA [71], the methylation patterns of ERα in tissue taken from the MPOA of the offspring of High and Low LG offspring can be compared. Graphically, these methylation patterns can be illustrated as bead-on-string figure, with the string representing the sequence of DNA being analyzed and the beads along the string representing each of the sites at which a methyl group can bind to the DNA. If 5-methylcytosine is detected the bead is colored black whereas white beads represent sites where no methylation is detected. Analysis of methylation patterns within the ERα promoter indicate that within the MPOA there are elevated levels of promoter methylation in the offspring of Low LG dams compared to the offspring of High LG dams [68] (Figure 1). This differential methylation occurs at several sites within the promoter. Interestingly, despite overall group differences in methylation, there is considerable within individual variation in the methylation patterns of ERα promoters within the MPOA suggesting that transcriptional activity of this gene in response to maternal care may vary between cell types.
Figure 1.
Bead-on-string illustration of methylation patterns within the 1b promotor region of ERα in MPOA tissue from offspring of High (n=2) and Low LG (n=2) dams. Black circles indicate the presence of 5-methylcytosine. The columns represent the 14 potential sites of differential methylation within the promotor sequence.
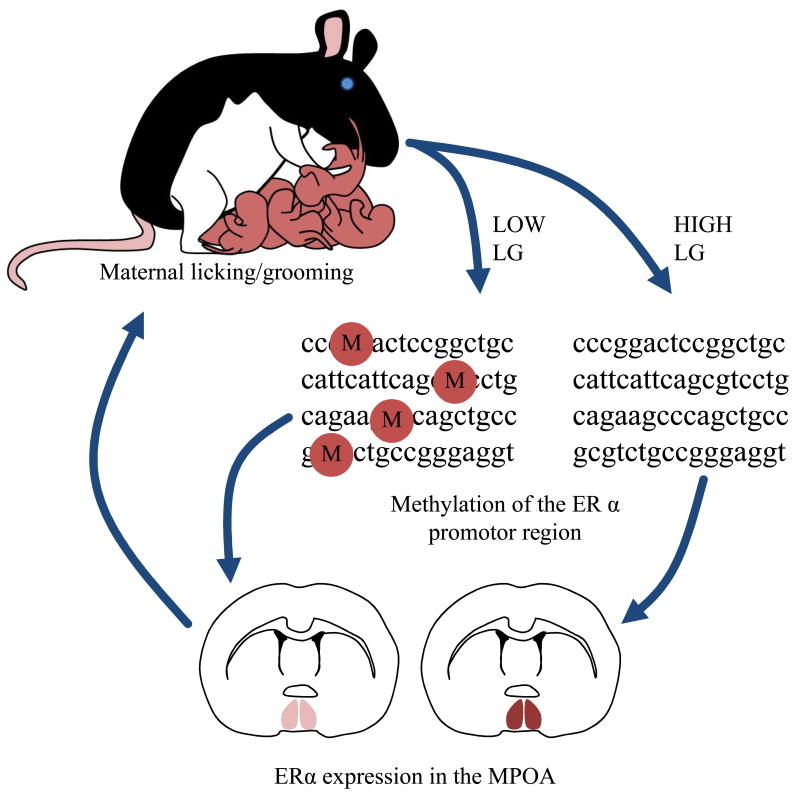
The ERα promotor sequence contains response elements and binding domains for many factors that serve to regulate the expression levels of this gene. One such factor is Stat5, which has been demonstrated to up-regulate ERα through activation of Jak/Stat signaling pathways [72]. Site specific analysis of ERα methylation patterns in the MPOA of offspring of High and Low LG dams indicates that one particular region of differential methylation contains a Stat5 response element. As such, this element is relatively unmethylated in the offspring of High LG dams whereas high levels of methylation are present in the offspring of Low LG dams [68]. To determine the functional consequence of this differential methylation of the Stat5 response element, one strategy is to use a chromatin immunoprecipitation assay (ChIP). Using ChIP it is possible to quantify the level of binding of a transcription factor to a region of differentially methylated DNA [73]. Comparison of binding of Stat5b to the ERα promotor in MPOA tissue from the adult offspring of High and Low LG dams indicates that levels of binding of this transcription factor are significantly reduced in the offspring of Low LG dams [68]. This finding may also implicate prolactin involvement in the regulation of ERα as this peptide hormone upregulates ERα expression through Stat5 pathways [72]. Thus high levels of maternal LG received in infancy are associated with decreased ERα promotor methylation and thus may increase transcriptional activity of this promotor in response to factors such as Stat5. It is hypothesized that this increased transcriptional activity leads to increased levels of ERα in the MPOA which serves to increase estrogen sensitivity in response to the rising hormone levels experienced in late gestation. Consequently, levels of hypothalamic oxytocin receptor binding may be increased potentially activating mesolimbic dopaminergic neurons which serve to increase the duration and frequency of LG provided towards pups. Through these pathways there may be a behavioral transmission of maternal care from mother to offspring through epigenetic modification to ERα (Figure 2).
Figure 2.
Illustration of the epigenetic transmission of maternal care from mother to offspring through the effects of LG on ERα promotor methylation and consequent ERα gene expression in the MPOA.
Implications of the Transgenerational Effects Maternal Care
The behavioral transmission of maternal care across generations provides a mechanism for the transmission of other maternally mediated effects such as stress responsivity, cognitive ability, and response to reward. Thus both the offspring and grand-offspring of High LG dams would be predicted to exhibit an attenuated behavioral and physiological response to stress. Interestingly, this transmission is also associated with epigenetic alterations to steroid receptors involved in stress responsivity. As mentioned previously, differential levels of maternal LG are associated with variation in the expression levels of hippocampal GR mRNA [36]. Analysis of the level of DNA methylation within the GR promoter region suggests that elevated levels of maternal LG are associated with decreased GR methylation corresponding to the elevated levels of receptor expression observed in the hippocampus [74]. Site-specific analysis of the methylation pattern in this region indicates that the NGF1-A (nerve growth factor) binding site is differentially methylated in the offspring of High and Low LG dams and subsequent analysis has indicated that the binding of NGF1-A to this region is reduced in hippocampal tissue taken from the offspring of Low LG dams [74]. Thus, the differential methylation of the GR promoter may prevent the binding of factors necessary for increased expression of the receptor. A temporal analysis of the methylation of the GR promoter indicates that differences between the offspring of High and Low emerge during the postpartum period and are sustained at weaning and into adulthood [74]. Moreover, cross-fostering studies confirm that these effects are indeed mediated by the quality of the postnatal environment [74]. Hypothetically, GR promotor methylation will be decreased in both offspring and grand-offspring of High LG mothers due to the transmission of levels of maternal LG from one generation to the next. This provides a dynamic mechanism for maintaining long-term changes in the gene expression and behavior of offspring.
