Abstract
A key cell type of the resident skin immune system is the dendritic cell, which in normal skin is located in two distinct microanatomical compartments: Langerhans cells (LC) mainly in the epidermis and dermal dendritic cells (DDC) in the dermis. Here, the lineage of dermal dendritic cells was investigated using monoclonal antibodies and immunohistology. We provide evidence that “dermal dendritic cells” comprise at least two major phenotypic populations of dendritic appearing cells: immature DC expressing CD1, CD11c and CD208, and macrophages expressing CD209, CD206, CD163 and CD68. These data suggest that dermal dendritic-appearing macrophages comprise a novel part of the innate immune response in the resident skin immune system.
Introduction
The resident skin immune system functions to defend the host against external threats including microbial pathogens and allergens, as well as physical assaults including trauma and ultraviolet light. The innate immune response is rapid, involving cells expressing germ-line encoded pattern recognition receptors, and has a role in directly dealing with the threat by triggering microbicidal mechanisms, inflammation and/or apoptosis. Cells of the innate immune response include keratinocytes, endothelial cells, Langerhans cells and dermal dendritic cells (DDC). In contrast, the adaptive immune response is slower in onset, involves receptors clonally selected after gene rearrangement, results in immunologic memory and is mediated by T and B lymphocytes.
A major skin resident innate immune cell subset is the “dermal dendritic cell”. Initially, DDC were identified using a polyclonal antibody against clotting factor XIIIa and thought to be a homogeneous population (Cerio et al., 1988; Cerio et al., 1989). As such, they have been isolated from skin using various markers and used in functional assays (Duraiswamy et al., 1994; Meunier et al., 1993; Nestle et al., 1993a; Tse and Cooper, 1990). Since DDC are classified as dendritic cells (DC), they are presumed to be professional antigen presenting cells (APCs) that have been recognized as crucial regulators of cutaneous adaptive immune response in humans and mice (Dupasquier et al., 2004). However, the homogeneity and origin of these cells has also been questioned (Adany et al., 1985; Adany et al., 1988; Muszbek et al., 1985).
One of the problems in determining whether DDC comprise a single homogeneous population or represent two or more subsets has been the lack of specific markers. We recently demonstrated that activation of Toll-like receptors causes the rapid differentiation of distinct precursors of human peripheral monocytes into CD1b+ and CD209+ (DC-SIGN) cells (Krutzik et al., 2005). CD1b+ cells had an immature dendritic cell phenotype, release pro-inflammatory cytokines and function as efficient antigen presenting cells. The CD209+ (DC-SIGN) cells had a macrophage-like phenotype, were phagocytic, and utilized CD209to facilitate the uptake of bacteria. These data provide evidence that the dual host defense roles of the innate immune system are mediated by the induction of two distinct phenotypic and functional populations, CD1b+ dendritic cells and CD209+ (DC-SIGN) macrophages. The surprising finding was that CD209+ (DC-SIGN), which was previously thought to be a marker for dendritic cells (Engering et al., 2002; Geijtenbeek et al., 2000), was found on a population of macrophages in tonsil and inflamed skin. Given this observation, we sought to investigate the phenotype of various “DC” populations in the skin with monoclonal antibodies that discriminate against dendritic cells and macrophages, by using immunoperoxidase and confocal laser microscopy.
RESULTS
Identification of cells bearing dendritic cell markers in normal human skin
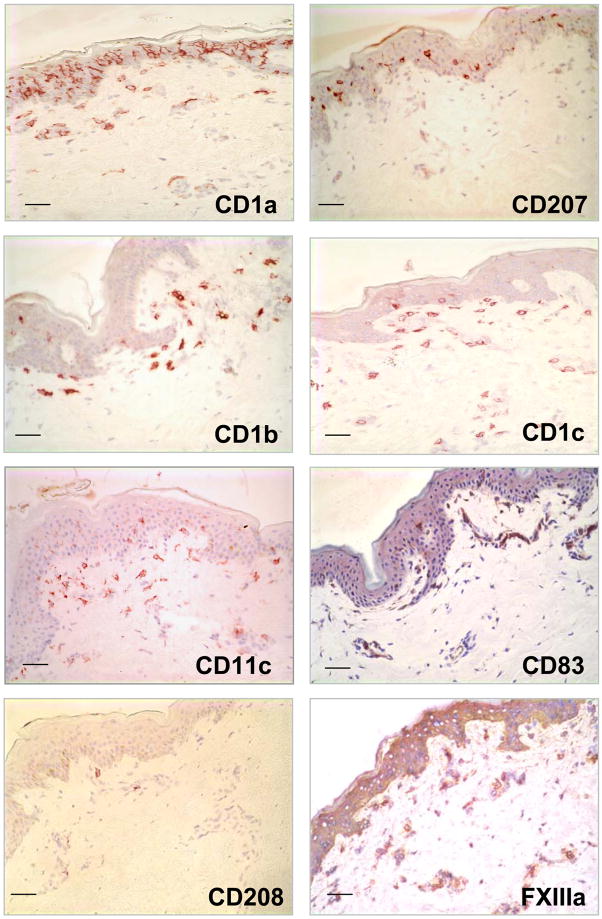
We investigated the in situ expression of different DC markers (Supplemental Table 1) in normal human skin tissue to determine their morphology, phenotype and distribution (Figure 1). Cells with a dendritic morphology were found in the epidermis and dermis. CD1a+ and CD207+ (Langerin) cells, presumably Langerhans cells, were located in the epidermis. Other DC markers including CD1b, CD1c and CD11c were found on dendritic-appearing cells mainly in the superior dermis. We also used markers for mature DC such as CD83 and CD208 (DC-LAMP), however very few DC expressing these markers could be identified in normal skin.
Figure 1. Identification of Dendritic cells in normal human skin.
