Abstract
Background:
The DASH diet effectively reduces blood pressure. In observational studies, the association between diets consistent with DASH and risk of coronary heart disease and stroke has been examined with varying results. We hypothesized that diets consistent with the DASH diet would be associated with lower incidence of heart failure (HF).
Methods:
We conducted a prospective, observational study of 36,019 participants in the Swedish Mammography Cohort who were 48-83 years old without baseline HF, diabetes, or myocardial infarction. Diet was measured using food-frequency questionnaires. We created a score to assess consistency with DASH by ranking the intake of DASH diet components and 3 additional scores based on food and nutrient guidelines. Cox models were used to calculate rate ratios of HF hospitalization or death determined through the Swedish inpatient and cause-of-death registers between January 1, 1998 and December 31, 2004.
Results:
Over 7 years, 443 women developed HF. Women in the top quartile of the DASH diet score based on ranking DASH diet components had a 37% lower rate of HF after adjustment for age, physical activity, energy intake, education, family history of myocardial infarction, cigarette smoking, postmenopausal hormone use, living alone, hypertension, high cholesterol, body mass index, and incident myocardial infarction. Rate ratios across quartiles were 1.00, 0.85 (95% CI 0.66-1.11), 0.69 (95% CI 0.54-0.88), and 0.63 (95% CI 0.48-0.81), p for trend <0.001. A similar pattern was seen with the guideline-based scores.
Conclusions:
In this population, diets consistent with DASH were associated with lower rates of HF.
INTRODUCTION
Although diet patterns and foods choices have been linked to risk factors for heart failure (HF), little is known about whether diet may help prevent or delay HF. A recent American Heart Association scientific statement stresses the importance of preventing HF through medical treatment and lifestyle interventions targeting HF risk factors, including the DASH diet.1 The DASH diet may contribute to prevention of some cases of HF because it effectively reduced blood pressure and LDL cholesterol in clinical trials.2-4 This diet featured high intakes of fruits, vegetables, low-fat dairy, and whole grains resulting in high potassium, magnesium, calcium, and fiber, moderately high protein, and low total and saturated fat consumption.5 The National Heart, Lung, and Blood Institute has made public recommendations on food and nutrient intake for the prevention and treatment of hypertension based on the DASH diet.6 However, trials of the diet have not been long enough to determine the overall effect on cardiovascular events.
In a recent observational study by Fung and colleagues, women with the highest values of a score designed to measure consistency with DASH had a 24% lower risk of coronary heart disease and a 18% lower risk of stroke.7 Using a different DASH score, Folsom and colleagues did not find statistically significant associations with cardiovascular events.8 However, women with diets most consistent with DASH had an 18% lower rate of death from coronary heart disease and a 14% lower rate of death from stroke, which is similar to the projected effect from the DASH trial.2, 8
We hypothesized that diets consistent with the DASH diet would be associated with lower rates of HF. We constructed 4 scores to assess consistency with DASH, 2 patterned on previously published scores,7, 8 a score based on food intake recommendations, and a score based on nutrient intake recommendations.6 We examined the associations between the DASH scores and incident HF in a population of middle-aged and older women.
METHODS
Study population
This study included 36,019 women who participated in the Swedish Mammography Cohort. The recruitment process, characteristics, and study methods of this population-based cohort have been previously described.9 The Swedish Mammography Cohort includes women born between 1914 and 1948 living in Västmanland and Uppsala counties in central Sweden who completed questionnaires with items on demographic, behavioral, and anthropometric factors and consumption of foods and beverages in late 1997 and early 1998. Participants who did not provide or provided incorrect national identification numbers, who reported implausible energy intakes (>3 standard deviations from the natural logarithm-transformed mean), who had a previous diagnosis of cancer (other than nonmelanoma skin cancer) or who left more than half of the food and beverage items blank were excluded. Additionally for these analyses, participants who at baseline had a history of HF, myocardial infarction (MI), or diabetes were excluded. Participants with baseline MI or diabetes were excluded because people who develop these diseases receive dietary counseling and may change both their diet and their reporting of diet. History of HF and MI were determined through linkage to the inpatient register; history of diabetes was assessed using self-report and linkage to the inpatient register. The study was approved by the Regional Ethical Review Board at Karolinska Institute, Stockholm, Sweden. Completion and return of the self-administered questionnaire was taken to imply consent.
Diet assessment and DASH diet scores
Self-administered food-frequency items in questionnaires asked participants to report usual frequency of consumption of 96 foods and beverages over the previous year. For foods such as milk, coffee, cheese, and bread that are commonly eaten in Sweden, participants reported their consumption in servings per day or per week. For other foods there were 8 predefined responses ranging from never to ≥3 times/day. Portion sizes for most foods were not specified. In validation studies using weighed diet records, habitual portion sizes were found to vary by age. The total consumption of foods and beverages was calculated by multiplying the frequency of consumption by age-specific portion sizes. To make the food intake data comparable to US dietary recommendations, we standardized portion sizes to those used in the US.10 Nutrient values were calculated using food composition data from the Swedish National Food Administration.11
The first score (DASH component score) was based on one proposed by Fung and colleagues which ranked participants on intake of 1) fruits, 2) vegetables, 3) nuts and legumes, 4) low-fat dairy, 5) whole grains, 6) sodium, 7) sweeten beverages, and 8) red and processed meats.7 Participants in the highest quintile of fruits, vegetables, nuts and legumes, low-fat dairy, and whole grains received a score of 5 and those in the lowest quintile received a score of 1. Participants in the highest quintile of sodium, sweeten beverages, and red and processed meats received a score of 1 and those in the lowest quintile received a score of 5. The score for each component was summed to get the overall score.
