Abstract
Haemophilus ducreyi causes chancroid, which facilitates transmission of HIV-1. To better understand the biology of H. ducreyi, we developed a human inoculation model. Here, we describe the clinical outcomes of 267 volunteers who were infected with H. ducreyi. There was a relationship between papule formation and dose. The outcome (pustule formation or resolution) of infected sites within a subject was not independent; the most important determinants of pustule formation were gender and host. When infected a second time, subjects (n = 41) segregated towards their initial outcome, confirming the host effect. Subjects with pustules developed local symptoms, requiring termination from the study after a mean of 8.6 days. Hypertrophic scars developed in 16.2% of volunteers who were biopsied, but the model was otherwise safe. Mutant-parent trials confirmed key features in H. ducreyi pathogenesis, and the model has provided an opportunity to study differential human susceptibility to a bacterial infection.
Keywords: H. ducreyi, GUD, chancroid, human, human challenge model, host effect, pathogenesis
Haemophilus ducreyi causes chancroid, a sexually transmitted genital ulcer disease that is endemic in regions of Africa and Asia. Although the World Health Organization estimated the annual global prevalence of chancroid to be 4-6 million cases in the late 1990s, the epidemiology of the disease is not well characterized due to syndromic management and a lack of diagnostic testing [1, 2]. Chancroid facilitates the transmission and acquisition of HIV-1 and is a public health concern [3].
H. ducreyi is a strict human pathogen that naturally infects genital and nongenital skin [4]. The bacteria presumably enter the skin by superficial abrasions that occur during intercourse [4]. Clinical disease is characterized initially by a painless papule that develops into a pustule at the site(s) of entry. Pustules erode into painful ulcers that prompt patients to seek medical attention 1-3 weeks after symptoms begin [2]. Purulent ulcers with ragged edges and suppurative lymphadenopathy typify the ulcerative stage [4].
During the 1980s and 1990s, chancroid outbreaks occurred in New York City and New Orleans [5]. At the time of these outbreaks, data regarding the pathogenesis of H. ducreyi infection in humans were scarce [4, 5] and findings from in vitro or animal models could not be correlated with human disease. Given the initial clinical course of natural disease, we reasoned that experimental infection up to the pustular stage would pose minimal risk to volunteers. Thus, a model of experimental infection with H. ducreyi in humans was developed to better define the pathogenesis of and host responses to the organism [6, 7].
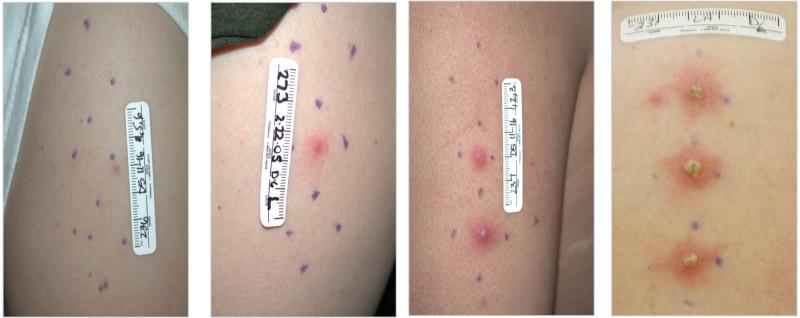
In this model, healthy adult volunteers are inoculated with H. ducreyi at multiple sites on the skin overlying the upper deltoid. Based on the delivery characteristics of the allergy testing device used for inoculation, the estimated delivered dose (EDD) is calculated by dividing the CFU loaded on the device by 1000 [8]. Papules develop within 24 hours, and either spontaneously resolve or evolve into pustules over the next 2-5 days. Volunteers remain infected until they reach clinical endpoint, which is defined as resolution of disease at all sites, development of a pustule that is either painful or > 4 mm in diameter, or 14 days after inoculation. At endpoint, all volunteers are treated with one dose of oral ciprofloxacin.
Within 24 hours of inoculation, fibrin and collagen are deposited in the wounds. PMN and macrophages traffic on the collagen and fibrin scaffolds, forming micropustules in the epidermis and dermis [9, 10]. By 48 hours, PMN form an abscess that ulcerates through the epidermis [9, 10]. Below the abscess is a dermal infiltrate of myeloid dendritic cells (DC), macrophages, memory and effector memory subsets of CD4 and CD8 T cells and activated NK cells [11-15] (unpublished data). The DC likely phagocytose H. ducreyi [14], migrate to regional nodes and sensitize naïve T cells to , as H. ducreyi-specific T cell lines can be propagated from pustules [16].
The histology of experimental pustules is nearly identical to natural ulcers [1]. In pustules, H. ducreyi are found in the abscess and dermis where they associate with collagen, fibrin, PMN and macrophages, which fail to ingest the organism [10]. H. ducreyi also colocalizes with fibrin and PMNs in natural ulcers [17]. Thus, evasion of phagocytosis and the bactericidal activity of serum that transudates into lesions are the major mechanisms of bacterial survival in experimental pustules and in natural disease.
Here, we report our cumulative 15-year experience with the experimental human infection model of H. ducreyi. We describe the clinical outcomes of the 267 volunteers who were infected with strain 35000 or its human passaged derivative, 35000HP. We describe the results obtained from 41 volunteers who were reinfected with 35000HP and of 20 mutant-parent comparison trials in the context of what is known about pathogenesis.
