Abstract
Discriminative stimulus effects of the serotonin (5-HT) receptor agonist 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM) have been studied in rats and, more recently, in rhesus monkeys. This study examined DOM, 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7), and dipropyltryptamine hydrochloride (DPT) alone and in combination with three antagonists, MDL100907 [(±)2,3-dimethoxyphenyl-1-[2-(4-piperidine)-methanol]], ketanserin [3-[2-[4-(4-fluorobenzoyl)piperidin-1-yl]ethyl]-1H-quinazoline-2,4-dione], and ritanserin [6-[2-[4-[bis(4-fluorophenyl)methylidene]piperidin-1-yl]ethyl]-7-methyl-[1,3]thiazolo[2,3-b]pyrimidin-5-one], to identify the 5-HT receptor subtype(s) that mediates the discriminative stimulus effects of these 5-HT receptor agonists. Four adult rhesus monkeys discriminated between 0.32 mg/kg s.c. DOM and vehicle while responding under a fixed ratio 5 schedule of stimulus shock termination. DOM, 2C-T-7, and DPT dose-dependently increased responding on the DOM-associated lever. MDL100907 (0.001-0.01 mg/kg), ketanserin (0.01-0.1 mg/kg), and ritanserin (0.01-0.1 mg/kg) each shifted the dose-response curves of DOM, 2C-T-7, and DPT rightward in a parallel manner. Schild analysis of each drug combination was consistent with a simple, competitive, and reversible interaction. Similar apparent affinity (pA2) values were obtained for MDL100907 in combination with DOM (8.61), 2C-T-7 (8.58), or DPT (8.50), for ketanserin with DOM (7.67), 2C-T-7 (7.75), or DPT (7.71), and for ritanserin with DOM (7.65), 2C-T-7 (7.75), or DPT (7.65). Potency of antagonists in this study was correlated with binding affinity at 5-HT2A receptors and not at 5-HT2C or α1 adrenergic receptors. This study used Schild analysis to examine receptor mechanisms mediating the discriminative stimulus effects of hallucinogenic drugs acting at 5-HT receptors; results provide quantitative evidence for the predominant, if not exclusive, role of 5-HT2A receptors in the discriminative stimulus effects of DOM, 2C-T-7, and DPT in rhesus monkeys.
Phenethylamines and tryptamines are two classes of drugs that act at serotonin (5-HT) receptors and can produce hallucinations; in general, agonists from these two classes have similar but not identical behavioral and neurochemical effects. For example, many phenethylamines bind relatively nonselectively to 5-HT2A and 5-HT2C receptors and have comparatively lower (e.g., 1000-fold) affinity for other (e.g., 5-HT1A) 5-HT receptors. Tryptamines, on the other hand, often display higher affinity than phenethylamines for 5-HT1A receptors (for review, see Nichols, 2004; Fantegrossi et al., 2008a) and comparatively lower affinity for 5-HT2A and 5-HT2C receptors. Despite differences between phenethylamines and tryptamines in their binding selectivity for different 5-HT receptors, with few exceptions (e.g., Winter et al., 2000), agonists from these chemical classes have similar effects that seem to be mediated predominantly by 5-HT2A receptors (e.g., Vollenweider et al., 1998).
Drug discrimination is used to study receptor mechanisms that mediate the effects of drugs from a variety of pharmacologic classes. Many drugs with hallucinogenic effects in humans have agonist actions at 5-HT receptors, and among those drugs, the discriminative stimulus effects of the phenethylamine 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM) have been studied extensively in rodents (Glennon et al., 1982; Fiorella et al., 1995a) and, more recently, in nonhuman primates (Li et al., 2008). Converging lines of evidence indicate that despite the nonselective binding of DOM to 5-HT2A and 5-HT2C receptors, the discriminative stimulus effects of DOM seem to be mediated predominantly by 5-HT2A receptors inasmuch as drugs that are antagonists at 5-HT2A receptors often block the discriminative stimulus effects of DOM (e.g., Glennon et al., 1983). Moreover, the ability of drugs to antagonize the discriminative stimulus of DOM is positively correlated with binding affinity at 5-HT2A receptors (Fiorella et al., 1995b). However, there are conditions under which other receptors seem to play a role in the discriminative stimulus effects of DOM. For example, the nonselective 5-HT2A/2C receptor agonist MK-212 substitutes for the discriminative stimulus effects of DOM in rats, and this effect of MK-212 is not fully antagonized by the 5-HT2A receptor antagonist pirenperone (Fiorella et al., 1995c). Collectively, these data suggest that other (e.g., 5-HT2C) receptors might play a role, directly or by modulation, in the discriminative stimulus effects of DOM and related 5-HT receptor agonists.
