Abstract
A possible role of allelic variation on chromosome 19q13 in multiple sclerosis (MS) susceptibility has been suggested. We tested association of sixteen 19q13 markers with MS in 459 families. Nominally significant associations were tested in an independent set of 323 families as well as in the pooled set of 782 families. We were not able to confirm previously suggested associations with APOE, GIPR, ZNF45, ILT6 and D19S585. In the screening dataset nominally significant associations were found with D19S867 and with APOE haplotype (p=0.007 in both), but these were not replicated in the independent dataset nor in the pooled analysis of 757 families. Thus, we were not able to detect any statistically significant allelic associations. Re-sequencing based approaches may be required for elucidating the role chromosome 19q13 with MS.
Keywords: Multiple sclerosis, APOE, HLA-DR15, ILT6
1. Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system resulting in demyelination and axonal damage (Compston and Coles, 2002). Its etiology is still unclear, but both genetic and environmental factors have been shown to be involved. Multiple genes, still mostly unknown, are likely to modify disease susceptibility. The most consistent genetic finding thus far is the association between MS and the HLA-DR15,DQ6 haplotype (Olerup and Hillert, 1991; Lincoln et al., 2005; Hafler et al., 2007). Other loci have been more difficult to detect, but recently associations with a small number of genes have been replicated in more than one population, such as protein kinase C alpha (PRKCA) (Barton et al., 2004; Saarela et al., 2006), IRF5 (Kristjansdottir et al., 2008), EVI5 (Hafler et al., 2007; Hoppenbrouwers et al., 2008), the interleukin 2 and 7 receptor alpha chain genes respectively (IL2RA and IL7RA) (Zhang et al., 2005; Gregory et al., 2007; Hafler et al., 2007).
Chromosome 19q13 is one of the genomic regions that reportedly exhibits clustering of predisposing loci in several putative autoimmune diseases (Becker et al., 1998). In systemic lupus erythematosus 19q13 has been suggested, based on linkage studies, as a susceptibility locus in European Americans (Xing et al., 2007). In MS the situation is presently ambiguous. There are several reports providing weak evidence for an MS susceptibility gene on 19q13, but there is lack of replication (especially in allelic associations), and the signals have been scattered in distinct subregions of 19q13. A meta-analysis of linkage screens identified 19q13 as one of the most interesting non-HLA loci (Wise et al., 1999). In our previous study in Finnish multiplex families we obtained weak linkage evidence for the 19q13.1 subregion (Reunanen et al., 2002). Here we have performed a larger follow-up study to test, via allelic association, whether we are able to obtain evidence for an MS susceptibility locus in 19q13.
2. Materials and methods
2.1. Families
In association analyses two independent Finnish MS family datasets were used. Datasets contained nuclear families with an MS index case diagnosed as clinically definite or laboratory supported definite MS according to Poser criteria. The patients included both relapsing-remitting and primary progressive MS cases.
Dataset-1 consisted of 459 MS trio families. The patients were ascertained in the University Central Hospitals of Helsinki (n = 130), Turku (n = 139), Tampere (n = 13), Kuopio (n = 62), and Oulu (n = 13), as well as in Seinäjoki Central Hospital (n = 102). Twelve of the families contained multiply affected offspring, in these cases the oldest affected sibling was chosen for association analysis. Patients mean year of birth was 1959 (range 1934-1983), female:male ratio 2.4 and the percentage of HLA-DR15-positive patients 57%.
Dataset-2 consisted of 323 MS trio families. The patients were ascertained in the University Central Hospitals of Helsinki (n = 109), Tampere (n = 89), Kuopio (n = 17), and Oulu (n = 60), as well as in Seinäjoki Central Hospital (n = 35). Patients mean year of birth was 1959 (range 1943-1982), female:male ratio 2.3 and the percentage of HLA-DR15-positive patients 58%.
Case-control association analysis was carried out for APOE and ILT6 to complement the family-based analysis. For the APOE analysis the controls constituted two non-selected population based cohorts of individuals from Southern Finland, previously genotyped for the APOE markers (Strandberg et al., 1996; Eriksson et al., 2001). For the ILT6 association analysis we genotyped 207 controls. These controls consisted of non-parkinsonian spouses of Parkinson disease patients collected in Helsinki University Central Hospital (n = 96) and Seinajoki Central Hospital (n = 41) (Eerola et al., 2003) as well as a sample of thymus specimen (n = 70) obtained in Helsinki University Central Hospital, Children’s Hospital from cardiac surgery of infants. Parts of thymus often need to be removed for technical reasons, normally these are discarded. We have collected these specimens to analyse the effect of common allelic variation on thymic mRNA expression. The thymus specimens were obtained without any personal identifications. This study has been approved by the Ethics Committee for the Department of Neurology, Helsinki University Central Hospital (Decision 63/1999) and has been updated by the Ethics Committee for Ophthalmology, Otorhinolaryngology, Neurology and Neurosurgery in the Hospital District of Helsinki and Uusimaa. (Decision 46/2002, Dnro 192/E9/02).
2.2. DNA analysis
DNA was extracted from peripheral blood leukocytes using standard procedures. The microsatellites D19S876 and D19S585 and their genotyping protocols were obtained from the genome database (http://gdbwww.dkfzheidelberg.de/). The microsatellites D19S1175 and D19S610 (Kestila et al., 1998), D19S400, D19S582 and ATP1A3 (Reunanen et al., 2002) and their genotyping protocols were obtained as described. Autoradiography of the polyacrylamide gels using [33P-a] dATP was used for microsatellite genotyping.
Two candidate genes TYRO protein tyrosine kinase binding protein/DNAX-activation protein 12 (TYROBP/DAP12, here called DAP12) and Homo sapiens hematopoietic cell signal transducer/DNAX-activation protein 10 (HCST/DAP10, here called DAP10) were re-sequenced. These genes are located close to the marker D19S1175 that provided a lod score of 1.8 in our previous study (Reunanen et al., 2002). We re-sequenced the proband of a multiplex family that individually contributed a lod score of 0.95. Of the DAP12 gene (5 exons), we sequenced 640 bp of the promoter, exons 1-4, introns 1-3, 152 bp of the distal intron-4 and the 140 bp of the proximal part of exon-5 (the 3′ untranslated region not fully sequenced). Of the DAP10 gene we sequenced 89 bp of the promoter, exons 1-3, introns 1-3 and 91 bp of the proximal part of exon-4 (the 3′ untranslated region not fully sequenced). Of the DAP10 gene (4 exons) we sequenced 89 bp of the promoter, exons 1-3, introns 1-3 and 91 bp of the proximal part of exon-4 (the 3′ untranslated region not fully sequenced). By re-sequencing no single nucleotide polymorphism (SNPs) were found in the DAP10 gene, whereas three non-coding SNPs were found in the DAP12 gene. These were located in the promoter (A->G at −493 bp from exon-1), in the exon-15′-untranslated region (G->A at position +32) and intron-1 (A->T at position 249 of intron-1). The heterozygocity for these SNPs rules out the deletion implicated in polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy (PLO-SL), a rare recessively inherited disorder with degeneration of brain white matter and bones (Paloneva et al., 2000). A more detailed analysis of PLO-SL mutations is reported in a separate manuscript, in which both of the two genes implicated in PLO-SL (TREM2 on chromosome 6p21 and DAP12 on 19q13) have been analysed by mutation screening (Sulonen et al., 2008).
