Abstract
Methamphetamine abuse is a significant public health concern. Although widely studied in laboratory animals, little is known about the abuse-related behavioral effects of methamphetamine relative to other abused stimulants in controlled laboratory settings in humans. The aim of this study was to examine the discriminative stimulus, subject-rated, performance, and cardiovascular effects of methamphetamine in humans. In the present study, subjects first learned to discriminate 10 mg of oral methamphetamine from placebo. After acquiring the discrimination (≥80% drug-appropriate responding on four consecutive sessions), a range of oral doses of methamphetamine (2.5-15 mg), d-amphetamine (2.5-15 mg), methylphenidate (5-30 mg), and triazolam (0.0625-0.375 mg) was tested. Methamphetamine functioned as a discriminative stimulus and produced prototypical stimulant-like subject-rated effects. d-Amphetamine and methylphenidate produced dose-related increases in methamphetamine-appropriate responding, whereas triazolam did not. d-Amphetamine and methylphenidate produced stimulant-like behavioral effects, whereas triazolam produced sedative-like effects. Methamphetamine, but no other drug, increased heart rate, systolic pressure, and diastolic pressure significantly above placebo levels. Performance in the Digit-Symbol Substitution Test was not affected by any of the drugs tested. Overall, these results demonstrate that the acute behavioral effects of methamphetamine, d-amphetamine, and methylphenidate overlap extensively in humans, which is concordant with findings from preclinical studies. Future studies should assess whether the similarity in the behavioral effects of methamphetamine and related stimulants can be extended to other behavioral assays, such as measures of reinforcement, in humans.
The illicit use of stimulants, in general, and methamphetamine, in particular, remains a significant public health concern. Multiple sources point to the problem of methamphetamine abuse in the United States. For example, an estimated 5.8% of the U.S. population 12 years or older used methamphetamine at least once in their lifetimes for nonmedical purposes, and this estimate remained unchanged from that reported in the past 4 years. Furthermore, the percentage of current (past month) methamphetamine users (i.e., 0.3% of the U.S. population) remained constant for the past 4 years (Substance Abuse and Mental Health Service Administration, 2006). In addition, the number of admissions to treatment programs for amphetamine abuse more than tripled from 1996 to 2006 (51,985 in 1996 to 158,880 in 2006; Treatment Episode Data Set). The growing problem of methamphetamine abuse also has been associated with significant health hazards, such as cardiovascular complications, sexually transmitted diseases, burns, and emergency department visits (Halkitis et al., 2001; Danks et al., 2004; Drug Abuse Warning Network, 2005).
Behavioral assays such as drug discrimination and self-administration have been used to assess the abuse-related behavioral effects of stimulants in animals and humans. For example, rodents and monkeys can be trained to discriminate methamphetamine from saline, and stimulants such as d-amphetamine, methylphenidate, and cocaine fully substitute for methamphetamine (Czoty et al., 2004a,b). Moreover, like other abused stimulants, methamphetamine is readily self-administered by laboratory animals (Clemens et al., 2006). Thus, results from animal studies indicate that methamphetamine can function as a discriminative and reinforcing stimulus and that the behavioral effects of methamphetamine overlap with other stimulant drugs of abuse.
Several studies conducted in humans have examined the subject-rated and reinforcing effects of methamphetamine. Methamphetamine increases subject ratings of high to a degree that is qualitatively similar to that observed with other abused stimulants such as cocaine (Newton et al., 2005). Positive subject-rated effects are thought to be associated with reinforcing effects of drugs of abuse, and previous studies have shown that a range of doses of methamphetamine is chosen over placebo by stimulant users (Hart et al., 2001) and abusers (De La Garza et al., 2008). These findings suggest that methamphetamine, like other abused stimulants, produces positive subject-rated and reinforcing effects in humans.
Although a number of studies have examined the subject-rated and reinforcing effects of methamphetamine in humans, laboratory studies assessing the discriminative stimulus effects of methamphetamine are relatively scarce. We know of only one published report that explicitly established methamphetamine as a discriminative stimulus in humans (Hart et al., 2002). The results indicated that methamphetamine functioned as a discriminative stimulus and dose-dependently produced drug-appropriate responding, whereas memantine, an N-methyl-d-aspartate receptor antagonist, did not. In addition, two other studies evaluated whether methamphetamine shares discriminative stimulus effects with d-amphetamine and cocaine (Lamb and Henningfield, 1994; Johanson et al., 2006). In these studies, methamphetamine engendered drug-appropriate responding as a function of dose and, with the highest dose tested, substituted completely for d-amphetamine or cocaine.
Although these studies provide valuable basic information on the abuse-related effects of methamphetamine in humans, it remains to be determined whether methamphetamine generalizes to other known stimulants in a drug discrimination assay. Methamphetamine is the primary form of amphetamine abused in the United States (Substance Abuse and Mental Health Services Administration. Drug and Alcohol Services Information System, 2004). Increased rates of abuse have been observed with methamphetamine, relative to other related stimulants, such as d-amphetamine and methylphenidate (Maxwell and Rutkowski, 2008). However, it is not clear whether the difference in relative abuse potential is related to differences in behavioral effects (e.g., discriminative stimulus effects) produced by the stimulants. Thus, to examine similarities and differences in the behavioral effects produced by methamphetamine and related stimulants, the present study determined the discriminative stimulus, subject-rated, performance, and cardiovascular effects of methamphetamine, d-amphetamine, and methylphenidate in humans. To examine the pharmacological specificity of the methamphetamine discrimination, the behavioral effects of a pharmacologically unrelated drug, triazolam (a GABAA receptor modulator), were evaluated in the present study. The behavioral effects of a 6-fold dose range of drugs across 5 h were determined.
Materials and Methods
Subjects
Ten healthy adult humans were recruited from the local community via newspaper advertisements, flyers, and word of mouth to participate in this experiment. Two of these subjects were discharged from the study because they were unable to acquire the discrimination, and a third was lost to follow-up. Data from these three subjects were not included in the analyses. Seven (four males, three females; all white) subjects completed this experiment. Subjects were paid $40 per session to participate in this experiment and received performance-based payment as outlined below.
Subjects ranged in age from 20 to 24 years (mean = 21.1 years) and in education from 13 to 16 years (mean = 14.4). Subjects ranged in weight from 54 to 110 kg (mean = 80.5 kg) and had a body mass index ranging from 21 to 32 (mean = 25.7). All subjects reported recreational stimulant use within the past year, but they did not meet Diagnostic and Statistical Manual—Version IV criteria for stimulant abuse. Of these, three subjects reported past year use of amphetamine (e.g., Adderall), one subject reported past year use of cocaine, two subjects reported past year use of amphetamine and cocaine, and one subject reported past year use of amphetamine, cocaine, and methylphenidate (Ritalin). Subjects reported lifetime use of amphetamine and related drugs (n = 6; total times used = 1-36, mean = 11.2; number of years used = 1-20, mean = 4.8) and cocaine (n = 4; total times used = 1-18, mean = 9.8; number of years used = 1-3, mean = 2.0). All subjects reported consuming alcohol-containing beverages weekly (range = 4-23; mean = 15.7). Three subjects reported daily tobacco cigarette use (range = 1-20; mean = 10.3). These three subjects were allowed to smoke one cigarette midway through each experimental session. All subjects reported previous marijuana use, and five of them reported current use at the time of screening (range = 1-21 days last month; mean = 6.2). Other lifetime nonmedical drug use included opioids (n = 7; range = 1-15 times; mean = 3.4), benzodiazepines (n = 5; range = 1-5 times; mean = 2.4), and hallucinogens (n = 3; range = 1-3 times; mean = 2.0).
Before enrollment, all potential subjects completed standard comprehensive medical, physical, and psychological screens. Individuals with current or past histories of axis I psychiatric disorder, including substance abuse or dependence disorders (except nicotine) and ADHD, were excluded from participating. All subjects were in good health, with no contraindications to stimulant or sedative medications. The Institutional Review Board of the University of Kentucky Chandler Medical Center approved this study and the informed consent document. Subjects signed the informed consent after passing appropriate sobriety tests and before enrolling in the study.