The second score (food and nutrient recommendations) was constructed based on one proposed by Folsom and colleagues with a combination of food and nutrient guidelines: 1) fruits, 2) vegetables, 3) nuts and legumes, 4) dairy products, 5) total grains, 6) whole grains, 7) sodium, 8) sweets, 9) meats, 10) total fat, and 11) saturated fat.8 Participants could receive 0, ½, or 1 point for each component.
The third score (food recommendations) was based on Nation Heart, Lung, and Blood Institute food intake recommendations.6 Participants were assigned to 1 of 4 sets of consumption goals based on their reported energy intake. We awarded a maximum of 1 point for meeting guidelines for 1) fruits, 2) vegetables, 3) nuts, seeds, and legumes, 4) low-fat dairy, 5) total grain, 6) whole grain, 7) sweets and added sugars, 8) lean meats, poultry, and fish, 9) fats and oils, and 10) alcoholic beverages. The guidelines state that most grain servings should be whole grain; we defined the whole grain target as half the recommended total number of grain servings. For total grain, dairy, lean meats, and nuts, seed, and legumes the maximum score was awarded for consumption near the guidelines, with partial points for consuming more or less depending on the percentage of deviation. For vegetables, fruits, and whole grains, we awarded 1 point for eating at least as many servings as recommended with partial points awarded for less consumption. For sweets and fats and oils, full points were awarded for consumption of no more than the recommended levels with partial points for more consumption. Full points for alcohol consumption were given for ≤2 drinks/day and no points for >2 drinks/day.
The final score (nutrient recommendations) was based on National Heart, Lung, and Blood Institute guidelines for consumption of nutrients: 1) total fat, 2) saturated fat, 3) protein, 4) carbohydrate, 5) cholesterol, 6) sodium, 7) potassium, 8) calcium, 9) magnesium, and 10) fiber.6 A maximum of 1 point was awarded for consumption of total fat, saturated fat, protein, and carbohydrate near energy-level specific guidelines. One point was awarded for cholesterol and sodium consumption less than or equal to the guideline with fewer points given for exceeding the guideline, depending on the percentage exceeded. One point was awarded for potassium, calcium, magnesium, and fiber consumption meeting or exceeding the energy-level specific levels with partial points awarded for less consumption.
HF follow-up
Participants were followed from January 1, 1998 until December 31, 2004 through record linkage to the Swedish inpatient and cause-of-death registers. The inpatient register captures more than 99% of inpatient care.12 Hospitalization for or death from HF was identified by codes 428 (International Classification of Disease-9), I50, or I11.0 (International Classification of Disease-10). A previous study found that 95% of people with these codes as primary diagnosis in the inpatient register had HF on medical record review using European Society of Cardiology criteria.13 We included only hospitalizations or deaths with HF listed as the primary diagnosis and only the first HF event recorded in the registers for each individual. Incident MI during follow-up was also assessed through the inpatient register.
Statistical analysis
Because some of the participants were missing data on body mass index (1.6%) and physical activity (22.1%), we used Markov chain Monte Carlo multiple imputation to simulate 5 complete datasets.14 All statistical analyses described, except for the penalized spline, were performed in each of the datasets separately. The results were averaged, and confidence intervals and p-values were calculated accounting for the uncertainty in the imputed estimates.14 Results from the complete case analyses were similar, though with wider confidence intervals; only the multiple imputation analyses are presented.
We calculated correlation coefficients between the DASH diet scores, and the mean food group and nutrient intake by approximate quartiles of each of the scores. We computed means and percentages of demographic, behavioral, and health covariates. We used Cox proportional hazards models that allowed the baseline rate to vary by age to calculate incidence rate ratios (RR) and associated 95% confidence intervals (CI).15 We examined the RR of HF associated with quartiles of each of the DASH scores. We tested for linear trend by entering the median value in each quartile as a continuous predictor. We adjusted for physical activity (linear), energy intake (linear), education (less than high school, high school, university), family history of myocardial infarction at less than 60 years (yes, no), cigarette smoking (current, past, never), postmenopausal hormone use (yes, no), living alone (yes, no), self-reported history of hypertension and high cholesterol, body mass index (linear), and incident MI as a time varying covariate (no MI, MI within one year, and more distant history of MI).
We examined the shape of the association between the DASH diet scores and incidence of HF using penalized splines.16, 17 Details of the spline analysis are as follows: the penalized spline model had 10 knots with piecewise cubic functions constrained to have approximately 3 degrees of freedom. We examined the associations between the DASH scores and HF by self-reported history of hypertension, self-reported history of high cholesterol, current smoking, and overweight (body mass index ≥ 25 kg/m2) and performed formal tests of interaction. In a sensitivity analysis, the outcome was defined as HF without preceding MI; women who experienced an MI were censored. We tested for violations of the proportional hazards assumption by entering the product of the DASH diet score and the natural logarithm of time in the model. The proportional hazards assumption did not appear to be violated in any of the models.
Statistical analyses were performed using SAS version 9.1 (Cary, NC) and R version 2.6 (Vienna, Austria). A 2-sided p-value < 0.05 was considered statistically significant.