METHODS
Study Population
Between February 25, 1993 and December 31, 2007, we infected 267 volunteers (162 females, 105 males; 212 whites, 49 blacks, 6 Asians; age range 18 to 68 years; mean age ± standard deviation, 33.7±10.3 years) at least once with H. ducreyi. Safety data, such as hypertrophic scar formation, are reported for the entire cohort. For analysis of initial infections, we included 220 subjects who were infected at 2 or 3 sites with the parent strain and who achieved clinical endpoint. If subjects participated in mutant-parent comparison trials, only sites inoculated with the parent strain were included in the analyses. Subjects (n=36) who participated in either dose-response [6] or chemoprophylaxis trials [18], or other time course studies [7, 10, 12], were excluded because of a short duration of infection (1-4 days). Subjects (n=11) who were HIV seropositive [15] were also excluded from the analysis.
Analysis of site independence
A total of 186 subjects, who received identical doses of the parent strain at both of two (n=106) or all of three (n=80) sites, were included in the analysis of site independence. Cumulative data for 90 of these subjects inoculated at two sites was reported previously [19]. The observed outcomes were compared to expected numbers under the assumption of site independence using chi square tests.
Papule and pustule formation rates
We estimated the odds of papule and pustule formation based on the EDD of 35000 and/or 35000HP, gender, race and age. For this analysis, we used logistic regression with generalized estimating equations (GEE) to predict papule and pustule formation rates. The GEE sandwich estimator for standard errors was used to calculate 95% confidence intervals for these rates.
Second Infections
Forty-one of the 220 subjects included in the analyses of initial infections were infected a second time [19, 22] (unpublished data). For these 41 subjects, we estimated the odds of pustule formation based on their previous outcome using logistic regression as described above.
Mutant versus Parent Comparison Trials
To test the role of putative bacterial virulence factors in disease, we performed isogenic mutant-parent comparison trials [8, 23-30]. These trials are double-blinded, multi-stage, dose ranging studies with at least 2 stages. The primary endpoint is the pustule formation rate. The first group of volunteers is inoculated at three sites with a fixed dose (x) of 35000HP on one arm and at three sites with varying doses (0.5 x, x, 2x) of an isogenic mutant on the other arm [23, 24]. If the mutant and parent sites form pustules at similar rates, we inoculate a second group with similar doses. If pustules do not develop at sites inoculated with the mutant during the first iteration, the doses of the mutant are increased for each subsequent group until the EDD of the mutant is approximately 10-fold higher that of the parent. Mutants are categorized as attenuated (unable to form pustules even at doses 10-fold that of the parent), partially attenuated (form pustules at doses 2- or 3-fold that of the parent, but not at doses equivalent to the parent) or virulent (form pustules at doses similar to the parent).
RESULTS
Outcomes of initial challenges
A total of 220 subjects (88 men, 132 women) contributed 538 sites (219 from men, 319 from women) to the analysis of papule formation. Of the 220, 192 subjects (76 men, 116 women) contributed 470 sites (192 from men, 278 from women) to the analysis of pustule formation.
Of the 192 subjects, 186 were infected with identical doses of 35000 or 35000HP at 2 or 3 sites, which were analyzed for independence. The expected number of people with 0, 1, 2 and 3 pustules (figure 1) was calculated based on the observed pustule formation rate and assumption of independence. A greater number of subjects than expected formed pustules at 0 or 2 sites for those inoculated at 2 sites (P=0.001) (table 1). Similarly, a greater number of subjects than expected formed pustules at 0 or 3 sites for those inoculated at a total of 3 sites (P<0.001) (table 1). Thus, the outcome of infected sites within a subject inoculated with identical doses of the parent strain was not independent, suggesting a host effect on outcome, as reported previously [19].
Figure 1.
Outcomes of volunteers who were infected at three sites with 35000HP and developed 0, 1, 2 or 3 pustules.
Table 1.
Observed vs. expected number of people forming 0, 1, 2, or 3 pustules.
| Subjects Inoculated at 2 sites | Subjects Inoculated at 3 sites | ||||
|---|---|---|---|---|---|
| No. of pustules |
No. of subjects observed |
No. of subjects expected |
No. of pustules |
No. of subjects observed |
No. of subjects expected |
| 0 | 33 | 22 | 0 | 18 | 7 |
| 1 | 31 | 53 | 1 | 19 | 27 |
| 2 | 42 | 31 | 2 | 16 | 33 |
| 3 | 27 | 13 | |||
| Total | 106 | 106 | Total | 80 | 80 |
NOTE: Expected numbers based on the observed overall pustule formation rate and the assumption of site independence.