The promoter (DAP12/StyI) and exon-1 (DAP12/HaeIII) SNPs found by re-sequencing were included to the association analysis. The other SNPs were obtained from the SNP homepage (http://www.ncbi.nlm.nih.gov/SNP/) or previous publications. The SNPs were amplified by PCR and genotyped by restriction enzyme digestion and agarose or by solid-phase minisequencing (Syvanen, 1999). The following primers were used: rs1371624 5-aaa tgg gtg att caa tac ag-3 and 5-ctt tat ttt taa tgg aat ata c-3 (minisequencing primer: 5-aca gag ggc tgc cag aag gg-3). rs3977: 5-cat aga aat gga aag cag gtg tag-3 and 5-gga agt aag tat tag cca gag gag-3 (detection with HaeIII). Rs2396449: 5-cat tag gcg gtt ggt tac ac-3 and 5-acg caa cat agg cca cat ac-3 (minisequencing primer 5-ttt gcc taa gcc aga tgt tt-3). rs1456554: 5-cat cgg agt gtt acc tta cc-3 and 5-tgc ctc cag tat gag tgt ag-3 (minisequencing primer: 5 ttc ttg agg aga cct ttt cc). rs917314: 5-gat caa gac agc ggt aga ag-3 and 5-gta cct cca cca ctt act tg-3 (detection with SspI). DAP12/StyI: 5-gac cct ggt cta gga act tta a-3 and 5-tta aca gga ggg tca gtg tc-3. DAP12/HaeIII: 5-ctc aga ctt cct cct tca ct-3 and 5-ccc taa ctc acc act tac ag-3. PVRL2 (rs394221): 5-tgg aag gcg gcc aca ttc tg-3 and 5-tct cca cct aga ccc act tg-3 (detection with SphI). GIPR (rs 1800437): 5-cgc att ctt ggc att ctc ct-3 and 5-att cag tgg ctg cac cag ag-3 (minisequencing primer: 5-cac ggg agc aaa cac cac ct-3). ZNF45 (rs 388706): 5-agc tct tgc cac atg cat tg-3 and 5-tgg gaa aag ctc aca ttg tc-3 (minisequencing primer: 5-aca ctt ata tgg ttt ctt tcc ag-3). The primers for the SNPs APOE-219, APOE+113 and APOE ε were obtained from previous publication (Reunanen et al., 2002) and the detection of the ILT6 deletion was performed as described (Koch et al., 2005). None of the markers deviated from Hardy-Weinberg equilibrium (p>0.05 for all markers). The genotyping success was >95% for all markers. All markers were in Hardy-Weinberg equilibrium at p>0.05.
2.3. Statistical analysis
We applied the transmission disequilibrium test (TDT) (Spielman et al., 1993) to analyse whether allelic association between MS and chromosome 19q13 markers could be found. The genotype data was analysed using the TRANSMIT 2.5.2 computer program package (Clayton, 1999). Alleles that were included to the association analysis had to be transmitted from at least 20 informative meioses, corresponding to the Var (O-E) value of 5 or more in the TRANSMIT analysis. In case-control association analysis the haplotype frequency estimation was performed using the expectation-maximisation algorithm as implemented in the HAPLO program (ftp:krunch.edu.etc.). Association analyses were carried out in the total material and in subgroups according to the patients’ carriage status of HLA-DR15 and according to the geographic origin of patients’ parents. The DR15 subdivision was based on prior observations that linkage evidence on 19q13 differed in DR15 positive and negative families (Green et al., 2001; Pericak-Vance et al., 2004; Reunanen et al., 2002). Subdivision on geographic origin was based on our previous observations on genetic founder effect in MS patients originating from the Southern Ostrobothnian high-risk region in Western Finland (Sumelahti et al., 2000; Pihlaja et al., 2003; Tienari et al., 2004). If at least one of the parents were born in this high-risk region, the family was included to the subgroups denoted here as Bothnia. Using the dataset-1 with 459 MS trio families we have approximately 80% power to detect a dominant predisposing variant with an odds ratio of 1.5 (marker and disease allele frequencies set to 0.20, D’ value 1.0). With lower disease allele frequencies the power decreases. With disease allele frequencies of 0.10, 0.05 and 0.01, the power is 72%, 52% and 17%, respectively. Power in the TDT was estimated using the Genetic Power Calculator (http://pngu.mgh.harvard.edu/~purcell/gpc/).
3. Results
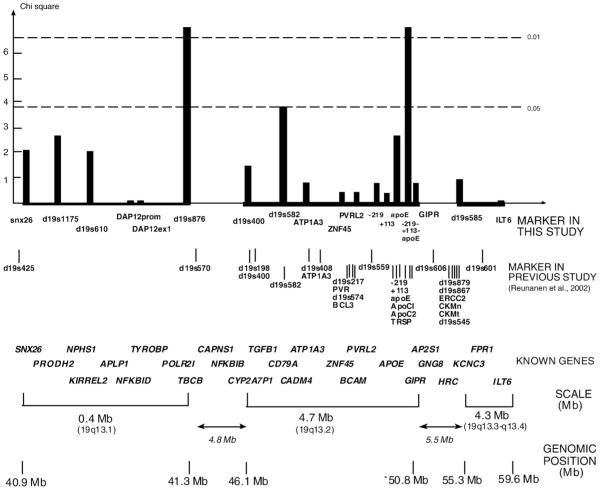
We analysed association of 19q13 marker with MS in 459 Finnish MS trio families using the transmission disequilibrium test (TDT). We tested 16 markers, 10 single nucleotide polymorphisms (SNPs) and 6 microsatellites (Fig. 1). The three APOE SNPs were analysed both singly and as 3-marker haplotypes. Two of the markers showed a nominally significant association with MS. The microsatellite D19S876 on 19q13.1 subregion, and APOE haplotype on 19q13.2 subregion both showed a nominal allele-wise p=0.007 for association with MS with (Fig. 1). Due to multiple comparisons these associations cannot be regarded as statistically significant.