Though experimental analysis of this type of behavioral inheritance has primarily been limited to rodents, there is certainly potential for this transmission to occur in primates and humans. Amongst postpartum rhesus and pigtail macaques abusive behavior has been demonstrated to be transmitted from mother to daughter and the experience of abuse influences multiple behavioral and neurobiological characteristics of offspring [21; 75; 76]. Abuse occurring during the first 3 months is associated with an increased frequency of screaming, yawning, and other indices of infant distress at 4–6 months. The high levels of maternal rejection exhibited by these females may have a particularly profound effect on offspring behavior and is correlated with increased solitary play and decreased CSF levels of 5-HIAA, implicating the role of serotonergic activity [75; 77].
In humans, ratings obtained from the PBI which indicate low scores for maternal care and high scores for overprotection, a ‘style’ referred to as ‘affectionless control’, is a risk factor for depression [78; 79; 80] adult antisocial personality traits [81], anxiety disorders, drug use, obsessive-compulsive disorder and attention-deficit disorders [82; 83; 84; 85]. Non-clinical subjects who reported high levels of maternal care on the PBI were found to have elevated self-esteem, reduced trait anxiety and decreased salivary cortisol in response to stress [86]. Elevated cortisol in the low maternal care subjects is associated with increased dopamine release in the ventral striatum in response to stress measured with [C11] raclopride during a positron emission tomography scan [86]. A significant linear negative correlation has also been found between cerebrospinal levels of CRH and reported levels of parental care [87]. Longitudinal studies have demonstrated that mother-child attachment is crucial in the shaping of the cognitive, emotional and social development of the child [15; 16]. Throughout childhood and adolescence, secure children are more self-reliant, have increased self-confidence and self-esteem than individuals classified as insecure. Secure infants also have improved emotional regulation, express more positive emotion and exhibit appropriate persistence and flexibility in response to stress. Infant disorganized attachment has been associated with the highest risk of developing later psychopathology [88], including dissociative disorders [89], aggressive behavior [90], conduct disorder and self-abuse [15]. Thus, aspects of the mother-infant interaction which have been demonstrated to be transmitted intergenerationally in humans and primates have profound effects on infant development and thus can mediate the inheritance by offspring of increased risk or resilience to physical or emotional disorder.
Environmental Regulation of Maternal Care
The quality of maternal care provided by a female to offspring can clearly be influenced by early environmental experiences. In the 1950s and 1960s Harlow examined the impact of complete maternal deprivation on the development of rhesus macaques. Females who spent the first 6 months of postnatal life in isolation rearing conditions were found to display impairments in maternal behavior as adults [91; 92; 93], including high rates of abuse, neglect, and infanticide. In rodents, the effects of complete maternal deprivation have been studied using an artificial rearing (AR) paradigm in which pups are removed from their mother on Day 3 postpartum and raised in complete social isolation (Hall, 1975). Adult offspring reared under these conditions are more fearful, engaging in fewer open-arm entries in an elevated plus maze, display hyperactive locomotor activity, display cognitive impairments related to attentional-shifting, and are impaired on measures of social behavior, including maternal care [26; 27; 94]. Females raised under these conditions display deficits in maternal licking/grooming and other forms of contact with their own pups [33] and may be less responsive to hormonal priming of maternal behavior [95]. Likewise, females separated from their mothers for 5 hours per day during the pre-weaning period display deficits in maternal licking/grooming toward their own offspring [26]. Thus, early environments that disrupt the mother-infant interaction can have a long-term influence on neuroendocrine function and adult maternal behavior.
Though the stability of these early environmental effects on maternal care has been clearly demonstrated there are social experiences that occur beyond the postnatal period that are capable of reversing these effects. In rodents, there is experimental evidence for the influence of post-weaning housing conditions on offspring development. Social isolation during the juvenile and adolescent period has been demonstrated to exert similar effects to early maternal separation or deprivation. Post-weaning social isolation is associated with increased HPA activity, cognitive impairment, and reduced social behavior [96; 97]. Conversely, post-weaning housing conditions that are characterized by social enrichment in the form of group housing with same-sex peers has been demonstrated to attenuate the HPA response to stress and improve cognitive performance [98; 99]. Moreover, amongst offspring exposed to perinatal alcohol or maternal separation, this enriched juvenile environment can ameliorate the deficits that would normally be observed [100; 101]. Thus the critical period for shaping development can be extended under specific environmental conditions.
Previous studies using these post-weaning environments to shift patterns of behavior have demonstrated a gene-environment interaction. In a classic demonstration of this interaction, mice selectively bred for maze-running ability, termed Maze-dull and Maze-bright were placed in either enriched or restricted post-weaning environments and then assessed for maze-running performance [102]. Following exposure to these environmental conditions, the genetically mediated difference in maze-running behavior was no longer apparent. Similar effects have been demonstrated in the female offspring of High and Low LG dams. Under standard laboratory housing conditions, offspring who receive high levels of LG are themselves High LG dams whereas offspring who receive low levels of LG are themselves Low LG dams. If these offspring are placed in socially isolated or enriched post-weaning housing conditions no group differences in LG are observed [103]. Moreover, levels of oxytocin receptor binding in hypothalamic regions such as the MPOA are increased in socially enriched offspring of Low LG dams and decreased in socially impoverished offspring of High LG dams. These findings complement earlier work indicating that offspring of females reared in these environments “inherit” the phenotype characteristic of animals housed under these conditions [104; 105]. Both the biological and foster offspring of female rats raised in socially enriched environments spend more time exploring a novel environment and require fewer trials to learn to bar press for reinforcement when compared to females raised in impoverished environments [104]. The demonstration that social enrichment and impoverishment alter maternal behavior suggests a mechanism for the inheritance of these environmental effects. In addition, rather than demonstrating a gene-environment interaction, these results provide evidence for an environment-environment interaction in which the epigenetic influences or early experiences interact with environmental conditions experienced later in development.