This study investigated the receptor mechanisms that mediate the discriminative stimulus effects of DOM and related drugs in rhesus monkeys. Each of three 5-HT receptor agonists was studied alone and in combination with each of three other drugs that are known to have antagonist actions at 5-HT2A receptors: MDL100907, ketanserin, and ritanserin. Schild analysis has been used to examine receptor mechanisms for drugs acting at other receptors (Dykstra et al., 1988; Dykstra, 1990; France et al., 1990; Gerak and France, 2007) and, in the current study, was used to quantitatively compare dose-response curves from combinations of 5-HT receptor agonists and antagonists. MDL100907 and ketanserin have higher affinity at 5-HT2A compared with 5-HT2C receptors, whereas ritanserin has similar affinity at 5-HT2A and 5-HT2C receptors (NIMH Psychoactive Drug Screening Program, http://pdsp.med.unc.edu). If 5-HT2C receptors are involved in the discriminative stimulus effects of DOM, then antagonists acting at 5-HT2A and 5-HT2C receptors (e.g., ritanserin) should more effectively block the effects of DOM compared with antagonists acting selectively at 5-HT2A receptors (e.g., MDL100907). The 5-HT2A receptor agonists studied included DOM, 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7), and dipropyltryptamine hydrochloride (DPT). 2C-T-7 is a “designer” phenethylamine with hallucinogenic activity and high affinity at 5-HT2A and 5-HT2C receptors; however, the behavioral effects of 2C-T-7, including discriminative stimulus effects in nonhuman primates (Li et al., 2008), seem to be mediated by 5-HT2A receptors (Fantegrossi et al., 2005). DPT, a tryptamine with hallucinogenic activity, recently was shown to have agonist activity at 5-HT2A and 5-HT1A receptors (Li et al., 2007; Fantegrossi et al., 2008b).
Materials and Methods
Subjects. Four adult rhesus monkeys weighing between 4 and 8 kg and previously trained to discriminate between saline and 0.32 mg/kg DOM (Li et al., 2008) were housed individually with unlimited access to water. Primate chow (Harlan Teklad High Protein Monkey Diet; Harlan Teklad, Madison, WI), fresh fruit, and peanuts were provided after daily sessions in amounts sufficient to maintain normal, age- and gender-appropriate weights. Monkeys were maintained on a 14-/10-h light/dark cycle. The animals used in these studies were maintained in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and with the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council, 1996).
Apparatus. During experimental sessions, subjects were seated in chairs (model R001; Primate Products, Inc., Woodside, CA) that provided restraint at the neck and arms and were placed within ventilated, sound-attenuating chambers. Chambers were equipped with response panels, each containing two stimulus lights and two response levers. The feet of monkeys were placed in shoes that were mounted to the front of chairs and equipped with brass electrodes to which a brief (250 ms, 3 mA) electric shock could be delivered from AC generators. Experiments were controlled and data recorded with a microprocessor and commercially available interface (MED Associates, St. Albans, VT).
Procedure. Daily training sessions comprised two to six 15-min cycles, with each cycle starting with a 10-min timeout period, during which stimulus lights were not illuminated and responding had no programmed consequence. This timeout period was followed by a 5-min response period during which stimulus lights were illuminated above the levers and a schedule of stimulus shock termination was active. Monkeys could extinguish stimulus lights and postpone scheduled shock for 30 s by responding five times [fixed ratio (FR) 5] consecutively on the lever designated correct by an injection administered during the 1st min of the cycle (e.g., right lever, saline; left lever, DOM). Incorrect responses reset the FR requirement on the correct lever. Failure to satisfy the FR requirement within 30 s resulted in the delivery of a brief (250 ms, 3 mA) stimulus. Thereafter, shock was delivered every 30 s until the response requirement was satisfied, the cycle ended, or a total of four shocks were delivered, whichever occurred first.
For drug training sessions, monkeys received an injection of 0.32 mg/kg s.c. DOM before one cycle followed by one sham (no injection) cycle. For vehicle training sessions, monkeys received an injection of saline (subcutaneously) before one cycle followed by between one and five sham (no injection) cycles. Monkeys had previously satisfied the following criteria for five consecutive or six of seven sessions (Li et al., 2008): at least 80% of the total responses on the correct lever and fewer than five responses (one FR requirement) on the incorrect lever before completion of the FR on the correct lever. Thereafter, monkeys were tested every 3rd day provided that the testing criteria were satisfied during intervening training sessions. If monkeys failed to satisfy these criteria, testing was postponed until the criteria were satisfied for two consecutive training sessions.
