Abstract
Previous research shows that nicotine increases dopamine (DA) clearance in rat prefrontal cortex (PFC) and striatum via a nicotinic receptor (nAChR)-mediated mechanism. The present study investigated whether activation of nAChRs regulates DA transporter (DAT) function through a trafficking-dependent mechanism. After nicotine administration (0, 0.3, and 0.8 mg/kg s.c., 15-1440 min after injection), DAT function and trafficking in synaptosomes of PFC and striatum were determined. nAChR mediation of the effect of nicotine on DAT function and trafficking in PFC was determined by pretreatment with mecamylamine, dihydro-β-erythroidine, or methyllycaconitine. Nicotine (0.8 mg/kg, 15 and 30 min after injection) increased the maximal velocity (Vmax) of [3H]DA uptake in PFC with no change in Km, compared with control. Biotinylation and Western blot assays showed that nicotine (0.8 mg/kg; 30 min) increased DAT cell surface expression in PFC. In contrast, a lower dose of nicotine (0.3 mg/kg; 30 min) did not alter DAT function and trafficking in PFC. Pretreatment with mecamylamine, dihydro-β-erythroidine, or methyllycaconitine (1.5, 8.0, and 10.0 mg/kg s.c., respectively) completely blocked the nicotine-induced increase in Vmax in PFC. In addition, mecamylamine completely blocked the nicotine-induced increase in DAT cell surface expression in PFC. Nicotine did not increase DAT function and cell surface expression in striatum, indicating that nicotine modulates DAT function in a brain region-specific manner. Thus, results from the present study suggest that the nicotine-induced increases in DAT function and cell surface expression in PFC may mediate some of the behavioral effects of nicotine.
Nicotine, a psychostimulant and the major alkaloidal constituent in tobacco, is believed to be responsible for the reward associated with tobacco use. Nicotine stimulates dopamine (DA) release from its presynaptic terminals by acting as an agonist at nicotinic acetylcholine receptors (nAChRs) located on dopaminergic cell bodies and terminals in both the mesocorticolimbic and nigrostriatal systems (Clarke and Pert, 1985). nAChRs are composed of α (α2-α10) and β (β2-β4) subunits, which assemble into pentameric structures (Anand et al., 1991); however, only α3 to α7 and β2 to β4 subunits are expressed in DA neurons (Klink et al., 2001). The exact composition of nAChR subtypes mediating nicotine-evoked DA release is controversial, although α4β2-, α6β2-, α4α6β2-, α4α6β2β3-, α6β2β3-, and α4α5β2-containing nAChRs may be involved (Salminen et al., 2004, 2007; Scholze et al., 2007).
Extracellular DA concentrations are the net result of release (exocytosis) and clearance (uptake) from the extracellular space. Uptake through the plasma membrane DA transporter (DAT) is the primary mechanism for regulation of extracellular DA concentration and, thus, the most effective means of terminating DA actions at postsynaptic and presynaptic receptors (Gainetdinov and Caron, 2003). Acute administration of nicotine has been shown to increase DAT function (i.e., enhance DA clearance) in striatum, nucleus accumbens (NAc), and PFC via an nAChR-mediated mechanism (Hart and Ksir, 1996; Middleton et al., 2004). Nicotine-induced increases in DA clearance would result in decreases in extracellular DA concentrations, thus tending to compensate for the nicotine-induced enhancement of DA release.
The clearance efficiency of DAT is governed by the transport rate of individual transporters, the number of transporters on the neuronal cell surface, and the modulation of cell surface transporter expression (Sorkina et al., 2005). DAT cell surface expression and activity are regulated by multiple receptor signal transduction pathways and interactions with cytosolic proteins (Bjerggaard et al., 2004; Melikian, 2004). In addition, DAT trafficking is regulated by substrates and inhibitors, i.e., exposure to DA or amphetamine decreases DAT cell surface expression (Chi and Reith, 2003; Kahlig et al., 2004), whereas cocaine exposure increases DAT cell surface expression in rat NAc (Daws et al., 2002). Thus, the shuttling of DAT protein between intracellular compartments and the plasma membrane is regulated by a number of different mechanisms.
Different mechanisms also probably underlie the effects of various psychostimulants on DAT function and trafficking. For example, in vivo voltammetry studies show that cocaine and amphetamine decrease DAT function (Zahniser et al., 1999), whereas nicotine increases DAT function (Hart and Ksir, 1996; Middleton et al., 2004). nAChR modulation of DAT function also is supported by results showing that nAChR stimulation augments amphetamine-induced reverse transport of DA by DAT in rat PFC slices, but not in striatum (Drew et al., 2000). Thus, in addition to stimulating DA release from presynaptic terminals, nAChRs modulate DAT function to regulate extracellular DA concentration.
Although nicotine has been shown to increase DAT function, the molecular mechanisms underlying this effect have not been elucidated. Moreover, previous results show that in vitro exposure to nicotine does not alter [3H]DA uptake into striatal synaptosomes (Carr et al., 1989; Zhu et al., 2003), suggesting that nicotine does not interact directly with the transporter to augment function. The current study determined whether the effect of nicotine to enhance DAT function is mediated by alterations in DAT trafficking. Thus, effects of acute systemic nicotine administration on synaptosomal [3H]DA uptake and DAT trafficking in PFC and striatum were determined. To determine the involvement of nAChRs, the ability of nAChR antagonists to inhibit the effect of nicotine on [3H]DA uptake and DAT trafficking in PFC also was investigated.
Materials and Methods
Materials. Antibodies recognizing rat DAT (C-20; goat polyclonal antibody) and protein phosphatase 2A (PP2A; sc-13601; mouse monoclonal antibody) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-Syntaxin 1A antibody (HPC-1; mouse monoclonal antibody), β-actin (A 5441, mouse monoclonal antibody), and anti-mouse IgG horseradish peroxidase (HRP) were purchased from Sigma-Aldrich (St. Louis, MO). Anti-goat IgG HRP and anti-mouse IgG HRP were purchased from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA). Sulfosuccinimidobiotin (sulfo-NHS-biotin) and immunoPure immobilized monomeric avidin gel were purchased from Pierce Chemical (Rockford, IL). Paroxetine hydrochloride was a generous gift from Beecham Pharmaceuticals (Surrey, UK). Desipramine hydrochloride, nomifensine maleate, S(-)-nicotine ditartrate (nicotine), mecamylamine, methyllycaconitine (MLA), dihydro-β-erythroidine (DHβE), pargyline, and ascorbic acid were purchased from Sigma-Aldrich. d-Glucose was purchased from Aldrich Chemical Co. (Milwaukee, WI). [3H]DA (3,4-ethyl-2 [N-3H] dihydroxyphenylethylamine; specific activity, 31 Ci/mmol) and [3H]WIN 35,428 (85 Ci/mmol) were purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA). All other chemicals were purchased from Thermo Fisher Scientific (Waltham, MA).
Subjects. Adult male, Sprague-Dawley rats (body weight, 200-225 g) were obtained from Harlan (Indianapolis, IN) and were housed in standard polyurethane cages with free access to food and water. The colony room was maintained at 24°C and 45% humidity, with lights on from 7:00 A.M. to 12:00 PM. The experimental procedures were approved by the University of Kentucky Institutional Animal Care and Use Committee and conformed to the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Drug Administration. For dose-response analysis, rats were administered nicotine (0.3 and 0.8 mg/kg freebase s.c.) or saline (1 ml/kg s.c.), and 30 min later, brain regions (PFC and striatum) were obtained. Doses of nicotine were chosen based on our previous reports showing dose-related effects of nicotine on DAT function using in vivo voltammetry (Middleton et al., 2004). In the time course experiments in the current study, rats were administrated nicotine (0.8 mg/kg s.c.) or saline, and brain regions (PFC and striatum) were obtained at 15, 30, 45, 60, and 1440 min after injection. A previous study (de Fiebre et al., 1991) reported that the high dose of nicotine (0.8 mg/kg) used in the current study produced seizures. However, nicotine-induced seizures were not observed during the 24-h observation period in the current study. Differences are noted between the current study, in which male Sprague-Dawley rats were administered nicotine subcutaneously, and that of de Fiebre et al. (1991), in which inbred lines of alcohol-sensitive female rats were used, and nicotine was administered intraperitoneally. Thus, differences in strain, sex, and route of administration may be responsible for the differential sensitivity to nicotine with respect to seizure induction.