Stress and Maternal Care
Psychosocial stress is an effective means for inducing a change in behavior. Amongst pregnant or post-parturient females this change in behavior can result in profound alterations in offspring development. In the case of prenatal stress, the neuroendocrine basis for these effects has been studied extensively in rodents and may involve changes to both the gestational and postpartum environment. Psychosocial stress experienced by pregnant females activates the maternal hypothalamic-pituitary-adrenal (HPA) axis resulting in the release of glucocorticoids which activate the parasympathetic nervous system [106]. Though there are enzymes within the placenta such as 11-β-hydroxysteroid dehydrogenase-2 (11-βHSD-2) that can inactivate glucocorticoids and thus buffer the exposure of the developing fetus to these steroid hormones, the experience of a severe stressor may exceed the capacity of the enzymatic conversion [107; 108]. Offspring exposed to high levels of glucocorticoids during fetal development have elevated plasma corticosterone [109] and increased CRH mRNA in the amygdala [110] resulting in hyperactivity, inhibition from exploring novelty, impairment on measures of cognitive and social behavior [111; 112]. Though evidence certainly implicates fetal exposure to prenatal maternal glucocorticoid secretion as a mediator of these effects [113], there is also the possibility that maternal stress experienced during the prenatal period will compromise maternal care during the postnatal period and thus influence offspring development [114; 115]. High LG females exposed to gestational stress during the last week of pregnancy exhibit low levels of maternal care during the postpartum period associated with decreased hypothalamic oxytocin receptor binding in both mothers and female offspring [114]. The relationship between individual differences in stress responsivity and maternal LG in post-partum females has yet to be fully elucidated and it will be interesting to explore the association between epigenetic modification of GR and ER in mediating transgenerational effects.
In both primates and rodents the level of stress experienced by a post-parturient mother can also be manipulated by altering foraging demand. Through varying the accessibility of food, the foraging effort of mothers can be adjusted to be high (food availability consistently low - HFD), low (food availability of consistently high - LFD) or unpredictable (food availability alternates randomly between high and low – VFD) [116]. Initial studies in bonnet macaques revealed that VFD alters mother-infant interactions. In addition to creating a prolonged maternal separation, VFD has been shown to reduce the maternal responsivity of mothers when they are in contact with offspring [116]. Consequently, offspring CSF levels of CRH, cortisol, dopamine, serotonin, and growth hormone are altered [117; 118] corresponding to decreased exploratory behavior [116], increased timidity, and excessive clinging and fearfulness when separated from the mother [119]. Congruent with primate studies, rat offspring born to VFD dams were found to be more fearful and have higher HPA activity than offspring born to either the HFD or LFD dams [120]. Thus it is not the level of demand that is critical for these effects but rather the variability of the demand that can profoundly alter mother-infant interactions.
Though these paradigms suggest that the experience of stress will induce reductions in the quality of mother-interactions, there is also evidence that activation of the maternal HPA axis can stimulate maternal responsiveness. In rodents, both tail pinch and repeated brief maternal separation, referred to as handling, have been found to stimulate maternal care [121; 122]. In particular, handling is associated with increases in maternal LG with attenuating effects on offspring stress responsivity [35; 36; 121]. Exposure to predator odor during late gestation has been found to increase postpartum maternal LG and frequency of arched-back nursing (ABN) [123]. In addition, females reared by these predator exposed dams also engage in higher levels of LG and ABN and have elevated levels of ERα and ERβ mRNA in the MPOA than control-reared females. Thus, activation of the HPA axis can increase maternal care. However, it is perhaps the nature of the stressor that will determine whether an increase or decrease in maternal behavior will be observed. Prolonged HPA activation induced by restraint stress or foraging demand may cause a down-regulation in neuroendocrine systems regulating maternal behavior whereas acute stress in the form of tail-pinch, handling, or exposure to predator odor may stimulate the activation of dopaminergic systems that consequently increase maternal response to pups.
Tactile Stimulation as a Mediator of Maternal Effects in Mammals
There is converging evidence that maternal LG can mediate the transmission of epigenetic changes to gene expression and behavior across generations and that the quality of the environment can influence frequency of LG and thus shape offspring development though variations in maternal care. However, not all species engage in LG and this form or maternal care is not typically observed in primates and humans. However, LG is also a very discrete form of tactile stimulation and though the form of tactile stimulation provided to infants may differ between species, there is typically substantial mother-infant contact early in development across mammalian species. The contribution of tactile stimulation to infant development has been studied in artificially-reared rat pups where other factors, such as milk quality and nest temperature are controlled. Providing pups with high levels of tactile stimulation (stroking with a paintbrush) during the postnatal period improves maternal responsivity and lessens fear-related behaviors [27]. In addition, this stimulation results in a rapid induction of Fos immunoreactivity in the ventral MPOA, and in PVN oxytocinergic neurons as well as increasing serum lactate, a major source of energy for the metabolic needs of the developing brain [124; 125]. Likewise, if pups are stroked with a paintbrush during periods of maternal separation, levels of growth hormone and ornithine decarboxylase (ODC) which normally decrease during separation are found to return to baseline levels [126; 127]. In humans, touch during the postnatal period results in increased weight gain and improved performance on development tasks by premature and low birth-weight babies [128; 129; 130]. Extended mother-infant contact during the postpartum period also increases maternal responsiveness to infants [131]. Secure infant attachment is thought to be dependent on physical contact between mother and infant [132] and Main [133] reported that infants of mothers with insecure attachments showed an aversion to physical contact. Moreover, stress and maternal depression are associated with decreased maternal responsivity and decreased initiation of contact with infants [134]. Thus, much like visual, olfactory, and auditory stimulation; tactile stimulation may serve as an important cue for brain development exerting specific effects of neuroendorine systems regulating social and emotional behavior which may have consequences for subsequent generations of offspring.