Test sessions were similar to training sessions, except that five consecutive responses on either lever postponed shock and increasing doses of drug were administered across cycles. For substitution studies, saline was administered in the first cycle, followed by increasing doses of drug in subsequent cycles, with the cumulative dose increasing by 0.25 or 0.5 log units per cycle. Drugs were studied up to doses that occasioned greater than 80% responding on the DOM lever. For antagonism studies, a single dose of antagonist was administered 5 min before the start of the first cycle.
Data Analyses. Drug discrimination data are expressed as a percentage of the total responses made on the DOM-paired lever averaged among four monkeys (±1 S.E.M.) and plotted as a function of dose. Rate of lever pressing is plotted as the average (±1 S.E.M.) number of responses per second on both levers. The control response rate is the average of the five vehicle training sessions before the test.
Doses of test drugs to occasion 50% drug lever responding (ED50) and 95% confidence limits (CLs) were estimated using interpolation or linear regression using the portion of the dose-effect curve spanning 50% drug-lever responding. Dose ratios were determined for each monkey by dividing the ED50 values for each agonist (DOM, 2C-T-7, and DPT) studied in combination with an antagonist (MDL100907, ketanserin, and ritanserin) by the ED50 values for each agonist studied alone. Schild analyses were conducted as described previously (e.g., Li et al., 2008) using the method of Arunlakshana and Schild (1959). Schild plots were constructed by plotting the log of the dose ratio (agonist with antagonist divided by agonist alone) - 1 as a function of the negative log dose of antagonist (moles per kilogram). Straight lines were simultaneously fitted to the individual Schild plots using GraphPad Prism version 5.00 for Windows (GraphPad Software Inc., San Diego, CA) and the following equation: log (dose ratio - 1) =-log (molar dose of antagonist) × slope + intercept. Apparent affinity (pA2) values and 95% CLs with unconstrained slopes and, when appropriate, with slopes constrained to -1 (unity) were determined for each agonist and antagonist combination. Slopes of Schild plots were considered to conform to unity when the 95% CL included -1 and did not include 0 (e.g., Paronis and Bergman, 1999).
Drugs. The compounds used in this study were as follows. DOM and 2C-T-7 were obtained from the National Institute on Drug Abuse (Research Technology Branch, Rockville, MD); DPT and MDL100907 were synthesized as described previously (Ullrich and Rice, 2000); and ketanserin tartrate and ritanserin were purchased from Sigma-Aldrich (St. Louis, MO). MDL100907 was dissolved in 20% dimethyl sulfoxide (v/v) and saline; other drugs were dissolved in sterile 0.9% saline. Doses are expressed as the form of the drug listed above in milligrams per kilogram of body weight or in Schild plots as moles per kilogram. Injection volumes were 0.1 to 1.0 ml.
Results
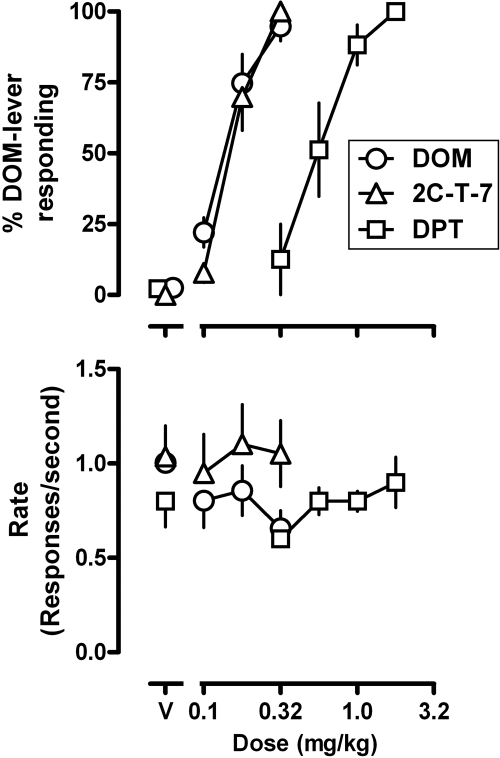
DOM, 2C-T-7, and DPT increased responding on the DOM associated lever in a dose-related manner (Fig. 1, top), with the largest dose of each occasioning more than 90% DOM-lever responding [DOM, ED50 (95% CL) = 0.158 (0.117, 0.194) mg/kg; 2C-T-7, 0.156 (0.145, 0.167) mg/kg; and DPT, 0.639 (0.436, 0.817) mg/kg]. DOM and 2C-T-7 were similar in potency, and both were 4-fold more potent than DPT. None of the compounds markedly altered rate of lever pressing at the doses studied (Fig. 1, bottom).