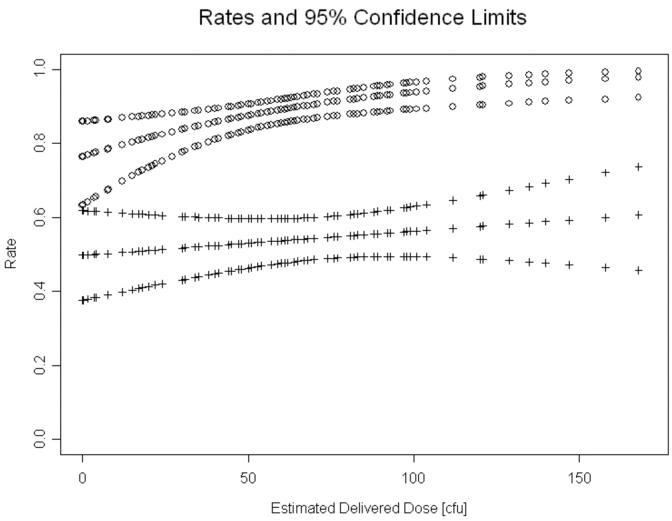
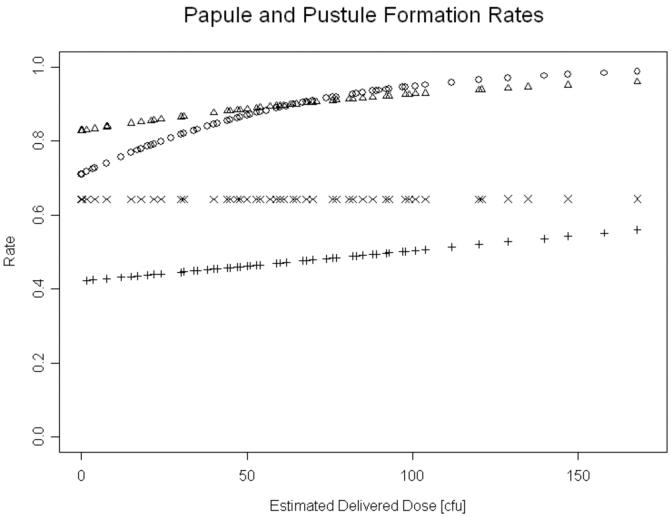
We calculated overall papule and pustule formation rates using logistic models and GEE to account for the within host effect on sites. Papules formed at 479 (89% [95% confidence interval (CI), 85.7%-91.9%]) of 538 sites (figure 2). There was a significant effect of dose on papule formation (P=0.0044); the odds of papule formation increased by 2% per unit increase in EDD (figure 2). After adjusting for dose, we found no association between the likelihood of papule formation and either gender (figure 3) or race (data not shown).
Figure 2.
The relationship of estimated delivered dose and overall probabilities (with 95% CI) of papule (open circles) and pustule (+) formation, as predicted by logistic regression.
Figure 3.
Papule formation rates for females (circle) and males (triangle) and pustule formation rates for females (+) and males (×) related to estimated delivered doses as predicted by logistic regression. For simplicity, the 95% CI are not shown.
A total of 470 sites were included in the calculation of pustule formation rates. The pustule formation rates for 85 sites inoculated with 35000 and 385 sites inoculated with 35000HP were not significantly different. Consequently, we analyzed results for the two strains together. Pustules formed at 256 of 470 sites for an overall pustule formation rate of 54.5% [95% CI, 48.7%-60.1%] (figure 2). There was no significant change in the odds of forming a pustule as the dose increased. When controlled for dose, men were significantly more likely than women to form pustules (OR 1.94; [95% CI, 1.23-3.09]; P=0.0048) (figure 3), consistent with previous reports [21]. There was no significant association between pustule formation and either race or age (data not shown). Thus, the most important determinants of pustule formation were host and gender.
Papules that progressed into pustules (n=256) did so within a mean ± SD of 3.8±2.2 days (range, 1-14 days). Papules that resolved (n=156) did so within 5.3±2.6 days (range, 2-14 days). Only 7 pustules (2.7% [95% CI, 1.2%-6.2%]) reverted into papules and subsequently resolved without treatment. Thus, spontaneous resolution of papules is common, while resolution of pustules is uncommon in the model.
Of the 140 subjects who formed at least 1 pustule, 122 (87.1% [95% CI, 81.6%-92.7%]) developed pain or pruritis at the pustule site. Infection in these subjects was terminated due to symptoms after 8.6±2.7 days. Only 18 subjects who developed pustules did not develop local symptoms and remained infected for 14 days.
Of the 192 volunteers, 139 (72.4% [95% CI, 66.1%-78.7%]) had at least 1 pustule at endpoint and were defined as a pustule former, while 51 (26.6% [95% CI, 20.3%-32.8%]) had all sites of infection resolve and were defined as resolvers. Two subjects had papules at 14 days and were not included in either category.
Outcomes of second challenges
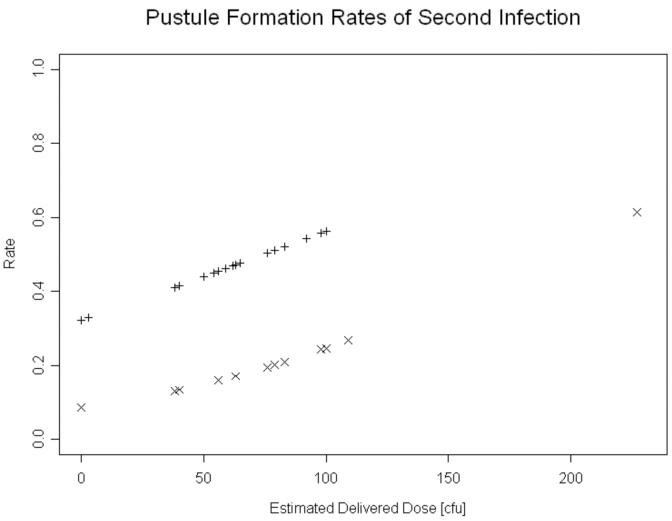
Of the subjects who were initially classified as pustule formers or resolvers, 41 volunteered to be infected a second time [19, 22] (unpublished observations). Of 15 (13 women, 2 men) resolvers challenged a second time, 7 (46.7%; 95% CI, 21.4% -71.9%) resolved all sites, 6 (40%; 95% CI, 15.2%-64.8%) formed at least 1 pustule, and 2 had papules at endpoint. Of 26 (16 women, 10 men) pustule formers challenged a second time, 23 (88.5% [95% CI, 76.2%-100 %]) formed at least 1 pustule, 2 (7.7% [95% CI, 1.0%-25.1%]) resolved at all sites, and 1 had papules at endpoint. Adjusted for gender, a subject was more likely to be a resolver in the second infection if he/she was a resolver in the first infection (P=0.017). Similarly, a pustule former was more likely to form at least one pustule during a second infection if he/she was a pustule former during the first infection (P=0.012). The odds ratio of forming a pustule during the second infection for an initial pustule former was 3.42 [95% CI, 1.32-8.87] times that of an initial resolver (figure 4). Thus, subjects segregate towards their initial outcome when rechallenged.
