I. OVERVIEW
As our society ages, age-related diseases assume increasing prominence as both personal and public health concerns. Disorders of cognition are particularly important in both regards, and Alzheimer’s disease (AD) is by far the most common cause of dementia of aging. In 2000, the prevalence of AD in the United States was estimated to be 4.5 million individuals, and this number has been projected to increase to 14 million by 2050 1. While AD is not an inevitable consequence of aging, these numbers speak to the dramatic scope of its impact. This review will focus on AD as well as the milder degrees of cognitive impairment that may precede the clinical diagnosis of probable AD such as mild cognitive impairment 2.
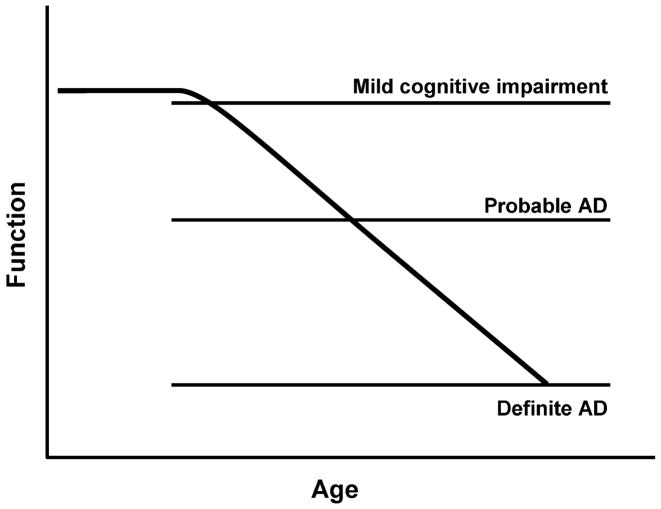
One presumes a gradual pathological progression which begins with normal aging, evolves through clinically probable AD and culminates in neuropathologically proven AD 3. It is likely that individuals pass through a transitional stage between normal aging and clinically probable AD, as illustrated in Figure 1. This phase of mild cognitive impairment may be characterized by memory impairment associated with minimal or no functional decline. These individuals do not meet criteria for clinically probable AD yet are worthy of identification and monitoring.
Figure 1.
Theoretical progression of cognitive function from normal through mild cognitive impairment to probable and definite Alzheimer’s Disease (AD) in persons destined to develop AD. Reprinted with permission from Saunders 2.
In 2001, the American Academy of Neurology published three practice parameters, evidence-based medicine analyses of the extant literature on dementia 4–6. One dealt with mild cognitive impairment 5, the second with diagnostic issues concerning AD and other dementias 4 and the third paper reviewed treatment recommendations for AD and other dementias 6. These documents provide current assessments of diagnostic and management issues regarding AD.
A. Normal Aging
Implicit to a discussion of AD and mild cognitive impairment is knowledge about cognitive changes of normal aging. Characterization of these cognitive changes remains an active area of research, with no agreement on the nature or degree of impairment or the pathological substrate of that clinical picture. Consequently, the characterization of early changes of mild cognitive impairment remains difficult 7. Normative data on a variety of neuropsychological tests for individuals up to age 100 years exists, as do criticisms of these data 8, 9. Some argue that existent normative data are contaminated by the inclusion of persons who would meet current definitions of mild cognitive impairment, and consequently the norms reflect more impairment than should be expected as a consequence of “normal aging” 9. Exclusion of these individuals from the normative data presents a conundrum, and the recursive logic necessary to do so makes this impractical if not impossible.
A meta-analysis investigating cognitive impairment prior to the diagnosis of AD indicated that preclinical deficits in global functioning, episodic memory, perceptual speed and executive functioning were indicative of the subsequent development of AD 10. Among episodic memory parameters, delayed recall procedures produced the largest effect sizes, and the authors concluded that deficits in multiple cognitive domains preceded the clinical development of AD.
Research to more precisely delineate cognitive changes associated with normal aging may allow more accurate interpretation of very early cognitive changes and prediction of their pathologic substrates. At present, clinical judgment remains the best means of assessing mild cognitive impairment.
B. Dementia
Dementia implies a cognitive decline of sufficient severity to compromise a person’s daily function. While diagnostic criteria vary depending upon dementia subtype, general features such as those found in the Diagnostic and Statistical Manual – III R (DSM III-R) remain useful 11. In general, they require memory impairment beyond what would be normal for aging and impairment of at least one other cognitive domain such as attention, language, visuospatial skills or problem solving. These deficits are of sufficient severity to compromise daily functional activities and do not occur in the setting of altered sensorium such as delirium or an acute confusional state. Once this type of cognitive impairment has been determined, the clinician must then determine the underlying nature of the dementia. In the DSM III-R definition, memory impairment is an essential feature of dementia. While this is true of many dementias, it is conceivable that patients with frontotemporal dementia or a Lewy body dementia might present with significant impairment of non-memory cognitive domains early in the disorder. Nevertheless, the DSM III-R criteria provide a practical reference point, particularly for Alzheimer’s disease.
In an elderly person with gradually progressive amnestic disorder which has advanced to involve non-memory cognitive domains to a degree that these changes affect daily functioning, AD is the most likely diagnosis.
II. ALZHEIMER’S DISEASE
This dementia is slowly progressive with prominent memory disturbance appearing early in the clinical presentation 12. As the disease progresses, other cognitive domains become involved and behavioral alterations also arise 13–16. Alzheimer’s disease is a degenerative disorder, and definitive diagnosis can only be made by post-mortem examination of the brain. The classic neuropathological features are neuritic plaques and neurofibrillary tangles 17.
A. Epidemiology
Alzheimer’s disease is an age-related phenomenon, and is the most common cause of dementia in the United States. The incidence of AD increases dramatically with age, doubling every five years after age 65 18. The prevalence of AD rises dramatically with age, becoming quite common in the 70s and more so into the 80s. While it is uncertain whether the incidence of Alzheimer’s disease continues to rise into the 90s; the incidence of dementia increases rapidly in that age range. The prevalence of AD doubles every five years, and there is a greater prevalence of AD in women, likely reflecting their greater longevity 19–25.
B. Clinical Diagnosis
The most commonly employed clinical criteria for diagnosis of AD are those listed in the Diagnostic and Statistical Manual, Fourth Revision (DSM-IV) 26 or those established by the National Institute of Neurologic, Communicative Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA) work group 27. The DSM-IV criteria for dementia of the Alzheimer’s type (Table 1) involve the development of memory impairment accompanied by impairment of one or more other cognitive domains including aphasia, apraxia, agnosia or disturbance of executive functioning. The cognitive impairments are gradually progressive, of sufficient severity to impair functional abilities, and cannot be accounted for by other neurologic or psychiatric disturbances. The American Academy of Neurology practice parameter found these criteria to be reliable 4.
TABLE 1.
DIAGNOSTIC CRITERIA FOR DEMENTIA OF THE ALZHEIMER’S TYPE
|
An adequate history is essential to establishing the diagnosis of dementia. It is critical to take the history from the patient as well as an informant who knows the patient well. Simple inquiry regarding changes in the patient’s ability to carry out typical activities of daily living provide a valuable gauge of the severity of cognitive impairment. In addition to the history, instruments designed to screen for cognitive impairment such as the Mini-Mental State Examination (MMSE) 28, the modified Mini-Mental State (3MS) 29, the Blessed Orientation Memory Concentration Test 30, the Kokmen Short Test of Mental Status 31 or the Clinical Dementia Rating scale (CDR) 32 can be quite useful. None has been demonstrated to be superior to the others.
C. General Neurologic Examination
In early AD, the general neurologic examination is typically normal with the exception of the mental status evaluation. Abnormalities such as parkinsonism, focal neurologic deficits, or deficits in other parts of the nervous system may suggest an alternative diagnosis. It is important to assess sensory functions since sensory deprivation can affect the mental status and neurologic examination. Finally, the neurologic examination should be complemented by a general medical examination looking for medical conditions which may contribute to cognitive impairment.
D. Laboratory Tests
The utility of a variety of laboratory tests in evaluating a patient with dementia was assessed in the American Academy of Neurology practice parameter 4. The authors concluded that vitamin B-12 levels and thyroid functions should be routinely assessed in cases of dementia.
In practice, a variety of tests may be considered in evaluation of dementia, as outlined in Table 2. Although few of these have been demonstrated to actually have an impact on improving the dementia, they can be helpful in the appropriate clinical setting since medical conditions may impact cognitive function.
TABLE 2.
EVALUATION OF PATIENTS WITH DEMENTIA
| ROUTINE | OPTIONAL |
|---|---|
| Electrolytes | Sedimentation rate |
| Complete blood count | Drug levels |
| Vitamin B12 level* | HIV testing |
| Thyroid function studies* | Lyme serology |
| Syphilis serology | Urinalysis |
| 24-urine for heavy metal | |
| CT/MRI* | Cerebrospinal fluid |
| Chest x-ray | |
| Electrocardiogram | |
| Electroencephalogram | |
| PET/SPECT | |
Suggested by the American Academy of Neurology 4.
E. Neuroimaging
The American Academy of Neurology practice parameter recommended that a CT or MRI be done in most circumstances at the initial dementia assessment to exclude potentially treatable structural lesions such as subdural hematoma, neoplasm, and stroke 4.
Atrophy of medial temporal lobe structures, e.g., the hippocampus, has been found in patients with AD, but this atrophy may be nonspecific and while consistent with AD, it may be seen in other conditions as well 33. Longitudinal volumetric measurements of the hippocampal formation have indicated more rapid progression of atrophy among persons with AD than normal controls 34. The utility of volumetric measurements of the entorhinal cortex remains controversial 35–37. While atrophy of the hippocampus, amygdala, and entorhinal cortex is established in AD, the role of these findings in the diagnostic evaluation of people having or at risk for developing AD remains to be defined 38.
Functional neuroimaging has been studied in AD, and while several studies have suggested that SPECT imaging augments the clinician’s acumen 39–43, added discriminability has yet to be definitively demonstrated. PET scanning has shown promise in differentiation amongst dementias 44 and FDG-PET may be a useful adjunct in the diagnosis of AD 45. There is literature validating the utility of FDG-PET in differentiating AD from frontotemporal dementia and the Centers for Medicare and Medicaid have approved for reimbursement the use of FDG-PET for this purpose. Some evidence suggests that FDG-PET may be useful in assessing people at risk for developing AD, but longitudinal outcome data is not yet available 45.
Proton MR spectroscopy has also shown promise in evaluating incipient cases of AD 46, and possibly in differentiating among the various types of dementias 47. This technique may be useful in assisting in the diagnosis of dementia in the future.
Recently, exciting new imaging techniques have been developed which allow antemortem detection of amyloid deposition in the brain through the use of the radioligand PET imaging studies 48–50. Pittsburgh Compound B (PIB), while not ready for clinical use, provides the potential for detection of the onset of amyloid deposition prior to the development of clinical symptoms, as well as the opportunity of following amyloid-targeted therapies 51, 52. Another compound, FDDNP, which binds to both amyloid and tau was recently reported to differentiate between normal controls, MCI and AD 50. These developments may lead to important new research opportunities in the early diagnosis and treatment of AD 52.
F. Neuropsychological Testing
Neuropsychological testing can help to determine if reported cognitive changes represent normal aging, MCI, or signify AD. In early AD, subjects commonly have deficits in delayed verbal recall and learning, and may also exhibit impaired naming. A depressed subject may generate a flat learning curve over multiple trials to learn a list of words, but will be able to retain the amount learned after a delay. Frontotemporal dementia patients may have profound difficulties with executive function, sustained attention and speed of processing with relative sparing of naming and memory. While not diagnostic, these neuropsychological profiles can help to distinguish among various dementias. Neuropsychological testing can also provide a baseline against which to compare future evaluations. Consequently, depending upon the particular clinical situation, neuropsychological testing can be an important adjunct.
G. Lumbar Puncture
Several retrospective studies have found little evidence to recommend spinal fluid analysis in the routine evaluation of dementia in elderly patients 53. Dementia characterized by a subacute mental status change, fever, nuchal rigidity, or in the setting of possible contributing processes such as systemic cancer or collagen vascular disease may warrant CSF analysis. Positive syphilis serology may be further evaluated by CSF examination. In immunocompromised patients, syphilis, fungal infections, lymphoma and other opportunistic infections must be considered. Individuals having a rapidly progressive or atypical clinical course and those presenting younger than age 60 may also prompt CSF analysis. Obviously, one must ascertain that there are no contraindications to the procedure.
H. Genetic Testing
Individuals presenting in their 30s, 40s or 50s with a family history suggestive of an autosomal dominant disease may merit testing for mutations on chromosomes 1, 14 or 21 54. Testing should only be undertaken in the setting of appropriate genetic counseling, since the results may have significant impact on the patient and family members. Genetic testing for specific mutations is not typically useful in typical late onset AD.
