Abstract
The dopamine transporter (DAT) is a key mediator of dopaminergic neurotransmission and a major target for amphetamine. We found previously that protein kinase C (PKC) β regulates amphetamine-mediated dopamine efflux. Here, using PKCβ wild-type (WT) and knockout (KO) mice, we report a novel role for PKCβ in amphetamine-induced regulation of DAT trafficking and activity. PKCβ KO mice have less striatal surface DAT, [3H]dopamine uptake, and amphetamine-stimulated dopamine efflux, yet higher novelty-induced locomotor activity than WT mice. Although a short exposure (≤90 s) to amphetamine rapidly increases striatal surface DAT and [3H]dopamine uptake in WT mice, this treatment decreases surface DAT and [3H]dopamine uptake in KO mice. Increases in surface DAT and [3H]dopamine uptake are not evident in KO mice until a longer exposure (60 min) to amphetamine, by which time WT mice exhibit decreased surface DAT and dopamine uptake. The slowness of amphetamine-induced striatal DAT trafficking in PKCβ KO mice was mimicked by the use of a specific PKCβ inhibitor, LY379196, in WT mice. Furthermore, PKCβ KO mice exhibit reduced locomotor responsiveness to amphetamine compared with WT, which could be explained by reduced surface DAT and delayed amphetamine-induced DAT trafficking in KO mice. Our results indicate that PKCβ is crucial for proper trafficking of DAT to the surface and for functioning of DAT and amphetamine signaling, providing new insight into the role of PKCβ as an important regulator of dopaminergic homeostasis.
The dopamine transporter (DAT) is an integral membrane protein containing 12 putative transmembrane domains and cytosolic amino and carboxyl termini. It belongs to a superfamily of Na+/Cl--dependent neurotransmitter transporters. Dopaminergic transmission in the synapse is primarily terminated by removal of dopamine (DA) from presynaptic nerve terminals via DAT. DAT is also a primary target of psychostimulants such as amphetamine (AMPH). As a substrate, AMPH elicits reverse transport of DA through DAT, a mechanism underlying AMPH addiction. Dysfunctional DAT signaling is also implicated in various other neurodegenerative and psychiatric disorders such as Parkinson's disease, attention deficit/hyperactive disorder, and schizophrenia.
DAT trafficking and activity are regulated by a complicated protein network involving multiple protein kinases and DAT-interacting proteins (Torres, 2006), among which protein kinase C (PKC) is the most characterized. Longer term activation of PKC (>20 min) by phorbol 12-myristate 13-acetate, a general PKC activator, leads to increased DAT endocytosis and reduced DAT recycling, resulting in reduced surface DAT expression and activity in rat synaptosomes and cultured cells (Daniels and Amara, 1999; Melikian and Buckley, 1999; Loder and Melikian, 2003). In accordance, PKC inhibitors block phorbol 12-myristate 13-acetate-induced DAT internalization (Daniels and Amara, 1999; Melikian and Buckley, 1999; Chang et al., 2001). An endocytic motif in the C terminus of DAT that dictates PKC-regulated DAT internalization has recently been identified (Holton et al., 2005).
DAT trafficking and activity are also regulated by substrates. DAT internalization can be induced by persistent exposure to DAT substrates such as DA, AMPH, and methamphetamine (Chi and Reith, 2003; Melikian, 2004; Zahniser and Sorkin, 2004; Cervinski et al., 2005; Gorentla and Vaughan, 2005). In contrast, our lab found that there is an up-regulation in surface DAT expression elicited by AMPH that occurs during the time frame of its initial physiological effect (≤1 min) (Johnson et al., 2005a). Thus, DAT trafficking is biphasically regulated by substrate in a time-dependent manner. What remains controversial is whether substrate-induced DAT trafficking is mediated by PKC. It has been reported that the PKC inhibitor bisindoylmaleimide I (BIM I) blocks methamphetamine-induced down-regulation of DAT in LLC-PK1 cells (Cervinski et al., 2005) but does not block AMPH-induced DAT internalization in PC12 cells (Boudanova et al., 2008). Furthermore, our previous results suggest that more immediate effects of AMPH, namely AMPH-stimulated DA efflux, are mediated by PKC (Kantor and Gnegy, 1998; Cowell et al., 2000), in particular, PKCβ (Johnson et al., 2005b). Because PKCβ coimmunoprecipitates with DAT from rat striatum and is important for the ability of AMPH to elicit DA efflux (Johnson et al., 2005b), the rapid delivery of DAT to the surface upon short-term AMPH exposure could also be importantly mediated by PKCβ. Therefore, it is imperative to define roles of specific PKC isoforms that are potentially involved in DAT trafficking.
