Abstract
Cocaine is a psychostimulant that inhibits the inward transport of dopamine (DA) via the neuronal DA transporter, thereby increasing DA concentrations in the synaptic cleft. Cocaine administration also causes a redistribution of striatal vesicular monoamine transporter (VMAT)-2-containing vesicles that co-fractionate with synaptosomal membranes after osmotic lysis (referred to herein as membrane-associated vesicles) to a nonmembrane-associated, cytoplasmic subcellular fraction. Although previous studies from our laboratory have focused on the impact of cocaine on cytoplasmic vesicles, the present report describes the pharmacological effects of cocaine on the membrane-associated vesicle population. Results revealed that the redistribution of VMAT-2 and associated vesicles away from synaptosomal membranes is associated with a decrease in total DA transport and DA content in the membrane-associated VMAT-2-containing subcellular fraction. Cocaine also decreases the velocity and magnitude of K+-stimulated exocytotic DA release from whole striatal suspensions. The cocaine-induced VMAT-2 redistribution, decrease in DA release, and decrease in total DA transport are mediated by D2 receptors as these events were prevented by pretreatment with the D2 receptor antagonist, eticlopride [S-(-)-3-chloro-5-ethyl-N-[(1-ethyl-2-pyrrolidinyl)methyl]-6-hydroxy-2-methoxybenzamide hydrochloride]. These data suggest that after cocaine administration, D2 receptors are activated because of increased synaptic DA, resulting in a redistribution of DA-containing vesicles away from synaptosomal membranes, thus leading to less DA released after a depolarizing stimulus. These findings provide insight into not only the mechanism of action of cocaine but also mechanisms underlying the regulation of dopaminergic neurons.
Synaptic vesicles sequester neurotransmitters for storage and subsequent release. The transport of catecholamines into vesicles is solely mediated by the vesicular monoamine transporter (VMAT)-2, so any alterations in VMAT-2 function can affect intra- and extra-neuronal dopamine (DA) concentrations. There are probably several different populations of VMAT-2-associated vesicles, including those that cofractionate with synaptosomal membranes after osmotic lysis (referred to herein as membrane-associated vesicles) and those that do not (referred to herein as cytoplasmic vesicles). These vesicle populations differ in that DA transport into cytoplasmic vesicles obeys Michaelis-Menten kinetics, whereas the DA transport profile of membrane-associated VMAT-2 is sigmoidal (Volz et al., 2007a). In addition, the membrane-associated vesicles are able to transport, in aggregate, 5- to 9-fold more DA in total capacity than cytoplasmic vesicles (Volz et al., 2007a); thus, they may have greater bearing on cytosolic DA concentrations than cytoplasmic vesicles.
Several investigators have assessed the impact of cocaine on stimulated dopamine release. For example, Wightman and coworkers demonstrated that cocaine increases DA release caused by electrical stimuli that mobilize vesicles from the reserve vesicular pool (Venton et al., 2006). However, others have reported decreases in K+-stimulated DA release after cocaine administration (Dembiec, 1980; Dembiec and Cohen, 1981). Still other studies have focused on the effects of cocaine within the nerve terminal and have shown that cocaine treatment rapidly (within 1 h) redistributes VMAT-2 and associated vesicles away from synaptosomal membranes into the cytoplasm, as assessed in the striatum of treated rats (Riddle et al., 2002). This latter trafficking event occurs concurrently with both increased Vmax of DA transport and binding of the VMAT-2 ligand, dihydrotetrabenazine, in cytoplasmic vesicles. Both the increase in cytoplasmic vesicular transport and dihydrotetrabenazine binding are inhibited by pretreatment with the D2 antagonist, eticlopride (Brown et al., 2001 and references cited therein).
Although the impact of cocaine on cytoplasmic vesicles has been investigated, the effects of cocaine on the membrane-associated vesicles described above have received less study. This is of importance because the finding that the membrane-associated pool contains the active zone marker, piccolo (Volz et al., 2007a), suggests that at least some of these vesicles belong to the readily releasable and/or recycling pool of vesicles. In accordance, the purpose of the present study was to investigate the impact of cocaine on this vesicle population. Results revealed cocaine treatment decreased total vesicular DA transport into, and DA content within, membrane-associated vesicles, an effect attributable to a decrease in VMAT-2 immunoreactivity. Cocaine treatment also decreased the velocity and magnitude of K+-stimulated DA release from whole striatal suspensions. The cocaine-induced VMAT-2 redistribution, decrease in release, and decrease in transport were prevented by pretreatment with the D2 receptor antagonist, eticlopride. Taken together, these findings indicate that although the overall extracellular DA concentration may be elevated because of DAT inhibition, cocaine administration also leads to decreased stimulated exocytotic DA release because of fewer vesicles and a lower DA content at the synaptosomal membrane. This latter phenomenon may reflect an acute compensatory mechanism that occurs by D2 autoreceptor feedback inhibition in striatal presynaptic neurons after cocaine administration. Thus, these findings have implications for understanding the mechanism of action of cocaine and the underlying regulation of dopaminergic neurons.