Among susceptibility polymorphisms for AD, the most recognized is the lipid carrying protein, apolipoprotein E (Apo E) 55, 56. A large neuropathological study investigating the utility of Apo E genotyping found that Apo E ε4 presence increased diagnostic accuracy for AD by about 4 percent and its absence increased the diagnostic accuracy of something other than AD by 8 percent 57. These percentages augmented the clinician’s diagnostic accuracy. Apo E testing in is not currently recommended for asymptomatic individuals who feel they may be at risk by virtue of a positive family history 56, 58. The American Academy of Neurology does not recommend evaluation of any genetic markers for AD at this time 4.
I. Biomarkers
Several studies have found that CSF levels of beta amyloid (Aβ) 1-42 are reduced relative to normal control subjects 59–62, but the utility of these measurements for early diagnosis remains unclear. Low Aβ 1-42 levels were not found to correlate with degree of cognitive impairment 63. A recent study found CSF Aβ 1-42 useful in distinguishing AD with white matter lesions from vascular dementia, with the acknowledgment that Aβ 1-42 serves simply as a surrogate marker in this context 64.
CSF tau levels in AD have been shown to be elevated relative to controls 65–68. Few studies have compared the CSF Aβ or tau levels to a clinical diagnosis. One study found that in 26 neuropathologically confirmed AD cases, CSF p-tau and brain homogenate p-tau correlated, as did the score of neuritic plaques 69. The combination of CSF Aβ 1-42 and tau, and in particular the species of tau phosphorylated at threonine 181 or 231 (p-tau), may be useful, and studies have indicated sensitivities and specificities of 85 and 87 percent, respectively 60, 62, 70–72. However, it is not known if these biomarkers augment the diagnostic accuracy of the clinician.
Another CSF marker, AD7c-NTP (neuronal thread protein), has shown high sensitivities and specificities, but due to technical limitations and the absence of studies of well delineated patient populations, the utility of quantitative measurement of this marker in CSF or urine is unclear 73–76.
The American Academy of Neurology practice parameter states that no biomarkers have emerged as being appropriate for routine use in the clinical evaluation of patients with suspected AD 4.
AD Pathophysiology
Most investigators believe that AD is all or in part due to abnormal processing or deposition of amyloid. The pathogenic form of amyloid is generated by abnormal cleavage of amyloid precursor protein (APP), and is referred to as Aβ 1-42. Normally, APP is cleaved by α-secretase. Aβ 1-42 is formed when APP is cleaved by β- and γ-secretases and then deposited in the brain as an insoluble aggregate. This deposition presumably initiates a cascade of events which result in inflammatory responses and cell destruction. While this may not be the only pathologic process, it is believed to be an important component of the degenerative cascade, and is the target for many new treatment interventions.
The other primary pathologic feature of AD involves the abnormal processing of tau and neurofibrillary tangle formation. The recent development of animal models exhibiting both Aβ deposition and neurofibrillary tangles holds promise of providing a better model of the fundamental pathological elements involved in human AD.
III. MILD COGNITIVE IMPAIRMENT
A. Conceptual Framework
Clinicians are faced with the dilemma of trying to determine the importance of a patient’s forgetfulness. Elderly individuals who feel that their memory has changed from a previous level of functioning are frequently concerned about developing AD. Mild cognitive impairment (MCI) refers to the clinical state in which a subject is cognitively impaired usually in the memory domain, but not demented.
The American Academy of Neurology practice parameter addressing MCI, concluded that persons with memory impairment who meet criteria for MCI have an increased risk of progressing to clinically probable AD and should be counseled and followed accordingly 5. Ideally, identification of these individuals would allow an effective treatment intervention to reduce this risk of progression to dementia, but at present no treatment exists that does so. Candidate treatments include cholinesterase inhibitors, antioxidants, anti-inflammatories and nootropics 77.
B. Clinical Criteria
While there are no accepted criteria for the diagnosis of MCI, most investigators have used the variation of those presented in Table 3. Longitudinal clinical studies indicate that subjects with amnestic MCI have an increased rate of progression to clinically probable AD 78. It should be emphasized that these criteria for MCI are clinical. While neuropsychological testing may help to differentiate these individuals from those who experience normal aging, MCI is not a neuropsychological diagnosis. A recent study from France demonstrated the unreliability of retrospective application of a neuropsychological test-based definition of MCI 79. This study found that application of arbitrary neuropsychological cutoffs in the absence of clinical judgment eliminated the predictive value of the MCI diagnosis. However, diagnosis based upon neuropsychological data used in conjunction with the clinical criteria shown in Table 3 can be reliable and predictive of eventual progression 80.
TABLE 3.
CLINICAL CRITERIA FOR MILD COGNITIVE IMPAIRMENT
|
The first diagnostic criterion is a cognitive complaint. Typically subjects are mildly affected and are aware of their deficit, and corroboration by an informant is particularly useful 81. The second criterion requires objective demonstration of cognitive impairment by the clinician and neuropsychologist, relative to their age- and education-mates 82, 83. As discussed, no particular reference point for normal aging is entirely accurate, but normative data on subjects of similar demographic characteristics may be useful. MCI subjects tend to fall 1.5 standard deviations below their age- and education-matched mates on measures of learning and recall, but it must be emphasized that these are only guidelines and not cutoff scores for assisting in the diagnosis of MCI. Some subjects who fall within the normal range of memory function may in fact have experienced a decline from their prior level of function, and it may be appropriate to diagnosis MCI.
The third criterion refers to relatively normal general cognition. Put simply, cognitive domains outside the one primarily impaired are relatively preserved. Close inspection of these subjects may demonstrate subtle deficits in other cognitive domains, but they are not of sufficient severity to suggest that the person is demented 80. Again, this is a clinical judgment. Similarly, activities of daily living are largely preserved. Subjects may experience minor difficulties due to memory deficits, so technically their activities of daily living are slightly impaired. However, the degree of impairment is insufficient to constitute dementia.
The last criterion is perhaps the most important. The clinician does not feel that the patient meets criteria for dementia or clinically probable AD. These individuals function independently in the community and carry out their routine daily activities. Most clinicians feel that it would be a disservice to label these patients with the diagnosis of AD at this very mild stage of impairment, and the concept of MCI has been developed to identify them.
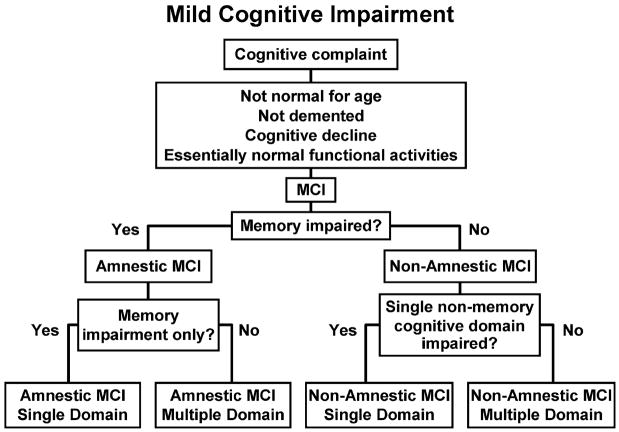
More recently, the construct of MCI has been extended beyond just memory deficit 84. As shown in Figure 2, the person can now have any type of cognitive complaint, although memory is most common. In the estimation of the clinician, this person is not normal for age but not demented, based upon the clinical history from the patient and an informant and office exam, possibly supplemented with additional cognitive testing. Despite the cognitive decline, activities of daily living remain essentially normal. Patients meeting these criteria merit consideration for the diagnosis of MCI. As Figure 2 illustrates, the major subtypes of MCI are amnestic MCI or non-amnestic MCI. Memory function is assessed by the mental status exam, which may be supplemented with neuropsychological testing. If memory impairment is present, cognitive testing can help to determine whether only memory is affected (amnestic MCI single domain) or whether other cognitive domains are also impaired (amnestic cognitive impairment-multiple domain). The other cognitive domains typically evaluated include language, attention/executive function and visuospatial skills. If the person meets MCI criteria and memory is not impaired, other cognitive domains need to be assessed to determine if it is an isolated problem (non-amnestic MCI single domain) or if multiple non-memory domains involved (non-amnestic MCI multiple domain).
Figure 2.
Flow diagram for diagnosing MCI. The cardinal feature is cognitive impairment intermediate between the cognitive changes of normal aging and those of early dementia. Subtyping of MCI is first made along the dimension of memory into amnestic and non-amnestic. These subtypes are further classified into single cognitive domain or multiple cognitive domains. See text for explanation.
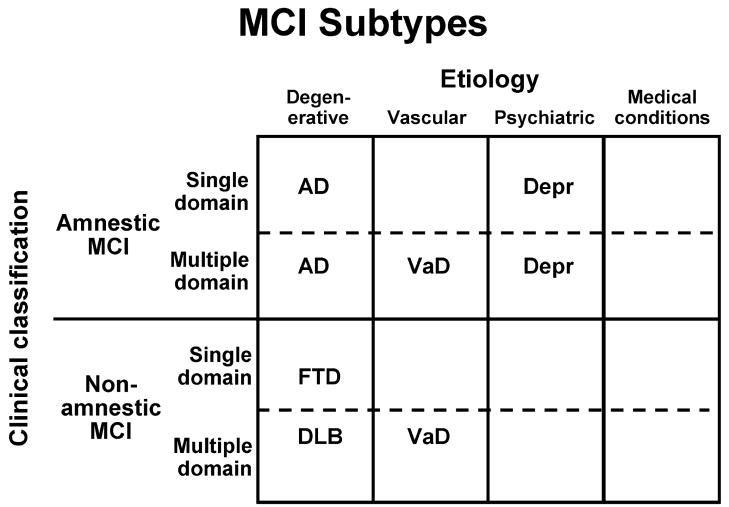
Similar to the evaluation of the etiology of dementia, the historical and neuroimaging data may suggest the cause of MCI is of a degenerative, vascular, psychiatric, or traumatic etiology or secondary to medical illnesses, as illustrated in Figure 3. In reality, part of the scheme shown in Figure 3 is theoretical and not yet validated, while other aspects are well documented in the literature 85–87. Amnestic MCI of a degenerative etiology is highly likely to progress to AD and the American Academy of Neurology endorsed this construct in its practice parameter 5. The corresponding outcome of non-amnestic MCI subjects is currently under investigation.
Figure 3.
The four clinical subtypes of MCI are then combined with the presumed etiology of the clinical syndrome. For example, amnestic MCI of single or multiple domain subtypes can be combined with the presumed degenerative etiology to result in the likely outcome of Alzheimer’s disease when the condition progresses to dementia. The other suggested clinical outcomes are theoretical and other outcomes may be possible.
C. Evaluation
The clinical evaluation of suspected MCI is virtually identical to that described above for clinically probable AD. As mentioned, the history is of particular importance and should be verified by an informant, if this is at all possible. The clinician should perform a mental status examination in addition to a general neurologic exam. Most of the commonly used instruments available, MMSE, 3MS, Kokmen Short Test of Mental Status 28, 29, 31, are relatively insensitive in this range of cognitive function, and in all likelihood performance on these measures will appear more normal than not. Subjects with MCI often score in the 26 to 28 range on the MMSE, which is typically reported as normal. If the mental status exam instrument does not have a significant memory component, subjects with relatively isolated memory impairment will not be differentiated from those who are aging normally. Consequently, the clinician may consider augmenting the clinical examination with an additional memory test 88, 89.
The medical laboratory tests are similar to those described above for clinically probable AD as well (Table 2). One should pay particular attention to subtle medical issues that could affect cognitive function. . While depression can often be differentiated from clinically probable AD, it could present with subtle memory impairment in its early stages. Consequently, the clinician should remain attuned to possible psychiatric components of subtle memory impairments as well.
D. Neuroimaging
Several recent MRI studies involving volumetric assessments of medial temporal lobe have been informative 33–36, 90. There is ongoing discussion of the relative utility of volumetric measurements of the hippocampal formation versus entorhinal cortex volumes 35–37. Measurements of whole brain atrophy are under investigation, although it remains to be determined if whole brain volume changes will be useful in assessing the early stages of AD. A 2001 study suggested that this may be the case 91 and was supported by a subsequent prospective study 92, although precise delineation of the rate of atrophy in early AD will require further study.
The utility of other imaging modalities, such as magnetic resonance spectroscopy, SPECT and PET, has not been definitively demonstrated in this population yet 93. Nevertheless, in selected instances, particularly in the setting of a normal structural imaging scan, functional imaging modalities may provide additional useful information 94. Patients with amnestic MCI are at an increased risk of progression to AD, and imaging agents such as PIB or FDDNP may augment this prognostic information, although this is not yet verified.