In this study, we used PKCβ knockout (KO) mice to study the role of PKCβ in AMPH-induced DAT trafficking and activity. We identify a novel role of PKCβ in the regulation of constitutive and AMPH-induced DAT trafficking and activity in vivo and in vitro.
Materials and Methods
Animals. Generation of PKCβ wild-type (WT) and KO mice was described previously (Leitges et al., 1996). PKCβ WT and KO mouse breeders, which had been backcrossed with C57BL/6J mice 10 times, were supplied by Dr. Robert W. Gereau IV (Washington University, St. Louis, MO). There were no significant differences in brain and body weight and life span and no brain abnormalities between WT and KO mice. Experimental WT and KO mice were age-(2-3 months old) and gender-matched. Mice had free access to standard Purina rodent chow and water and were maintained in a temperature- and humidity-controlled environment on a 12-h dark/light cycle with lights on at 7:00 AM. All animal use procedures complied with the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) and were approved by the University of Michigan Committee on the Use and Care of Animals.
Basal Surface DAT Expression in Striatal Synaptosomes. The basal surface DAT expression was determined in mouse striatal synaptosomes by sulfo-NHS-SS-biotin (Pierce Chemical, Rockford, IL) labeling and streptavidin bead (Pierce Chemical) pull-down as described previously (Johnson et al., 2005a). In brief, mice were sacrificed by cervical dislocation, and brains were rapidly removed. Mouse striatal tissues were dissected and homogenized in 0.32 M sucrose containing a cocktail of protease inhibitors (Complete Mini; Roche Diagnostics, Indianapolis, IN). Homogenates were centrifuged at 800g for 10 min at 4°C to remove cell debris. The supernatant was further centrifuged at 12,000g for 15 min at 4°C. The supernatant was removed, and the pellet was saved as crude synaptosomes. Striatal synaptosomes were suspended in oxygenated Krebs-Ringer bicarbonate (KRB) containing 24.9 mM NaHCO3, 1.2 mM KH2PO4, 146.2 mM NaCl, 2.7 mM KCl, 1.0 mM MgCl2, 10 mM glucose, 0.05 mM ascorbic acid, and 50 μM pargyline. One hundred percent of the biotinylated fraction and 1/10 of the total lysate fraction were resolved by SDS-polyacrylamide gel electrophoresis and immunoblotted using 1:2000 dilution of DAT antibody (MAB369; Millipore Bioscience Research Reagents, Temecula, CA). The membranes were further blotted with a goat anti-rat horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) with a 1:10,000 dilution. Total lysates were also blotted for actin as an internal control. Bands were visualized using the Pico Enhanced Chemiluminescence detection method (Millipore Corporation, Billerica, MA). Quantification of the bands was performed by the Scion Image software (Scion Corporation, Frederick, MD), and the mouse genotype difference in band density was analyzed by Student's t test using Prism 5 (GraphPad Software Inc., San Diego, CA).
Saturation Analysis of [3H]WIN 35,428 Binding in Striatal Synaptosomes. Binding of the DAT antagonist [3H]WIN 35,428 (specific activity, 85 Ci/mmol; PerkinElmer Life and Analytical Sciences, Waltham, MA) to striatal synaptosomes was determined as described previously (Reith and Coffey, 1994). Striatal synaptosomes were suspended in the assay buffer containing 0.32 M sucrose, 30 mM sodium phosphate, pH 7.4, and a cocktail of protease inhibitors (Roche Diagnostics). The binding assay was conducted in the presence of concentrations of [3H]WIN 35,428 ranging from 3 to 30 nM for 1 h at 4°C. Nonspecific binding was performed in the presence of cocaine (30 μM). Binding was terminated by the addition of 5 ml of ice-cold assay buffer, rapid filtration, and two more 5-ml washes. The radioactivity on the filters was measured by a Beckman LS5801 liquid scintillation counter (Beckman Coulter, Fullerton, CA). The maximal numbers of [3H]WIN 35,428 binding sites (Bmax) and affinities for radioligand binding to DAT (Kd) were determined by nonlinear regression analysis in Prism 5.0. The genotype difference in Bmax or Kd was analyzed by Student's t test.