Materials and Methods
Drugs and Chemicals. (-)-Cocaine hydrochloride was supplied by the National Institute on Drug Abuse (Bethesda, MD). Eticlopride hydrochloride and SCH-23390 were purchased from Sigma-Aldrich (St. Louis, MO). All drugs were administered at doses and times previously used to investigate receptor-mediated effects of psychostimulants in rat brain (Brown et al., 2001; Sandoval et al., 2002; Truong et al., 2004; Riddle et al., 2007). Drug doses were calculated as the free base, dissolved in 0.9% (w/v) saline, and administered at 1 ml/kg as stated in the figure legends. VMAT-2 antibody (AB1767) was purchased from Millipore (Temecula, CA).
The pH 7.4 sucrose buffer used had a final concentration of 320 mM sucrose, 3.8 mM NaH2PO4, and 12.7 mM Na2HPO4. The VMAT-2 assay buffer contained 25 mM HEPES, 100 mM potassium tartrate, 0.05 mM EGTA, 0.1 mM EDTA, and 2 mM ATP-Mg2+, with a final pH of 7.5. The DAT assay buffer, pH 7.4, contained 126 mM NaCl, 4.8 mM KCl, 1.3 mM CaCl2, 16 mM sodium phosphate, 1.4 mM MgSO4, and 11 mM dextrose. The pH 2.5 tissue buffer consisted of 50 mM sodium phosphate, 30 mM citric acid, and 10% (v/v) methanol.
Animals. Male Sprague-Dawley rats (300-360 g) were purchased from Charles River Laboratories, Inc. (Raleigh, NC) and housed in a light- and temperature-controlled room, with food and water provided ad libitum. All animal procedures were conducted in compliance with the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) and were approved by the University of Utah Institutional Animal Care and Use Committee.
Subcellular Fractionation and Measurement of DA Transport Velocities. Rotating disk electrode (RDE) voltammetry (Schenk et al., 2005; Volz et al., 2006) was used to measure the initial velocities of inwardly directed vesicular DA transport into membrane-associated vesicles purified from rat striata as described previously (Volz et al., 2007a). Each sample consisted of both striata (∼60-70 mg total wet weight) from a rat that were homogenized in ice-cold sucrose buffer and then centrifuged (800g for 12 min at 4°C) to remove nuclei and large debris. The resulting supernatant was centrifuged (22,000g for 15 min at 4°C) to obtain the synaptosomal pellet. This synaptosomal pellet was then resuspended and homogenized in ice-cold water to lyse the synaptosomal membranes. Ice-cold 25 mM HEPES and 100 mM potassium tartrate, pH 7.5, were then added to the homogenate and centrifuged (20,000g for 20 min at 4°C) to form the membrane-associated vesicle pellet. To isolate cytoplasmic vesicles, 1 mM ice-cold MgSO4, pH 7.5, was added to the supernatant, and the resulting mixture was centrifuged (100,000g for 45 min at 4°C) to obtain the cytoplasmic vesicle pellet.
To measure DA transport velocities, the membrane-associated vesicle pellet was resuspended in 500 μl of VMAT-2 assay buffer and placed in a cylindrical glass chamber (10-mm internal diameter with a height of 20 mm) maintained at 37°C by a VWR (West Chester, PA) model 1104 Heating Recirculator. A Pine Instrument Company (Grove City, PA) AFMD03GC glassy carbon electrode (5-mm total diameter with a 3-mm-diameter glassy carbon electrode shrouded in Teflon) attached to a Pine Instruments MSRX high-precision rotator was lowered into the glass chamber and rotated at 2000 rpm. A Bioanalytical Systems (West Lafayette, IN) LC3D (Petite Ampere) potentiostat was used to apply a potential of +450 mV relative to a Ag/AgCl reference electrode and a detection current baseline was obtained in approximately 5 min. An aqueous DA (10.2 μl) solution was then injected into the resuspended tissue sample using a Hamilton Co. (Reno, NV) CR-700-20 constant rate syringe for a final concentration of 1.5 μM DA inside the glass incubation chamber. The current outputs were recorded onto a Tektronix (Beaverton, OR) TDS 1002 digital storage oscilloscope, and the initial velocities of DA transport were calculated from the linear slope of the initial apparent zero order portion of a plot of DA concentration versus time as described previously (Volz et al., 2006). A Bradford protein assay (Bio-Rad, Hercules, CA) was conducted to measure protein concentrations in each sample. VMAT-2-mediated DA transport velocities were normalized to protein as reported previously (Volz et al., 2006, 2007a, 2008) to allow for comparison with values previously published in the scientific literature.
VMAT-2 Immunoreactivity. After DA transport velocities were measured with RDE, SDS-polyacrylamide gel electrophoresis and Western blot analysis were completed on the membrane-associated vesicle samples as described previously (Riddle et al., 2002, 2007; Volz et al., 2007a). Forty micrograms of protein (as determined with a Bradford protein assay) from each membrane-associated vesicle sample was loaded onto the 10% gel. Bound VMAT-2 antibody was visualized with horseradish peroxidase-conjugated rabbit secondary antibody (BioSource International, Camarillo, CA). Bands on all Western blots were quantified by densitometry using a FluorChem SP Imaging System from Alpha Innotech (San Leandro, CA).
