Abstract
Purpose of review
Though designed to target only the HIV protease, HIV protease inhibitors (PIs) induce toxicities in patients such as insulin resistance and lipodystrophy that suggest that PIs have other targets in mammalian cells. Akt controls insulin signaling and is an important target in cancer, but no Akt inhibitors are approved as cancer therapeutics. These observations have prompted study of HIV protease inhibitors as inhibitors of Akt and possible cancer therapeutics. This review will highlight the latest advances in repositioning HIV PIs as cancer therapeutics.
Recent findings
Although PIs can inhibit Akt activation and inhibit the proliferation of over 60 cancer cell lines, as well as improve sensitivity to radiation or chemotherapy, these effects do not always correlate with Akt inhibition. Other important processes such as the induction of endoplasmic reticulum stress appear critical to the biological activity of PIs. These impressive and surprising preclinical data have prompted clinical testing of nelfinavir as a lead HIV PI in cancer patients.
Summary
While mechanism of actions for the anti-tumor effects of HIV PIs are complex, their broad spectrum of activity, minimal toxicity, and wide availability make PIs ideal candidates for repositioning as cancer therapeutics.
Keywords: protease inhibitors, Akt, apoptosis, ER stress, autophagy
Introduction
The development of cancer drugs is slow and costly. The repositioning of HIV PIs as cancer therapeutics has been based on two facts. First, Akt is an important target in cancer, yet no Akt inhibitors are clinically available. Second, HIV PIs inhibit Akt activation, which likely explains the clinical toxicities of insulin resistance and lipodystrophy that are associated with their use. Recent studies show that HIV PIs are established broad-spectrum anti-cancer agents that work through pleiotropic mechanisms in cancer cells. The clinical efficacy of PIs is now being evaluated in cancer patients.
Protease inhibitors target more than HIV protease
The protease inhibitors were rationally designed to block HIV aspartyl protease, an enzyme that cleaves the gag and gag-pol precursor polyproteins, arresting maturation and preventing the generation of infectious virions [1, 2]. Because this class of drugs is only weakly active against human aspartyl proteases [2, 3], minimal toxicity was anticipated. Shortly after their introduction, however, off-target effects began to be described.
Some of the earliest data came from Kempf et al. [3] who found that treating mice with ritonavir could prevent the expansion of cytotoxic T cells against lymphocytic choriomeningitis virus (LCMV) epitopes. This was not because of inhibition of LCMV replication, but rather because of decreased presentation of LCMV antigen. The mechanism proposed by Schmidtke et al. [4] was inhibition of antigen processing via ritonavir-induced inhibition of the 20S proteasome chymotrypsin activity. Consistent with this, early HIV trials reported on the ability of saquinavir and ritonavir to affect immune reconstitution before virus replication was suppressed [5] and to maintain immune reconstitution in face of persistent viremia [6–10]. In 1999, a report of a patient with regression of Kaposi’s sarcoma (KS) following therapy with a PI-containing regimen was published [11], and improvements in response rates and relapsed free survival in many AIDS associated malignancies followed [12]. While these findings were initially attributed to immune reconstitution and better control of oncogenic viral infections, the number of reports in solid tumors, KS [13], lymphoma, fibrosarcoma [14], multiple myeloma [15], and prostate cancer [16, 17] suggested other mechanisms for the anti-neoplastic activity of PIs.
Lipodystrophy and insulin resistance
Another indication that PIs target more than the HIV protease was based on reports of lipodystrophy and insulin resistance in PI-treated HIV patients [18–21]. Explanations for lipodystrophy centered on adipocyte transcription factors such as peroxisome proliferators activated receptor γ (PPAR-γ) and sterol regulatory element binding protein-1 (SREBP-1). Carr et al. [22] proposed that through one of several mechanisms, adipocyte differentiation was blocked, ultimately resulting in apoptosis. While controversy exists regarding adipocyte differentiation [23–27], a consensus that PIs increase SREBP-1 expression developed [26, 28]. SREBP-1 expression has been shown to be increased 2.6 fold in adipocytes of HIV infected persons after treatment with ritonavir [28]. Increased expression of SREBP-1, especially SREBP-1c, is a feature of the congenital lipoatrophy syndrome, an autosomal recessive disorder, characterized by unregulated expression of SREBP-1c, loss of subcutaneous fat, insulin resistance, and dyslipidemia [29], phenotypic features of patients with PI-induced lipodystrophy.
HIV PIs may also induce insulin resistance through multiple mechanisms. PIs inhibit release of insulin by pancreatic beta cells [30], and inhibit the response of skeletal muscle cells, adipocytes, and hepatocytes to insulin. This occurs through diminished signaling through Akt and isoforms of protein kinase C [31, 32], as well as through direct binding to glucose transporters such as Glut1 and Glut4 [32, 33]. The role of Akt in mediating the effects of insulin was confirmed in studies of mice that lack specific isoforms of Akt. Mice lacking Akt2 are insulin resistant and have higher fasting and post-prandial glucose levels than heterozygous or wild type mice, and show compensatory but inadequate hyperinsulinemia [34]. Inhibition of Akt signaling by PIs not only provided a mechanism for a commonly observed toxicity, but also provided strong rationale to test PIs as cancer therapeutics.
The Akt pathway in cancer
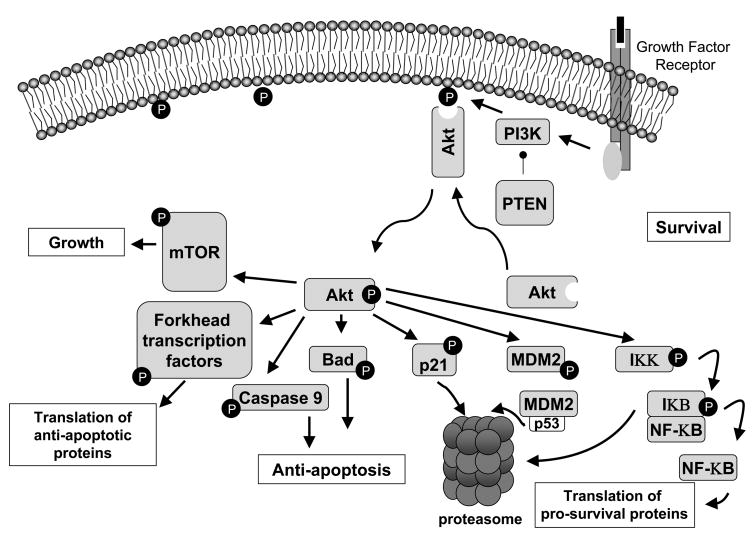
The Akt pathway is the prototypic survival pathway and is constitutively activated in a number of malignancies (Figure 1) [35]. In preclinical studies, Akt promotes cellular transformation, cellular proliferation, and drives tumor formation in mice. In addition, Akt activation promotes resistance to chemotherapy as well as radiation therapy, and portends a poor prognosis for patients with many types of cancer [36]. The Akt signaling cascade is initiated with the activation of phosphatidylinositol-3-kinase (PI3K) following cross-linking of a growth factor with its cell surface receptor. Activated PI3K phosphorylates membrane bound phosphoinositides. Phosphorylated phosphoinositides bind to Akt, leading to its translocation to the inner cell surface where it can be phosphorylated by many mechanisms [37]. Following phosphorylation, activated Akt moves to the cytosol and nucleus to activate its many substrates, which control important cellular processes such as cell cycle progression, apoptosis, transcription and translation [37, 38]. Regulation of the PI3K/Akt pathway is complex, and an important negative regulator is the tumor suppressor PTEN, a lipid phosphatase that de-phosphorylates the products of PI3K.
