Abstract
Sulfur mustards (SMs) have been used as warfare agents since World War I and still pose a significant threat against civilian and military personnel. SM exposure can cause significant blistering of the skin, respiratory injury, and fibrosis. No antidote currently exists for SM exposure, but recent studies, using the SM analog 2-chloroethyl ethyl sulfide (CEES), have focused on the ability of antioxidants to prevent toxicity. Although antioxidants can prevent CEES-induced toxicity, the mechanisms by which these compounds are effective against SM agents are largely unknown. Using human bronchial epithelial (16HBE) cells and primary small airway epithelial cells, we show that CEES causes a significant increase in mitochondrial dysfunction as early as 4 h, which is followed by increases in mitochondrial reactive oxygen species (ROS), peaking 12 h after exposure. We also have identified a catalytic antioxidant metalloporphyrin that can rescue airway cells from CEES-induced toxicity when added 1 h after CEES exposure. In addition, the cytoprotective effects of the catalytic antioxidant are associated with correcting mitochondrial dysfunction ROS, DNA oxidation, and decreases in intracellular GSH. These findings suggest a role for oxidative stress in CEES toxicity and provide a rationale to investigate antioxidants as rescue agents in SM exposures.
Bis(2-chloroethyl sulfide) or sulfur mustard (SM) was first synthesized in the late 1880s and since has been used as a warfare agent on a number of occasions. SM was first used in World War I and has been used in warfare as recently as the Iran-Iraq conflict of the late 1980s (Blanc, 1999). Although SM is less of a threat in warfare as it once was, it still posses a threat to military and civilian personnel because of current concerns for its deployment in a terrorist attack.
Sulfur mustards are classic vesicating agents that mainly affect the skin, eyes, and respiratory system. There is no known antidote or specific treatment for SM exposure, and the current therapy is largely supportive. SM on the skin can be decontaminated with soap and water or a dilute bleach solution, but internal exposure, such as the respiratory system, is considerably more difficult to treat (Munro et al., 1990; Watson and Griffin, 1992). SM produces airway damage that includes necrosis, inflammation, and edema (Kehe and Szinicz, 2005). The exact mechanism of SM toxicity is unknown. 2-Chloroethyl ethyl sulfide (CEES; half mustard) is a monofunctional analog of SM (Fig. 1) that provides a useful model for SM injury without the need for a specialized containment facility. CEES, like SM, is an alkylating agent that can bind DNA and other macromolecules within the cell. Recent research into counteragents has focused on bolstering the endogenous antioxidant defenses by supplementation with N-acetyl-cysteine (McClintock et al., 2002; McClintock et al., 2006; Hoesel et al., 2008), vitamin E (Hoesel et al., 2008), GSH (Han et al., 2004), or addition of exogenous SOD or catalase (McClintock et al., 2002, 2006; Das et al., 2003). Although bolstering endogenous antioxidants can be effective, it is largely unknown how or why this protection occurs. We have developed a class of small-molecule metalloporphyrin catalytic antioxidants that possess both high SOD and catalase activities among other detoxifying properties (Day, 2008). Metalloporphyrins have also shown promise as therapeutic agents in several ROS-mediated animal models of human disease states (Day, 2004).
Fig. 1.
Structures of bis(2-chloroethyl sulfide), also known as SM, and its analog CEES.
In this study, we show that CEES not only causes an increase in ROS but that it is a delayed response that may involve the mitochondria of the airway epithelium. We hypothesize that CEES is causing mitochondrial dysfunction that drives increased ROS production and resulting oxidative stress. We also identify a catalytic antioxidant metalloporphyrin, AEOL 10150, which is able to rescue airway epithelial cells from CEES-induced cytotoxicity when treated 1 h after CEES exposure. Our findings support the rationale for antioxidant therapy in the treatment of SM exposures.
Materials and Methods
Cell Culture. Human primary small airway epithelial (SAE) cells (Lonza Walkersville, Inc., Walkersville, MD) were grown in SAGM media (Lonza Walkersville, Inc.) supplemented with bovine pituitary extract, insulin, hydrocortisone, gentamicin sulfate, amphotericin B, retinoic acid, bovine serum albumin, transferrin, triiodothyronine, epinephrine, and recombinant human epidermal growth factor. To preserve the characteristics of a primary cell, SAE cells were not used after approximately 10 doubling times. 16HBE, a transformed human bronchial epithelial cell, was grown in minimal essential medium α+ Glutamax (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum and penicillin/streptomycin.
Cell Treatments. Cells were plated in 24-well plates and grown to approximately 90% confluence before treatment. CEES (TCI America, Portland, OR) was freshly diluted from the stock in DMSO, which was then further diluted into the media to the desired final concentration. The amount of DMSO did not exceed 0.1% in any treatment. The metalloporphyrins were dissolved in distilled H2O and added directly to the treatment media to the desired final concentration. SAE cells were treated with 900 μM CEES for 48 h. Because of the increased sensitivity of the 16HBE cells, they were treated with the same concentration of CEES for only 24 h.
Cell Viability. Cell viability was measured using the calcein AM dye (Invitrogen), a cell-permeable nonfluorescent probe that once cleaved by intracellular esterases becomes fluorescent. The treatment media were removed, cells were washed with PBS to remove remaining compound or extracellular esterases, and fresh media were added back to the cells containing 1 μM calcein AM dye and allowed to incubate for 1 h. Fluorescence was measured with an excitation wavelength of 485 nm and emission wavelength of 530 nm using a CytoFluor fluorescent plate reader (Appplied Biosystems, Foster City, CA). Calcein AM results were verified using the MTT assay as a second cell viability test. In brief, 200 μl of fresh media were added to the cells, and 50 μl of MTT reagent (2 mg/ml) was added to each well and incubated for 1 h at 37°C. Media were then removed, and 200 μl of DMSO was added followed by 25 μl of Sorensen's glycine buffer (0.75g glycine and 0.58g NaCl in 1 liter of H2O, pH to 10.5 using 1 N NaOH). Samples were then transferred to a 96-well plate and read in triplicate at 550 nm.