Fig. 1.
Association of chromosome 19q13 markers with MS. The most significant allele-wise (1 df) chi-square values are shown. Known genes of the regions are positioned in the figure as landmarks. The markers were genotyped in 459 MS trio families, except ILT6, GIPR, and ZNF45, which were genotyped in 186 families. Linkage disequilibrium (LD) between pairs of SNP markers was analysed with Haploview program. Lod score ≥3 was used as criterion for significant LD. Microsatellite-microsatellite and microsatellite-SNP LD was analysed by comparing the observed haplotype distribution in fully informative families to the distribution expected on the basis of marker allele frequencies using the χ2 test. The p-value p<0.001 was used as criterion for significant LD. In the 19q13.1 subregion all the markers exhibited significant LD except the markers pairs SNX26/DAP12-StyI and DAP12-StyI/DAP12-HaeIII (data not shown). In the 19q13.2 subregion the APOE SNPs were the only markers that showed significant LD with each other, and 19q13.3-q13.4 subregion markers were not in significant LD (data not shown). There was no LD between the subregions.
Subgroup analysis in Bothnia (n=94) and non-Bothnia families (n=365) (see Materials and methods: Section 2.3) did not reveal any nominally significant associations in either subgroup (data not shown). Subgroup analysis according to the presence or absence of HLA-DR15 in the patients showed that the association signal for the microsatellite D19S876 slightly strengthened in DR15-negative families (p=0.002).
We re-sequenced two candidate genes, DAP12 and DAP10, of the 19q13.1 region in the proband of the multiplex family that contributed mostly to the lod score of 1.8 with D19s1175 (Reunanen et al., 2002). No insertions/deletions or non-synonymous substitutions were found. Three SNPs were found (at the promoter, exon-1, intron-1) in the DAP12 gene (see more details in Materials and methods: Section 2.2). The DAP12 promoter and exon-1 SNPs were included in the above analysis and did not provide any evidence for allelic association.
ILT6 null alleles on 19q13.4 were also tested in case-control setting in addition to TDT. ILT6 deficiency (−/− genotype) has been found in increased frequency in German MS patients (7.1%) vs. controls (3.8%) (Koch et al., 2005). The frequency of the −/− genotype was 14.0% in MS patients, 14.0% in the parents of MS patients and 11.6% in the controls (MS patients vs. controls p=0.34), indicating no association between ILT6 deficiency and MS susceptibility.
D19S876 microsatellite and APOE haplotypes have been previously associated with MS but the associated alleles were different than in the present study (D’Alfonso et al., 2000; Schmidt et al., 2002). Both association signals, we obtained, were negative association (under-transmission of the associated allele/haplotype). The APOE haplotype association was mainly due to the under-transmission of −219T/+113C/ε3 haplotype to MS patients. Interestingly, the same APOE haplotype has been previously associated with protection against Alzheimer’s disease in Finns (Myllykangas et al., 2002). In order to further verify thesefindings, we analysed these markers in another independent dataset of 323 MS families. The association with D19S876 was not replicated (Table 1A). When the two datasets were pooled the association was of borderline nominal significance (p=0.05, Table 1A), while subpopulation analysis according to DR15 stratification yielded p=0.03 in DR15-negative and p=0.17 in DR 15-positive families. However, due to the number of comparisons made, the obtained nominal p-values cannot be regarded statistically significant. The APOE haplotype association was not replicated either (Table 1B). Furthermore, APOE haplotype analysis in case-control setting in 737 MS patients and 1156 controls did not provide any evidence for an association (Table 1B).
Table 1.
Association of marker d19s876 and −219/+113/APOE haplotype with MS
| Allele | Allele |
Transmit |
Transmit |
Transmit |
|---|---|---|---|---|
| Frequencya |
(1df) p-value |
(1df) p-value |
(1df) p-value |
|
| (n=733 families) | Dataset-1 (n=418 families) | Dataset-2 (n=315 families) | Pooled (n=733 families) | |
|
| ||||
| A) Family-based association analysis of marker D19S867 | ||||
| 1 | 0.006 | na | na | na |
| 2 | 0.010 | na | na | 0.69 |
| 3 | 0.034 | 0.85 | 0.77 | 0.91 |
| 4 | 0.112 | 0.84 | 0.06b | 0.32 |
| 5 | 0.236 | 0.007b | 0.86 | 0.05b |
| 6 | 0.331 | 0.24 | 0.67 | 0.31 |
| 7 | 0.219 | 0.27 | 0.39 | 0.12 |
| 8 | 0.014 | 0.48 | 0.82 | 0.54 |
| 11 | 0.035 | 0.96 | 0.34 | 0.51 |
| Global p=0.43 (12df) | Global p=0.57 (10df) | Global p=0.45 (12df) | ||
| B) Family-based association analysis of APOE haplotypes | ||||
|---|---|---|---|---|
| Haplotype | Haplotype frequency1 |
Transmit |
Transmit |
Transmit |
| (n=753 families) | (1df) p-value |
(1df) p-value |
(1df) p-value |
|
| Dataset-1 (n=440 families) | Dataset-2 (n=317 families) | Pooled (n=753 families) | ||
| G-G-ε2 | 0.053 | 0.07c | 0.78c | 0.11c |
| G-C-ε2 | 0.001 | na | na | na |
| G-G-ε3 | 0.474 | 0.58 | 0.86 | 0.74 |
| T-G-ε3 | 0.008 | na | na | na |
| G-C-ε3 | 0.004 | na | na | na |
| T-C-ε3 | 0.280 | 0.007b | 0.30b | 0.22b |
| G-G-ε4 | 0.032 | 0.21 | 0.51 | 0.13 |
| T-G-ε4 | 0.149 | 0.11 | 0.55 | 0.37 |
| Global p=0.03 (7df) | Global p=0.64 (9df) | Global p=0.08 (9df) | ||
| C) Case-control association analysis of APOE haplotypes | ||
|---|---|---|
| Haplotype | MS patients % |
Controls % |
| (n=737d) | (n=1156) | |
| G-G-ε2 | 0.059 | 0.062 |
| G-C-ε2 | 0 | 0 |
| G-G-ε3 | 0.478 | 0.447 |
| T-G-ε3 | 0.006 | 0.004 |
| G-C-ε3 | 0.005 | 0.003 |
| T-C-ε3 | 0.271 | 0.295 |
| G-G-ε4 | 0.022 | 0.022 |
| T-G-ε4 | 0.157 | 0.161 |
| Global p=0.16 (8df) | ||
The haplotype frequency estimate in parental chromosomes.