Figure 4.
The likelihood of forming a pustule on the second infection for volunteers who formed pustules (+) or who resolved (×) during their first infections.
Outcomes of sites inoculated with mutants
In isogenic mutant-parent comparison trials, subjects serve as their own controls for the host and gender effects. Two important themes emerge from the 20 trials published to date (table 2). First, the trials have validated the importance of the association of H. ducreyi with fibrin and collagen and evasion of phagocytosis [9, 10]. Mutants that lack expression of OMPs involved in serum resistance (DsrA and DltA), adherence to collagen (NcaA), fibrinogen binding (FgbA) or secreted proteins that are antiphagocytic (LspA1 and LspA2) are fully or partially attenuated in vivo [8, 24-26, 30]. In contrast, mutants whose gene products target host cells or compartments that do not have major roles in pathogenesis in vivo are dispensable for virulence [8]. For example, mutants that do not make paragloboside-like lipooligosaccharide (LOS), hemolysin and cytolethal distending toxin, which primarily target adherence to and invasion of keratinocytes or killing of keratinocytes and fibroblasts, are virulent [31-33]. A mutant in superoxide dismutase C, which promotes survival in phagolysosomes, a compartment that H. ducreyi evades in vivo, is virulent [34].
Table 2.
H. ducreyi mutants tested in the human challenge model.
| Source | Gene | Definition | Function | No. of Subjects |
Results [ref.] |
|---|---|---|---|---|---|
| Elkins | dsrA | ducreyi serum resistance A |
OMP; major role in serum resistance |
6 | Attenuated [46] |
| Kawula | ncaA | necessary for collagen adherence |
OMP; confers collagen binding |
10 | Attenuated [26] |
| Hansen |
lspA1 lspA2 |
double mutant, large supernatant proteins |
secreted proteins; prevent Fc-g mediated phagocytosis |
6 | Attenuated [24] |
| Spinola | pal | peptidoglycan associated lipoprotein |
lipoprotein; OM stability | 9 | Attenuated [47] |
| Hansen | tadA | tight adherence protein A | type IV secretion; secretion of Flp proteins |
15 | Attenuated [23] |
| Bauer Janowicz |
fgbA | putative fibrinogen binding adhesin |
OMP that binds to fibrinogen in ligand blot |
6 | Partially Attenuated [30] |
| Elkins | dltA | ducreyi lectin A | OMP; partial serum resistance |
7 | Partially Attenuated [25] |
| Munson | hhdB | secretion/activation of hemolysin |
lyses fibroblasts | 6 | Virulent [32] |
| Munson | losB | D-glycero-D-manno- heptosyltransferase |
extends LOS beyond KDO-triheptose-glucose |
5 | Virulent [31] |
| Munson | lst | lipooligosaccharide sialyltransferase |
adds sialic acid to major glycoform of LOS |
5 | Virulent [31] |
| Campagnari Munson |
glu | glucosyltransferase gene | adds glucose to KDO- triheptose LOS core |
5 | Virulent [48] |
| Hansen | cdtC | cytolethal distending toxin | toxic for T cells, epithelial cells, fibroblasts |
6 | Virulent [33] |
| Hansen Munson | cdtC hhdB | double mutant, cytolethal distending toxin, hemolysin |
see above | 5 | Virulent [33] |
| Kawula | sodC | superoxide dismutase C | detoxifies ROS for bacteria in phagolysosomes |
6 | Virulent [34] |
| Spinola Hansen |
momp | major outer membrane protein |
OmpA homologue; minor role in fibronectin binding |
6 | Virulent [49] |
| Campagnari |
ompP2A ompP2B |
double mutant, porin proteins |
encode known classical trimeric porins |
8 | Virulent [27] |
| Spinola | ftpA | fine tangled pilus major subunit |
unknown | 7 | Virulent [50] |
| Elkins | hgbA | hemoglobin binding outer membrane protein |
heme/iron acquisition and transport from hemoglobin |
9 | Attenuated [35] |
| Elkins | tdX/tdhA | double mutant; ton B dependent receptors |
heme uptake | 6 | Virulent [28] |
| Munson | wecA | first enzyme in the ECA biosynthetic pathway |
initiates synthesis of putative ECA glycoconjugate |
5 | Partially Attenuated [29] |
Second, the trials have helped identify vaccine candidates. Although some gram negative organisms contain as many as 40 TonB-dependent receptors, H. ducreyi contains only the hemoglobin receptor (HgbA), the heme receptor (TdhA), and TdX, whose function is not known [28]. A HgbA mutant is attenuated for pustule formation in the model, while a TdhA and TdX mutant is virulent [28, 35]. Thus, HgbA is both necessary and sufficient for heme/iron acquisition by H. ducreyi in humans. HgbA is conserved in H. ducreyi strains and does not undergo phase or antigenic variation. Immunization with purified HgbA elicits protective antibody-mediated immunity in the swine model [28, 36]. The results of these trials strengthen the idea that HgbA may be an excellent vaccine candidate in humans.