Figure 1. Activation of Akt promotes cellular survival through multiple mechanisms.
Akt inhibits apoptosis by phosphorylation of many substrates, including 1. the FoxO subfamily of forkhead family transcription factors that inhibits the transcription of pro-apoptotic genes, 2. pro-apoptotic proteins such as BAD and caspase 9, which inactivates them, and 3. IKK, which indirectly increases the activity of NF-κB and stimulates the transcription of pro-survival genes. Cell cycle progression is promoted by phosphorylation of the cdk inhibitor p21, which is subsequently cleared via the proteasome. Phosphorylation of MDM2 by Akt leads to increased ubiquitinylation of p53 and increased cleared by the proteasome.
Because Akt promotes the formation, maintenance, and therapeutic resistance of cancer, the development of Akt inhibitors has become a major effort within industry and academia. Given the inherent delay of developing Akt inhibitors de novo, the immediate availability of the PIs made them logical candidates to study in cancer.
The efficacy of PIs as anti-cancer agents
The results of several studies that assessed the efficacy of PIs in cancer cells are summarized in Table 1. HIV PIs have a very broad spectrum of activity, and can inhibit the proliferation and/or cause the death of virtually every cancer cell line tested in a dose dependent manner. Many investigators have confirmed the in vitro efficacy of PIs by demonstrating inhibition of growth of human tumors in mice when transplanted as xenografts. Cytotoxic chemotherapies such as docetaxel and targeted therapies such as imatinib have been successfully combined with PIs [16, 39, 40], suggesting that HIV PIs have properties that are shared with traditional cancer therapeutics.
Table 1.
| Protease Inhibitor | Cell Lines | Cell Type | Mechanism | p-Akt Inhibition | Other Observations | Xenograft data | Increased efficacy of chemoradiation therapy? | Ref |
|---|---|---|---|---|---|---|---|---|
| RIT | EL4-T, Jurkat, T1 Meth-A, P815, 3T3 | Thymoma, leukemia, T/B lymphoblastoid hybird, Murine Fibrosarcoma, Murine Mastocytoma, Non transformed fibroblasts | Inhibition of 20S proteasome | Not studied | Apoptosis, accumulation of cell cycle inhibitors | RIT induced growth inhibition in C57BL/6 with ELT-4 tumors | Not studied | 14 |
| SAQ | LnCaP, DU-145, PC-3, U373, K562, Jurkat | Prostate (LnCaP, DU-145, PC-3) Glioblastoma (U373) Leukemia (K562, Jurkat) |
Inhibition of 20S and 26S proteasome, inhibition of NF-κB activity | Not studied | Apoptosis | None | Radiation | 55 |
| RIT, SAQ, NEL, IND | U266, RPMI8226, ARH77 | Multiple myeloma | Inactivation of STAT3 and ERK1/2 | Not studied | Growth arrest, apoptosis, and anti- angiogenesis | None | Not studied | 15 |
| RIT, SAQ, IND | PC-3, DU-145 | Androgen independent prostate | Inhibition of NF-κB binding activity, inhibition of CYP 3A4 by RIT | Not studied | Apoptosis | RIT induced growth inhibition in BNX nu/nu mice with DU145 tumors | Docetaxel | 16 |
| NEL, RIT, SAQ, IND, AMP | SQ20B, T24, MIAPACA2, A549 | SCCa head and neck, Bladder, Pancreatic, Lung | Inhibition of Akt activation | Decreased p-Akt | AMP, NEL induced growth inhibition via decr p-Akt in Ncr nude mice with SQ20B and T24 tumors | Radiation | 45 | |
| NEL, SAQ | HL-60 | Leukemia | Inhibition of 20S proteasome | Not studied | None | Not studied | 57 | |
| NEL, RIT, SAQ, IND, AMP | SW872, LiSa-2, HT1080 | Liposarcoma | Up regulation SREBP-1 expression | Not studied | G1 cell cycle arrest, apoptosis | None | Not studied | 51 |
| SAQ, RIT, NEL IND, AMP, LOP, TAZ | E6 transfected C33A, SiHa | SCCa Cervix | Inhibition of 20S proteasome preventing E6- induced clearance of p53 | Not studied | Apoptosis | None | Not studied | 58 |
| NEL | SQ20B, A549 | SCCa head and neck, Lung | Decreased VEGF expression, decreased hypoxic induction of HIF1α via inactivated Akt pathway | Decreased p-Akt | Increased tumor oxygenation | NEL induced decreased VEGF expression in nude mice with SQ20 or A549 tumors | Radiation | 48 |
| RIT | MCF7, T47D, MDA-MB-436, MDA- MB-231 | Breast cancer | Inhibition of chaparone function of Hsp90 | Decreased p-Akt | G1 arrest, depletion of cyclin dependent kinases 2,4,6 and Cyclin D1, and p-Rb | RIT induced growth inhibition in nude mice with MDA-MB- 231 tumors | Not studied | 49 |
| NEL, SAQ, RIT | H460, H520, A549, EBC-1, ABC-1 | Non small cell lung cancer | Inhibition of Akt activation | Decreased p-Akt | Apoptosis, accumulation of cell cycle inhibitors | NEL induced growth inhibition and increased apoptosis in BALB/c nude mice with H460 tumors | Docetaxel | 40 |
| NEL, SAQ, AMP | NA | Human umbilical vein endotheial cells (HUVEC) | Inhibition of Akt activation | Decreased p-Akt | Apoptosis | NEL induced increased sensitivity to radiation in vascular window model | Radiation | 46 |