AMPH-Induced DAT Trafficking in Striatal Synaptosomes. Based on our studies in the rat (Johnson et al., 2005a), short-term treatment (<1.5 min) with AMPH would induce greater DAT trafficking toward the surface (exocytosis) in WT mice, whereas longer AMPH treatment (≥15 min) would result in greater DAT trafficking away from surface (endocytosis) than Veh treatment. Therefore, the time course for AMPH-induced DAT trafficking was investigated in KO mice using WT mice as controls. Mouse striatal synaptosomes were prewarmed for 5 min at 37°C before the treatment with AMPH (10 μM) or KRB (Veh) for 0.5, 1.5, 15, or 60 min. The reaction was stopped by adding 1 ml of cold KRB and immediate centrifugation. Samples were washed two more times with 1 ml of cold KRB before biotinylation for surface DAT determination as described above. Surface DAT expression was calculated as the ratio of biotinylated DAT to total DAT. Data were also expressed as the relative surface DAT expression upon AMPH treatment versus Veh treatment (percentage of Veh) in the same time frame of drug exposure for comparison of genotype difference in DAT trafficking. Data were analyzed by a two-way analysis of variance (ANOVA) (genotype × time) with post hoc Bonferroni analysis.
To exclude potential developmental effects on basal and AMPH-induced DAT trafficking because of the constitutive knockout of PKCβ, striatal synaptosomes from WT mice were pretreated with either the PKCβ-specific inhibitor LY379196 (LY; 100 nM) or Veh for 1 h at 37°C before 0.5- or 60-min AMPH (10 μM) or Veh treatment. Surface DAT expression was determined by biotinylation as described above. Data were expressed as percentage of the Veh treatment or the LY treatment to investigate the effect of the specific PKCβ inhibitor on basal and AMPH-induced DAT trafficking in WT mice. Paired Student's t test was used to analyze the difference between the LY and the Veh treatment on DAT trafficking.
DA Uptake in Striatal Synaptosomes. Basal DA uptake kinetics was measured in striatal synaptosomes prepared from WT and KO mice. The uptake assay was performed in KRB with various concentrations of [3H]DA (specific activity, 23.5 Ci/mmol; PerkinElmer Life and Analytical Sciences) ranging from 30 nM to 3 μM for 1 min at 37°C. Nonspecific uptake of [3H]DA was determined by incubation of a parallel set of samples in the presence of 30 μM cocaine. The assay was terminated by the addition of 5 ml of cold KRB, rapid filtration over GF/C Whatman filters (Whatman, Clifton, NJ), followed by two more 5-ml cold KRB washes. The radioactivity on the filters was determined by a Beckman liquid scintillation counter. DA binding affinity for DAT (Km) and DA uptake velocity (Vmax) values were determined by nonlinear regression analysis using Prism 5. The mouse genotype difference in DA uptake kinetics (Vmax and Km) was analyzed by Student's t test.
[3H]DA uptake was also measured in striatal synaptosomes pretreated with Veh or AMPH (10 μM) for either 0.5 or 60 min. After the AMPH or Veh pretreatment, striatal synaptosomes were washed six times with 1 ml of cold KRB each time by centrifugation to remove residual AMPH. Synaptosomes were prewarmed at 37°C for 5 min, and then DA uptake was measured for 15 s at 37°C in the presence of 500 nM [3H]DA. Nonspecific DA uptake was determined in the presence of 30 μM cocaine. Data were expressed as picomoles of DA per milligram of protein per 15 s for each treatment. The difference in DA uptake between AMPH and Veh pretreated groups was analyzed by paired Student's t test for each genotype.
AMPH-Induced DA Efflux from Striatal Synaptosomes. Mouse striatal synaptosomes were prepared and loaded in chambers of a Brandel perfusion apparatus (Brandel SF-12; Brandel Inc., Gaithersburg, MD) onto Whatman GF/B filter disks. The chambers were perfused at 37°C with oxygenated KRB at a rate of 450 μl/min. AMPH-stimulated DA efflux does not require extracellular calcium (Kantor et al., 2001), so calcium was omitted from the perfusion buffer. After 20 min of perfusion to achieve a steady baseline of DA efflux, 1 μM, 10 and 100 μM AMPH were introduced to the synaptosomes sequentially (at fractions number 2, 8, and 14) for 2 min followed by perfusion with KRB. Perfusates (20 total) were collected at 2-min intervals into vials containing a final concentration of 50 mM HClO4, 25 μM sodium bisulfate, 25 μM EDTA, and 10 nM 2-aminophenol (an internal standard). DA was measured by high-performance liquid chromatography with electrochemical detection. Data were represented as picomoles of DA per milligram of protein per 2 min. A two-way ANOVA with repeated measures over time intervals was performed to analyze the mouse genotype difference in AMPH-induced DA efflux.