E. Neuropsychological Testing
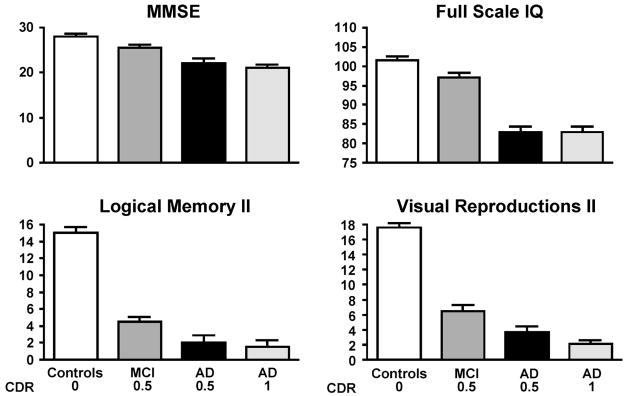
Neuropsychological testing can aid in differentiating subjects with MCI from normal aging, but the testing battery must involve sufficiently difficult learning and recall tasks to tease apart these subtle deficits. Neuropsychological testing will not make the diagnosis of MCI, but can be suggestive in the appropriate clinical context 95. Figure 4 presents typical neurocognitive profiles of subjects with MCI, normal aging and very mild clinically probable Alzheimer’s disease (CDR 0.5). On measures of general cognitive function such as the MMSE and full scale IQ, the individual with MCI performs more like the normal elderly subject, while memory function on delayed verbal recall (Logical Memory II) and non-verbal delayed recall (Visual Reproductions II) more closely resembles mild AD 80.
Figure 4.
Cognitive profile of persons with a mild cognitive impairment. The top two panels, Mini-Mental State Exam (MMSE) and Full Scale IQ represent measures of general intellectual function. The bottom two panels represent memory function for verbal memory (Logical Memory II) and non-verbal memory (Visual Reproductions II). Reprinted with permission from the American Medical Association 80.
F. Biomarkers
As in Alzheimer’s disease, biomarkers are in the early stages of development. There are some indications that the CSF measures of Aβ and tau may be useful at differentiating subjects with MCI from normal aging 60, 66, 96. These markers may have utility in predicting progression from MCI to AD63, 97. A multinational study found that baseline CSF levels of tau phosphorylated at theorinine 231, but not total tau protein levels, correlated with cognitive decline and conversion from MCI to AD 72. Another recent study that investigated the utility of CSF concentrations of Aβ 1-42, total tau (T-tau) and tau phosphorylated at threonine 181 (p-tau) in predicting progression from MCI to AD reported a sensitivity of 95% and specificity of 83% using the combination of elevated T-tau and lowered Aβ 1-42 98. This is an exciting area of research, but the present data is insufficient to recommend use of CSF biomarkers in evaluation of MCI.
G. Genetics
The genetic features of MCI are similar to those of clinically probable AD. There appears to be a higher representation of Apo E ε4 carriers in MCI, and some studies suggest that presence of the ε4 allele may be predict a higher rate of progression 99, 100. However, these data are only weakly positive, and Apo E genotyping is not currently recommended as a diagnostic or prognostic indicator in MCI.
H. Neuropathology
Several studies have reported on the neuropathology of subjects who died with the clinical classification of MCI 101, 102. Neuropathology in subjects with MCI in the Religious Orders Study was intermediate between the neuropathological changes of normal aging and fully developed AD 101. This study also indicated that vascular features were present and suggested that both neurodegenerative and vascular changes may account for the clinical features of MCI. Another report from the Nun Study indicated that individuals who were retrospectively classified as having MCI primarily had neuropathologic changes of AD at autopsy 103. This study found that patients with MCI had significantly more neuritic plaques relative to controls, and that these findings were more similar to those of early AD than normal aging. However, a study from the Mayo Clinic on MCI demonstrated that subjects had intermediate neuropathologic changes significantly different from both controls and AD 102. MCI subjects in this series more closely resembled normal control subjects than AD subjects, implying that these MCI subjects were diagnosed at an earlier stage in the disease process. Two small neuropathological studies demonstrated neurofibrillary tangle pathology in the nucleus basalis 104 and locus ceruleus 105 in MCI and early AD.
A Washington University study found that when dementia subjects classified as having AD and a Clinical Dementia Rating of 0.5 progressed, 84% of them had the neuropathologic features of AD 106. A Mayo Clinic study of 34 subjects previously diagnosed with MCI who had progressed to dementia demonstrated that while approximately 75% of these subjects went on to have AD, the other 25% developed other forms of dementia 107. Therefore, while amnestic MCI criteria are predictive of developing AD, they are not absolute and when patients are diagnosed at an early stage of impairment, neuropathological features do not correspond to AD at that point in time.
I. Summary
The construct of mild cognitive impairment identifies individuals at increased risk of developing AD. The early identification of these more subtle impairments may allow clinicians to counsel and follow their patients more effectively. Many subjects with amnestic MCI will ultimately progress to probable AD. Identification at this earlier point in the progression may ultimately allow intervention to slow or halt progression. Mild cognitive impairment is an important area for clinical research, and work characterizing the clinical features of these subjects and documenting their outcome is progressing at a rapid rate.
IV. TREATMENTS
A. Alzheimer’s Disease
1. Symptomatic
Five drugs are approved by the Food and Drug Administration (FDA) for the treatment of clinically probable AD (Table 4), although only four are commonly used. Three of these four are acetylcholinesterase inhibitors, used in response to research indicating a cholinergic deficit in AD 108. Acetylcholine is involved in many aspects of cognition including memory and attention. Cholinergic neurons in the basal forebrain project to many regions of the neocortex and to the medial temporal lobe including the hippocampus. Anticholinergic drugs, such as scopolamine, can impair learning and recall, reminiscent of the cognitive changes seen in AD 109. Choline acetyltransferase, the synthetic enzyme for acetylcholine, is reduced in the brains of subjects with AD 110, 111. In the past decade, acetylcholinesterase inhibitors have been shown to be effective at modulating the symptoms of AD and currently form the mainstay of treatment in clinically probable AD.
TABLE 4.
SYMPTOMATIC MEDICATIONS FOR ALZHEIMER’S DISEASE
| Drug | Mechanism | Initial Dose | Target Dose | Titration Interval | Side Effects |
|---|---|---|---|---|---|
| Donepezil (Aricept) | AChI | 5 mg QD | 10 mg QD | 4–6 wks | Nausea, vomiting, diarrhea, muscle cramps, anorexia, vivid dreaming |
| Rivastigmine (Exelon) | AChI | 1.5 mg BID | 6 mg BID | 2–4 wks | Nausea, vomiting, diarrhea, weight loss, dizziness |
| Galantamine (Reminyl) | AChI | 4 mg BID | 12 mg BID | 4 wks | Nausea, vomiting, diarrhea, anorexia, dizziness |
| Tacrine (Cognex) | AChI | 10 mg QID | 40 mg QID | 4 wks | Liver function test evaluations, diarrhea, anorexia, nausea, vomiting, myalgia |
| Memantine (Namenda) | NMDA-A | 5 mg QD | 10 mg BID | 1–2 wks | dizziness, confusion, headache, constipation, nausea, agitation, hallucination, seizure |
AChI – Acetylcholinesterase inhibitor, NMDA-A –NMDA antagonist
The first compound approved was tacrine (Cognex), which led the way for treatment of AD. However, tacrine required four times a day dosing and liver toxicity necessitated regular monitoring of liver enzymes. Newer drugs without these limiting features were introduced, and tacrine is now only rarely used for the treatment of AD.
Donepezil (Aricept) became available in the mid-1990s as the next acetylcholinesterase inhibitor approved by the FDA. It is administered once daily and does not require any laboratory monitoring. It is heavily bound to plasma proteins, and has a half-life of approximately 70 hours. Typically, it is started at 5 mg daily and the dose is increased to 10 mg daily after 4 to 6 weeks if the drug is well tolerated. The most common side effects include nausea, increased bowel frequency and vomiting. Occasionally patients experience vivid dreaming, which may be reduced by morning administration of the drug. Cholinesterase inhibitors could theoretically influence cardiac rhythm, but this is not commonly encountered in the absence of an underlying disturbance of cardiac conduction. They may also have an effect on respiratory conditions such as chronic obstructive pulmonary disease or asthma and could theoretically interfere with the administration of anesthesia during surgery. The absorption of donepezil is not influenced by food intake.
Several studies of the efficacy of donepezil show a modest improvement in cognitive function as measured by scales such as the Alzheimer’s Disease Assessment Scale – Cognitive Subscale (ADAS-Cog) and the Clinician’s Interview-Based Impression of Change (CIBIC Plus) 6. The drug is approved for mild to moderate AD, and the length of the response has been documented up to 52 weeks. It is uncertain if the benefit persists longer than this. Studies indicate that performance of the subject returns to the same as in the untreated state when donepezil is discontinued, suggesting that donepezil has a symptomatic effect on the disease but does not affect the underlying pathophysiologic process.
A large study of donepezil in amnestic MCI found a reduced rate of progression to AD during the first 12 months of the study, but no overall impact on rate of progression at the 3 year endpoint 112. This suggests a transient symptomatic benefit without modification of disease course in amnestic MCI, similar to its effect in AD.
Rivastigmine (Exelon) is another acetylcholinesterase inhibitor approved by the FDA 113. Rivastigmine is a pseudo-irreversible inhibitor of acetylcholinesterase and dissociates from the enzyme slowly. Dosing begins with 1.5 mg twice a day and increases in increments of 1.5 mg per dose to a maximum of 6 mg twice daily. While this offers greater dosing flexibility, the twice daily dosing may make it more difficult for memory impaired patients to adhere to therapy. It may provide greater cholinesterase inhibition at the highest dose, but is also prone to an increased frequency of side effects possibly related to its inhibition of butyrylcholinesterase. Side effects are similar to those of donepezil, although with a somewhat higher incidence of GI side effects 113. To encourage adherence to dose increases, titration is recommended to be advanced on a 2 to 4 week basis. The effect size of rivastigmine on the ADAS-Cog and the CIBIC Plus is approximately the same as donepezil 6.
The fourth cholinesterase inhibitor approved by the FDA, galantamine (Reminyl), is a reversible inhibitor of cholinesterase which also has some nicotinic receptor activity. This mechanism has been proposed to provide an additional benefit over the other cholinesterase inhibitors. The initial starting dose of galantamine is 4 mg twice daily, which is increased to 8 mg twice daily and ultimately 12 mg twice daily if tolerated. This dose escalation is done at four week intervals to minimize side effects. Galantamine has similar potential for GI, cardiac and pulmonary concerns as the other cholinesterase inhibitors. The effect size of galantamine on the ADAS-Cog and the CIBIC Plus is similar to donepezil and rivastigmine. One study found that galantamine had an effect on activities of daily living and behavior 114. Concern about an increase in mortality, presumably from cardiac deaths, has arisen in clinical trials of galantamine in MCI. The FDA has expressed caution about the use of galantamine for that indication.
Memantine (Namenda) is a NMDA antagonist which has been approved by the FDA for the treatment of moderate to severe AD. This drug can be used either alone or in combination with a cholinesterase inhibitor to help improve the symptoms of AD, but has not been shown to have an effect on the underlying disease process.
In summary, donepezil, rivastigmine, galantamine and memantine are the most commonly used drugs in the treatment of AD. They appear to be equally efficacious, have a similar side effect profiles, their cost is approximately equivalent and there is little evidence to recommend one over the other. They appear to provide a modest enhancement of cognitive function in subjects with AD but do not seem to have a significant impact on the underlying pathophysiology of AD. The American Academy of Neurology’s practice parameter on treatment of dementia recommended that cholinesterase inhibitors be considered in mild to moderate AD patients, although the effect size is modest 6. The gastrointestinal side effects can be minimized with slow titration of the drug. Their role in MCI is incompletely defined, with a large trial suggesting transient symptomatic benefit in amnestic MCI, but no lasting impact on progression to AD 112.
Cost effectiveness of the cholinesterase inhibitors has been debated with one study suggesting a modest cost savings in favor of donepezil over placebo with a reduction in the use of residential care 115 and another study coming to the opposite conclusion 116. Comparable studies have not been done with the other cholinesterase inhibitors or with memantine. Thus, the long-term benefit remains questionable from an economic perspective.