To determine whether nAChRs mediate the effect of nicotine to enhance DAT function, rats were administered mecamylamine (1.5 mg/kg s.c., salt weight) or saline 20 min before nicotine (0.8 mg/kg s.c.). The dose of mecamylamine was chosen based on our previous in vivo voltammetry studies, in which this dose completely blocked the effect of nicotine on DA clearance in medial prefrontal cortex (mPFC) and striatum (Middleton et al., 2004). In the current study, rats were randomly assigned to four treatment groups (saline/saline, saline/nicotine, mecamylamine/saline, and mecamylamine/nicotine). To further determine the nAChR subtype(s) mediating the nicotine-induced enhancement of DAT function, in separate experiments, rats were pretreated 15 min before nicotine (0.8 mg/kg s.c.) or saline administration with DHβE (8.0 mg/kg salt weight s.c.), an antagonist at β2-containing nAChRs, or with MLA (10.0 mg/kg salt weight s.c.), an antagonist at α7-containing nAChRs, using the same design as described for the mecamylamine antagonism study. DHβE and MLA doses were chosen based on previous studies showing antagonist-induced decreases in i.v. nicotine self-administration (Watkins et al., 1999; Markou and Paterson, 2001). All drugs were dissolved in saline and prepared immediately before injection; pH was adjusted to 7.0 with 1 M NaOH.
[3H]DA Uptake Assay. To determine the effect of nicotine administration on DAT function in PFC and striatum, the kinetic parameters (Vmax and Km) of synaptosomal [3H]DA uptake were determined using a previously published method (Zhu et al., 2004). Brain regions from each rat were homogenized immediately in 20 ml of ice-cold sucrose solution (0.32 M sucrose and 5 mM sodium bicarbonate, pH 7.4) with 16 passes of a Teflon pestle homogenizer. Homogenates were centrifuged at 2000g for 10 min at 4°C, and resulting supernatants were centrifuged at 20,000g for 15 min at 4°C. Resulting pellets from PFC and striatum were resuspended in 4.8 and 3.0 ml, respectively, of ice-cold assay buffer (125 mM NaCl, 5 mM KCl, 1.5 mM MgSO4, 1.25 mM CaCl2, 1.5 mM KH2PO4, 10 mM glucose, 25 mM HEPES, 0.1 mM EDTA, 0.1 mM pargyline, and 0.1 mM l-ascorbic acid, saturated with 95% O2/5% CO2, pH 7.4). Nonspecific [3H]DA uptake in PFC and striatum was determined in the presence of 10 μM nomifensine.
Because DA is transported by DAT, the norepinephrine transporter (NET), and the serotonin transporter (SERT) in PFC (Morón et al., 2002; Williams and Steketee, 2004), kinetic analysis of [3H]DA uptake by DAT in the PFC was assessed in the presence of desipramine (1 μM) and paroxetine (5 nM) to prevent [3H]DA uptake into norepinephrine- and serotonin-containing nerve terminals, respectively, thereby isolating uptake of DA into DAT (Zhu et al., 2004). Desipramine is a potent reuptake inhibitor at NET with an IC50 value of 1.2 nM, whereas desipramine has a lower potency inhibiting DA uptake at DAT (IC50 = 9.3 μM; Roubert et al., 2001). At 1 μM concentration, desipramine does not exhibit an inhibitory effect on [3H]DA uptake into rat striatal synaptosomes, which are rich in DAT protein relative to PFC (Zhu et al., 2004). Paroxetine is a potent serotonin reuptake inhibitor (IC50 = 0.3 nM) that has low potency for inhibiting DA uptake at DAT (IC50 = 50 μM; Nemeroff and Owens, 2003; Norrholm et al., 2007). In the current study, the potential role of NET in DA uptake in PFC from rats administered nicotine or saline was determined. Kinetic analysis of [3H]DA uptake by NET was assessed in the presence of GBR 12909 (50 nM) and paroxetine (5 nM) to prevent [3H]DA uptake into DA- and serotonin-containing nerve terminals, respectively, thereby isolating uptake of DA into NET. For these experiments, nonspecific uptake of [3H]DA into NET was determined in the presence of 1 μM desipramine.
PFC and striatal synaptosomes containing ∼150 μg protein/100 μl and ∼50 μg protein/30 μl, respectively, were incubated in a metabolic shaker for 5 min at 34°C and then incubated for 10 min at 34°C after adding one of 10 [3H]DA concentrations (0.1 nM-5 μM) in a 500-μl total volume. To determine the effect of in vitro nicotine exposure on synaptosomal [3H]DA uptake in PFC, synaptosomes were incubated with nicotine (1 nM-10 μM) for 5 min, and then 0.1 μM [3H]DA (final concentration, in 50 μl) was added, and incubation continued for 10 min.
Incubation was terminated by the addition of 3 ml of ice-cold assay buffer, followed by immediate filtration through Whatman GF/B glass fiber filters (presoaked with 1 mM pyrocatechol for 3 h). Filters were washed three times with 3 ml of ice-cold buffer containing pyrocatechol using a Brandel cell harvester (model MP-43RS; Brandel Inc., Gaithersburg, MD). Radioactivity was determined by liquid scintillation spectrometry (model B1600TR; PerkinElmer Life and Analytical Sciences). Protein concentrations were determined, with bovine serum albumin as the standard. Kinetic parameters (Vmax and Km) were determined using the commercially available GraphPad Prism 4.0 program (GraphPad Software Inc., San Diego, CA).
[3H]WIN 35,428 Binding Assay. Brain regions were homogenized in 80 volumes (w/v) of ice-cold sodium-phosphate buffer (2.1 mM NaH2PO4, 7.3 mM Na2HPO4·7H2O, 320 mM sucrose, pH 7.4) with seven passes of a Teflon pestle homogenizer. Homogenates were centrifuged at 40,000g for 20 min at 4°C, and resulting pellets were washed twice by resuspension in ice-cold buffer and centrifuged at 40,000g at 4°C for 20 min. Final pellets from PFC and striatum were resuspended in fresh buffer at a concentration of 10 mg/ml original wet weight and homogenized using a Polytron homogenizer (Brinkmann Instruments, Westbury, NY; setting 5 for 15 s). Saturation binding assays were conducted in duplicate in a final volume of 250 μl for PFC and 500 μl for striatum, containing membrane homogenate (185-200 μg protein/200 μl and 35-40 μg/100 μl for PFC and striatum, respectively). Samples were incubated with seven concentrations of [3H]WIN 35,428 (0.1-30 nM) on ice for 2 h. Nonspecific binding was determined in the presence of 10 μM cocaine. Assays were terminated by rapid filtration onto Whatman GF/B glass fiber filters, presoaked for 2 h with assay buffer containing 0.5% polyethylenimine. Filters were washed three times with 4 ml of ice-cold assay buffer. Radioactivity remaining on the filters was determined using the liquid scintillation spectrometry (model B1600TR; PerkinElmer Life and Analytical Sciences).