Vesicular DA Content. Vesicular DA content was measured using high-performance liquid chromatography as described previously (Volz et al., 2007a). The membrane-associated and cytoplasmic vesicle pellets were prepared as explained above and resuspended in ice-cold tissue buffer at 50 and 100 mg original striatal wet weight/ml tissue buffer, respectively. The resuspended vesicle preparations were then sonicated for ∼10 s and centrifuged (22,000g for 15 min at 4°C). A 100-μl aliquot of the resulting supernatant from each sample was then injected onto a high-performance liquid chromatograph system (4.6 × 250 mm; Whatman, Maidstone, UK; Partisphere C18 column) that was coupled to an electrochemical detector (+730 mV relative to an Ag/AgCl reference electrode). The pH 2.86 mobile phase contained 50 mM sodium phosphate, 30 mM citric acid, 0.16 mM EDTA, 1.5 mM sodium octyl sulfate, and 10% (v/v) methanol (Chapin et al., 1986).
Measurement of K+-Stimulated DA Release. RDE voltammetry (Schenk et al., 2005; Volz et al., 2006) was used to measure K+-stimulated DA release in striatal suspensions prepared from treated rats as described before (McElvain and Schenk, 1992; Volz et al., 2007a). Each sample consisted of one striatum (∼27-38 mg wet weight) that was placed on an ice-cold watch glass and chopped by hand with an ice-cold razor blade for ∼30 s. The chopped striatum was then placed in 500 μl of DAT assay buffer inside the RDE glass chamber and was resuspended by repetitive pipetting for approximately 1 min. The resulting striatal suspension was allowed to stand for 12 min and was then washed by the addition and subsequent removal of 250 μl of fresh DAT assay buffer six times. The washes were done without disrupting the settled striatal tissue. A detection current baseline was obtained as described above in approximately 18 min, and then a small quantity of DAT assay buffer containing an elevated KCl concentration (for a final concentration of 40 mM K+ inside the RDE glass chamber) was added to the striatal suspension to stimulate DA release. The initial velocity of K+-stimulated DA release (measured from the first 3 s of release), the magnitude of K+-stimulated DA release (the maximum amount of DA released), and the duration of K+-stimulated DA release (the amount of time for the maximal amount of DA to be released) were calculated and normalized to striatal wet weight as described previously (McElvain and Schenk, 1992; Volz et al., 2007a). These values are reported per milligram of wet weight to allow for comparison with values previously published in the scientific literature.
Results
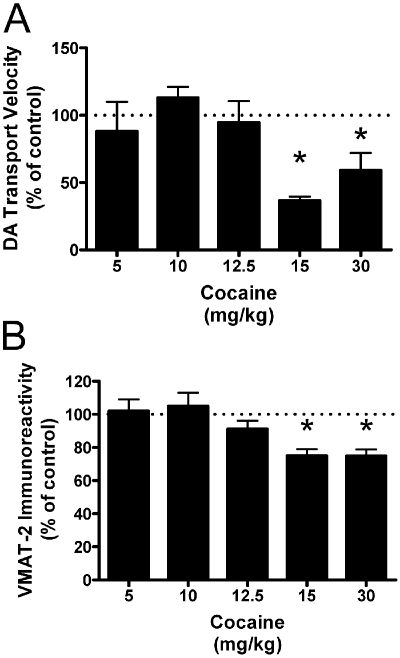
These studies were conducted to determine the pharmacological impact of cocaine on membrane-associated vesicles. It is important that it has been established that the DAT does not contribute to DA binding or transport measured in this preparation; thus, DA transport in membrane-associated vesicles reported is VMAT-2-mediated (Volz et al., 2007a). Results presented in Fig. 1 demonstrate that a single administration of cocaine decreases VMAT-2-mediated DA transport into, and VMAT-2 immunoreactivity in, the entire synaptosomal membrane-associated vesicular subcellular fraction as assessed 1 h after treatment. The lowest effective dose determined in this study (15 mg/kg i.p.) was used in all subsequent studies.
Fig. 1.
Cocaine decreases DA transport velocity (A) and VMAT-2 immunoreactivity (B) in the membrane-associated vesicle fraction. Rats received a single intraperitoneal injection of cocaine at the doses indicated or saline vehicle (1 ml/kg i.p.). All animals were killed 1 h after the last injection. Each column represents the mean ± S.E.M. of four independent determinations, and an asterisk indicates a statistical difference, p < 0.05 via a one-way ANOVA with a Tukey post-test, from the 5 mg/kg cocaine group.