2. Disease Modifying
a. Vitamin E
Considerable research supports a role of oxidative damage in the pathophysiology of AD, and the use of antioxidants as a treatment of AD remains an area of active research. Epidemiologic data suggest that anti-oxidants may be associated with a lower incidence of AD 117–121. A large clinical trial of moderate AD patients found that vitamin E and selegiline were effective at slowing the progression of moderate AD 122. The primary endpoint of this study were death, institutionalization, loss of basic activities of daily living or a progression on the CDR from 2 to 3, and both vitamin E and selegiline reduced the rate of progression. The dosage of vitamin E was 1,000 IU twice daily and that of selegiline was 10 mg daily. Due to its drug interactions and other potential toxicities as well as a suggestion of a very mild superiority of vitamin E to selegeline, the AAN practice parameter recommended that vitamin E at 1000 IU twice daily can be considered as a treatment to slow the progression of AD 6. This finding has not been replicated, nor has the optimal dose of vitamin E been determined by additional studies. A single large study which investigated vitamin E in amnestic MCI found no benefit over placebo 112. Theoretical concerns of GI toxicity and bleeding exist with vitamin E, but generally it is well tolerated.
This single positive study for vitamin E needs to be interpreted in the context of a recent meta-analysis that suggests an increased risk of death among those taking vitamin E at 400 IU or more per day 123. These deaths were mostly cardiovascular, and overall medical condition of the participants in the studies pooled for meta-analysis may not accurately represent typical AD patients.
3. Under Investigation
a. Anti-inflammatory Medications
Research implicates an inflammatory component to the neurodegenerative process in AD 124, 125 and epidemiologic studies have suggested that nonsteroidal anti-inflammatory drug (NSAID) use may protect against developing AD 126–130. Given this background, it is not surprising that several studies have been undertaken to determine the possible efficacy of anti-inflammatories in treating AD 131–133.
Studies using glucocorticoids such as prednisone or NSAIDs have been negative to date 131, 134. The cyclooxygenase (COX) –2 inhibitors have not been demonstrated to be beneficial and cardiovascular concerns exist regarding the prolonged use of these medications 135–139. Thus, while the epidemiologic data and theoretical considerations remain intriguing, the benefit of anti-inflammatory agents for the treatment or prevention of AD has yet to be demonstrated 140.
There is speculation that some NSAIDs may have specific Aβ lowering properties and hence might be useful in the treatment of AD through an alternative mechanism, and this remains an area of ongoing research 141–143.
b. Estrogen Replacement Therapy
Some epidemiologic evidence suggests that post-menopausal women who take estrogen replacement may be protected from developing AD 144–147. More recently, the Women’s Health Initiative Memory Study has demonstrated that post-menopausal estrogen use might actually be a risk factor for developing AD and MCI rather than a protective factor 148–151. There are longitudinal studies underway concerning possible prophylactic effect of estrogen in reducing the risk of developing dementia, but the data from these studies are pending 152, 153. A large trial of estrogen replacement in mild to moderate AD failed to demonstrate benefit over the course of 12 months 154. A smaller 16-week trial was also negative 155. A case-control study found no overall correlation between estrogen replacement and incident cases of AD, although it did suggest that this factor interacted with smoking history 156. At present there are no data which suggest that estrogen is useful as a treatment or prophylactic for AD and it is currently not recommended for that purpose.
c. Amyloid Treatments
Deposition of beta amyloid (Aβ) is considered to be intrinsic to the pathophysiology of AD and several research strategies are underway that address this mechanism 157. Beta amyloid is a major component of the neuritic plaques in AD, and fibrillar Aβ has been shown to be neurotoxic. Beta amyloid is processed by several proteases to various amylogenic and non-amylogenic pathways 158. The protease α-secretase produces the non-amylogenic fragments and is the preferred pathway, while β-secretase and γ-secretase cleavage results in the generation of Aβ. Consequently, strategies which attempt to inhibit the activities of β-secretase and γ-secretase have been developed 159–162. The β-secretase enzyme BACE is one target 163 and γ-secretase is another 164–167. A 12 month clinical trial of an NSAID-derived γ-secretase inhibitor suggested benefit in daily activities and psychiatric events 168, and a larger phase III trial of this agent is ongoing. Thus, while candidate compounds have been identified, only limited clinical trial data are currently available.
Immunization was proposed as a treatment in 1999, based upon mouse data suggesting that immunization of Aβ in early life reduced Aβ plaque formation later in life 169. Mid-life immunization of these mice showed a reduced progression of the disease as well as a suggesting some regression of the underlying pathology 169.
A multicenter Phase II clinical trial of active immunotherapy with a vaccine against Aβ-42 (AN1792) plus adjuvant QS-21 in humans began in 2001. This study was halted in early 2002 after a subacute meningoencephalitis developed in approximately 5% of the immunized subjects 170. Active immunization was discontinued; however there was a suggestion that the subset of subjects who developed sufficient antibody levels may have had a reduced progression of the disease 171. The few autopsies performed on trial participants have suggested some clearance of neuritic plaques 172. Subjects who produced antibody showed more brain volume reduction on MRI, hinting that some degree of amyloid clearing process may have affected brain volumes 173.
Polyclonal antibodies against Aβ can be found in human immunoglobulins, and IVIg has been advanced as a method of passive immunization for the treatment of AD 174, 175. One study of five patients suggested that monthly IVIg reduced CSF Aβ concentrations while increasing serum levels of Aβ 175. A phase I trial of IVIg in eight patients suggested sustained cognitive benefit among some patients 176 and a small phase II trial is currently underway. Passive immunization using monoclonal antibodies produced in cell culture has also been proposed, and a phase IIa trial of bapineuzumab (a humanized antibody against Aβ) is expected to be completed in 2008.
4. Non-cognitive symptoms
Non-cognitive symptoms of AD such as anxiety, depression, psychosis and sleep disturbances may prove more bothersome to the patient and family than cognitive impairment and can cause considerable stress on both the patient and the caregiver. These non-cognitive symptoms often require ongoing active management by the physician and are the motivating factor for many telephone calls. Many of these symptoms can be ameliorated, and consequently they deserve significant attention on the part of the treating physician. Increased recognition of these non-cognitive symptoms has fostered increasing research attention in this area 177.
An increased risk of cardiovascular symptoms, glucose intolerance, stroke, and death has been associated with the use atypical antipsychotics in some psychiatric disorders, and this has resulted in an FDA “black box warning” on the use of atypical antipsychotics in older subjects with dementia 178. Caution should be exercised in using these drugs, but their judicious use can have a dramatically positive impact on problematic behavioral symptoms 6.
a. Frequency of Symptoms
Estimates of the frequency of non-cognitive symptoms in AD vary from virtually absent to over 80 percent (Table 5) 179–183. One study using the Neuropsychiatric Inventory (a questionnaire established to assess an array of neuropsychiatric symptoms and their impact as estimated by caregivers), found apathy to be the most common symptom, followed by agitation, anxiety, irritability, dysphoria, disinhibition, delusions, hallucinations and euphoria, respectively 182. Co-occurrence of non-cognitive symptoms is also common, and the symptom complexes tend to fluctuate. Consequently, treatment strategies require ongoing adjustment.
TABLE 5.
FREQUENCY OF BEHAVIORAL DISORDERS IN ALZHEIMER’S DISEASE
| Agitation | 50–70% |
| Anxiety | 30–50% |
| Depression | 25–50% |
| Disinhibition | 20–35% |
| Aggression | 25% |
| Delusions | 15–50% |
| Hallucinations | 10–25% |
| Sexual Disinhibition | 5–10% |
(Please see Reference 183)
b. Assessment
It should not be presumed that all non-cognitive symptoms in an AD patients are a result of progression of the underlying neurodegenerative process. Medical problems may manifest as a behavioral change and screening for urinary tract infection, pneumonia, congestive heart failure and electrolyte abnormality should be considered. Treatment of the underlying medical issues may improve or eliminate the problematic behaviors.
c. Behavioral Management
Non-pharmacologic methods should be considered, and can avoid potential side effects of additional medications 16. Environmental changes such as family crises, new caregivers or altered surroundings may exacerbate or cause behavioral problems. Often, simple strategies such as distraction, redirection or exercise can ameliorate the behaviors.
d. Pharmacologic Treatments
Behaviors of sufficient severity to disrupt a patient’s (or caregiver’s) quality of life may require pharmacologic intervention. Accurate assessment of the underlying condition is a crucial first step 182 and several scales exist to assist the clinician in addressing these behaviors, including the BEHAVE-AD, 183 the Cohen-Mansfield Agitation Inventory (CMAI) 184 and the Neuropsychiatric Inventory (NPI) 185. The NPI is one of the most commonly used instruments, and assesses ten commonly encountered behaviors including delusions, hallucinations, agitation, dysphoria, anxiety, apathy, irritability, euphoria, disinhibition and aberrant motor behavior. The frequency and severity of the symptoms are reported by a caregiver, allowing computation of a final index for each behavior. The impact on the caregiver is also assessed. The NPI-Q is an abbreviated version designed for assessment in the office setting 186. Table 5 shows the frequency of non-cognitive behaviors in AD.
d. Depression
Depression or dysphoria is common in AD, and can herald the onset of the disorder 187 or develop as the dementia progresses 182. It commonly worsens cognitive symptoms and places greater stress on the caregiver. Selective serotonin reuptake inhibitors (SSRIs) are the preferred treatments for depression in AD. Tricyclic antidepressants may also be effective, but anti-cholinergic side effects may worsen cognition and act at cross-purpose to cholinesterase inhibitors (Table 6).
TABLE 6.
MEDICATIONS FOR NON-COGNITIVE SYMPTOMS IN DEMENTIA
| Class | Agents | Usual Daily Dose |
|---|---|---|
| Delusions | Risperidone (Risperdal) Olanzapine (Zyprexa) Quetiapine (Seroquel) Aripiprazole (Abilify) Haloperidol (Haldol) |
1 mg (.05–2 mg) 5 mg (5–10 mg) 400 mg (50–400 mg) 5 mg (5–20 mg) 1 mg (.5–3 mg) |
| Agitation/aggression | Risperidone (Risperdal) Olanzapine (Zyprexa) Quetiapine (Seroquel) Aripiprazole (Abilify) Haloperidol (Haldol) Trazodone (Desyrel) Buspirone (Buspar) Propranolol (Inderal) Carbamazepine (Tegretol) Divalproex (Depakote) Lorazepam (Ativan) |
1 mg (.05–2 mg) 5 mg (5–10 mg) 400 (50–400 mg) 5 mg (5–20 mg) 1 mg (.5–3 mg) 100 mg (100–400 mg) 15 mg (15–30 mg) 120 mg (80–240 mg) 400 mg (200–1200 mg) 500 mg (250–2000 mg) 1 mg (0.5–6 mg) |
| Depression | Fluoxetine (Prozac) Sertraline (Zoloft) Paroxetine (Paxil) Citalopram (Celexa) Venlafaxin (Effexor) Nefazodone (Serzone) Mirtazepine (Remeron) Duloxetine (Cymbalta) Nortriptyline (Pamelor) Trazodone (Desyrel) |
20 mg (20–40 mg) 50 mg (50–200 mg) 20 mg (10–50 mg) 20 mg (10–30 mg) 100 mg (50–225 mg) 400 mg (200–600 mg) 15 mg (7.5–30 mg) 20 mg (20–60 mg) 50 mg (50–100 mg) 50 mg (100–400 mg) |
| Anxiety | Oxazepam (Serax) Lorazepam (Ativan) Buspirone (Buspar) Propranolol (Inderal) |
30 mg (20–60 mg) 1 mg (0.5–6 mg) 30 mg (15–45 mg) 120 mg (80–240) |
| Insomnia | Trazodone (Desyrel) Zolpidem (Ambien) Temazepam (Restoril) Zoloplon (Sonata) |
50 mg (50–200 mg) 10 mg (5–10 mg) 15 mg (15–30 mg) 10 mg |
| Apathy | Donepezil (Aricept) Rivastigmine (Exelon) |
10 mg (5–10 mg) 9 mg (6–12 mg) |
f. Psychosis
Delusions are common in AD, often with coexistent paranoia 188, 189. Hallucinations can be seen in AD, particularly if Lewy bodies are present, and misidentification syndromes can occur when the right hemisphere is predominantly involved. Psychosis can also indicate a more rapid decline in function 15, 190.