Striatal Dopamine Content. Striatal DA content was measured as described previously (Chen et al., 2007). In brief, mice were sacrificed by cervical dislocation, and striata were homogenized in 0.1 M perchloric acid containing 10 nM 2-aminophenol as an internal standard. Homogenates were centrifuged for 10 min at 10,000g. The supernatants were filtered through 0.2-μm filters, and DA was analyzed using high-performance liquid chromatography with electrochemical detection. Mouse strain differences in the striatal DA content were analyzed by Student's t test.
Locomotor Stimulation by Acute AMPH Treatment. Locomotor stimulation in response to AMPH treatment was measured in mouse home cages using radiotransmitter implantation and the Vital view data recording and analyzing system (Philips Respironics, Bend, OR) as described previously (Chen et al., 2007). In brief, transmitter-produced locomotor activity signals (both horizontal and vertical locomotion) were sent to receivers (model ER-4000 Receiver; Philips Respironics) placed underneath mouse home cages. Signals from the receivers were sent to a computer, and data were collected and processed by the Vital view data acquisition system (Philips Respironics). Locomotor activity is expressed in gross activity counts. This measurement does not differentiate high doses of AMPH-induced stereotyped behavior such as licking, sniffing, or focused head movement from locomotion. AMPH-stimulated activity from WT and KO mice in the first 30 min was also watched to determine whether prominent stereotyped behavior occurred.
A radiotransmitter (Philips Respironics) was implanted into the peritoneal cavity of each mouse as described previously (Chen et al., 2007). Surgery was performed under xylazine (10 mg/kg) and ketamine (100 mg/kg). Mice were individually housed after transmitter implants and allowed to recover for 7 days. One day before behavioral testing, mice housed in their home cages, with food and water ad libitum, were placed on the top of the transmitter receivers to allow for habituation to the testing environment.
For determination of genotype difference in locomotor activity induced by acute AMPH treatment, WT and KO mice were given an injection of saline (Sal) 2 h before either AMPH or Sal treatment to allow for habituation to the injection procedure. Then, mice were given either AMPH (1, 2, 3, 5, or 7 mg/kg i.p.) or Sal (intraperitoneal) injection at a volume of 10 mg/kg, and locomotor activity was recorded immediately after the injection. Data were expressed as the total locomotor counts in 80 min. A two-way ANOVA (genotype × AMPH dose) using Prism 5.0 was performed to analyze the main effect of genotype and AMPH dose and their interaction effect.
Novelty-Induced Locomotor Stimulation. To measure genotype difference in novelty-induced locomotor activity, WT and KO mice implanted with radiotransmitters were taken out of their home cages and placed in unfamiliar rectangular boxes without bedding and food. Locomotor activity was monitored immediately after the exposure to the novel environment. Data were expressed as the locomotor activity summed over every 5 min for a total of 120 min. The genotype difference in locomotor activity was analyzed by a two-way ANOVA with repeated measures (genotype × time).
Chemicals. All chemicals used were from Sigma-Aldrich (St. Louis, MO), except those specified. AMPH sulfate was dissolved in 0.9% saline and injected at a volume of 10 ml/kg to mice. LY379196 was a generous gift from Eli Lilly & Co. (Indianapolis, IN).
Results
Reduced Surface DAT Expression in KO Mice. Surface DAT expression was determined by surface biotinylation and confirmed by binding of the DAT antagonist [3H]WIN 35,428 using striatal synaptosomes prepared from WT and KO mice. As indicated by biotinylation (Fig. 1A), KO mice had a significantly lower surface DAT expression than WT mice (Student's t test, p < 0.05, n = 6). There was no genotype difference in the total DAT expression (21.3 ± 2 and 20.7 ± 2 OD units in WT and KO mice, respectively). In agreement with the biotinylation data, the maximal binding of [3H]WIN 35,428 to intact striatal synaptosomes was reduced in KO mice compared with WT mice (Fig. 1B). The Bmax in WT mice (6.9 ± 0.4 pmol/mg protein, n = 5) was significantly greater than that in KO mice (4.2 ± 0.5 pmol/mg protein, n = 6; p < 0.05, Student's t test). There was no significant genotype difference in Kd values (7.3 ± 2 and 5.3 ± 3 nM for WT and KO mice, respectively).