RESULTS
The DASH diet scores were moderately correlated with each other (r = 0.44-0.68) (Table 1). Women in the top quartile of the DASH component score ate an average of 3.0 servings of fruit, 3.5 servings of vegetables, 5.1 servings of whole grains, 1.6 servings of low-fat dairy, 0.1 servings of sweetened beverages, and 0.8 servings of red meat or processed meat per day. In comparison, women in the bottom quartile of the score ate an average of 1.4 servings of fruit, 1.8 servings of vegetables, 3.3 servings of whole grains, 0.6 servings of low-fat dairy, 0.4 servings of sweetened beverages, and 1.3 servings of red or processed meat per day. For all of the DASH scores, higher scores appeared to be associated with food and nutrient intake patterns that were closer to the DASH diet than those with lower scores.
Table 1.
DASH diet scores, nutrient intake, and food intake
| DASH component score |
Food and nutrient recommendations |
Food recommendations |
Nutrient recommendations |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Correlations with other scores | |||||||||
| DASH component score | 1 | ||||||||
| Food and nutrient recommendations | 0.60 | 1 | |||||||
| Food recommendations | 0.57 | 0.44 | 1 | ||||||
| Nutrient recommendations | 0.61 | 0.68 | 0.46 | 1 | |||||
|
Mean nutrient intake in lowest/highest quartilea |
Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | |
| Total fat, g/d | 64.4 | 54.4 | 68.0 | 50.5 | 64.1 | 54.8 | 68.7 | 49.8 | |
| Saturated fat, g/d | 29.8 | 24.4 | 31.7 | 22.2 | 30.1 | 24.2 | 32.3 | 21.7 | |
| Protein, g/d | 67.9 | 72.6 | 71.2 | 69.9 | 64.7 | 76.6 | 70.6 | 70.1 | |
| Carbohydrate, g/d | 203 | 221 | 192 | 232 | 208 | 215 | 191 | 233 | |
| Cholesterol, mg/d | 254 | 209 | 267 | 197 | 233 | 231 | 272 | 188 | |
| Sodium, mg/d | 2,542 | 2,496 | 2,572 | 2,491 | 2,378 | 2,686 | 2,566 | 2,471 | |
| Potassium, mg/d | 2,723 | 3,413 | 2,761 | 3,448 | 2,652 | 3,493 | 2,662 | 3,516 | |
| Calcium, mg/d | 963 | 1,104 | 1,039 | 1,035 | 963 | 1,104 | 1,004 | 1,063 | |
| Magnesium, mg/d | 288 | 350 | 289 | 354 | 289 | 350 | 283 | 358 | |
| Fiber, g/d | 18.0 | 26.3 | 17.8 | 27.2 | 19.1 | 25.5 | 17.9 | 26.8 | |
| Alcohol, g/d | 4.1 | 4.2 | 4.3 | 4.0 | 3.9 | 4.4 | 4.0 | 4.3 | |
|
Mean food group intake in lowest/highest quartile |
Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | Q1 | Q4 | |
| Fruit (servings/d) | 1.4 | 3.0 | 1.5 | 3.0 | 1.4 | 3.0 | 1.2 | 3.1 | |
| Vegetables (servings/d) | 1.8 | 3.5 | 2.0 | 3.4 | 1.6 | 3.7 | 1.8 | 3.4 | |
| Whole grain (servings/d) | 3.3 | 5.1 | 3.2 | 5.2 | 4.2 | 4.4 | 2.9 | 5.4 | |
| Low-fat dairy (servings/d) | 0.6 | 1.6 | 0.8 | 1.4 | 0.6 | 1.6 | 0.6 | 1.7 | |
| Sweetened beverages (servings/d) | 0.4 | 0.1 | 0.2 | 0.2 | 0.4 | 0.1 | 0.2 | 0.2 | |
| Red or processed meat (servings/d) | 1.3 | 0.8 | 1.3 | 0.8 | 1.0 | 1.1 | 1.1 | 0.9 | |
Nutrients (except alcohol) were adjusted for energy intake using the residuals method.
Over 7 years of follow-up, 443 of 36,019 women developed HF (415 hospitalizations and 28 deaths with HF as the primary diagnosis), corresponding to a rate of 18.1 per 10,000 women per year. Participants who developed HF tended to be older and to have higher body mass index, were more likely to be current smokers, and to have a history of hypertension and high cholesterol (Table 2).
Table 2.
Characteristics of study participants by heart failure incidence
| Incident heart failure | No heart failure | ||
|---|---|---|---|
| N | 443 | 35,576 | |
| Age, ya | 73.4 (7.2) | 61.4 (9.1) | |
| Body mass index, kg/m2 a | 25.2 (4.3) | 25.0 (3.9) | |
| History of hypertension (%) | 189 (42.7) | 6,999 (19.7) | |
| History of high cholesterol (%) | 44 (9.9) | 2,799 (7.9) | |
| Family history of myocardial infarction at < 60 y (%) |
76 (17.2) | 4,816 (13.5) | |
| Physical activity (Met-hr/d)a | 41.0 (5.2) | 42.5 (4.8) | |
| Education (%) | |||
| Less than high school | 397 (89.6) | 26,096 (73.4) | |
| High school | 17 (3.8) | 2,853 (8.0) | |
| University | 29 (6.6) | 6,627 (18.6) | |
| Cigarette smoking (%) | |||
| Current | 94 (21.2) | 8,126 (22.8) | |
| Past | 69 (15.6) | 8,091 (22.7) | |
| Never | 280 (63.2) | 19,359 (54.4) | |
| Postmenopausal hormone use (%) | 182 (41.1) | 17,657 (49.6) | |
| Living alone (%) | 188 (42.4) | 8,207 (23.1) | |
| DASH diet scoresa | |||
| DASH component score | 23.9 (4.1) | 24.9 (4.1) | |
| Food and nutrient recommendations | 4.9 (1.3) | 4.9 (1.2) | |
| Food recommendations | 5.2 (1.0) | 5.4 (1.0) | |
| Nutrient recommendations | 7.1 (0.8) | 7.2 (0.8) | |
Mean (standard deviation)
After control for potential confounders, the score based on ranking participants intake of DASH diet components was significantly associated with a 37% lower incidence of HF (RR comparing top to bottom quartiles = 0.63, 95% CI 0.48-0.81, p for linear trend <0.001) (Table 3). The women with scores in the upper 10% had half the rate of HF as the women with scores in the lowest quartile (RR = 0.49, 95% CI 0.34-0.71). The association appeared to be linear across the score range (p for linear trend < 0.001, p for deviation from linearity = 0.97) (Figure 1). The other scores also appeared to be associated with lower rates of HF, though the food and nutrient recommendation score did not reach statistical significance. We did not find evidence for deviation from linearity for any of the scores.