| NEL, SAQ, RIT, AMP, TAZ | NCI-60, H157, A549 | NCI-60 (leukemia, lung, colon, CNS, melanoma, ovarian, renal, prostate, breast); H157, A549 Lung | ER stress | Varies among cell lines | G1 cell cycle arrest, apoptosis, autophagy | NEL induced growth inhibtion in BAL/c AnNCr nude mice with H157 tumors | Not studied | 52 |
| NEL | SQ20B | SCCa | Proteasome inhibition, ER stress, and unfolded protein response causing dephosphorylation of p-Akt by PP1 | Decreased p-Akt | None | Radiation | 50 | |
| NEL, RIT, SAQ, IND | 1205 LU, A375, C8161, NIH1286, WM35, WM115 | Melanoma | Cell cycle arrest via inhibition of CDK2, dephosphorylation of Rb; proteasome induced degradation of Cdc25A phosphatase | No change in p- Akt | Growth inhibition and apoptosis | None | Not studied | 53 |
| NEL | U251MG, U87MG | PTEN deficient glioblastoma | Inhibition of Akt activation | Decreased p-Akt, p-S6 | Radiation sensitization in nude mice with U87 tumors | Radiation and temozolomide | 47 | |
| RIT | HUVEC, KSIIM | Normal endothelial cells, KS | Anti-angiogenesis, inhibition of NF-κ B activity | Not studied | Inhibiton of proliferation of HUVEC, inhibition of KS promoting inflammatory proteins | RIT induced growth inhibition in BNX mice with KSIMM tumors | Not studied | 13 |
| NEL, TAZ | T98G, LN229, U251, U87 | Glioblastoma | Proteasome inhibition, ER stress, and unfolded protein response | Not studied | Apoptosis | NEL induced growth inhibition in nude mice with U87 tumors | Not studied | 54 |
| SAQ + Lovastatin | Raj, Daudi | Lymphoma | Proteasome inhibition for both, no data presented | Not studied | Additive effect on growth inhibition | No | Not studied | 56 |
| NEL + Imatinib | IOMM - Lee | Meningioma cell line and Fresh meningioma tissue | Increased Bax:Bcl- 2 and decreased survivin leading to apoptosis | Not studied | Additive effect on growth inhibition | NEL + Imatinib induced growth inhibition in athymic nude mice and IOMM-Lee tumors | Imatinib | 39 |
p-Akt is phosphorylated Akt; Hsp90 is heat shock protein 90
Bold face indicates most active agent
NEL nelfinavir, SAQ saquinavir, RIT ritonavir, IND indinavir, AMP amprenavir, TAZ tazanavir, LOP lopinavir
SCCa is squamous cell carcinoma
Despite this wide spectrum of activity, PIs are not very potent. Most studies require ≥10 μM for cellular activity. This is relevant because pharmacokinetic studies performed in HIV patients revealed that the maximum concentrations achieved for nelfinavir were 7–9 μM. Of HIV PIs tested, nelfinavir appears to be most potent, which has led to its consideration as a lead HIV PI for cancer therapy. The recognition of PIs as broadly cytotoxic agents to cancer cells has intensified efforts to understand how PIs work, given the absence of HIV protease.
Is activated Akt the critical molecular target for protease inhibitors?
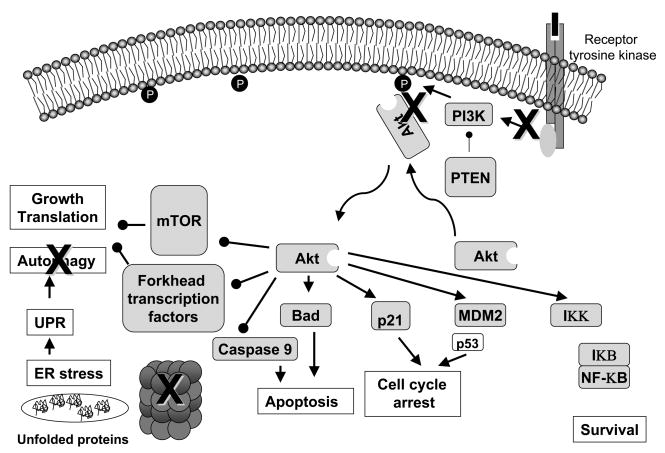
Constitutive activation of Akt protects cancer cells from apoptosis, making them resistant to the effects of ionizing radiation and/or chemotherapy [41–44]. Several groups [40, 45–47] have suggested that inhibition of PI3K-induced activation of Akt by HIV PIs is an important mechanism by which the class exerts anti-tumor effect. These investigators showed that decreased phosphorylation of Akt correlated with increased sensitivity to ionizing radiation [40, 45–47] and chemotherapy [40]. However, this has not been observed in all studies, and in many cases inhibition of Akt activation lags behind other effects such as induction of ER stress and inhibition of cell cycle progression. Moreover, other groups hypothesize that the anti-tumor effects of PIs are related to other mechanisms such inhibition of VEGF and HIF1α expression [48] (which may be secondary to Akt inactivation), direct inhibition of the chaperone function of Hsp90 [49], or inhibition of proteasome function [50] (Figure 2).
Figure 2. Potential sites of action of HIV PIs.
The black Xs represent possible sites of action of protease inhibitors. The inhibition of Akt phosphorylation results in a loss translation of the pro-survival genes and a loss in the inhibitory phosphorylation of the pro-apoptotic proteins caspase 9 and BAD. p21 and p53 may induce cell cycle arrest. Inhibition of the proteasome results in the accumulation of unfolded proteins and the unfolded protein response. To permit cell recovery, a global decrease in protein synthesis or autophagy may follow.
Administration of a PI to a cancer cell may not only result in direct Akt inhibition, but also indirect inhibition. For example, Akt inhibition has been observed in some studies after cell cycle arrest with apoptosis [14, 15, 40, 49, 51–53] cell cycle arrest without apoptosis [46, 48, 52], or only apoptosis [39, 54]. More recently, studies that identified cell cycle arrest as a consequence of administration of PIs also showed a correlation with the accumulation of proteins that control cell cycle progression [14, 40, 49, 53]. This led to the important observation that protein degradation, and more generally, protein homeostasis is altered by PIs.