Under-transmission.
Over-transmission.
Only patients with 100% complete genotype information were included.
4. Discussion
Chromosome 19q13 has provided evidence for a genetic effect in MS repeatedly (Table 2). In our earlier linkage study, we obtained a LOD score of 1.8 in DR15-negative multiplex families, peaking at the marker D19S1175 on 19q13.1 (Reunanen et al., 2002). The 19q13.1 subregion has also been suggested in an association study, in which the microsatellite marker D19S876 was associated with MS in continental Italian patients (D’Alfonso et al., 2000). Here, we were not able to detect statistically significant allelic association with D19S1175, D19S876 or other 19q13.1 subregion markers with MS. The subregion 19q13.2 has been suggested by allelic associations with markers within or close to APOE (Barcellos et al., 1997; Schmidt et al., 2002; Burton et al., 2007) and by linkage evidence (Pericak-Vance et al., 2001). In our previous analysis (Reunanen et al., 2002) we analysed microsatellite markers of 19q13.2, which did not associate with MS. Here, we carried out a larger study to analyse the APOE locus more thoroughly but, again, did not find any evidence for a significant allelic association. The 19q13.4 subregion has been suggested by two studies. In a German study a null allele of ILT6 was associated with MS, and a British study utilising pooled DNA provided signal at the microsatellite D19S585 (Koch et al., 2005; Yeo et al., 2003).
Table 2.
Previous studied on the role of chromosome 19q13 in MS susceptibility
| Reference | Type of study | Best marker/subregion | Country | Subjects | Significance |
|---|---|---|---|---|---|
| Sawcer et al. (1996) | Linkage | d19s246/19q13.4 | UK | 227 multiplex families | LOD=1 |
| Haines et al. (1996) | Linkage | d19s219/19q13.2 | USA | 52 multiplex families | LOD=1.13 |
| Ebers et al. (1996) | Linkage | d19s47/19q13.2 | Canada | 100 sibling pairs | LOD=0.73 |
| Kuokkanen et al. (1997) | Linkage | d19s246/19q13.4 | Finland | 16 multiples families | LOD=1 |
| D’Alfonso et al. (1999) | Linkage | 19q13.3 | Italy | 69 families | LOD<0.7 |
| Coraddu et al. (2001) | Linkage | 19q13 | Italy/Sardinia | 49 multiplex families | LOD<0.7 |
| Broadley et al. (2001) | Linkage | 19q13 | Italy | 40 multiplex families | LOD<0.7 |
| Green et al. (2001) | Linkage | CEA/19q13.2 | USA | 161 multiplex | LOD=1.25 |
| Xu et al. (2001) | Linkage | D19S246/19q13.4 | Sweden | 46 multiplex families | NPL score -0.46 |
| Reunanen et al. (2002) | Linkage | d19s876/19q13.1 | Finland | 27 multiplex families | LOD=1.8 |
| Haines et al. (2002) | Linkage | d19s879/19q13.4 | USA | 98 multiplex families | LOD=3.01 |
| Lucotte et al. (2002) | Linkage | 19q13.3 | France | 18 multiplex families | LOD=2.1 |
| Pericak-Vance et al. (2004) | Linkage | D19S217/19q13.2 | USA | 98 multiplex families | LOD=2.17 |
| Pericak-Vance et al. (2004) | Linkage | D19S217/19q13.2 | USA | 53 families, HLA-DR15+ | LOD=2.37 |
| Pericak-Vance et al. (2004) | Linkage | D19S217/19q13.2 | France | 90 families | LOD<0.7 |
| Haghighi et al. (2006) | Linkage with OCBa | D2S219/19q13.2 | Sweden | 2 extended families | LOD=1.8 |
| Schmidt et al. (2002) | Association | APOE ε2-haplotype | USA | 328 families | p=0.005 |
| Yeo et al. (2003) | Association | d19s585/19q13.4 | UK | 961 patients | p=0.12 |
| Ban et al. (2003) | Association | d19s219/19q13.2 | Australia | 217 patients | p=0.009 |
| Koch et al. (2005) | Association | ILT6/19q13.4 | Germany, France | 751 patients | p=0.009 |
| Burwick et al. (2006) | Association | APOE/19q13.2 | Meta-analysis | 3200 patients | p>0.05 |
| Burton et al. (2007) | Association | ZNF45 and GIPR/19q13.2 | UK | 1000 patients | p=0.00005 and p=0.0008 |
| Hafler et al. (2007) | Associationb | MAG, CD22, TYROBP/19q13.1c | UK, USA | 931 trios/2431 controls | p>0.05 |
| Hafler et al. (2007) | Associationb | APOE, ZNF45, GIPR/19q13.2c | UK, USA | 931 trios/2431 controls | p>0.05 |
| Hafler et al. (2007) | Associationb | synaptogyrin4/13q13.3d | UK, USA | 931 trios/2431 controls | p=0.02/0.0003 |
| Hafler et al. (2007) | Associationb | ZNF577/19q13.4d | UK, USA | 931 trios/2431 controls | p=0.04/0.001 |
OCB = oligoclonal bands in cerebrospinal fluid.
We search for association on chromosome 19q13 between positions 40 Mb and 60 Mb for the following criteria: TDT statistics have a p-value<0.05 or CMH statistics have a p-value<0.001 (https://imsgc.org/php/results.php).
The 19q13.1 region harbouring MAG, CD22, TYROBP (40-42 Mb) and the 19q13.2 region harbouring APOE, ZNF45 and GIPR (49-51 Mb) were analysed with 128 and 177 markers, respectively, all of which failed to show any association (in both TDT and CMH statistics).
These markers fulfilled the association criteria.