Normal human skin tissue was labeled with monoclonal antibodies (mAbs) specific for CD207 (Langerin), CD1a, CD1b, CD1c, CD83, CD208 (DC-LAMP), CD11c using the immunoperoxidase method. Original magnification 200×. Scale bar: 50um
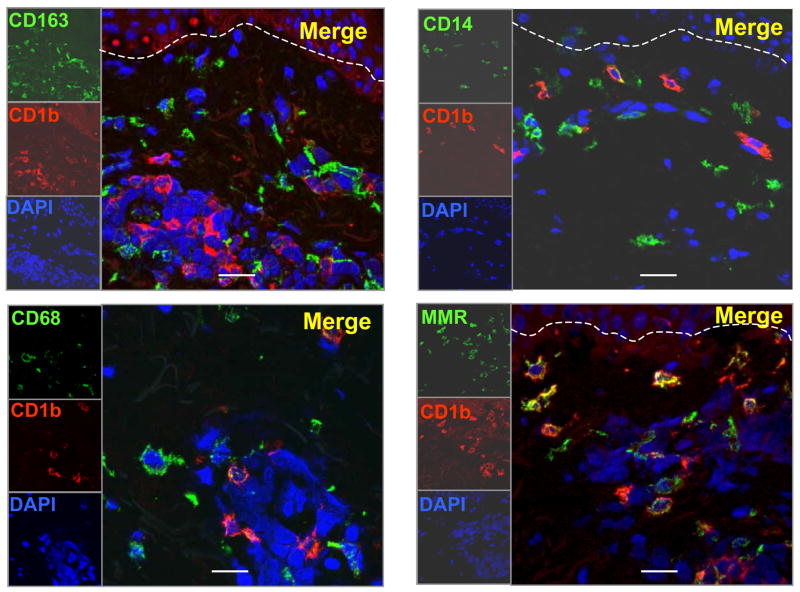
Identification of cells expressing monocyte/macrophage markers in normal human skin
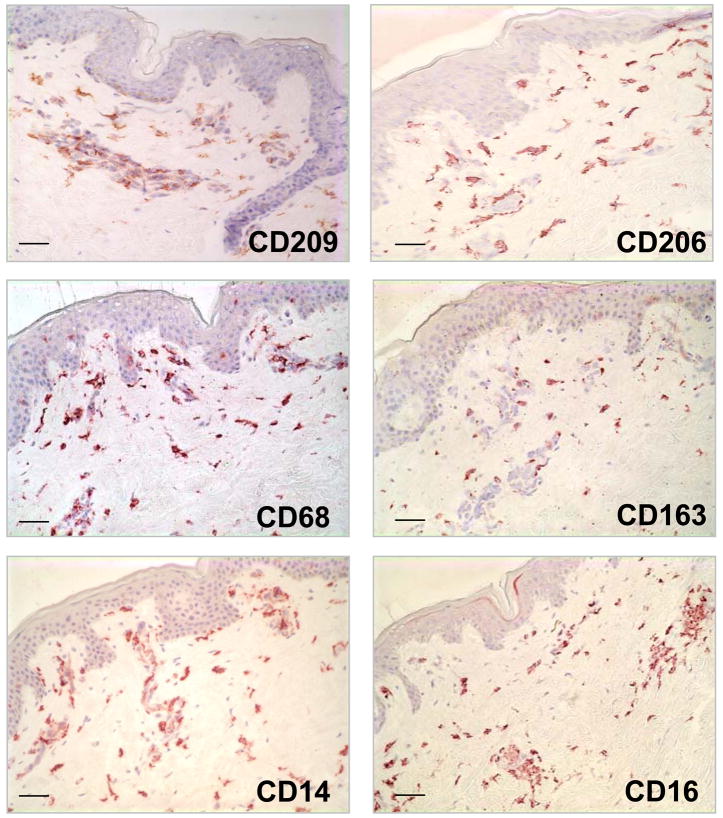
The highest level of expression (i.e. greatest frequency of cells) using characteristic monocyte/macrophage markers such as CD14, CD68 and CD163 (Supplemental Table 1) was detected in the dermis. Similar to the DC, cells bearing monocyte/macrophage markers were also characterized by a dendritic morphology and were located at the level of the capillaries and in the higher part of the reticular dermis. The distribution of CD209+ cells were similar to the distribution of cells expressing CD14, CD68, CD163 and CD206 (mannose receptor) (Figure 2).
Figure 2. Identification of Monocyte/Macrophage cells in normal human skin.