Fig. 1.
Reduced striatal surface DAT expression in KO compared with WT mice. A, representative Western blot of the striatal surface and total DAT expression for WT and KO mice. Striatal synaptosomes were biotinylated, and bands were quantified to determine the surface and total DAT expression as described under Materials and Methods. KO mice showed less surface (biotinylated) DAT level than WT mice (Student's t test, p < 0.05, n = 6), whereas no significant genotype difference in the expression of the total DAT was observed. B, surface DAT was also compared between genotypes by determining binding of the DAT antagonist [3H]WIN 35,428 with striatal synaptosomes. Striatal synaptosomes from WT and KO mice were incubated with different concentrations of [3H]WIN35,428 for 1 h at 4 °C. Bmax was significantly lower for KO mice than for WT mice (Student's t test, p < 0.05, n = 5-6), whereas Kd values did not differ. Data are represented as mean ± S.E.M.
Reduced DAT Activity in KO Mice. To examine whether the reduction in striatal surface DAT in KO mice compared with WT mice results in reduced DAT function, both uptake of [3H]DA and AMPH-stimulated DA efflux were measured in striatal synaptosomes. As shown in Fig. 2A, the maximal DA uptake velocity (Vmax) was significantly lower in KO mice than in WT mice (23.0 ± 1.0 versus 32.4 ± 2.4 pmol/mg protein/min, n = 6, respectively, t test, p < 0.01). There was no significant genotype difference in DA binding affinities (0.12 ± 0.02 versus 0.20 ± 0.05 μM, n = 6 for KO and WT mice, respectively, p = 0.25).
Fig. 2.
Reduced DAT activity in KO compared with WT mice. KO mice showed significantly less DA uptake (A) and AMPH-stimulated DA efflux (B) from striatal synaptosomes than WT mice. A, striatal synaptosomes from WT and KO mice were incubated with various concentrations of [3H]DA for 1 min at 37°C. The Vmax value for [3H]DA uptake was significantly lower in KO mice than in WT mice (Student's t test, p < 0.05, n = 6), whereas there was no difference in Km values. B, striatal synaptosomes from WT and KO mice were perfused (37°C) for 20 min with KRB at a speed of 450 μl/min before sample collections (2 min interval). Then, 1, 10, and 100 μM AMPH were introduced to the synaptosomes at fraction numbers 2, 8, and 14, respectively, and KRB was perfused for the rest of the fractions. A two-way ANOVA indicated a significant genotype effect [F(1,191) = 5.18, p < 0.01]. Post hoc Bonferroni analysis indicated that a significant reduction in AMPH induced DA efflux at fractions 3, 4, 9, 10, 15, and 16. *, p < 0.05; **, p < 0.01 WT versus KO. Data are represented as mean ± S.E.M.
To examine whether DAT-mediated DA efflux was similarly reduced, DA efflux in response to various concentrations of AMPH was measured in striatal synaptosomes from WT and KO mice. As shown in Fig. 2B, efflux of DA in response to 1 (fraction numbers 3 and 4), 10 (fraction numbers 9 and 10), and 100 (fraction numbers 15 and 16) μM AMPH was lower in striatal synaptosomes from KO mice compared with WT mice. A two-way ANOVA (genotype × fraction) revealed a significant main effect of genotype [F(1,191) = 5.18, p < 0.01] on AMPH-induced DA efflux. There was also a significant interaction effect of genotype and fraction [F(18, 191) = 3.476, p < 0.01]. The total striatal content of DA did not differ between WT and KO mice (103 ± 10 and 93 ± 19 pmol/mg protein, respectively, n = 4).