Beyond the Akt pathway: proteasome inhibition and ER stress
The 20S proteasome was one of the first “off target” activities of the HIV PIs [3]. Several investigators [14, 50, 54–58] have proposed direct inhibition of the proteasome as the mechanism for anti-neoplastic activity. In these studies, proteasome inhibition resulted in the accumulation of cell cycle inhibitors, cell cycle arrest and apoptosis [14,] or decrease in NF-κB activity [55]. Hampson et al. [58] showed that proteasome inhibition by lopinavir prevented E6 induced clearance of p53 in an HPV-16 cervical cancer cell line. Other investigators [50, 52, 54, 59] have extended these studies and observed that PIs induce ER stress.
The endoplasmic reticulum (ER), responsible for protein folding and maturation, is also capable of signal transduction for cell homeostasis. Any condition that alters function of the ER is called ER stress. Such conditions include accumulation of misfolded proteins resulting from proteasome inhibition, lipid or glycolipid imbalances, and changes in the ionic balance of the ER lumen [60, 61]. ER stress as a result of abnormal glucose transport and proteasome inhibition has been shown to be part of PI-induced lipodystrophy [62]. The accumulation of unfolded protein aggregates prompts signaling through an evolutionarily conserved pathway called the unfolded protein response, in which misfolded proteins directly activate specific ER kinases such as PERK that cause a decrease in protein synthesis and thereby give the cell a chance to clear the denatured proteins. As part of a feedback loop, protein phosphatase 1 (PP1) can be expressed, which can de-phosphorylate and inactivate Akt, further decreasing protein synthesis. If a cell cannot recover from ER stress, it can directly undergo apoptosis via a caspase dependent pathway or can attempt survival through autophagy. Invariably, prolonged autophagy leads to cell death.
The studies of Gupta et al. [50], Gills et al. [52], and Pyrko et al. [54] have provided the most thorough analysis of how PIs induce ER stress in cancer cell lines. Gupta described induction of ER stress as a means by which Akt was de-phosphorylated by PP1. Gills et al. [52] suspected ER stress when vacuolization and cellular detachment in nelfinavir treated cell lines was observed. Transmission electron microscopy revealed marked dilatation of ER, and two markers of ER stress, phosphorylation of eIF2α and increased expression of ATF3, were increased within hours after treatment with nelfinavir, suggesting ER stress preceded other cellular responses. Similar results were observed by Pyrko et al. [54], who treated glioma cell lines with nelfinavir and atazanavir and showed that expression of ER stress markers and dilatation of ER was critical to the response to PIs.
Autophagy
Autophagy is a tightly regulated catabolic process in which a cell degrades long-lived proteins or organelles as part of normal homeostasis or as a means to survive a period of nutrient depletion. Autophagy enables the cell to transfer nutrients from less essential locations to those vital for survival [63]. Autophagy can be induced by starvation, mTOR inhibition (secondary to Akt inhibition), ER stress and the unfolded protein response, as well as inhibition of growth factor receptor signaling [64]. Several investigators [30, 31, 40] reported that pre-treating cells with nelfinavir blocks growth factor receptor signaling, ultimately blocking Akt phosphorylation and activation of downstream substrates such as mTOR. However, autophagy and ER stress were not evaluated in these early papers. More recently, Gills et al. [52] demonstrated evidence of autophagy in nelfinavir treated cells. Because the effects of nelfinavir on Akt inhibition were transient and cell line specific in their studies, mTOR inhibition was an unlikely mechanism for the induction of autophagy. ER stress and the UPR were more likely based upon rapid upregulation of eIF2α and ATF3 (markers of ER stress) by nelfinavir, and EM and immunofluorescence data showed that nelfinavir caused dilatation of ER and accumulation of an ER-specific fluorescent marker in vesicles, respectively. Though a pathway leading to the induction of autophagy was identified, the expression of autophagy markers was atypical. Expression of LC3-II, a prototypic marker of autophagy, was increased and autophagosomes were observed using EM, but induction of beclin-1 was not observed. Nonetheless, an inhibitor of autophagy increased the cytotoxicity of nelfinavir in cancer cell lines. Thus, a practical concern is whether the induction of autophagy could be a means by which cancer cells could survive nelfinavir treatment.
From the bench to the bedside
There are currently two clinical trials evaluating nelfinavir in solid tumors and two as a radiation/chemotherapy sensitizer (Clinicaltrials.gov). Results are available from a phase I trial using nelfinavir as a radiation sensitizer in locally advanced pancreatic cancer [65]. Investigators treated 12 subjects with advanced pancreatic carcinoma with nelfinavir 1250 mg orally twice daily starting 3 days before radiation therapy. Subjects received cisplatin and one of two dose levels of gemcitabine concurrently with 59.4 Gy radiation over 6 weeks. Tumor response was determined using Response Evaluation Criteria in Solid Tumors (RECIST). None of the observed toxicities were attributed to nelfinavir. Partial responses were seen in five of 10 subjects. Negative resection margins were obtained six of the 10 responders who underwent surgical resection. These data compare favorably with historical controls, as tumor responses rates following combined modality therapy for pancreatic cancer are approximately 30%. Interestingly, inhibition of Akt phosphorylation did not correlate with clinical responses, although this was a preliminary analysis.
Two other Phase I dose escalation studies using nelfinavir as a single agent in solid tumor patients are underway. In each, the objectives are to establish the maximum tolerated dose and define dose-limiting toxicities of nelfinavir. One study at the National Cancer Institute and National Naval Medical Center is open to patients with any solid tumor, and one study at City of Hope is open to patients with liposarcomas. These dose escalation studies are important because when nelfinavir was originally developed, a maximum tolerated dose was never established. Given the dose dependent cytotoxic effects of nelfinavir in preclinical studies, it is possible that higher doses could yield greater clinical responses. Currently, each trial is at a dose level where over 3,000 mg are being administered bid without significant toxicities. In addition to clinical endpoints, each study is assessing biomarkers for nelfinavir administration. The NCI study is focusing on assessment of Akt activation, and expression of markers of apoptosis, ER stress, and autophagy. The City of Hope trial is focusing on SREBP-1 expression, which explains the restriction to patients with liposarcomas, and is based on preclinical data from these investigators [51].