Risks and Adverse Events
We typically infect groups of subjects with a common inoculum. In 128 iterations, two groups (6 subjects) were infected with inocula that contained low levels of fungi or bacteria other than H. ducreyi. The subjects developed papules, were treated with ciprofloxacin once the contaminants were detected, and had no adverse outcomes. No subject developed fever or systemic symptoms due to experimental infection. Secondary transmission of H. ducreyi from a volunteer to another person has not occurred in 2,123 subject-days of infection.
Although we exclude persons who form keloids and hypertrophic scars, some subjects developed hypertrophic scars at sites of infection that were biopsied. Hypertrophic scars have not formed at infected sites that were not biopsied or uninfected sites that were biopsied. Subjects were followed for six months post-inoculation. Of 191 volunteers who were biopsied, 18 were lost to follow-up. Of the 173 remaining subjects, 21 women and 7 men (16.2% [95% CI, 10.7%-21.7%]) developed hypertrophic scars. More women (21.7% [95% CI 13.4-29.8%]) than men (9.2% [95% CI, 2.7%-15.7%]) developed hypertrophic scars (P=0.028). Of 31 African Americans, 12 (38.7% [95% CI, 21.6%-55.9%]) developed scars, while 14 of 140 Whites (10% [95% CI, 5%-15%]) developed scars (P≤0.0001). Both Asians who participated in the study developed hypertrophic scars. All subjects who developed hypertrophic scars received an intradermal injection of triamcinolone, and all scars became flat within 2 months.
DISCUSSION
If done under carefully regulated conditions, experimental infection of humans with H. ducreyi is safe, with minimal risk. No subject developed fever, lymphadenopathy or disseminated lesions. Most volunteers achieved clinical endpoint prior to 14 days because of local symptoms. Hypertrophic scars developed at infected sites that were biopsied in 16% of subjects. Although we did not assess lesional TGF-β levels in volunteers who formed hypertrophic scars, TGF-β transcripts are readily amplified from pustules (unpublished data), and expression of TGF-β may contribute to hypertrophic scar formation by enhancing localized production of collagen and fibronectin [37].
In the limited dose range employed in the model, the odds of forming a papule was dose dependent, while the odds of forming a pustule was not dose-dependent. The latter result differs from analyses reported previously, in which site outcomes within a subject were assumed to be independent [8, 20, 21]. However, the outcome of multiple infected sites within a subject was not independent, indicating a host effect [19]. When reinfected, subjects segregated towards their initial outcome (pustule formation or resolution). The mechanism of disease resolution is unknown, but is likely due to enhanced phagocytic clearance in those subjects who resolve. Some naturally infected patients have spontaneous clearance of ulcers [2], while others have multiple recurrences of ulcers [38]. Taken together, the data suggest that some persons are prone to clearing infection while others are prone to disease progression, and that protective immunity does not develop in those who progress to pustules or ulcers [19, 22].
The mechanisms of disease progression versus resolution may be determined in part by the microenvironment at the infected site. Gene expression profiles of infected sites from volunteers who were infected a third time after resolving infection twice are consistent with an effective immune response [39]. In contrast, profiles indicative of a hyperinflammatory, dysregulated immune response are present in persons infected a third time after developing pustules twice [39]. Monocyte-derived myeloid DC obtained from the volunteers who resolved twice expressed transcripts that should promote Th1 and Th17 responses in response to H. ducreyi, while DC from the volunteers who formed pustules twice express transcripts consistent with Th1 and regulatory T cell responses [39]. Th1 and Th17 responses may lead to a cytokine environment that promotes phagocytosis, while a combined Th1 and regulatory response may inhibit phagocytosis [39]. Alternatively, DC from the resolver group might direct NK cells to make an appropriate amount of IFNγ during early stages of infection, which augments phagocytosis.
Men and women formed papules, or became infected, at equal rates; however, men were two times more likely to form pustules. The male to female ratio of natural chancroid in endemic areas is 3:1, which likely reflects gender differences in susceptibility to disease progression [4]. In vitro, H. ducreyi dies at temperatures above 35°C. We were unable to assess whether gender differences in susceptibility were associated with elevated body temperatures at the time of ovulation in women because menstrual cycles of female participants were not assessed in detail [21]. Alternatively, the gender effect may reflect the fact that women are more prone to proinflammatory than tolerizing responses compared to men [40]. If women are less prone to tolerizing responses than men, and a tolerizing response promotes phagocytic failure, women should be less susceptible to pustule formation than men.