General Procedures
Subjects enrolled as outpatients at the Laboratory of Human Behavioral Pharmacology at the University of Kentucky Chandler Medical Center. Subjects completed one “practice” session to familiarize them with the behavioral measures and daily laboratory routine. Experimental drugs were not administered on this day. Subjects then completed 33 to 45 (mean = 38) experimental sessions.
Subjects were informed that during their participation, they would receive various drugs, administered orally, which would include placebo, methamphetamine, d-amphetamine, methylphenidate, and triazolam. Other than this general information, subjects were blind to the type of drug administered. Subjects were told that the purpose of the study was to see how different drugs affect mood and behavior and whether people are able to detect the presence of a drug. Subjects were given no instruction of what they were “supposed” to do or of what outcomes might be expected. Subjects were asked to abstain from all drug (illicit and licit) use for the duration of the experiment, with the exception of nonsteroidal anti-inflammatory analgesics and nicotine. In addition, subjects also were asked not to ingest food or caffeine for 4 h before each experimental session and alcohol for 12 h before and after each experimental session.
Experimental sessions were generally conducted daily Monday through Friday. The time of day at which each session began ranged from 8:00 to 10:00 AM but was generally held constant for individual subjects. On experimental session days, subjects followed a daily routine. Each experimental session day, subjects were first provided a light breakfast. Subjects then provided an expired air sample, which was assayed for the presence of alcohol using an Alco-Sensor breathalyzer (Intoximeters, Inc., St. Louis, MO). Expired air samples provided by subjects had to be negative for an experimental session to continue. Each morning, subjects provided a urine sample that was screened for the presence of amphetamines, barbiturates, benzodiazepines, cocaine, opioids, and THC. If a urine sample was positive for any drug, other than THC or compounds administered experimentally, the session was canceled until the subject provided a drug-free urine sample. Urine samples occasionally tested positive for amphetamines and benzodiazepines during the conduct of the experimental protocol, which could be explained by experimental drug administration; therefore, on these days, sessions proceeded as scheduled. Five subjects sporadically tested positive for THC throughout the study. One subject tested positive for benzodiazepines on one occasion that could not be explained by experimental drug administration. Experimental sessions were canceled for this subject until a benzodiazepine-free urine sample was provided. On experimental session days, subjects completed the self-reported drug-effect questionnaires and a performance task approximately 30 min before drug administration and then completed the drug-discrimination, self-reported drug-effect questionnaires, and performance task 1, 2, 3, 4, and 5 h after drug administration.
Drug Discrimination Procedure
This experiment consisted of three phases, which were completed in a fixed order: 1) sampling phase, 2) acquisition phase, and 3) test phase.
Sampling Phase. All subjects completed two sampling sessions to acquaint them with the drug effects. During each sampling session, subjects ingested three capsules that contained a total of 10 mg of methamphetamine. Methamphetamine was identified by letter code (e.g., drug A), but the subjects were not explicitly informed of the contents of the capsules. Methamphetamine (10 mg) is identified as drug A here for illustrative purposes only. A unique letter code was used for each subject. Subjects read a set of instructions before receiving the capsules, and a research assistant also read them aloud. In brief, subjects were told that they would be receiving drug A and that they could earn money by allocating their available points to the drug A option on the drug discrimination task. Subjects also were told that they should pay close attention to the effects of drug A because in future sessions, they would no longer be told whether they had received drug A. For the exact instruction set, see Rush et al. (2003). During sampling sessions, all subjects were instructed to distribute 100 points to the drug A option and 0 points to the not drug A option in the drug discrimination task, which is described below.
Drug Discrimination Task. A point distribution drug discrimination task was completed 1, 2, 3, 4, and 5 h after oral drug administration on an Apple Macintosh computer (Apple Computer, Cupertino, CA). In this procedure, subjects were required to distribute 100 points between two options (i.e., drug A or not drug A) (e.g., Rush et al., 2003). During sampling and acquisition sessions, points accumulated on the correct option were exchangeable for money at a rate of $0.08/point. During test sessions, subjects were credited with the greater number of points allocated to the drug A or not drug A option, which were exchangeable at the same rate. Thus, subjects were able to earn a maximum of $40/session on this task. The dependent measure in this procedure was percentage methamphetamine-appropriate responding.
Acquisition Phase. After the sampling phase, an acquisition phase was conducted to determine whether subjects could discriminate 10 mg of methamphetamine. During this phase, subjects ingested capsules under double-blind conditions, but they were not told whether the capsules contained 10 mg of methamphetamine (e.g., drug A) or placebo (e.g., not drug A). Subjects were not explicitly instructed that they would be attempting to acquire a drug versus placebo discrimination. Subjects read a set of instructions before receiving the capsules, and a research assistant also read them aloud. In brief, subjects were told that they would not be informed of the identity of the drug administered that day. They were instructed further that if they felt they received drug A, they should allocate their available points to the drug A option on the drug discrimination task. The instruction set also directed subjects to allocate their points to the not drug A option if they felt that they had received anything other than drug A. For the exact instruction set, see Rush et al. (2003). These instructions were also used during the test phase described below. At the end of each experimental session, subjects opened a sealed envelope that informed them and the research assistant of the identity of the drug administered (e.g., drug A or not drug A). The points that subjects allocated to the correct option were converted to bonus money at the rate of $0.08/point, which they received at the end of the entire experiment. Subjects were considered to have acquired the discrimination if they allocated 80% or more points to the correct option on the drug discrimination task across four consecutive sessions. Subjects were discharged if they did not meet the discrimination criterion within 12 sessions. The order of drug administration during the acquisition phase was random except that each subject received each training condition, 10 mg of methamphetamine and placebo, at least twice.
Test Phase. After successfully completing the acquisition phase, subjects entered a test phase. The test phase consisted of test days interspersed with acquisition sessions. The test sessions were identical to the acquisition sessions except that subjects did not receive any feedback concerning their drug discrimination performance. On the test sessions, subjects earned the bonus money allocated to the drug A or not drug A option, whichever was greater. Subjects were not told the purpose of these test sessions, nor did they know when they were scheduled until after they opened the sealed envelope.
Additional acquisition sessions were interspersed among the test days to ensure subjects continued to accurately discriminate 10 mg of methamphetamine. Acquisition sessions comprised approximately 42% of sessions during the test phase. These acquisition sessions were identical to those in the acquisition phase (i.e., 10 mg of methamphetamine or placebo). If a subject responded incorrectly on an acquisition session (i.e., <80% correct), additional acquisition sessions were scheduled. These additional acquisition sessions continued until the subject correctly identified both of the training conditions once each.
Drug doses tested during the test phase included methamphetamine (2.5, 5, 10, and 15 mg), d-amphetamine (2.5, 5, 10, and 15 mg), methylphenidate (5, 10, 20, and 30 mg), triazolam (0.0625, 0.125, 0.25, and 0.375 mg), and placebo. The recommended dose range of methamphetamine for treatment of ADHD is 20 to 25 mg daily (Physicians' Desk Reference, 2006). The recommended dose of d-amphetamine for the treatment of ADHD is 20 mg daily. The recommended dose range of methylphenidate for the treatment of ADHD is 20 to 30 mg daily. The recommended dose range of triazolam for the treatment of insomnia is 0.125 to 0.5 mg. Each drug dose was administered once in the present study. The order of drug administration was random during this phase, except that an active drug dose was never administered on more than three consecutive sessions. The chemical structures of all four drugs used in the present study are known and published (see Patrick and Markowitz, 1997; Fustero et al., 2006; Sun et al., 2008).
Self-Reported Questionnaires, Performance Task, Cardiovascular Measures
Four self-reported drug-effect questionnaires and a performance task were completed in fixed order approximately 30 min before drug administration and 1, 2, 3, 4, and 5 h after drug administration.
Stimulant-Sensitive Adjective-Rating Scale. The Stimulant-Sensitive Adjective-Rating Scale consisted of 21 items (Di Marino et al., 1998). Subjects rated each item using a computer mouse to point to and select among one of five response options: not at all, a little bit, moderately, quite a bit, and extremely (scored numerically from 0-4, respectively; similar to that reported by Rush et al., 2003). Responses to individual items are summed to create a composite score, with a maximum total score of 84.