Table 3.
Association of DASH scores with incident heart failure
| DASH Scores |
||||||
|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | Pa | ||
| DASH component score | ||||||
| Range | 9-22 | 23-24 | 25-27 | 28-39 | ||
| Cases | 162 | 87 | 105 | 89 | ||
| Person-years | 68,296 | 44,213 | 66,395 | 66,231 | ||
| Model 1 RR (95% CI)b | 1.00 (Reference) | 0.81 (0.63-1.06) | 0.67 (0.53-0.86) | 0.59 (0.46-0.77) | <0.001 | |
| Model 2 RR (95% CI)c | 1.00 (Reference) | 0.85 (0.66-1.11) | 0.69 (0.54-0.88) | 0.63 (0.48-0.81) | <0.001 | |
|
Food and nutrient recommendations |
||||||
| Range | 0.5-4.0 | 4.5-4.5 | 5.0-5.5 | 6.0-10.5 | ||
| Cases | 138 | 73 | 126 | 106 | ||
| Person-years | 69,565 | 38,495 | 77,090 | 59,983 | ||
| Model 1 RR (95% CI)b | 1.00 (Reference) | 0.89 (0.67-1.19) | 0.74 (0.58-0.94) | 0.80 (0.62-1.03) | 0.06 | |
| Model 2 RR (95% CI)c | 1.00 (Reference) | 0.92 (0.69-1.23) | 0.76 (0.60-0.97) | 0.83 (0.64-1.08) | 0.12 | |
| Food recommendations | ||||||
| Range | 2.0-4.7 | 4.8-5.4 | 5.5-6.1 | 6.2-9.5 | ||
| Cases | 157 | 100 | 105 | 81 | ||
| Person-years | 60,666 | 61,308 | 61,453 | 61,708 | ||
| Model 1 RR (95% CI)b | 1.00 (Reference) | 0.71 (0.55-0.92) | 0.78 (0.61-1.00) | 0.69 (0.53-0.90) | 0.008 | |
| Model 2 RR (95% CI)c | 1.00 (Reference) | 0.70 (0.55-0.91) | 0.76 (0.59-0.98) | 0.69 (0.52-0.90) | 0.007 | |
| Nutrient recommendations | ||||||
| Range | 2.3-6.8 | 6.9-7.3 | 7.4-7.7 | 7.8-9.9 | ||
| Cases | 134 | 128 | 106 | 75 | ||
| Person-years | 60,809 | 61,080 | 61,484 | 61,762 | ||
| Model 1 RR (95% CI)b | 1.00 (Reference) | 1.05 (0.82-1.34) | 0.87 (0.67-1.12) | 0.67 (0.51-0.90) | 0.007 | |
| Model 2 RR (95% CI)c | 1.00 (Reference) | 1.10 (0.86-1.40) | 0.91 (0.70-1.18) | 0.69 (0.51-0.93) | 0.02 | |
P for linear trend
Model 1: Cox proportional hazards models with baseline hazard allowed to vary by age.
Model 2: Model 1 additionally adjusted for physical activity (linear), energy intake (linear), education (less than high school, high school, university), family history of myocardial infarction at less than 60 years (yes, no), cigarette smoking (current, past, never), living alone (yes, no), postmenopausal hormone use (yes, no), self-reported history of hypertension and high cholesterol, body mass index (linear), and incident myocardial infarction (time varying: no myocardial infarction, myocardial infarction in the previous year, more distant history of myocardial infarction).
Figure 1.