Mitochondrial Reactive Oxygen Species. Mitochondrial ROS was measured using MitoSOX red (Invitrogen) added to cells at a final concentration of 5 μM and incubated for 1 h at 37°C. Media were then removed, the cells were washed with warm PBS (500 μl), and the cells were scraped and placed into 1.2-ml microtiter tubes (Life Science Products, Frederick, CO). Fluorescence was measured using a BD Biosciences FACScalibur cytometer running Cellquest Pro version 4.0.2 (BD Biosciences, San Jose, CA). Results were obtained by gating on FSC-H versus SSC-H live cell population. Mean fluorescence was measured on FL2 and expressed as percentage of the controls. The mean control fluorescence values in the SAE and 16HBE cells were 142 and 134, respectively.
Determination of Mitochondrial Dysfunction. Mitochondrial membrane potential was measured using the cell-permeant fluorescent dye Rhodamine 123 (Rho 123) (Eastman Kodak, Rochester, NY). Cells were treated with Rho 123 dissolved in DMSO for 30 min at a final concentration of 20 μM in the media. Media were then removed, and the cells were washed once with PBS and scraped in 500 μl of PBS for analysis via flow cytometry. Fluorescence was measured using an Accuri C6 cytometer (Accuri Cytometers, Ann Arbor, MI; running Cflow software or a BD Biosciences FACScalibur cytometer running Cellquest Pro version 4.0.2). The mean fluorescence was quantified and expressed as percentage of the controls. Fluorescence is inversely correlated with mitochondrial membrane potential (Darzynkiewicz et al., 1981).
Total Glutathione Levels. Human lung 16HBE cells were exposed to CEES for a total of 12 h, after which the treatment media were removed and replaced with 300 μl of room temperature PBS. The cells were lysed by sonication, and samples were centrifuged at 12,000g for 10 min to pellet cell debris. Total GSH was measured spectrophotometrically using a modified Tietze assay (Tietze, 1969) described by Rahman et al. (2006). Essentially, 5,5′-dithio-bis(2-nitrobenzoic acid) solution (1.33 mg/ml), glutathione reductase (13.3 μl/ml), and NADPH (1.33 mg/ml) is dissolved in KPE buffer (0.1 M potassium phosphate buffer with 5 mM EDTA, pH 7.5). Standard or sample (20 μl) was added in triplicate to a 96-well plate along with 100-μl equal part mixture of 5,5′-dithio-bis(2-nitrobenzoic acid) and glutathione reductase, left to stand for 1 min, and then 50 μl of NADPH was added, shaken, and read at 412 nm for 5 min. GSH concentration is determined using a GSH standard curve run in tandem with the samples. Protein was measured using Coomassie Blue (Thermo Fisher Scientific, Waltham, MA), and GSH was normalized to the amount of protein per sample; results are expressed as nanomoles of GSH per milligram of protein.
DNA Oxidation. Human lung 16HBE cells were exposed to CEES for a total of 12 h, after which the treatment media were removed and replaced with 300 μl of room temperature PBS. The cells were lysed by sonication, and samples were centrifuged at 12,000g for 10 min to pellet cell debris. DNA from 16HBE cells was extracted using DNeasy tissue kit (QIAGEN, Valencia, CA). DNA purity was measured using a Nanodrop 1000 spectrophotometer (Thermo Fisher Scientific). Approximately 6 μg of purified DNA was incubated with 4 units of Nuclease P1 (US Biological, Swampscott, MA) at 60°C for 20 min, then 4 units of Alkaline Phosphatase (Sigma-Aldrich, St. Louis, MO) at 37°C for 60 min. The samples where then analyzed for 8-hydroxy-2-deoxyguanosine (8OHdG) and 2-deoxyguanosine (2dG), respectively, by high-performance liquid chromatography coupled with UV and electrochemical detection (CoulArray model 5600; ESA Inc., Chelmsford, MA). Mobile phase A consisting of 50 mM sodium acetate, pH 4.0, and mobile phase B consisting of 50 mM sodium acetate with acetonitrile 85:15 (v/v), pH 4.2, with a flow rate of 1 ml/min using a gradient of 100% A for 5 min; 60% A, 40% B for 12 min; 20% A, 80% B for 5 min; and 100% A for 8 min. Analysis consisted of a 4.6- by 250-mm, C18 reverse phase column (Tosoh Bioscience LLC, Montgomeryville, PA) with the detection of 2dG by UV and 8OHdG using electrode potentials of 140, 200, 260, and 320 mV. The retention times for 2dG and 8OHdG where 13.0 and 14.1 min, respectively. Concentrations were determined using an 11-point standard curve containing increasing concentrations of 8OHdG and 2dG and expressed as a ratio of 8OHdG/105 2dG.
Statistics. Results are expressed as mean ± S.E.M. One-way ANOVA with Dunnett's comparison test or two-way ANOVA with Bonferroni post-test was performed using Prism version 5 (Graph-Pad Software Inc., San Diego, CA). A p value < 0.05 was considered statistically significant.