Fig. 1.
Discriminative stimulus and rate effects of DOM, 2C-T-7, and DPT in four rhesus monkeys discriminating between vehicle and 0.32 mg/kg DOM. Abscissae, dose in milligrams per kilogram of body weight; V, vehicle. Ordinates, mean (±S.E.M.) percentage of responses on the DOM lever (top) and mean (±S.E.M.) rate of responding in responses per second (bottom).
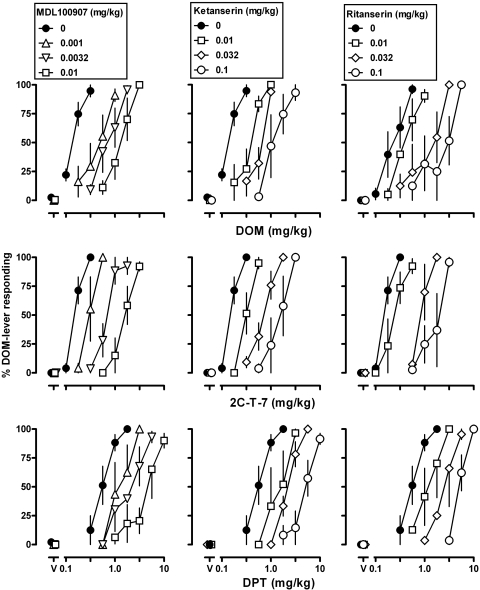
MDL100907 (Fig. 2, left), ketanserin (Fig. 2, middle), and ritanserin (Fig. 2, right) antagonized the discriminative stimulus effect of all three agonists, in each case shifting the dose-response curves to the right in a dose-related manner. For example, under-control condition doses of 0.32 to 1.0 mg/kg DOM, 0.32 mg/kg 2C-T-7, and 1.0-3.2 mg/kg DPT occasioned greater than 90% drug lever responding (filled symbols, top, middle, and bottom, respectively, Fig. 2); in monkeys that received 0.01 mg/kg MDL100907, doses of 3.2 mg/kg DOM, 3.2 mg/kg 2C-T-7, and 10 mg/kg DPT were required to produce at least 90% responding on the DOM lever (triangles, left, Fig. 2). In the presence of larger doses of antagonists, larger doses of agonists were required to obtain responding on the drug lever. For example, after administration of 0.1 mg/kg ritanserin, doses of 5.6 mg/kg DOM, 3.2 2C-T-7, and 10.0 mg/kg DPT were required to obtain greater than 90% drug lever responding (open circles, right, Fig. 2). For all drug combinations, the antagonism was surmountable, with larger doses of agonists occasioning responding on the DOM-associated lever. When administered alone, none of the antagonists occasioned responding on the DOM associated lever (points above “V”; Fig. 2, all panels).
Fig. 2.
Discriminative stimulus effects of DOM (top), 2C-T-7 (middle), and DPT (bottom) administered alone (filled symbols) and in combination with different doses of MDL100907 (left), ketanserin (center), and ritanserin (right). For other details, see Fig. 1.