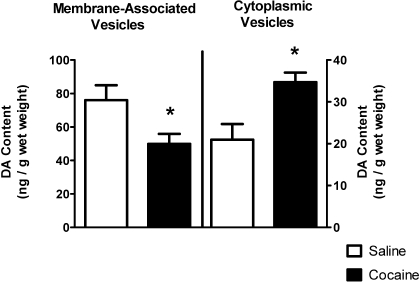
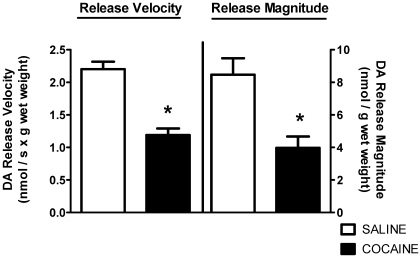
Results presented in Fig. 2 show that concurrent with these decreases in DA transport and VMAT-2 immunoreactivity, cocaine treatment decreased and increased total membrane-associated and cytoplasmic vesicular DA content, respectively. A single administration of cocaine decreased the velocity and magnitude of K+-stimulated DA release from striatal suspensions 1 h after treatment as well (Fig. 3). The duration of K+-stimulated DA release was not affected by drug treatment (7.6 ± 1.3 s for saline, 7.3 ± 1.9 s for cocaine; n = 4).
Fig. 2.
Cocaine decreases and increases DA content in the membrane-associated and cytoplasmic vesicle fractions, respectively. Rats received a single administration of cocaine (15 mg/kg i.p.) or saline vehicle (1 ml/kg s.c.) and were killed 1 h later. Each column represents the mean ± S.E.M. of six independent determinations, and an asterisk indicates a statistical difference, p < 0.05, via a two-tailed Student's t test, between DA content in saline- and cocaine-treated animals.
Fig. 3.
Cocaine decreases both the velocity and magnitude of K+-stimulated DA release from striatal suspensions. Rats received a single administration of cocaine (15 mg/kg i.p.) or saline vehicle (1 ml/kg i.p.) and were killed 1 h later. Each column represents the mean ± S.E.M. of four independent determinations, and an asterisk indicates a statistical difference, p < 0.05, via a two-tailed Student's t test, between DA release in saline- and cocaine-treated animals.
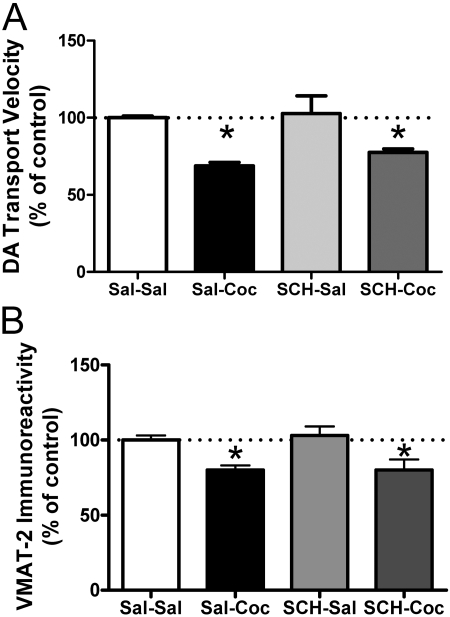
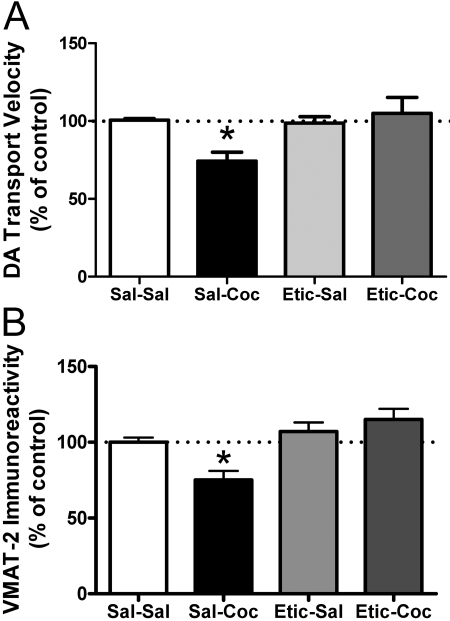
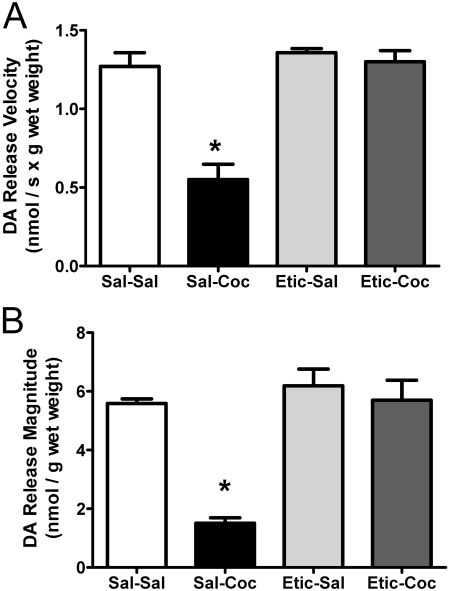
Results shown in Fig. 4 demonstrate that administration of the D1 receptor antagonist SCH-23390 (0.5 mg/kg i.p.) 15 min before cocaine treatment had no effect on cocaine-induced decreases on DA transport (Fig. 4A) or VMAT-2 immunoreactivity (Fig. 4B) in the membrane-associated vesicular fraction. However, pretreatment with the D2 receptor antagonist eticlopride (0.5 mg/kg i.p.) 15 min before cocaine administration inhibited cocaine-induced decreases in DA transport (Fig. 5A) and VMAT-2 immunoreactivity (Fig. 5B) in membrane-associated vesicles 1 h after treatment. Eticlopride pretreatment also prevented cocaine-induced decreases in K+-stimulated DA release (Fig. 6A) and magnitude (Fig. 6B). Again, the duration of DA release was not changed by drug treatment (6.6 ± 0.87 s for saline/saline, 6.1 ± 0.35 s for saline/cocaine, 6.5 ± 0.35 s for eticlopride/saline, and 6.4 ± 0.39 s for eticlopride/cocaine).