Non-pharmacologic interventions are the preferable first-line treatment for psychosis, agitation, aggression and other problematic behaviors which may compromise the health and safety of patients and those around them. Atypical anti-psychotic medications remain the preferred treatment of psychosis in AD. Risperidone was demonstrated to improve psychosis and aggression, but did produce somnolence and extrapyramidal symptoms 191. Quetiapine has been shown to reduce psychotic symptoms with relatively few side effects 192, as has olanzapine 193. The results of the CATIE-AD trial generated significant publicity in suggesting that potential adverse effects of atypical antipsychotics may offset potential benefits from these agents 194. A subsequent meta-analysis of placebo-controlled trials of atypical neuroleptic medications found only modest group efficacy and echoed concerns about potential adverse effects 195. In this analysis, the authors recommend that deliberate consideration and discussion with patients and their families should precede initiation of antipsychotic medications. These medications should be discontinued if improvement has not been seen within 10–12 weeks and should be adjusted to the minimum effective dosage. Periodic medication withdrawal trials may be used to evaluate for continued necessity. These authors also caution against the imprudence of prescribing alternative medications as first-line agents due to a perception that they are safer or as effective as antipsychotics. Atypical antipsychotics can be expensive, but are generally felt to be preferable to typical anti-psychotic agents such as haloperidol (Table 6).
g. Apathy
Apathy may be the most common non-cognitive symptoms in AD 177, 182 and causes diminished quality of life for both patient and caregiver. Acetylcholinesterase inhibitors may be useful, but pharmacological management of these symptoms is not well developed. Other medications such as methylphenidate, bromocriptine, pramipexole, ropinerole or activating anti-depressants such as fluoxetine may theoretically be useful, although benefit has not been established in the literature 196.
h. Agitation
Agitation can be quite bothersome to caregivers since the patient appears to be in distress 197. Trazodone can useful, and has a better side effect profile than haloperidol 198. Atypical anti-psychotic agents such as risperidone, olanzapine and quetiapine can be considered, as can anti-convulsants such as carbamazepine or valproic acid 199, 200.
i. Summary
Non-cognitive symptoms of AD can be responsible for significant distress for both patients and caregivers, and their impact can be ameliorated using both pharmacologic and behavioral interventions. Interventions are being studied more extensively, with the recognition that successful intervention can result in significant improvement of the qualities of lives for all involved.
Acknowledgments
We would like to acknowledge the support for preparation of this chapter and research reported herein from the National Institute on Aging P50 AG16574, U01 AG06786, and the Robert H. and Clarice Smith and Abigail van Buren Alzheimer’s Disease Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998 Sep;88(9):1337–1342. doi: 10.2105/ajph.88.9.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petersen RC. Aging, mild cognitive impairment, and Alzheimer’s disease. Neurol Clin. 2000 Nov;18(4):789–806. doi: 10.1016/s0733-8619(05)70226-7. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC. Mild Cognitive Impairment: Transtion from Aging to Alzheimer’s Disease. In: Iqbal KSS, Winblad B, editors. Alzheimer’s Disease: Advances in Etiology, Pathogenesis and Therapeutics. West Sussex, England: J Wiley & Sons, Ltd; 2001. pp. 141–151. [Google Scholar]
- 4.Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001 May 8;56(9):1143–1153. doi: 10.1212/wnl.56.9.1143. [DOI] [PubMed] [Google Scholar]
- 5.Petersen RC, Stevens JC, Ganguli M, Tangalos EG, Cummings JL, DeKosky ST. Practice parameter: early detection of dementia: mild cognitive impairment (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001 May 8;56(9):1133–1142. doi: 10.1212/wnl.56.9.1133. [DOI] [PubMed] [Google Scholar]
- 6.Doody RS, Stevens JC, Beck C, et al. Practice parameter: management of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001 May 8;56(9):1154–1166. doi: 10.1212/wnl.56.9.1154. [DOI] [PubMed] [Google Scholar]
- 7.Ivnik RJ, Smith GE, Lucas JA, et al. Testing normal older people three or four times at 1- to 2-year intervals: defining normal variance. Neuropsychology. 1999 Jan;13(1):121–127. doi: 10.1037//0894-4105.13.1.121. [DOI] [PubMed] [Google Scholar]
- 8.Petersen RC. Mild cognitive impairment: aging to Alzheimer’s disease. Oxford; New York: Oxford University Press; 2003. [Google Scholar]
- 9.Sliwinski M, Lipton RB, Buschke H, Stewart W. The effects of preclinical dementia on estimates of normal cognitive functioning in aging. J Gerontol B Psychol Sci Soc Sci. 1996 Jul;51(4):P217–225. doi: 10.1093/geronb/51b.4.p217. [DOI] [PubMed] [Google Scholar]
- 10.Backman L, Jones S, Berger AK, Laukka EJ, Small BJ. Cognitive impairment in preclinical Alzheimer’s disease: a meta-analysis. Neuropsychology. 2005 Jul;19(4):520–531. doi: 10.1037/0894-4105.19.4.520. [DOI] [PubMed] [Google Scholar]
- 11.aaa. American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. 3. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 12.Fleming KC, Adams AC, Petersen RC. Dementia: diagnosis and evaluation. Mayo Clin Proc. 1995 Nov;70(11):1093–1107. doi: 10.4065/70.11.1093. [DOI] [PubMed] [Google Scholar]
- 13.Burns A, Jacoby R, Levy R. Psychiatric phenomena in Alzheimer’s disease. IV: Disorders of behaviour. Br J Psychiatry. 1990 Jul;157:86–94. doi: 10.1192/bjp.157.1.86. [DOI] [PubMed] [Google Scholar]
- 14.Drevets WC, Rubin EH. Psychotic symptoms and the longitudinal course of senile dementia of the Alzheimer type. Biol Psychiatry. 1989 Jan;25(1):39–48. doi: 10.1016/0006-3223(89)90145-5. [DOI] [PubMed] [Google Scholar]
- 15.Rosen J, Zubenko GS. Emergence of psychosis and depression in the longitudinal evaluation of Alzheimer’s disease. Biol Psychiatry. 1991 Feb 1;29(3):224–232. doi: 10.1016/0006-3223(91)91284-x. [DOI] [PubMed] [Google Scholar]
- 16.Teri L, Larson EB, Reifler BV. Behavioral disturbance in dementia of the Alzheimer’s type. J Am Geriatr Soc. 1988 Jan;36(1):1–6. doi: 10.1111/j.1532-5415.1988.tb03426.x. [DOI] [PubMed] [Google Scholar]
- 17.The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. Neurobiol Aging. 1997 Jul-Aug;18(4 Suppl):S1–2. [PubMed] [Google Scholar]
- 18.Kukull WA, Ganguli M. Epidemiology of dementia: concepts and overview. Neurol Clin. 2000 Nov;18(4):923–950. doi: 10.1016/s0733-8619(05)70233-4. [DOI] [PubMed] [Google Scholar]
- 19.Bachman DL, Wolf PA, Linn R, et al. Prevalence of dementia and probable senile dementia of the Alzheimer type in the Framingham Study. Neurology. 1992 Jan;42(1):115–119. doi: 10.1212/wnl.42.1.115. [DOI] [PubMed] [Google Scholar]
- 20.Canadian study of health and aging: study methods and prevalence of dementia. Cmaj. 1994 Mar 15;150(6):899–913. [PMC free article] [PubMed] [Google Scholar]
- 21.Evans DA, Funkenstein HH, Albert MS, et al. Prevalence of Alzheimer’s disease in a community population of older persons. Higher than previously reported. Jama. 1989 Nov 10;262(18):2551–2556. [PubMed] [Google Scholar]
- 22.Hendrie HC, Osuntokun BO, Hall KS, et al. Prevalence of Alzheimer’s disease and dementia in two communities: Nigerian Africans and African Americans. Am J Psychiatry. 1995 Oct;152(10):1485–1492. doi: 10.1176/ajp.152.10.1485. [DOI] [PubMed] [Google Scholar]
- 23.Hofman A, Ott A, Breteler MM, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet. 1997 Jan 18;349(9046):151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- 24.Kokmen E, Beard CM, Offord KP, Kurland LT. Prevalence of medically diagnosed dementia in a defined United States population: Rochester, Minnesota, January 1, 1975. Neurology. 1989 Jun;39(6):773–776. doi: 10.1212/wnl.39.6.773. [DOI] [PubMed] [Google Scholar]
- 25.White L, Petrovitch H, Ross GW, et al. Prevalence of dementia in older Japanese-American men in Hawaii: The Honolulu-Asia Aging Study. Jama. 1996 Sep 25;276(12):955–960. [PubMed] [Google Scholar]
- 26.aaa-apa. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) Washington DC: American Psychiatric Association; 2000. [Google Scholar]
- 27.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984 Jul;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 28.Folstein M, Folstein S, McHugh P. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987 Aug;48(8):314–318. [PubMed] [Google Scholar]
- 30.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short Orientation-Memory-Concentration Test of cognitive impairment. Am J Psychiatry. 1983 Jun;140(6):734–739. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 31.Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC. The short test of mental status. Correlations with standardized psychometric testing. Arch Neurol. 1991 Jul;48(7):725–728. doi: 10.1001/archneur.1991.00530190071018. [DOI] [PubMed] [Google Scholar]
- 32.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993 Nov;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 33.Jack CR, Jr, Petersen RC, Xu YC, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology. 1997 Sep;49(3):786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jack CR, Jr, Petersen RC, Xu YC, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999 Apr 22;52(7):1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Killiany RJ, Gomez-Isla T, Moss M, et al. Use of structural magnetic resonance imaging to predict who will get Alzheimer’s disease. Ann Neurol. 2000 Apr;47(4):430–439. [PubMed] [Google Scholar]
- 36.Xu Y, Jack CR, Jr, O’Brien PC, et al. Usefulness of MRI measures of entorhinal cortex versus hippocampus in AD. Neurology. 2000 May 9;54(9):1760–1767. doi: 10.1212/wnl.54.9.1760. [DOI] [PubMed] [Google Scholar]
- 37.Wahlund LO, Almkvist O, Blennow K, et al. Evidence-based evaluation of magnetic resonance imaging as a diagnostic tool in dementia workup. Top Magn Reson Imaging. 2005 Dec;16(6):427–437. doi: 10.1097/01.rmr.0000245463.36148.12. [DOI] [PubMed] [Google Scholar]
- 38.Ramani A, Jensen JH, Helpern JA. Quantitative MR imaging in Alzheimer disease. Radiology. 2006 Oct;241(1):26–44. doi: 10.1148/radiol.2411050628. [DOI] [PubMed] [Google Scholar]
- 39.Van Gool WA, Walstra GJ, Teunisse S, Van der Zant FM, Weinstein HC, Van Royen EA. Diagnosing Alzheimer’s disease in elderly, mildly demented patients: the impact of routine single photon emission computed tomography. J Neurol. 1995 Jun;242(6):401–405. doi: 10.1007/BF00868397. [DOI] [PubMed] [Google Scholar]
- 40.Claus JJ, van Harskamp F, Breteler MM, et al. The diagnostic value of SPECT with Tc 99m HMPAO in Alzheimer’s disease: a population-based study. Neurology. 1994 Mar;44(3 Pt 1):454–461. doi: 10.1212/wnl.44.3_part_1.454. [DOI] [PubMed] [Google Scholar]
- 41.Johnson KA, Kijewski MF, Becker JA, Garada B, Satlin A, Holman BL. Quantitative brain SPECT in Alzheimer’s disease and normal aging. J Nucl Med. 1993 Nov;34(11):2044–2048. [PubMed] [Google Scholar]
- 42.Johnson KA, Holman BL, Rosen TJ, Nagel JS, English RJ, Growdon JH. Iofetamine I 123 single photon emission computed tomography is accurate in the diagnosis of Alzheimer’s disease. Arch Intern Med. 1990 Apr;150(4):752–756. [PubMed] [Google Scholar]