Biotinylation and Western Blot Assay. Biotinylation assays were performed as described previously (Zhu et al., 2005). Synaptosomes from PFC (2500 μg protein/sample) and striatum (500 μg protein/sample) were incubated for 1 h at 4 °C with sulfo-NHS-biotin and continual shaking in 500 μl of 1.5 mg/ml sulfo-NHS-biotin in PBS/Ca/Mg buffer (138 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4, 9.6 mM Na2HPO4, 1 mM MgCl2, 0.1 mM CaCl2, pH 7.3). After incubation, samples were centrifuged at 8000g for 4 min at 4°C. To remove the free sulfo-NHS-biotin, the resulting pellets were resuspended and centrifuged three times with 1 ml of ice-cold 100 mM glycine in PBS/Ca/Mg buffer and centrifuged at 8000g for 4 min at 4°C. Final pellets were resuspended in 1 ml of ice-cold 100 mM glycine in PBS/Ca/Mg buffer and incubated with continual shaking for 30 min at 4°C. Samples were centrifuged subsequently at 8000g for 4 min at 4°C, the resulting pellets were resuspended in 1 ml of ice-cold PBS/Ca/Mg buffer, and the resuspension and centrifugation step was repeated twice. Final pellets were lysed by sonication for 2 to 4 s in 300 μl of Triton X-100 buffer (10 mM Tris, 150 mM NaCl, 1 mM EDTA, 1.0% Triton X-100, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μM pepstatin, 250 μM phenylmethysulfonyl fluoride, pH 7.4), followed by incubation and continual shaking for 20 min at 4°C. Lysates (300 μl) were centrifuged at 21,000g for 20 min at 4°C. Pellets were discarded, and 100 μl of the supernatants was stored at -20°C for determination of immunoreactive DAT in the total synaptosomal fraction. Remaining supernatants were incubated with continuous shaking in the presence of monomeric avidin beads in Triton X-100 buffer (100 μl/tube) for 1 h at room temperature. Samples were centrifuged subsequently at 17,000 g for 4 min at 4°C, and supernatants (containing the nonbiotinylated, intracellular protein fraction) were stored at -20°C. The efficiency of avidin to isolate the biotinylated and nonbiotinylated proteins across the protein range in the synaptosomal extract was verified using the avidin-conjugated antibody. The avidin-conjugated antibody showed that biotinylated proteins were completely absorbed by the avidin and were not present in the supernatant. Resulting pellets containing the avidin-absorbed biotinylated proteins (cell-surface fraction) were resuspended in 1 ml of 1.0% Triton X-100 buffer and centrifuged at 17,000g for 4 min at 4°C, and pellets were resuspended and centrifuged twice. Final pellets consisted of the biotinylated proteins adsorbed to monomeric avidin beads. Biotinylated proteins were eluted by incubating with 50 μl of Laemmli buffer (62.5 mM Tris-HCl, 20% glycerol, 2% SDS, 0.05% β-mercaptoethanol, and 0.05% bromphenol blue, pH 6.8) for 20 min at room temperature. Samples were stored at -20°C.
To obtain immunoreactive DAT protein in total synaptosomal, intracellular, and cell surface fractions, samples were thawed and subjected to gel electrophoresis and Western blotting. Proteins were separated by 10% SDS-polyacrylamide gel electrophoresis for 90 min at 150 V and transferred to Immobilon-P transfer membranes (0.45-μm pore size; Millipore Corporation, Billerica, MA) in transfer buffer (50 mM Tris, 250 mM glycine, 3.5 mM SDS) using a Mini Trans-Blot Electrophoretic Transfer Cell (Bio-Rad, Hercules, CA) for 110 min at 72 V. Transfer membranes were incubated with blocking buffer (5% dry milk powder in PBS containing 0.5% Tween 20) for 1 h at room temperature, followed by incubation with goat polyclonal DAT antibody (1 μg/ml in blocking buffer) overnight at 4°C. Transfer membranes were washed five times with wash buffer (PBS containing 0.5% Tween 20) at room temperature and then incubated with rabbit anti-goat DAT antibody (1:2500 dilution in blocking buffer) for 1 h at 22°C. Blots on transfer membranes were detected using enhanced chemiluminescence and developed on Hyperfilm (ECL-plus; Amersham Bioscience UK Ltd., Chalfont St. Giles, UK). After detection and quantification of DAT protein, each blot was stripped in 10% Re-blot plus mild antibody stripping solution (Chemicon, Temecula, CA) for 20 min at room temperature and reprobed for detection of PP2A, syntaxin 1A, and β-actin.
PP2A, β-actin, and syntaxin 1A were used as control proteins to monitor differences in the intactness of the sample preparation between nicotine- and saline-treated groups and to monitor protein loading between samples. PP2A and β-actin are predominantly located in the intracellular compartment, and syntaxin 1A is a plasma membrane protein. Because of the potential presence of broken membrane fragments and leaky synaptosomes in crude synaptosomal preparations, the membrane-impermeant biotinylation reagent was expected to produce some degree of biotinylation of the control proteins associated with the intracellular compartment. Hence, these proteins were monitored mainly to report differences in the intactness of the synaptosomal preparations between the nicotine- and saline-treated groups. PP2A, β-actin, and syntaxin 1A were determined using mouse monoclonal PP2A antibody (1:1000 dilution in blocking buffer), mouse monoclonal syntaxin 1A antibody (1:5000 dilution in blocking buffer), and mouse monoclonal β-actin antibody (1:1000 dilution in blocking buffer), respectively. Multiple autoradiographs were obtained using different exposure times, and immunoreactive bands within the linear range of detection were quantified by densitometric scanning using Scion image software (Scion Corporation, Frederick, MD). Band density measurements, expressed as relative optical density, were used to determine levels of DAT in the total synaptosomal fraction, the intracellular fraction (nonbiotinylated), and the cell surface fraction (biotinylated).
Data Analysis. Data are presented as mean ± S.E.M., and n represents the number of independent experiments for each treatment group. To analyze the kinetic parameters, Vmax and Km, for [3H]DA uptake and the parameters, Bmax and Kd, for [3H]WIN 34,428 binding in nicotine-treated and saline-control groups, separate unpaired Student's t tests were performed. Log-transformed Km and Kd values were used for statistical analyses. To determine the dose-related effect of nicotine on [3H]DA uptake, one-way ANOVA was conducted on Vmax values and on log-transformed Km values from PFC and striatum. To determine the time-related effect of nicotine in PFC, a one-way ANOVA also was performed using Vmax values for [3H]DA uptake. To determine the effect of nicotine on DAT distribution, separate one-way ANOVAs were conducted on DAT immunoreactivity for each subcellular fraction [total synaptosomal fraction, intracellular fraction (nonbiotinylated) and cell surface fraction (biotinylated)]. To determine the ability of each nAChR antagonist (mecamylamine, DHβE, or MLA) to inhibit effects of nicotine on [3H]DA uptake and on DAT subcellular distribution, separate two-way ANOVAs were performed with two between-group factors (antagonist treatment and nicotine treatment). Simple effect comparisons (unpaired Student's t test) were made for post hoc analyses. All statistical analyses were performed using SPSS (standard version 14.0; SPSS Inc., Chicago, IL), and differences were considered significant at p < 0.05.
Results
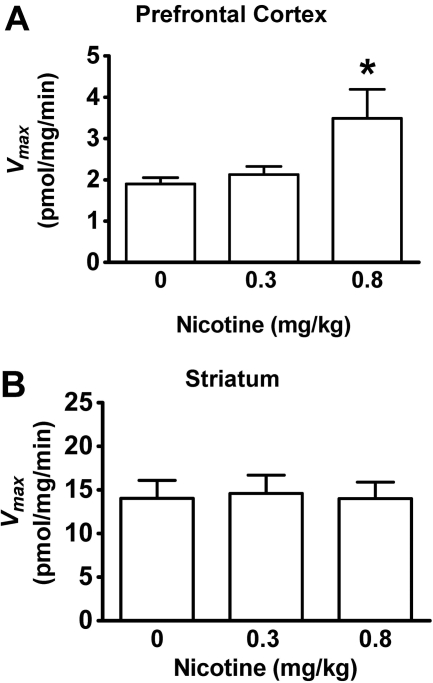
Effect of Acute Nicotine Administration on Synaptosomal [3H]DA Uptake in PFC and Striatum. To determine the effect of systemic administration of nicotine on [3H]DA uptake into dopaminergic terminals in the PFC, kinetic analyses of synaptosomal [3H]DA uptake were performed in the presence of specific inhibitors of NET and SERT, i.e., desipramine and paroxetine, respectively. The dose-related effects of nicotine (0, 0.3, and 0.8 mg/kg) on Vmax and Km values for [3H]DA uptake in PFC and striatum initially were determined 30 min after injection. One-way ANOVAs revealed significant differences only for Vmax in PFC (F2,23 = 5.13, p < 0.05). The low nicotine dose (0.3 mg/kg) did not alter either Vmax (Fig. 1) or Km (PFC: nicotine, 23.1 ± 2.7 nM and saline, 21.0 ± 1.9 nM; striatum: nicotine, 28.2 ± 3.9 nM and saline, 26.0 ± 4.5 nM). The current results from striatum with the low dose of nicotine are different from those reported previously (Middleton et al., 2007), in which nicotine (0.32 mg/kg s.c.) produced a small increase (23%) in Vmax in striatum (for explanation of the discrepancy in the results, see Discussion). In the current study, a higher dose of nicotine (0.8 mg/kg) also did not alter Vmax (Fig. 1B) or Km (nicotine, 18.9 ± 2.4 nM and saline, 16.6 ± 3.9 nM) in striatum. However, the higher nicotine dose (0.8 mg/kg) resulted in an 83 ± 15% increase in Vmax in PFC compared with the saline control (Fig. 1A), with no change in Km (nicotine, 14.4 ± 1.5 nM and saline, 12.4 ± 2.4 nM). Therefore, nicotine dose-dependently increased the maximal velocity of DA uptake in PFC, but not in striatum.