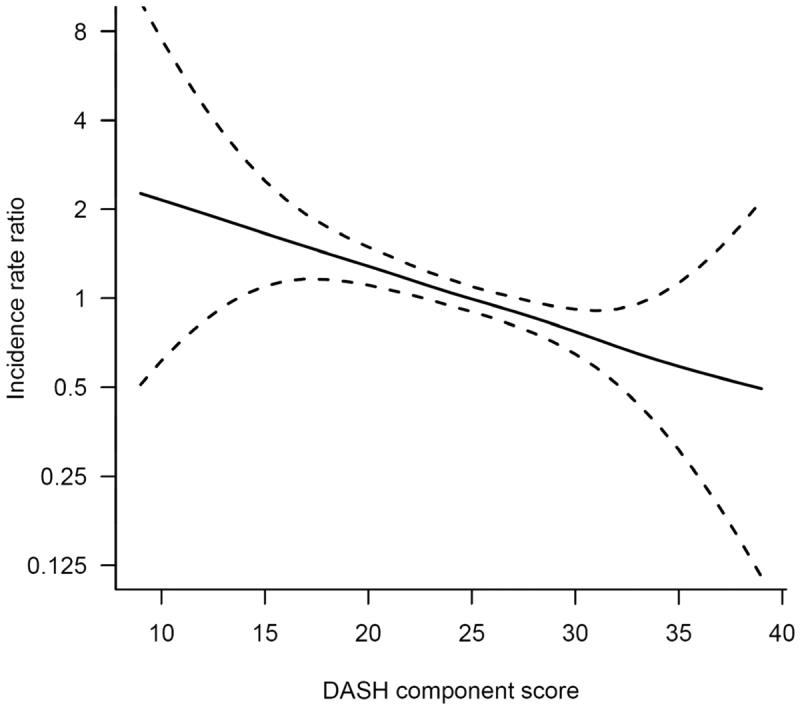
The solid line represents the incidence rate ratio of heart failure and dashed lines represent 95% confidence intervals. Penalized cubic splines with 3 degrees of freedom were used to flexibly model the shape of the association. Cox proportional hazards models with baseline hazard allowed to vary by age and additionally adjusted for physical activity (linear), energy intake (linear), education (less than high school, high school, university), family history of myocardial infarction at less than 60 years (yes, no), cigarette smoking (current, past, never), living alone (yes, no), postmenopausal hormone use (yes, no), self-reported history of hypertension and high cholesterol, body mass index (linear), and incident myocardial infarction (time varying: no myocardial infarction, myocardial infarction in the previous year, more distant history of myocardial infarction).
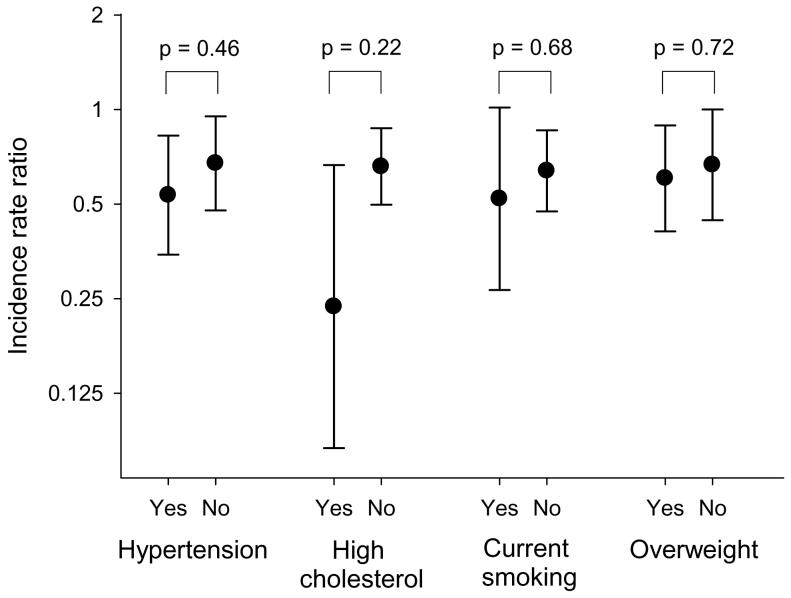
Although the association between the DASH component score and incidence of HF appeared stronger in those who reported hypertension, high cholesterol, or current cigarette smoking, the interactions were not statistically significant (Figure 2). There were 387 cases of HF that were not preceded by a recognized MI. When women were censored at the time of MI, the RR comparing top to bottom quartiles of the DASH component score was 0.60 (95% CI 0.45-0.80, p for linear trend <0.001).
Figure 2.
The circles represent the incidence rate ratios of heart failure comparing the top to bottom quartiles of the DASH component score, and lines represent 95% confidence intervals. P-values are for tests of the difference in estimates between those with and without self-reported hypertension, self-reported high cholesterol, current cigarette smoking, and body mass index ≥ 25 kg/m2. Cox proportional hazards models with baseline hazard allowed to vary by age and additionally adjusted for physical activity (linear), energy intake (linear), education (less than high school, high school, university), family history of myocardial infarction at less than 60 years (yes, no), cigarette smoking (current, past, never), living alone (yes, no), postmenopausal hormone use (yes, no), self-reported history of hypertension and high cholesterol, body mass index (linear), and incident myocardial infarction (time varying: no myocardial infarction, myocardial infarction in the previous year, more distant history of myocardial infarction).
COMMENT
We used 4 different approaches to create diet scores to assess consistency with the DASH diet. We found a 37% lower rate of HF in the 25% of women whose diets were closest to the DASH diet as assessed by ranking intake of key components of the diet; a similar pattern was observed with the other scores.