- 43.Bartenstein P, Minoshima S, Hirsch C, et al. Quantitative assessment of cerebral blood flow in patients with Alzheimer’s disease by SPECT. J Nucl Med. 1997 Jul;38(7):1095–1101. [PubMed] [Google Scholar]
- 44.Mielke R, Heiss WD. Positron emission tomography for diagnosis of Alzheimer’s disease and vascular dementia. J Neural Transm Suppl. 1998;53:237–250. doi: 10.1007/978-3-7091-6467-9_21. [DOI] [PubMed] [Google Scholar]
- 45.Silverman DH, Small GW, Chang CY, et al. Positron emission tomography in evaluation of dementia: Regional brain metabolism and long-term outcome. Jama. 2001 Nov 7;286(17):2120–2127. doi: 10.1001/jama.286.17.2120. [DOI] [PubMed] [Google Scholar]
- 46.Kantarci K, Jack CR, Jr, Xu YC, et al. Regional metabolic patterns in mild cognitive impairment and Alzheimer’s disease: A 1H MRS study. Neurology. 2000 Jul 25;55(2):210–217. doi: 10.1212/wnl.55.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kantarci K, Petersen RC, Boeve BF, et al. 1H MR spectroscopy in common dementias. Neurology. 2004 Oct 26;63(8):1393–1398. doi: 10.1212/01.wnl.0000141849.21256.ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neuro. 2004 Mar;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 49.Shoghi-Jadid K, Small GW, Agdeppa ED, et al. Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with Alzheimer disease. Am J Geriatr Psychiatry. 2002 Jan–Feb;10(1):24–35. [PubMed] [Google Scholar]
- 50.Small GW, Kepe V, Ercoli LM, et al. PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med. 2006 Dec 21;355(25):2652–2663. doi: 10.1056/NEJMoa054625. [DOI] [PubMed] [Google Scholar]
- 51.Nordberg A. PET imaging of amyloid in Alzheimer’s disease. Lancet Neurol. 2004 Sep;3(9):519–527. doi: 10.1016/S1474-4422(04)00853-1. [DOI] [PubMed] [Google Scholar]
- 52.Johnson KA. Amyloid imaging of Alzheimer’s disease using Pittsburgh Compound B. Curr Neurol Neurosci Rep. 2006 Nov;6(6):496–503. doi: 10.1007/s11910-006-0052-5. [DOI] [PubMed] [Google Scholar]
- 53.Becker PM, Feussner JR, Mulrow CD, Williams BC, Vokaty KA. The role of lumbar puncture in the evaluation of dementia: the Durham Veterans Administration/Duke University Study. J Am Geriatr Soc. 1985 Jun;33(6):392–396. doi: 10.1111/j.1532-5415.1985.tb07148.x. [DOI] [PubMed] [Google Scholar]
- 54.Hardy J, Gwinn-Hardy K. Genetic classification of primary neurodegenerative disease. Science. 1998 Nov 6;282(5391):1075–1079. doi: 10.1126/science.282.5391.1075. [DOI] [PubMed] [Google Scholar]
- 55.Roses AD. Apolipoprotein E and Alzheimer’s disease. In: Rosenberg RN, Prusiner SB, DiMaur oS, Barchi RL, editors. The Molecular and Genetic Basis of Neurological Disease. 2. Boston: Butterworth-Heinemann; 1997. [Google Scholar]
- 56.Farrer LA, Cupples LA, Haines JL, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Jama. 1997 Oct 22–29;278(16):1349–1356. [PubMed] [Google Scholar]
- 57.Mayeux R, Saunders AM, Shea S, et al. Utility of the apolipoprotein E genotype in the diagnosis of Alzheimer’s disease. Alzheimer’s Disease Centers Consortium on Apolipoprotein E and Alzheimer’s Disease. N Engl J Med. 1998 Feb 19;338(8):506–511. doi: 10.1056/NEJM199802193380804. [DOI] [PubMed] [Google Scholar]
- 58.Roses AD. The predictive value of APOE genotyping in the early diagnosis of dementia of the Alzheimer type: Data from three independent series. In: Iqbal K, Winblad B, Nishimura T, Takeda M, Wisniewski HM, editors. Alzheimer’s Disease: Biology, Diagnosis and Therapeutics. West Sussex, England: John Wiley & Sons; 1997. pp. 85–91. [Google Scholar]
- 59.Andreasen N, Hesse C, Davidsson P, et al. Cerebrospinal fluid beta-amyloid(1-42) in Alzheimer disease: differences between early- and late-onset Alzheimer disease and stability during the course of disease. Arch Neurol. 1999 Jun;56(6):673–680. doi: 10.1001/archneur.56.6.673. [DOI] [PubMed] [Google Scholar]
- 60.Galasko D, Chang L, Motter R, et al. High cerebrospinal fluid tau and low amyloid beta42 levels in the clinical diagnosis of Alzheimer disease and relation to apolipoprotein E genotype. Arch Neurol. 1998 Jul;55(7):937–945. doi: 10.1001/archneur.55.7.937. [DOI] [PubMed] [Google Scholar]
- 61.Hulstaert F, Blennow K, Ivanoiu A, et al. Improved discrimination of AD patients using beta-amyloid(1-42) and tau levels in CSF. Neurology. 1999 May 12;52(8):1555–1562. doi: 10.1212/wnl.52.8.1555. [DOI] [PubMed] [Google Scholar]
- 62.Shoji M, Matsubara E, Kanai M, et al. Combination assay of CSF tau, A beta 1-40 and A beta 1-42(43) as a biochemical marker of Alzheimer’s disease. J Neurol Sci. 1998 Jun 30;158(2):134–140. doi: 10.1016/s0022-510x(98)00122-1. [DOI] [PubMed] [Google Scholar]
- 63.Stefani A, Martorana A, Bernardini S, et al. CSF markers in Alzheimer disease patients are not related to the different degree of cognitive impairment. J Neurol Sci. 2006 Dec 21;251(1–2):124–128. doi: 10.1016/j.jns.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 64.Stenset V, Johnsen L, Kocot D, et al. Associations between white matter lesions, cerebrovascular risk factors, and low CSF Abeta42. Neurology. 2006 Sep 12;67(5):830–833. doi: 10.1212/01.wnl.0000234030.77831.5a. [DOI] [PubMed] [Google Scholar]
- 65.Arai H, Higuchi S, Sasaki H. Apolipoprotein E genotyping and cerebrospinal fluid tau protein: implications for the clinical diagnosis of Alzheimer’s disease. Gerontology. 1997;43 (Suppl 1):2–10. doi: 10.1159/000213879. [DOI] [PubMed] [Google Scholar]
- 66.Galasko D, Clark C, Chang L, et al. Assessment of CSF levels of tau protein in mildly demented patients with Alzheimer’s disease. Neurology. 1997 Mar;48(3):632–635. doi: 10.1212/wnl.48.3.632. [DOI] [PubMed] [Google Scholar]
- 67.Kurz A, Riemenschneider M, Buch K, et al. Tau protein in cerebrospinal fluid is significantly increased at the earliest clinical stage of Alzheimer disease. Alzheimer Dis Assoc Disord. 1998 Dec;12(4):372–377. doi: 10.1097/00002093-199812000-00020. [DOI] [PubMed] [Google Scholar]
- 68.Andreasen N, Minthon L, Clarberg A, et al. Sensitivity, specificity, and stability of CSF-tau in AD in a community-based patient sample. Neurology. 1999 Oct 22;53(7):1488–1494. doi: 10.1212/wnl.53.7.1488. [DOI] [PubMed] [Google Scholar]
- 69.Buerger K, Ewers M, Pirttila T, et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer’s disease. Brain. 2006 Nov;129(Pt 11):3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- 70.Clark CM, Xie S, Chittams J, et al. Cerebrospinal fluid tau and beta-amyloid: how well do these biomarkers reflect autopsy-confirmed dementia diagnoses? Arch Neurol. 2003 Dec;60(12):1696–1702. doi: 10.1001/archneur.60.12.1696. [DOI] [PubMed] [Google Scholar]
- 71.Schoonenboom NS, Pijnenburg YA, Mulder C, et al. Amyloid beta(1–42) and phosphorylated tau in CSF as markers for early-onset Alzheimer disease. Neurology. 2004 May 11;62(9):1580–1584. doi: 10.1212/01.wnl.0000123249.58898.e0. [DOI] [PubMed] [Google Scholar]
- 72.Buerger K, Teipel SJ, Zinkowski R, et al. CSF tau protein phosphorylated at threonine 231 correlates with cognitive decline in MCI subjects. Neurology. 2002 Aug 27;59(4):627–629. doi: 10.1212/wnl.59.4.627. [DOI] [PubMed] [Google Scholar]
- 73.de la Monte SM, Volicer L, Hauser SL, Wands JR. Increased levels of neuronal thread protein in cerebrospinal fluid of patients with Alzheimer’s disease. Ann Neurol. 1992 Dec;32(6):733–742. doi: 10.1002/ana.410320606. [DOI] [PubMed] [Google Scholar]
- 74.de la Monte SM, Wands JR. The AD7c-ntp neuronal thread protein biomarker for detecting Alzheimer’s disease. Front Biosci. 2002 Apr 1;7:d989–996. doi: 10.2741/monte. [DOI] [PubMed] [Google Scholar]
- 75.Monte SM, Ghanbari K, Frey WH, et al. Characterization of the AD7C-NTP cDNA expression in Alzheimer’s disease and measurement of a 41-kD protein in cerebrospinal fluid. J Clin Invest. 1997 Dec 15;100(12):3093–3104. doi: 10.1172/JCI119864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ghanbari K, Ghanbari HA. A sandwich enzyme immunoassay for measuring AD7C-NTP as an Alzheimer’s disease marker: AD7C test. J Clin Lab Anal. 1998;12(4):223–226. doi: 10.1002/(SICI)1098-2825(1998)12:4<223::AID-JCLA6>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Geda YE, Petersen RC. Clinical trials in mild cognitive impairment. In: Gauthier S, Cummings JL, editors. Alzheimer’s Disease and Related Disorders Annual 2001. Vol. 103. London: Martin Dunitz; 2001. pp. 69–83. [Google Scholar]
- 78.Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001 Dec;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 79.Ritchie K, Artero S, Touchon J. Classification criteria for mild cognitive impairment: a population-based validation study. Neurology. 2001 Jan 9;56(1):37–42. doi: 10.1212/wnl.56.1.37. [DOI] [PubMed] [Google Scholar]
- 80.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999 Mar;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 81.Daly E, Zaitchik D, Copeland M, Schmahmann J, Gunther J, Albert M. Predicting conversion to Alzheimer disease using standardized clinical information. Arch Neurol. 2000 May;57(5):675–680. doi: 10.1001/archneur.57.5.675. [DOI] [PubMed] [Google Scholar]
- 82.Smith GE, Petersen RC, Parisi JE, Ivnik RJ, Tangalos E, Waring S. Definition, course, and outcome of mild cognitive impairment. Aging Neuropsychology & Cognition. 1996;3(2):141–147. [Google Scholar]
- 83.Ivnik RJ, Malec JF, Smith GE, et al. Mayo’s Older Americans Normative Studies: WAIS-R, WMS-R and AVLT norms for ages 56 to 97. Clin Neuropsychol. 1992;6(Suppl):1–104. [Google Scholar]
- 84.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004 Sep;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 85.Bennett DA, Wilson RS, Schneider JA, et al. Natural history of mild cognitive impairment in older persons. Neurology. 2002 Jul 23;59(2):198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 86.Ganguli M, Dodge HH, Shen C, DeKosky ST. Mild cognitive impairment, amnestic type: an epidemiologic study. Neurology. 2004 Jul 13;63(1):115–121. doi: 10.1212/01.wnl.0000132523.27540.81. [DOI] [PubMed] [Google Scholar]
- 87.Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 1. Arch Neurol. 2003 Oct;60(10):1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 88.Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol. 1989 Feb;46(2):141–145. doi: 10.1001/archneur.1989.00520380041011. [DOI] [PubMed] [Google Scholar]
- 89.Petersen RC. Memory assessment at the bedside. In: Yanagihara TRCP, editor. Memory Disorders. New York: Dekker; 1991. pp. 137–152. [Google Scholar]
- 90.Fox NC, Warrington EK, Freeborough PA, et al. Presymptomatic hippocampal atrophy in Alzheimer’s disease. A longitudinal MRI study. Brain. 1996 Dec;119 (Pt 6):2001–2007. doi: 10.1093/brain/119.6.2001. [DOI] [PubMed] [Google Scholar]
- 91.Fox NC, Crum WR, Scahill RI, Stevens JM, Janssen JC, Rossor MN. Imaging of onset and progression of Alzheimer’s disease with voxel-compression mapping of serial magnetic resonance images. Lancet. 2001 Jul 21;358(9277):201–205. doi: 10.1016/S0140-6736(01)05408-3. [DOI] [PubMed] [Google Scholar]
- 92.Schott JM, Price SL, Frost C, Whitwell JL, Rossor MN, Fox NC. Measuring atrophy in Alzheimer disease: a serial MRI study over 6 and 12 months. Neurology. 2005 Jul 12;65(1):119–124. doi: 10.1212/01.wnl.0000167542.89697.0f. [DOI] [PubMed] [Google Scholar]
- 93.Small GW, Mazziotta JC, Collins MT, et al. Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. Jama. 1995 Mar 22–29;273(12):942–947. [PubMed] [Google Scholar]
- 94.Reiman EM, Caselli RJ, Yun LS, et al. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996 Mar 21;334(12):752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 95.Petersen RC. Mild cognitive impairment: transition between aging and Alzheimer’s disease. Neurologia. 2000 Mar;15(3):93–101. [PubMed] [Google Scholar]