Adjective-Rating Scale. The Adjective-Rating Scale consisted of 32 items and contained two subscales: sedative and stimulant (Oliveto et al., 1992). Subjects rated each item using the computer mouse to point to and select among one of five response options: not at all, a little bit, moderately, quite a bit, and extremely (scored numerically from 0-4, respectively).
ARCI. The short form of the Addiction Research Center Inventory (ARCI) consisted of 49 true/false questions and contained five major subscales: the morphine-Benzedrine group (MBG; a measure of euphoria); the pentobarbital, chlorpromazine, alcohol group (PCAG; a measure of sedation); the d-lysergic acid diethylamide (a measure of dysphoria); and the Benzedrine group (BG) and amphetamine (A) scales (stimulant-sensitive scales) (Martin et al., 1971; Jasinski, 1977).
Drug-Effect Questionnaire. The Drug-Effect Questionnaire consisted of 20 items (for the items rated, see Rush et al., 2003). Each item was presented on the video screen, one at a time. Subjects rated each item using the computer mouse to point to and select among one of five response options: not at all, a little bit, moderately, quite a bit, and extremely (scored numerically from 0-4, respectively).
Digit-Symbol Substitution Test. A computerized version of the Digit-Symbol Substitution Test (DSST), which has been described previously, was used in this experiment (McLeod et al., 1982). In brief, subjects used a numeric keypad to enter a geometric pattern associated with one of nine digits displayed on a video screen. Subjects had 90 s to enter as many geometric patterns as possible. The dependent measure was the percentage of trials correct (i.e., number of patterns the subject entered correctly divided by number of patterns attempted then multiplied by 100).
Heart Rate and Blood Pressure. Heart rate and blood pressure were recorded using an automated blood pressure monitor (DINAMAP; Johnson and Johnson, Alexandria, TX). Heart rate and blood pressure were monitored for approximately 30 min before drug administration and at hourly intervals for 5 h afterward. Heart rate and blood pressure were recorded immediately before subjects completed the drug discrimination, self-reported drug-effect questionnaires and performance task.
Drug Administration
All drug conditions were administered in a double-blind fashion under medical supervision. Methamphetamine (Abbott Laboratories, Abbott Park, IL), d-amphetamine (Barr Pharmaceuticals, Inc., Montvale, NJ), methylphenidate (Mallinckrodt, Hazelwood, MO), and triazolam (Roxane Laboratories, Inc., Columbus, OH) were prepared by encapsulating commercially available tablets in an opaque blue/white size 0 capsule. Each methamphetamine capsule contained 2.5 or 5 mg, each d-amphetamine capsule contained 2.5 or 5 mg, each methylphenidate capsule contained 5 or 10 mg, and each triazolam capsule contained 0.0625 or 0.125 mg. Cornstarch was used to fill the remainder of all capsules. Placebo capsules contained only cornstarch. Capsules were prepared by the University of Kentucky Chandler Medical Center Investigational Pharmacy.
During each experimental session, subjects ingested three capsules. Administering the appropriate number of active and placebo capsules varied the dose of methamphetamine, d-amphetamine, methylphenidate, and triazolam. Capsules were taken orally with water. Drug administration procedures were designed to ensure that subjects swallowed the capsules and did not open them in their mouths to taste the contents (Abreu and Griffiths, 1996). Drug administration sessions were separated at least by 24 h. In general, subjects completed three to four sessions per week. An active drug dose was never administered on more than three consecutive days.
Data Analyses
Statistical analyses of group data were conducted to examine drug effects on the drug-discrimination task, self-reported drug-effect questionnaires, DSST, heart rate, and blood pressure. Data were analyzed statistically as raw scores. For all measures, effects were considered significant at p ≤ 0.05.
Drug discrimination (i.e., percentage of drug-appropriate responding), drug-effect questionnaire, performance, and cardiovascular data obtained during the acquisition phase were averaged across all exposures to placebo and 10 mg of methamphetamine on the four sessions that subjects met the discrimination criteria. These data were then analyzed by two-factor repeated-measure ANOVA, with drug (placebo and 10 mg of methamphetamine) and time (1, 2, 3, 4, and 5 h for drug-discrimination data; pre-, 1, 2, 3, 4, and 5 h for all other measures) as factors. If the interaction of drug and time attained statistical significance, the mean square error term was used to conduct Dunnett's post hoc tests, comparing 10 mg of methamphetamine with placebo at each time point.
Discrimination data from the test phase were analyzed as the total percent of points allocated to the drug option across the 5-h session. Data from self-reported drug-effect questionnaire, performance, and cardiovascular measures were analyzed as peak effect (i.e., maximal value observed for each subject 1-5 h after oral drug administration for each measure). These data were analyzed initially with one-factor repeated-measure ANOVA with dose (i.e., placebo and each of the 16 active dose conditions) as the factor. If the effect of dose attained significance, separate one-factor repeated-measure ANOVA were conducted for each drug with dose (placebo, dose 1×, dose 2×, dose 4×, and dose 6×) as the factor. Subsequent analyses employed two-factor repeated-measure ANOVA to determine between-drug differences. Factors for these analyses were drug (i.e., methamphetamine versus d-amphetamine; methamphetamine versus methylphenidate; methamphetamine versus triazolam; d-amphetamine versus methylphenidate; d-amphetamine versus triazolam; methylphenidate versus triazolam) and dose (dose 1×, dose 2×, dose 4×, and dose 6×). Placebo data were omitted from these analyses. Between-drug differences were inferred if the main effect of drug or the interaction of drug and dose attained statistical significance.
Finally, time course analyses of the data obtained during the test phase used two-factor repeated-measure ANOVA with dose (methamphetamine, 2.5, 5, 10, 15 mg; d-amphetamine, 2.5, 5, 10, 15 mg; methylphenidate, 5, 10, 20, 30 mg; triazolam, 0.0625, 0.125, 0.25, 0.375 mg; and placebo) and time (1, 2, 3, 4, 5 h for drug discrimination data; pre-, 1, 2, 3, 4, 5 h for all other measures) as factors. If a significant interaction of dose and time was detected, Dunnett's post hoc tests were conducted to determine differences between the actual dose and the corresponding placebo value at the same time point. When a significant interaction between factors is observed for an analysis, significant main effects are not reported in the text for the sake of brevity.
Results
Acquisition Phase
Drug Discrimination Performance. Subjects met the discrimination criterion in an average of six sessions (range = 4-12). The two-factor repeated-measure ANOVA revealed a significant interaction of drug and time on percentage of methamphetamine-appropriate responding (F5,30 = 10.2, p < 0.05). Methamphetamine (10 mg, the training dose) increased drug-appropriate responding significantly above placebo levels 1 to 5 h after drug administration (Dunnett's critical value = 13.4; data not shown). Greater than 80% of drug-appropriate responding was observed 2 to 5 h after methamphetamine administration.
Stimulant-Sensitive Adjective-Rating Scale. A significant interaction of drug and time was detected on the Stimulant-Sensitive Adjective-Rating Scale (F5,30 = 6.2, p < 0.05). Methamphetamine increased scores on this scale significantly above placebo levels 1 to 5 h after drug administration (Dunnett's = 1.8; data not shown).
Adjective-Rating Scale. A significant interaction of drug and time was detected on the stimulant and sedative subscales of the Adjective-Rating Scale (F5,30 ≥ 8.3, p < 0.05). Relative to placebo, methamphetamine significantly increased scores on the stimulant subscale (Dunnett's = 1.8) and decreased scores on the sedative subscale (Dunnett's = 1.7) 1 to 5 h after drug administration (data not shown).
ARCI. A significant interaction of Drug and Time was detected on four subscales from the ARCI (A, BG, MBG, and PCAG; F5,30 ≥ 7.4, p < 0.05). Methamphetamine increased scores on the A, BG, and MBG subscales significantly above the corresponding placebo levels 1 to 4 h after drug administration (Dunnett's = 0.6, 0.9, and 1.2, respectively; data not shown). Relative to placebo, methamphetamine decreased PCAG scores 1 to 5 h after drug administration (Dunnett's = 1.0; data not shown).