Fig. 1.
Nicotine dose-dependently increased the Vmax of [3H]DA uptake in the prefrontal cortex, but not in the striatum. Nicotine (0, 0.3, and 0.8 mg/kg s.c.) was administered, and brain regions were obtained 30 min later. Data from two independent control groups administered saline contemporaneously with each nicotine dose were pooled for graphical presentation. Kinetic analysis of synaptosomal [3H]DA uptake was determined in the presence of desipramine (1 μM) and paroxetine (5 nM). Vmax values (picomoles per milligram per minute) in the prefrontal cortex (A) and striatum (B) are expressed as mean ± S.E.M. Both brain regions were obtained from each subject (n = 7 rats/treatment group). *, p < 0.05 different from saline-control group.
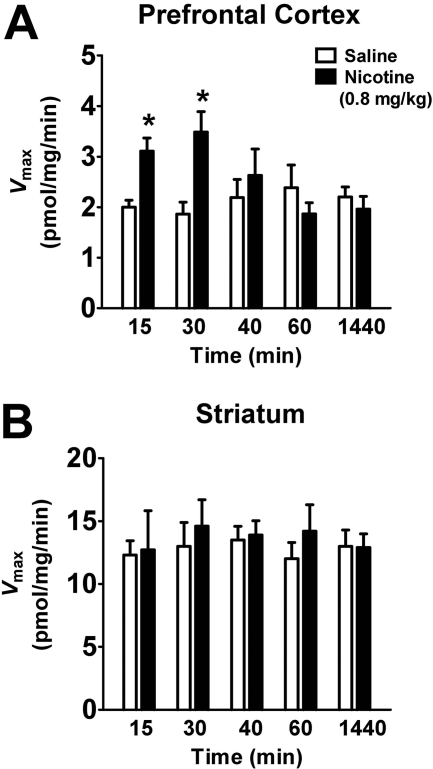
To determine whether the nicotine-induced increase in [3H]DA uptake in PFC was time-dependent, the Vmax and Km of [3H]DA uptake were determined 15 to 1440 min after nicotine (0.8 mg/kg) or saline injection. At 15 and 30 min, Vmax from the nicotine-treated group was increased (55 ± 2.1% and 87 ± 9.2%, respectively) compared with the respective saline-control group [15 min, t(12) = 2.3, p < 0.05; 30 min, t(12) = 2.2; p < 0.05; unpaired Student's t test, Fig. 2A], with no change in Km at any time point (data not shown). However, nicotine (0.8 mg/kg) did not alter Vmax (Fig. 2B) or Km (data not shown) in striatum at any time point evaluated.
Fig. 2.
In a time-dependent manner, nicotine increased the Vmax of [3H]DA uptake in the prefrontal cortex, but not in the striatum. Rats received nicotine (0.8 mg/kg s.c.) or saline, and brain regions were obtained at various times (15-1440 min) after injection. Kinetic analysis of synaptosomal [3H]DA uptake in prefrontal cortex (A) was determined in the presence of desipramine (1 μM) and paroxetine (5 nM) and in the absence of these uptake inhibitors in striatum (B). Vmax values (picomoles per milligram per minute) are expressed as mean ± S.E.M. Both brain regions were obtained from each subject (n = 7 rats/treatment group). *, p < 0.05, different from the respective saline-control group.
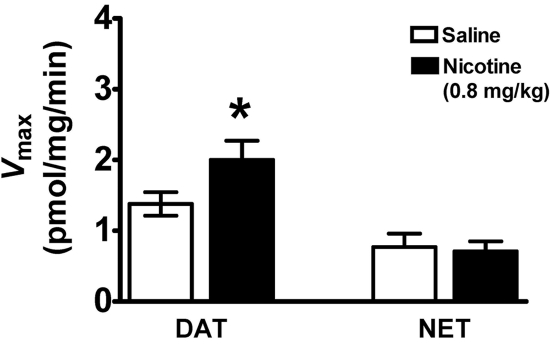
To determine the role of NET in DA uptake in PFC after nicotine or saline injection, [3H]DA uptake assays were conducted in the presence of desipramine (1 μM) plus paroxetine (5 nM) or GBR 12909 (50 nM) plus paroxetine (5 nM); paroxetine was used to inhibit SERT. Figure 3 shows that nicotine (0.8 mg/kg s.c., 30 min after injection) increased (31 ± 1.7%) the Vmax of [3H]DA uptake under conditions in which NET and SERT (i.e., desipramine plus paroxetine) were inhibited [t(12) = 2.6, p < 0.05, unpaired Student's t test], with no change in Km (nicotine, 21.2 ± 5.0 nM; saline, 18.7 ± 4.1 nM). In addition, nicotine did not alter Vmax when both DAT and SERT (i.e., GBR 12909 plus paroxetine) were inhibited (Fig. 3), with no change in Km (nicotine, 60.2 ± 5.2 nM; saline, 55.4 ± 3.1 nM). To determine the effect of nicotine exposure in vitro on [3H]DA uptake in PFC synaptosomes, [3H]DA uptake assays were conducted during exposure to a range of nicotine concentrations (1 nM-10 μM). Results revealed that in vitro exposure to nicotine did not alter [3H]DA uptake in PFC (data not shown).
Fig. 3.
Nicotine increased the Vmax of [3H]DA uptake via DAT, but not via NET, in prefrontal cortex. Rats were injected with nicotine (0.8 mg/kg s.c.) or saline, and prefrontal cortex was obtained 30 min later. To assess DAT and NET function simultaneously, synaptosomes from two rats treated either with nicotine or saline were pooled, and one half of the pooled sample was used for the kinetic analysis of [3H]DA uptake via each transporter. [3H]DA uptake via DAT was determined in the presence of 1 μM desipramine and 5 nM paroxetine. [3H]DA uptake via NET was determined in the presence of 50 nM GBR 12909 and 5 nM paroxetine. Vmax values (picomoles per milligram per minute) are expressed as mean ± S.E.M. n = 7 Independent experiments using pooled prefrontal cortex from each treatment group. *, p < 0.05, different from saline control.
Effect of Acute Nicotine Administration on DAT Subcellular Distribution in PFC and Striatum. To determine whether the nicotine-induced increase in Vmax for [3H]DA uptake in PFC was associated with an alteration in DAT protein levels, saturation analysis of [3H]WIN 35,428 binding was performed to determine the maximal number (Bmax) of DAT binding sites and affinity (Kd) in PFC and striatum 30 min after nicotine (0.8 mg/kg s.c.) or saline injection. No differences in Bmax and Kd between nicotine-treated and saline-control groups were found in either PFC or striatum (Table 1), suggesting that the nicotine-induced increase in Vmax in PFC was not accompanied by a change in DAT protein levels.
TABLE 1.