Results
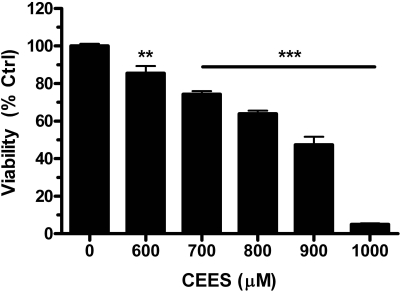
CEES-Induced Airway Epithelial Cell Injury. Human lung 16HBE cells were grown to approximately 90% confluence and treated with increasing concentrations of CEES, ranging from 600 to 1000 μM. Cell viability was determined by measuring the fluorescence of calcein AM and was found to decrease in a dose-dependent manner from 80% with the 600 μM CEES to below 10% with 1000 μM CEES (Fig. 2). We used 900 μM CEES as the optimal dose to carry out our cytoprotection studies because it provides enough cell injury (∼50%) for potential therapeutics to demonstrate efficacy and the most consistent cell injury response in the two cell systems. Because of observed increased resistance of SAE cells to CEES toxicity as seen with 16HBE cells, these exposures were prolonged to 48 h in the SAE cells to provide similar injury responses for comparison of antioxidant protective effects between cell systems.
Fig. 2.
CEES exposure causes a concentration-dependent injury of human airway epithelial cells. Human lung 16HBE cells were grown to approximately 90% confluence and treated with concentrations of CEES ranging from 600 to 1000 μM for 24 h. Cell viability decreased in a dose-dependent manner as measured by quantifying calcein AM fluorescence. Data represented as mean ± S.E.M., n = 4 where control group fluorescence was defined as 100% viability.
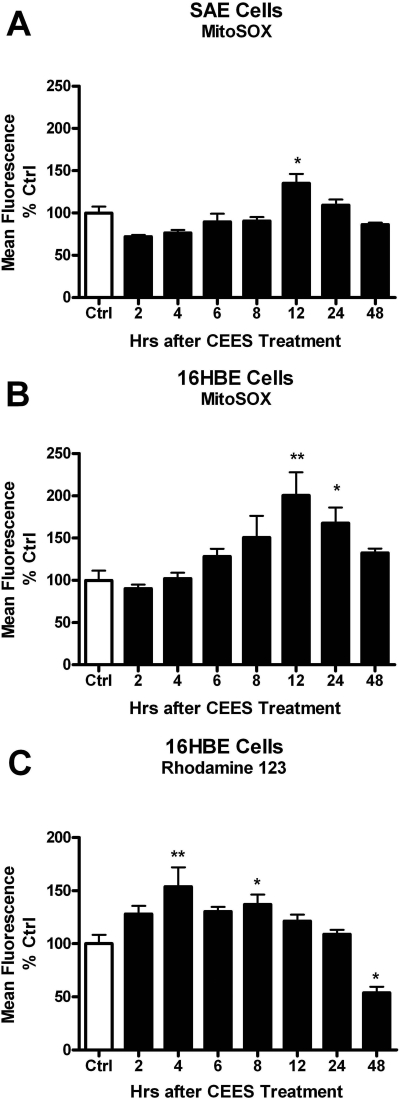
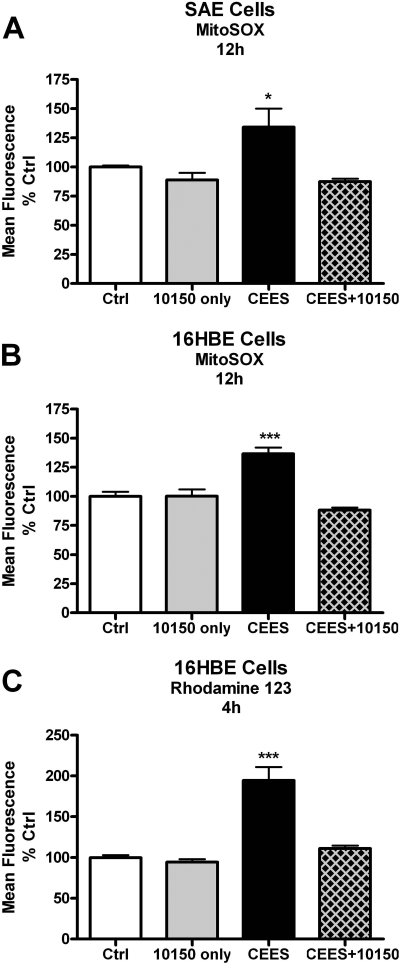
Delayed Increase in Mitochondrial ROS and Dysfunction with CEES Exposure. Mitochondria are a major source of cellular ROS production, and we sought to determine whether CEES exposure increases mitochondrial ROS production using the mitochondrially targeted ROS probe, mitoSOX. Both SAE and 16HBE cells were exposed to 900 μM CEES for 2, 4, 6, 8, 12, 24, and 48 h, after which the cells were incubated with MitoSOX, and fluorescence was measured using flow cytometry. CEES exposure increased ROS levels that peaked at 12 h, and this time-dependent increase was seen in both SAE (Fig. 3A) and 16HBE (Fig. 3B) cells. As a consequence, further exposure studies measuring markers of cellular stress were examined after 12 h of exposure.
Fig. 3.
CEES exposure produces increased levels of mitochondrial ROS and dysfunction. SAE (A) and 16HBE (B) cells were treated with 900 μM CEES for 2, 4, 6, 8, 12, 24, and 48 h, after which cells were incubated with the mitochondrial ROS probe MitoSOX (A and B) for 1 h or mitochondrial membrane potential indicator Rhodamine 123 (C) for 30 min. Cells were washed then scraped and placed in microtiter tubes. Fluorescence was determined via flow cytometry gated on the live cell population. MitoSOX fluorescence correlated with increased ROS, whereas Rhodamine 123 fluorescence is inversely correlated with mitochondrial membrane potential. Data are expressed as percentage of the control, where the control fluorescence was set to 100%. *, p < 0.05; **, p < 0.01 compared with control group; n = 3.