Drug-Effect Questionnaire. A significant interaction of drug and time was detected on 14 items on the Drug-Effect Questionnaire: any effect; good effects; like drug; stimulated; talkative, friendly; willing to pay for; performance improved; rush; sluggish, fatigued, lazy; active, alert, energetic; willing to take again; irregular, racing heart beat; shaky, jittery, and high (F5,30 ≥ 2.6, p < 0.05). In general, the effects of methamphetamine differed significantly from placebo 1 h after drug administration, peaked 2 h after drug administration, and returned to baseline 4 to 5 h after drug administration (data not shown).
DSST. There were no significant effects detected on the percentage of trials completed correctly on the DSST (F5,30 = 0.1, p > 0.05; data not shown).
Heart Rate and Blood Pressure. A significant interaction of drug and time was detected for heart rate and systolic and diastolic blood pressure (F5,30 ≥ 6.7, p < 0.05). Methamphetamine increased heart rate significantly above placebo levels 2 to 5 h after administration (Dunnett's = 3.6; data not shown). Systolic and diastolic blood pressure was increased significantly above placebo levels 1 to 5 h after drug administration (Dunnett's = 3.9 and 2.7, respectively; data not shown).
Test Phase
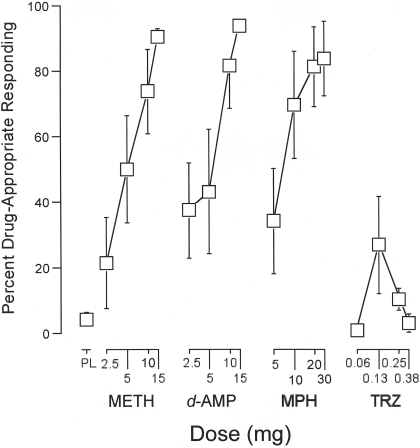
Drug Discrimination Performance. The one-factor repeated-measure ANOVA that included all 16 drug doses and placebo revealed a significant effect of dose on percentage of methamphetamine-appropriate responding (F16,96 = 8.0, p < 0.05). Subsequent one-factor repeated-measure ANOVA indicated that methamphetamine, d-amphetamine, and methylphenidate (F4,24 ≥ 7.1, p < 0.05), but not triazolam (F4,24 = 2.3, p > 0.05), increased drug-appropriate responding as an orderly function of dose (Fig. 1). Two-factor repeated-measure ANOVA indicated that the dose-related effects of methamphetamine, d-amphetamine, and methylphenidate did not differ significantly (F4,24 ≤ 1.1, p > 0.05). The effects of methamphetamine, d-amphetamine, and methylphenidate differed significantly from those of triazolam (F1,6 ≥ 37.3, p < 0.05).
Fig. 1.
Dose effects for METH, d-AMP, MPH, and TRZ for percentage of methamphetamine-appropriate responding on the point-distribution drug-discrimination task. x-axis, dose in milligrams. Data points above PL designate values from the placebo “test” session. Data points for 10 above METH designate values from the methamphetamine (10 mg) test session. Data points show means of seven subjects; brackets show 1 S.E.M. Unidirectional error bars are shown in some instances for clarity.
It is worth noting that the highest dose of methamphetamine, d-amphetamine, and methylphenidate engendered ≥80% drug-appropriate responding, which traditionally is considered full substitution. The average maximal methamphetamine-appropriate responding observed with any triazolam dose was approximately 27%. During the acquisition sessions that were interspersed in the test phase, 10 mg of methamphetamine engendered 85% of drug-appropriate responding, whereas placebo engendered 24% of drug-appropriate responding, indicating that the discrimination was maintained throughout the test phase.
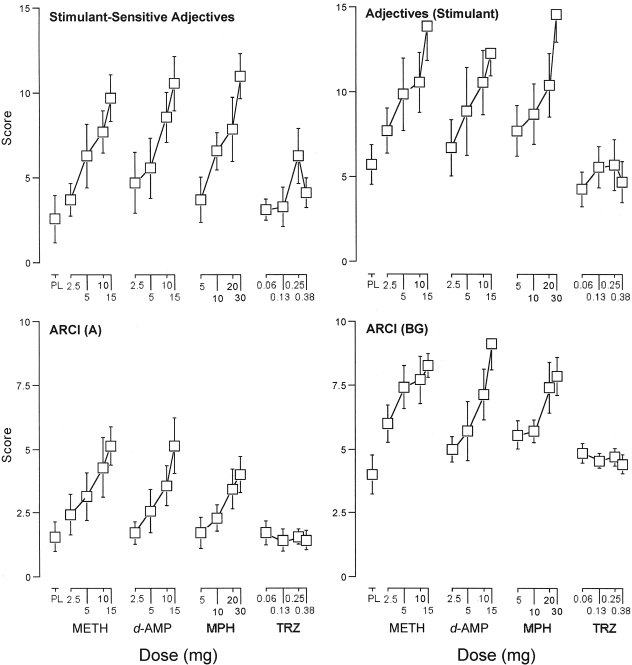
Stimulant-Sensitive Adjective-Rating Scale. The one-factor repeated-measure ANOVA that included all 16 drug doses and placebo revealed a significant effect of dose on scores on the Stimulant-Sensitive Adjective-Rating Scale (F16,96 = 5.3, p < 0.05). Subsequent one-factor repeated-measure ANOVA indicated that methamphetamine, d-amphetamine, and methylphenidate (F4,24 ≥ 6.5, p < 0.05), but not triazolam (F4,24 = 2.2, p > 0.05), significantly increased scores on this scale as an orderly function of dose (Fig. 2, top left). Two-factor repeated-measure ANOVA indicated that the dose-related effects of methamphetamine, d-amphetamine, and methylphenidate did not differ significantly on this scale (F4,24 ≤ 0.4, p > 0.05). The effects of methamphetamine, d-amphetamine, and methylphenidate differed significantly from those of triazolam (F1,6 ≥ 5.7, p < 0.05).
Fig. 2.
Dose effects for METH, d-AMP, MPH, and TRZ for scores on the Stimulant-Sensitive Adjective-Rating Scale and stimulant subscale of the Adjective-Rating Scale, along with the A and the BG subscales of the ARCI. x-axes, dose in milligrams. Data points above PL designate values from the placebo test session. Data points for 10 above METH designate values from the methamphetamine (10 mg) test session. Data points show means of seven subjects; brackets show 1 S.E.M. Unidirectional error bars are shown in some instances for clarity.
Adjective-Rating Scale. The one-factor repeated-measure ANOVA that included all 16 drug doses and placebo revealed a significant effect of dose on the stimulant and sedative subscales of the Adjective-Rating Scale (F16,96 ≥ 5.7, p < 0.05). For the stimulant subscale, subsequent one-factor repeated-measure ANOVA indicated that methamphetamine, d-amphetamine, and methylphenidate (F4,24 ≥ 5.1, p < 0.05), but not triazolam (F4,24 = 0.8, p > 0.05), significantly increased scores as an orderly function of dose (Fig. 2, top right). Two-factor repeated-measure ANOVA indicated that the dose-related effects of methamphetamine, d-amphetamine, and methylphenidate did not differ significantly on this scale (F4,24 ≤ 0.6, p > 0.05). The effects of methamphetamine, d-amphetamine, and methylphenidate differed significantly from those of triazolam (F1,6 ≥ 19.3, p < 0.05).
For the sedative subscale, subsequent one-factor repeated-measure ANOVA indicated that methamphetamine, d-amphetamine, and methylphenidate decreased scores as an orderly function of dose (F4,24 ≥ 3.7, p < 0.05; data not shown). Triazolam did not significantly affect scores on this subscale (F4,24 = 1.7, p > 0.05). Two-factor repeated-measure ANOVA indicated that the dose-related effects of methamphetamine, d-amphetamine, and methylphenidate did not differ significantly on this subscale (F4,24 ≤ 2.0, p > 0.05). The effects of methamphetamine, d-amphetamine, and methylphenidate differed significantly from those of triazolam (F1,6 ≥ 21.7, p < 0.05).
ARCI. The one-factor repeated-measure ANOVA that included all 16 drug doses and placebo revealed a significant effect of dose on four subscales from the ARCI: A, BG, MBG, and PCAG (F16,96 ≥ 5.1, p < 0.05). For the A, BG, and MBG subscales, subsequent one-factor repeated-measure ANOVA indicated that methamphetamine, d-amphetamine, and methylphenidate (F4,24 ≥ 3.9, p < 0.05), but not triazolam (F4,24 ≤ 1.2, p > 0.05), significantly increased scores as an orderly function of dose (Fig. 2, bottom). Two-factor repeated-measure ANOVA indicated that the dose-related effects of methamphetamine, d-amphetamine, and methylphenidate did not differ significantly on these subscales (F4,24 ≤ 0.03, p > 0.05). The effects of methamphetamine, d-amphetamine, and methylphenidate differed significantly from those of triazolam (F1,6 ≥ 5.7, p < 0.05).