Normal human skin tissue was labeled with monoclonal antibodies (mAbs) specific for CD209 (DC-SIGN), CD206 (mannose receptor), CD163, CD14, CD16 and CD68) using the immunoperoxidase method. Original magnification 200×. Scale bar: 50um
Dendritic cells and macrophages in the dermis have similar morphology
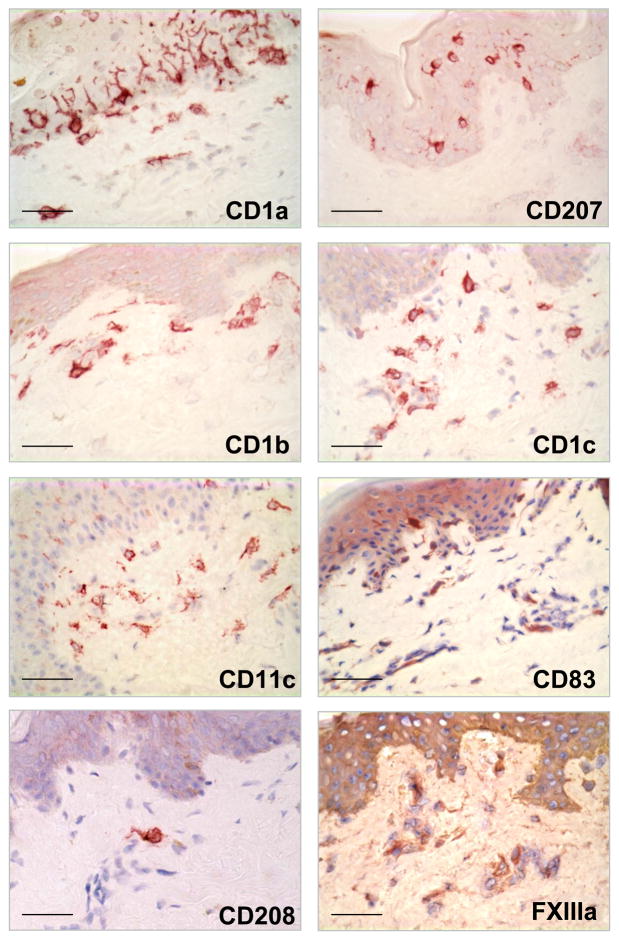
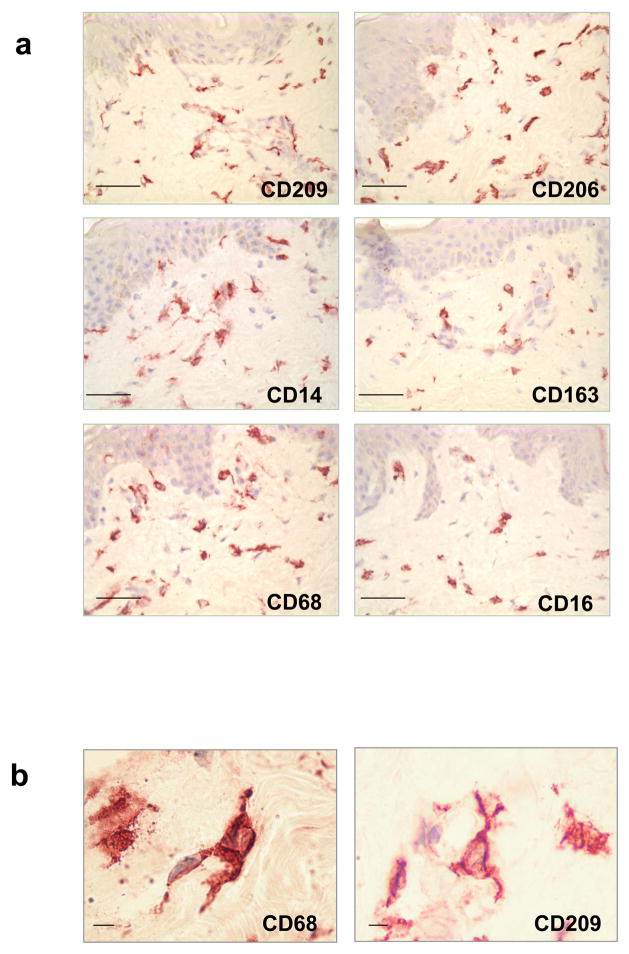
Although DC can be distinguished from monocytes/macrophages by their dendritic morphology, we found that cells expressing characteristic macrophage markers can also display a dendritic morphology (Figure 3 and 4a). The morphology of the macrophages in the papillary and reticular dermis ranged from elongated and spindled to compact and stellate-shaped. (Figure 4b)
Figure 3. Morphology and distribution of Dendritic cell markers in normal human skin.
Dendritic cell markers as CD207 (Langerin), CD1a, CD1b, CD1c, CD83, CD208 (DC-LAMP), CD11c were located in the epidermis and the superior dermis. Original magnification 400×. Scale bar: 50um
Figure 4. Morphology and distribution of Monocyte/Macrophage cell markers in normal human skin.
(a) Monocyte/Macrophage cell markers as CD209 (DC-SIGN), CD206 (mannose receptor), CD163, CD14, CD16 and CD68 were only located in the superior papillary and reticular dermis, not in the epidermis. Original magnification 400×. Scale bar: 50um. (b) Monocyte/Macrophage cells display dendritic morphology. Original magnification 1000×. Scale bar: 5um
Macrophage and dendritic cell markers are expressed on distinct subsets of cells in normal human skin
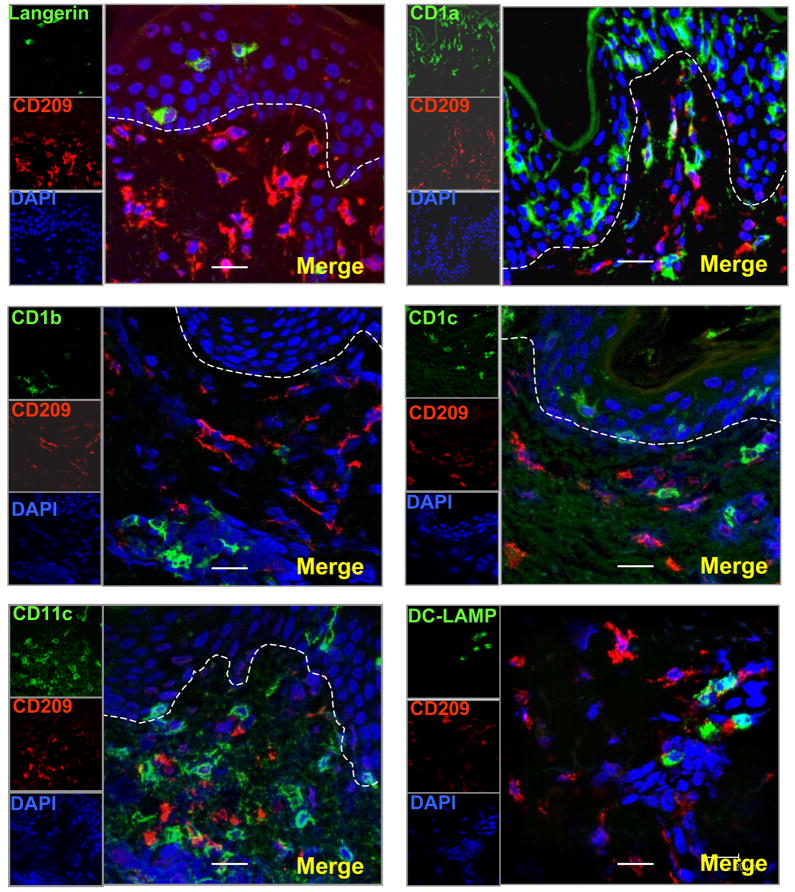
To determine the phenotype of CD209+ (DC-SIGN) cells in normal human skin tissue, frozen tissue sections were labelled with antibodies to CD209 (DC-SIGN) and paired with the different DC and macrophage markers. CD209+ (DC-SIGN) cells did not express CD207+ (Langerin), CD1a, CD1b, CD1c, CD11c, CD83 and CD208 (DC-LAMP). These data indicate that CD209+ (DC-SIGN) expression is not restricted to DCs in the skin (Fig. 5a). In addition CD1b+ cells did not express macrophage markers (CD163, CD68 and CD14) suggesting that CD1 expression is restricted to DCs in the skin (Fig. 5b).
