Abstract
The predominantly human sequence anti-cocaine monoclonal antibody (mAb), 2E2, has high affinity and specificity for cocaine and antagonizes cocaine distribution to the brain in mice. To determine whether 2E2 can alter the self-administration of cocaine in rats, both cocaine-induced reinstatement (priming) of self-administration, and the rates of cocaine consumption were assessed during daily sessions. After self-administration training, the rats' cocaine priming threshold values were stable over a 2-week baseline period. Furthermore, the rates of cocaine consumption at unit doses of 0.3 and 3.0 μmol/kg were steady within sessions and stable between sessions. Then, 2E2 (120 mg/kg i.v.) or an equivalent dose of nonspecific human polyclonal IgG (control) was infused and daily sessions continued. 2E2 produced an initial, approximately 3-fold, increase in the cocaine priming threshold that declined toward baseline values over the subsequent 3 weeks, with an effect t½ of approximately 4 days. In contrast to the substantial increase in the cocaine priming threshold, 2E2 produced only modest dose-dependent increases (42 and 18%) in the cocaine consumption rates, and these also gradually declined toward baseline values. There was no significant effect of the control IgG on the priming threshold or rates of consumption of cocaine. After infusion, antibody blood concentrations declined over time, and a two-compartment pharmacokinetic model generated values for the distribution and elimination half-lives of 0.5 and 11.6 days for 2E2 and 0.4 and 6.0 days for control IgG. 2E2 had a long-lasting effect on cocaine-induced priming, which may predict its efficacy as an immunotherapy for cocaine abuse.
The drug-induced reinstatement (priming) of drug self-administration behavior represents an animal model of some aspects of the relapse process (de Wit and Stewart, 1981; Shalev et al., 2002) in addicts. The cumulative concentration of cocaine is a critical determinant of the probability of reinstating cocaine self-administration in rats (Norman et al., 1999, 2002). Because the site of action for cocaine is presumably in the brain, decreasing the drug concentrations reaching the brain would be expected to decrease the probability of relapse. Antibodies with high affinity and specificity for cocaine are hypothesized to sequester cocaine in the peripheral circulation and reduce its entry to the brain (Kosten and Owens, 2005). Pharmacokinetic antagonism is defined as a decrease in the concentration of an agonist at its site of action. Typically, the mechanism by which this is achieved is by increasing the rate of agonist clearance (Rang et al., 2007). Although this may occur with antibody Fab fragments targeting slowly cleared drugs such as digoxin (Bateman, 2004), this would not occur with monoclonal antibodies (mAb) targeting rapidly cleared drugs such as cocaine or nicotine (Keyler et al., 2005). Antibodies generally act as chemical antagonists by binding to and thereby reversibly inactivating drugs. As a consequence, anti-drug antibodies alter drug distribution and in so doing reduce the concentration of a drug at its site of action, which also meets the definition of pharmacokinetic antagonism (Rang et al., 2007). Therefore, the action of anti-drug antibodies has aspects of both pharmacokinetic and chemical antagonism.
Anti-cocaine antibodies have been demonstrated to antagonize the effects of cocaine in vivo. Thus, active immunization of animals with hapten-carrier conjugates can elicit the production of polyclonal anti-cocaine antibodies with sufficient levels and affinity for cocaine that they can reduce the amounts entering the brain (Fox et al., 1996). Furthermore, in rats, active immunization to cocaine has also been shown to attenuate the behavioral effects (Carrera et al., 1995, 2001; Fox et al., 1996; Ettinger et al., 1997; Koetzner et al., 2003) and the priming effects (Carrera et al., 2000) of systemically administered cocaine. It is important that active immunization in humans has been shown to produce levels of polyclonal anti-cocaine antibodies (Kosten et al., 2002) that were associated with a decrease in cocaine use (Martell et al., 2005). These results have demonstrated the potential efficacy of immunotherapy for cocaine abuse.
Passive immunization, which entails the systemic administration of an mAb with a defined affinity, specificity, and dose, should be even more efficacious. Passive immunization with murine anti-cocaine mAb has been demonstrated to attenuate the behavioral effects of cocaine (Fox et al., 1996; Mets et al., 1998; Carrera et al., 2000) in animals and therefore represents an alternative or adjunct to active immunization (Kosten and Owens, 2005). Recently, we reported the generation and characterization of an anti-cocaine mAb, designated 2E2 (Paula et al., 2004), that was generated in transgenic mice engineered to produce human sequence mAb (Lonberg, 2005). The mAb 2E2 has been determined to have a human sequence γ1 heavy chain and a murine λ light chain (Norman et al., 2007) and a high affinity (Kd, approximately 4 nM) and specificity for cocaine over its inactive metabolites (Paula et al., 2004). This unique mAb has been demonstrated to change the in vivo distribution of cocaine in mice such that cocaine is sequestered in the plasma with a concomitant dramatic decrease in brain cocaine concentrations (Norman et al., 2007). Based on these results, it was hypothesized that in the presence of the anti-cocaine mAb, the plasma cocaine concentration required to induce self-administration behavior (the cocaine priming threshold) would be increased. This hypothesis was tested in a group of rats trained to self-administer cocaine.
Materials and Methods
Cocaine Self-Administration Training. Male Sprague-Dawley rats (Harlan, Indianapolis, IN; approximately 400-500 g over the duration of the studies) were housed individually and kept on a 12-h light/dark cycle (lights on at 6:00 AM) with food and water available ad libitum. All studies were conducted in accordance with the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996). Rats were surgically implanted with an indwelling catheter into the right jugular vein while under halothane anesthesia.
Twelve test chambers (modified chambers from Lafayette Instrument Company, Lafayette, IN) were each equipped with active and inactive levers and a signal light. Each chamber was situated inside a laminated wooden compartment (43 × 61 × 35 cm) that provided sound attenuation and was equipped with a house light (7 W). Syringe pumps (model PHM-100; MED Associates, St. Albans, VT) were situated outside of the laminated compartments and additionally insulated to attenuate pump noise and vibration. The unit doses of cocaine were regulated by the duration of the injection (the cocaine concentration was 40 nmol/μl in saline and the rate of injection was 2.7 μl/s), which was controlled by computers using a program written in Medstate Notation language (MED Associates). The signal light was illuminated for the duration of the injection and any subsequent time-out. Therefore, each drug injection was initiated whenever the active lever was pressed, and the signal light was off. Each session began with an activation of the pump for 4.6 s, which filled the dead volume of the catheter (13 μl) with the cocaine solution. Catheter patency was evaluated by administration of short-acting barbiturate methohexital (Brevital, 6 mg/kg i.v.) as described previously (Norman et al., 2002).