H. ducreyi is more closely related to the animal pathogens Mannheimia haemolytica and Actinobacillus pleuropneumoniae than human pathogens within the Pasteurellaceae [41]. Rabbit, primate, swine and murine models of chancroid were developed; the infectious doses in these models range from 104 to 107 CFU [2, 8]. Murine lesions are due to the content of LOS in the inoculum, not viable bacteria. Bacteria are cleared over time in swine [34], and lesions in rabbits also spontaneously clear. In rabbits and swine, 1 to 2 weeks of infection evoke serum antibody responses and provide protection against repeated exposure; bactericidal activity develops in repeatedly infected swine [42, 43]. The animal models do not simulate human disease sufficiently to study pathogenesis or host responses, reflecting the fact that H. ducreyi diverged from other pathogens to its unique niche in the human host [41].
In summary, experimental infection with H. ducreyi clinically and histologically mirrors natural infection and provides an excellent model for studying the pathogenesis of the infection. Since H. ducreyi naturally infects nongenital skin and causes a chronic limb ulceration syndrome [44, 45], infection of the arm is not a major limitation of the model. Although our model is limited to the pustular stage of disease, the relationships between H. ducreyi and human cells in the model are maintained in natural ulcers [17], suggesting that the duration of infection is also a minor limitation. The model has significantly contributed to the understanding of H. ducreyi pathogenesis and led to the discovery of immune mechanisms that may underlie differential human susceptibility to H. ducreyi infection.
Acknowledgments
This work was supported by grants AI31494 and AI27863 to S.M.S. and AI074657 to D.M.J. from the National Institutes of Allergy and Infectious Diseases (NIAID). The human challenge trials were supported by grant MO1RR00750 to the GCRC at Indiana University. All authors have no relevant financial relationships to disclose. We thank Kate Fortney and Stan Taylor for their assistance with the database as well as Byron Batteiger and Barbara Van Der Pol for their thoughtful critiques of the manuscript. We thank our many colleagues and collaborators and the volunteers who have participated in human challenge trials.
References
- 1.Bong CTH, Bauer ME, Spinola SM. Haemophilus ducreyi: clinical features, epidemiology, and prospects for disease control. Microb. Infect. 2002;4:1141–1148. doi: 10.1016/s1286-4579(02)01639-8. [DOI] [PubMed] [Google Scholar]
- 2.Spinola SM. Chancroid and Haemophilus ducreyi. In: Holmes KK, Sparling PF, Stamm WE, et al., editors. Sexually Transmitted Diseases. 4th ed. McGraw-Hill; New York: 2008. pp. 689–699. [Google Scholar]
- 3.Orroth KK, White RG, Korenromp EL, et al. Empirical observations underestimate the proportion of human immunodeficiency virus infections attributable to sexually transmitted diseases in the Mwanza and Rakai sexually transmitted disease treatment trials: Simulation results. Sex. Trans. Dis. 2006;33:536–44. doi: 10.1097/01.olq.0000204667.11192.71. [DOI] [PubMed] [Google Scholar]
- 4.Morse SA. Chancroid and Haemophilus ducreyi. Clin. Microbiol. Rev. 1989;2:137–157. doi: 10.1128/cmr.2.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trees DL, Morse SA. Chancroid and Haemophilus ducreyi: an update. Clin. Microbiol. Rev. 1995;8:357–375. doi: 10.1128/cmr.8.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spinola SM, Wild LM, Apicella MA, Gaspari AA, Campagnari AA. Experimental human infection with Haemophilus ducreyi. J. Infect. Dis. 1994;169:1146–1150. doi: 10.1093/infdis/169.5.1146. [DOI] [PubMed] [Google Scholar]
- 7.Al-Tawfiq JA, Thornton AC, Katz BP, et al. Standardization of the experimental model of Haemophilus ducreyi infection in human subjects. J. Infect. Dis. 1998;178:1684–1687. doi: 10.1086/314483. [DOI] [PubMed] [Google Scholar]
- 8.Spinola SM, Bauer ME, Munson RS., Jr. Immunopathogenesis of Haemophilus ducreyi infection (chancroid) Infect. Immun. 2002;70:1667–1676. doi: 10.1128/IAI.70.4.1667-1676.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer ME, Spinola SM. Localization of Haemophilus ducreyi at the pustular stage of disease in the human model of infection. Infect. Immun. 2000;68:2309–2314. doi: 10.1128/iai.68.4.2309-2314.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bauer ME, Goheen MP, Townsend CA, Spinola SM. Haemophilus ducreyi associates with phagocytes, collagen, and fibrin and remains extracellular throughout infection of human volunteers. Infect. Immun. 2001;69:2549–2557. doi: 10.1128/IAI.69.4.2549-2557.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palmer KL, Schnizlein-Bick CT, Orazi A, et al. The immune response to Haemophilus ducreyi resembles a delayed-type hypersensitivity reaction throughout experimental infection of human subjects. J. Infect. Dis. 1998;178:1688–1697. doi: 10.1086/314489. [DOI] [PubMed] [Google Scholar]