Nicotine (0.8 mg/kg s.c.) did not alter Bmax and Kd values for [3H]WIN 35,428 binding in prefrontal cortex and striatum
Bmax and Kd data are presented as mean ± S.E.M. for n = 5 independent experiments/region/treatment group.
|
Bmax
|
Kd
|
|||
|---|---|---|---|---|
| Saline | Nicotine | Saline | Nicotine | |
| fmol/mg protein | nM | |||
| PFC | 734 ± 64 | 828 ± 57 | 15.9 ± 2.0 | 17.2 ± 1.1 |
| Striatum | 1190 ± 165 | 1290 ± 110 | 69.0 ± 8.9 | 64.0 ± 7.6 |
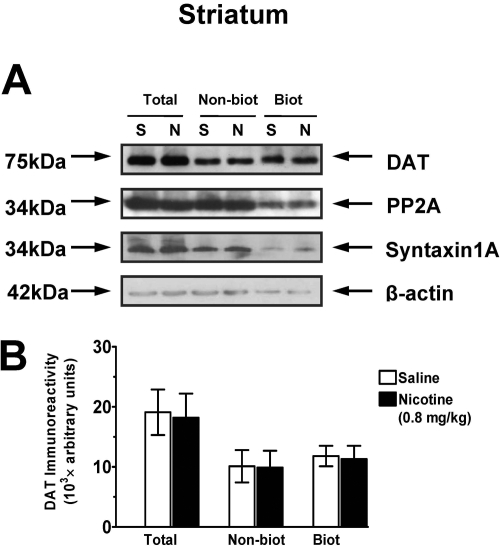
To determine whether the systemic nicotine-induced increase in Vmax for [3H]DA uptake in PFC was associated with an alteration of the subcellular distribution of DAT, biotinylation and immunoblot assays were performed to assess cell surface and intracellular DAT localization. Three subcellular fractions were prepared from rat PFC and striatum 30 min after nicotine (0, 0.3, and 0.8 mg/kg s.c.). The low dose (0.3 mg/kg) of nicotine did not alter DAT immunoreactivity in the total synaptosomal fraction, the intracellular fraction (nonbiotinylated), or cell surface fraction (biotinylated) of PFC compared with the saline-control group (data not shown). Figure 4 shows that the higher dose (0.8 mg/kg) of nicotine did not alter total synaptosomal DAT immunoreactivity; however, an increase (32 ± 2.5%) in DAT immunoreactivity in the cell surface fraction and a decrease (34 ± 3.1%) in the intracellular fraction were found in the nicotine-treated group compared with the saline-control group. In the striatum, neither the low dose nor the high dose of nicotine altered DAT immunoreactivity in the total synaptosomal fraction, intracellular fraction, or the cell surface fraction relative to the saline-control group (Fig. 5). There was no significant difference between nicotine- and saline-treated groups in the levels of control proteins, PP2A, β-actin, and syntaxin 1A in the total synaptosomal fraction, intracellular fraction (nonbiotinylated), and cell surface fraction (biotinylated). These results suggest that the nicotine-induced increase in Vmax in PFC may be the result of DAT trafficking from the intracellular pool to the cell surface of DA terminals in the PFC.
Fig. 4.
Nicotine dose-dependently increased cell surface DAT expression in prefrontal cortex 30 min after injection. A, representative immunoblots of total synaptosomal fraction (Total), intracellular fraction (nonbiotinylated, Nonbiot), and cell surface fraction (biotinylated, Biot) from prefrontal cortex from nicotine-treated (N; 0.8 mg/kg) and saline-control (S) groups. PP2A, β-actin, and syntaxin 1A were used as control proteins (see Materials and Methods). B, DAT immunoreactivity expressed as mean ± S.E.M. densitometry units, n = 6 rats/group. *, p < 0.05, different from respective saline-control group.
Fig. 5.
Nicotine did not alter cell surface DAT expression in striatum 30 min after injection. A, representative immunoblots of total synaptosomal fraction (Total), intracellular fraction (nonbiotinylated, Nonbiot), and cell surface fraction (biotinylated, Biot) from striatum from nicotine-treated (N, 0.8 mg/kg) and saline-control (S) groups. PP2A, β-actin, and syntaxin 1A were used as control proteins (see Materials and Methods). B, DAT immunoreactivity expressed as mean ± S.E.M. densitometry units, n = 6 rats/group.
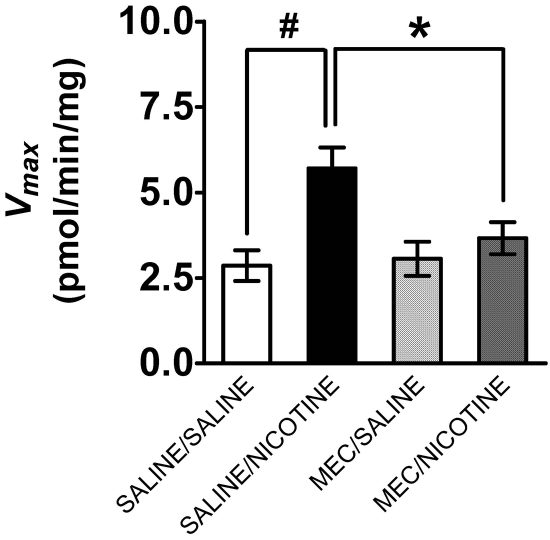
Effect of nAChR Antagonists on Nicotine-Induced Changes in [3H]DA Uptake and DAT Cell Surface Expression in PFC. To determine whether the nicotine-induced increase in DAT function and DAT cell surface expression are mediated by nAChRs, the ability of mecamylamine to inhibit these effects was determined. Groups of rats were pretreated with mecamylamine (1.5 mg/kg s.c.) or saline and 20 min later treated with nicotine (0.8 mg/kg s.c.) or saline. Thirty minutes after the last injection, PFC was obtained for saturation analysis of [3H]DA uptake or for biotinylation and Western blot assays. Two-way ANOVA revealed that the main effect of mecamylamine on Vmax was not significant (F1,28 = 3.5, p > 0.05), whereas the main effect of nicotine was significant (F1,28 = 13.3, p < 0.05). Moreover, a significant mecamylamine × nicotine interaction (F1,28 = 8.52, p < 0.05) was observed (Fig. 6). Comparison of the saline/nicotine group with the saline/saline group revealed a 2-fold increase in Vmax [t(14) = 3.7, p < 0.05, unpaired Student's t test]. Vmax for the mecamylamine/nicotine group was decreased compared with the saline/nicotine [t(14) = 2.3; p < 0.05, unpaired Student's t test], indicating that mecamylamine inhibited the effect of nicotine on DAT function (Fig. 6). No differences in Km among the treatment groups were found (data not shown).
Fig. 6.
Mecamylamine inhibited the nicotine-induced increase in Vmax for [3H]DA uptake in prefrontal cortex. Rats were pretreated with mecamylamine (MEC; 1.5 mg/kg s.c.) or saline 20 min before nicotine (0.8 mg/kg s.c.) or saline injection. Synaptosomes were prepared 30 min after nicotine or saline injection. [3H]DA uptake was determined in the presence of desipramine (1 μM) and paroxetine (5 nM) to prevent [3H]DA uptake into norepinephrine- and serotonin-containing nerve terminals, respectively. Data are expressed as picomoles per milligram per minute (mean ± S.E.M.; n = 8 rats/treatment group). #, p < 0.05, different from saline/saline group. *, p < 0.05, different from saline/nicotine group.
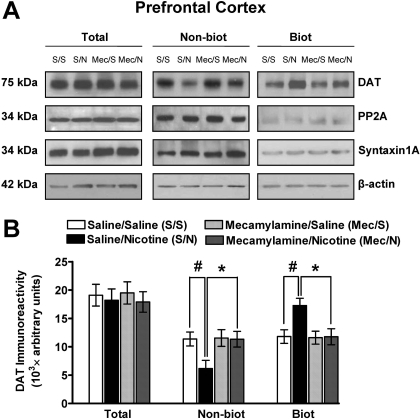
To determine whether nAChRs mediate the nicotine-induced increase in DAT cell surface expression in PFC, the ability of mecamylamine (1.5 mg/kg) to inhibit this effect of nicotine (0.8 mg/kg) was determined (Fig. 7). Again, DAT immunoreactivity in the total synaptosomal fraction was not altered by any treatment condition. Two-way ANOVA revealed that the main effect of mecamylamine on DAT immunoreactivity in the cell surface fraction was not significant (F1,24 = 1.2, p > 0.05), whereas the main effect of nicotine was significant (F1,24 = 4.0, p < 0.05). Moreover, a significant mecamylamine × nicotine interaction for DAT immunoreactivity in the cell surface fraction was found (biotinylated, F1,24 = 4.43, p < 0.05). DAT immunoreactivity in the cell surface fraction was increased (46 ± 1.4%) in the saline/nicotine group compared with the saline/saline group [t(12) = 3.0, p < 0.05, unpaired Student's t test]. DAT immunoreactivity in the cell surface fraction from the mecamylamine/nicotine group was lower (41 ± 1.6%) than that in the saline/nicotine group [t(12) = 3.1, p < 0.05, unpaired Student's t test]. In addition, no main effect of mecamylamine on DAT immunoreactivity in the intracellular fraction was found (F1,24 = 2.2, p > 0.05), whereas the main effect of nicotine was significant (F1,24 = 3.5, p < 0.05). Moreover, a significant mecamylamine × nicotine interaction for DAT immunoreactivity in the intracellular fraction (nonbiotinylated, F1,24 = 4.47, p < 0.05) was found. DAT immunoreactivity in the intracellular fraction was decreased (45 ± 2.1%) in the saline/nicotine group compared with the saline/saline group [t(12) = 4.0, p < 0.05, unpaired Student's t test]. DAT immunoreactivity in the intracellular fraction in the mecamylamine/nicotine group was greater (42 ± 1.9%) than that in the saline/nicotine group [t(12) = 3.5, p < 0.05, unpaired Student's t test]. There were no significant differences between nicotine- and saline-treated groups in the levels of control proteins, PP2A, β-actin, and syntaxin 1A in the total synaptosomal fraction, intracellular fraction (nonbiotinylated), and cell surface fraction (biotinylated).