Beginning 6 or 7 days after the surgery, rats were trained to self-administer cocaine HCl by pressing an active lever, and, except for time-out periods, every lever press resulted in an injection of cocaine [fixed ratio (FR) = 1 schedule] fixed at a unit dose of 1.5 μmol/kg (approximately 0.5 mg/kg cocaine HCl). After each injection of cocaine, there was a 5-s time-out period when cocaine was not available. This restriction on access to cocaine allows time for injected cocaine to partially distribute to the brain. Daily sessions lasted for 3 h or until 25 injections of 1.5 μmol/kg cocaine were self-administered. Training at this standard unit dose continued until individual rats met the criterion for stable maintained self-administration. This criterion was no significant change of the rat's mean rate of cocaine self-administration between five consecutive sessions.
Priming Threshold Measured by Escalating Doses of Cocaine. In trained animals, cocaine reinstates self-administration behavior when a minimal threshold concentration is reached in an animal's body (Norman et al., 1999, 2002). To measure this threshold level, a titration method is used where the cumulative cocaine concentration in the animal's body is gradually raised until the animal's lever-pressing response is reinstated.
In this study, at least 2 weeks before the infusion of antibody, test sessions with trained animals commenced and were run 6 days/week throughout the studies. During all sessions, both before and after the infusion of the antibodies, at the start of each daily session (starting between 8:00 and 10:00 AM), rats were placed in individual test chambers, and the times of all lever presses were recorded. Any initial lever-pressing activity occurring before the delivery of cocaine, presumably related to environmental cues, complicates the measurement of cocaine-induced responses. To minimize responses not induced by administered cocaine, the lever-pressing responses to environmental cues were extinguished by programming the initial lever presses to produce no activation of the pump despite the signal light being activated. To extinguish the animal's activity related to the signal light, this light then was activated at pseudorandom intervals, with no solution being injected. Thirty minutes after the animal's last press on either the active or the inactive lever, the catheter was filled with cocaine, and noncontingent infusions of escalating doses of cocaine were administered every 2 min until each animal's self-administration behavior was reinstated. Each subsequent infusion of cocaine was at a dose calculated to raise the peak cocaine level in vivo by 0.06 μmol/kg above the previous calculated peak level. This escalating drug dose regimen resulted in a linear increase in the peak cocaine level as a function of time at the rate of 0.03 μmol/kg/min (Norman et al., 2002). Our criterion for the reinstatement of cocaine self-administration was defined as the animal's maintained lever pressing subsequent to the first five consecutive lever presses that occurred at intervals not longer than 2 min apart. Once lever-pressing behavior was reinstated, the calculated peak cumulative cocaine levels resulting from the ultimate and penultimate priming infusions were recorded. The mean of these calculated values represented the priming threshold and for simplicity are presented as the single dose of cocaine that would achieve this peak level.
After reinstatement of self-administration behavior, the cocaine unit dose injected was initially the same as the dose of the ultimate priming infusion. After approximately 5 to 15 min, when the calculated cumulative level of cocaine approached the anticipated maximal level at which lever-pressing behavior is typically observed (Tsibulsky and Norman, 1999), the unit dose was then switched to 0.3 μmol/kg for the next 50 self-administrations. The unit dose then was again changed to 3.0 μmol/kg for the last 15 self-administrations. As described above, the rats self-administered cocaine under the schedule FR = 1, time-out = 5 s. After these defined numbers of cocaine self-administrations, the program terminated access to cocaine, but all subsequent lever presses were recorded. The session was considered ended 30 min after the animal's last lever press, and the time from the termination of cocaine access to the last lever press of the session typically was 20 to 40 min.
Calculation of Cocaine Levels in the Body. As stated above, the cocaine priming threshold was defined as the minimal concentration of cocaine in the body that reinstated cocaine self-administration behavior. This value was calculated by monitoring the amount of cocaine that was administered to the animals and then using predetermined pharmacokinetic values to estimate the resulting cocaine levels in the animals over time. The calculated values for the whole body cocaine levels depend upon the amount injected per kilogram of body weight minus the amount eliminated per unit time and cocaine's volume of distribution (Vd), which is assumed to be constant for each animal. Therefore, the cocaine level in the body (L) was calculated every Δt seconds, according to the simplified linear equation for the zero-order input/first-order elimination kinetics for a two-compartment model (Tsibulsky and Norman, 2005):
 |
(Eq.1) |
 |
(Eq.2) |
where Lc1 and Lp1 are cocaine levels at the time t1 in central and peripheral compartments, respectively; Lc0 and Lp0 are cocaine level at the time t0; Δt = t1-t0 is a computation interval, and the value for Δt was set at 1 s; k10, k20, k12, and k21 are the cocaine elimination rate constants and, V01 is the rate of cocaine injection; and V01·Δt represents the amount of cocaine administered. The group mean value for the cocaine k10 was 0.0014 s-1 [k = ln(2)/t½, t½ was 480 s], which was calculated as described previously (Tsibulsky and Norman, 1999), and k12 = k21 = 0.0116 s-1 (t½ = 60 s), which are consistent with literature values for these parameters (Booze et al., 1997). In this two-compartment model, it is assumed that there is no elimination of cocaine from the peripheral (second) compartment; therefore, k20 = 0.
Infusion of Antibodies. The anti-cocaine mAb 2E2 or the control IgG were administered by intravenous infusion via the same catheter through which cocaine was administered. The dose of each antibody was 120 mg/kg, which is the same dose of mAb 2E2 that was previously demonstrated to significantly alter the distribution of a single bolus injection of cocaine in mice (Norman et al., 2007). This corresponds to a dose of 0.8 μmol/kg 2E2 or control IgG molecules, which because antibodies are bivalent, corresponds to 1.6 μmol/kg 2E2 cocaine recognition sites. The mAb 2E2 was purified, both antibody samples were prepared in phosphate-buffered normal saline as described previously (Norman et al., 2007), and the solutions were sterilized via passage through a 0.2-μm filter before infusion. The antibody solutions were infused intravenously at an average rate of 968 μg/kg/min over 124 min. The concentration of mAb 2E2 ranged from 7 to 8 μg/μl, and the concentration of the control IgG ranged from 7 to 12 μg/μl. After the infusion was complete, the rats were returned to their home cages, and blood samples were obtained 2 h later and after the self-administration sessions on the days indicated in Fig. 1.