Figure 5. CD209 +(DC-SIGN) cells and Dendritic cell markers are expressed on distinct subsets of cells in normal human skin.
(a) Normal human skin tissue sections were labeled with specific antibodies for DCs and visualized using confocal laser microscopy. CD209 positive cells (red images) did not co-localize with other DCs markers (CD207 (Langerin), CD1a, CD1b, CD1c, CD11c, CD208 (DC lamp)) (green images)). (b) CD1b+ cells did not express Monocyte/Macrophage markers (CD163, CD68, CD14). Nuclei were labeled with DAPI (blue images). Magnification 400×. Scale bar: 20um
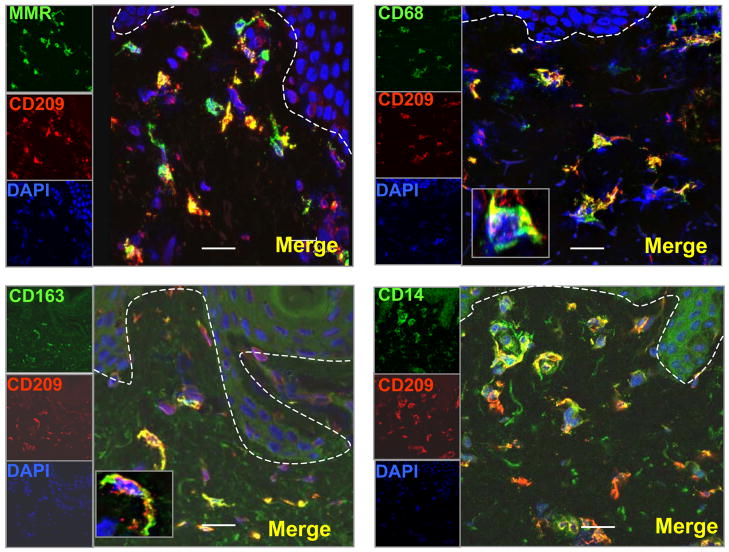
We reasoned that CD209+ (DC-SIGN) and other monocyte/macrophage markers might colocalize on cells in the dermis of normal human skin. Therefore the expression of CD209+ (DC-SIGN) and CD68, CD14, CD163 and CD206+ (macrophage mannose receptor or MMR) was examined in normal human skin tissue using immunofluorescence. A major population of dendritic appearing cells was located at the level of the capillaries and in the higher part of the reticular dermis and co-expressed CD209+ (DC-SIGN), CD206+ (macrophage mannose receptor or MMR) and the monocyte/macrophage markers CD14, CD163, CD68, but not DC markers (CD1a, CD1b, CD1c, CD11c, DC-LAMP, Langerin). (Fig 6).
Figure 6. CD209 + (DC-SIGN) cells and Monocyte/Macrophage cell markers are expressed on the same subsets of cells in normal human skin.
Normal human skin tissue sections were labeled with specific antibodies for Monocyte/Macrophage markers and visualized using confocal laser microscopy. CD209 (DC-SIGN) positive cells (red images) co-localize with other Monocyte/Macrophage markers (CD206 (mannose receptor), CD163, CD68, CD14) (green images). Nuclei were labeled with DAPI (blue images). Magnification 400×. Scale bar: 20um. The box in panel 2 and 3 indicates a magnified positive cell.
Therefore, we conclude that CD209+ (DC-SIGN) macrophages, expressing CD68, CD14, and CD163, markers typically found on monocytes/macrophages, comprise a major subset of dendritic-appearing dermal cells.