Although the association between the DASH diet and HF is not known, several observational studies have been conducted examining other cardiovascular outcomes. Fung and colleagues found statistically significant associations between a DASH diet score and incidence of coronary heart disease and stroke.7 Folsom and colleagues found that a different DASH diet score was not statistically significantly associated with incident hypertension, coronary heart disease mortality, stroke mortality, or cardiovascular mortality, though there was a trend towards decreased risk.8 In a cross-sectional analysis, people in the top quartile of a DASH diet score that measured intake of fruits, vegetables, and dairy had 1.5 mmHg lower systolic blood pressure and 1.4 mmHg lower diastolic blood pressure.18 After a mean of 5.4 years of follow-up, the blood pressure of those in the top quartile of the DASH score had increased less than those with lower DASH scores. The PREMIER trial of multiple lifestyle interventions showed that a DASH adherence index based on intake of fruits and vegetables, dairy, and percent of calories from saturated fat was associated with lower systolic blood pressure, but the association was not statistically significant after control for weight change.19 In a nationally representative study of US adults, a nutrient-based DASH score was associated with older age, a diagnosis of diabetes, and higher education.20
In this study, the DASH score based on ranking components of diet was a stronger predictor of HF than the other scores that were based on absolute intake. A likely explanation for this is that the food-frequency questionnaire methodology is designed to rank individuals' dietary intake rather than determine absolute intake.21 Although studies of cardiovascular outcomes in the Swedish Mammography Cohort are limited, a healthy dietary pattern constructed using factor analysis and characterized by high intake of fruit, vegetables, whole grains, and legumes, like the DASH diet, was associated with reduced rate of MI.22
Research into diet and HF has been understudied to date;23 however, the relationship between several components of the DASH diet and HF have been investigated in human and animal studies. In prospective studies of free-living individuals, daily consumption of whole grain breakfast cereals was been associated with 30% lower rate of HF compared to no consumption,24 while consumption of eggs more than twice per day was associated with a 64% higher rate of HF 25 and 100 mmol/d higher sodium consumption was associated with a 26% higher rate of HF,26 though nut consumption27 was not associated with rate of HF. In rat models of heart failure, macronutrient intake modified the course of cardiac dysfunction. High fat diets reduced cardiac remodeling and contractile dysfunction, but animals fed high linoleic acid diets had longer survival than those fed high carbohydrate diets or high lard diets.28, 29 When high starch, high fructose, and high fat diets were compared, the animals on the high fructose diet had more cardiac remodeling and worse survival.30
In the DASH randomized trial of 459 adults with normal or modestly elevated blood pressure, 2 months of the DASH diet reduced mean systolic blood pressure by 5.5 mmHg and mean diastolic blood pressure by 3.0 mmHg.2 A second trial demonstrated that 1 month of sodium restriction lead to a further reduction in blood pressure.4 The DASH diet also reduced LDL cholesterol, which would tend to decrease risk of cardiovascular disease, but it decreased HDL cholesterol and may have increased triglycerides.3 Based data from the Framingham Heart Study,31 the 5.5 mmHg decrease in systolic blood pressure observed in the DASH trial might be expected to reduce the rate of HF by approximately 12%.
In addition to the blood-pressure effect, several potential beneficial physiologic effects of the DASH diet have been outlined including estrogenic effects of phytochemicals32, 33 and decreased oxidative stress.34 Overabundance of circulating fatty acids and glucose caused by poor diet quality may mediate the association between diet and HF through decreased mechanical efficiency of the heart, increased myocardial triglyceride content, cardiac hypertrophy and fibrosis, and perturbed mitochondrial function.23
Because the DASH diet is recommended to the public for the prevention and treatment of hypertension, a metric to measure adherence to this diet may be a useful in clinical practice. The scores presented in this study were derived from a semi-quantitative food-frequency questionnaires which may be too burdensome for routine clinical use. A shorter instrument may be more useful in this setting but would require validation.
The rate of HF hospitalization or death in this study (18.1 cases per 10,000 women per year) was similar to the age-standardized rate in Sweden as a whole (17.1 cases per 10,000 women per year).35 These rates were lower than those seen in Olmsted County, Minnesota, (28.9 cases per 10,000 women per year) or in the Cardiovascular Health Study (146 cases per 10,000 women per year); however, 44% of the cases in Olmsted County were not hospitalized at the time of diagnosis and the Cardiovascular Health Study participants were all ≥ 65 years old (mean 73 years old) at enrollment.36, 37 In this study, rates of HF were higher in women with major risk factors for HF including older age, cigarette smoking, and hypertension, as expected.
There are several important limitations of this study. HF is a heterogeneous syndrome, and risk factors may not be identical for all HF subtypes.38 We were not able to determine HF etiology or subtype. Although Swedish inpatient and cause-of-death registers are almost complete and the accuracy of HF diagnosis has been shown to be high,13, 39 the registers only capture cases of HF that result in hospitalization or death. Therefore, our results may not be generalizable to less severe cases of HF treated exclusively on an outpatient basis. In addition, this study did not include populations with known high rates of hypertensive heart disease and HF, such as African Americans.1 Hypertension was measured by self-report, which is inherently less reliable than clinical measurement. The DASH diet scores have not been validated against an external standard; however, the food-frequency questionnaire used in this population has been validated against diet records and 24-hour recalls.40 Correlations between food frequency questionnaires and diet records were 0.5-0.7 for fruit items,41 0.4-0.6 for vegetable items,41 0.5-0.7 for whole grain items,42 0.4-0.6 for dairy items,43 and 0.6 for sweetened beverages.44 Using food-frequency questionnaires resulted in some exposure misclassification. If the misclassification of diet was unrelated to HF incidence, the results would likely be biased towards the null. However, this assumption was not verifiable with available data.
In summary, greater consistency with the DASH diet as measured using food-frequency questionnaires was associated with lower rates HF in middle-aged and elderly women living in Sweden.
ACKNOWLEDGEMENTS
Funding/Support: Grants from the Swedish Research Council/Committee for infrastructure supplied funding for maintenance of the cohort. Dr. Levitan was supported by a grant from the Swedish Foundation for International Cooperation in Research and Higher Education (STINT) and National Institutes of Health grant F32 HL091683.