- 12.Humphreys TL, Schnizlein-Bick CT, Katz BP, et al. Evolution of the cutaneous immune response to experimental Haemophilus ducreyi infection and its relevance to HIV-1 acquisition. J. Immunol. 2002;169:6316–6323. doi: 10.4049/jimmunol.169.11.6316. [DOI] [PubMed] [Google Scholar]
- 13.Humphreys TL, Baldridge LA, Billings SD, Campbell JJ, Spinola SM. Trafficking pathways and characterization of CD4 and CD8 cells recruited to the skin of humans experimentally infected with Haemophilus ducreyi. Infect. Immun. 2005;73:3896–3902. doi: 10.1128/IAI.73.7.3896-3902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banks KE, Humphreys T, Li W, Katz BP, Wilkes DS, Spinola SM. Haemophilus ducreyi Partially Activates Human Myeloid Dendritic Cells. Infect. Immun. 2007;75:5678–5685. doi: 10.1128/IAI.00702-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janowicz DM, Tenner-Racz K, Racz P, et al. Experimental Infection with Haemophilus ducreyi in Persons Who Are Infected with HIV Does Not Cause Local or Augment Systemic Viral Replication. J Infect Dis. 2007;195:1443–51. doi: 10.1086/513877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gelfanova V, Humphreys TL, Spinola SM. Characterization of Haemophilus ducreyi-specific T cell lines from lesions of experimentally infected human subjects. Infect. Immun. 2001;69:4224–4231. doi: 10.1128/IAI.69.7.4224-4231.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauer ME, Townsend CA, Ronald AR, Spinola SM. Localization of Haemophilus ducreyi in naturally acquired chancroidal ulcers. Microbe. Infect. 2006;8:2465–2468. doi: 10.1016/j.micinf.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Thornton AC, O'Mara EM, Jr., Sorensen SJ, et al. Prevention of experimental Haemophilus ducreyi infection: a randomized, controlled clinical trial. J. Infect. Dis. 1998;177:1608–1613. doi: 10.1086/515320. [DOI] [PubMed] [Google Scholar]
- 19.Spinola SM, Bong CTH, Faber AL, et al. Differences in host susceptibility to disease progression in the human challenge model of Haemophilus ducreyi infection. Infect. Immun. 2003;71:6658–6663. doi: 10.1128/IAI.71.11.6658-6663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Tawfiq JA, Harezlak J, Katz BP, Spinola SM. Cumulative experience with Haemophilus ducreyi in the human model of experimental infection. Sex Transm Dis. 2000;27:111–114. doi: 10.1097/00007435-200002000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Bong CTH, Harezlak J, Katz BP, Spinola SM. Men are more susceptible to pustule formation than women in the experimental model of Haemophilus ducreyi infection. Sex Transm Dis. 2002;29:114–118. doi: 10.1097/00007435-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Al-Tawfiq JA, Palmer KL, Chen C-Y, et al. Experimental infection of human volunteers with Haemophilus ducreyi does not confer protection against subsequent challenge. J. Infect. Dis. 1999;179:1283–1287. doi: 10.1086/314732. [DOI] [PubMed] [Google Scholar]
- 23.Spinola SM, Fortney KR, Katz BP, et al. Haemophilus ducreyi requires an intact flp gene cluster for virulence in humans. Infect. Immun. 2003;71:7178–7182. doi: 10.1128/IAI.71.12.7178-7182.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janowicz DM, Fortney KR, Katz BP, et al. Expression of the LspA1 and LspA2 proteins by Haemophilus ducreyi is required for virulence in human volunteers. Infect. Immun. 2004;72:4528–4533. doi: 10.1128/IAI.72.8.4528-4533.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janowicz D, Leduc I, Fortney KR, Katz BP, Elkins C, Spinola SM. A DltA mutant of Haemophilus ducreyi is partially attenuated in its ability to cause pustules in human volunteers. Infect. Immun. 2006;74:1394–1397. doi: 10.1128/IAI.74.2.1394-1397.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fulcher RA, Cole LE, Janowicz DM, et al. Expression of Haemophilus ducreyi collagen binding outer membrane protein NcaA is required for virulence in swine and human challenge models of chancroid. Infect. Immun. 2006;74:2651–8. doi: 10.1128/IAI.74.5.2651-2658.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janowicz D, Luke NR, Fortney KR, Katz BP, Campagnari AA, Spinola SM. Expression of OmpP2A and OmpP2B is not required for pustule formation by Haemophilus ducreyi in human volunteers. Microb. Path. 2006;40:110–115. doi: 10.1016/j.micpath.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Leduc I, Banks KE, Fortney KR, et al. Evaluation of the repertoire of the TonB-dependent receptors of Haemophilus ducreyi for their role in virulence in humans. J. Infect. Dis. 2008;197:1103–9. doi: 10.1086/586901. [DOI] [PubMed] [Google Scholar]
- 29.Banks KE, Fortney KR, Baker B, et al. The enterobacterial common antigen-like gene cluster of Haemophilus ducreyi contributes to virulence in humans. J. Infect. Dis. 2008;197:1531–6. doi: 10.1086/588001. [DOI] [PubMed] [Google Scholar]