For the PCAG subscale, subsequent one-factor repeated-measure ANOVA indicated that methamphetamine, d-amphetamine, and methylphenidate decreased scores as an orderly function of dose (F4,24 ≥ 2.9, p < 0.05; data not shown). Triazolam did not significantly affect scores on this subscale (F4,24 = 2.5, p > 0.05). Two-factor repeated-measure ANOVA indicated that the dose-related effects of methamphetamine, d-amphetamine, and methylphenidate did not differ significantly on this subscale (F4,24 ≤ 2.0, p > 0.05). The effects of methamphetamine, d-amphetamine, and methylphenidate differed significantly from those of triazolam (F1,6 ≥ 20.1, p < 0.05).
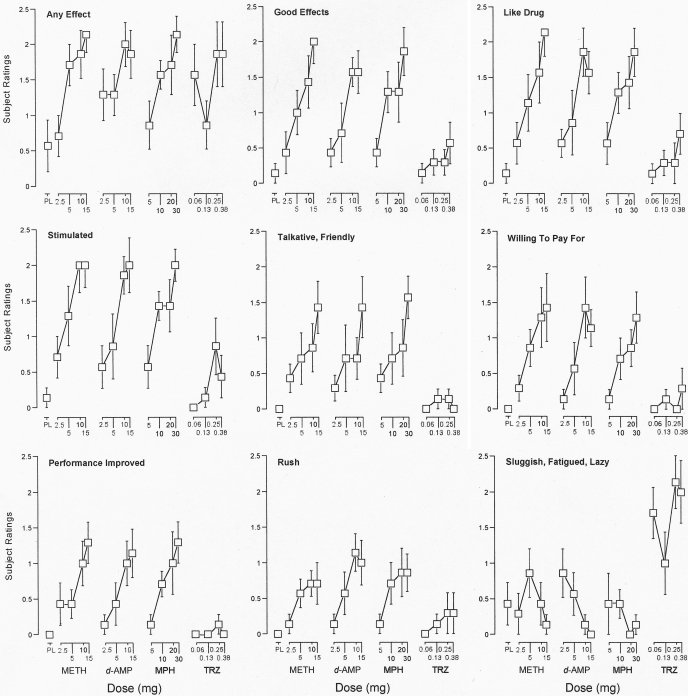
Drug-Effect Questionnaire. The one-factor repeated-measure ANOVA that included all 16 drug doses and placebo revealed a significant effect of dose on 16 items from the Drug-Effect Questionnaire: any effect; good effects; like drug; stimulated; talkative, friendly; willing to pay for; performance improved; rush; sluggish, fatigued, lazy; active, alert, energetic; willing to take again; irregular, racing heart beat; nervous, anxious; shaky, jittery; performance impaired; and high (F16,96 ≥ 1.8, p < 0.05). Subsequent one-factor repeated-measure ANOVA indicated that all drugs significantly increased ratings of any effect (F4,24 ≥ 4.2, p < 0.05; Fig. 3). Methamphetamine, d-amphetamine, and methylphenidate increased ratings of any effect as an orderly function of dose. The effects of triazolam on ratings of any effect were not as orderly in that the 0.0625 mg dose produced a greater increase than the 0.125 mg dose. The two-factor repeated-measure ANOVA indicated that the dose-related effects of methamphetamine, d-amphetamine, and methylphenidate did not differ significantly on ratings of any effect (F4,24 ≤ 2.2, p > 0.05). The effects of methamphetamine, but not d-amphetamine and methylphenidate, differed significantly from those of triazolam (F4,24 = 5.5, p < 0.05).
Fig. 3.
Dose effects for METH, d-AMP, MPH, and TRZ for subject ratings of any effect; good effects; like drug; stimulated; talkative, friendly; willing to pay for; performance improved; rush and sluggish, fatigued, lazy on the Drug-Effect Questionnaire. x-axes, dose in milligrams. Data points above PL designate values from the placebo test session. Data points for 10 above METH designate values from the methamphetamine (10 mg) test session. Data points show means of seven subjects; brackets show 1 S.E.M. Unidirectional error bars are shown in some instances for clarity.
Methamphetamine, d-amphetamine, and methylphenidate (F4,24 ≥ 3.9, p < 0.05), but not triazolam (F4,24 ≤ 2.5, p > 0.05), significantly increased ratings of good effects; like drug; stimulated; talkative, friendly; willing to pay for; performance improved and rush (Fig. 3); along with the measures of active, alert, energetic, and willing to take again (data not shown in Fig. 3) as an orderly function of dose. Two-factor repeated-measure ANOVA indicated that the dose-related effects of methamphetamine, d-amphetamine, and methylphenidate did not differ significantly on these items (F4,24 ≤ 2.7, p > 0.05). The effects of methamphetamine, d-amphetamine, and methylphenidate differed significantly from those of triazolam (F1,6 ≥ 6.9, p < 0.05).
Triazolam, but no other drug, significantly increased ratings of sluggish, fatigued, lazy (F4,24 = 4.2, p < 0.05; Fig. 3). Two-factor repeated-measure ANOVA indicated that the effects of triazolam differed significantly from those of methamphetamine, d-amphetamine, and methylphenidate (F1,6 ≥ 36.0, p < 0.05).
Methylphenidate, and no other drugs, significantly increased ratings of irregular, racing heart beat and nervous, anxious (F4,24 ≥ 2.9, p < 0.05; data not shown). d-Amphetamine, and no other drugs, significantly increased ratings of shaky, jittery (F4,24 = 3.7, p < 0.05; data not shown). Although one-factor repeated-measure ANOVA that included all 16 drug doses and placebo revealed a significant effect of dose on the ratings of performance impaired and high, the subsequent one-factor repeated-measure ANOVA indicated that no drug significantly increased the ratings on these two items (F4,24 ≤ 2.4, p > 0.05; data not shown).
DSST. The one-factor repeated-measure ANOVA that included all 16 drug doses and placebo did not reveal a significant effect of dose on the percentage of trials completed correctly on the DSST (F16,96 = 0.7, p > 0.05; data not shown).
Heart Rate and Blood Pressure. The one-factor repeated-measure ANOVA that included all 16 drug doses and placebo revealed a significant effect of dose on heart rate, systolic pressure, and diastolic pressure (F16,96 ≥ 1.8, p < 0.05). Subsequent one-factor repeated-measure ANOVA indicated that methamphetamine, and no other drugs, increased heart rate, systolic pressure, and diastolic pressure significantly above placebo levels (F4,24 ≥ 2.7, p < 0.05; data not shown). The peak heart rate was 91.1 ± 5.1 (mean ± S.E.M.) beats/min after methamphetamine administration. The peak systolic pressure was 147.1 ± 1.7 mm Hg, and diastolic pressure was 81.7 ± 3.7 mm Hg after methamphetamine administration. Although methamphetamine, but not d-amphetamine, increased heart rate, the two-factor repeated-measure ANOVA indicated that the dose-related effects of these drugs did not differ significantly (F4,24 = 1.9, p > 0.05). The dose-related effects of methamphetamine differed significantly from those of methylphenidate and triazolam (F4,24 ≥ 3.0, p < 0.05), in part, because methylphenidate and triazolam produced a greater increase in heart rate than what was observed after low doses of methamphetamine (i.e., 2.5 and 5 mg). The dose-related effects of d-amphetamine, methylphenidate, and triazolam did not differ significantly on heart rate (F4,24 ≤ 2.0, p > 0.05).
Although methamphetamine, but not d-amphetamine and methylphenidate, increased systolic blood pressure, the two-factor repeated-measure ANOVA indicated that the dose-related effects of methamphetamine, d-amphetamine, and methylphenidate did not differ significantly (F4,24 ≤ 1.6, p > 0.05). The effects of methamphetamine, d-amphetamine, and methylphenidate differed significantly from those of triazolam (F1,6 ≥ 7.4, p < 0.05).