In the present study, utilising 459 Finnish MS families in screening and 782 in thefinal analysis, we did not find statistically significant evidence for allelic association with any of the markers tested. Although the statistical power might not be enough for detecting very small effects, it has to be noted that due to the characteristics of the Finnish population, the statistical power is at a much higher level than the number of families reveals. The Finnish population has a relatively small number of founders and has remained relatively isolated for centuries (Peltonen et al., 2000). This type of populations poses certain advantages in genetic association studies. First, there is higher level of linkage disequilibrium. This has been exemplified by the finding that the r2 measure of LD between SNPs that are more than 200 kb apart has been shown to be 4.7-fold greater in the Finnish population compared to the CEPH population (Shifman and Darvasi, 2001). Second, there is less genetic heterogeneity and polymorphism in an isolated population. For the Finnish population reduced genetic heterogeneity has been confirmed by the reduced number of polymorphic SNPs: 13% of SNPs which were polymorphic in the CEPH families were non-polymorphic in the Finns (Taillon-Miller et al., 2000). As the result of the above points, we can anticipate that there is less polymorphism among the Finns (less allelic heterogeneity), fewer causative loci (less locus heterogeneity) and more LD, all increasing the power of the present study.
The putative MS susceptibility locus on chromosome 19q13 remains elusive. Our present results indicate that the indirect approach (via allelic association) for the detection of the disease predisposing variants might be of limited value. There is more consistency in the linkage results (the majority of the studies are modestly positive) than in the association results. Association studies, including high-density genome-wide association studies, are well suited, when a relatively common major allele underlines the disease risk (as is the case with HLA-DR15,DQ6 haplotype in MS). The situation is likely to be more complex, if there are multiple predisposing variants in a gene (allelic heterogeneity), either common or rare, or if multiple genes within the same region are involved. Re-sequencing-based approaches are probably needed to complement linkage and association studies, in order to clarify the role of 19q13 in MS susceptibility.
Acknowledgements
We are indebted to the patients and their families for participation to the study. We thank Ms. Lilja Jansson for excellent technical assistance. This study was financially supported by grants from the Finnish Academy, The Center of Excellence for Disease Genetics of the Academy of Finland, the Sigrid Juselius Foundation, Helsinki University Central Hospital, the Paulo Foundation, and the Finnish Cultural Foundation. AB has been a recipient of a CIMO fellowship and is currently a member of the Helsinki Biomedical Graduate School (HBGS).
References
- Ban M, Sawcer SJ, Heard RNS, Bennetts BH, Adams S, Booth D, Perich V, Setakis E, Compston A, Stewart GJ. A genome-wide screen for linkage disequilibrium in Australian Hla-DRB1*1501 positive multiple sclerosis patients. J. Neuroimmunol. 2003;143:60–64. doi: 10.1016/j.jneuroim.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Barcellos LF, Thomson G, Carrington M, Schafer J, Begovich AB, Lin P, Xu XH, Min BQ, Marti D, Klitz W. Chromosome 19 single-locus and multilocus haplotype associations with multiple sclerosis. Evidence of a new susceptibility locus in Caucasian and Chinese patients. JAMA. 1997;278:1256–1261. [PubMed] [Google Scholar]
- Barton A, Woolmore JA, Ward D, Eyre S, Hinks A, Ollier WE, Strange RC, Fryer AA, John S, Hawkins CP, Worthington J. Association of protein kinase C alpha (PRKCA) gene with multiple sclerosis in a UK population. Brain. 2004;127:1717–1722. doi: 10.1093/brain/awh193. [DOI] [PubMed] [Google Scholar]
- Becker KG, Simon RM, Bailey-Wilson RM, Freidlin B, Biddison WE, McFarland HF, Trent JM. Clustering of non-major histocompatibility complex susceptibility candidate loci in human autoimmune diseases. Proc. Natl. Acad. Sci. U. S. A. 1998;95:9979–9984. doi: 10.1073/pnas.95.17.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadley S, Sawcer S, D’Alfonso S, Hensiek A, Coraddu F, Gray J, Roxburgh R, Clayton D, Buttinelli C, Quattrone A, Trojano M, Massacesi L, Compston A. A genome screen for multiple sclerosis in Italian families. Genes Immun. 2001;2:205–210. doi: 10.1038/sj.gene.6363758. [DOI] [PubMed] [Google Scholar]
- Burton PR, Clayton DG, Cardon LR, Craddock N, Deloukas P, Duncanson A, Kwiatkowski DP, McCarthy MI, Ouwehand WH, Samani NJ, Todd JA, Donnelly P, Barrett JC, Davison D, Easton D, Evans DM, Leung HT, Marchini JL, Morris AP, Spencer CC, Tobin MD, Attwood AP, Boorman JP, Cant B, Everson U, Hussey JM, Jolley JD, Knight AS, Koch K, Meech E, Nutland S, Prowse CV, Stevens HE, Taylor NC, Walters GR, Walker NM, Watkins NA, Winzer T, Jones RW, McArdle WL, Ring SM, Strachan DP, Pembrey M, Breen G, St Clair D, Caesar S, Gordon-Smith K, Jones L, Fraser C, Green EK, Grozeva D, Hamshere ML, Holmans PA, Jones IR, Kirov G, Moskivina V, Nikolov I, O’Donovan MC, Owen MJ, Collier DA, Elkin A, Farmer A, Williamson R, McGuffin P, Young AH, Ferrier IN, Ball SG, Balmforth AJ, Barrett JH, Bishop TD, Iles MM, Maqbool A, Yuldasheva N, Hall