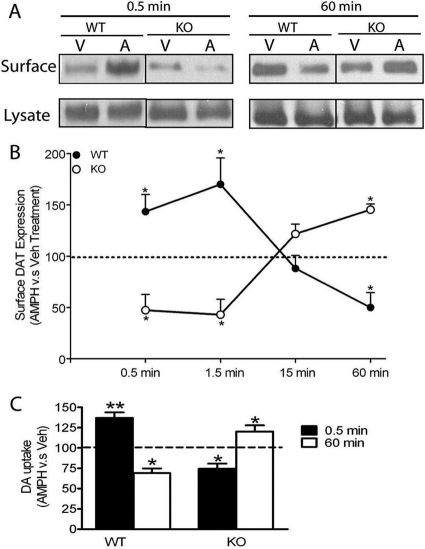
Differential Time-Dependent DAT Trafficking and Activity Induced by AMPH Treatment in Striatal Synaptosomes of WT and KO Mice. Our previous data suggested that PKCβ played a role in AMPH-stimulated DAT activity (Browman et al., 1998; Johnson et al., 2005b) and that PKCβ and DAT could be coimmunoprecipitated (Johnson et al., 2005b). The reduction in surface, but not total, DAT in KO mice compared with WT mice, as shown here, suggests that PKCβ could affect DAT activity by altering DAT trafficking. Therefore, we investigated whether substrate (AMPH)-induced changes in DAT surface content were altered in KO mice compared with WT mice. Striatal synaptosomes prepared from WT and KO mice were incubated with 10 μM AMPH at 37°C for either short times (0.5 and 1.5 min), when AMPH is known to increase surface DAT (Johnson et al., 2005a), or longer times (15 and 60 min), when AMPH-induced DAT internalization is detected (Sulzer and Galli, 2003). As shown in Fig. 3, A and B, there was a radically different time-dependent pattern of AMPH-induced DAT trafficking in striatal synaptosomes between WT and KO mice. A two-way ANOVA (time × genotype) revealed a significant interaction effect of genotype and time [F(3,42) = 10.90, p < 0.01]. As expected, a short exposure (i.e., 0.5 and 1.5 min) of AMPH to striatal synaptosomes significantly increased DAT surface levels in WT mice compared with the Veh treatment (paired Student's t test for AMPH versus Veh, p < 0.05, n = 8). Longer treatment (60 min) of synaptosomes from WT mice with AMPH switched from an increase in surface DAT content to a reduction compared with the Veh treatment (internalization) (paired Student's t test for AMPH versus Veh, p < 0.05, n = 5).
Fig. 3.
Differential time-dependent DAT trafficking and DAT activity upon short- or long-term AMPH treatment between WT and KO mice. A, representative blots of surface (biotinylated fraction) and total DAT expression upon short- and long-term A (10 μM) or V treatment for WT and KO mice. B, surface DAT level was expressed as the ratio of biotinylated DAT versus the total DAT. The data shown in B were calculated as the percentage of surface DAT level upon the AMPH treatment (biotinylated DAT/lysate DAT) relative to the Veh treatment for WT and KO mice and graphically demonstrated as a time course. A two-way ANOVA (time × genotype) revealed a significant interaction effect of genotype and time [F(3,42) = 10.90, p < 0.01]. Upon short-term AMPH exposure (0.5 and 1.5 min), there was a significant increase in surface DAT expression in striatal synaptosomes for WT mice (n = 8) and a significant decrease for KO mice (n = 5) compared with their control (Veh-treated) groups (AMPH versus Veh, paired Student's t test; *, p < 0.05). Upon long-term AMPH exposure (60 min), there was a significant decrease in surface DAT expression for WT mice (n = 5) and a significant increase for KO mice (n = 3) (AMPH versus Veh, paired Student's t test; *, p < 0.05). C, striatal synaptosomes were pretreated with AMPH or Veh for 0.5 or 60 min. Then, synaptosomes were washed extensively to remove residual AMPH before measurement of [3H]DA uptake. Data were expressed as the percentage of the [3H]DA uptake into synaptosomes pretreated with AMPH relative to Veh. Upon short-term AMPH exposure (0.5 min), there was a significant increase in [3H]DA uptake into synaptosomes from WT mice compared with their Veh-treated control groups (paired Student's t test, p < 0.01, n = 4) and a significant decrease for KO mice (paired Student's t test, p < 0.05, n = 4). Upon long-term AMPH exposure (60 min), there was a significant decrease in [3H]DA uptake into synaptosomes from WT mice (n = 6) and a significant increase for KO mice (n = 5) compared with control synaptosomes. *, p < 0.05; **, p < 0.01. Data are represented as mean ± S.E.M.
The effect of the AMPH treatment compared with Veh on surface DAT in PKCβ KO mice, however, was exactly the inverse of that in WT mice. In KO mice, very brief treatment (i.e., 0.5 or 1.5 min) with 10 μM AMPH decreased the surface DAT levels by 50% compared with the Veh treatment (paired Student's t test, p < 0.05, n = 5). Conversely, long-time AMPH exposure (i.e., 15 or 60 min) induced a significant increase in surface DAT in KO mice compared with the Veh treatment (paired Student's t test, p < 0.05, n = 3-5).