We next examined whether CEES exposure was associated with any mitochondrial dysfunction. Mitochondria need to maintain a membrane potential to actively make ATP. To examine this, we measured Rho 123 fluorescence, which is inversely correlated with mitochondrial membrane potential (Darzynkiewicz et al., 1981). Human lung 16HBE cells were exposed to CEES for 2, 4, 6, 8, 12, 24, and 48 h, after which the cells were incubated with Rho 123, and fluorescence was measured using flow cytometry. We found that CEES produced a decrease in mitochondrial membrane potential by 4 h, which persisted for 24 h as evidenced by the increase in Rho 123 fluorescence (Fig. 3C). It is interesting that there was a significant decrease in Rho 123 fluorescence at 48 h, which can be attributed to the cell death that would be expected to occur based on previous cell viability tests.
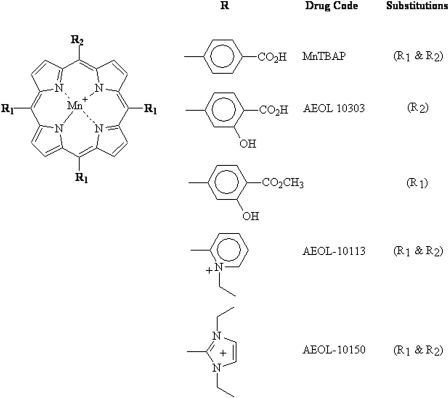
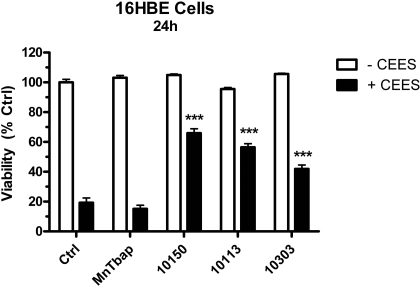
Metalloporphyrins Rescue Human Lung Cells from CEES-Induced Toxicity. We hypothesized that catalytic antioxidant metalloporphyrins would be able to rescue the cells from CEES-induced toxicity based on the delayed mitochondrial ROS and dysfunction response to CEES. Several structurally different metalloporphyrins (AEOL 10150, AEOL 10113, AEOL 10303, and MnTBAP) were screened in 16HBE cells for efficacy against CEES toxicity 1 h after the initial exposure (Fig. 4). Our previous experiences with these compounds have found that the maximal tolerated concentration of metalloporphyrins is around 50 μM and this concentration was used for screening. Cells were treated with CEES for 1 h at 37°C, after which AEOL 10150, AEOL 10113, AEOL 10303, and MnTBAP were added at a final concentration of 50 μM. After 24 h, cell viability was measured using calcein AM fluorescence. Three catalytic antioxidant compounds significantly increased cell viability in CEES-exposed cells to 60, 56, and 41% in AEOL 10150, AEOL 10113, and AEOL 10303 groups compared with only 20% in CEES-only exposed cells (Fig. 5). Of the four compounds tested, only MnTBAP did not show any protection. None of the compounds significantly changed cell viability by themselves.
Fig. 4.
Chemical structures of several catalytic antioxidant metalloporphyrins.
Fig. 5.
The protective effects of metalloporphyrins on CEES-induced cell injury. 16HBE cells were grown to 90% confluence and exposed to 900 μM CEES for a total of 24 h. Cells were treated 1 h after the initial CEES exposure with AEOL 10150, AEOL 10113, AEOL 10303, or MnTBAP at a final concentration of 50 μM in the presence (black bars) or absence (white bars) of 900 μM CEES. Data represented as mean ± S.E.M., n = 4. ***, p < 0.001 compared with CEES-only treatment group.
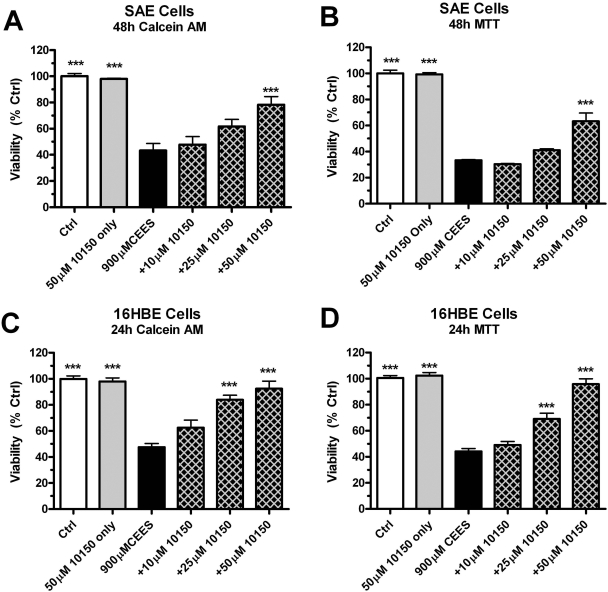
AEOL 10150 Rescues Human Primary Airway Cells from CEES-Induced Toxicity. Primary human lung SAE cells and 16HBE cells were exposed to 900 μM CEES for 48 h. Treatment with AEOL 10150 (10, 25, and 50 μM) occurred 1 h after the initial CEES exposure. AEOL 10150 (50 μM) alone did not change the viability of the cells, as measured by both the calcein AM (Fig. 6, A and C) and the MTT (Fig. 6, B and D) assays. CEES alone caused a 50% decrease in cell viability, and this was significantly attenuated at the highest concentration of AEOL 10150, to 80% of the control in SAE cells (Fig. 6, A and B) and nearly 90% in 16HBE cells (Fig. 6, C and D). Although neither 10 nor 25 μM AEOL 10150 showed a significant increase in viability in the SAE cells, 25 μM AEOL 10150 did show a significant increase in viability in the 16HBE cells. Similar results were obtained in both the calcein AM and the MTT assays used to assess cell viability.
Fig. 6.