Summary
Traditionally, definitions of inheritance have been limited to the passing of genetic information from one generation to the next. However, it is not simply the presence of genes but rather levels of gene expression that lead to individual variations in offspring characteristics. Levels of gene expression can be regulated by genetic polymorphisms however there is also growing evidence that through epigenetic modification to gene promotor regions, environmentally mediated effects can be transmitted across generations. In rodents, the epigenetic influence of maternal care on offspring levels of steroid receptors provides a mechanism through which maternal care can be passed from mother to daughter and grand-daughter with implications for the inheritance of multiple aspects of offspring phenotype. These epigenetic effects, in the form of DNA methylation, exert stable effects on gene expression and behavior that permit the experiences of early infancy to influence adult reproductive behavior. However, maternal behavior and the neuroendocrine systems that regulate this aspect of reproduction display a high degree of plasticity in response to experiences beyond the postnatal environment and there is evidence for an interaction between the effects of early and later environments. Are the neuroendocrine effects of these experiences across the lifespan also mediated by DNA methylation? The answer to this question is not yet known. However, previous studies have illustrated that pharmacological targeting of the epigenome in adulthood using compounds that either increase or decrease DNA methylation can reverse the effects of early life experiences [74; 135; 136]. Moreover, DNA methylation has been found to be dynamically altered during learning tasks [137], suggesting that this epigenetic modification certainly has the capacity to shift in response to environmental cues beyond the postnatal period. Finally, the epigenetic modification of DNA through maternal care leading to transgenerational effects on offspring behavior provides evidence for the inheritance of acquired traits. The Lamarckian theory that traits acquired in response to the environment experienced over the lifetime will be transmitted to offspring was initially overlooked as a potential mechanism of inheritance. However, current research on the role of epigenetic modifications in mediating environmentally induced changes in maternal care that are transmitted across generations provides a mechanisms though which Lamarckian inheritance is possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. 2005;19:296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Barker DJ, Clark PM. Fetal undernutrition and disease in later life. Rev Reprod. 1997;2:105–12. doi: 10.1530/ror.0.0020105. [DOI] [PubMed] [Google Scholar]
- 3.Martin-Gronert MS, Ozanne SE. Maternal nutrition during pregnancy and health of the offspring. Biochem Soc Trans. 2006;34:779–82. doi: 10.1042/BST0340779. [DOI] [PubMed] [Google Scholar]
- 4.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–92. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 5.Chapman D, Scott K. The impact of maternal intergenerational risk factors on adverse developmental outcomes. Developmental Review. 2001;21:305–325. [Google Scholar]
- 6.Egeland B, Jacobvitz D, Papatola K. Child Abuse and Neglect: Biosocial Dimensions. Aldine; New York: 1987. [Google Scholar]
- 7.Dowdney L, Skuse D, Rutter M, Quinton D, Mrazek D. The nature and qualities of parenting provided by women raised in institutions. J Child Psychol Psychiatry. 1985;26:599–625. doi: 10.1111/j.1469-7610.1985.tb01644.x. [DOI] [PubMed] [Google Scholar]
- 8.Parker G. The Parental Bonding Instrument: psychometric properties reviewed. Psychiatr Dev. 1989;7:317–35. [PubMed] [Google Scholar]
- 9.Miller L, Kramer R, Warner V, Wickramaratne P, Weissman M. Intergenerational transmission of parental bonding among women. J Am Acad Child Adolesc Psychiatry. 1997;36:1134–9. doi: 10.1097/00004583-199708000-00022. [DOI] [PubMed] [Google Scholar]
- 10.Benoit D, Parker KC. Stability and transmission of attachment across three generations. Child Dev. 1994;65:1444–56. doi: 10.1111/j.1467-8624.1994.tb00828.x. [DOI] [PubMed] [Google Scholar]
- 11.Main M, Hesse E. Parents’ unresolved traumatic experiences are related to infant disorganized attachment status: Is frightened and/or frightening parental behavior the linking mechanism? In: Greenberg M, Cicchetti D, Cummings E, editors. Attachment in the preschool years. University of Chicago Press; Chicago: 1990. pp. 161–182. [Google Scholar]
- 12.Pederson DR, Gleason KE, Moran G, Bento S. Maternal attachment representations, maternal sensitivity, and the infant-mother attachment relationship. Dev Psychol. 1998;34:925–33. doi: 10.1037//0012-1649.34.5.925. [DOI] [PubMed] [Google Scholar]
- 13.van I, Jzendoorn MH. Adult attachment representations, parental responsiveness, and infant attachment: a meta-analysis on the predictive validity of the Adult Attachment Interview. Psychol Bull. 1995;117:387–403. doi: 10.1037/0033-2909.117.3.387. [DOI] [PubMed] [Google Scholar]
- 14.Ainsworth M, Wittig B. Attachment and exploratory behavior of one-year-olds in a strange situation. In: Foss B, editor. Determinants of infant behavior. Barnes & Noble; New York: 1969. [Google Scholar]
- 15.Sroufe LA. Attachment and development: a prospective, longitudinal study from birth to adulthood. Attach Hum Dev. 2005;7:349–67. doi: 10.1080/14616730500365928. [DOI] [PubMed] [Google Scholar]
- 16.Sroufe LA, Egeland B, Carlson E, Collins W. The development of the person: the Minnesota study of risk and adpatation from birth to adulthood. The Guildford Press; New York: 2005. [Google Scholar]
- 17.Maestripieri D. Parenting styles of abusive mothers in group-living rhesus macaques. Anim Behav. 1998;55:1–11. doi: 10.1006/anbe.1997.0578. [DOI] [PubMed] [Google Scholar]
- 18.Maestripieri D. Fatal attraction: interest in infants and infant abuse in rhesus macaques. Am J Phys Anthropol. 1999;110:17–25. doi: 10.1002/(SICI)1096-8644(199909)110:1<17::AID-AJPA2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 19.Maestripieri D, Carroll KA. Child abuse and neglect: usefulness of the animal data. Psychol Bull. 1998;123:211–23. doi: 10.1037/0033-2909.123.3.211. [DOI] [PubMed] [Google Scholar]