Conclusion
Are the data convincing enough to conclude that HIV PIs could be repositioned as anti-cancer agents? Clearly, PIs inhibit a variety of malignant cell lines and xenografts, with nelfinavir consistently being the most potent and effective at clinically achievable concentrations. Although lipodystrophy and insulin resistance in PI-treated HIV patients originally linked these agents to the Akt signaling pathway, induction of other molecular processes such as proteasome inhibition [50], ER stress, the unfolded protein response, and autophagy [52, 59] must now also be considered as being critical to the effects of PI in cancer cells. Despite the uncertainty of a unifying mechanism of action for PIs, their track record of minimal toxicity, FDA approved status, and readily availability makes them excellent candidates for further evaluation as cancer therapeutics.
Acknowledgments
The author would like to thank Dr. Joell Gills for helpful reading and discussion of the manuscript.
Funding: This research was supported by the Intramural Research Program of the NIH, Center for Cancer Research, National Cancer Institute. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services or Department of Defense, nor does mention of trade names, commercial products, or organization imply endorsement by the United States Government.
References
- 1.Karacostas V, Nagashima K, Gonda MA, Moss B. Human immunodeficiency virus-like particles produced by a vaccinia virus expression vector. Proceedings of the National Academy of Sciences of the United States of America. 1989 Nov;86(22):8964–7. doi: 10.1073/pnas.86.22.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts NA, Martin JA, Kinchington D, Broadhurst AV, Craig JC, Duncan IB, et al. Rational design of peptide-based HIV proteinase inhibitors. Science (New York, NY. 1990 Apr 20;248(4953):358–61. doi: 10.1126/science.2183354. [DOI] [PubMed] [Google Scholar]
- 3.Kempf DJ, Marsh KC, Denissen JF, McDonald E, Vasavanonda S, Flentge CA, et al. ABT-538 is a potent inhibitor of human immunodeficiency virus protease and has high oral bioavailability in humans. Proceedings of the National Academy of Sciences of the United States of America. 1995 Mar 28;92(7):2484–8. doi: 10.1073/pnas.92.7.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidtke G, Holzhutter HG, Bogyo M, Kairies N, Groll M, de Giuli R, et al. How an inhibitor of the HIV-I protease modulates proteasome activity. The Journal of biological chemistry. 1999 Dec 10;274(50):35734–40. doi: 10.1074/jbc.274.50.35734. [DOI] [PubMed] [Google Scholar]
- 5.Badley AD, Dockrell DH, Algeciras A, Ziesmer S, Landay A, Lederman MM, et al. In vivo analysis of Fas/FasL interactions in HIV-infected patients. The Journal of clinical investigation. 1998 Jul 1;102(1):79–87. doi: 10.1172/JCI2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufmann D, Pantaleo G, Sudre P, Telenti A. CD4-cell count in HIV-1-infected individuals remaining viraemic with highly active antiretroviral therapy (HAART). Swiss HIV Cohort Study. Lancet. 1998 Mar 7;351(9104):723–4. doi: 10.1016/s0140-6736(98)24010-4. [DOI] [PubMed] [Google Scholar]
- 7.Levitz SM. Improvement in CD4+ cell counts despite persistently detectable HIV load. The New England journal of medicine. 1998 Apr 9;338(15):1074–5. doi: 10.1056/NEJM199804093381517. [DOI] [PubMed] [Google Scholar]
- 8.Deeks SG, Barbour JD, Martin JN, Swanson MS, Grant RM. Sustained CD4+ T cell response after virologic failure of protease inhibitor-based regimens in patients with human immunodeficiency virus infection. The Journal of infectious diseases. 2000 Mar;181(3):946–53. doi: 10.1086/315334. [DOI] [PubMed] [Google Scholar]
- 9.Lecossier D, Bouchonnet F, Schneider P, Clavel F, Hance AJ. Discordant increases in CD4+ T cells in human immunodeficiency virus-infected patients experiencing virologic treatment failure: role of changes in thymic output and T cell death. The Journal of infectious diseases. 2001 Apr 1;183(7):1009–16. doi: 10.1086/319285. [DOI] [PubMed] [Google Scholar]
- 10.Piketty C, Weiss L, Thomas F, Mohamed AS, Belec L, Kazatchkine MD. Long-term clinical outcome of human immunodeficiency virus-infected patients with discordant immunologic and virologic responses to a protease inhibitor-containing regimen. The Journal of infectious diseases. 2001 May 1;183(9):1328–35. doi: 10.1086/319861. [DOI] [PubMed] [Google Scholar]
- 11.Niehues T, Horneff G, Megahed M, Schroten H, Wahn V. Complete regression of AIDS-related Kaposi’s sarcoma in a child treated with highly active antiretroviral therapy. AIDS (London, England) 1999 Jun 18;13(9):1148–9. doi: 10.1097/00002030-199906180-00026. [DOI] [PubMed] [Google Scholar]
- 12.Monini P, Sgadari C, Toschi E, Barillari G, Ensoli B. Antitumour effects of antiretroviral therapy. Nature reviews. 2004 Nov;4(11):861–75. doi: 10.1038/nrc1479. [DOI] [PubMed] [Google Scholar]
- 13.Pati S, Pelser CB, Dufraine J, Bryant JL, Reitz MS, Jr, Weichold FF. Antitumorigenic effects of HIV protease inhibitor ritonavir: inhibition of Kaposi sarcoma. Blood. 2002 May 15;99(10):3771–9. doi: 10.1182/blood.v99.10.3771. [DOI] [PubMed] [Google Scholar]
- 14.Gaedicke S, Firat-Geier E, Constantiniu O, Lucchiari-Hartz M, Freudenberg M, Galanos C, et al. Antitumor effect of the human immunodeficiency virus protease inhibitor ritonavir: induction of tumor-cell apoptosis associated with perturbation of proteasomal proteolysis. Cancer research. 2002 Dec 1;62(23):6901–8. [PubMed] [Google Scholar]
- 15.Ikezoe T, Saito T, Bandobashi K, Yang Y, Koeffler HP, Taguchi H. HIV-1 protease inhibitor induces growth arrest and apoptosis of human multiple myeloma cells via inactivation of signal transducer and activator of transcription 3 and extracellular signal-regulated kinase 1/2. Molecular cancer therapeutics. 2004 Apr;3(4):473–9. [PubMed] [Google Scholar]