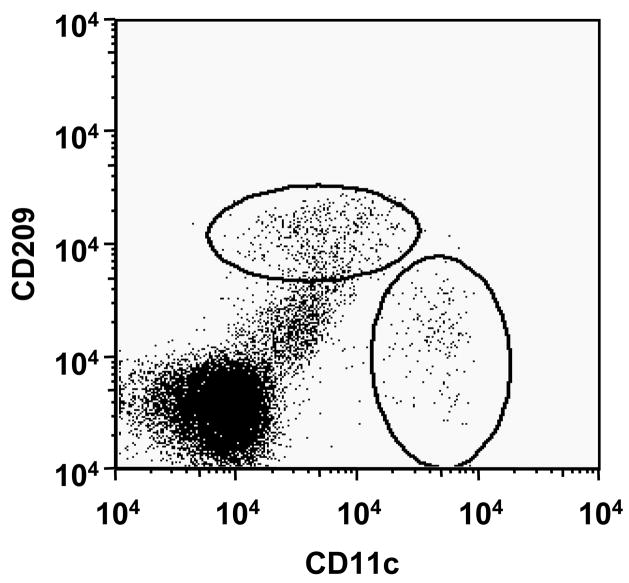
Isolated dermal cells from normal human skin contain CD209+CD11clo cells
To substantiate the immunohistologic identification of distinct populations of macrophages and DC, dermal cells were isolated from normal human skin and analyzed them directly by flow cytometry. Distinct populations of CD209+CD11clo and CD209-CD11chi+ cells were identified (Figure 7). These data were obtained by separating epidermis from dermis, then dispersing cells in the dermis using a scalpel, which resulted in a low yield of mononuclear cells. Treatment of the dermis with collagenase overnight as published (Zaba et al., 2007) resulted in loss of CD209 expression as did treatment of peripheral blood derived CD209+ cells (data not shown). The loss of CD209 expression may be due to dedifferentiation of macrophages during the collagenase treatment or the proteolytic digestion of the CD209 epitope. Therefore, additional technologic considerations will be required to obtain sufficient numbers of CD209+ cells from normal skin for functional study
Figure 7. Dermal cells isolated from normal human skin contain CD209 + CD11clo+ cells.
Distinct populations of CD209+CD11clo and CD209-CD11chi+ cells were identified in dermal single-cell suspensions from normal human skin.
Discussion
The resident skin immune system stands guard to deal with attacks from the outside environment including microbes, allergens and ultraviolet light. The epidermis serves as the initial barrier, however the resident immune cells in the dermis must serve to deal with host threats that either escape detection by the epidermis or enter via the cutaneous vasculature. The dermal immune system is known to involve circulating T cells that survey the skin for foreign antigens, but also a population of “dermal dendritic cells”. Here we provide evidence that “dermal dendritic cells” comprise at least two major phenotypic populations of dendritic appearing cells, immature DC that express CD1, CD11c and CD208, and macrophages that express CD209, CD206, CD163 and CD68. We conclude that dermal dendritic-appearing macrophages are a novel component of the skin immune system.
DDCs were initially identified and characterized using a polyclonal antibody that recognizes clotting factor XIIIa. This antibody was used to identify spindle or stellate-shaped cells in human connective tissue (Fear et al., 1984). Later on, Reid et al. called them fibroblasts (Reid et al., 1986). But it was Headington who in1986 named them “Dermal Dendrocytes” –or uncommitted stem cells – using a polyclonal antibody for FXIIIa (Cerio et al., 1989; Headington, 1986). Unfortunately, Factor XIIIa is not a specific marker, as it can also be expressed on several cell types, including endothelial cells (Suvarna and Cotton, 1993). However, in 1987, using macrophage markers such as RFD7, RFDR2 (HLA-DR), KiM7 (CD68) (Adany and Muszbek, 1989; Adany and Muszbek, 1987) suggested that these cells were tissue macrophages. Functionally, DDCs demonstrate features of both macrophages and dendritic cells. A key macrophage function of DDCs is their ability to efficiently phagocytose hemosiderin, melanin and bacteria (Altman et al., 1992; Filgueira et al., 1996; Nestle et al., 1998). A key dendritic cell function of DDCs is their ability to take up and process antigens, migrate to lymphatic organs and activate naive T cells (Nestle et al., 1993b; Nestle and Nickoloff, 1995). Our data indicate that in fact resident DDCs in normal skin are comprised of at least two phenotypically distinct populations of CD209+ macrophages and CD1+ DCs.
The CD209+ dermal dendritic-appearing macrophage population is also present in inflamed skin, as we reported in leprosy lesions (Krutzik et al., 2005). The phenotype of these dermal dendritic-appearing macrophages includes several markers of phagocytic function, CD209 (DC-SIGN), CD206 (macrophage mannose receptor) and CD163 (Figure 5). Both CD209 and CD206 are pattern recognition receptors; CD209 (DC-SIGN) is a C-type lectin that binds various glycoproteins and glycolipids (Ebner et al., 2004; Geijtenbeek et al., 2000). CD209 has been shown to facilitate the uptake of bacteria including Mycobacterium tuberculosis and viruses including HIV (Pohlmann et al., 2001). CD206 (macrophage mannose receptor) is known to facilitate uptake of microbial molecules containing a repeating mannose motif (Sallusto et al., 1995; Wollenberg et al., 2002). CD163 is the haptoglobin receptor, involved in the transport of iron into cells (Kristiansen et al., 2001). CD206 (mannose receptor) and CD11c (myeloid marker) can be expressed in the two populations –dendritic cells and macrophages- as we previously reported (Krutzik et al., 2005). Together, the co-expression of these molecules indicates that dermal dendritic-appearing macrophages are resident in normal skin, and are likely to recognize microbial pathogens via multiple pattern recognition receptors and phagocytize them. The present study points the way to devise strategies to isolate these cells from normal skin and study their function in vitro.
The function of dermal CD1+ DC, in terms of antigen presentation activity has been demonstrated in earlier studies (Meunier et al., 1993; Nestle et al., 1993a; Nestle et al., 1993b; Turville et al., 2002). It has also been suggested that human Langerhans cells are replenished by precursors in the dermis and that CD14+ dermal antigen presenting cells are the main precursor population (Larregina et al., 2001). Further examination of leprosy skin lesions using confocal laser microscopy revealed that the distribution and frequency of these resident skin populations may correlate with disease status (Krutzik et al., 2005). Within self-limited tuberculoid leprosy lesions, CD1a+ cells were frequent in the epidermis, and CD209(DC-SIGN) and CD1b are expressed on distinct, non-overlapping dermal cell populations. In contrast, within the progressive lepromatous leprosy lesions, the frequency of CD1a+ Langerhans cells and CD1b+ dermal dendritic cells was fewer. The CD209+ cells in tuberculoid leprosy lesions were found to express the monocyte/macrophage markers CD14, CD16, CD64 and CD68 but did not express the dendritic cells markers CD1a, CD1b, CD63, CD83, or DC-LAMP. These data indicate that the majority of CD209+ cells express cell surface markers typically found on monocytes/macrophages, and reflect the phenotype found here in normal skin. Future studies should be directed towards identifying the phenotype of CD209+ cells in other disease states.