Fig. 4.
The DA D1 receptor antagonist, SCH-23390, does not attenuate the decreases in DA transport velocity (A) or VMAT-2 immunoreactivity (B) in the membrane-associated vesicle fraction caused by cocaine treatment. Rats received a single administration of SCH-23390 (SCH; 0.5 mg/kg i.p.) or saline vehicle (Sal; 1 ml/kg i.p.) 15 min before a single administration of cocaine (Coc; 15 mg/kg i.p.) or saline vehicle (1 ml/kg i.p.). All animals were killed 1 h after the last injection. Each column represents the mean ± S.E.M. of four independent determinations, and an asterisk indicates a statistical difference, p < 0.05, via a one-way ANOVA with a Tukey post-test, versus Sal-Sal and SCH-Sal groups.
Fig. 5.
The D2 receptor antagonist, eticlopride, blocks the cocaine-induced decrease in DA transport velocity (A) and in VMAT-2 immunoreactivity (B) in the membrane-associated vesicle fraction. Rats received a single administration of eticlopride (Etic; 0.5 mg/kg i.p.) or saline vehicle (Sal; 1 ml/kg i.p.) 15 min before a single injection of cocaine (Coc; 15 mg/kg i.p.) or saline vehicle (1 ml/kg i.p.). All animals were killed 1 h after the last injection. Each column represents the mean ± S.E.M. of four independent determinations, and an asterisk indicates a statistical difference, p < 0.05, via a one-way ANOVA with a Tukey post-test, versus other-treated groups.
Fig. 6.
The DA D2 receptor antagonist, eticlopride, prevents cocaine-induced decreases in both the velocity (A) and magnitude (B) of K+-stimulated DA release from striatal suspensions. Rats received a single administration of eticlopride (Etic; 0.5 mg/kg i.p.) or saline vehicle (Sal; 1 ml/kg i.p.) 15 min before a single injection of cocaine (Coc; 15 mg/kg i.p.) or saline vehicle (1 ml/kg s.c.). All animals were killed 1 h after the last injection. Each column represents the mean ± S.E.M. of four independent determinations, and an asterisk indicates a statistical difference, p < 0.05, via a one-way ANOVA with a Tukey post-test, versus groups treated otherwise.
Discussion
Previous investigations have examined the effects of cocaine on nonmembrane-associated cytoplasmic vesicles, wherein it was determined that cocaine administration increases DA transport into this cytoplasmic vesicular fraction (Brown et al., 2001). This effect was attributed to a redistribution of VMAT-2 and associated vesicles from synaptosomal membranes into the cytoplasm. Although several studies have focused on the pharmacological regulation of these cytoplasmic vesicles, effects on vesicles that remained associated with the synaptosomal membranes have been less described. Hence, the present study determined the pharmacological impact of cocaine on vesicles that remain associated with the synaptosomal membranes after osmotic lysis. This is of significance because of the unique kinetic properties and sequestration potential of this population of vesicles (see Introduction). Furthermore, at least some of these vesicles correspond to the “readily releasable” pool of vesicles; thus, alterations in their function will probably affect exocytotic DA release (Volz et al., 2007a).
Results revealed that cocaine treatment decreases DA transport into membrane-associated vesicles, as assessed by measuring DA transport into the entire synaptosomal membrane-associated subcellular fraction. This occurs concurrently with a decrease in VMAT-2 immunoreactivity and in total DA content in the membrane-associated fraction. In contrast, cocaine increases cytoplasmic vesicular DA uptake (Brown et al., 2001), VMAT-2 immunoreactivity (Riddle et al., 2002), and DA content (Fig. 2). Taken together, these data suggest that cocaine administration leads to a redistribution of vesicle populations between the two subcellular fractions. This effect on membrane-associated VMAT-2 is of importance because the rate that the readily releasable and/or recycling pool of vesicles is replenished and refilled is a significant determinant of presynaptic efficacy and of several types of both short- and long-term synaptic plasticity (Sudhof, 2000; Liu, 2003). In addition, trafficking of VMAT-2 and associated vesicles away from the plasma membrane may have implications for the probability of exocytotic release because the number of vesicles in the readily releasable pool affects this probability and thus has a significant effect on DA neurotransmission (Sudhof, 2000; Kavalali, 2006).