- 20.Maestripieri D. Early experience affects the intergenerational transmission of infant abuse in rhesus monkeys. Proc Natl Acad Sci U S A. 2005;102:9726–9. doi: 10.1073/pnas.0504122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maestripieri D, Wallen K, Carroll KA. Infant abuse runs in families of group-living pigtail macaques. Child Abuse Negl. 1997;21:465–71. doi: 10.1016/s0145-2134(97)00006-9. [DOI] [PubMed] [Google Scholar]
- 22.Fairbanks LA. Early experience and cross-generational continuity of mother-infant contact in vervet monkeys. Dev Psychobiol. 1989;22:669–81. doi: 10.1002/dev.420220703. [DOI] [PubMed] [Google Scholar]
- 23.Berman C. Intergenerational transmission of maternal rejection rates among free-ranging rheus monkeys on Cayo Santiago. Animal Behavior. 1990;44:247–258. [Google Scholar]
- 24.Simpson M, Howe S. Group and matriline differences in the behaviour of rhesus monkey infants. Anim Behav. 1986;34:444–459. [Google Scholar]
- 25.Kikusui T, Isaka Y, Mori Y. Early weaning deprives mouse pups of maternal care and decreases their maternal behavior in adulthood. Behav Brain Res. 2005;162:200–6. doi: 10.1016/j.bbr.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Lovic V, Gonzalez A, Fleming AS. Maternally separated rats show deficits in maternal care in adulthood. Dev Psychobiol. 2001;39:19–33. doi: 10.1002/dev.1024. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez A, Lovic V, Ward GR, Wainwright PE, Fleming AS. Intergenerational effects of complete maternal deprivation and replacement stimulation on maternal behavior and emotionality in female rats. Dev Psychobiol. 2001;38:11–32. doi: 10.1002/1098-2302(2001)38:1<11::aid-dev2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 28.Gubernick DJ, Alberts JR. Maternal licking of young: resource exchange and proximate controls. Physiol Behav. 1983;31:593–601. [PubMed] [Google Scholar]
- 29.Gubernick DJ, Alberts JR. Maternal licking by virgin and lactating rats: water transfer from pups. Physiol Behav. 1985;34:501–6. doi: 10.1016/0031-9384(85)90040-x. [DOI] [PubMed] [Google Scholar]
- 30.Sullivan RM, Shokrai N, Leon M. Physical stimulation reduces the body temperature of infant rats. Dev Psychobiol. 1988;21:225–35. doi: 10.1002/dev.420210304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan RM, Wilson DA, Leon M. Physical stimulation reduces the brain temperature of infant rats. Dev Psychobiol. 1988;21:237–50. doi: 10.1002/dev.420210305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Champagne FA, Francis DD, Mar A, Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79:359–71. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 33.Fleming AS, Kraemer GW, Gonzalez A, Lovic V, Rees S, Melo A. Mothering begets mothering: the transmission of behavior and its neurobiology across generations. Pharmacol Biochem Behav. 2002;73:61–75. doi: 10.1016/s0091-3057(02)00793-1. [DOI] [PubMed] [Google Scholar]
- 34.Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–8. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 35.Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U S A. 1998;95:5335–40. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 37.Sapolsky RM, Meaney MJ, McEwen BS. The development of the glucocorticoid receptor system in the rat limbic brain. III. Negative-feedback regulation. Brain Res. 1985;350:169–73. doi: 10.1016/0165-3806(85)90261-5. [DOI] [PubMed] [Google Scholar]
- 38.Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology. 2000;22:219–29. doi: 10.1016/S0893-133X(99)00110-4. [DOI] [PubMed] [Google Scholar]
- 39.Francis DD, Caldji C, Champagne F, Plotsky PM, Meaney MJ. The role of corticotropin-releasing factor--norepinephrine systems in mediating the effects of early experience on the development of behavioral and endocrine responses to stress. Biol Psychiatry. 1999;46:1153–66. doi: 10.1016/s0006-3223(99)00237-1. [DOI] [PubMed] [Google Scholar]
- 40.Liu D, Caldji C, Sharma S, Plotsky PM, Meaney MJ. Influence of neonatal rearing conditions on stress-induced adrenocorticotropin responses and norepinepherine release in the hypothalamic paraventricular nucleus. J Neuroendocrinol. 2000;12:5–12. doi: 10.1046/j.1365-2826.2000.00422.x. [DOI] [PubMed] [Google Scholar]
- 41.Caldji C, Diorio J, Meaney MJ. Variations in maternal care alter GABA(A) receptor subunit expression in brain regions associated with fear. Neuropsychopharmacology. 2003;28:1950–9. doi: 10.1038/sj.npp.1300237. [DOI] [PubMed] [Google Scholar]
- 42.Liu D, Diorio J, Day JC, Francis DD, Meaney MJ. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat Neurosci. 2000;3:799–806. doi: 10.1038/77702. [DOI] [PubMed] [Google Scholar]
- 43.Bredy TW, Grant RJ, Champagne DL, Meaney MJ. Maternal care influences neuronal survival in the hippocampus of the rat. Eur J Neurosci. 2003;18:2903–9. doi: 10.1111/j.1460-9568.2003.02965.x. [DOI] [PubMed] [Google Scholar]
- 44.Weaver IC, Grant RJ, Meaney MJ. Maternal behavior regulates long-term hippocampal expression of BAX and apoptosis in the offspring. J Neurochem. 2002;82:998–1002. doi: 10.1046/j.1471-4159.2002.01054.x. [DOI] [PubMed] [Google Scholar]
- 45.Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24:4113–23. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang TY, Chretien P, Meaney MJ, Gratton A. Influence of naturally occurring variations in maternal care on prepulse inhibition of acoustic startle and the medial prefrontal cortical dopamine response to stress in adult rats. J Neurosci. 2005;25:1493–502. doi: 10.1523/JNEUROSCI.3293-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Numan M. Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behav Cogn Neurosci Rev. 2006;5:163–90. doi: 10.1177/1534582306288790. [DOI] [PubMed] [Google Scholar]
- 48.Numan M, Sheehan TP. Neuroanatomical circuitry for mammalian maternal behavior. Ann N Y Acad Sci. 1997;807:101–25. doi: 10.1111/j.1749-6632.1997.tb51915.x. [DOI] [PubMed] [Google Scholar]
- 49.Numan M, Numan MJ. Expression of Fos-like immunoreactivity in the preoptic area of maternally behaving virgin and postpartum rats. Behav Neurosci. 1994;108:379–94. doi: 10.1037//0735-7044.108.2.379. [DOI] [PubMed] [Google Scholar]