AS, Braund PS, Dixon RJ, Mangino M, Stevens S, Thompson JR, Bredin F, Tremelling M, Parkes M, Drummond H, Lees CW, Nimmo ER, Satsangi J, Fisher SA, Forbes A, Lewis CM, Onnie CM, Prescott NJ, Sanderson J, Matthew CG, Barbour J, Mohiuddin MK, Todhunter CE, Mansfield JC, Ahmad T, Cummings FR, Jewell DP, Webster J, Brown MJ, Lathrop MG, Connell J, Dominiczak A, Marcano CA, Burke B, Dobson R, Gungadoo J, Lee KL, Munroe PB, Newhouse SJ, Onipinla A, Wallace C, Xue M, Caulfield M, Farrall M, Barton A, Bruce IN, Donovan H, Eyre S, Gilbert PD, Hilder SL, Hinks AM, John SL, Potter C, Silman AJ, Symmons DP, Thomson W, Worthington J, Dunger DB, Widmer B, Frayling TM, Freathy RM, Lango H, Perry JR, Shields BM, Weedon MN, Hattersley AT, Hitman GA, Walker M, Elliott KS, Groves CJ, Lindgren CM, Rayner NW, Timpson NJ, Zeggini E, Newport M, Sirugo G, Lyons E, Vannberg F, Hill AV, Bradbury LA, Farrar C, Pointon JJ, Wordsworth P, Brown MA, Franklyn JA, Heward JM, Simmonds MJ, Gough SC, Seal S, Stratton MR, Rahman N, Ban M, Goris A, Sawcer SJ, Compston A, Conway D, Jallow M, Newport M, Sirugo G, Rockett KA, Bumpstead SJ, Chaney A, Downes K, Ghori MJ, Gwilliam R, Hunt SE, Inouye M, Keniry A, King E, McGinnis R, Potter S, Ravindrarajah R, Whittaker P, Widden C, Withers D, Cardin NJ, Davison D, Ferreira T, Pereira-Gale J, Hallgrimsdottir IB, Howie BN, Su Z, Teo YY, Vukcevic D, Bentley D, Brown MA, Compston A, Farrall M, Hall AS, Hattersley AT, Hill AV, Parkes M, Pembrey M, Stratton MR, Mitchell SL, Newby PR, Brand OJ, Carr-Smith J, Pearce SH, McGinnis R, Keniry A, Deloukas P, Reveille JD, Zhou X, Sims AM, Dowling A, Taylor J, Doan T, Davis JC, Savage L, Ward MM, Learch TL, Weisman MH, Brown M. Association scan of 14,500 nonsynonymous SNPs in four diseases identifies autoimmunity variants. Nat. Genet. 2007;39:1329–1337. doi: 10.1038/ng.2007.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwick RM, Ramsay PP, Haines JL, Hauser SL, Oksenberg JR, Pericak-Vance MA, Schmidt S, Compston A, Sawcer S, Cittadella R, Savettieri G, Quattrone A, Polman CH, Uitdehaag BM, Zwemmer JN, Hawkins CP, Ollier WE, Weatherby S, Enzinger C, Fazekas F, Schmidt H, Schmidt R, Hillert J, Masterman T, Hogh P, Niino M, Kikuchi S, Maciel P, Santos M, Rio ME, Kwiecinski H, Zakrzewska-Pniewska B, Evangelou N, Palace J, Barcellos LF. APOE epsilon variation in multiple sclerosis susceptibility and disease severity: some answers. Neurology. 2006;661:1373–1383. doi: 10.1212/01.wnl.0000210531.19498.3f. [DOI] [PubMed] [Google Scholar]
- Clayton D. A Generalization of the transmission/disequilibrium test for uncertain haplotype transmission. Am. J. Hum. Genet. 1999;65:1170–1177. doi: 10.1086/302577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston A, Coles A. Multiple sclerosis. Lancet. 2002;359:1221–1231. doi: 10.1016/S0140-6736(02)08220-X. [DOI] [PubMed] [Google Scholar]
- Coraddu F, Sawcer S, D’Alfonso S, Lai M, Hensiek A, Solla E, Broadley S, Mancosu C, Pugliatti M, Marrosu MG, Compston A. A genome screen for multiple sclerosis in Sardinian multiplex families. Eur. J. Hum. Genet. 2001;9:621–626. doi: 10.1038/sj.ejhg.5200680. [DOI] [PubMed] [Google Scholar]
- D’Alfonso S, Nistico L, Zavattari P, Marrosu MG, Murru R, Lai M, Massacesi L, Ballerini C, Gestri D, Salvetti M, Ristori G, Bomprezzi R, Trojano M, Liguori M, Gambi D, Quattrone A, Fruci D, Cucca F, Richiardi PM, Tosi R. Linkage analysis of multiple sclerosis candidate region markers in Sardinian and Continental Italian families. Eur. J. Hum. Genet. 1999;7:377–385. doi: 10.1038/sj.ejhg.5200301. [DOI] [PubMed] [Google Scholar]
- D’Alfonso S, Nisitco L, Bocchio D, Bomprezzi R, Marrosu MG, Murru MR, Lai M, Massacesi L, Ballerini C, Repice A, Salvetti M, Montesperelli C, Ristori G, Trojano M, Liguori M, Gambi D, Quattrone A, Tosi R, Momigliano Richiardi P. An attempt of identifying MS-associated loci as a follow-up of a genomic linkage study in the Italian population. J. Neurovirol. 2000;6:S18–22. [PubMed] [Google Scholar]
- Ebers GC, Kukay K, Bulman DE, Sadovnick AD, Rice G, Anderson C, Armstrong H, Cousin K, Bell RB, Hader W, Paty DW, Hashimoto S, Oger J, Duquette P, Warren S, Gray T, O’Connor P, Nath A, Auty A, Metz L, Francis G, Paulseth JE, Murray TJ, Pryse-Phillips W, Risch N, et al. A full genome search in multiple sclerosis. Nat. Genet. 1996;13:472–476. doi: 10.1038/ng0896-472. [DOI] [PubMed] [Google Scholar]
- Eerola J, Hernandez D, Launes J, Hellström O, Hague S, Gulick C, Johnson J, Peuralinna T, Hardy J, Tienari PJ, Singleton AB. Assessment of a DJ-1 (PARK7) polymorphism in Finnish Parkinson’s disease. Neurology. 2003;61:1000–1002. doi: 10.1212/01.wnl.0000083992.28066.7e. [DOI] [PubMed] [Google Scholar]
- Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJ. Early growth and coronary heart disease in later life: a longitudinal study. BMJ. 2001;323:572–573. doi: 10.1136/bmj.322.7292.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AJ, Barcellos LF, Rimmler JB, Garcia ME, Caillier S, Lincoln RR, Bucher P, Pericak-Vance MA, Haines JL, Hauser SL, Oksenberg JR. Sequence variation in the transforming growth factor-beta1 (TGFB1) gene and multiple sclerosis susceptibility. J. Neuroimmunol. 2001;116:116–124. doi: 10.1016/s0165-5728(01)00283-1. [DOI] [PubMed] [Google Scholar]