It is probable that because of the decrease in VMAT-2-associated vesicles and DA content at the synaptosomal membrane due to a redistribution of DA in the nerve terminal, cocaine treatment decreased both the velocity and magnitude of K+-stimulated DA release from whole striatal suspensions. The observation of cocaine-induced decreases in K+-stimulated release is consistent with data obtained in striatal slice (Dembiec, 1980; Dembiec and Cohen, 1981) and pheochromocytoma (PC12) cell lines (Pothos and Sulzer, 1998). However, Wightman and coworkers (Venton et al., 2006) reported that cocaine administration increased electrically stimulated DA release in an intact animal by recruiting a reserve pool of vesicles. Possible explanations for this discrepancy include: 1) differences in paradigms used to stimulate release (i.e., electrical stimulation versus K+-stimulated release) and 2) the absence of residual cocaine in our ex vivo protocol and in the other studies that demonstrated cocaine-induced decreases in DA release (Dembiec, 1980; Dembiec and Cohen, 1981) versus the studies that found an increase in DA release wherein cocaine was present.
D2, but not D1, antagonist pretreatment inhibited the cocaine-induced decrease in K+-stimulated release reported herein. This permits speculation of a compensatory mechanism wherein D2 receptors are activated in response to cocaine-enhanced extracellular DA and that this receptor activation decreases exocytotic DA release (Stark et al., 1989; Pothos et al., 1998; Liu, 2003). However, it should be noted that Pothos and Sulzer (1998) found that preapplication of eticlopride did not prevent the cocaine-induced decreases in stimulated DA release when incubated in a PC12 cell line (i.e., a cell line that expresses D2 receptors; see Zhu et al., 1997; Kobayashi et al., 1999). These dissimilar results may be attributable to the differences in experimental protocols (i.e., an in vitro versus in vivo administration of eticlopride and cocaine). Moreover, acute cocaine administration also has been shown to decrease phosphorylation of tyrosine hydroxylase and thus its activity (Jedynak et al., 2002). D2 agonists such as quinpirole have also been demonstrated to reduce tyrosine hydroxylase activity (Onali et al., 1988; Starke et al., 1989; O'Hara et al., 1996). Hence, it is likely that another compensatory mechanism whereby cocaine affects DA vesicular transport and release is by decreasing DA biosynthesis via autoreceptor activation.
The impact of cocaine is strikingly different from the pharmacological effects of another DAT inhibitor, methylphenidate (MPD), despite the fact that these agents: 1) have a similar binding site on the DAT (Schweri et al., 1985; Schenk, 2002), 2) block the inward transport of DA via the plasmalemmal DAT (Ritz et al., 1987; Wayment et al., 1999), and 3) redistribute VMAT-2 and associated vesicles away from synaptosomal membranes and into the cytoplasm (Riddle et al., 2002, 2007; Sandoval et al., 2002; Volz et al., 2007a,b). In particular, cocaine and MPD differentially affect the transport of DA into vesicles that remain associated with the synaptosomal membranes in that cocaine decreases DA transport because of vesicle trafficking, whereas MPD kinetically up-regulates the activity of the VMAT-2 protein per se in this subcellular fraction (Volz et al., 2007a,b). Furthermore, the effects of cocaine on DA release resemble the effects of the D2 agonist, quinpirole, thus supporting the suggestion that cocaine decreases K+-stimulated DA release via an indirect effect on D2 autoreceptors (see above). Of relevance are recent studies demonstrating that MPD has affinity for muscarinic receptors (Markowitz et al., 2006) and that muscarinic activation contributes to the MPD-induced decrease in K+-stimulated DA release (Volz et al., 2008). In contrast, cocaine exhibits micromolar affinity as a muscarinic receptor antagonist (Sharkey et al., 1988), thus permitting speculation that the difference between these agents in terms of their differential impact on stimulated DA release and VMAT-2-mediated DA transport in membrane-associated vesicles is a consequence, at least in part, of their differential impact on muscarinic receptors.
It is of interest to note that one pharmacological consequence of the difference in the effects of cocaine and MPD on DA sequestration by membrane-associated VMAT-2 may be that MPD attenuates persistent (e.g., 7 days) dopaminergic deficits caused by methamphetamine (Sandoval et al., 2003; Volz et al., 2007b), whereas cocaine does not, as assessed 18 to 24 h after treatment (Hanson et al., 1987; Klongpanichapak et al., 2006). Methamphetamine-induced DA deficits have been suggested to occur as a consequence of improper vesicular DA sequestration and resulting DA-associated reactive species formation (Cubells et al., 1994; Cadet and Brannock, 1998; see also Fleckenstein et al., 2008 and references therein). In accordance, because MPD kinetically up-regulates membrane-associated VMAT-2 such that significantly more DA is sequestered than after cocaine treatment, MPD affords neuroprotection.