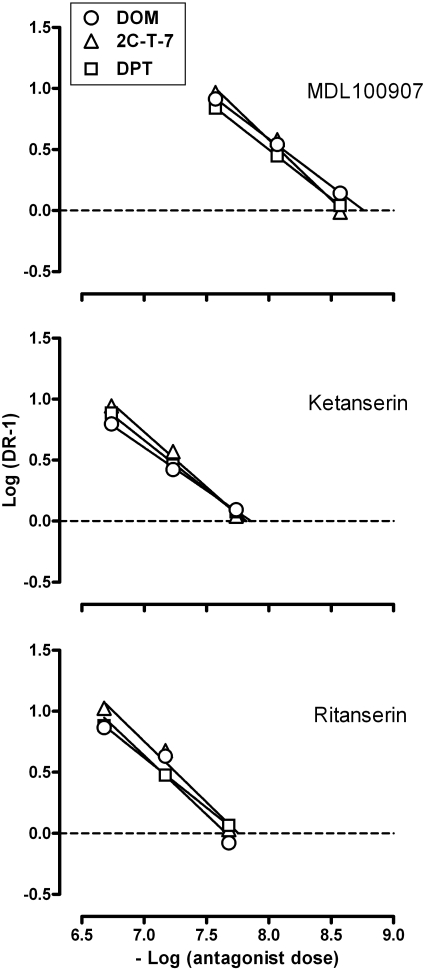
The same data shown in Fig. 2 as dose-response curves are presented in Fig. 3 as Schild plots, expressing the magnitude of antagonism (ordinate; log [dose ratio - 1]) as a function of the -log of antagonist dose (abscissa). The similarity among the three regression lines in each panel (i.e., for each antagonist combined with each of three different agonists) reflects the similar potency for each antagonist in attenuating the discriminative stimulus effects of DOM, 2C-T-7, and DPT. The intercept of each regression line with the horizontal dashed line (0 on the ordinate) indicates the apparent pA2 or estimated dose of antagonist to shift the agonist dose-response curve 2-fold to the right. The apparent pA2 values were similar for each antagonist studied in combination with each of the three agonists (Table 1). For example, the pA2 values (unconstrained slopes) for MDL100907 were as follows: 8.77 with DOM, 8.59 with 2C-T-7, and 8.62 with DPT. None of the slopes of the Schild regression lines was significantly different from -1 (unity); thus, Table 1 also shows apparent pA2 values determined with slopes constrained to -1. With the constrained slope, the pA2 values for MDL100907 were as follows: 8.61 with DOM, 8.50 with 2C-T-7, and 8.58 with DPT. Overall, MDL 100907 was 7.1- and 7.6-fold more potent than ketanserin and ritanserin, respectively, in antagonizing the discriminative stimulus effects of DOM, 2C-T-7, and DPT.
Fig. 3.
Schild plots constructed from the same data shown in Fig. 2. Abscissa, negative log of the dose of antagonist in moles per kilogram of body weight. Ordinate, log of the dose ratio - 1.
TABLE 1.
Results of Schild analyses for combinations of 5-HT2 receptor antagonists and agonists in rhesus monkeys (n = 4)
| Drugs | Slope (Unconstrained) | 95% CL | pA2 (Unconstrained) | 95% CL | pA2 (Constrained) | 95% CL |
|---|---|---|---|---|---|---|
| MDL100907 and DOM | −0.77 | (−0.53, −1.03) | 8.77 | (8.51, 9.03) | 8.61 | (8.46, 8.76) |
| MDL100907 and 2C-T-7 | −0.98 | (−0.60, −1.36) | 8.59 | (8.38, 8.80) | 8.58 | (8.44, 8.72) |
| MDL100907 and DPT | −0.80 | (−0.36, −1.24) | 8.62 | (8.31, 8.94) | 8.50 | (8.33, 8.68) |
| Ketanserin and DOM | −0.70 | (−0.33, −1.08) | 7.86 | (7.54, 8.01) | 7.67 | (7.51, 7.84) |
| Ketanserin and 2C-T-7 | −0.90 | (−0.53, −1.27) | 7.81 | (7.57, 8.05) | 7.75 | (7.61, 7.90) |
| Ketanserin and DPT | −0.81 | (−0.45, −1.16) | 7.78 | (7.50, 8.06) | 7.71 | (7.57, 7.85) |
| Ritanserin and DOM | −0.97 | (−0.61, −1.33) | 7.67 | (7.48, 7.86) | 7.65 | (7.52, 7.79) |
| Ritanserin and 2C-T-7 | −1.00 | (−0.65, −1.35) | 7.76 | (7.59, 7.92) | 7.75 | (7.63, 7.88) |
| Ritanserin and DPT | −0.81 | (−0.38, −1.23) | 7.76 | (7.45, 8.07) | 7.65 | (7.48, 7.82) |
Discussion
Reliable stimulus control between DOM and saline was maintained in rhesus monkeys responding under a two-choice, multiple-cycle, cumulative-dosing procedure, and the potency of DOM under these conditions was similar to its potency determined when the same monkeys responded under a single-cycle, acute-dosing procedure (Li et al., 2008). Under this multiple-cycle, cumulative-dosing procedure, 2C-T-7 and DPT also increased responding on the DOM-associated lever, with potencies similar to their potencies under the single-cycle, acute-dosing procedure (Li et al., 2008). One general feature of drug discrimination procedures is pharmacological selectivity such that, in general, only drugs that share a mechanism of action with the training drug occasion responding on the drug-associated lever. In that regard, the apparent qualitative similarity in discriminative stimulus effects among these three compounds is consistent with actions at 5-HT2A receptors. Although DPT also binds to 5-HT1A receptors, and 2C-T-7 has similar affinity for 5-HT2A and 5-HT2C receptors (Fantegrossi et al., 2005), results of these substitution studies indicate that agonist activity at 5-HT2A receptors accounts for the DOM-like discriminative stimulus effects of these drugs.