- Gregory SG, Schmidt S, Seth P, Oksenberg JR, Hart J, Prokop A, Caillier SJ, Ban M, Goris A, Barcellos LF, Lincoln R, McCauley JL, Sawcer SJ, Compston DA, Dubois B, Hauser SL, Garcia-Blanco MA, Pericak-Vance MA, Haines JL. Interleukin 7 receptor alpha chain (IL7R) shows allelic and functional association with multiple sclerosis. Nat. Genet. 2007;39:1083–1091. doi: 10.1038/ng2103. [DOI] [PubMed] [Google Scholar]
- Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL, de Bakker PI, Gabriel SB, Mirel DB, Ivinson AJ, Pericak-Vance MA, Gregory SG, Rioux JD, McCauley JL, Haines JL, Barcellos LF, Cree B, Oksenberg JR, Hauser SL. Risk Alleles For Multiple Sclerosis IdentifiEd By A Genomewide Study. N. Engl. J. Med. 2007;357:851–862. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- Haghighi S, Andersen O, Nilsson S, Rydberg L, Wahlstrom J. A linkage study in two families with multiple sclerosis and healthy members with oligoclonal CSF immunopathy. Mult. Scler. 2006;12:723–730. doi: 10.1177/1352458506070972. [DOI] [PubMed] [Google Scholar]
- Haines JL, Ter-Minassian M, Bazyk A, Gusella JF, Kim DJ, Terwedow H, Pericak-Vance MA, Rimmler JB, Haynes CS, Roses AD, Lee A, Shaner B, Menold M, Seboun E, Fitoussi RP, Gartioux C, Reyes C, Ribierre F, Gyapay G, Weissenbach J, Hauser SL, Goodkin DE, Lincoln R, Usuku K, Oksenberg JR, et al. The Multiple Sclerosis Genetics Group A complete genomic screen for multiple sclerosis underscores a role for the major histocompatibility complex. Nat. Genet. 1996;13:469–471. doi: 10.1038/ng0896-469. [DOI] [PubMed] [Google Scholar]
- Haines JL, Bradford Y, Garcia ME, Reed AD, Neumeister E, Pericak-Vance MA, Rimmler JB, Menold MM, Martin ER, Oksenberg JR, Barcellos LF, Lincoln R, Hauser SL. Multiple susceptibility loci for multiple sclerosis. Hum. Mol. Genet. 2002;11:2251–2256. doi: 10.1093/hmg/11.19.2251. [DOI] [PubMed] [Google Scholar]
- Hoppenbrouwers IA, Aulchenko YS, Ebers GC, Ramagopalan SV, Oostra BA, van Dujin CM, Hintzen RQ. EVI5 is a risk gene for multiple sclerosis. Genes Immun. 2008;9:334–337. doi: 10.1038/gene.2008.22. [DOI] [PubMed] [Google Scholar]
- Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K. Positionally cloned gene for a novel glomerular protein-nephrin-is mutated in congenital nephrotic syndrome. Mol. Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- Koch S, Goedde R, Nigmatova V, Epplen JT, Muller N, de Seze J, Vermersch P, Momot T, Schmidt RE, Witte T. Association of multiple sclerosis with ILT6 deficiency. Genes Immun. 2005;6:445–447. doi: 10.1038/sj.gene.6364187. [DOI] [PubMed] [Google Scholar]
- Kristjansdottir G, Sandling JK, Bonetti A, Roos IM, Milani L, Wang C, Gustafsdottir SM, Sigurdsson S, Lundmark A, Tienari PJ, Koivisto K, Elovaara I, Pirttila T, Reunanen M, Peltonen L, Saarela J, Hillert J, Olsson T, Landegren U, Alcina A, Fernandez O, Leyva L, Guerrero M, Lucas M, Izquierdo G, Matesanz F, Syvanen AC. Interferon regulatory factor 5 (IRF5) gene variants are associated with multiple sclerosis in three distinct populations. J. Med. Genet. 2008;45:362–369. doi: 10.1136/jmg.2007.055012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuokkanen S, Gschwend M, Rioux JD, Daly MJ, Terwilliger JD, Tienari PJ, Wikstrom J, Palo J, Stein LD, Hudson TJ, Lander ES, Peltonen L. Genomewide scan of multiple sclerosis in Finnish multiplex families. Am. J. Hum. Genet. 1997;61:1379–1387. doi: 10.1086/301637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln MR, Montpetit A, Cader MZ, Saarela J, Dyment DA, Tiislar M, Ferretti V, Tienari PJ, Peltonen L, Ebers GC, Hudson TJ. A predominant role for the HLA class II region in the association of the MHC region with multiple sclerosis. Nature Genet. 2005;37:1108–1112. doi: 10.1038/ng1647. [DOI] [PubMed] [Google Scholar]
- Lucotte GL, French MS Consortium Confirmation of a gene for multiple scleriosis to chromosome 19q13.3. Genet. Couns. 2002;13:133–138. [PubMed] [Google Scholar]
- Myllykangas L, Polvikoski T, Reunanen K, Wavrant-De Vrieze F, Ellis C, Hernandez D, Sulkava R, Kontula K, Verkkoniemi A, Notkola IL, Hardy J, Perez-Tur J, Haltia MJ, Tienari PJ. ApoE epsilon3-haplotype modulates Alzheimer beta-amyloid deposition in the brain. Am. J. Med. Genet. 2002;114:288–291. doi: 10.1002/ajmg.10202. [DOI] [PubMed] [Google Scholar]
- Olerup O, Hillert J. HLA class II-associated genetic susceptibility in multiple sclerosis: a critical evaluation. Tissue Antigens. 1991;38:1–15. doi: 10.1111/j.1399-0039.1991.tb02029.x. [DOI] [PubMed] [Google Scholar]
- Paloneva J, Kestilä M, Wu J, Salminen A, Bohling T, Ruotsalainen V, Hakola P, Bakker AB, Phillips JH, Pekkarinen P, Lanier LL, Timonen T, Peltonen L. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat. Genet. 2000;25:357–361. doi: 10.1038/77153. [DOI] [PubMed] [Google Scholar]
- Peltonen L, Palotie A, Lange K. Use of population isolates for mapping complex traits. Nat. Rev. Genet. 2000;1:182–190. doi: 10.1038/35042049. [DOI] [PubMed] [Google Scholar]
- Pericak-Vance MA, Rimmler JB, Martin ER, Haines JL, Garcia ME, Oksenberg JR, Barcellos LF, Lincoln R, Goodkin DE, Hauser SL. Linkage and association analysis of chromosome 19q13 in multiple sclerosis. Neurogenetics. 2000;3:195–201. doi: 10.1007/s100480100119. [DOI] [PubMed] [Google Scholar]
- Pericak-Vance MA, Rimmler JB, Haines JL, Garcia ME, Oksenberg JR, Barcellos LF, Lincoln R, Hauser SL, Cournu-Rebeix I, Azoulay-Cayla A, Lyon-Caen O, Fontaine B, Duhamel E, Coppin H, Brassat D, Roth MP, Clanet M, Alizadeh M, Yaouanq J, Quelvennec E, Semana G, Edan G, Babron MC, Genin E, Clerget-Darpoux F. Investigation of seven proposed regions of linkage in multiple sclerosis: an American and French collaborative study. Neurogenetics. 2004;5:45–48. doi: 10.1007/s10048-003-0163-y. [DOI] [PubMed] [Google Scholar]