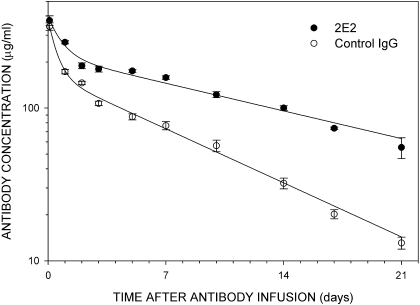
Fig. 1.
The pharmacokinetics of the anti-cocaine mAb 2E2 and human polyclonal antibodies. Rats received an intravenous infusion of either 120 mg/kg mAb 2E2 or human polyclonal antibodies. Blood samples (10 μl) were obtained from tail veins at the indicated times after the antibody infusions. Concentrations of antibodies in blood were determined using an ELISA. Data points represent the mean ± S.E.M. from seven 2E2-treated or eight control IgG-treated rats. The Vdss were approximately 0.48 and 0.63 l/kg for 2E2 and the control IgG, respectively. A two-compartment pharmacokinetic model with t½α and t½β of 0.5 and 11.6 days, respectively, was used to fit the data. A similar pharmacokinetic model with t½α and t½β values of 0.4 and 6.0 days, respectively, approximated the time course of the disappearance of the control IgG from rat blood. The models are represented by the best fit regression lines through the data points.
In Vivo 2E2 Pharmacokinetic Studies: Sample Preparation. Blood samples were obtained from the same rats used in the cocaine self-administration studies, except for one rat that was excluded from the self-administration study because of a failed catheter that occurred before the completion of the study. The blood 2E2 concentrations from this rat were included in the 2E2 pharmacokinetic data set. Blood samples were taken immediately before the infusion of 2E2 or control IgG and then 2 h after the infusion and periodically for at least 21 days. A sterile 27-gauge hypodermic needle was used to puncture a tail vein, and 10 μl of blood was collected using a heparinized capillary pipette tip. The blood was immediately placed in a 1.5-ml polypropylene microcentrifuge tube containing 40 μl of 0.1 M sodium citrate/0.1% sodium azide at pH 4.75. These samples were rapidly placed on ice and then stored at 4°C until use.
Antibody Quantification. The in vivo concentrations of 2E2 and control IgG were determined using a previously described enzyme-linked immunosorbent assay (ELISA) (Paula et al., 2004) that compared the quantity of mAb in varying dilutions of the rat blood samples with that quantified by a standard curve generated using known concentrations of purified 2E2 or control IgG. In brief, for 2E2, the conjugate benzoylecgonine-ovalbumin (3 μg/ml, 100 μl/well) in 1 mM EGTA, pH 7.4, was adsorbed onto polyvinyl chloride 96-well microtiter plates for 1 h. For the human polyclonal antibodies, goat anti-human Fc region-specific IgG antibodies (3 μg/ml, 100 μl/well in 1 mM EGTA, pH 7.4; Cappel Laboratories, Durham, NC) were adsorbed onto the 96-well microtiter plates. The plates were then washed three times, and all wells were exposed for 10 min to 0.5% BSA in Tris-buffered saline (10 mM Tris, 140 mM NaCl, and 0.02% NaN3, pH 6.9) to block nonspecific protein binding. The second layer added then was 100 μl/well blood samples, diluted (1:500) into BSA-Tris-buffered saline and incubated for 2 h. Serving as quantitation standards, duplicate 100 μl/well aliquots of 2E2 or the control IgG over a range of concentrations from 0.003 to 3.0 μg/ml were also similarly plated and incubated. The plates were washed with solution A (containing 0.5% BSA, 10 mM sodium phosphate, 145 mM NaCl, 1.5 mM MgCl2, 0.05% Triton X-100, and 0.02% NaN3, pH 7.2), and 50 μl/well affinity-purified biotinylated goat anti-human IgG diluted 1:500 in solution A was added and incubated for 1 h. After washing with solution A, 50 μl/well streptavidin-alkaline phosphatase, diluted (1:200) in solution A, was added, incubated for 1 h, and removed. Then, 50 μl/well colorimetric reaction mixture, comprised of the substrate para-nitrophenylphosphate (1 mg/ml) in substrate buffer (50 mM Na2CO3, 50 mM NaHCO3, and 1 mM MgCl2 at pH 9.8), was added. After 6 to 8 min, the reaction was stopped with 1 M sodium hydroxide (50 μl/well). All steps were performed at room temperature. The reaction endpoint was measured with an ELISA reader (Molecular Devices, Sunnyvale, CA) at a wavelength of 405 nm. Each determination was done in duplicate.
Antibodies. The hybridoma cell line secreting mAb 2E2 was generated as described previously (Paula et al., 2004; Norman et al., 2007) using standard hybridoma technology by fusing splenocytes obtained from a transgenic mouse, strain HCo7/Ko5, after immunization with benzoylecgonine coupled to 1,4-butanediamine-derivatized keyhole limpet hemocyanin with the mouse cell line P3 × 63-Ag8.653. Production of mAb 2E2 was accomplished by growing hybridomas in severe combined immunodeficient mice (Biodesign International, Kennebunk, ME) and collecting the fluid of the ascites. The hybridoma-secreted mAb was purified from ascites by sodium sulfate precipitation and a multistep protein A-Sepharose column chromatography procedure adapted from that described previously (Ball et al., 1999). As a control, to test for nonspecific in vivo effects resulting from infusion of mAb, human polyclonal IgG (Sigma-Aldrich, St. Louis, MO), having no measurable affinity for cocaine or its major metabolites (data not shown), was administered to a group of rats.