- 50.Numan M, Smith HG. Maternal behavior in rats: evidence for the involvement of preoptic projections to the ventral tegmental area. Behav Neurosci. 1984;98:712–27. doi: 10.1037//0735-7044.98.4.712. [DOI] [PubMed] [Google Scholar]
- 51.Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A. 2001;98:12736–41. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12:1145–8. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- 53.Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev Psychobiol. 2007;49:12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- 54.Bale TL, Dorsa DM. Transcriptional regulation of the oxytocin receptor gene. Adv Exp Med Biol. 1998;449:307–15. doi: 10.1007/978-1-4615-4871-3_38. [DOI] [PubMed] [Google Scholar]
- 55.Zingg HH, Rozen F, Breton C, Larcher A, Neculcea J, Chu K, Russo C, Arslan A. Gonadal steroid regulation of oxytocin and oxytocin receptor gene expression. Adv Exp Med Biol. 1995;395:395–404. [PubMed] [Google Scholar]
- 56.Young LJ, Wang Z, Donaldson R, Rissman EF. Estrogen receptor alpha is essential for induction of oxytocin receptor by estrogen. Neuroreport. 1998;9:933–6. doi: 10.1097/00001756-199803300-00031. [DOI] [PubMed] [Google Scholar]
- 57.Kato S, Sato T, Watanabe T, Takemasa S, Masuhiro Y, Ohtake F, Matsumoto T. Function of nuclear sex hormone receptors in gene regulation. Cancer Chemother Pharmacol. 2005;56(Suppl 1):4–9. doi: 10.1007/s00280-005-0102-8. [DOI] [PubMed] [Google Scholar]
- 58.McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–84. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- 59.Champagne FA, Weaver IC, Diorio J, Sharma S, Meaney MJ. Natural variations in maternal care are associated with estrogen receptor alpha expression and estrogen sensitivity in the medial preoptic area. Endocrinology. 2003;144:4720–4. doi: 10.1210/en.2003-0564. [DOI] [PubMed] [Google Scholar]
- 60.Turner B. Chromatin and Gene Regulation. Blackwell Science Ltd; Oxford: 2001. [Google Scholar]
- 61.Russo E, Martienssen R, Riggs AD. Epigenetic Mechanisms of Gene Regulation. Cold Spring Harbor Lab; Plainview, NY: 1996. [Google Scholar]
- 62.Razin A. CpG methylation, chromatin structure and gene silencing-a three-way connection. Embo J. 1998;17:4905–8. doi: 10.1093/emboj/17.17.4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Strathdee G, Brown R. Aberrant DNA methylation in cancer: potential clinical interventions. Expert Rev Mol Med. 2002;2002:1–17. doi: 10.1017/S1462399402004222. [DOI] [PubMed] [Google Scholar]
- 64.Jones PA, Taylor SM. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980;20:85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- 65.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–9. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–54. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 67.Waterland RA. Assessing the effects of high methionine intake on DNA methylation. J Nutr. 2006;136:1706S–1710S. doi: 10.1093/jn/136.6.1706S. [DOI] [PubMed] [Google Scholar]
- 68.Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147:2909–15. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- 69.Freyschuss B, Grandien K. The 5′ flank of the rat estrogen receptor gene: structural characterization and evidence for tissue- and species-specific promoter utilization. J Mol Endocrinol. 1996;17:197–206. doi: 10.1677/jme.0.0170197. [DOI] [PubMed] [Google Scholar]
- 70.Grandien K, Berkenstam A, Gustafsson JA. The estrogen receptor gene: promoter organization and expression. Int J Biochem Cell Biol. 1997;29:1343–69. doi: 10.1016/s1357-2725(97)89967-0. [DOI] [PubMed] [Google Scholar]
- 71.Clark SJ, Harrison J, Paul CL, Frommer M. High sensitivity mapping of methylated cytosines. Nucleic Acids Res. 1994;22:2990–7. doi: 10.1093/nar/22.15.2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Frasor J, Gibori G. Prolactin regulation of estrogen receptor expression. Trends Endocrinol Metab. 2003;14:118–23. doi: 10.1016/s1043-2760(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 73.Umlauf D, Goto Y, Feil R. Site-specific analysis of histone methylation and acetylation. Methods Mol Biol. 2004;287:99–120. doi: 10.1385/1-59259-828-5:099. [DOI] [PubMed] [Google Scholar]
- 74.Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 75.Maestripieri D, Lindell SG, Ayala A, Gold PW, Higley JD. Neurobiological characteristics of rhesus macaque abusive mothers and their relation to social and maternal behavior. Neurosci Biobehav Rev. 2005;29:51–7. doi: 10.1016/j.neubiorev.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 76.Maestripieri D, Tomaszycki M, Carroll KA. Consistency and change in the behavior of rhesus macaque abusive mothers with successive infants. Dev Psychobiol. 1999;34:29–35. [PubMed] [Google Scholar]
- 77.Maestripieri D, Higley JD, Lindell SG, Newman TK, McCormack KM, Sanchez MM. Early maternal rejection affects the development of monoaminergic systems and adult abusive parenting in rhesus macaques (Macaca mulatta) Behav Neurosci. 2006;120:1017–24. doi: 10.1037/0735-7044.120.5.1017. [DOI] [PubMed] [Google Scholar]
- 78.Parker G. Parental reports of depressives. An investigation of several explanations. J Affect Disord. 1981;3:131–40. doi: 10.1016/0165-0327(81)90038-0. [DOI] [PubMed] [Google Scholar]
- 79.Parker G. Parental ‘affectionless control’ as an antecedent to adult depression. A risk factor delineated. Arch Gen Psychiatry. 1983;40:956–60. doi: 10.1001/archpsyc.1983.01790080038005. [DOI] [PubMed] [Google Scholar]
- 80.Sato T, Sakado K, Uehara T, Narita T, Hirano S, Nishioka K, Kasahara Y. Dysfunctional parenting as a risk factor to lifetime depression in a sample of employed Japanese adults: evidence for the ‘affectionless control’ hypothesis. Psychol Med. 1998;28:737–42. doi: 10.1017/s0033291797006430. [DOI] [PubMed] [Google Scholar]
- 81.Reti IM, Samuels JF, Eaton WW, Bienvenu OJ, 3rd, Costa PT, Jr, Nestadt G. Adult antisocial personality traits are associated with experiences of low parental care and maternal overprotection. Acta Psychiatr Scand. 2002;106:126–33. doi: 10.1034/j.1600-0447.2002.02305.x. [DOI] [PubMed] [Google Scholar]