- Pihlaja H, Rantamaki-Hakkinen T, Wikström J, Sumelahti ML, Pirttila T, Elovaara I, Reunanen M, Laaksonen M, Ilonen J, Ruutiainen J, Kuokkanen S, Peltonen L, Koivisto K, Tienari PJ. Linkage disequilibrium between myelin basic protein (MBP) microsatellite and multiple sclerosis is restricted to a geographically-defined subpopulation in Finland. Genes Immun. 2003;4:138–146. doi: 10.1038/sj.gene.6363943. [DOI] [PubMed] [Google Scholar]
- Reunanen K, Finnila S, Laaksonen M, Sumelahti ML, Wikstrom J, Pastinen T, Kuokkanen S, Saarela J, Uimari P, Ruutiainen J, Ilonen J, Peltonen L, Tienari PJ. Chromosome 19q13 and multiple sclerosis susceptibility in Finland: a linkage and two-stage association study. J. Neuroimmunol. 2002;126:134–142. doi: 10.1016/s0165-5728(02)00051-6. [DOI] [PubMed] [Google Scholar]
- Saarela J, Kallio SP, Chen D, Montpetit A, Jokiaho A, Choi E, Asselta R, Bronnikov D, Lincoln MR, Sadovnick AD, Tienari PJ, Koivisto K, Palotie A, Ebers GC, Hudson TJ, Peltonen L. PRKCA and multiple sclerosis: association in two independent populations. PloS Genet. 2006;2:e42. doi: 10.1371/journal.pgen.0020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawcer S, Jones HB, Feakes R, Gray J, Smaldon N, Chataway J, Robertson N, Clayton D, Goodfellow PN, Compston A. A genome screen in multiple sclerosis reveals susceptibility loci on chromosome 6p21 and 17q22. Nat. Genet. 1996;13:464–468. doi: 10.1038/ng0896-464. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Barcellos LF, DeSombre K, Rimmler JB, Lincoln RR, Bucher P, Saunders AM, Lai E, Martin ER, Vance JM, Oksenberg JR, Hauser SL, Pericak-Vance MA, Haines JL. Association of polymorphisms in the apolipoprotein E region with susceptibility to and progression of multiple sclerosis. Am. J. Hum. Genet. 2002;70:708–717. doi: 10.1086/339269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shifman S, Darvasi A. The value of isolated populations. Nat. Genet. 2001;28:309–310. doi: 10.1038/91060. [DOI] [PubMed] [Google Scholar]
- Spielman RS, McGinnis RE, Ewens WJ. Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM) Am. J. Hum. Genet. 1993;52:506–516. [PMC free article] [PubMed] [Google Scholar]
- Strandberg TE, Tilvis RS, Lindberg O, Valvanne J, Sairanen S, Ehnholm C, Tuomilehto J. High plasma insulin is associated with lower LDL cholesterol in elderly individuals. Atherosclerosis. 1996;121:267–273. doi: 10.1016/0021-9150(95)05733-1. [DOI] [PubMed] [Google Scholar]
- Sulonen AM, Kallio SP, Ellonen P, Suvela M, Elovaara I, Koivisto K, Pirttila T, Reunanen M, Tienari PJ, Palotie A, Peltonen L, Saarela J. No Evidence for Shared Etiology in Two Demyelinative Disorders, MS and PLOSL. J. Neuroimmunol. 2008 Nov 17; doi: 10.1016/j.jneuroim.2008.10.005. Electronic publication ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumelahti ML, Tienari PJ, Wikstrom J, Palo J, Hakama M. Regional and temporal variation in multiple sclerosis incidence in Finland during 1979-93. Neuroepidemiology. 2000;19:67–75. doi: 10.1159/000026241. [DOI] [PubMed] [Google Scholar]
- Syvanen AC. From gels to chips: “minisequencing” primer extension for analysis of point mutations and single nucleotide polymorphisms. Hum. Mutat. 1999;13:1–10. doi: 10.1002/(SICI)1098-1004(1999)13:1<1::AID-HUMU1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Taillon-Miller P, Bauer-Sardiña I, Saccone NL, Putzel J, Laitinen T, Cao A, Kere J, Pilia G, Rice JP, Kwok PY. Juxtaposed regions of extensive and minimal linkage disequilibrium in human Xq25 and Xq28. Nat. Genet. 2000;25:324–328. doi: 10.1038/77100. [DOI] [PubMed] [Google Scholar]
- Tienari PJ, Sumelahti ML, Rantamaki-Hakkinen T, Wikstrom J. Multiple sclerosis in western Finland: evidence for founder effect. Clin. Neurol. Neurosurg. 2004;106:175–179. doi: 10.1016/j.clineuro.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Yeo TW, Roxburgh R, Maranian M, Singlehurst S, Gray J, Hensiek A, Setakis E, Compston A, Sawcer S. Refining the analysis of a whole genome linkage disequilibrium association map: the United Kingdom results. J. Neuroimmunol. 2003;143:53–59. doi: 10.1016/j.jneuroim.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Xing C, Sestak AL, Kelly JA, Nguyen KL, Bruner GR, Harley JB, Gray-McGuire C. Localization and replication of the system lupus erythematosus linkage signal at 4p16: interaction with 2p11, 12q24 and 19q13 in European Americans. Hum. Genet. 2007;120:623–631. doi: 10.1007/s00439-006-0248-4. [DOI] [PubMed] [Google Scholar]
- Wise LH, Lanchbury JS, Lewis CM. Meta-analysis of genome searches. Ann. Hum. Genet. 1999;63:263–272. doi: 10.1046/j.1469-1809.1999.6330263.x. [DOI] [PubMed] [Google Scholar]
- Xu C, Dai Y, Lorentzen JC, Dahlman I, Olsson T, Hillert J. Linkage analysis in multiple sclerosis of chromosomal regions syntenic to experimental autoimmune disease loci. Eur. J. Hum. Genet. 2001;9:458–463. doi: 10.1038/sj.ejhg.5200653. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Duvefelt K, Svensson F, Masterman T, Jonasdottir G, Salter H, Emahazion T, Hellgren D, Falk G, Olsson T, Hillert J, Anvret M. Two genes encoding immune-regulatory molecules (LAG3 and IL7R) confer susceptibility to multiple sclerosis. Genes Immun. 2005;6:145–152. doi: 10.1038/sj.gene.6364171. [DOI] [PubMed] [Google Scholar]