In conclusion, these results elucidate mechanisms whereby cocaine alters DA signaling within the striatum. The data described herein permit speculation that after cocaine administration, D2 receptors are activated because of an increase in synaptic DA caused by DAT inhibition. This, in turn, causes a redistribution of VMAT-2 and associated vesicles away from the synaptosomal membranes, leading to a lower overall DA content at the membrane and less DA released after a depolarizing stimulus. These phenomena may represent a compensatory or homeostatic mechanism that striatal neurons employ in response to increases in synaptic DA concentrations caused by DAT inhibition by cocaine. These findings aid in the understanding of the mechanism of action of cocaine and, thus, have important implications for psychostimulant abuse and the neurochemical changes that occur in addiction.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA00869, DA04222, DA13367, DA11389, DA019447, DA00378].
doi:10.1124/jpet.108.146159.
ABBREVIATIONS: VMAT, vesicular monoamine transporter; DA, dopamine; eticlopride, S-(-)-3-chloro-5-ethyl-N-[(1-ethyl-2-pyrrolidinyl)methyl]-6-hydroxy-2-methoxybenzamide hydrochloride; DAT, dopamine transporter; SCH-23390, R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride; RDE, rotating disk electrode; MPD, methylphenidate; ANOVA, analysis of variance.
References
- Brown JM, Hanson GR, and Fleckenstein AE (2001) Cocaine-induced increases in vesicular dopamine uptake: role of dopamine receptors. J Pharmacol Exp Ther 298 1150-1153. [PubMed] [Google Scholar]
- Cadet JL and Brannock C (1998) Free radicals and the pathobiology of brain dopamine systems. Neurochem Int 32 117-131. [DOI] [PubMed] [Google Scholar]
- Chapin DS, Lookingland KJ, and Moore KE (1986) Effects of lc mobile phase composition on retention times for biogenic amines, and their precursors and metabolites. Curr Sep 7 68-70. [Google Scholar]
- Cubells JF, Rayport S, Rajendran G, and Sulzer D (1994) Methamphetamine neurotoxicity involves vacuolation of endocytic organelles and dopamine-dependent intracellular oxidative stress. J Neurosci 14 2260-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dembiec D (1980) Inhibition of potassium-stimulated release of [3H]dopamine from rat striata as a result of prior exposure to cocaine, nomifensine, or mazindol. Neurochem Res 5 345-349. [DOI] [PubMed] [Google Scholar]
- Dembiec D and Cohen G (1981) Potassium-induced release of [3H]catecholamine from brain: effects of pre-exposure to catecholamine uptake inhibitors. J Pharmacol Exp Ther 217 727-732. [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, and Hanson GR (2008) Psychostimulant-induced alterations in vesicular monoamine transporter-2 function: neurotoxic and therapeutic implications. Neuropharmacology 56 (Suppl 1): 133-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson GR, Matsuda LA, and Gibb JW (1987) Effects of cocaine on methamphetamine-induced neurochemical changes: characterization of cocaine as a monoamine uptake blocker. J Pharmacol Exp Ther 242 507-513. [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed. Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC.
- Jedynak JP, Ali SF, Haycock JW, and Hope BT (2002) Acute administration of cocaine regulates the phosphorylation of serine-19, -31 and -40 in tyrosine hydroxylase. J Neurochem 82 382-388. [DOI] [PubMed] [Google Scholar]
- Kavalali ET (2006) Synaptic vesicle reuse and its implications. Neuroscientist 12 57-66. [DOI] [PubMed] [Google Scholar]
- Klongpanichapak S, Govitrapong P, Sharma SK, and Ebadi M (2006) Attenuation of cocaine and methamphetamine neurotoxicity by coenzyme Q10. Neurochem Res 31 303-311. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Conforti L, Zhu WH, Beitner-Johnson D, and Millhorn DE (1999) Role of the D2 dopamine receptor in molecular adaptation to chronic hypoxia in PC12 cells. Pflugers Arch 438 750-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G (2003) Presynaptic control of quantal size: kinetic mechanisms and implications for synaptic transmission and plasticity. Curr Opin Neurobiol 13 324-331. [DOI] [PubMed] [Google Scholar]
- Markowitz JS, DeVane CL, Pestreich LK, Patrick KS, and Muniz R (2006) A comprehensive in vitro screening of d-, l-, and dl-threo-methylphenidate: an exploratory study. J Child Adolesc Psychopharmacol 16 687-698. [DOI] [PubMed] [Google Scholar]
- McElvain JS and Schenk JO (1992) Blockade of dopamine autoreceptors by haloperidol and the apparent dynamics of potassium-stimulated endogenous release of dopamine from and reuptake into striatal suspensions in the rat. Neuropharmacology 31 649-659. [DOI] [PubMed] [Google Scholar]
- O'Hara CM, Uhland-Smith A, O'Malley KL, and Todd RD (1996) Inhibition of dopamine synthesis by D2 and D3 but not D4 receptors. J Pharmacol Exp Ther 277 186-192. [PubMed] [Google Scholar]