Data Analysis and Statistics. 2E2 and control IgG pharmacokinetic data were analyzed using the program SigmaPlot (Systat Software, Inc., San Jose, CA). Data were first analyzed according to a single exponential decay mathematical model, representing a one-compartment pharmacokinetic model. In these experiments a one-compartment model gave a poor fit to the 2E2 and the control IgG pharmacokinetic data, as assessed by a systematic deviation of the model from the data. In these cases, the fits to the data were improved by applying a biexponential decay mathematical model representing a two-compartment pharmacokinetic model that assumed that the antibodies distributed between a central and a second compartment. The improvement in the fit of the model to the data was evaluated by a reduction in the sum of squares residuals. In the data sets presented here, the decreases in the sum of square residuals were 3.4- and 5.9-fold for 2E2 and the control IgG, respectively.
During the 2-week period of baseline measurements of the values for the cocaine priming threshold, the rate of cocaine consumption and the number of presses during the extinction phase of the session were assessed by linear regression analysis. A slope of the regression line close to zero indicated the lack of systematic change in the values over time. Any effect of antibody infusion on these same measures was determined using a one-way analysis of variance (ANOVA) with repeated measures and post hoc Tukey's test.
Results
Pharmacokinetics of mAb 2E2 and Human Polyclonal Antibodies. The in vivo concentrations of mAb 2E2 and the control IgG were first determined in samples of tail vein blood taken at 2 h after the completion of their intravenous infusion. The mean ± S.E.M. blood concentrations of 2E2 and control IgG at that time point were 375 ± 27 (n = 7 rats) and 342 ± 19 (n = 8 rats) μg/ml, respectively. As shown in Fig. 1, for both antibodies, there was an initial rapid decline in their blood concentrations over the first 2 days, followed by a slower decline over the subsequent 2 weeks. A two-compartment pharmacokinetic model was the simplest that approximated the biexponential time course of the decreases of both 2E2 and the control IgG over the duration of the behavioral studies. This pharmacokinetic model generated values for the distribution t½α and terminal elimination t½β for 2E2 of 0.5 and 11.6 days, respectively, and values generated for the control IgG for these same parameters were 0.4 and 6.0 days, respectively. Although there was a distribution phase for the antibodies as monitored from venous blood, the antibodies were not widely distributed within the body. This was indicated by the relatively small calculated volume of distribution at steady state (Vdss) of the 2E2 and the control IgG of 0.48 l/kg and 0.63 l/kg, respectively, which would probably indicate antibody distribution from circulating blood to the interstitial space.
The Self-Administration of Cocaine. The cumulative event records for representative cocaine self-administration sessions (Fig. 2) show sequentially the times of programmed illuminations of the signal light with no cocaine injection and then programmed cocaine priming infusions, self-administrations (at each lever press; FR = 1 schedule) and, finally, lever presses that do not result in cocaine injections. Each testing session began, after the extinction of any environment-induced lever-pressing behavior, by administering programmed escalating doses of cocaine until self-administration behavior was reinstated. Typically, there were a few or no cue-induced lever presses when the rats were first placed in the experimental chambers, and programmed cocaine injections generally began after 30 to 40 min. The range of doses delivered noncontingently started at 0.06 μmol/kg, and the last (and highest) dose of the series was 0.2 μmol/kg. Once active lever pressing was reinstated, rats self-administered cocaine at a steady rate at the last programmed dose. Then, when the unit dose was increased to 0.3 μmol/kg, self-administration continued but at a less frequent, slower rate. Although slower, the rat's rate of self-administration was steady as demonstrated by the relatively small S.E.M. value for the variance in interinjection intervals. Then, the unit dose was again increased, this time to 3 μmol/kg, and the rate of self-administration again decreased to a new rate that was also steady and with little variance, as demonstrated by the relatively small S.E.M. value for these longer interinjection intervals. When the access to cocaine was terminated, there was always an abrupt increase in the rate of lever pressing by the animals until this behavior was extinguished. As shown in Fig. 2B, for this same animal, 1 day after the infusion of 2E2, programmed injections of cocaine were able to reinstate cocaine self-administration; however, the level of cocaine required was increased almost 3-fold compared with that in the absence of 2E2. The range of doses delivered noncontingently started at 0.06 μmol/kg, and the last dose of the series was 0.5 μmol/kg. Once reinstated, the frequency of self-administration of the 0.3 μmol/kg cocaine unit dose and the concomitant rate of cocaine consumption were steady. When the unit dose of cocaine was then increased to 3.0 μmol/kg, a similar decrease in the frequency of self-administration as occurred with this dose before mAb infusion was observed, and the rate of cocaine consumption was steady. Finally, when access to cocaine was terminated, the typical temporary increase in the rate of lever-pressing behavior was observed, followed by its subsequent extinction (Fig. 2B), similar to what happened in the absence of 2E2.
Fig. 2.
Cumulative record of events during representative self-administration sessions before (A) and after (B) the infusion of 2E2. Each vertical increment (with its corresponding symbol) represents an event (signal light, cocaine injection, or lever press), and the horizontal distances represent the time between consecutive events. Large cross-hatched circles represent signal light activations with no drug injection, and large open circles represent the priming injections of the escalating doses of cocaine. In these representative sessions, when the animal was initially placed in the chamber, there were no environmental cue-induced lever presses. All self-administration events were under an FR 1 schedule. After self-administration was reinstated, the last priming dose was self-administered (indicated by the filled triangles) until the unit dose was programmed to be changed to 0.3 μmol/kg (indicated by the open squares) and then to 3.0 μmol/kg (indicted by the closed circles). A, the calculated cocaine priming threshold was 1.1 μmol/kg, and the mean ± S.E.M. interinjection intervals were 49.9 ± 4.4 s (n = 50 self-injections) and 411.9 ± 13.6 s (n = 15 self-injections) at unit doses of 0.3 and 3 μmol/kg, respectively. B, the calculated cocaine priming threshold was 3.2 μmol/kg, and the mean ± S.E.M. interinjection intervals were 33.5 ± 4.2 s (n = 50 self-injections) and 311.0 ± 22.9 s (n = 15 self-injections) at unit doses of 0.3 and 3 μmol/kg, respectively. Lever presses occurring after access to cocaine was terminated (extinction phase of the session) are indicated by the open triangles.