- 16.Ikezoe T, Hisatake Y, Takeuchi T, Ohtsuki Y, Yang Y, Said JW, et al. HIV-1 protease inhibitor, ritonavir: a potent inhibitor of CYP3A4, enhanced the anticancer effects of docetaxel in androgen-independent prostate cancer cells in vitro and in vivo. Cancer research. 2004 Oct 15;64(20):7426–31. doi: 10.1158/0008-5472.CAN-03-2677. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Ikezoe T, Takeuchi T, Adachi Y, Ohtsuki Y, Takeuchi S, et al. HIV-1 protease inhibitor induces growth arrest and apoptosis of human prostate cancer LNCaP cells in vitro and in vivo in conjunction with blockade of androgen receptor STAT3 and AKT signaling. Cancer science. 2005 Jul;96(7):425–33. doi: 10.1111/j.1349-7006.2005.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carr A, Samaras K, Burton S, Law M, Freund J, Chisholm DJ, et al. A syndrome of peripheral lipodystrophy, hyperlipidaemia and insulin resistance in patients receiving HIV protease inhibitors. AIDS (London, England) 1998 May 7;12(7):F51–8. doi: 10.1097/00002030-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Walli R, Herfort O, Michl GM, Demant T, Jager H, Dieterle C, et al. Treatment with protease inhibitors associated with peripheral insulin resistance and impaired oral glucose tolerance in HIV-1-infected patients. AIDS (London, England) 1998 Oct 22;12(15):F167–73. doi: 10.1097/00002030-199815000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Hadigan C, Miller K, Corcoran C, Anderson E, Basgoz N, Grinspoon S. Fasting hyperinsulinemia and changes in regional body composition in human immunodeficiency virus-infected women. The Journal of clinical endocrinology and metabolism. 1999 Jun;84(6):1932–7. doi: 10.1210/jcem.84.6.5738. [DOI] [PubMed] [Google Scholar]
- 21.Vigouroux C, Gharakhanian S, Salhi Y, Nguyen TH, Chevenne D, Capeau J, et al. Diabetes, insulin resistance and dyslipidaemia in lipodystrophic HIV-infected patients on highly active antiretroviral therapy (HAART) Diabetes & metabolism. 1999 Sep;25(3):225–32. [PubMed] [Google Scholar]
- 22.Carr A, Samaras K, Chisholm DJ, Cooper DA. Pathogenesis of HIV-1-protease inhibitor-associated peripheral lipodystrophy, hyperlipidaemia, and insulin resistance. Lancet. 1998 Jun 20;351(9119):1881–3. doi: 10.1016/S0140-6736(98)03391-1. [DOI] [PubMed] [Google Scholar]
- 23.Gagnon A, Angel JB, Sorisky A. Protease inhibitors and adipocyte differentiation in cell culture. Lancet. 1998 Sep 26;352(9133):1032. doi: 10.1016/S0140-6736(05)60074-8. [DOI] [PubMed] [Google Scholar]
- 24.Zhang B, MacNaul K, Szalkowski D, Li Z, Berger J, Moller DE. Inhibition of adipocyte differentiation by HIV protease inhibitors. The Journal of clinical endocrinology and metabolism. 1999 Nov;84(11):4274–7. doi: 10.1210/jcem.84.11.6234. [DOI] [PubMed] [Google Scholar]
- 25.Dowell P, Flexner C, Kwiterovich PO, Lane MD. Suppression of preadipocyte differentiation and promotion of adipocyte death by HIV protease inhibitors. The Journal of biological chemistry. 2000 Dec 29;275(52):41325–32. doi: 10.1074/jbc.M006474200. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen AT, Gagnon A, Angel JB, Sorisky A. Ritonavir increases the level of active ADD-1/SREBP-1 protein during adipogenesis. AIDS (London, England) 2000 Nov 10;14(16):2467–73. doi: 10.1097/00002030-200011100-00007. [DOI] [PubMed] [Google Scholar]
- 27.Caron M, Auclair M, Vigouroux C, Glorian M, Forest C, Capeau J. The HIV protease inhibitor indinavir impairs sterol regulatory element-binding protein-1 intranuclear localization, inhibits preadipocyte differentiation, and induces insulin resistance. Diabetes. 2001 Jun;50(6):1378–88. doi: 10.2337/diabetes.50.6.1378. [DOI] [PubMed] [Google Scholar]
- 28.Bastard JP, Caron M, Vidal H, Jan V, Auclair M, Vigouroux C, et al. Association between altered expression of adipogenic factor SREBP1 in lipoatrophic adipose tissue from HIV-1-infected patients and abnormal adipocyte differentiation and insulin resistance. Lancet. 2002 Mar 23;359(9311):1026–31. doi: 10.1016/S0140-6736(02)08094-7. [DOI] [PubMed] [Google Scholar]
- 29.Seip M, Trygstad O. Generalized lipodystrophy, congenital and acquired (lipoatrophy) Acta Paediatr Suppl. 1996 Jun;413:2–28. doi: 10.1111/j.1651-2227.1996.tb14262.x. [DOI] [PubMed] [Google Scholar]
- 30.Schutt M, Zhou J, Meier M, Klein HH. Long-term effects of HIV-1 protease inhibitors on insulin secretion and insulin signaling in INS-1 beta cells. The Journal of endocrinology. 2004 Dec;183(3):445–54. doi: 10.1677/joe.1.05620. [DOI] [PubMed] [Google Scholar]
- 31.Ben-Romano R, Rudich A, Tirosh A, Potashnik R, Sasaoka T, Riesenberg K, et al. Nelfinavir-induced insulin resistance is associated with impaired plasma membrane recruitment of the PI 3-kinase effectors Akt/PKB and PKC-zeta. Diabetologia. 2004 Jun;47(6):1107–17. doi: 10.1007/s00125-004-1408-5. [DOI] [PubMed] [Google Scholar]
- 32.Ben-Romano R, Rudich A, Torok D, Vanounou S, Riesenberg K, Schlaeffer F, et al. Agent and cell-type specificity in the induction of insulin resistance by HIV protease inhibitors. AIDS (London, England) 2003 Jan 3;17(1):23–32. doi: 10.1097/00002030-200301030-00005. [DOI] [PubMed] [Google Scholar]
- 33.Murata H, Hruz PW, Mueckler M. The mechanism of insulin resistance caused by HIV protease inhibitor therapy. The Journal of biological chemistry. 2000 Jul 7;275(27):20251–4. doi: 10.1074/jbc.C000228200. [DOI] [PubMed] [Google Scholar]