The studies by Zaba et al (Zaba et al., 2007) support the presence of distinct populations of macrophages and dendritic cells in normal human dermis, but differ in identifying the specific phenotypic of the dermal macrophages. Our study clearly identifies a CD209+CD163+ macrophage population in normal skin that do not express characteristic DC markers CD1a, CD1b, CD1c and DC-LAMP, in addition to CD11c. The restricted expression of CD209 is of particular interest because it mediates the phagocytosis of a range of microbial pathogens. Zaba et al. demonstrated CD209 expression on both macrophage and dendritic cell populations, but utilized fluorescence microscopy for their analysis. Our studies used confocal microscopy, which has the capability to analyze thin optical sections that helps differentiate epitope colocalization from cell overlap.
The mechanism by which these dermal DC and macrophages differentiate from monocyte precursors is fundamental to understanding the resident skin immune system. We previously demonstrated that human peripheral monocytes activated with a Toll-like receptor 2/1 ligand were differentiated into two distinct subsets: CD209+ CD16+ macrophages and CD1b+ CD209− dendritic cells. CD209+ cells were expanded by TLR-mediated upregulation of interleukin (IL)-15, had a macrophage-like phenotype, were phagocytic, and used CD209+ to facilitate the uptake of bacteria. In contrast, CD1b+ DC were expanded by TLR-mediated upregulation of granulocyte-macrophage colony-stimulating factor (GM-CSF), promoted T cell activation and secreted pro-inflammatory cytokines. The mechanisms by which these dermal DC and dendritic appearing macrophages accumulate in normal skin and disease lesions, as well as their functional role in disease pathogenesis should provide new therapeutic targets for skin disease.
Materials and Methods
Human normal skin tissue
Human normal skin tissue (obtained after breast reduction and abdominal plastic surgery) from healthy volunteers was obtained from the UCLA Tissue Procurement & Histology Core Laboratory (HTLPC) at the UCLA School of Medicine. The acquisition of all skin biopsy specimens from human donors was reviewedand approved by the committees on investigations involving humansubjects of the University of California, Los Angeles and according to the Helsinki Guidelines.
Biopsy specimens were embedded in OCT medium (Ames Co., Elkhart, IN) and snap-frozen in liquid nitrogen. Sections (4μm thick) were acetone-fixed and kept frozen (−80°) until use.
Immunohistochemical studies of dendritic cells and monocytes/macrophages expression in human normal skin tissue
Antibodies used for immunohistochemical studies are shown in Table 1. Frozen tissue sections were blocked with normal horse serum before incubation with the monoclonal antibodies (mAbs) for 60 min, followed by incubation with biotinylated horse anti-mouse immunoglobulin G (IgG) for 30 min. Primary antibody was visualized using the ABC Elite system (Vector Laboratories, Burlingame, CA), which uses avidin and a biotin-peroxidase conjugate for signal amplification. ABC reagent was incubated for 30 min followed by the addition of substrate (3-amino-9-ethylcarbazole) for 10 min. Slides were counterstained with haematoxylin and mounted in crystal mounting medium (Biomeda, Foster City, CA).
Two-colour immunofluorescence staining of cryostat sections
Double immunofluorescence was performed by serially incubating cryostat tissue sections with mouse anti-human mAbs of different isotypes [e.g. O10 (anti-CD1a, IgG2a), and DCN46 (anti-CD209, IgG2b)], followed by incubation with isotype-specific, fluorochrome (A488) or (A568) -labelled goat anti-mouse immunoglobulin antibodies (Molecular Probes). Controls included staining with isotype-matched antibodies, as well as with CD1a or CD209, followed by secondary antibodies mismatched to the primary antibody isotype, to demonstrate the isotype specificity of the secondary labeled antibodies. Images were obtained using confocal laser microscopy. Nuclei was stained with 4′,6-diamidino-2-phenylindole (DAPI).
Confocal microscopy
Double immunofluorescence of normal skin sections was examined using a Leica-TCS-SP MP inverted single confocal laser-scanning and two-photon laser microscope (Heidelberg, Germany) fitted with DPSS-diode (561 nm), argon (488 nm) and two photon lasers (Spectra-Physics Millenia X 532 diode pump laser and Tsunami picosecond Ti-sapphire laser), at the Carol Moss Spivak Cell Imaging Facility in the UCLA Brain Research Institute. Sections were illuminated with 488 and 561 nm for red and green labels and Ti-sapphire laser set at 770 nm for DAPI excitation. Images of specimens with Alexa 488, or Alexa 568, (Molecular Probes) or DAPI were recorded sequentially through the spectral emission filters set from 500–550 nm for Alexa 488, from 580–700 nm for Alexa 568 and from 410–490 nm for DAPI. Pairs of single images were superimposed for co-localization analysis. All micrographs are compiled serial Z-stack images 1 μm apart.
Isolation of Dermal cells
Human normal skin was placed in cold RPMI and minced using two scalpels until single cells were obtained. Non-specific staining was blocked by incubating with non-specific mouse IgG for 10 minutes in FACS buffer (PBS 0.1% sodium azide and 2% FBS). Cells were then incubated with fluorescently-labeled primary antibodies (CD209-FITC and CD11c-PE) (BD Biosciences). Cells were washed two times before fixing in 1% paraformaldehyde. Cells were then acquired using a FACSCalibur (BD Biosciences) in the UCLA Flow Cytometry Core Facility. Data analysis was done using FlowJo software (Treestar).