Time Course of the Effect of 2E2 on the Cocaine Priming Threshold. As shown in Fig. 3, A and B, over the 2-week period before the infusion of the antibodies, the geometric mean priming threshold values for both groups of rats was stable, as demonstrated by linear regression analysis of these values versus time gave slopes not significantly different from zero (2E2 group, p = 0.751; control IgG group, p = 0.113). One day after the infusion of 2E2, there was a significant (p < 0.01, one-way ANOVA with repeated measures and post hoc Tukey's test), almost 3-fold increase in the mean priming threshold value (Fig. 3A). Over subsequent days, the magnitude of the effect declined, and the mean priming threshold values gradually returned toward baseline values. The mean priming threshold values were significantly higher than baseline values for almost 2 weeks, with the rate of decline in the mean priming threshold to baseline values approximated by a simple first-order exponential process, with an apparent effect t½ of approximately 4 days. As shown in Fig. 4, there was a significant correlation between the decreasing blood concentrations of 2E2 and the decreasing mean priming thresholds over the 3-week period after the infusion of 2E2. In contrast, after the infusion of the control IgG, there was no significant change in the initial geometric mean priming threshold values (p > 0.05, one-way ANOVA with repeated measures) and no evidence for any systematic change over the subsequent 3 weeks (Fig. 3B).
Fig. 3.
The effect of the anti-cocaine mAb, 2E2, on the cocaine priming threshold. The cocaine priming threshold was determined for individual rats during daily sessions both before and after the infusion of mAb 2E2 (A) or control IgG (B). The symbols represent the geometric means with their associated S.E.s for six rats infused with 2E2 and eight rats with the control IgG. The best fit linear regression lines through the relevant data sets are shown. The curve through the post-2E2 data points represents the best fit nonlinear regression, according to an exponential decay model. The model generated a value for the t½ for the decrease in effect of 4 days. *, mean values significantly different (at least p < 0.05) from pre-2E2 infusion values, as determined by one-way ANOVA with repeated measures and post hoc Tukey's test.
Fig. 4.
Correlation between the 2E2 concentration and the cocaine priming threshold. The symbols represent the geometric mean of the calculated priming threshold (taken from Fig. 3A) and the mean blood concentration of mAb 2E2 from the same rats on the days when both parameters were measured. The linear regression analysis indicates a significant (p < 0.001) positive correlation between the two parameters; correlation coefficient, r = 0.904.
Time Course of the Effect of 2E2 on the Rate of Cocaine Self-Administration. As shown in Fig. 5, A and B, over the 2-week period before the infusion of the antibodies, the mean rates of consumption of cocaine were stable for both the 0.3 and 3.0 μmol/kg cocaine unit doses and for both groups of rats as demonstrated by linear regressions with slopes not significantly different from zero (2E2 group, unit doses of 0.3 and 3.0 μmol/kg, p = 0.58 and 0.33, respectively; control IgG group, unit doses of 0.3 and 3.0 μmol/kg, p = 0.82 and 0.27, respectively). It is well documented in rats that the rate of cocaine consumption is greater at higher unit doses (Pickens and Thompson, 1968; Tsibulsky and Norman, 1999). This phenomenon was also observed in these groups of rats. For example, in the group of rats that received the infusion of 2E2, in the 11 self-administration sessions conducted before the mAb infusion, their mean ± S.E.M. rates of cocaine consumption at the unit doses of 0.3 and 3.0 μmol/kg were 21.5 ± 0.3 and 29.5 ± 0.3 μmol/kg/h, respectively. Therefore, at the 10-fold higher unit dose, the rate of cocaine consumption was approximately 33 to 37% higher.
Fig. 5.
The effect of 2E2 on the rate of cocaine consumption. The rate of cocaine consumption was measured during the maintenance phase of each session at unit doses of 0.3 and 3.0 μmol/kg. The rate of consumption was calculated by the number of self-injections multiplied by the unit dose divided by the time over which the injections occurred. The symbols represent the mean ± S.E.M. (from six rats for 2E2 and eight rats for the control IgG) consumption rate at unit doses of 0.3 or 3.0 μmol/kg before and after the infusion of 2E2 (A) or control IgG (B). The best fit linear regression lines are presented for the data before and after the antibody infusions. *, significantly different (at least p < 0.05) from the corresponding pre-2E2 baseline values, one-way ANOVA with repeated measures and post hoc Tukey's test.
As shown in Fig. 5A, 1 day after the infusion of 2E2, in addition to a higher threshold for reinstatement of cocaine self-administration, the rates of consumption of cocaine at 0.3 and 3.0 μmol/kg unit doses increased from their mean baseline values stated above to 30.5 ± 1.8 and 34.7 ± 1.6 μmol/kg/h, respectively. These rates of cocaine consumption observed after the 2E2 infusion were significantly different (both p < 0.01, one-way ANOVA with repeated measures and post hoc Tukey's test) from those observed before 2E2 infusion. Comparing these values with the mean rates of cocaine consumption during the baseline period, the magnitude of the mAb effect was approximately a 42 and 18% increase at the unit doses of 0.3 and 3.0 μmol/kg, respectively. These initial increased rates of cocaine consumption during the maintenance phase of the sessions then steadily decreased toward the baseline values over the subsequent 2 weeks (Fig. 5A). However, although the mean consumption rates of cocaine were increased by 2E2, the frequencies of self-administration within each session were steady at all unit doses (an example is shown in Fig. 2B). In contrast to the effect of 2E2, the infusion of the control IgG produced no significant change in the rates of consumption of cocaine at any unit dose (Fig. 5B).
2E2 Has No Effect on Extinction Responding. Over the 2-week baseline period before the infusion of 2E2, the mean number of lever presses carried out by the animals in the 20 to 40 min after the termination of access to cocaine during each session was stable between sessions (data not shown). Over the 3-week period after the infusion, the mean number of lever presses was also stable. Likewise, the mean number of lever presses was also relatively stable over the days before the infusion of the control IgG and over the 3-week period after the antibody infusion. In contrast to the effects of mAb 2E2 on the cocaine priming threshold and the rate of cocaine consumption, there was no evidence for any 2E2-induced change in the number of lever presses during the extinction process. The mean ± S.E.M. number of presses over the days before and after 2E2 infusion was 24 ± 1 and 27 ± 2, respectively (data not shown). In addition, there was no evidence for any control IgG-induced change in the number of extinction lever presses, with the mean ± S.E.M. number of presses over the days before and after the control IgG infusion being 23 ± 2 and 23 ± 1, respectively (data not shown).