- 34.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science (New York, NY. 2001 Jun 1;292(5522):1728–31. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 35.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005 Nov 14;24(50):7455–64. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 36.Tsurutani J, Fukuoka J, Tsurutani H, Shih JH, Hewitt SM, Travis WD, et al. Evaluation of two phosphorylation sites improves the prognostic significance of Akt activation in non-small-cell lung cancer tumors. J Clin Oncol. 2006 Jan 10;24(2):306–14. doi: 10.1200/JCO.2005.02.4133. [DOI] [PubMed] [Google Scholar]
- 37.Thompson JE, Thompson CB. Putting the rap on Akt. J Clin Oncol. 2004 Oct 15;22(20):4217–26. doi: 10.1200/JCO.2004.01.103. **A comprehensive review of the Akt pathway in malignancy with emphasis on proposed mechanisms for the anti-apoptotic effects of activated Akt. [DOI] [PubMed] [Google Scholar]
- 38.LoPiccolo J, Blumenthal GM, Bernstein WB, Dennis PA. Targeting the PI3K/Akt/mTOR pathway: effective combinations and clinical considerations. Drug Resist Updat. 2008 Feb–Apr;11(1–2):32–50. doi: 10.1016/j.drup.2007.11.003. **An overview of the Akt pathway, mechanisms of activation in malignancy and the use of various agents either alone or in combination to inhibit the pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta V, Samuleson CG, Su S, Chen TC. Nelfinavir potentiation of imatinib cytotoxicity in meningioma cells via survivin inhibition. Neurosurgical focus. 2007;23(4):E9. doi: 10.3171/FOC-07/10/E9. [DOI] [PubMed] [Google Scholar]
- 40.Yang Y, Ikezoe T, Nishioka C, Bandobashi K, Takeuchi T, Adachi Y, et al. NFV, an HIV-1 protease inhibitor, induces growth arrest, reduced Akt signalling, apoptosis and docetaxel sensitisation in NSCLC cell lines. British journal of cancer. 2006 Dec 18;95(12):1653–62. doi: 10.1038/sj.bjc.6603435. *Using silencing RNA these investigators propose that Akt may be the molecular marker of nelfinavir. By inhibiting Akt, they were able to reverse resistance to docetaxel in non small cell lung cancer cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grana TM, Rusyn EV, Zhou H, Sartor CI, Cox AD. Ras mediates radioresistance through both phosphatidylinositol 3-kinase-dependent and Raf-dependent but mitogen-activated protein kinase/extracellular signal-regulated kinase kinase-independent signaling pathways. Cancer research. 2002 Jul 15;62(14):4142–50. [PubMed] [Google Scholar]
- 42.Gupta AK, Bakanauskas VJ, Cerniglia GJ, Cheng Y, Bernhard EJ, Muschel RJ, et al. The Ras radiation resistance pathway. Cancer research. 2001 May 15;61(10):4278–82. [PubMed] [Google Scholar]
- 43.Gupta AK, Cerniglia GJ, Mick R, Ahmed MS, Bakanauskas VJ, Muschel RJ, et al. Radiation sensitization of human cancer cells in vivo by inhibiting the activity of PI3K using LY294002. International journal of radiation oncology, biology, physics. 2003 Jul 1;56(3):846–53. doi: 10.1016/s0360-3016(03)00214-1. *Investigators show inhibition of Akt results in reversal of radiation resistance in a variety of cancer cell lines. No mechanism is proposed. [DOI] [PubMed] [Google Scholar]
- 44.Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004 Mar 18;428(6980):332–7. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 45.Gupta AK, Cerniglia GJ, Mick R, McKenna WG, Muschel RJ. HIV protease inhibitors block Akt signaling and radiosensitize tumor cells both in vitro and in vivo. Cancer research. 2005 Sep 15;65(18):8256–65. doi: 10.1158/0008-5472.CAN-05-1220. [DOI] [PubMed] [Google Scholar]
- 46.Cuneo KC, Tu T, Geng L, Fu A, Hallahan DE, Willey CD. HIV protease inhibitors enhance the efficacy of irradiation. Cancer research. 2007 May 15;67(10):4886–93. doi: 10.1158/0008-5472.CAN-06-3684. *Instead of a focus on the cancer cell, these investigators focus on the ability of protease inhibitor to sensitize human vascular endothelial cells to radiation. The effect was associated with inhibition of Akt phosphorylation. Ionizing radiation plus nelfinavir had more than an additive effect in vivo. [DOI] [PubMed] [Google Scholar]
- 47.Jiang Z, Pore N, Cerniglia GJ, Mick R, Georgescu MM, Bernhard EJ, et al. Phosphatase and tensin homologue deficiency in glioblastoma confers resistance to radiation and temozolomide that is reversed by the protease inhibitor nelfinavir. Cancer research. 2007 May 1;67(9):4467–73. doi: 10.1158/0008-5472.CAN-06-3398. [DOI] [PubMed] [Google Scholar]
- 48.Pore N, Gupta AK, Cerniglia GJ, Jiang Z, Bernhard EJ, Evans SM, et al. Nelfinavir down-regulates hypoxia-inducible factor 1alpha and VEGF expression and increases tumor oxygenation: implications for radiotherapy. Cancer research. 2006 Sep 15;66(18):9252–9. doi: 10.1158/0008-5472.CAN-06-1239. [DOI] [PubMed] [Google Scholar]
- 49.Srirangam A, Mitra R, Wang M, Gorski JC, Badve S, Baldridge L, et al. Effects of HIV protease inhibitor ritonavir on Akt-regulated cell proliferation in breast cancer. Clin Cancer Res. 2006 Mar 15;12(6):1883–96. doi: 10.1158/1078-0432.CCR-05-1167. *Investigators tested ritonavir on a series of breast cancer cell lines and showed that growth inhibition was mediated by Akt inhibition. Because ritonavir blocked he association of Hsp 90 and Akt, they proposed that growth inhibition from ritonavir was in part due to blocking the interaction of Hsp 90 and its substrates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta AK, Li B, Cerniglia GJ, Ahmed MS, Hahn SM, Maity A. The HIV protease inhibitor nelfinavir downregulates Akt phosphorylation by inhibiting proteasomal activity and inducing the unfolded protein response. Neoplasia (New York, NY. 2007 Apr 9;(4):271–8. doi: 10.1593/neo.07124. *Expanding on early work, investigators propose the unfolded protein response following protease inhibition as the means by which inhibition of Akt reverses radiation resistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chow WA, Guo S, Valdes-Albini F. Nelfinavir induces liposarcoma apoptosis and cell cycle arrest by upregulating sterol regulatory element binding protein-1. Anti-cancer drugs. 2006 Sep 17;(8):891–903. doi: 10.1097/01.cad.0000224448.08706.76. **Investigators study another target, the sterol receptor binding protein-1, known to be up regulated in HIV patients treated with protease inhibitors. They observed decreased proliferation in liposarcoma cell lines tested against nelfinavir. This study led to the development of their on going phase I study looking at nelfinavir in liposarcoma patients. [DOI] [PubMed] [Google Scholar]