- Onali P, Olianas MC, and Bunse B (1988) Evidence that adenosine A2 and dopamine autoreceptors antagonistically regulate tyrosine hydroxylase activity in rat striatal synaptosomes. Brain Res 456 302-309. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Przedborski S, Davila V, Schmitz Y, and Sulzer D (1998) D2-like dopamine autoreceptor activation reduces quantal size in PC12 cells. J Neurosci 18 5575-5585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothos EN and Sulzer D (1998) Modulation of quantal dopamine release by psychostimulants. Adv Pharmacol 42 198-202. [DOI] [PubMed] [Google Scholar]
- Riddle EL, Hanson GR, and Fleckenstein AE (2007) Therapeutic doses of amphetamine and methylphenidate selectively redistribute the vesicular monoamine transporter-2. Eur J Pharmacol 571 25-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle EL, Topham MK, Haycock JW, Hanson GR, and Fleckenstein AE (2002) Differential trafficking of the vesicular monoamine transporter-2 by methamphetamine and cocaine. Eur J Pharmacol 449 71-74. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, and Kuhar MJ (1987) Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237 1219-1223. [DOI] [PubMed] [Google Scholar]
- Sandoval V, Riddle EL, Hanson GR, and Fleckenstein AE (2002) Methylphenidate redistributes vesicular monoamine transporter-2: role of dopamine receptors. J Neurosci 22 8705-8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval V, Riddle EL, Hanson GR, and Fleckenstein AE (2003) Methylphenidate alters vesicular monoamine transport and prevents methamphetamine-induced dopaminergic deficits. J Pharmacol Exp Ther 304 1181-1187. [DOI] [PubMed] [Google Scholar]
- Schenk JO (2002) The functioning neuronal transporter for dopamine: kinetic mechanisms and effects of amphetamines, cocaine and methylphenidate. Prog Drug Res 59 111-131. [DOI] [PubMed] [Google Scholar]
- Schenk JO, Wright C, and Bjorklund N (2005) Unraveling neuronal dopamine transporter mechanisms with rotating disk electrode voltammetry. J Neurosci Methods 143 41-47. [DOI] [PubMed] [Google Scholar]
- Schweri MM, Skolnick P, Rafferty MF, Rice KC, Janowsky AJ, and Paul SM (1985) [3H]Threo-(±)-methylphenidate binding to 3,4-dihydroxyphenylethylamine uptake sites in corpus striatum: correlation with the stimulant properties of Ritalinic acid esters. J Neurochem 45 1062-1070. [DOI] [PubMed] [Google Scholar]
- Sharkey J, Ritz MC, Schenden JA, Hanson RC, and Kuhar MJ (1988) Cocaine inhibits muscarinic cholinergic receptors in heart and brain. J Pharmacol Exp Ther 246 1048-1052. [PubMed] [Google Scholar]
- Starke K, Göthert M, and Kilbinger H (1989) Modulation of neurotransmitter release by presynaptic autoreceptors. Physiol Rev 69 864-989. [DOI] [PubMed] [Google Scholar]
- Südhof TC (2000) The synaptic vesicle cycle revisited. Neuron 28 317-320. [DOI] [PubMed] [Google Scholar]
- Truong JG, Newman AH, Hanson GR, and Fleckenstein AE (2004) Dopamine D2 receptor activation increases vesicular dopamine uptake and redistributes vesicular monoamine transporter-2 protein. Eur J Pharmacol 504 27-32. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Seipel AT, Phillips PE, Wetsel WC, Gitler D, Greengard P, Augustine GJ, and Wightman RM (2006) Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. J Neurosci 26 3206-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz TJ, Hanson GR, and Fleckenstein AE (2006) Measurement of kinetically resolved vesicular dopamine uptake and efflux using rotating disk electrode voltammetry. J Neurosci Methods 155 109-115. [DOI] [PubMed] [Google Scholar]
- Volz TJ, Farnsworth SJ, King JL, Riddle EL, Hanson GR, and Fleckenstein AE (2007a) Methylphenidate administration alters vesicular monoamine transporter-2 function in cytoplasmic and membrane-associated vesicles. J Pharmacol Exp Ther 323 738-745. [DOI] [PubMed] [Google Scholar]
- Volz TJ, Hanson GR, and Fleckenstein AE (2007b) The role of the plasmalemmal dopamine and vesicular monoamine transporters in methamphetamine-induced dopaminergic deficits. J Neurochem 101 883-888. [DOI] [PubMed] [Google Scholar]
- Volz TJ, Farnsworth SJ, Rowley SD, Hanson GR, and Fleckenstein AE (2008) Methylphenidate-induced increases in vesicular dopamine sequestration and dopamine release in the striatum: the role of muscarinic and dopamine D2 receptors. J Pharmacol Exp Ther 327 161-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayment HK, Deutsch H, Schweri MM, and Schenk JO (1999) Effects of methylphenidate analogues on phenethylamine substrates for the striatal dopamine transporter: potential as amphetamine antagonists? J Neurochem 72 1266-1274. [DOI] [PubMed] [Google Scholar]
- Zhu WH, Conforti L, and Millhorn DE (1997) Expression of dopamine D2 receptor in PC-12 cells and regulation of membrane conductances by dopamine. Am J Physiol 273 C1143-C1150. [DOI] [PubMed] [Google Scholar]