Discussion
The slow elimination of 2E2 in rats was similar to that we previously observed in mice (Norman et al., 2007). However, in this group of rats, there was evidence for an initial distribution of 2E2 from the circulating blood, presumably to interstitial spaces, that was not observed in mice (Norman et al., 2007). The initial distribution (t½α) and terminal elimination (t½β) values of both mAb 2E2 and the control IgG were consistent with those previously reported for murine mAbs and human polyclonal antibodies in rats (Bazin-Redureau et al., 1997). Despite the initial distribution from plasma, the low Vdss of both 2E2 and the control IgG indicated that the antibodies were predominantly restricted to the blood volume. This is also consistent with that observed for several murine monoclonal IgG1 antibodies (Valentine and Owens, 1996; Bazin-Redureau et al., 1997; Keyler et al., 2005) and human polyclonal IgG1 antibodies (Bazin-Redureau et al., 1997) in rats.
Consistent with the relatively slow elimination of mAb 2E2 from the rats, the effect of a single dose of 2E2 on the cocaine priming threshold, although steadily decreasing, persisted for almost 2 weeks. The protracted effects of 2E2 predict that an effective dose of this anti-cocaine mAb will provide long-lasting clinical efficacy. Furthermore, the observed decrease in efficacy indicated that the magnitude of 2E2's effect is proportional to its in vivo concentration. This is consistent with the dose-dependent effect of 2E2 on brain cocaine concentrations observed previously in mice (Norman et al., 2007).
Despite the consensus that anti-cocaine antibodies do decrease the concentrations of cocaine in the brain, there is no consensus about their effects on self-administration behavior. Passive immunization with murine anti-cocaine mAbs (Fox et al., 1996; Fox, 1997; Carrera et al., 2000; Kantak et al., 2000) or moderate doses of a catalytic anti-cocaine mAb (Mets et al., 1998; Baird et al., 2000) has been reported to decrease the self-administration of cocaine. Conversely, passive immunization with a higher dose of a catalytic anti-cocaine mAb (100 mg/kg) modestly increased the rate of cocaine self-administration (Baird et al., 2000). In the present studies using the highest dose of anti-cocaine anti-body used to date in rats (120 mg/kg), the results were consistent with the results reported by Baird et al. (2000) using the high dose of mAb. Discrepant results are reported for studies where vaccinations producing high titers of anti-cocaine polyclonal antibodies were reported to decrease (Kantak et al., 2000) and to increase (Carrera et al., 2000) the rate of cocaine self-administration. Furthermore, such discrepant results are not limited to anti-cocaine antibodies. Two anti-methamphetamine mAb with 20-fold different affinities for methamphetamine decreased, had both no effect, or increased the rate of consumption of methamphetamine, depending on the methamphetamine unit doses (McMillan et al., 2004). The reasons for these varied results are not clear presently but seem to be related to the different schedules of drug delivery, the varying doses of mAb, and differing unit doses of the self-administered drug.
It should be emphasized that the conditions of these self-administration studies were not designed to mimic the conditions under which humans consume cocaine. In the present studies, cocaine was, by design, delivered noncontingently in sufficient quantities to induce the reinstatement of self-administration behavior. It would be expected that under conditions where access to cocaine is restricted by more time-consuming dosing schedules or where only a single priming dose of cocaine is administered (Carrera et al., 2000), the probability of reinstating or maintaining self-administration behavior will probably be substantially decreased. However, once self-administration is reinstated, it may be expected that cocaine consumption in the presence of mAb would increase. This latter effect is not a desirable clinical outcome. It is important that in these studies, we found that the increase in the rate of consumption of cocaine during the maintenance phase of the sessions was quite modest (approximately 42 and 18%) compared with the 3-fold increase in the priming threshold. Furthermore, frequencies of cocaine self-administration were drug dose-dependent and steady, indicating that the animal's self-administration behavior was similarly regulated in the presence and absence of 2E2. In addition, the fact that mAb 2E2 did not alter the rat's lever-pressing behavior after access to cocaine was terminated indicates that 2E2 did not exacerbate any cocaine withdrawal symptoms.
We have demonstrated previously that 2E2 sequesters a single dose of cocaine in the peripheral circulation, resulting in a concomitant decrease in the brain cocaine concentrations (Norman et al., 2007). Because the pharmacodynamic mechanisms that mediate cocaine's effects in the brain should be unaffected by mAb 2E2, to maintain cocaine self-administration in 2E2's presence, the concentration of cocaine in the periphery must be increased to levels that can achieve effective brain concentrations. According to first-order kinetics, the rate of plasma cocaine elimination is increased as cocaine concentrations increase. This would result in the elevated plasma cocaine concentrations decreasing more rapidly, therefore shortening each interinjection interval. This pharmacokinetic model requires that 2E2 binding of cocaine does not slow the rate of elimination of cocaine in rats, which is consistent with what was observed in mice (Norman et al., 2007). Because anti-nicotine antibodies have been reported to decrease nicotine clearance in some studies (Keyler et al., 1999, 2005) but not in others (de Villiers et al., 2004), verification of our prediction will require additional studies that will be conducted as additional supplies of 2E2 are available.