We examined whether there were functional consequences of the altered patterns of AMPH-induced DAT trafficking on DAT activity (i.e., DA uptake) in striatal synaptosomes from WT and KO mice. Striatal synaptosomes were exposed to 10 μM AMPH for either 0.5 or 60 min, followed by extensive washout of AMPH and measurement of [3H]DA uptake. [3H]DA uptake was measured at a short time (15 s) to minimize DA-induced trafficking, which could occur during the incubation period. As shown in Fig. 3C, consistent with the pattern of AMPH on DAT trafficking (Fig. 3B), pretreatment of striatal synaptosomes from WT mice with AMPH for 0.5 min significantly increased [3H]DA uptake compared with the Veh treatment (n = 4, paired Student's t test, p < 0.01). Conversely, pretreatment of striatal synaptosomes from KO mice for 0.5 min with AMPH significantly decreased [3H]DA uptake (n = 4, paired Student's t test, p < 0.05). Long-term treatment (60 min) with AMPH compared with Veh significantly decreased [3H]DA uptake in striatal synaptosomes from WT mice (n = 6, paired Student's t test, p < 0.05) but significantly increased [3H]DA uptake in synaptosomes of KO mice compared with the Veh treatment (n = 5, paired Student's t test, p < 0.05).
To confirm that the difference in basal and AMPH-induced DAT trafficking between WT and KO mice was due to the absence of PKCβ, rather than developmental adaptations, basal and AMPH-induced alterations in surface DAT were further investigated in the presence and absence of the specific PKCβ inhibitor, LY379196 (Jirousek et al., 1996). To determine whether a reduction in PKCβ activity would result in a change in surface DAT, striatal synaptosomes were preincubated with 100 nM LY or Veh (V) for 1 h, and surface DAT expression was measured. Incubation of WT synaptosomes with LY379196 mice significantly reduced basal surface DAT expression (LY versus Veh, paired Student's t test, n = 9, p < 0.05, Fig. 4A), mimicking the consequence of loss of PKCβ activity in KO mice. We then investigated, in WT synaptosomes incubated with LY379196, whether AMPH would demonstrate the reversed pattern of AMPH-stimulated DAT trafficking exhibited by KO mice. WT mouse striatal synaptosomes that were pretreated for 1 h with 100 nM LY379196 were challenged with either AMPH (A) or V for either 0.5 or 60 min. It is important that in WT mouse striatal synaptosomes pretreated with the PKCβ inhibitor, a 0.5-min challenge with 10 μM AMPH significantly reduced DAT trafficking to the surface compared with Veh controls (LY + V versus LY + 30-s AMPH, paired Student's t test, n = 4, p < 0.05, Fig. 4B), whereas a challenge with AMPH for 60 min increased surface DAT expression compared with controls (LY + V versus LY + 60-min AMPH, paired Student's t test, n = 6, p < 0.01, Fig. 4B). Thus, preincubation of synaptosomes from WT mice with the PKCβ inhibitor mimicked the pattern of AMPH-induced time-dependent DAT trafficking found in PKCβ KO mice (Fig. 3B).
Fig. 4.
Effect of PKCβ inhibition on basal and AMPH-induced DAT trafficking in WT mice. Striatal synaptosomes from WT mice were pretreated with either the PKCβ-specific inhibitor LY (100 nM) or V for 1 h at 37°C. Surface DAT expression was determined by biotinylation and quantified as described under Materials and Methods. Data were expressed as percentage of DAT surface expression (biotinylated DAT/lysate DAT) relative to control. A, incubation with 100 nM LY for 1 h at 37°C significantly reduced basal surface DAT expression compared with Veh treatment (LY versus V, paired Student's t test, p < 0.05, n = 9). B, striatal synaptosomes from WT mice were preincubated with 100 nM LY379196, then challenged with 10 μM A or V for either 0.5 or 60 min. Short-term (0.5 min) AMPH exposure resulted in a significant reduction in surface DAT expression compared with Veh exposure (LY + V versus LY + 30-sec AMPH, paired Student's t test, p < 0.05, n = 4), mimicking short-term AMPH-induced DAT trafficking in KO mice. Conversely, long-term AMPH treatment (60 min) increased surface DAT expression compared with Veh controls (LY + V versus LY + 60-min AMPH, paired Student's t test, p < 0.01, n = 6), a similar pattern to KO mice. Data are represented as mean ± S.E.M.