Drugs with affinity for and no apparent efficacy at 5-HT2A receptors can attenuate the discriminative stimulus effects of DOM and related agonists in rats (Glennon et al., 1983) and in nonhuman primates (Li et al., 2008). Likewise, in the current study, drugs that are known to have antagonist actions at 5-HT2A receptors antagonized the discriminative stimulus effects of all three 5-HT receptor agonists, in each case shifting the discrimination dose-response curve to the right. MDL100907 and ketanserin bind selectively to 5-HT2A receptors, compared with 5-HT2C receptors, whereas ritanserin has similar affinity for 5-HT2A and 5-HT2C receptors. Despite differences in their binding selectivity for different 5-HT receptors, all three antagonists blocked the effects of all three agonists in a dose-related and surmountable manner.
Schild analysis has been used to evaluate the behavioral effects of drugs acting at various different receptors, including opioid (Woods et al., 1988; France et al., 1990), GABAA (Paronis and Bergman, 1999), and 5-HT1A receptors (Koek et al., 2000); however, this approach has not been used widely to examine the behavioral effects of drugs acting at 5-HT2A receptors. Despite the challenges inherent with this analysis (e.g., Kenakin, 1982), particularly in vivo when assumptions (e.g., equilibrium) cannot be confirmed, it is noteworthy that orderly data can be obtained with this approach using behavioral data (Dykstra et al., 1988; Paronis and Bergman, 1999). Likewise, in the current study, the dose-response curves of each agonist were shifted to the right in an orderly dose-related manner by each of the antagonists. Moreover, for each drug combination, the Schild analysis yielded slopes that were not significantly different from unity (-1), a result that is consistent with a simple, competitive, and reversible interaction, probably at a single 5-HT receptor subtype (e.g., 5-HT2A).
One value of Schild analysis is that the role of a particular receptor in the observed response can be confirmed quantitatively by comparing families of dose-response curves for combinations of agonists and antagonists that vary in selectivity for different receptors. Each of the agonists used in this study (DOM, 2C-T-7, and DPT) has activity at 5-HT2A receptors, but each also has activity at other receptors. Likewise, each of the antagonists used in this study (MDL100907, ketanserin, and ritanserin) has affinity for 5-HT2A receptors, but each also has affinity for other receptors. If only one receptor type mediates the effects of all drugs under a particular set of conditions, then under those conditions, the potency of an antagonist should be the same in blocking the actions of all agonists that have activity at that receptor. As shown by the convergence of regression lines on Schild plots (Fig. 3) and the estimated apparent pA2 values (Table 1), the potency of each antagonist was remarkably similar with each of three different agonists. For example, the (unconstrained) apparent pA2 values for ritanserin in combination with DOM, 2C-T-7, and DPT were 7.67, 7.76, and 7.76, respectively. Constraining the slope of the Schild plot to unity (-1) had little effect on the absolute value of the apparent pA2 values or on the high degree of consistency among these values across agonists (Table 1), and this was the case for all three antagonists. Collectively, these results strongly suggest that a single receptor type mediates the effects of all three agonists and antagonists under these in vivo conditions and that the interaction of these drugs with that receptor type is simple, competitive, and reversible.
To the extent that only one receptor type mediates the effects of drugs under the conditions used in this discrimination study, the relative potency or affinity of these drugs for that receptor should predict their effects in this assay. That seems to be the case both for agonists and for antagonists. DOM and 2C-T-7 have similar potency, and both are 3-fold more potent than DPT in producing head twitching in mice (Fantegrossi et al., 2005, 2008b), an effect that is thought to be mediated by 5-HT2A receptors. Based on apparent pA2 values, ketanserin and ritanserin have very similar potency in antagonizing the discriminative stimulus effects of each agonist, being 10- to 17-fold less potent than MDL100907 in this regard. This potency relationship among these three antagonists parallels their relative potencies in blocking (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane-induced head twitching (Table 2). Moreover, the potency of MDL100907, ketanserin, and ritanserin in antagonizing the discriminative stimulus effects of DOM, 2C-T-7, and DPT parallels their relative binding affinities for 5-HT2A receptors and not their relative binding affinities for 5-HT2C or α1 adrenergic receptors (Table 2). This striking similarity between antagonist potencies in the present study and receptor binding affinities in other studies provides strong evidence for these discriminative stimulus effects and, perhaps, the discriminative stimulus effects of other related drugs with hallucinogenic actions in humans being mediated by a single receptor type (5-HT2A).