Fig. 7.
Mecamylamine inhibited the nicotine-induced increase in DAT cell surface expression in prefrontal cortex. Rats were pretreated with mecamylamine (Mec; 1.5 mg/kg s.c.) or saline (S) 20 min before nicotine (N; 0.8 mg/kg s.c.) or saline injection. A, representative immunoblots of total synaptosomal fraction (Total), intracellular fraction (nonbiotinylated, Nonbiot), and cell surface fraction (biotinylated, Biot) from prefrontal cortex from the four treatment groups. PP2A, β-actin, and syntaxin 1A were used as control proteins (see Materials and Methods). B, DAT immunoreactivity is expressed as mean ± S.E.M. densitometry units (n = 7 rats/group). #, p < 0.05 indicates difference from the saline-control group. *, p < 0.05 indicates difference from the saline/nicotine group.
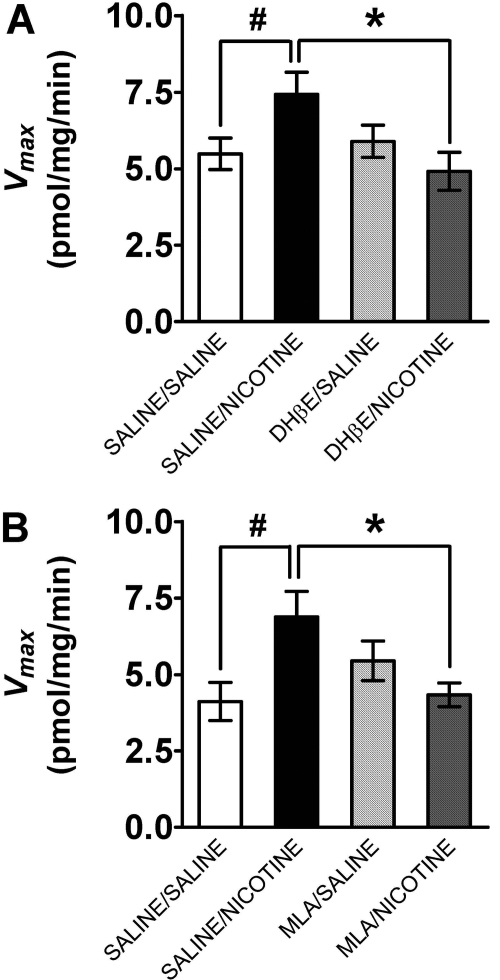
To begin to determine the specific nAChR subtypes involved in the nicotine-induced increase in Vmax for [3H]DA uptake in PFC, the ability of DHβE and MLA to inhibit this effect of nicotine was determined. Groups of rats were preinjected with DHβE (8.0 mg/kg s.c.), MLA (10.0 mg/kg s.c.), or saline and 15 min later with nicotine (0.8 mg/kg s.c.) or saline. Thirty minutes after the last injection, saturation analysis of [3H]DA uptake in PFC was conducted. Separate two-way ANOVAs were performed to determine the inhibitory effect of DHβE and MLA pretreatment. No main effect of DHβEon Vmax was found (F1,28 = 0.63, p > 0.05), whereas the main effect of nicotine was significant (F1,28 = 3.8, p < 0.05). Moreover, a significant DHβE × nicotine interaction was found (F1,28 = 5.87, p < 0.05, Fig. 8). Vmax values for the saline/nicotine group were greater (35 ± 1.7%) than for the saline/saline group [t(14) = 4.8, p < 0.05, unpaired Student's t test]. Vmax values for the DHβE/nicotine group were 34 ± 1.9% less than those for the saline/nicotine group [t(14) = 6.6, p < 0.05, unpaired Student's t test], demonstrating that DHβE inhibited the nicotine-induced increase in DAT function. Likewise, no main effect of MLA on Vmax was observed (F1,28 = 1.36, p > 0.05), whereas the main effect of nicotine was significant (F1,28 = 4.22, p < 0.05). Moreover, a significant MLA × nicotine interaction was found (F1,28 = 7.44, p < 0.05, Fig. 8). Vmax values for the saline/nicotine group were greater (40 ± 2.2%) than those for the saline/saline group [t(14) = 5.1; p < 0.05, unpaired Student's t test]. Vmax values for the MLA/nicotine group were less (36 ± 1.5%) than those for the saline/nicotine group [t(14) = 7.0, p < 0.05, unpaired Student's t test], demonstrating that MLA also inhibited the nicotine-induced increase in DAT function.
Fig. 8.
DHβE and MLA inhibited the nicotine-induced increase in Vmax for [3H]DA uptake in prefrontal cortex. Rats were administered DHβE (8 mg/kg s.c.; A) or MLA (10 mg/kg s.c.; B) or saline 15 min before nicotine (0.8 mg/kg s.c.) or saline. Synaptosomes were prepared 30 min after the last injection. [3H]DA uptake was determined in the presence of desipramine (1 μM) and paroxetine (5 nM) to prevent [3H]DA uptake into norepinephrine- and serotonin-containing nerve terminals, respectively. Vmax values are expressed as mean ± S.E.M. picomoles per milligram per minute (n = 8 rats/group). #, p < 0.05 different from saline/saline control. *, p < 0.05 different from saline/nicotine group.
Discussion
The current study investigated the mechanism by which nicotine activation of nAChRs enhances DAT function in PFC and striatum. Dose- and time-dependent nicotine-induced increases in Vmax for [3H]DA uptake in PFC were observed, consistent with previous findings that nicotine increases DA clearance in mPFC using in vivo voltammetry (Middleton et al., 2004; Zhu et al., 2007). Current results show that nicotine increased DAT expression at the cell surface in PFC, with a corresponding decrease in DAT expression in the intracellular compartment, suggesting that nicotine-induced increases in DAT cell surface expression underlie increases in Vmax for [3H]DA uptake in PFC. Moreover, the nicotine-induced increases in Vmax and surface expression were blocked completely by mecamylamine pretreatment, indicating that nAChRs mediate nicotine effects on DAT function and trafficking in PFC. Furthermore, the nicotine-induced increase in Vmax was blocked by DHβE or MLA pretreatment, suggesting that multiple nAChR subtypes play a role in the trafficking-dependent effects of nicotine on DAT function in PFC.
Results show that nicotine-induced enhancement of PFC DAT function is accompanied by increases in DAT cell surface expression, with no change in levels of total DAT protein assessed using Western blot analysis or [3H]WIN 35,428 binding. Previous studies have shown that DAT trafficking is regulated by exposure to psychostimulants and novel environmental stimuli during development (Daws et al., 2002; Zhu et al., 2005). In addition, DAT function is regulated by activation of protein kinase C, which decreases Vmax of [3H]DA uptake and decreases DAT surface expression in cell expression systems (Melikian, 2004) and in striatum (Chi and Reith, 2003). Changes in posttranslational modifications of DAT protein, including phosphorylation state and protein-protein interactions, contribute to changes in DAT function via trafficking-independent mechanisms (Torres, 2006). In the current study, an 83% increase in Vmax of DA transport was accompanied only by a 32% increase in DAT surface expression. Thus, differences in the magnitude of these effects may represent contributions of both trafficking-dependent and -independent mechanisms in the nicotine-induced increase in DAT function. A greater understanding of the molecular mechanisms underlying nicotine-induced alterations in DAT cellular localization would provide a greater understanding of the neurochemical effects of nicotine.