Role of the Sponsor: The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Presented in poster form at the 2008 Annual Conference on Cardiovascular Disease Epidemiology and Prevention of the American Heart Association, Colorado Springs, CO, March 12, 2008.
Author Contributions: Dr. Wolk had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Levitan, Wolk, Mittleman
Acquisition of data: Wolk
Analysis and interpretation of data: Levitan, Wolk, Mittleman
Drafting of the manuscript: Levitan
Critical revision of the manuscript for important intellectual content: Wolk, Mittleman
Statistical analysis: Levitan, Wolk, Mittleman
Obtained funding: Levitan, Wolk, Mittleman
Study supervision: Wolk, Mittleman
Financial Disclosures: The authors have no relevant financial disclosures.
REFERENCES
- 1.Schocken DD, Benjamin EJ, Fonarow GC, et al. Prevention of heart failure: a scientific statement from the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117:2544–2565. doi: 10.1161/CIRCULATIONAHA.107.188965. [DOI] [PubMed] [Google Scholar]
- 2.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 3.Obarzanek E, Sacks FM, Vollmer WM, et al. Effects on blood lipids of a blood pressure-lowering diet: the Dietary Approaches to Stop Hypertension (DASH) Trial. Am J Clin Nutr. 2001;74:80–89. doi: 10.1093/ajcn/74.1.80. [DOI] [PubMed] [Google Scholar]
- 4.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 5.Karanja NM, Obarzanek E, Lin PH, et al. Descriptive characteristics of the dietary patterns used in the Dietary Approaches to Stop Hypertension Trial. DASH Collaborative Research Group. J Am Diet Assoc. 1999;99(8 Suppl):S19–27. doi: 10.1016/s0002-8223(99)00412-5. [DOI] [PubMed] [Google Scholar]
- 6.Your Guide to Lowering Your Blood Pressure with DASH: U.S. Department of Health and Human Services . National Institutes of Health. National Heart, Lung, and Blood Institute; 2006. [Google Scholar]
- 7.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168:713–720. doi: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 8.Folsom AR, Parker ED, Harnack LJ. Degree of Concordance With DASH Diet Guidelines and Incidence of Hypertension and Fatal Cardiovascular Disease. Am J Hypertens. 2007;20:225–232. doi: 10.1016/j.amjhyper.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolk A, Larsson SC, Johansson JE, Ekman P. Long-term fatty fish consumption and renal cell carcinoma incidence in women. JAMA. 2006;296:1371–1376. doi: 10.1001/jama.296.11.1371. [DOI] [PubMed] [Google Scholar]
- 10.Pennington JAT. Bowes and Church's Food Values of Portions Commonly Used. 15 ed. Harper & Row; New York: 1989. [Google Scholar]
- 11.Bergström L, Kylberg E, Hagman U, Erikson H, Bruce Å. The food composition database KOST: the National Administration's information system for nutritive values of food. Vår Föda. 1991;43:439–447. [Google Scholar]
- 12.The Nation Board of Health and Welfare . The Swedish Hospital Discharge Registry 1964-2003. Stockholm: 2005. [Google Scholar]
- 13.Ingelsson E, Arnlov J, Sundstrom J, Lind L. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail. 2005;7:787–791. doi: 10.1016/j.ejheart.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Schafer JL. Analysis of Incomplete Multivariate Data. CRC Press; Boca Raton: 1997. [Google Scholar]
- 15.Collett D. Modelling survival data in medical research. 2 ed. Chapman & Hall/CRC; Boca Raton: 2003. [Google Scholar]
- 16.Eilers PHC, Marx BD. Flexible smoothing with B-splines and penalties. Stat Sci. 1996;11:89–102. [Google Scholar]
- 17.Thurston SW, Eisen EA, Schwartz J. Smoothing in survival models: an application to workers exposed to metalworking fluids. Epidemiology. 2002;13:685–692. doi: 10.1097/00001648-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Dauchet L, Kesse-Guyot E, Czernichow S, et al. Dietary patterns and blood pressure change over 5-y follow-up in the SU.VI.MAX cohort. Am J Clin Nutr. 2007;85:1650–1656. doi: 10.1093/ajcn/85.6.1650. [DOI] [PubMed] [Google Scholar]
- 19.Obarzanek E, Vollmer WM, Lin PH, et al. Effects of individual components of multiple behavior changes: the PREMIER trial. Am J Health Behav. 2007;31:545–560. doi: 10.5555/ajhb.2007.31.5.545. [DOI] [PubMed] [Google Scholar]
- 20.Mellen PB, Gao SK, Vitolins MZ, Goff DC., Jr Deteriorating Dietary Habits Among Adults With Hypertension: DASH Dietary Accordance, NHANES 1988-1994 and 1999-2004. Arch Intern Med. 2008;168:308–314. doi: 10.1001/archinternmed.2007.119. [DOI] [PubMed] [Google Scholar]
- 21.Willett WC. Nutritional Epidemiology. 2 ed. Oxford University Press; New York: 1998. [Google Scholar]
- 22.Akesson A, Weismayer C, Newby PK, Wolk A. Combined effect of low-risk dietary and lifestyle behaviors in primary prevention of myocardial infarction in women. Arch Intern Med. 2007;167:2122–2127. doi: 10.1001/archinte.167.19.2122. [DOI] [PubMed] [Google Scholar]
- 23.Chess DJ, Stanley WC. Role of diet and fuel overabundance in the development and progression of heart failure. Cardiovasc Res. 2008;79:269–278. doi: 10.1093/cvr/cvn074. [DOI] [PubMed] [Google Scholar]