Although methamphetamine, but not methylphenidate, increased diastolic blood pressure, two-factor repeated-measure ANOVA indicated that the dose-related effects of methamphetamine and methylphenidate did not differ significantly (F4,24 = 1.9, p > 0.05). The dose-related effects of methamphetamine differed significantly from those of d-amphetamine on diastolic blood pressure (F1,6 = 5.9, p < 0.05), in part, because d-amphetamine produced a greater increase than that was observed after low doses of methamphetamine (i.e., 2.5 and 5 mg). The effects of methamphetamine, d-amphetamine, and methylphenidate differed significantly from those of triazolam (F1,6 ≥ 7.8, p < 0.05).
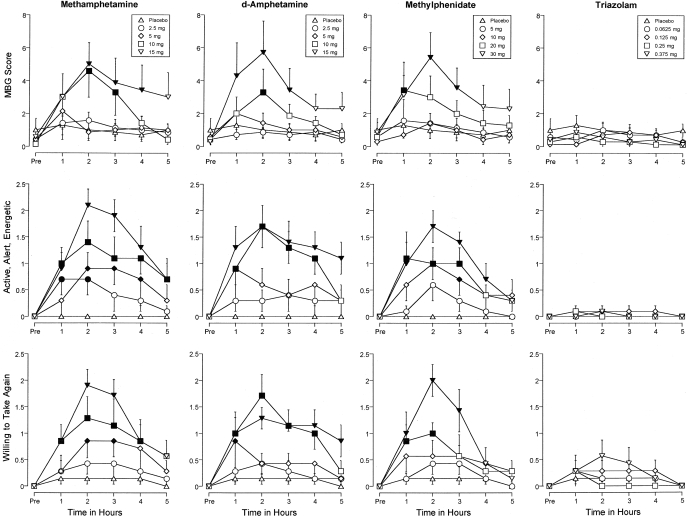
Time Course. Figure 4 shows the time-action curves for methamphetamine, d-amphetamine, methylphenidate, and triazolam on three measures: MBG scores from the ARCI, along with the ratings of active, alert, energetic, and willing to take again from the Drug-Effect Questionnaire. A significant interaction of dose and time was detected on these measures and on several other dependent measures (Table 1). Figure 4 shows that methamphetamine, d-amphetamine, and methylphenidate increased the ratings on these items significantly above placebo levels as an orderly function of dose and time (Dunnett's critical values = 0.7, 0.6, and 2.0, respectively). The effects of methamphetamine, d-amphetamine, and methylphenidate generally differed significantly from placebo 1 h after drug administration, peaked 2 h after drug administration, and returned to baseline 4 to 5 h after drug administration (Fig. 4). The effects of triazolam did not differ from those of placebo at any time point.
Fig. 4.
Time-action curves for methamphetamine, d-amphetamine, methylphenidate, and triazolam on the MBG subscale from the ARCI, along with ratings of active, alert, energetic, and willing to take again from the Drug-Effect Questionnaire. Filled symbols, values that are significantly different from the corresponding placebo value at the same time point (Dunnett's post hoc tests, p ≤ 0.05). x-axes, time in hours. Data points above “Pre” designate values from the predrug observation. Data points show means of seven subjects; brackets show 1 S.E.M. Unidirectional error bars are shown in some instances for clarity.
TABLE 1.
Summary of F statistics of the time-course analyses from the test phase
Summary statistics are not included for a particular measure if the two-factor ANOVA failed to reveal a significant effect of dose or interaction of dose and time.
| Measure | Dose (df16,96) | Time (df5,30) | Dose × Time (df80,480) |
|---|---|---|---|
| Discrimination performance | |||
| Drug-appropriate responding | 8.0 | 1.3 | 1.8 |
| Stimulant-sensitive adjectives | |||
| Score | 6.25 | 18.6 | 2.4 |
| Adjective rating scale | |||
| Stimulant scale | 7.5 | 6.4 | 3.3 |
| Sedative scale | 5.4 | 2.1 | 2.6 |
| Addiction Research Center Inventory | |||
| A | 4.7 | 7.1 | 1.8 |
| BG | 5.4 | 1.4 | 2.1 |
| MBG | 4.9 | 4.7 | 1.7 |
| PCAG | 5.9 | 0.3 | 2.7 |
| Drug effect questionnaire | |||
| Any effect | 4.0 | 19.0 | 1.6 |
| Good effects | 8.1 | 11.3 | 2.5 |
| Like drug | 6.5 | 12.8 | 2.1 |
| Stimulated | 8.5 | 15.4 | 2.7 |
| Talkative, friendly | 4.0 | 5.3 | 1.7 |
| Willing to pay for | 4.7 | 7.2 | 2.1 |
| Performance improved | 5.6 | 7.6 | 2.4 |
| Rush | 4.6 | 6.3 | 1.5 |
| Sluggish, fatigued, lazy | 4.1 | 6.9 | 2.3 |
| Active, alert, energetic | 8.1 | 11.7 | 2.7 |
| Willing to take again | 6.1 | 11.6 | 2.4 |
| Irregular racing heartbeat | 3.9 | 3.8 | 1.8 |
| Shaky, jittery | 2.3 | 3.1 | 1.5 |
| Performance impaired | 3.0 | 5.9 | 1.6 |
| High | 2.4 | 5.7 | 1.4 |
| Bad Effects | 1.1 | 5.8 | 1.5 |
| Digit-Symbol Substitution Test | |||
| Percentage of trials correct | 0.7 | 2.6 | 1.4 |
| Cardiovascular measures | |||
| Heart rate | 2.2 | 46.7 | 1.2 |
| Systolic pressure | 5.2 | 5.7 | 1.3 |
| Diastolic pressure | 3.0 | 7.5 | 1.2 |
Boldface values are statistically significant at p ≤ 0.05.
Discussion
The present study examined the discriminative stimulus, subject-rated, and cardiovascular effects of methamphetamine, d-amphetamine, methylphenidate, and triazolam in recreational stimulant users, who had learned to discriminate 10 mg of methamphetamine from placebo. Methamphetamine, d-amphetamine, and methylphenidate dose-dependently increased methamphetamine-appropriate responding and produced a similar constellation of stimulant-like subject-rated effects. In contrast, triazolam engendered low levels of methamphetamine-appropriate responding and produced sedative-like effects.
The finding that 10 mg of methamphetamine served as a discriminable dose is concordant with the results from a previous study that established 10 mg of methamphetamine as a discriminative stimulus in humans (Hart et al., 2002). In that study, methamphetamine dose-dependently increased drug-appropriate responding, and the N-methyl-d-aspartate receptor antagonist memantine did not occasion methamphetamine-like responding. The current results confirm and extend the findings of Hart et al. (2002) by showing that methamphetamine functioned as a discriminative stimulus and d-amphetamine and methylphenidate substituted for methamphetamine. The present results are also concordant with the findings from the animal studies demonstrating that d-amphetamine and methylphenidate substituted for methamphetamine (Czoty et al., 2004a,b; Desai et al., 2007). Thus, in agreement with the previous findings in humans and animals, results from the current study indicate that methamphetamine functions as a discriminative stimulus, and the behavioral effects of methamphetamine are similar to those of the other commonly abused stimulants.
Several lines of evidence indicate that methamphetamine shares discriminative stimulus effects with several abused stimulants in humans and animals. For instance, methamphetamine dose-dependently increased drug-appropriate responding in rats discriminating d-amphetamine or cocaine (Huang and Ho, 1974; Holtzman, 2001). A recent study by Johanson et al. (2006) demonstrated that intravenous cocaine functioned as a discriminative stimulus in humans, and methamphetamine substituted completely for cocaine at the highest dose tested. Earlier, Lamb and Henningfield (1994) had reported that methamphetamine substituted for d-amphetamine in a drug discrimination assay in humans. Taking together the findings of Lamb and Henningfield (1994) and that of the current study (i.e., d-amphetamine substituted for methamphetamine), it can be inferred that a symmetrical generalization exists between d-amphetamine and methamphetamine. Thus, in addition to the similar structures and similar profiles of neurochemical effects, the behavioral effects of methamphetamine and d-amphetamine overlap extensively in humans.