Rescue effect of AEOL 10150 on CEES-induced cell death. SAE cells (A and B) and 16HBE cells (C and D) were exposed to 900 μM CEES with AEOL 10150 at 10, 25, and 50 μM concentrations added 1 h after CEES. Cell viability was measured using both calcein AM (A and C) and MTT (B and D) staining with control values being defined as 100% viability. Data represented as mean ± S.E.M., n = 4. **, p < 0.01; ***, p < 0.001 compared with CEES-only treated group.
AEOL 10150 Prevents CEES-Mediated Mitochondrial ROS and Dysfunction. We next assessed whether the cytoprotective effects of AEOL 10150 are associated with CEES-mediated changes in mitochondrial ROS and dysfunction. Cells were grown to approximately 90% confluence and exposed to 900 μM CEES with and without AEOL 10150 (50 μM). Cells were incubated with MitoSOX 12 h after CEES exposure, and fluorescence was measured using flow cytometry. AEOL 10150 added 1 h after CEES treatments significantly decreased mitochondrial ROS compared with CEES exposed cells in both SAE (Fig. 7A) and 16HBE (Fig. 7B) cells. AEOL 10150 alone did not cause a change in mitochondrial ROS. We also wanted to determine whether AEOL 10150 can protect the mitochondria from CEES-induced dysfunction. Lung 16HBE cells were exposed to 900 μM CEES for 4 h with 50 μM AEOL 10150 added 1 h after the initial CEES exposure. The CEES-only treated groups showed an increase in Rhodamine 123 fluorescence, indicating a significant loss of mitochondrial membrane potential that was attenuated in the AEOL 10150-treated cells (Fig. 7C).
Fig. 7.
AEOL 10150 rescues CEES-induced increases in mitochondrial ROS and dysfunction. SAE (A) and 16HBE (B) cells were exposed to 900 μM CEES for 12 h. AEOL 10150 (50 μM) was added 1 h after CEES exposure, and ROS was determined using MitoSOX with flow cytometry. C, 16HBE Cells were exposed similar as before except for 4 h. Mitochondrial membrane potential was determined using Rhodamine 123, where fluorescence is inversely correlated with mitochondrial membrane potential. Mean fluorescence was normalized to control levels with controls being 100%. Data represents mean ± S.E.M., n = 3 to 6; *, p < 0.05; ***, p < 0.001 compared with control values. Two-way ANOVA of AEOL 10150, p = 0.0563; CEES, p = 0.0033; interaction, p = 0.042 (A); AEOL 10150, p = 0.1073; CEES, p = 0.0004; interaction, p < 0.0001 (B); and AEOL 10150, p = 0.2876; CEES, p = 0.0007; interaction, p = 0.0051 (C).
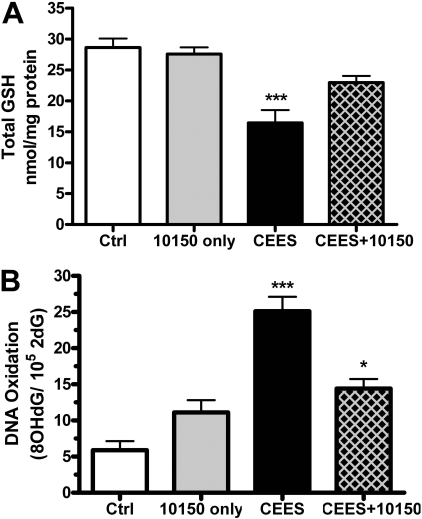
AEOL 10150 Prevents CEES-Induced Oxidative Stress. Oxidative stress can result from an imbalance between oxidant production and antioxidant defense. GSH is a major cellular antioxidant, and we sought to determine the effect of CEES on total cellular GSH levels and whether AEOL 10150 altered CEES-mediated changes in GSH levels. Human lung 16HBE cells were exposed for 12 h to CEES, and AEOL 10150 (50 μM) was added 1 h post-CEES treatment. AEOL 10150 alone did not alter intracellular GSH levels, whereas CEES caused a significant decrease in intracellular GSH levels (Fig. 8A). AEOL 10150 treatment prevented the CEES-induced decrease in GSH, further implicating an imbalance in redox status of the cells caused by CEES that was reversible by AEOL 10150.
Fig. 8.
The effects of CEES on markers of cellular oxidative stress and prevention by AEOL 10150 in 16HBE cells. Cells exposed to 900 μM CEES for 12 h had decreased total cellular GSH levels (A), and AEOL 10150 (50 μM) rescued this decrease when treated 1 h after CEES exposure. Total GSH levels were normalized to the amount of protein and expressed as nanomoles of GSH per milligram of protein. CEES also increased the levels of the DNA oxidation marker 8OHdG (B), and AEOL 10150 (50 μM) post-CEES treatment decreased the levels of DNA oxidation. Data expressed as a ratio of 8OHdG per 105 2dG. Data presented as mean ± S.E.M., n = 4 to 8; *, p < 0.05; ***, p < 0.001 compared with control levels. Two-way ANOVA of AEOL 10150, p = 0.1444; CEES, p = 0.0001; interaction, p = 0.0481 (A); and AEOL 10150, p = 0.1394; CEES, p < 0.0001; interaction, p = 0.0004 (B).
One consequence of oxidative stress is an increase in the oxidation of cellular macromolecules. A classic marker for DNA oxidation is the formation of 8OHdG, which we determined 12 h after CEES exposure. CEES caused a significant increase in 8OHdG levels in lung 16HBE cells as measured by high-performance liquid chromatography (Fig. 8B). We also found that AEOL 10150 added 1 h post-CEES exposure decreased CEES-mediated DNA oxidation. These data further support the role of oxidative stress in CEES-mediated injury that is ameliorated by the catalytic antioxidant metalloporphyrin, AEOL 10150.