- 96.Growdon JH. Biomarkers of Alzheimer disease. Arch Neurol. 1999 Mar;56(3):281–283. doi: 10.1001/archneur.56.3.281. [DOI] [PubMed] [Google Scholar]
- 97.Sunderland T, Wolozin B, Galasko D, et al. Longitudinal stability of CSF tau levels in Alzheimer patients. Biol Psychiatry. 1999 Sep 15;46(6):750–755. doi: 10.1016/s0006-3223(99)00143-2. [DOI] [PubMed] [Google Scholar]
- 98.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006 Mar;5(3):228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 99.Petersen RC, Smith GE, Ivnik RJ, et al. Apolipoprotein E status as a predictor of the development of Alzheimer’s disease in memory-impaired individuals. Jama. 1995 Apr 26;273(16):1274–1278. [PubMed] [Google Scholar]
- 100.Tierney MC, Szalai JP, Snow WG, et al. A prospective study of the clinical utility of ApoE genotype in the prediction of outcome in patients with memory impairment. Neurology. 1996 Jan;46(1):149–154. doi: 10.1212/wnl.46.1.149. [DOI] [PubMed] [Google Scholar]
- 101.Bennett DA, Schneider JA, Bienias JL, Evans DA, Wilson RS. Mild cognitive impairment is related to Alzheimer disease pathology and cerebral infarctions. Neurology. 2005 Mar 8;64(5):834–841. doi: 10.1212/01.WNL.0000152982.47274.9E. [DOI] [PubMed] [Google Scholar]
- 102.Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006 May;63(5):665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- 103.Riley KP, Snowdon DA, Markesbery WR. Alzheimer’s neurofibrillary pathology and the spectrum of cognitive function: findings from the Nun Study. Ann Neurol. 2002 May;51(5):567–577. doi: 10.1002/ana.10161. [DOI] [PubMed] [Google Scholar]
- 104.Mesulam M, Shaw P, Mash D, Weintraub S. Cholinergic nucleus basalis tauopathy emerges early in the aging-MCI-AD continuum. Ann Neurol. 2004 Jun;55(6):815–828. doi: 10.1002/ana.20100. [DOI] [PubMed] [Google Scholar]
- 105.Grudzien A, Shaw P, Weintraub S, Bigio E, Mash DC, Mesulam MM. Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer’s disease. Neurobiol Aging. 2006 Mar 27; doi: 10.1016/j.neurobiolaging.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 106.Morris JC, Storandt M, Miller JP, et al. Mild cognitive impairment represents early-stage Alzheimer disease. Arch Neurol. 2001 Mar;58(3):397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- 107.Jicha GA, Parisi JE, Dickson DW, et al. Neuropathologic outcome of mild cognitive impairment following progression to clinical dementia. Arch Neurol. 2006 May;63(5):674–681. doi: 10.1001/archneur.63.5.674. [DOI] [PubMed] [Google Scholar]
- 108.Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR. Alzheimer disease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol. 1981 Aug;10(2):122–126. doi: 10.1002/ana.410100203. [DOI] [PubMed] [Google Scholar]
- 109.Petersen RC. Scopolamine induced learning failures in man. Psychopharmacology (Berl) 1977 May 9;52(3):283–289. doi: 10.1007/BF00426713. [DOI] [PubMed] [Google Scholar]
- 110.Bowen DM, Smith CB, White P, Davison AN. Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain. 1976 Sep;99(3):459–496. doi: 10.1093/brain/99.3.459. [DOI] [PubMed] [Google Scholar]
- 111.Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet. 1976 Dec 25;2(8000):1403. doi: 10.1016/s0140-6736(76)91936-x. [DOI] [PubMed] [Google Scholar]
- 112.Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005 Jun 9;352(23):2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 113.Rosler M, Anand R, Cicin-Sain A, et al. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. Bmj. 1999 Mar 6;318(7184):633–638. doi: 10.1136/bmj.318.7184.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tariot PN, Solomon PR, Morris JC, Kershaw P, Lilienfeld S, Ding C. A 5-month, randomized, placebo-controlled trial of galantamine in AD. The Galantamine USA-10 Study Group. Neurology. 2000 Jun 27;54(12):2269–2276. doi: 10.1212/wnl.54.12.2269. [DOI] [PubMed] [Google Scholar]
- 115.Feldman H, Gauthier S, Hecker J, et al. Economic evaluation of donepezil in moderate to severe Alzheimer disease. Neurology. 2004 Aug 24;63(4):644–650. doi: 10.1212/01.wnl.0000134663.79663.6e. [DOI] [PubMed] [Google Scholar]
- 116.Courtney C, Farrell D, Gray R, et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. Lancet. 2004 Jun 26;363(9427):2105–2115. doi: 10.1016/S0140-6736(04)16499-4. [DOI] [PubMed] [Google Scholar]
- 117.Gale CR, Martyn CN, Cooper C. Cognitive impairment and mortality in a cohort of elderly people. Bmj. 1996 Mar 9;312(7031):608–611. doi: 10.1136/bmj.312.7031.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Goodwin JS, Goodwin JM, Garry PJ. Association between nutritional status and cognitive functioning in a healthy elderly population. Jama. 1983 Jun 3;249(21):2917–2921. [PubMed] [Google Scholar]
- 119.La Rue A, Koehler KM, Wayne SJ, Chiulli SJ, Haaland KY, Garry PJ. Nutritional status and cognitive functioning in a normally aging sample: a 6-y reassessment. Am J Clin Nutr. 1997 Jan;65(1):20–29. doi: 10.1093/ajcn/65.1.20. [DOI] [PubMed] [Google Scholar]
- 120.Morris MC, Beckett LA, Scherr PA, et al. Vitamin E and vitamin C supplement use and risk of incident Alzheimer disease. Alzheimer Dis Assoc Disord. 1998 Sep;12(3):121–126. doi: 10.1097/00002093-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 121.Perrig WJ, Perrig P, Stahelin HB. The relation between antioxidants and memory performance in the old and very old. J Am Geriatr Soc. 1997 Jun;45(6):718–724. doi: 10.1111/j.1532-5415.1997.tb01476.x. [DOI] [PubMed] [Google Scholar]
- 122.Sano M, Ernesto C, Thomas RG, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. The Alzheimer’s Disease Cooperative Study. N Engl J Med. 1997 Apr 24;336(17):1216–1222. doi: 10.1056/NEJM199704243361704. [DOI] [PubMed] [Google Scholar]
- 123.Miller ER, 3rd, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005 Jan 4;142(1):37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 124.Aisen PS, Davis KL. Inflammatory mechanisms in Alzheimer’s disease: implications for therapy. Am J Psychiatry. 1994 Aug;151(8):1105–1113. doi: 10.1176/ajp.151.8.1105. [DOI] [PubMed] [Google Scholar]
- 125.McGeer PL, Rogers J. Anti-inflammatory agents as a therapeutic approach to Alzheimer’s disease. Neurology. 1992 Feb;42(2):447–449. doi: 10.1212/wnl.42.2.447. [DOI] [PubMed] [Google Scholar]
- 126.Andersen K, Launer LJ, Ott A, Hoes AW, Breteler MM, Hofman A. Do nonsteroidal anti-inflammatory drugs decrease the risk for Alzheimer’s disease? The Rotterdam Study. Neurology. 1995 Aug;45(8):1441–1445. doi: 10.1212/wnl.45.8.1441. [DOI] [PubMed] [Google Scholar]
- 127.Breitner JC, Welsh KA, Helms MJ, et al. Delayed onset of Alzheimer’s disease with nonsteroidal anti-inflammatory and histamine H2 blocking drugs. Neurobiol Aging. 1995 Jul-Aug;16(4):523–530. doi: 10.1016/0197-4580(95)00049-k. [DOI] [PubMed] [Google Scholar]
- 128.Breitner JC, Gau BA, Welsh KA, et al. Inverse association of anti-inflammatory treatments and Alzheimer’s disease: initial results of a co-twin control study. Neurology. 1994 Feb;44(2):227–232. doi: 10.1212/wnl.44.2.227. [DOI] [PubMed] [Google Scholar]
- 129.The Canadian Study of Health and Aging: risk factors for Alzheimer’s disease in Canada. Neurology. 1994 Nov;44(11):2073–2080. doi: 10.1212/wnl.44.11.2073. [DOI] [PubMed] [Google Scholar]
- 130.Stewart WF, Kawas C, Corrada M, Metter EJ. Risk of Alzheimer’s disease and duration of NSAID use. Neurology. 1997 Mar;48(3):626–632. doi: 10.1212/wnl.48.3.626. [DOI] [PubMed] [Google Scholar]
- 131.Aisen PS, Davis KL, Berg JD, et al. A randomized controlled trial of prednisone in Alzheimer’s disease. Alzheimer’s Disease Cooperative Study. Neurology. 2000 Feb 8;54(3):588–593. doi: 10.1212/wnl.54.3.588. [DOI] [PubMed] [Google Scholar]
- 132.Rogers J, Kirby LC, Hempelman SR, et al. Clinical trial of indomethacin in Alzheimer’s disease. Neurology. 1993 Aug;43(8):1609–1611. doi: 10.1212/wnl.43.8.1609. [DOI] [PubMed] [Google Scholar]
- 133.Scharf S, Mander A, Ugoni A, Vajda F, Christophidis N. A double-blind, placebo-controlled trial of diclofenac/misoprostol in Alzheimer’s disease. Neurology. 1999 Jul 13;53(1):197–201. doi: 10.1212/wnl.53.1.197. [DOI] [PubMed] [Google Scholar]
- 134.Aisen PS, Schafer KA, Grundman M, et al. Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. Jama. 2003 Jun 4;289(21):2819–2826. doi: 10.1001/jama.289.21.2819. [DOI] [PubMed] [Google Scholar]
- 135.Ho L, Pieroni C, Winger D, Purohit DP, Aisen PS, Pasinetti GM. Regional distribution of cyclooxygenase-2 in the hippocampal formation in Alzheimer’s disease. J Neurosci Res. 1999 Aug 1;57(3):295–303. doi: 10.1002/(SICI)1097-4547(19990801)57:3<295::AID-JNR1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 136.Pasinetti GM, Aisen PS. Cyclooxygenase-2 expression is increased in frontal cortex of Alzheimer’s disease brain. Neuroscience. 1998 Nov;87(2):319–324. doi: 10.1016/s0306-4522(98)00218-8. [DOI] [PubMed] [Google Scholar]
- 137.Sainati SM, Ingram DM, Talwalker S, Geis G. Results of a double-blind, randomized, placebo-controlled study of celecoxib in the treatment of progression of Alzheimer’s disease. Paper presented at: Sixth International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; April 5–8, 2000; Stockholm, Sweden. 2000. [Google Scholar]
- 138.Mukherjee D, Nissen SE, Topol EJ. Risk of cardiovascular events associated with selective COX-2 inhibitors. Jama. 2001 Aug 22–29;286(8):954–959. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- 139.Topol EJ. Failing the public health--rofecoxib, Merck, and the FDA. N Engl J Med. 2004 Oct 21;351(17):1707–1709. doi: 10.1056/NEJMp048286. [DOI] [PubMed] [Google Scholar]
- 140.Launer L. Nonsteroidal anti-inflammatory drug use and the risk for Alzheimer’s disease: dissecting the epidemiological evidence. Drugs. 2003;63(8):731–739. doi: 10.2165/00003495-200363080-00001. [DOI] [PubMed] [Google Scholar]
- 141.in t’ Veld BA, Ruitenberg A, Hofman A, et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N Engl J Med. 2001 Nov 22;345(21):1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- 142.Weggen S, Eriksen JL, Das P, et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001 Nov 8;414(6860):212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 143.Weggen S, Eriksen JL, Sagi SA, et al. Evidence that nonsteroidal anti-inflammatory drugs decrease amyloid beta 42 production by direct modulation of gamma-secretase activity. J Biol Chem. 2003 Aug 22;278(34):31831–31837. doi: 10.1074/jbc.M303592200. [DOI] [PubMed] [Google Scholar]
- 144.Henderson VW, Paganini-Hill A, Emanuel CK, Dunn ME, Buckwalter JG. Estrogen replacement therapy in older women. Comparisons between Alzheimer’s disease cases and nondemented control subjects. Arch Neurol. 1994 Sep;51(9):896–900. doi: 10.1001/archneur.1994.00540210068014. [DOI] [PubMed] [Google Scholar]
- 145.Kawas C, Resnick S, Morrison A, et al. A prospective study of estrogen replacement therapy and the risk of developing Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 1997 Jun;48(6):1517–1521. doi: 10.1212/wnl.48.6.1517. [DOI] [PubMed] [Google Scholar]
- 146.Paganini-Hill A, Henderson VW. Estrogen deficiency and risk of Alzheimer’s disease in women. Am J Epidemiol. 1994 Aug 1;140(3):256–261. doi: 10.1093/oxfordjournals.aje.a117244. [DOI] [PubMed] [Google Scholar]
- 147.Paganini-Hill A, Henderson VW. Estrogen replacement therapy and risk of Alzheimer disease. Arch Intern Med. 1996 Oct 28;156(19):2213–2217. [PubMed] [Google Scholar]