A single dose of 2E2 remained effective despite the animals self-administering cumulative doses of cocaine that were approximately 40-fold greater than the initial dose of mAb. This could potentially saturate the mAb and render the treatment ineffective. However, our data are consistent with the observation that the effects of anti-drug mAbs are not simply overcome by large stoichiometric doses of drugs (Pitas et al., 2006). This phenomenon may be explained by considering the effect of differing molar ratios between the antibody and drug. The magnitude of the decrease in brain cocaine concentrations after a bolus injection of cocaine is proportional to the dose of 2E2 (Norman et al., 2007), demonstrating that the ratio between 2E2's drug binding sites and cocaine molecules determines the magnitude of effect. The relatively large 2E2-induced increase in the cocaine priming threshold occurred at an approximately equimolar ratio. When the cocaine concentration increased as the animal's self-administration of cocaine proceeded, the resulting decrease in the ratio of 2E2/cocaine should decrease 2E2's effects. As a consequence, the effect of mAbs on the rates of consumption of cocaine during self-administration was proportionally less than on the reinstatement. It is interesting that the effect of the mAb did not diminish as more cocaine was consumed, as evidenced by the consistent frequency of self-administration during each session. This indicates that the ratio between 2E2 and cocaine must remain relatively constant during maintained self-administration. Because the concentration of 2E2 did not change appreciably for the duration of each session, this, in turn, implies that a pseudo-steady-state concentration of cocaine was established at this time. Because the mean pseudo-steady-state concentration of cocaine is proportional to the unit dose, at higher cocaine unit doses, the 2E2 and cocaine molar ratios are reduced. That the effect of 2E2 on the rate of cocaine consumption was proportionally less at the 3.0 compared with the 0.3 μmol/kg unit dose is consistent with this hypothesis. It is obvious that the lack of effects of the control IgG on the priming threshold or on the rate of maintained cocaine self-administration demonstrate that there is no non-specific IgG effect, and antibody binding of cocaine is required for efficacy.
The effects our anti-cocaine mAb in rats can be compared with those of competitive antagonists of dopamine receptors that also have been demonstrated to increase the rate of self-administration of cocaine (Koob et al., 1987; Gerber and Wise, 1989; Corrigall and Coen, 1991; Caine and Koob, 1994; Mello and Negus, 1996; Norman et al., 1999) and increase the cocaine priming threshold (Norman et al., 2002). Although the effects of mAb 2E2, in acting as a pharmacokinetic/chemical antagonist, were qualitatively similar to those of competitive dopamine receptor antagonists, there were noticeable quantitative differences between these two different types of antagonists. In studies analogous to those reported here, a dose of the competitive D1/D5 dopamine receptor antagonist, SCH23390, that almost doubled the cocaine priming threshold in rats also more than doubled the rate of cocaine self-administration (Norman et al., 2002). In contrast, in this study, a dose of 2E2 that approximately tripled the cocaine priming threshold only produced 42 and 18% increases in the rate of cocaine self-administration. Therefore, anti-cocaine antibodies may have advantages over competitive antagonists as potential treatments for cocaine abuse.
In summary, 2E2 greatly increased the level of cocaine required to reinstate cocaine self-administration. However, once reinstated, the pattern of self-administration was similar to that observed in the absence of 2E2, with the animals responding with regular interinjection intervals that were proportional to the unit doses of cocaine. The effects of 2E2 on cocaine-induced behaviors of rats observed in this study may predict clinical efficacy for the prevention of relapse in cocaine abusers.
Acknowledgments
We thank Purabi Dey for additional technical assistance and Nora E. Stevenson for invaluable program coordination.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant DA018538].
doi:10.1124/jpet.108.146407.
ABBREVIATIONS: mAb, monoclonal antibody (antibodies); FR, fixed ratio; ELISA, enzyme-linked immunosorbent assay; BSA, bovine serum albumin; ANOVA, analysis of variance; t½α, distribution half-life in a two-compartment pharmacokinetic model; t½β, terminal elimination half-life in a two-compartment pharmacokinetic model; Vdss, volume of distribution at steady state; SCH23390, R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine.
References
- Baird TJ, Deng SX, Landry DW, Winger G, and Woods JH (2000) Natural and artificial enzymes against cocaine: I. Monoclonal antibody 15A10 and the reinforcing effects of cocaine in rats. J Pharmacol Exp Ther 295 1127-1134. [PubMed] [Google Scholar]
- Ball WJ Jr, Kasturi R, Dey P, Tabet M, O'Donnell S, Hudson D, and Fishwild D (1999) Isolation and characterization of human monoclonal antibodies to digoxin. J Immunol 163 2291-2298. [PubMed] [Google Scholar]
- Bateman DN (2004) Digoxin-specific antibody fragments: how much and when? Toxicol Rev 23 135-143. [DOI] [PubMed] [Google Scholar]
- Bazin-Redureau MI, Renard CB, and Scherrmann JM (1997) Pharmacokinetics of heterologous and homologous immunoglobulin G, F(ab′)2 and Fab after intravenous administration in the rat. J Pharm Pharmacol 49 277-281. [DOI] [PubMed] [Google Scholar]
- Booze RM, Lehner AF, Wallace DR, Welch MA, and Mactutus CF (1997) Dose-response cocaine pharmacokinetics and metabolite profile following intravenous administration and arterial sampling in unanesthetized, freely moving male rats. Neurotoxicol Teratol 19 7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine SB and Koob GF (1994) Effects of dopamine D-1 and D-2 antagonists on cocaine self-administration under different schedules of reinforcement in the rat. J Pharmacol Exp Ther 270 209-218. [PubMed] [Google Scholar]
- Carrera MR, Ashley JA, Parsons LH, Wirsching P, Koob GF, and Janda KD (1995) Suppression of psychoactive effects of cocaine by active immunization. Nature 378 727-730. [DOI] [PubMed] [Google Scholar]
- Carrera MR, Ashley JA, Wirsching P, Koob GF, and Janda KD (2001) A second generation vaccine protects against the psychoactive effects of cocaine. Proc Natl Acad Sci U S A 98 1988-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrera MR, Ashley JA, Zhou B, Wirsching P, Koob GF, and Janda KD (2000) Cocaine vaccines: antibody protection against relapse in a rat model. Proc Natl Acad Sci U S A 97 6202-6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA and Coen KM (1991) Cocaine self-administration is increased by both D1 and D2 dopamine antagonists. Pharmacol Biochem Behav 39 799-802. [DOI] [PubMed] [Google Scholar]
- de Villiers SH, Lindblom N, Kalayanov G, Gordon S, Johansson AM, and Svensson TH (2004) Active immunization against nicotine alters the distribution of nicotine but not the metabolism to cotinine in the rat. Naunyn-Schmiedebergs Arch Pharmacol 370 299-304. [DOI] [PubMed] [Google Scholar]
- de Wit H and Stewart J (1981) Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology 75 134-143. [DOI] [PubMed] [Google Scholar]
- Ettinger RH, Ettinger WF, and Harless WE (1997) Active immunization with cocaine-protein conjugate attenuates cocaine effects. Pharmacol Biochem Behav 58 215-220. [DOI] [PubMed] [Google Scholar]
- Fox BS (1997) Development of a therapeutic vaccine for the treatment of cocaine addiction. Drug Alcohol Depend 48 153-158. [DOI] [PubMed] [Google Scholar]
- Fox BS, Kantak KM, Edwards MA, Black KM, Bollinger BK, Botka AJ, French TL, Thompson TL, Schad VC, Greenstein JL, et al. (1996) Efficacy of a therapeutic cocaine vaccine in rodent models. Nat Med 2 1129-1132. [DOI] [PubMed] [Google Scholar]
- Gerber GJ and Wise RA (1989) Pharmacological regulation of intravenous cocaine and heroin self-administration in rats: A variable dose paradigm. Pharmacol Biochem Behav 32 527-531. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC.