Discussion
These studies suggest that CEES produces mitochondrial dysfunction that is followed by an increase in mitochondrial ROS production and cellular oxidative stress. In addition, we have identified a small-molecule catalytic antioxidant, AEOL 10150, that can rescue airway epithelial cells from CEES-induced toxicity and oxidative stress. These studies further shed insight into the mechanism of CEES toxicity and suggest that it is due, at least in part, to a delayed mitochondrial dysfunction and oxidative stress. These studies provide a rationale for the protective effects of antioxidants in CEES injury and why there may be a rescue window opportunity for therapeutics.
There is currently no antidote for SM poisoning. Upon exposure, the best recourse is decontamination and supportive treatment (Munro et al., 1990; Watson and Griffin, 1992). Decontamination of the skin is relatively straightforward and beneficial, whereas an internal exposure such as inhalation of sulfur mustards is much more difficult to treat (Munro et al., 1990). Medical surveillance of individuals exposed to mustard gas in the early 1980s has documented a number of respiratory conditions including bronchiolitis obliterans, asthma, and lung fibrosis that can persist throughout the victims' lifetimes (Ghanei and Harandi, 2007).
CEES is a close SM analog and provides a useful model for SM toxicity. CEES is termed monofunctional because of the single terminal chlorine, whereas SM has two chlorines (Fig. 1), giving it the additional ability to cross-link biological molecules (Watson and Griffin, 1992). Depending on route of administration, CEES is approximately 10 times less toxic than SM (Gautam et al., 2006). It is important to note that studies done with CEES, especially with therapeutics, need to be viewed with caution and are only a first step that needs to be repeated using SM.
Not much is known about the mechanism of SM toxicity despite its use for over a century. Early work focused on the alkylation of DNA and corresponding activation of PARP (Korkmaz et al., 2006), depletion of cellular NAD+ (Brookes and Lawley, 1961; Lawley and Brookes, 1967; Papirmeister et al., 1985), and inhibition of transcription factor binding (Gray, 1995) as a mechanism of toxicity. Papirmeister et al. (1985) showed that the difference in amount of radioactively labeled DNA that is degraded because of alkylation by SM or CEES in Escherichia coli crude extract is only approximately 10%. This indicated that CEES may be a useful model to study molecular effects of SM despite the lack of crosslinking and lower toxicity. It was also suggested that PARP would be activated, which in turn leads to a decrease in cellular NAD+ that would inhibit cellular energy processes and eventually lead to toxicity. It was found that the SM-induced depletion of NAD+ is time-dependent and does not occur until at least 1 h (Meier et al., 1987), being maximal by 4 h after exposure (Papirmeister et al., 1985). This research is some of the first to suggest a delayed nature of the cellular response to SM exposure and some of the earliest characterization of cellular effects of SM. It is unfortunate that the majority of earlier work was done in skin exposure models, and it is not clear whether it is relevant to the lung's response to SM.
More recent work with SM on DNA has characterized genomic changes that show increases in markers of apoptosis, cell cycle regulation, and various other response genes (Dillman et al., 2005). Furthermore, exploring the role of oxidative stress in CEES-mediated injury and supplementation with antioxidants as a treatment has been a major area of interest. Studies have indicated that TNF-α is increased in lung macrophages of guinea pigs 1 h after intratracheal instillation with CEES (Chatterjee et al., 2003). Other studies suggest a role for oxidative stress mediated by CEES that include changes in SOD (Mukhopadhyay et al., 2006), catalase, glutathione reductase (Gautam et al., 2006), and glutathione transferase activities (Kim et al., 1996; Jafari, 2007), inhibition of iNOS (Qui et al., 2006), depletion of glutathione (Elsayed et al., 1989), and increases in ROS (Elsayed et al., 1992; Gautam et al., 2006). As a consequence, supplementation with catalase, resveratrol, N-acetyl-l-cysteine, and GSH can be effective in treating mustard exposures (McClintock et al., 2002, 2006; Hoesel et al., 2008; Paromov et al., 2008). In rats, liposomes containing N-acetylcysteine, GSH, or a combination of the two can provide protection when administered between 1 to 1.5 h after CEES exposure (McClintock et al., 2006; Hoesel et al., 2008). Although these studies have suggested an oxidative stress environment, there is little mechanistic characterization of the increase in ROS levels caused by CEES. Recent reported finding suggest that CEES can alter cellular electron transfer systems as a mechanism for increased ROS production (Brimfield et al., 2009). In the current study, we have shown that there is delayed ROS production that is maximal 12 h after CEES exposure that may be localized to the mitochondria. We have also shown that in addition to the formation of ROS, there is also significant mitochondrial dysfunction. The delayed nature of the ROS response suggests a reason why antioxidants such as N-acetylcysteine, GSH, or AEOL 10150 have beneficial effects even when administered after CEES exposure. The ability of a catalytic antioxidant like AEOL 10150 that has been show to have both SOD and catalase activity (Milano and Day, 2000; Day, 2008) to rescue cells from CEES further demonstrates a role for ROS in CEES injury.
In addition to the ability of CEES to alkylate DNA, we also have demonstrated that CEES exposure can lead to DNA oxidation. Whether this oxidation is directly because of CEES, its metabolites, or the ROS that is formed is yet to be determined. The increased ROS we have seen in mitochondria may be because of mitochondrial dysfunctional, which we have seen in our CEES model and what others have seen after SM exposure (Sourdeval et al., 2006). The mitochondrion, specifically the respiratory chain, can produce a substantial amount of endogenous ROS (Fridovich, 1978; Drose and Brandt, 2008), and, if CEES causes mitochondrial uncoupling, this could explain oxidation products seen in other areas of the cell and markers of apoptosis (Dillman et al., 2005; Sourdeval et al., 2006). This idea is supported by recent findings that suggest sulfur and nitrogen mustards can react with cellular reductases and increase free radical production (Brimfield et al., 2009).