- 30.Bauer ME, Townsend CA, Doster RS, et al. A fibrinogen-binding lipoprotein contributes to virulence of Haemophilus ducreyi in humans. J Infect Dis. doi: 10.1086/596656. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young RS, Fortney K, Haley JC, et al. Expression of sialylated or paragloboside-like lipooligosaccharides are not required for pustule formation by Haemophilus ducreyi in human volunteers. Infect. Immun. 1999;67:6335–6340. doi: 10.1128/iai.67.12.6335-6340.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmer KL, Thornton AC, Fortney KR, Hood AF, Munson RS, Jr., Spinola SM. Evaluation of an isogenic hemolysin-deficient mutant in the human model of Haemophilus ducreyi infection. J. Infect. Dis. 1998;178:191–199. doi: 10.1086/515617. [DOI] [PubMed] [Google Scholar]
- 33.Young RS, Fortney KR, Gelfanova V, et al. Expression of cytolethal distending toxin and hemolysin are not required for pustule formation by Haemophilus ducreyi in human volunteers. Infect. Immun. 2001;69:1938–1942. doi: 10.1128/IAI.69.3.1938-1942.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bong CTH, Fortney KR, Katz BP, et al. A superoxide dismutase C mutant of Haemophilus ducreyi is virulent in human volunteers. Infect. Immun. 2002;70:1367–1371. doi: 10.1128/IAI.70.3.1367-1371.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Tawfiq JA, Fortney KR, Katz BP, Elkins C, Spinola SM. An isogenic hemoglobin receptor-deficient mutant of Haemophilus ducreyi is attenuated in the human model of experimental infection. J. Infect. Dis. 2000;181:1049–1054. doi: 10.1086/315309. [DOI] [PubMed] [Google Scholar]
- 36.Afonina G, Leduc I, Nepluev I, et al. Immunization with the Haemophilus ducreyi Hemoglobin Receptor HgbA Protects against Infection in the Swine Model of Chancroid. Infect. Immun. 2006;74:2224–32. doi: 10.1128/IAI.74.4.2224-2232.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niessen FB, Andriessen MP, Schalkwijk J, Visser L, Timens W. Keratinocyte-derived growth factors play a role in the formation of hypertrophic scars. J.Pathol. 2001;194:207–216. doi: 10.1002/path.853. [DOI] [PubMed] [Google Scholar]
- 38.Hammond GW, Slutchuk M, Scatliff J, Sherman E, Wilt JC, Ronald AR. Epidemiologic, clinical, laboratory, and therapeutic features of an urban outbreak of chancroid in North America. Rev. Infect. Dis. 1980;2:867–879. doi: 10.1093/clinids/2.6.867. [DOI] [PubMed] [Google Scholar]
- 39.Humphreys T, Li L, Li X, et al. Dysregulated Immune Profiles for Skin and Dendritic Cells Are Associated with Increased Host Susceptibility to Haemophilus ducreyi Infection in Human Volunteers. Infect. Immun. 2007;75:5686–5697. doi: 10.1128/IAI.00777-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beagley KW, Gockel CM. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol Med Microbiol. 2003;38:13–22. doi: 10.1016/S0928-8244(03)00202-5. [DOI] [PubMed] [Google Scholar]
- 41.Gioia J, Qin X, Jiang H, et al. The genome sequence of Mannheimia haemolytica A1: insights into virulence, natural competence, and Pasteurellaceae phylogeny. Journal of Bacteriology. 2006;188:7257–66. doi: 10.1128/JB.00675-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cole LE, Toffer KL, Fulcher RA, San Mateo LR, Orndorff PE, Kawula TH. A humoral immune response confers protection against Haemophilus ducreyi infection. Infect. Immun. 2003;71:6971–6977. doi: 10.1128/IAI.71.12.6971-6977.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hansen EJ, Lumbley SR, Richardson JA, et al. Induction of protective immunity to Haemophilus ducreyi in the temperature-dependent rabbit model of experimental chancroid. J. Immunol. 1994;152:184–192. [PubMed] [Google Scholar]
- 44.Ussher JE, Wilson E, Campanella S, Taylor SL, Roberts SA. Haemophilus ducreyi causing chronic skin ulceration in children visiting Samoa. Clin Infect Dis. 2007;44:e85–7. doi: 10.1086/515404. [DOI] [PubMed] [Google Scholar]
- 45.McBride WJ, Hannah RC, Le Cornec GM, Bletchly C. Cutaneous chancroid in a visitor from Vanuatu. Australas J Dermatol. 2008;49:98–9. doi: 10.1111/j.1440-0960.2008.00439.x. [DOI] [PubMed] [Google Scholar]
- 46.Bong CTH, Throm RE, Fortney KR, et al. A DsrA-deficient mutant of Haemophilus ducreyi is impaired in its ability to infect human volunteers. Infect. Immun. 2001;69:1488–1491. doi: 10.1128/IAI.69.3.1488-1491.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fortney KR, Young RS, Bauer ME, et al. Expression of peptidoglycan-associated lipoprotein is required for virulence in the human model of Haemophilus ducreyi infection. Infect. Immun. 2000;68:6441–6448. doi: 10.1128/iai.68.11.6441-6448.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Young RS, Filiatrault MJ, Fortney KR, et al. Haemophilus ducreyi lipooligosaccharide mutant defective in expression of B1,4-glucosyltransferase is virulent in humans. Infect. Immun. 2001;69:4810–4814. doi: 10.1128/IAI.69.6.4180-4184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Throm RE, Al-Tawfiq JA, Fortney KR, et al. Evaluation of an isogenic MOMP-deficient mutant in the human model of Haemophilus ducreyi infection. Infect Immun. 2000;68:2602–2607. doi: 10.1128/iai.68.5.2602-2607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al-Tawfiq JA, Bauer ME, Fortney KR, et al. A pilus-deficient mutant of Haemophilus ducreyi is virulent in the human model of experimental infection. J. Infect. Dis. 2000;181:1176–1179. doi: 10.1086/315310. [DOI] [PubMed] [Google Scholar]