Methylphenidate is also structurally related to amphetamines, and it shares neurochemical (i.e., enhancement in dopaminergic transmission) and therapeutic (i.e., treatment for ADHD) effects with amphetamines (Patrick and Markowitz, 1997; Carboni and Silvagni, 2004; Fone and Nutt, 2005). Along these lines, the current findings indicate that methylphenidate shares discriminative stimulus effects with methamphetamine. It is worth noting that methylphenidate also substitutes for d-amphetamine and cocaine in humans (Rush et al., 1998; Rush and Baker, 2001). The similarity between the discriminative stimulus effects of methylphenidate and other abused stimulants is consistent with the documented abuse potential of methylphenidate (Rush et al., 2001).
Triazolam produced prototypical sedative-like effects (i.e., increased ratings of sluggish, fatigued, lazy) in the present experiment. These findings are concordant with earlier studies that used a similar dose range and reported that triazolam produced a profile of sedative effects (Rush et al., 1998; Stoops et al., 2005). In the current study, triazolam engendered low levels of methamphetamine-appropriate responding and produced subject-rated effects that were markedly different from those observed with methamphetamine. Thus, the present results demonstrate that the methamphetamine-placebo discrimination in humans is pharmacologically selective and that the GABAA receptor modulator triazolam is an appropriate negative control in studies of stimulant discrimination in humans (Rush et al., 1998; Stoops et al., 2005).
Subject-rated effects are the most widely used behavioral measures in human laboratory experiments for assessing the abuse-related effects of stimulants. In the present study, methamphetamine, d-amphetamine, and methylphenidate produced an array of positive subject-rated effects across the range of doses tested (e.g., increased ratings of good effects, like drug), and these findings are consistent with previous reports (Martin et al., 1971; Mayfield, 1973; Rush et al., 1998). Hart et al. (2001) reported that methamphetamine increased subject ratings (e.g., good effects) that were dose- and time-dependent. The present study confirms and extends those findings by demonstrating that methamphetamine, d-amphetamine, and methylphenidate increased stimulant-like subject-rated effects (e.g., active, alert, energetic) as an orderly function of dose and time. The similarity observed in the subject-rated effects of methamphetamine, d-amphetamine, and methylphenidate is consistent with the findings of Martin et al. (1971). In that study, the relative potencies of amphetamines and methylphenidate in producing positive subject-rated effects (e.g., like drug; A, BG scales) were similar to those observed here. In addition, the present results demonstrating that the onset of action, peak effects, and duration of the effects overlapped among methamphetamine, d-amphetamine, and methylphenidate are consistent with the findings of Martin et al. (1971). Thus, in agreement with the previous reports, results from the current study indicate that subject-rated effects of methamphetamine and related stimulants overlap extensively.
The behavioral effects of methamphetamine, d-amphetamine, and methylphenidate are believed to be mediated through their interaction with transporters for monoamines, such as dopamine, norepinephrine, and serotonin (Fleckenstein et al., 2000). Amphetamines and methylphenidate elevate synaptic monoamine levels, albeit through somewhat different mechanisms; amphetamines primarily act as substrates for monoamine transporters to promote reverse transport of monoamines, whereas methylphenidate primarily blocks the reuptake of monoamines back to the neuronal terminal (Fleckenstein et al., 2000). Despite these subtle differences in molecular mechanisms, a common neurochemical effect of amphetamines and methylphenidate is to elevate the synaptic concentration of monoamines in the brain. Therefore, it is likely that the similarity in the neurochemical actions of methamphetamine, d-amphetamine, and methylphenidate contributed, at least in part, to the similar behavioral effects of these drugs observed in the present study.
The current results add to the body of information on the discriminative stimulus effects of stimulants in humans. However, we acknowledge that there were some limitations to the present study. The doses of methamphetamine used in this study were relatively small. The recommended daily dose range of stimulants for the treatment of ADHD is 20 to 25 mg of methamphetamine, 20 mg of d-amphetamine, or 20 to 30 mg of methylphenidate (Physicians' Desk Reference, 2006). Moreover, it is worth noting that the subjects in the present study were not stimulant abusers, and methamphetamine-dependent individuals typically use much greater amounts. Therefore, the small doses used in this study limit the implications of current findings and warrant the need for the future studies to assess the behavioral effects of higher doses of stimulants in methamphetamine-dependent individuals. Another limitation of the current study is that the drugs were administered orally, which is not commonly used for the illicit drug taking. Administering methamphetamine intranasally, intravenously, or by smoking are most often associated with its abuse, in part because of a rapid onset of effects. Thus, the present results should be interpreted cautiously, considering the variation in the emergence of the drug effects by different routes. Another limitation worth noting is the small sample size, signaling the need to extend these findings by assessing the effects of stimulants in a larger cohort.
Previous human laboratory studies have shown that stimulants can increase heart rate and blood pressure (e.g., Martin et al., 1971; Stoops et al., 2005; Lile et al., 2006). For example, Martin et al. (1971) reported that amphetamines and methylphenidate increased heart rate and systolic and diastolic pressure, and there was a good concordance between the potency estimates for these stimulants. In the current study, two high doses of methamphetamine (i.e., 10 and 15 mg) increased heart rate and blood pressure significantly above placebo levels, whereas other drugs did not affect cardiovascular parameters across the range of doses tested. It is worth noting that the methamphetamine-induced enhancement in heart rate and blood pressure was not clinically significant, which suggests that the dose range of methamphetamine studied was safe and tolerable. Moreover, methamphetamine did not alter performance in the DSST, which indicates that the doses of methamphetamine used in the current study did not impair psychomotor performance.
In summary, the present study demonstrates that methamphetamine functions as a discriminative stimulus and produces prototypical stimulant-like subject-rated effects in humans. The behavioral effects of methamphetamine, d-amphetamine, and methylphenidate were remarkably similar. This similarity in the behavioral effects of stimulants tested in the present experiment is discordant with the perceived notion that methamphetamine has greater abuse potential than d-amphetamine and methylphenidate. Increased rates of abuse observed with methamphetamine relative to d-amphetamine and methylphenidate suggest that behavioral pharmacologic effects of stimulants may not be the sole determinant of their abuse potential (Maxwell and Rutkowski, 2008). Social variables and drug availability, for example, could influence the relative abuse potential of stimulants. Therefore, the present data should be viewed cautiously in the context of epidemiological findings delineating nonpharmacological factors contributing to methamphetamine abuse (Gonsalves et al., 2007; Degenhardt et al., 2008; Maxwell and Rutkowski, 2008). Future studies should determine whether the behavioral effects of methamphetamine, d-amphetamine, and methylphenidate overlap under different experimental conditions (e.g., across a wider range of doses) and behavioral assays (e.g., drug self-administration).
Acknowledgments
We thank Frances Wagner for expert medical assistance. We thank Neena Khanna, Josh Barnett, Doris Castellana-Cruz, Derek Roe, and Kristi Yingling for skilled technical assistance.
This work was supported by the National Institutes of Health National Institute of Drug Abuse [Grant DA017711].
doi:10.1124/jpet.108.147124.
ABBREVIATIONS: ADHD, attention deficit hyperactivity disorder; THC, tetrahydrocannabinol; ARCI, Addiction Research Center Inventory; MBG, morphine-benzedrine group; PCAG, pentobarbital, chlorpromazine, alcohol group; BG, Benzedrine group; A, amphetamine; DSST, Digit-Symbol Substitution Test; ANOVA, analysis of variance; METH, methamphetamine; d-AMP, d-amphetamine; MPH, methylphenidate; TRZ, triazolam.