Supplementary Material
Table 1. Antibodies used to evaluate dendritic cells (DC) and macrophage (MΦ) expression in normal human skin.
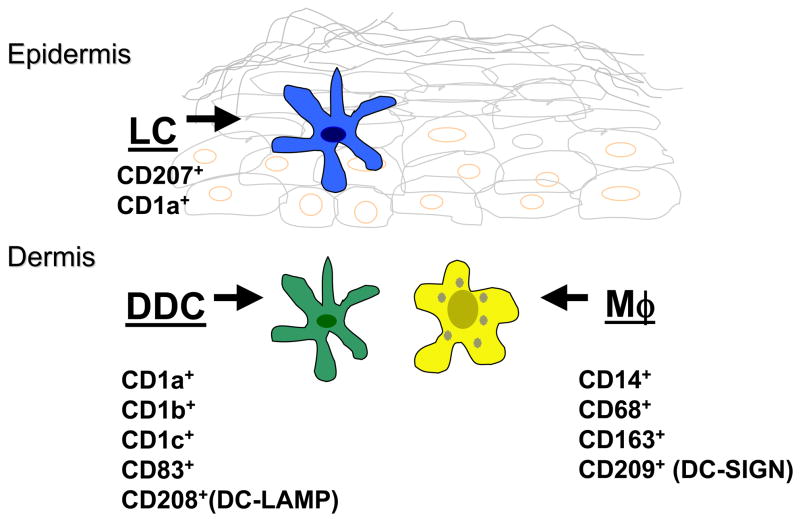
Figure 8. Subsets of Dendritic cells and Macrophages in normal human skin.
“Dermal dendritic cells” include at least two major populations of dendritic appearing cells: immature DC that express CD1and CD208, and macrophages that express CD209, CD163, CD14 and CD68.
Acknowledgments
We thank Dr. Matthew Schibler and the Carol Moss Spivak Cell Imaging Facility in the UCLA Brain Research Institute for the use of the confocal laser microscope, Ping Fu, Christopher Creencia, and Delia Adefuin at the UCLA Tissue Procurement & Histology Core Laboratory for providing normal skin and preparation of tissue sections, Dr. Jenny Kim, Jenny Phan and Dr. Peter Sieling for helpful technical assistance. We acknowledge the financial support received from the National Institutes of Health (AI 22553, AI 056489, AI 47868) to R. L. M
Footnotes
Conflict of Interest: The authors state no conflict of interest
Reference List
- Adany R, Belkin A, Vasilevskaya T, Muszbek L. Identification of blood coagulation factor XIII in human peritoneal macrophages. Eur J Cell Biol. 1985;38:171–173. [PubMed] [Google Scholar]
- Adany R, Glukhova MA, Kabakov AY, Muszbek L. Characterisation of connective tissue cells containing factor XIII subunit a. J Clin Pathol. 1988;41:49–56. doi: 10.1136/jcp.41.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adany R, Muszbek L. Immunohistochemical detection of factor XIII subunit a in histiocytes of human uterus. Histochemistry. 1989;91:169–174. doi: 10.1007/BF00492391. [DOI] [PubMed] [Google Scholar]
- Adany R, Muszbek L. Cells containing factor XIII subunit a in benign and soft tissue tumours. Histopathology. 1987;11:1341–1343. doi: 10.1111/j.1365-2559.1987.tb01878.x. [DOI] [PubMed] [Google Scholar]
- Altman DA, Fivenson DP, Lee MW. Minocycline hyperpigmentation: model for in situ phagocytic activity of factor XIIIa positive dermal dendrocytes. J Cutan Pathol. 1992;19:340–345. doi: 10.1111/j.1600-0560.1992.tb01372.x. [DOI] [PubMed] [Google Scholar]
- Behar SM, Porcelli SA, Beckman EM, Brenner MB. A pathway of costimulation that prevents anergy in CD28- T cells: B7-independent costimulation of CD1-restricted T cells. J Exp Med. 1995;182:2007–2018. doi: 10.1084/jem.182.6.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerio R, Griffiths CE, Cooper KD, Nickoloff BJ, Headington JT. Characterization of factor XIIIa positive dermal dendritic cells in normal and inflamed skin. Br J Dermatol. 1989;121:421–431. doi: 10.1111/j.1365-2133.1989.tb15509.x. [DOI] [PubMed] [Google Scholar]
- Cerio R, Spaull J, Jones EW. Identification of factor XIIIa in cutaneous tissue. Histopathology. 1988;13:362–364. doi: 10.1111/j.1365-2559.1988.tb02051.x. [DOI] [PubMed] [Google Scholar]
- Dupasquier M, Stoitzner P, van OA, Romani N, Leenen PJ. Macrophages and dendritic cells constitute a major subpopulation of cells in the mouse dermis. J Invest Dermatol. 2004;123:876–879. doi: 10.1111/j.0022-202X.2004.23427.x. [DOI] [PubMed] [Google Scholar]
- Duraiswamy N, Tse Y, Hammerberg C, Kang S, Cooper KD. Distinction of class II MHC+ Langerhans cell-like interstitial dendritic antigen-presenting cells in murine dermis from dermal macrophages. J Invest Dermatol. 1994;103:678–683. doi: 10.1111/1523-1747.ep12398513. [DOI] [PubMed] [Google Scholar]
- Ebner S, Ehammer Z, Holzmann S, Schwingshackl P, Forstner M, Stoitzner P, Huemer GM, Fritsch P, Romani N. Expression of C-type lectin receptors by subsets of dendritic cells in human skin. Int Immunol. 2004;16:877–887. doi: 10.1093/intimm/dxh088. [DOI] [PubMed] [Google Scholar]
- Engering A, Geijtenbeek TB, Van Vliet SJ, Wijers M, van Liempt E, Demaurex N, Lanzavecchia A, Fransen J, Figdor CG, Piguet V, Van Kooyk Y. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J Immunol. 2002;168:2118–2126. doi: 10.4049/jimmunol.168.5.2118. [DOI] [PubMed] [Google Scholar]
- Fear JD, Jackson P, Gray C, Miloszewski KJ, Losowsky MS. Localisation of factor XIII in human tissues using an immunoperoxidase technique. J Clin Pathol. 1984;37:560–563. doi: 10.1136/jcp.37.5.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filgueira L, Nestle FO, Rittig M, Joller HI, Groscurth P. Human dendritic cells phagocytose and process Borrelia burgdorferi. J Immunol. 1996;157:2998–3005. [PubMed] [Google Scholar]