Our study shows that 900 μM CEES causes a reduction of cell viability to approximately 50%. It is interesting that in our cell model, we found that the transformed cell line 16HBE seems more susceptible to CEES toxicity than primary human SAE cells. 16HBE cells showed only approximately 50% change in cell viability when exposed to CEES for 24 h, whereas the same concentration in SAE cells took 48 h to decrease viability to approximately 55%. Recent studies have also seen this effect with SM in two other cell systems because of the degree of proteolytic processing of caspases induced by sulfur mustards (Ray et al., 2008). Another factor could be that the genetic background of the SAE cell donors was unknown. It also supports the notion that CEES may be bioactivated by specific cellular protein systems and different lung cell types, and cell lines may vary in their expression levels of these proteins.
In summary, we have established that there is delayed production of ROS and mitochondrial damage caused by CEES, which was prevented by AEOL 10150. We have established a role for CEES in initiating an oxidative stress environment within the cell by causing an overall decrease in intracellular GSH and increased DNA oxidation. Our results suggest that metalloporphyrins and other antioxidants can prevent oxidative stress caused by CEES in vitro through the delayed production of ROS in response to CEES.
This work was supported by the National Institutes of Health [Grant U54-ES015678]; and Aeolus Pharmaceuticals [Research Grant].
B.J.D. is a consultant for and holds equity in Aeolus Pharmaceuticals, which is commercially developing catalytic antioxidant mimetics as therapeutic agents.
doi:10.1124/jpet.108.145037.
ABBREVIATIONS: SM, sulfur mustard; CEES, 2-chloroethyl ethyl sulfide; SOD, superoxide dismutase; ROS, reactive oxygen species; AEOL 10150 or MnTDE-1,3-IP5+, manganese(III) meso-tetrakis(N,N′-diethylimidazolium-2-yl)porphyrin; SAE, small airway epithelial; DMSO, dimethyl sulfoxide; AM, acetoxymethyl ester; PBS, phosphate-buffered saline; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium; Rho 123, Rhodamine 123; 8OHdG, 8-hydroxy-2-deoxyguanosine; 2dG, 2-deoxyguanosine; ANOVA, analysis of variance; AEOL 10113 or MnTE-2-PyP5+, manganese(III) meso-tetrakis(N-ethylpyridinium-2-yl)porphyrin; AEOL 10303, manganese(III) meso-[5-(4-carboxy-3-hydroxyphenyl)-10,15,20-tris(4-carboxymethyl-3-hydroxyphenyl) porphyrin; MnTBAP, manganese(III) meso-tetrakis(4-benozic acid) porphyrin or manganese(III) meso-tetrakis(4-carboxyphenyl)porphyrin.
References
- Blanc PD (1999) The legacy of war gas. Am J Med 6 689-690. [DOI] [PubMed] [Google Scholar]
- Brimfield AA, Mancebo AM, Mason RP, Jiang JJ, Siraki AG, and Novak MJ (2009) Free radical production from the interaction of 2-chloroethyl vesicants (mustard gas) with pyridine nucleotide-driven flavoprotein electron transport systems. Toxicol Appl Pharmacol 234 128-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookes P and Lawley P (1961) The reaction of mono- and di-functional alkylating agents with nucleic acids. J Biochem 80 496-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee D, Mukherjee S, Smith MG, and Das SK (2003) Signal transduction events in lung injury induced by 2-chloroethyl ethyl sulfide, a mustard analog. J Biochem Mol Toxicol 17: 114-121. [DOI] [PubMed] [Google Scholar]
- Darzynkiewicz Z, Staiano-Coico L, and Melamed MR (1981) Increased mitochondrial uptake of rhodamine 123 during lymphocyte stimulation. Proc Natl Acad Sci U S A 78 2383-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Mukherjee S, Smith MG, and Chatterjee D (2003) Prophylactic protection by N-acetylcysteine against the pulmonary injury induced by 2-chloroethyl ethyl sulfide, a mustard analogue. J Biochem Mol Toxicol 17 177-184. [DOI] [PubMed] [Google Scholar]
- Day BJ (2004) Catalytic antioxidants: a radical approach to new therapeutics. Drug Discov Today 9 557-566. [DOI] [PubMed] [Google Scholar]
- Day BJ (2008) Antioxidants as potential therapeutics for lung fibrosis. Antioxid Redox Signal 10 355-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillman JF 3rd, Phillips CS, Dorsch LM, Croxton MD, Hege AI, Sylvester AJ, Moran TS, and Sciuto AM (2005) Genomic analysis of rodent pulmonary tissue following bis-(2-chloroethyl) sulfide exposure. Chem Res Toxicol 18 28-34. [DOI] [PubMed] [Google Scholar]
- Drose S and Brandt U (2008) The mechanism of mitochondrial superoxide production by the cytochrome bc1 complex. J Biol Chem 31 21649-21654. [DOI] [PubMed] [Google Scholar]
- Elsayed NM, Omaye ST, Klain GJ, Inase JL, Dahlberg ET, Wheeler CR, and Korte DW Jr (1989) Response of mouse brain to a single subcutaneous injection of the monofunctional sulfur mustard, butyl 2-chloroethyl sulfide. Toxicology 58 11-20. [DOI] [PubMed] [Google Scholar]
- Elsayed NM, Omaye ST, Klain GJ, and Korte DW Jr (1992) Free radical-mediated lung response to the monofunctional sulfur mustard butyl 2-chloroethyl sulfide after subcutaneous injection. Toxicology 72 153-165. [DOI] [PubMed] [Google Scholar]
- Fridovich I (1978) The biology of oxygen radicals. Science 201 875-880. [DOI] [PubMed] [Google Scholar]