- 52.Gills JJ, Lopiccolo J, Tsurutani J, Shoemaker RH, Best CJ, Abu-Asab MS, et al. Nelfinavir, A lead HIV protease inhibitor, is a broad-spectrum, anticancer agent that induces endoplasmic reticulum stress, autophagy, and apoptosis in vitro and in vivo. Clin Cancer Res. 2007 Sep 1;13(17):5183–94. doi: 10.1158/1078-0432.CCR-07-0161. **Investigators showed that nelfinavir was consistently the most potent of the 6 protease inhibitors tested in terms of growth inhibition. Caspase dependent apoptosis, ER stress, and autophagy were all proposed as mechanisms of action of the protease inhibitors. Akt activation was not consistently observed. [DOI] [PubMed] [Google Scholar]
- 53.Jiang W, Mikochik PJ, Ra JH, Lei H, Flaherty KT, Winkler JD, et al. HIV protease inhibitor nelfinavir inhibits growth of human melanoma cells by induction of cell cycle arrest. Cancer research. 2007 Feb 1;67(3):1221–7. doi: 10.1158/0008-5472.CAN-06-3377. *Investigators tested melanoma cell lines against nelfinavir. They proposed that the cell cycle arrest was due to enhanced proteasome mediated degradation of cdk25A. [DOI] [PubMed] [Google Scholar]
- 54.Pyrko P, Kardosh A, Wang W, Xiong W, Schonthal AH, Chen TC. HIV-1 protease inhibitors nelfinavir and atazanavir induce malignant glioma death by triggering endoplasmic reticulum stress. Cancer research. 2007 Nov 15;67(22):10920–8. doi: 10.1158/0008-5472.CAN-07-0796. *Investigators showed that the growth inhibition of nelfinavir and atazanavir in glioma cell lines was due to proteasome inhibtion resulting in a accumulation of unfolded proteins and activation of the unfolded protein response. [DOI] [PubMed] [Google Scholar]
- 55.Pajonk F, Himmelsbach J, Riess K, Sommer A, McBride WH. The human immunodeficiency virus (HIV)-1 protease inhibitor saquinavir inhibits proteasome function and causes apoptosis and radiosensitization in non-HIV-associated human cancer cells. Cancer research. 2002 Sep 15;62(18):5230–5. [PubMed] [Google Scholar]
- 56.Issat T, Nowis D, Jakobisiak M, Golab J. Lovastatin potentiates antitumor effects of saquinavir against human lymphoma cells. Oncology reports. 2004 Dec;12(6):1371–5. [PubMed] [Google Scholar]
- 57.Piccinini M, Rinaudo MT, Anselmino A, Buccinna B, Ramondetti C, Dematteis A, et al. The HIV protease inhibitors nelfinavir and saquinavir, but not a variety of HIV reverse transcriptase inhibitors, adversely affect human proteasome function. Antiviral therapy. 2005;10(2):215–23. [PubMed] [Google Scholar]
- 58.Hampson L, Kitchener HC, Hampson IN. Specific HIV protease inhibitors inhibit the ability of HPV16 E6 to degrade p53 and selectively kill E6-dependent cervical carcinoma cells in vitro. Antiviral therapy. 2006;11(6):813–25. [PubMed] [Google Scholar]
- 59.Gills JJ, Lopiccolo J, Dennis PA. Nelfinavir, a new anti-cancer drug with pleiotropic effects and many paths to autophagy. Autophagy. 2008 Jan–Feb;4(1):107–9. doi: 10.4161/auto.5224. *This is a follow up report looking more closely at the effects of nelfinavir on autophagy. They raise the concern that despite the presence of other markers of authphagy, beclin-1 was not expressed. [DOI] [PubMed] [Google Scholar]
- 60.Boyce M, Yuan J. Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell death and differentiation. 2006 Mar;13(3):363–73. doi: 10.1038/sj.cdd.4401817. **Overview of endoplasmic reticulum stress, the process, interactions with other cellular metabolism, especially apoptosis. [DOI] [PubMed] [Google Scholar]
- 61.Wu J, Kaufman RJ. From acute ER stress to physiological roles of the Unfolded Protein Response. Cell death and differentiation. 2006 Mar;13(3):374–84. doi: 10.1038/sj.cdd.4401840. **An overview of the unfolded protein response with focus on the mechanisms of activation, signal transduction, effects on protein translation, and termination of the response. [DOI] [PubMed] [Google Scholar]
- 62.Parker RA, Flint OP, Mulvey R, Elosua C, Wang F, Fenderson W, et al. Endoplasmic reticulum stress links dyslipidemia to inhibition of proteasome activity and glucose transport by HIV protease inhibitors. Molecular pharmacology. 2005 Jun;67(6):1909–19. doi: 10.1124/mol.104.010165. [DOI] [PubMed] [Google Scholar]
- 63.Cuervo AM. Autophagy: many paths to the same end. Molecular and cellular biochemistry. 2004 Aug;263(1–2):55–72. doi: 10.1023/B:MCBI.0000041848.57020.57. [DOI] [PubMed] [Google Scholar]
- 64.Kelekar A. Autophagy. Annals of the New York Academy of Sciences. 2005 Dec;1066:259–71. doi: 10.1196/annals.1363.015. **An overview of autophagy with details on the process, the different types of autophagy and the possible outcome of autophagy. [DOI] [PubMed] [Google Scholar]
- 65.Brunner TB, Geiger M, Grabenbauer GG, Lang-Welzenbach M, Mantoni TS, Cavallaro A, et al. Phase I trial of the human immunodeficiency virus protease inhibitor nelfinavir and chemoradiation for locally advanced pancreatic cancer. J Clin Oncol. 2008 Jun 1;26(16):2699–706. doi: 10.1200/JCO.2007.15.2355. *Investigators report data from the first ever clinical trial testing nelfinavir as a radiation sensitizer in locally advanced pancreatic cancer. They found no significant toxicity attributable to nelfinavir and had a response rate of 50% to chemoradiation therapy. Responses did not correlate with Akt activation status. [DOI] [PubMed] [Google Scholar]