- 24.Djousse L, Gaziano JM. Breakfast cereals and risk of heart failure in the Physicians' Health Study I. Arch Intern Med. 2007;167:2080–2085. doi: 10.1001/archinte.167.19.2080. [DOI] [PubMed] [Google Scholar]
- 25.Djousse L, Gaziano JM. Egg consumption and risk of heart failure in the Physicians' Health Study. Circulation. 2008;117:512–516. doi: 10.1161/CIRCULATIONAHA.107.734210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Dietary sodium intake and incidence of congestive heart failure in overweight US men and women: first National Health and Nutrition Examination Survey Epidemiologic Follow-up Study. Arch Intern Med. 2002;162:1619–1624. doi: 10.1001/archinte.162.14.1619. [DOI] [PubMed] [Google Scholar]
- 27.Djousse L, Rudich T, Gaziano JM. Nut consumption and risk of heart failure in the Physicians' Health Study I. Am J Clin Nutr. 2008;88:930–933. doi: 10.1093/ajcn/88.4.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chicco AJ, Sparagna GC, McCune SA, et al. Linoleate-rich high-fat diet decreases mortality in hypertensive heart failure rats compared with lard and low-fat diets. Hypertension. 2008;52:549–555. doi: 10.1161/HYPERTENSIONAHA.108.114264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okere IC, Chess DJ, McElfresh TA, et al. High-fat diet prevents cardiac hypertrophy and improves contractile function in the hypertensive dahl salt-sensitive rat. Clin Exp Pharmacol Physiol. 2005;32:825–831. doi: 10.1111/j.1440-1681.2005.04272.x. [DOI] [PubMed] [Google Scholar]
- 30.Sharma N, Okere IC, Duda MK, et al. High fructose diet increases mortality in hypertensive rats compared to a complex carbohydrate or high fat diet. Am J Hypertens. 2007;20:403–409. doi: 10.1016/j.amjhyper.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 31.Haider AW, Larson MG, Franklin SS, Levy D. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2003;138:10–16. doi: 10.7326/0003-4819-138-1-200301070-00006. [DOI] [PubMed] [Google Scholar]
- 32.Konhilas JP, Leinwand LA. The effects of biological sex and diet on the development of heart failure. Circulation. 2007;116:2747–2759. doi: 10.1161/CIRCULATIONAHA.106.672006. [DOI] [PubMed] [Google Scholar]
- 33.Most MM. Estimated phytochemical content of the dietary approaches to stop hypertension (DASH) diet is higher than in the Control Study Diet. J Am Diet Assoc. 2004;104:1725–1727. doi: 10.1016/j.jada.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Lopes HF, Martin KL, Nashar K, Morrow JD, Goodfriend TL, Egan BM. DASH diet lowers blood pressure and lipid-induced oxidative stress in obesity. Hypertension. 2003;41:422–430. doi: 10.1161/01.HYP.0000053450.19998.11. [DOI] [PubMed] [Google Scholar]
- 35.Schaufelberger M, Swedberg K, Koster M, Rosen M, Rosengren A. Decreasing one-year mortality and hospitalization rates for heart failure in Sweden; Data from the Swedish Hospital Discharge Registry 1988 to 2000. Eur Heart J. 2004;25:300–307. doi: 10.1016/j.ehj.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 36.Roger VL, Weston SA, Redfield MA, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 37.Gottdiener JS, Arnold AM, Aurigemma GP, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 38.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult--Summary Article. Circulation. 2005;112:1825–1852. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 39.McCullough PA, Philbin EF, Spertus JA, Kaatz S, Sandberg KR, Weaver WD. Confirmation of a heart failure epidemic: findings from the Resource Utilization Among Congestive Heart Failure (REACH) study. J Am Coll Cardiol. 2002;39:60–69. doi: 10.1016/s0735-1097(01)01700-4. [DOI] [PubMed] [Google Scholar]
- 40.Messerer M, Johansson SE, Wolk A. The validity of questionnaire-based micronutrient intake estimates is increased by including dietary supplement use in Swedish men. J Nutr. 2004;134:1800–1805. doi: 10.1093/jn/134.7.1800. [DOI] [PubMed] [Google Scholar]
- 41.Larsson SC, Hakansson N, Naslund I, Bergkvist L, Wolk A. Fruit and vegetable consumption in relation to pancreatic cancer risk: a prospective study. Cancer Epidemiol Biomarkers Prev. 2006;15:301–305. doi: 10.1158/1055-9965.EPI-05-0696. [DOI] [PubMed] [Google Scholar]
- 42.Larsson SC, Giovannucci E, Bergkvist L, Wolk A. Whole grain consumption and risk of colorectal cancer: a population-based cohort of 60,000 women. Br J Cancer. 2005;92:1803–1807. doi: 10.1038/sj.bjc.6602543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Larsson SC, Bergkvist L, Wolk A. Milk and lactose intakes and ovarian cancer risk in the Swedish Mammography Cohort. Am J Clin Nutr. 2004;80:1353–1357. doi: 10.1093/ajcn/80.5.1353. [DOI] [PubMed] [Google Scholar]
- 44.Larsson SC, Bergkvist L, Wolk A. Consumption of sugar and sugar-sweetened foods and the risk of pancreatic cancer in a prospective study. Am J Clin Nutr. 2006;84:1171–1176. doi: 10.1093/ajcn/84.5.1171. [DOI] [PubMed] [Google Scholar]