- Kantak KM, Collins SL, Lipman EG, Bond J, Giovanoni K, and Fox BS (2000) Evaluation of anti-cocaine antibodies and a cocaine vaccine in a rat self-administration model. Psychopharmacology 148 251-262. [DOI] [PubMed] [Google Scholar]
- Keyler DE, Hieda Y, St Peter J, and Pentel PR (1999) Altered disposition of repeated nicotine doses in rats immunized against nicotine. Nicotine Tob Res 1 241-249. [DOI] [PubMed] [Google Scholar]
- Keyler DE, Roiko SA, Benlhabib E, LeSage MG, St Peter JV, Stewart S, Fuller S, Le CT, and Pentel PR (2005) Monoclonal nicotine-specific antibodies reduce nicotine distribution to brain in rats: Dose- and affinity-response relationships. Drug Metab Dispos 33 1056-1061. [DOI] [PubMed] [Google Scholar]
- Koetzner L, Deng S, Sumpter TL, Weisslitz M, Abner RT, Landry DW, and Woods JH (2001) Titer-dependent antagonism of cocaine following active immunization in rhesus monkeys. J Pharmacol Exp Ther 296 789-796. [PubMed] [Google Scholar]
- Koob GF, Le HT, and Creese I (1987) The D1 dopamine receptor antagonist SCH 23390 increases cocaine self-administration in the rat. Neurosci Lett 79 315-320. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Rosen M, Bond J, Settles M, Roberts JS, Shields J, Jack L, and Fox B (2002) Human therapeutic cocaine vaccine: safety and immunogenicity. Vaccine 20 1196-1204. [DOI] [PubMed] [Google Scholar]
- Kosten T and Owens SM (2005) Immunotherapy for the treatment of drug abuse. Pharmacol Ther 108 76-85. [DOI] [PubMed] [Google Scholar]
- Lonberg N (2005) Human antibodies from transgenic animals. Nat Biotechnol 23 1117-1125. [DOI] [PubMed] [Google Scholar]
- Martell BA, Mitchell E, Poling J, Gonsai K, and Kosten TR (2005) Vaccine pharmacotherapy for the treatment of cocaine dependence. Biol Psychiatry 58 158-164. [DOI] [PubMed] [Google Scholar]
- McMillan DE, Hardwick WC, Li M, Gunnell MG, Carroll FI, Abraham P, and Owens SM (2004) Effects of murine-derived anti-methamphetamine monoclonal antibodies on (+)-methamphetamine self-administration in the rat. J Pharmacol Exp Ther 309 1248-1255. [DOI] [PubMed] [Google Scholar]
- Mello NK and Negus SS (1996) Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology 14 375-424. [DOI] [PubMed] [Google Scholar]
- Mets B, Winger G, Cabrera C, Seo S, Jamdar S, Yang G, Zhao K, Briscoe RJ, Almonte R, Woods JH, et al. (1998) A catalytic antibody against cocaine prevents cocaine's reinforcing and toxic effects in rats. Proc Natl Acad Sci U S A 95 10176-10181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman AB, Norman MK, Hall JF, and Tsibulsky VL (1999) Priming threshold: A novel quantitative measure of the reinstatement of cocaine self-administration. Brain Res 831 165-174. [DOI] [PubMed] [Google Scholar]
- Norman AB, Tabet MR, Norman MK, Buesing WR, Pesce AJ, and Ball WJ (2007) A chimeric human/murine anticocaine monoclonal antibody inhibits the distribution of cocaine to the brain in mice. J Pharmacol Exp Ther 320 145-153. [DOI] [PubMed] [Google Scholar]
- Norman AB, Welge JA, and Tsibulsky VL (2002) Characterization of the distribution of the cocaine priming threshold and the effect of SCH23390. Brain Res 946 253-261. [DOI] [PubMed] [Google Scholar]
- Paula S, Tabet MR, Farr CD, Norman AB, and Ball WJ Jr (2004) Modeling of cocaine binding by a novel human monoclonal antibody. J Med Chem 47 133-142. [DOI] [PubMed] [Google Scholar]
- Pickens R and Thompson T (1968) Cocaine reinforced behavior in rats: effects of reinforcement magnitude and fixed ratio size. J Pharmacol Exp Ther 161 122-129. [PubMed] [Google Scholar]
- Pitas G, Laurenzana EM, Williams DK, Owens SM, and Gentry WB (2006) Antiphencyclidine monoclonal antibody binding capacity is not the only determinant of effectiveness, disproving the concept that antibody capacity is easily surmounted. Drug Metab Dispos 34 906-912. [DOI] [PubMed] [Google Scholar]
- Rang HP, Dale MM, Ritter JM, and Flower RJ (2007) Pharmacology, 6th ed, Churchill Livingstone Elsevier, Philadelphia, PA.
- Shalev U, Grimm JW, and Shaham Y (2002) Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev 54 1-42. [DOI] [PubMed] [Google Scholar]
- Tsibulsky VL and Norman AB (1999) Satiety threshold: a quantitative model of maintained cocaine self-administration. Brain Res 839 85-93. [DOI] [PubMed] [Google Scholar]
- Tsibulsky VL and Norman AB (2005) Real time computation of in vivo drug levels during drug self-administration experiments. Brain Res Brain Res Protoc 15 38-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine JL and Owens SM (1996) Antiphencyclidine monoclonal antibody therapy significantly changes phencyclidine concentrations in brain and other tissues in rats. J Pharmacol Exp Ther 278 717-724. [PubMed] [Google Scholar]