- Geijtenbeek TB, Torensma R, Van Vliet SJ, van Duijnhoven GC, Adema GJ, Van Kooyk Y, Figdor CG. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575–585. doi: 10.1016/s0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- Headington JT. The dermal dendrocyte. Adv Dermatol. 1986;1:159–171. [PubMed] [Google Scholar]
- Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK, Moestrup SK. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- Krutzik SR, Tan B, Li H, Ochoa MT, Liu PT, Sharfstein SE, Graeber TG, Sieling PA, Liu YJ, Rea TH, Bloom BR, Modlin RL. TLR activation triggers the rapid differentiation of monocytes into macrophages and dendritic cells. Nat Med. 2005;11:653–660. doi: 10.1038/nm1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larregina AT, Morelli AE, Spencer LA, Logar AJ, Watkins SC, Thomson AW, Falo LD., Jr Dermal-resident CD14+ cells differentiate into Langerhans cells. Nat Immunol. 2001;2:1151–1158. doi: 10.1038/ni731. [DOI] [PubMed] [Google Scholar]
- Melian A, Geng YJ, Sukhova GK, Libby P, Porcelli SA. CD1 expression in human atherosclerosis. A potential mechanism for T cell activation by foam cells. Am J Pathol. 1999;155:775–786. doi: 10.1016/S0002-9440(10)65176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier L, Gonzalez-Ramos A, Cooper KD. Heterogeneous populations of class II MHC+ cells in human dermal cell suspensions. Identification of a small subset responsible for potent dermal antigen-presenting cell activity with features analogous to Langerhans cells. J Immunol. 1993;151:4067–4080. [PubMed] [Google Scholar]
- Muszbek L, Adany R, Szegedi G, Polgar J, Kavai M. Factor XIII of blood coagulation in human monocytes. Thromb Res. 1985;37:401–410. doi: 10.1016/0049-3848(85)90069-6. [DOI] [PubMed] [Google Scholar]
- Nestle FO, Filgueira L, Nickoloff BJ, Burg G. Human dermal dendritic cells process and present soluble protein antigens. J Invest Dermatol. 1998;110:762–766. doi: 10.1046/j.1523-1747.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- Nestle FO, Nickoloff BJ. A fresh morphological and functional look at dermal dendritic cells. J Cutan Pathol. 1995;22:385–393. doi: 10.1111/j.1600-0560.1995.tb00753.x. [DOI] [PubMed] [Google Scholar]
- Nestle FO, Zheng XG, Thompson CB, Turka LA, Nickoloff BJ. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J Immunol. 1993b;151:6535–6545. [PubMed] [Google Scholar]
- Nestle FO, Zheng XG, Thompson CB, Turka LA, Nickoloff BJ. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J Immunol. 1993a;151:6535–6545. [PubMed] [Google Scholar]
- Pohlmann S, Baribaud F, Lee B, Leslie GJ, Sanchez MD, Hiebenthal-Millow K, Munch J, Kirchhoff F, Doms RW. DC-SIGN interactions with human immunodeficiency virus type 1 and 2 and simian immunodeficiency virus. J Virol. 2001;75:4664–4672. doi: 10.1128/JVI.75.10.4664-4672.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MB, Gray C, Fear JD, Bird CC. Immunohistological demonstration of factors XIIIa and XIIIs in reactive and neoplastic fibroblastic and fibro-histiocytic lesions. Histopathology. 1986;10:1171–1178. doi: 10.1111/j.1365-2559.1986.tb02557.x. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Cella M, Danieli C, Lanzavecchia A. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvarna SK, Cotton DW. Dermal dendrocytes and other factor XIIIa-positive cells. J Pathol. 1993;171:251–252. doi: 10.1002/path.1711710403. [DOI] [PubMed] [Google Scholar]
- Tse Y, Cooper KD. Cutaneous dermal Ia+ cells are capable of initiating delayed type hypersensitivity responses. J Invest Dermatol. 1990;94:267–272. doi: 10.1111/1523-1747.ep12874114. [DOI] [PubMed] [Google Scholar]
- Turville SG, Cameron PU, Handley A, Lin G, Pohlmann S, Doms RW, Cunningham AL. Diversity of receptors binding HIV on dendritic cell subsets. Nat Immunol. 2002;3:975–983. doi: 10.1038/ni841. [DOI] [PubMed] [Google Scholar]
- Wollenberg A, Mommaas M, Oppel T, Schottdorf EM, Gunther S, Moderer M. Expression and function of the mannose receptor CD206 on epidermal dendritic cells in inflammatory skin diseases. J Invest Dermatol. 2002;118:327–334. doi: 10.1046/j.0022-202x.2001.01665.x. [DOI] [PubMed] [Google Scholar]
- Zaba LC, Fuentes-Duculan J, Steinman RM, Krueger JG, Lowes MA. Normal human dermis contains distinct populations of CD11c+BDCA-1+ dendritic cells and CD163+FXIIIA+ macrophages. J Clin Invest. 2007;117:2517–2525. doi: 10.1172/JCI32282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1. Antibodies used to evaluate dendritic cells (DC) and macrophage (MΦ) expression in normal human skin.