- 82.Gerra G, Zaimovic A, Garofano L, Ciusa F, Moi G, Avanzini P, Talarico E, Gardini F, Brambilla F, Manfredini M, Donnini C. Perceived parenting behavior in the childhood of cocaine users: Relationship with genotype and personality traits. Am J Med Genet B Neuropsychiatr Genet. 2006 doi: 10.1002/ajmg.b.30388. [DOI] [PubMed] [Google Scholar]
- 83.Parker G. The measurement of pathogenic parental style and its relevance to psychiatric disorder. Soc Psychiatry. 1984;19:75–81. doi: 10.1007/BF00583818. [DOI] [PubMed] [Google Scholar]
- 84.Parker G, Kiloh L, Hayward L. Parental representations of neurotic and endogenous depressives. J Affect Disord. 1987;13:75–82. doi: 10.1016/0165-0327(87)90076-0. [DOI] [PubMed] [Google Scholar]
- 85.Torresani S, Favaretto E, Zimmermann C. Parental representations in drug-dependent patients and their parents. Compr Psychiatry. 2000;41:123–9. doi: 10.1016/s0010-440x(00)90145-7. [DOI] [PubMed] [Google Scholar]
- 86.Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. J Neurosci. 2004;24:2825–31. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee RJ, Gollan J, Kasckow J, Geracioti T, Coccaro EF. CSF corticotropin-releasing factor in personality disorder: relationship with self-reported parental care. Neuropsychopharmacology. 2006;31:2289–95. doi: 10.1038/sj.npp.1301104. [DOI] [PubMed] [Google Scholar]
- 88.van Ijzendoorn MH, Schuengel C, Bakermans-Kranenburg MJ. Disorganized attachment in early childhood: meta-analysis of precursors, concomitants, and sequelae. Dev Psychopathol. 1999;11:225–49. doi: 10.1017/s0954579499002035. [DOI] [PubMed] [Google Scholar]
- 89.Carlson EA. A prospective longitudinal study of attachment disorganization/disorientation. Child Dev. 1998;69:1107–28. [PubMed] [Google Scholar]
- 90.Lyons-Ruth K, Bronfman E, Parsons E. Atypical attachment in infancy and early childhood among children at developmental risk. IV. Maternal frightened, frightening, or atypical behavior and disorganized infant attachment patterns. Monogr Soc Res Child Dev. 1999;64:67–96. doi: 10.1111/1540-5834.00034. discussion 213–20. [DOI] [PubMed] [Google Scholar]
- 91.Arling GL, Harlow HF. Effects of social deprivation on maternal behavior of rhesus monkeys. J Comp Physiol Psychol. 1967;64:371–7. doi: 10.1037/h0025221. [DOI] [PubMed] [Google Scholar]
- 92.Harlow HF, Suomi SJ. Induced depression in monkeys. Behav Biol. 1974;12:273–96. doi: 10.1016/s0091-6773(74)91475-8. [DOI] [PubMed] [Google Scholar]
- 93.Seay B, Alexander BK, Harlow HF. Maternal Behavior of Socially Deprived Rhesus Monkeys. J Abnorm Psychol. 1964;69:345–54. doi: 10.1037/h0040539. [DOI] [PubMed] [Google Scholar]
- 94.Gonzalez A, Fleming AS. Artificial rearing causes changes in maternal behavior and c-fos expression in juvenile female rats. Behav Neurosci. 2002;116:999–1013. doi: 10.1037//0735-7044.116.6.999. [DOI] [PubMed] [Google Scholar]
- 95.Novakov M, Fleming AS. The effects of early rearing environment on the hormonal induction of maternal behavior in virgin rats. Horm Behav. 2005;48:528–36. doi: 10.1016/j.yhbeh.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 96.Heidbreder CA, Weiss IC, Domeney AM, Pryce C, Homberg J, Hedou G, Feldon J, Moran MC, Nelson P. Behavioral, neurochemical and endocrinological characterization of the early social isolation syndrome. Neuroscience. 2000;100:749–68. doi: 10.1016/s0306-4522(00)00336-5. [DOI] [PubMed] [Google Scholar]
- 97.Weiss IC, Pryce CR, Jongen-Relo AL, Nanz-Bahr NI, Feldon J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav Brain Res. 2004;152:279–95. doi: 10.1016/j.bbr.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 98.Mohammed AH, Henriksson BG, Soderstrom S, Ebendal T, Olsson T, Seckl JR. Environmental influences on the central nervous system and their implications for the aging rat. Behav Brain Res. 1993;57:183–91. doi: 10.1016/0166-4328(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 99.Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- 100.Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J Neurosci. 2002;22:7840–3. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hannigan JH, O’Leary-Moore SK, Berman RF. Postnatal environmental or experiential amelioration of neurobehavioral effects of perinatal alcohol exposure in rats. Neurosci Biobehav Rev. 2007;31:202–11. doi: 10.1016/j.neubiorev.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 102.Cooper RM, Zubek JP. Effects of enriched and restricted early environments on the learning ability of bright and dull rats. Can J Psychol. 1958;12:159–64. doi: 10.1037/h0083747. [DOI] [PubMed] [Google Scholar]
- 103.Champagne FA, Meaney MJ. Transgenerational Effects of Social Environment on Variations in Maternal Care and Behavioral Response to Novelty. Behav Neurosci. 2007;121:1353–1363. doi: 10.1037/0735-7044.121.6.1353. [DOI] [PubMed] [Google Scholar]
- 104.Dell PA, Rose FD. Transfer of effects from environmentally enriched and impoverished female rats to future offspring. Physiol Behav. 1987;39:187–90. doi: 10.1016/0031-9384(87)90008-4. [DOI] [PubMed] [Google Scholar]
- 105.Kiyono S, Seo ML, Shibagaki M, Inouye M. Facilitative effects of maternal environmental enrichment on maze learning in rat offspring. Physiol Behav. 1985;34:431–5. doi: 10.1016/0031-9384(85)90207-0. [DOI] [PubMed] [Google Scholar]
- 106.Steckler TK, NH, Reul JMHM. Handbook of Stress and the Brain Part 1: The Neuobiology of Stress. Part 1. Vol. 15. Elsevier; New York: 2005. [Google Scholar]
- 107.Edwards CR, Benediktsson R, Lindsay RS, Seckl JR. 11 beta-Hydroxysteroid dehydrogenases: key enzymes in determining tissue-specific glucocorticoid effects. Steroids. 1996;61:263–9. doi: 10.1016/0039-128x(96)00033-5. [DOI] [PubMed] [Google Scholar]
- 108.Seckl JR, Nyirenda MJ, Walker BR, Chapman KE. Glucocorticoids and fetal programming. Biochem Soc Trans. 1999;27:74–8. doi: 10.1042/bst0270074. [DOI] [PubMed] [Google Scholar]
- 109.Stohr T, Schulte Wermeling D, Szuran T, Pliska V, Domeney A, Welzl H, Weiner I, Feldon J. Differential effects of prenatal stress in two inbred strains of rats. Pharmacol Biochem Behav. 1998;59:799–805. doi: 10.1016/s0091-3057(97)00541-8. [DOI] [PubMed] [Google Scholar]