TABLE 2.
In vivo antagonism potencies and in vitro receptor binding affinities of MDL100907
| MDL100907 | Ketanserin | Ritanserin | |
|---|---|---|---|
| In vivo antagonism | |||
| DOM discriminative stimulus (pA2, mg/kg) | 0.0006a (0.0003-0.0012) | 0.0075a (0.0053-0.0157) | 0.0102a (0.0071-0.0158) |
| (±)-1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane -induced head twitch (ED50, mg/kg) | 0.005b | 0.029c (0.009-0.096) | 0.027c (0.008-0.091) |
| In vitro binding | |||
| 5-HT2A receptor (Ki, nM) | 0.85d | 3.16c | 3.80c |
| 5-HT2C receptor (Ki, nM) | 88d | 186c | 2.3c |
| α1 Adrenergic (Ki, nM) | 128d | 15c | 190c |
Acknowledgments
We thank John Bernal, Blake Harrington, and Christopher Cruz for expert technical assistance.
This work was supported in part by the Intramural Research program of the National Institutes of Health National Institute on Drug Abuse and National of Institutes of Health National Institute on Alcohol Abuse and Alcoholism; and by National Institutes of Health National Institute on Drug Abuse [Grant DA17918].
doi:10.1124/jpet.108.145458.
ABBREVIATIONS: 5-HT, serotonin; DOM, 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane; MDL100907, (±)2,3-dimethoxyphenyl-1-[2-(4-piperidine)-methanol]; ketanserin, 3-[2-[4-(4-fluorobenzoyl)piperidin-1-yl]ethyl]-1H-quinazoline-2,4-dione; ritanserin, 6-[2-[4-[bis(4-fluorophenyl)-methylidene]piperidin-1-yl]ethyl]-7-methyl-[1,3]thiazolo[2,3-b]pyrimidin-5-one; 2C-T-7, 2,5-dimethoxy-4-(n)-propylthiophenethylamine; DPT, dipropyltryptamine hydrochloride; FR, fixed ratio; CL, confidence limit; MK-212, 6-chloro-2(1-piperazinyl) pyrozine hydrochloride.
References
- Arunlakshana O and Schild HO (1959) Some quantitative uses of drug antagonists. Br J Pharmacol Chemother 14 48-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykstra LA (1990) Butorphanol, levallorphan, nalbuphine and nalorphine as antagonists in the squirrel monkey. J Pharmacol Exp Ther 254 245-252. [PubMed] [Google Scholar]
- Dykstra LA, Bertalmio AJ, and Woods JH (1988) Discriminative and analgesic effects of mu and kappa opioids: in vivo pA2 analysis. Psychopharmacol Ser 4 107-121. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Harrington AW, Eckler JR, Arshad S, Rabin RA, Winter JC, Coop A, Rice KC, and Woods JH (2005) Hallucinogen-like actions of 2,5-dimethoxy-4-(n)-propylthiophenethylamine (2C-T-7) in mice and rats. Psychopharmacology (Berl) 181 496-503. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Murnane KS, and Reissig CJ (2008a) The behavioral pharmacology of hallucinogens. Biochem Pharmacol 75 17-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Reissig CJ, Katz EB, Yarosh HL, Rice KC, and Winter JC (2008b) Hallucinogen-like effects of N,N-dipropyltryptamine (DPT): possible mediation by serotonin 5-HT1A and 5-HT2A receptors in rodents. Pharmacol Biochem Behav 88 358-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorella D, Palumbo PA, Rabin RA, and Winter JC (1995a) The time-dependent stimulus effects of R(-)-2,5-dimethoxy-4-methamphetamine (DOM): implications for drug-induced stimulus control as a method for the study of hallucinogenic agents. Psychopharmacology (Berl) 119 239-245. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, and Winter JC (1995b) The role of the 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs: I. Antagonist correlation analysis. Psychopharmacology (Berl) 121 347-356. [DOI] [PubMed] [Google Scholar]
- Fiorella D, Rabin RA, and Winter JC (1995c) Role of 5-HT2A and 5-HT2C receptors in the stimulus effects of hallucinogenic drugs: II. Reassessment of LSD false positives. Psychopharmacology (Berl) 121 357-363. [DOI] [PubMed] [Google Scholar]
- France CP, de Costa BR, Jacobson AE, Rice KC, and Woods JH (1990) Apparent affinity of opioid antagonists in morphine-treated rhesus monkeys discriminating between saline and naltrexone. J Pharmacol Exp Ther 252 600-604. [PubMed] [Google Scholar]
- Gerak LR and France CP (2007) Time-dependent decreases in apparent pA2 values for naltrexone studied in combination with morphine in rhesus monkeys. Psychopharmacology 193 315-321. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, and Rosecrans JA (1982) Discriminative stimulus properties of DOM and several molecular modifications. Pharmacol Biochem Behav 16 553-556. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Young R, and Rosecrans JA (1983) Antagonism of the effects of the hallucinogen DOM and the purported 5-HT agonist quipazine by 5-HT2 antagonists. Eur J Pharmacol 91 189-196. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC.