- Gautam A, Vijayaraghavan R, Sharma M, and Ganesan K (2006) Comparative toxicity studies of sulfur mustard (2,2′-dichloro diethyl sulfide) and monofunctional sulfur mustard (2-chloroethyl ethyl sulfide), administered through various routes in mice. J Med CBR Def 4 1-21. [Google Scholar]
- Ghanei M and Harandi AA (2007) Long term consequences from exposure to sulfur mustard: a review. Inhal Toxicol 19 451-456. [DOI] [PubMed] [Google Scholar]
- Gray PJ (1995) Sulphur mustards inhibit binding of transcription factor AP2 in vitro. Nucleic Acids Res 23 4378-4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Espinoza LA, Liao H, Boulares AH, and Smulson ME (2004) Protection by antioxidants against toxicity and apoptosis induced by the sulphur mustard analog 2-chloroethylethyl sulphide (CEES) in Jurkat T cells and normal human lymphocytes. Br J Pharmacol 141 795-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoesel LM, Flierl MA, Niederbichler AD, Rittirsch D, McClintock SD, Reuben JS, Pianko MJ, Stone W, Yang H, Smith M, et al. (2008) Ability of antioxidant liposomes to prevent acute and progressive pulmonary injury. Antioxid Redox Signal 10: 978-981. [DOI] [PubMed] [Google Scholar]
- Jafari M (2007) Dose- and time-dependent effects of sulfur mustard on antioxidant system in liver and brain of rat. Toxicology 231 30-39. [DOI] [PubMed] [Google Scholar]
- Kehe K and Szinicz L (2005) Medical aspects of sulphur mustard poisoning. Toxicology 214 198-209. [DOI] [PubMed] [Google Scholar]
- Kim YB, Lee YS, Choi DS, Cha SH, and Sok DE (1996) Change in glutathione S-transferase and glyceraldehyde-3-phosphate dehydrogenase activities in the organs of mice treated with 2-chloroethyl ethyl sulfide or its oxidation products. Food Chem Toxicol 34 259-265. [DOI] [PubMed] [Google Scholar]
- Korkmaz A, Yaren H, Topal T, and Oter S (2006) Molecular targets against mustard toxicity: implication of cell surface receptors, peroxynitrite production, and PARP activation. Arch Toxicol 80 662-670. [DOI] [PubMed] [Google Scholar]
- Lawley PD and Brookes P (1967) Interstrand cross-linking of DNA by difunctional alkylating agents. J Mol Biol 25 143-160. [DOI] [PubMed] [Google Scholar]
- McClintock SD, Hoesel LM, Das SK, Till GO, Neff T, Kunkel RG, Smith MG, and Ward PA (2006) Attenuation of half sulfur mustard gas-induced acute lung injury in rats. J Appl Toxicol 26 126-131. [DOI] [PubMed] [Google Scholar]
- McClintock SD, Till GO, Smith MG, and Ward PA (2002) Protection from half-mustard-gas-induced acute lung injury in the rat. J Appl Toxicol 22 257-262. [DOI] [PubMed] [Google Scholar]
- Meier HL, Gross CL, and Papirmeister B (1987) 2,2′-Dichlorodiethyl sulfide (sulfur mustard) decreases NAD+ levels in human leukocytes. Toxicol Lett 39 109-122. [DOI] [PubMed] [Google Scholar]
- Milano J and Day BJ (2000) A catalytic antioxidant metalloporphyrin blocks hydrogen peroxide-induced mitochondrial DNA damage. Nucleic Acids Res 28 968-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Rajaratnam V, Mukherjee S, Smith M, and Das SK (2006) Modulation of the expression of superoxide dismutase gene in lung injury by 2-chloroethyl ethyl sulfide, a mustard analog. J Biochem Mol Toxicol 20 142-149. [DOI] [PubMed] [Google Scholar]
- Munro NB, Watson AP, Ambrose KR, and Griffin GD (1990) Treating exposure to chemical warfare agents: implications for health care providers and community emergency planning. Environ Health Perspect 89 205-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papirmeister B, Gross CL, Meier HL, Petrali JP, and Johnson JB (1985) Molecular basis for mustard-induced vesication. Fundam Appl Toxicol 5 S134-S149. [PubMed] [Google Scholar]
- Paromov V, Qui M, Yang H, Smith M, and Stone WL (2008) The influence of N-acetyl-l-cysteine on oxidative stress and nitric oxide synthesis in stimulated macrophages treated with a mustard gas analogue. BMC Cell Biol 9 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qui M, Paromov VM, Yang H, Smith M, and Stone WL (2006) Inhibition of inducible Nitric Oxide Synthase by a mustard gas analog in murine macrophages. BMC Cell Biol 7 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman I, Kode A, and Biswas SK (2006) Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc 1 3159-3165. [DOI] [PubMed] [Google Scholar]
- Ray R, Keyser B, Benton B, Daher A, Simbulan-Rosenthal CM, and Rosenthal DS (2008) Sulfur mustard induces apoptosis in cultured normal airway epithelial cells: evidence of a dominant caspase-8-mediated pathway and differential cellular responses. Drug Chem Toxicol 31 137-148. [DOI] [PubMed] [Google Scholar]
- Sourdeval M, Lemaire C, Deniaud A, Taysse L, Daulon S, Breton P, Brenner C, Boisvieux-Ulrich E, and Marano F (2006) Inhibition of caspase-dependent mitochondrial permeability transition protects airway epithelial cells against mustardinduced apoptosis. Apoptosis 11 1545-1559. [DOI] [PubMed] [Google Scholar]
- Tietze F (1969) Enzymatic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 27 502-522. [DOI] [PubMed] [Google Scholar]
- Watson AP and Griffin GD (1992) Toxicity of vesicant agents scheduled for destruction by the Chemical Stockpile Disposal Program. Environ Health Perspect 98 259-280. [DOI] [PMC free article] [PubMed] [Google Scholar]