A caveat, however, is that nicotine effects on DAT function and trafficking were observed only at the higher dose of nicotine and, thus, may have greater implications for individuals who are heavy smokers or who self-administer relatively high nicotine doses. Although a lower dose of nicotine (0.3 mg/kg) used in the current study is within the range frequently used to evaluate behavioral effects of nicotine in animal models, the higher nicotine dose (0.8 mg/kg) is used less commonly. Nevertheless, it is important to assess the pharmacological effects of a wide range of nicotine doses because smokers vary considerably in the amount of nicotine self-administered.
In the current study, biotinylation assays were conducted using brain synaptosomes that probably contain broken membrane fragments and leaky synaptosomes. Unlike cell surface biotinylation experiments carried out using recombinant cell systems, some level of biotinylation of PP2A and β-actin, which are predominantly intracellular proteins, was observed. Although the presence of membrane fragments or leaky synaptosomes was not investigated, three different control proteins, PP2A, β-actin, and syntaxin 1A, were monitored. If nicotine produced an unexpected change in the amount of membrane fragments or leaky synaptosomes, differences in biotinylation levels of control proteins would have been observed. However, the lack of any significant differences between nicotine- and saline-treated groups in the three control proteins in any of the cellular fractions suggests that levels of membrane fragments and leaky synaptosomes obtained between groups are comparable. Moreover, an effect of nicotine on DAT surface expression was observed in PFC, but not in striatum, indicating that nicotine did not produce a global or nonspecific change but rather a restricted brainregion specific change in DAT trafficking in PFC. These results suggest that nicotine regulates DAT function in striatum and PFC by two different mechanisms, i.e., trafficking-independent and -dependent mechanisms, respectively.
In the current study, nicotine increased Vmax for [3H]DA uptake and cell surface DAT expression in PFC, but not in striatum. We reported recently that nicotine (0.3 mg/kg) produced a small, but significant, 23% increase in Vmax in rat striatum at 10 and 40 min after injection, which was not accompanied by an increase in cell surface DAT expression (Middleton et al., 2007). In the current study, no significant effect of this nicotine dose was observed during the 60-min period after injection; however, there was a tendency for a nicotine-induced increase (∼20%) in Vmax in striatum (Fig. 2B), which was near the threshold for detecting a significant effect.
Differences in the magnitude of the effect of nicotine on DAT function in PFC measured as increases (83%) in Vmax for [3H]DA uptake and increases (46%) in DA clearance using in vivo voltammetry may be explained in part based on the different methods used (current results; Middleton et al., 2004). [3H]DA uptake was measured using synaptosomes, providing an averaged effect across this heterogeneous brain region, whereas DA clearance using in vivo voltammetry was measured within specific localized areas of this heterogeneous region. Furthermore, brain regions assessed in these studies were not identical. DA clearance was determined in mPFC, whereas Vmax was determined in PFC (including orbitofrontal, insular, and frontal cortex areas 1-3). In addition, in the previous voltammetry study, rats were anesthetized when DA clearance was measured, whereas anesthesia was not used in the current study. Thus, various methodological differences may have contributed to the observed differences in magnitude of effect of nicotine between current and previous work.
The present results indicate that nAChRs mediate nicotine-induced increases in Vmax of [3H]DA uptake and cell surface DAT expression in PFC because pretreatment with the nonselective nAChR antagonist, mecamylamine, completely inhibited these nicotine effects. These results are consistent with previous finding from in vivo voltammetry studies demonstrating that nAChRs mediate nicotine-induced increases in DA clearance in mPFC (Middleton et al., 2004; Zhu et al., 2007). In contrast to increases in DAT function observed after in vivo nicotine administration, in vitro exposure to a range of nicotine concentrations (1 nM-10 μM) did not alter [3H]DA uptake in either PFC or striatal synaptosomes (Carr et al., 1989; Zhu et al., 2003). Response to in vivo nicotine administration may be the result of activation of nAChRs at dopaminergic ventral tegmental cell bodies or elsewhere within the neuronal circuitry. In synaptosomal preparations, this neuronal circuitry is disrupted. If nAChRs mediating the response to nicotine were located on dopaminergic terminals, then effects of nicotine on DAT function would be expected to be observed in synaptosomal preparations exposed to nicotine. In general, nicotine is accepted as stimulating several different subtypes of nAChR located on dopaminergic terminals to evoke DA release (see Introduction). The nicotine-induced increase in DA release activates both pre- and postsynaptic D2 DA receptors, which may indirectly increase DAT function. The latter interpretation is supported by previous results showing that blockade of D2 DA receptors by locally applied raclopride (a D2 DA receptor antagonist) decreased DA clearance in PFC (Cass and Gerhardt, 1994) and results from D2 receptor knockout mice showing decreased striatal DAT function (Dickinson et al., 1999). Thus, nAChR-mediated modulation of DAT function may be by an indirect mechanism, such that activation of nAChRs on DA terminals in PFC directly stimulates DA release, activating presynaptic D2 DA receptors to indirectly increase DAT function.
To determine the role of β2- and α7-containing nAChRs in the mediation of nicotine effects on DAT function in PFC, rats were pretreated with either DHβE or MLA, which are thought to be primarily selective for α4β2 and α7 nAChRs, respectively (Chavez-Noriega et al., 1997; Papke et al., 2008). Results show that DHβE and MLA inhibited completely the nicotine-induced increase in Vmax of [3H]DA uptake in PFC, suggesting that both β2- and α7-containing nAChRs are involved. This finding is in contrast to reports that only DHβE-sensitive nAChRs, and not MLA-sensitive nAChRs, mediate nicotine-evoked DA release in PFC (Cao et al., 2005). A caveat, however, is that at high concentrations, DHβE and MLA are not subtype selective (Mogg et al., 2002; Papke et al., 2008); DHβE also inhibits α3β4 and α7 subtypes (Papke et al., 2008), and MLA also inhibits α4β2 (Buisson et al., 1996). Subtype selectivity is based on in vitro studies in which the antagonist concentration is known; however, because the antagonist concentration in brain after in vivo administration is not known, these antagonists may not be subtype selective at the doses used. In summary, the current study shows that nicotine increases DAT function and cell surface expression in PFC and that these effects are mediated by activation of nAChRs.
Acknowledgments
We thank Ronald C. Bruntz for technical assistance.
This study was supported by the National Institutes of Health National Institute on Drug Abuse [Grant DA-018372].
doi:10.1124/jpet.108.147025.
ABBREVIATIONS: DA, dopamine; nAChR, nicotinic acetylcholine receptor; DAT, dopamine transporter; NAc, nucleus accumbens; PFC, prefrontal cortex; PP2A, protein phosphatase 2A; HRP, horseradish peroxidase; sulfo-NHS-biotin, sulfosuccinimidobiotin; MLA, methyllycaconitine; DHβE, dihydro-β-erythroidine; [3H]WIN 35,428, (-)-3β-(4-fluorophentl)-tropan-2β-carboxylic acid methyl ester tartrate; mPFC, medial prefrontal cortex; NET, norepinephrine transporter; SERT, serotonin transporter; GBR 12909, 1-{2-[bis-(4-fluorophenyl)methoxy]ethyl}-4-(3-phenylpropyl)piperazine; PBS, phosphate-buffered saline; ANOVA, analysis of variance.