- Kehne JH, Baron BM, Carr AA, Chaney SF, Elands J, Feldman DJ, Frank RA, van Giersbergen PL, McCloskey TC, Johnson MP, et al. (1996) Preclinical characterization of the potential of the putative atypical antipsychotic MDL 100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. J Pharmacol Exp Ther 277 968-981. [PubMed] [Google Scholar]
- Kenakin TP (1982) The Schild regression in the process of receptor classification. Can J Physiol Pharmacol 60 249-265. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Assié MB, and Koek W (1997) Pharmacological characterization of in vivo properties of putative mixed 5-HT1A agonist/5-HT(2A/2C) antagonist anxiolytics. II. Drug discrimination and behavioral observation studies in rats. J Pharmacol Exp Ther 282 747-759. [PubMed] [Google Scholar]
- Koek W, Assié MB, Zernig G, and France CP (2000) In vivo estimates of efficacy at 5-HT1A receptors: effects of EEDQ on the ability of agonists to produce lower-lip retraction in rats. Psychopharmacology (Berl) 149 377-387. [DOI] [PubMed] [Google Scholar]
- Li JX, Rice KC, and France CP (2007) Behavioral effects of dipropyltryptamine in rats: evidence for 5-HT1A and 5-HT2A agonist activity. Behav Pharmacol 18 283-288. [DOI] [PubMed] [Google Scholar]
- Li JX, Rice KC, and France CP (2008) Discriminative stimulus effects of 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane in rhesus monkeys. J Pharmacol Exp Ther 324 827-833. [DOI] [PubMed] [Google Scholar]
- Nichols DE (2004) Hallucinogens. Pharmacol Ther 101 131-181. [DOI] [PubMed] [Google Scholar]
- Paronis CA and Bergman J (1999) Apparent pA2 values of benzodiazepine antagonists and partial agonists in monkeys. J Pharmacol Exp Ther 290 1222-1229. [PubMed] [Google Scholar]
- Ullrich T and Rice KC (2000) A practical synthesis of the serotonin 5-HT2A receptor antagonist MDL 100907, its enantiomer and their 3-phenolic derivatives as precursors for [11C]labeled PET ligands. Bioorg Med Chem 8 2427-2432. [DOI] [PubMed] [Google Scholar]
- Vickers SP, Easton N, Malcolm CS, Allen NH, Porter RH, Bickerdike MJ, and Kennett GA (2001) Modulation of 5-HT(2A) receptor-mediated head-twitch behaviour in the rat by 5-HT(2C) receptor agonists. Pharmacol Biochem Behav 69 643-652. [DOI] [PubMed] [Google Scholar]
- Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Bäbler A, Vogel H, and Hell D (1998) Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9 3897-3902. [DOI] [PubMed] [Google Scholar]
- Winter JC, Filipink RA, Timineri D, Helsley SE, and Rabin RA (2000) The paradox of 5-methoxy-N,N-dimethyltryptamine: an indoleamine hallucinogen that induces stimulus control via 5-HT1A receptors. Pharmacol Biochem Behav 65 75-82. [DOI] [PubMed] [Google Scholar]
- Woods JH, Bertalmio AJ, Young AM, Essman WD, and Winger G (1988) Receptor mechanisms of opioid drug discrimination. Psychopharmacol Ser 4 95-106. [DOI] [PubMed] [Google Scholar]