References
- Abreu ME and Griffiths RR (1996) Drug tasting may confound human drug discrimination studies. Psychopharmacology (Berl) 125 255-257. [DOI] [PubMed] [Google Scholar]
- Carboni E and Silvagni A (2004) Experimental investigations on dopamine transmission can provide clues on the mechanism of the therapeutic effect of amphetamine and methylphenidate in ADHD. Neural Plast 11 77-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens KJ, Cornish JL, Hunt GE, and McGregor IS (2006) Intravenous methamphetamine self-administration in rats: effects of intravenous or intraperitoneal MDMA co-administration. Pharmacol Biochem Behav 85 454-463. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Makriyannis A, and Bergman J (2004a) Methamphetamine discrimination and in vivo microdialysis in squirrel monkeys. Psychopharmacology (Berl) 175 170-178. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Ramanathan CR, Mutschler NH, Makriyannis A, and Bergman J (2004b) Drug discrimination in methamphetamine-trained monkeys: effects of monoamine transporter inhibitors. J Pharmacol Exp Ther 311 720-727. [DOI] [PubMed] [Google Scholar]
- Danks RR, Wibbenmeyer LA, Faucher LD, Sihler KC, Kealey GP, Chang P, Amelon M, and Lewis RW 3rd (2004) Methamphetamine-associated burn injuries: a retrospective analysis. J Burn Care Rehabil 25 425-429. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Baker A, and Maher L (2008) Methamphetamine: geographic areas and populations at risk, and emerging evidence for effective interventions. Drug Alcohol Rev 27 217-219. [DOI] [PubMed] [Google Scholar]
- De La Garza R 2nd, Mahoney JJ 3rd, Culbertson C, Shoptaw S, and Newton TF (2008) The acetylcholinesterase inhibitor rivastigmine does not alter total choices for methamphetamine, but may reduce positive subjective effects, in a laboratory model of intravenous self-administration in human volunteers. Pharmacol Biochem Behav 89 200-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RI, Paronis CA, Makriyannis A, Thakur GA, and Bergman J (2007) Discriminative-stimulus effects of methamphetamine and in vivo microdialysis in rats. FASEB J 21 715.3. [Google Scholar]
- Di Marino ME, Haberny KA, Felch LJ, Walsh SL, Preston KL, and Bigelow GE (1998) Development of a subjective rating scale sensitive to acute cocaine administration, in Problems of Drug Dependence 1997: Proceedings of the 59th Annual Scientific Meeting of the College on Problems of Drug Dependence. National Institute on Drug Abuse Research Monograph Series no. 178 (Harris LS ed) pp 139. National Institute on Drug Abuse, Rockville, MD.
- Fleckenstein AE, Gibb JW, and Hanson GR (2000) Differential effects of stimulants on monoaminergic transporters: pharmacological consequences and implications for neurotoxicity. Eur J Pharmacol 406 1-13. [DOI] [PubMed] [Google Scholar]
- Fone KC and Nutt DJ (2005) Stimulants: use and abuse in the treatment of attention deficit hyperactivity disorder. Curr Opin Pharmacol 5 87-93. [DOI] [PubMed] [Google Scholar]
- Fustero S, González J, and del Pozo C (2006) 1,4-Benzodiazepine N-nitrosoamidines: useful intermediates in the synthesis of tricyclic benzodiazepines. Molecules 11 583-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalves VM, Sapp JL, and Huss MT (2007) A comparison of methamphetamine and nonamphetamine users in a dual diagnosis facility. Addict Res Theory 15 277-284. [Google Scholar]
- Halkitis PN, Parsons JT, and Stirratt MJ (2001) A double epidemic: crystal methamphetamine drug use in relation to HIV transmission among gay men. J Homosex 41 17-35. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Foltin RW, and Fischman MW (2002) Effects of the NMDA antagonist memantine on human methamphetamine discrimination. Psychopharmacology (Berl) 164 376-384. [DOI] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Foltin RW, and Fischman MW (2001) Methamphetamine self-administration by humans. Psychopharmacology (Berl) 157 75-81. [DOI] [PubMed] [Google Scholar]
- Holtzman SG (2001) Differential interaction of GBR 12909, a dopamine uptake inhibitor, with cocaine and methamphetamine in rats discriminating cocaine. Psychopharmacology (Berl) 155 180-186. [DOI] [PubMed] [Google Scholar]
- Huang JT and Ho BT (1974) Discriminative stimulus properties of d-amphetamine and related compounds in rats. Pharmacol Biochem Behav 2 669-673. [DOI] [PubMed] [Google Scholar]
- Jasinski D (1977) Assessment of the abuse potentiality of morphine-like drugs (methods used in man), in Drug Addiction I (Martin WR ed) pp 197-258, Springer-Verlag New York Inc., New York.
- Johanson CE, Lundahl LH, Lockhart N, and Schubiner H (2006) Intravenous cocaine discrimination in humans. Exp Clin Psychopharmacol 14 99-108. [DOI] [PubMed] [Google Scholar]
- Lamb RJ and Henningfield JE (1994) Human d-amphetamine drug discrimination: methamphetamine and hydromorphone. J Exp Anal Behav 61 169-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Durell TM, Glaser PE, and Rush CR (2006) Discriminative-stimulus, self-reported, performance, and cardiovascular effects of atomoxetine in methylphenidate-trained humans. Exp Clin Psychopharmacol 14 136-147. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, and Jasinski DR (1971) Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther 12 245-258. [DOI] [PubMed] [Google Scholar]
- Maxwell JC and Rutkowski BA (2008) The prevalence of methamphetamine and amphetamine abuse in North America: a review of the indicators, 1992-2007. Drug Alcohol Rev 27 229-235. [DOI] [PubMed] [Google Scholar]
- Mayfield DG (1973) The effect of intravenous methamphetamine on mood. Int J Addict 8 565-568. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, and Yingling JE (1982) An automated version of the digit symbol substitution test (DSST). Behav Res Methods Instr 14 463-466. [Google Scholar]
- Newton TF, De La Garza R 2nd, Kalechstein AD, and Nestor L (2005) Cocaine and methamphetamine produce different patterns of subjective and cardiovascular effects. Pharmacol Biochem Behav 82 90-97. [DOI] [PubMed] [Google Scholar]
- Oliveto AH, Bickel WK, Hughes JR, Shea PJ, Higgins ST, and Fenwick JW (1992) Caffeine drug discrimination in humans: acquisition, specificity and correlation with self-reports. J Pharmacol Exp Ther 261 885-894. [PubMed] [Google Scholar]
- Patrick KS and Markowitz JS (1997) Pharmacology of methylphenidate, amphetamine enantiomers and pemoline in attention-deficit hyperactivity disorder. Hum Psychopharmacol Clin Exp 12 527-546. [Google Scholar]
- Rush CR and Baker RW (2001) Behavioral pharmacological similarities between methylphenidate and cocaine in cocaine abusers. Exp Clin Psychopharmacol 9 59-73. [DOI] [PubMed] [Google Scholar]
- Rush CR, Essman WD, Simpson CA, and Baker RW (2001) Reinforcing and subjectrated effects of methylphenidate and d-amphetamine in non-drug-abusing humans. J Clin Psychopharmacol 21 273-286. [DOI] [PubMed] [Google Scholar]
- Rush CR, Kollins SH, and Pazzaglia PJ (1998) Discriminative-stimulus and participant-rated effects of methylphenidate, bupropion, and triazolam in d-amphetamine-trained humans. Exp Clin Psychopharmacol 6 32-44. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Hays LR, Glaser PE, and Hays LS (2003) Risperidone attenuates the discriminative-stimulus effects of d-amphetamine in humans. J Pharmacol Exp Ther 306 195-204. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Glaser PE, and Rush CR (2005) Discriminative stimulus and self-reported effects of methylphenidate, d-amphetamine, and triazolam in methylphenidate-trained humans. Exp Clin Psychopharmacol 13 56-64. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Service Administration (2006) Results from the National Survey on Drug Use and Health. NSDUH Series H-32, DHHS Publication No. SMA 07-4293, National Findings Office of Applied Sciences, Rockville, MD.
- Substance Abuse and Mental Health Service Administration. Drug Abuse Warning Network (2005) National Estimates of Drug-Related Emergency Department visits Office of Applied Studies, DAWN Series D-29, DHHS Publication No (SMA) 07-4256, Rockville, MD.
- Substance Abuse and Mental Health Service Administration Drug and Alcohol Services Information System (2004) Primary methamphetamine/amphetamin treatment admissions increase: 1992-2002 report, Office of Applied Studies Publications and Data Dissemination, Rockville, MD.
- Sun J, Xu X, Wang C, and You T (2008) Analysis of amphetamines in urine with liquid-liquid extraction by capillary electrophoresis with simultaneous electrochemical and electrochemiluminescence detection. Electrophoresis 29 3999-4007. [DOI] [PubMed] [Google Scholar]