References
- Anand R, Conroy WG, Schoepfer R, Whiting P, and Lindstrom J (1991) Neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes have a pentameric quaternary structure. J Biol Chem 266 11192-11198. [PubMed] [Google Scholar]
- Bjerggaard C, Fog JU, Hastrup H, Madsen K, Loland CJ, Javitch JA, and Gether U (2004) Surface targeting of the dopamine transporter involves discrete epitopes in the distal C terminus but does not require canonical PDZ domain interactions. J Neurosci 24 7024-7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buisson B, Gopalakrishnan M, Arneric SP, Sullivan JP, and Bertrand D (1996) Human alpha4beta2 neuronal nicotinic acetylcholine receptor in HEK 293 cells: a patch-clamp study. J Neurosci 16 7880-7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao YJ, Surowy CS, and Puttfarcken PS (2005) Different nicotinic acetylcholine receptor subtypes mediating striatal and prefrontal cortical [3H]dopamine release. Neuropharmacology 48 72-79. [DOI] [PubMed] [Google Scholar]
- Carr LA, Rowell PP, and Pierce WM Jr (1989) Effects of subchronic nicotine administration on central dopaminergic mechanisms in the rat. Neurochem Res 14: 511-515. [DOI] [PubMed] [Google Scholar]
- Cass WA and Gerhardt GA (1994) Direct in vivo evidence that D2 dopamine receptors can modulate dopamine uptake. Neurosci Lett 176 259-263. [DOI] [PubMed] [Google Scholar]
- Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, and Johnson EC (1997) Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors ha2b2, ha2b4, ha3b2, ha3b4, ha4b2, ha4b4 and ha7 expressed in Xenopus oocytes. J Pharmacol Exp Ther 280 346-356. [PubMed] [Google Scholar]
- Chi L and Reith ME (2003) Substrate-induced trafficking of the dopamine transporter in heterologously expressing cells and in rat striatal synaptosomal preparations. J Pharmacol Exp Ther 307 729-736. [DOI] [PubMed] [Google Scholar]
- Clarke PB and Pert A (1985) Autoradiographic evidence for nicotine receptors on nigrostriatal and mesolimbic dopaminergic neurons. Brain Res 348 355-358. [DOI] [PubMed] [Google Scholar]
- Daws LC, Callaghan PD, Morón JA, Kahlig KM, Shippenberg TS, Javitch JA, and Galli A (2002) Cocaine increases dopamine uptake and cell surface expression of dopamine transporters. Biochem Biophys Res Commun 290 1545-1550. [DOI] [PubMed] [Google Scholar]
- de Fiebre CM, Romm E, Collins JT, Draski LJ, Deitrich RA, and Collins AC (1991) Responses to cholinergic agonists of rats selectively bred for differential sensitivity to ethanol. Alcohol Clin Exp Res 15 270-276. [DOI] [PubMed] [Google Scholar]
- Dickinson SD, Sabeti J, Larson GA, Giardina K, Rubinstein M, Kelly MA, Grandy DK, Low MJ, Gerhardt GA, and Zahniser NR (1999) Dopamine D2 receptor-deficient mice exhibit decreased dopamine transporter function but no changes in dopamine release in dorsal striatum. J Neurochem 72 148-156. [DOI] [PubMed] [Google Scholar]
- Drew AE, Derbez AE, and Werling LL (2000) Nicotinic receptor-mediated regulation of dopamine transporter activity in rat prefrontal cortex. Synapse 38 10-16. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR and Caron MG (2003) Monoamine transporters: from genes to behavior. Annu Rev Pharmacol Toxicol 43 261-284. [DOI] [PubMed] [Google Scholar]
- Hart C and Ksir C (1996) Nicotine effects on dopamine clearance in rat nucleus accumbens. J Neurochem 66 216-221. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC.
- Kahlig KM, Javitch JA, and Galli A (2004) Amphetamine regulation of dopamine transport. Combined measurements of transporter currents and transporter imaging support the endocytosis of an active carrier. J Biol Chem 279 8966-8975. [DOI] [PubMed] [Google Scholar]
- Klink R, de Kerchove d'Exaerde A, Zoli M, and Changeux JP (2001) Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci 21 1452-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A and Paterson NE (2001) The nicotinic antagonist methyllycaconitine has differential effects on nicotine self-administration and nicotine withdrawal in the rat. Nicotine Tob Res 3 361-373. [DOI] [PubMed] [Google Scholar]
- Melikian HE (2004) Neurotransmitter transporter trafficking: endocytosis, recycling, and regulation. Pharmacol Ther 104 17-27. [DOI] [PubMed] [Google Scholar]
- Middleton LS, Apparsundaram S, King-Pospisil KA, and Dwoskin LP (2007) Nicotine increases dopamine transporter function in rat striatum through a trafficking-independent mechanism. Eur J Pharmacol 554 128-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton LS, Cass WA, and Dwoskin LP (2004) Nicotinic receptor modulation of dopamine transporter function in rat striatum and medial prefrontal cortex. J Pharmacol Exp Ther 308 367-377. [DOI] [PubMed] [Google Scholar]
- Mogg AJ, Whiteaker P, McIntosh JM, Marks M, Collins AC, and Wonnacott S (2002) Methyllycaconitine is a potent antagonist of alpha-conotoxin-MII-sensitive presynaptic nicotinic acetylcholine receptors in rat striatum. J Pharmacol Exp Ther 302 197-204. [DOI] [PubMed] [Google Scholar]
- Morón JA, Brockington A, Wise RA, Rocha BA, and Hope BT (2002) Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci 22: 389-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB and Owens MJ (2003) Neuropharmacology of paroxetine. Psychopharmacol Bull 37 8-18. [PubMed] [Google Scholar]
- Norrholm SD, Horton DB, and Dwoskin LP (2007) The promiscuity of the dopamine transporter: implications for the kinetic analysis of [3H]serotonin uptake in rat hippocampal and striatal synaptosomes. Neuropharmacology 53 982-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Dwoskin LP, Crooks PA, Zheng G, Zhang Z, McIntosh JM, and Stokes C (2008) Extending the analysis of nicotinic receptor antagonists with the study of α6 nicotinic receptor subunit chimeras. Neuropharmacology 54 1189-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubert C, Cox PJ, Bruss M, Hamon M, Bönisch H, and Giros B (2001) Determination of residues in the norepinephrine transporter that are critical for tricyclic antidepressant affinity. J Biol Chem 276 8254-8260. [DOI] [PubMed] [Google Scholar]
- Salminen O, Drapeau JA, McIntosh JM, Collins AC, Marks MJ, and Grady SR (2007) Pharmacology of alpha-conotoxin MII-sensitive subtypes of nicotinic acetylcholine receptors isolated by breeding of null mutant mice. Mol Pharmacol 71 1563-1571. [DOI] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, and Grady SR (2004) Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol 65: 1526-1535. [DOI] [PubMed] [Google Scholar]
- Scholze P, Orr-Urtreger A, Changeux JP, McIntosh JM, and Huck S (2007) Catecholamine outflow from mouse and rat brain slice preparations evoked by nicotinic acetylcholine receptor activation and electrical field stimulation. Br J Pharmacol 151 414-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkina T, Hoover BR, Zahniser NR, and Sorkin A (2005) Constitutive and protein kinase C-induced internalization of the dopamine transporter is mediated by a clathrin-dependent mechanism. Traffic 6 157-170. [DOI] [PubMed] [Google Scholar]
- Torres GE (2006) The dopamine transporter proteome. J Neurochem 97 3-10. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Epping-Jordan MP, Koob GF, and Markou A (1999) Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav 62 743-751. [DOI] [PubMed] [Google Scholar]
- Williams JM and Steketee JD (2004) Characterization of dopamine transport in crude synaptosomes prepared from rat medial prefrontal cortex. J Neurosci Methods 137 161-165. [DOI] [PubMed] [Google Scholar]
- Zahniser NR, Larson GA, and Gerhardt GA (1999) In vivo dopamine clearance rate in rat striatum: regulation by extracellular dopamine concentration and dopamine transporter inhibitors. J Pharmacol Exp Ther 289 266-277. [PubMed] [Google Scholar]
- Zhu J, Apparsundaram S, Bardo MT, and Dwoskin LP (2005) Environmental enrichment decreases cell surface expression of the dopamine transporter in rat medial prefrontal cortex. J Neurochem 93 1434-1443. [DOI] [PubMed] [Google Scholar]
- Zhu J, Bardo MT, Green TA, Wedlund PJ, and Dwoskin LP (2007) Nicotine increases dopamine clearance in medial prefrontal cortex in rats raised in an enriched environment. J Neurochem 103 2575-2588. [DOI] [PubMed] [Google Scholar]
- Zhu J, Crooks PA, Ayers JT, Sumithran SP, and Dwoskin LP (2003) N-n-Alkylnicotinium and N-n-alkylpyridinium analogs, a novel class of nicotinic receptor antagonists: inhibition of the dopamine transporter. Drug Dev Res 61 1-15. [Google Scholar]
- Zhu J, Green T, Bardo MT, and Dwoskin LP (2004) Environmental enrichment enhances sensitization to GBR 12935-induced activity and decreases dopamine transporter function in the medial prefrontal cortex. Behav Brain Res 148 107-117. [DOI] [PubMed] [Google Scholar]