- 148.Shumaker SA, Legault C, Rapp SR, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. Jama. 2003 May 28;289(20):2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 149.Rapp SR, Espeland MA, Shumaker SA, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. Jama. 2003 May 28;289(20):2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- 150.Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. Jama. 2004 Jun 23;291(24):2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 151.Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. Jama. 2004 Jun 23;291(24):2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 152.Sano M. Moving from treatment to prevention in Alzheimer’s disease with vitamin E and estrogen. Psychiatric Times. 1999;16:1–4. [Google Scholar]
- 153.McBee WL, Dailey ME, Dugan E, Shumaker SA. Hormone replacement therapy and other potential treatments for dementias. Endocrinol Metab Clin North Am. 1997 Jun;26(2):329–345. doi: 10.1016/s0889-8529(05)70250-4. [DOI] [PubMed] [Google Scholar]
- 154.Mulnard RA, Cotman CW, Kawas C, et al. Estrogen replacement therapy for treatment of mild to moderate Alzheimer disease: a randomized controlled trial. Alzheimer’s Disease Cooperative Study. Jama. 2000 Feb 23;283(8):1007–1015. doi: 10.1001/jama.283.8.1007. [DOI] [PubMed] [Google Scholar]
- 155.Henderson VW, Paganini-Hill A, Miller BL, et al. Estrogen for Alzheimer’s disease in women: randomized, double-blind, placebo-controlled trial. Neurology. 2000 Jan 25;54(2):295–301. doi: 10.1212/wnl.54.2.295. [DOI] [PubMed] [Google Scholar]
- 156.Roberts RO, Cha RH, Knopman DS, Petersen RC, Rocca WA. Postmenopausal estrogen therapy and Alzheimer disease: overall negative findings. Alzheimer Dis Assoc Disord. 2006 Jul-Sep;20(3):141–146. doi: 10.1097/00002093-200607000-00004. [DOI] [PubMed] [Google Scholar]
- 157.Masters CL, Beyreuther K. Alzheimer’s centennial legacy: prospects for rational therapeutic intervention targeting the Abeta amyloid pathway. Brain. 2006 Nov;129(Pt 11):2823–2839. doi: 10.1093/brain/awl251. [DOI] [PubMed] [Google Scholar]
- 158.Selkoe DJ. The genetics and molecular pathology of Alzheimer’s disease: roles of amyloid and the presenilins. Neurol Clin. 2000 Nov;18(4):903–922. doi: 10.1016/s0733-8619(05)70232-2. [DOI] [PubMed] [Google Scholar]
- 159.Hussain I, Powell D, Howlett DR, et al. Identification of a novel aspartic protease (Asp 2) as beta-secretase. Mol Cell Neurosci. 1999 Dec;14(6):419–427. doi: 10.1006/mcne.1999.0811. [DOI] [PubMed] [Google Scholar]
- 160.Sinha S, Anderson JP, Barbour R, et al. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999 Dec 2;402(6761):537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- 161.Vassar R, Bennett BD, Babu-Khan S, et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999 Oct 22;286(5440):735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 162.Yan R, Bienkowski MJ, Shuck ME, et al. Membrane-anchored aspartyl protease with Alzheimer’s disease beta-secretase activity. Nature. 1999 Dec 2;402(6761):533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- 163.Garino C, Tomita T, Pietrancosta N, et al. Naphthyl and coumarinyl biarylpiperazine derivatives as highly potent human beta-secretase inhibitors. Design, synthesis, and enzymatic BACE-1 and cell assays. J Med Chem. 2006 Jul 13;49(14):4275–4285. doi: 10.1021/jm0602864. [DOI] [PubMed] [Google Scholar]
- 164.Siemers ER, Quinn JF, Kaye J, et al. Effects of a gamma-secretase inhibitor in a randomized study of patients with Alzheimer disease. Neurology. 2006 Feb 28;66(4):602–604. doi: 10.1212/01.WNL.0000198762.41312.E1. [DOI] [PubMed] [Google Scholar]
- 165.Dovey HF, John V, Anderson JP, et al. Functional gamma-secretase inhibitors reduce beta-amyloid peptide levels in brain. J Neurochem. 2001 Jan;76(1):173–181. doi: 10.1046/j.1471-4159.2001.00012.x. [DOI] [PubMed] [Google Scholar]
- 166.Eriksen JL, Sagi SA, Smith TE, et al. NSAIDs and enantiomers of flurbiprofen target gamma-secretase and lower Abeta 42 in vivo. J Clin Invest. 2003 Aug;112(3):440–449. doi: 10.1172/JCI18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Anderson JJ, Holtz G, Baskin PP, et al. Reductions in beta-amyloid concentrations in vivo by the gamma-secretase inhibitors BMS-289948 and BMS-299897. Biochem Pharmacol. 2005 Feb 15;69(4):689–698. doi: 10.1016/j.bcp.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 168.Black S, Wilcock G, Haworth J, et al. Efficacy and safety of MPC-7869 (R-flurbiprofen), a selective ab42-lowering agent, in mild Alzheimer’s disease (AD): Results of a 12-month Phase 2 trial and 1-year follow-on study. Neurology. 2006 MAR 14 2006;66(5 Suppl 2):A347–A347. [Google Scholar]
- 169.Schenk D, Barbour R, Dunn W, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999 Jul 8;400(6740):173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 170.Orgogozo JM, Gilman S, Dartigues JF, et al. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003 Jul 8;61(1):46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- 171.Gilman S, Koller M, Black RS, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005 May 10;64(9):1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- 172.Masliah E, Hansen L, Adame A, et al. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005 Jan 11;64(1):129–131. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- 173.Fox NC, Black RS, Gilman S, et al. Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005 May 10;64(9):1563–1572. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]
- 174.Dodel R, Hampel H, Depboylu C, et al. Human antibodies against amyloid beta peptide: a potential treatment for Alzheimer’s disease. Ann Neurol. 2002 Aug;52(2):253–256. doi: 10.1002/ana.10253. [DOI] [PubMed] [Google Scholar]
- 175.Dodel RC, Du Y, Depboylu C, et al. Intravenous immunoglobulins containing antibodies against beta-amyloid for the treatment of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004 Oct;75(10):1472–1474. doi: 10.1136/jnnp.2003.033399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Adamiak B, Monthe C, Bender H, et al. Intravenous immunoglobulin (IVIg) maintains cognition over 18 months in patients with Alzheimer’s disease (AD) Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 2006/07. 2006;2(3):S62–S63. [Google Scholar]
- 177.Chung JA, Cummings JL. Neurobehavioral and neuropsychiatric symptoms in Alzheimer’s disease: characteristics and treatment. Neurol Clin. 2000 Nov;18(4):829–846. doi: 10.1016/s0733-8619(05)70228-0. [DOI] [PubMed] [Google Scholar]
- 178.Henderson DC, Cagliero E, Copeland PM, et al. Glucose metabolism in patients with schizophrenia treated with atypical antipsychotic agents: a frequently sampled intravenous glucose tolerance test and minimal model analysis. Arch Gen Psychiatry. 2005 Jan;62(1):19–28. doi: 10.1001/archpsyc.62.1.19. [DOI] [PubMed] [Google Scholar]
- 179.Ballard C, Bannister C, Solis M, Oyebode F, Wilcock G. The prevalence, associations and symptoms of depression amongst dementia sufferers. J Affect Disord. 1996 Jan 22;36(3–4):135–144. doi: 10.1016/0165-0327(95)00072-0. [DOI] [PubMed] [Google Scholar]
- 180.Borson S, Raskind MA. Clinical features and pharmacologic treatment of behavioral symptoms of Alzheimer’s disease. Neurology. 1997 May;48(5 Suppl 6):S17–24. doi: 10.1212/wnl.48.5_suppl_6.17s. [DOI] [PubMed] [Google Scholar]
- 181.Devanand DP, Jacobs DM, Tang MX, et al. The course of psychopathologic features in mild to moderate Alzheimer disease. Arch Gen Psychiatry. 1997 Mar;54(3):257–263. doi: 10.1001/archpsyc.1997.01830150083012. [DOI] [PubMed] [Google Scholar]
- 182.Mega MS, Cummings JL, Fiorello T, Gornbein J. The spectrum of behavioral changes in Alzheimer’s disease. Neurology. 1996 Jan;46(1):130–135. doi: 10.1212/wnl.46.1.130. [DOI] [PubMed] [Google Scholar]
- 183.Reisberg B, Borenstein J, Salob SP, Ferris SH, Franssen E, Georgotas A. Behavioral symptoms in Alzheimer’s disease: phenomenology and treatment. J Clin Psychiatry. 1987 May;48 (Suppl):9–15. [PubMed] [Google Scholar]
- 184.Cohen-Mansfield J, Deutsch LH. Agitation: Subtypes and Their Mechanisms. Semin Clin Neuropsychiatry. 1996 Oct;1(4):325–339. doi: 10.1053/SCNP00100325. [DOI] [PubMed] [Google Scholar]
- 185.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994 Dec;44(12):2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 186.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000 Spring;12(2):233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 187.Devanand DP, Sano M, Tang MX, et al. Depressed mood and the incidence of Alzheimer’s disease in the elderly living in the community. Arch Gen Psychiatry. 1996 Feb;53(2):175–182. doi: 10.1001/archpsyc.1996.01830020093011. [DOI] [PubMed] [Google Scholar]
- 188.Cooper JK, Mungas D, Verma M, Weiler PD. Psychotic symptoms in Alzheimer’s disease. Int J Geriatr Psychiatry. 1991;6(10):721–726. [Google Scholar]
- 189.Rubin EH, Drevets WC, Burke WJ. The nature of psychotic symptoms in senile dementia of the Alzheimer type. J Geriatr Psychiatry Neurol. 1988 Jan;1(1):16–20. doi: 10.1177/089198878800100104. [DOI] [PubMed] [Google Scholar]
- 190.Stern Y, Albert M, Brandt J, et al. Utility of extrapyramidal signs and psychosis as predictors of cognitive and functional decline, nursing home admission, and death in Alzheimer’s disease: prospective analyses from the Predictors Study. Neurology. 1994 Dec;44(12):2300–2307. doi: 10.1212/wnl.44.12.2300. [DOI] [PubMed] [Google Scholar]
- 191.Katz IR, Jeste DV, Mintzer JE, Clyde C, Napolitano J, Brecher M. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. Risperidone Study Group. J Clin Psychiatry. 1999 Feb;60(2):107–115. doi: 10.4088/jcp.v60n0207. [DOI] [PubMed] [Google Scholar]
- 192.McManus DQ, Arvanitis LA, Kowalcyk BB. Quetiapine, a novel antipsychotic: experience in elderly patients with psychotic disorders. Seroquel Trial 48 Study Group. J Clin Psychiatry. 1999 May;60(5):292–298. [PubMed] [Google Scholar]
- 193.Street JS, Clark WS, Gannon KS, et al. Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing care facilities: a double-blind, randomized, placebo-controlled trial. The HGEU Study Group. Arch Gen Psychiatry. 2000 Oct;57(10):968–976. doi: 10.1001/archpsyc.57.10.968. [DOI] [PubMed] [Google Scholar]
- 194.Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006 Oct 12;355(15):1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- 195.Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry. 2006 Mar;14(3):191–210. doi: 10.1097/01.JGP.0000200589.01396.6d. [DOI] [PubMed] [Google Scholar]
- 196.McAllister TW. Apathy. Semin Clin Neuropsychiatry. 2000 Oct;5(4):275–282. doi: 10.1053/scnp.2000.9557. [DOI] [PubMed] [Google Scholar]
- 197.Deutsch LH, Bylsma FW, Rovner BW, Steele C, Folstein MF. Psychosis and physical aggression in probable Alzheimer’s disease. Am J Psychiatry. 1991 Sep;148(9):1159–1163. doi: 10.1176/ajp.148.9.1159. [DOI] [PubMed] [Google Scholar]
- 198.Sultzer DL, Gray KF, Gunay I, Berisford MA, Mahler ME. A double-blind comparison of trazodone and haloperidol for treatment of agitation in patients with dementia. Am J Geriatr Psychiatry. 1997 Winter;5(1):60–69. [PubMed] [Google Scholar]
- 199.Tariot PN, Erb R, Podgorski CA, et al. Efficacy and tolerability of carbamazepine for agitation and aggression in dementia. Am J Psychiatry. 1998 Jan;155(1):54–61. doi: 10.1176/ajp.155.1.54. [DOI] [PubMed] [Google Scholar]
- 200.Porsteinsson AP, Tariot PN, Erb R, Gaile S. An open trial of valproate for agitation in geriatric neuropsychiatric disorders. Am J Geriatr Psychiatry. 1997 Fall;5(4):344–351. doi: 10.1097/00019442-199700540-00010. [DOI] [PubMed] [Google Scholar]