- 110.Cratty MS, Ward HE, Johnson EA, Azzaro AJ, Birkle DL. Prenatal stress increases corticotropin-releasing factor (CRF) content and release in rat amygdala minces. Brain Res. 1995;675:297–302. doi: 10.1016/0006-8993(95)00087-7. [DOI] [PubMed] [Google Scholar]
- 111.Patin V, Lordi B, Vincent A, Caston J. Effects of prenatal stress on anxiety and social interactions in adult rats. Brain Res Dev Brain Res. 2005;160:265–74. doi: 10.1016/j.devbrainres.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 112.Weinstock M, Fride E, Hertzberg R. Prenatal stress effects on functional development of the offspring. Prog Brain Res. 1988;73:319–31. doi: 10.1016/S0079-6123(08)60513-0. [DOI] [PubMed] [Google Scholar]
- 113.Barbazanges A, Piazza PV, Le Moal M, Maccari S. Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. J Neurosci. 1996;16:3943–9. doi: 10.1523/JNEUROSCI.16-12-03943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Champagne FA, Meaney MJ. Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol Psychiatry. 2006;59:1227–35. doi: 10.1016/j.biopsych.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 115.Moore CL, Power KL. Prenatal stress affects mother-infant interaction in Norway rats. Dev Psychobiol. 1986;19:235–45. doi: 10.1002/dev.420190309. [DOI] [PubMed] [Google Scholar]
- 116.Rosenblum LA, Paully GS. The effects of varying environmental demands on maternal and infant behavior. Child Dev. 1984;55:305–14. [PubMed] [Google Scholar]
- 117.Coplan JD, Smith EL, Altemus M, Scharf BA, Owens MJ, Nemeroff CB, Gorman JM, Rosenblum LA. Variable foraging demand rearing: sustained elevations in cisternal cerebrospinal fluid corticotropin-releasing factor concentrations in adult primates. Biol Psychiatry. 2001;50:200–4. doi: 10.1016/s0006-3223(01)01175-1. [DOI] [PubMed] [Google Scholar]
- 118.Coplan JD, Smith EL, Trost RC, Scharf BA, Altemus M, Bjornson L, Owens MJ, Gorman JM, Nemeroff CB, Rosenblum LA. Growth hormone response to clonidine in adversely reared young adult primates: relationship to serial cerebrospinal fluid corticotropin-releasing factor concentrations. Psychiatry Res. 2000;95:93–102. doi: 10.1016/s0165-1781(00)00173-6. [DOI] [PubMed] [Google Scholar]
- 119.Andrews MW, Rosenblum LA. The development of affiliative and agonistic social patterns in differentially reared monkeys. Child Dev. 1994;65:1398–404. doi: 10.1111/j.1467-8624.1994.tb00824.x. [DOI] [PubMed] [Google Scholar]
- 120.Macri S, Wurbel H. Effects of variation in postnatal maternal environment on maternal behaviour and fear and stress responses in rats. Anim Behav. 2007;73:171–184. [Google Scholar]
- 121.Lee M, Williams D. Changes in licking behaviour of rat mother following handling of young. Animal Behavior. 1974;22:679–681. [Google Scholar]
- 122.Szechtman H, Siegel HI, Rosemblatt JS, Komisaruk BR. Tail-pinch facilitates onset of maternal behavior in rats. Physiol Behav. 1977;19:807–9. doi: 10.1016/0031-9384(77)90319-5. [DOI] [PubMed] [Google Scholar]
- 123.McLeod J, Sinal CJ, Perrot-Sinal TS. Evidence for non-genomic transmission of ecological information via maternal behavior in female rats. Genes Brain Behav. 2007;6:19–29. doi: 10.1111/j.1601-183X.2006.00214.x. [DOI] [PubMed] [Google Scholar]
- 124.Alasmi MM, Pickens WL, Hoath SB. Effect of tactile stimulation on serum lactate in the newborn rat. Pediatr Res. 1997;41:857–61. doi: 10.1203/00006450-199706000-00010. [DOI] [PubMed] [Google Scholar]
- 125.McCarthy MM, Besmer HR, Jacobs SC, Keidan GM, Gibbs RB. Influence of maternal grooming, sex and age on Fos immunoreactivity in the preoptic area of neonatal rats: implications for sexual differentiation. Dev Neurosci. 1997;19:488–96. doi: 10.1159/000111246. [DOI] [PubMed] [Google Scholar]
- 126.Evoniuk GE, Kuhn CM, Schanberg SM. The effect of tactile stimulation on serum growth hormone and tissue ornithine decarboxylase activity during maternal deprivation in rat pups. Commun Psychopharmacol. 1979;3:363–70. [PubMed] [Google Scholar]
- 127.Pauk J, Kuhn CM, Field TM, Schanberg SM. Positive effects of tactile versus kinesthetic or vestibular stimulation on neuroendocrine and ODC activity in maternally-deprived rat pups. Life Sci. 1986;39:2081–7. doi: 10.1016/0024-3205(86)90359-0. [DOI] [PubMed] [Google Scholar]
- 128.Field TM, Schanberg SM, Scafidi F, Bauer CR, Vega-Lahr N, Garcia R, Nystrom J, Kuhn CM. Tactile/kinesthetic stimulation effects on preterm neonates. Pediatrics. 1986;77:654–8. [PubMed] [Google Scholar]
- 129.Kuhn CM, Schanberg SM, Field T, Symanski R, Zimmerman E, Scafidi F, Roberts J. Tactile-kinesthetic stimulation effects on sympathetic and adrenocortical function in preterm infants. J Pediatr. 1991;119:434–40. doi: 10.1016/s0022-3476(05)82059-1. [DOI] [PubMed] [Google Scholar]
- 130.Solkoff N, Matuszak D. Tactile stimulation and behavioral development among low-birthweight infants. Child Psychiatry Hum Dev. 1975;6:33–7. doi: 10.1007/BF01434430. [DOI] [PubMed] [Google Scholar]
- 131.Kennell JH, Jerauld R, Wolfe H, Chesler D, Kreger NC, McAlpine W, Steffa M, Klaus MH. Maternal behavior one year after early and extended post-partum contact. Dev Med Child Neurol. 1974;16:172–9. doi: 10.1111/j.1469-8749.1974.tb02738.x. [DOI] [PubMed] [Google Scholar]
- 132.Ainsworth M, Blehar M, Waters E, Wall S. Patterns of attachment. Erlbaum; Hillsdale, MJ: 1978. [Google Scholar]
- 133.Main M, Solomon J. Procedures for identifying infants as disorganized/disoriented during the Ainsworth Strange Situation. In: Greenberg M, Cicchetti D, Cummings E, editors. Attachment in the preschool years. University of Chicago Press; Chicago: 1990. pp. 121–160. [Google Scholar]
- 134.Campbell SB, Matestic P, von Stauffenberg C, Mohan R, Kirchner T. Trajectories of maternal depressive symptoms, maternal sensitivity, and children’s functioning at school entry. Dev Psychol. 2007;43:1202–15. doi: 10.1037/0012-1649.43.5.1202. [DOI] [PubMed] [Google Scholar]
- 135.Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, Szyf M. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci. 2005;25:11045–54. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Weaver IC, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci U S A. 2006;103:3480–5. doi: 10.1073/pnas.0507526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007;53:857–69. doi: 10.1016/j.neuron.2007.02.022. [DOI] [PubMed] [Google Scholar]