Increased Novelty-Induced Locomotor Stimulation in KO Mice. The profound differences in surface DAT between WT and KO mice should be apparent upon measurement of locomotor behavior in these mice. One might expect a baseline difference in locomotor behavior between genotypes because the surface level of striatal DAT was reduced in KO mice compared with WT mice. The resultant increase in synaptic DA in KO mice compared with WT mice could be expressed as hyperactivity. Although KO mice showed some hyperactivity in their home cages, the difference in activity between WT and KO mice was more clearly visualized when they were exposed to novelty. KO mice exhibited significantly more locomotor activity than WT mice when exposed to a novel environment (Fig. 5). A two-way ANOVA with repeated measurement of novelty-induced locomotor activity indicated a significant main effect of mouse genotype [F(1,207) = 20.27, p < 0.01, n = 6].
Fig. 5.
Increased novelty-induced locomotor activity in KO mice compared with WT mice. WT and KO mice (n = 6) were placed in a novel environment, and locomotor activity was recorded over 2 h. A two-way ANOVA (genotype × time) revealed a significant main effect of genotype [F(1,207) = 20.27, p < 0.01]. KO mice showed significantly higher locomotor activity than WT mice. Data are represented as mean ± S.E.M.
Reduced Behavioral Response to Acute AMPH Treatment in KO Mice. To minimize exaggerated responses to injection stress, mice were given an injection of Sal 2 h before the test injection of Sal or different doses of AMPH. Representative time courses over 4 h of locomotor activity for WT and KO mice were shown in Fig. 6A using data from the 3 mg/kg AMPH treatment or the Veh treatment. A two-way ANOVA (genotype × time) comparing AMPH-treated WT and KO mice revealed a significant main effect of genotype [F(1,1076) = 28.31, p < 0.0001] and time [F(75,1076) = 17.45, p < 0.0001] and a significant interaction effect of genotype and time [F(75,1076) = 1.602, p < 0.001]. KO mice responded to 3 mg/kg AMPH, but the WT mice showed significantly greater responsiveness. As shown in Fig. 6A and the legend to Fig. 6, post hoc Bonferroni analysis demonstrated a significant difference in locomotor activity stimulated by 3 mg/kg AMPH between WT and KO mice from 45 to 65 min after AMPH injection.
Fig. 6.
Decreased responsiveness to AMPH in KO mice compared with WT mice. A, time course of locomotor activity induced by 3 mg/kg AMPH or Sal in WT and KO mice. Mice were given an injection of Sal to reduce injection stress 2 h before either AMPH (3 mg/kg) or Sal, and locomotor activity was recorded for 4 h as described under Materials and Methods. A two-way ANOVA (genotype × time) comparing AMPH-treated WT and KO mice revealed a significant main effect of genotype [F(1,1076) = 28.31, p < 0.0001], time [F(75,1076) = 17.45, p < 0.0001], and a significant interaction effect of genotype and time [F(75,1076) = 1.602, p < 0.001]. KO mice exhibited significantly lower locomotor activity in response to 3 mg/kg AMPH than WT mice (n = 8-9). Bar, significant values determined by post hoc Bonferroni analysis. *, p < 0.05 for all values except for 50 min (**, p < 0.01). B, mice were given an injection of Sal 2 h before the test injection of Sal or AMPH at doses of 1, 2, 3, 5, or 7 mg/kg. Locomotor activity was summed over 80 min. A two-way ANOVA (genotype × dose) revealed a significant main effect of AMPH dose [F(5, 87) = 61.49, p < 0.01] and a significant interaction effect of genotype and dose [F(5, 87) = 4.79, p < 0.05]. Post hoc Bonferroni analysis indicated that AMPH significantly stimulated locomotor activity in WT mice at doses of 1, 2, 3, 5, and 7 mg/kg compared with their Sal injection (n = 6-12), whereas KO mice showed stimulation only at doses of 3, 5, and 7 mg/kg AMPH (n = 6-13). In addition, the Sal-treated KO mice consistently exhibited higher locomotor activity than Sal-treated WT mice (Student's t test, p < 0.05, n = 11-13). *, p < 0.05; **, p < 0.01, AMPH versus Sal within each genotype; #, p < 0.05, WT versus KO within each dose of AMPH.
We then assessed responsiveness of PKCβ KO mice to different doses of AMPH, summing the activity over 80 min, the area showing the biggest difference in activity in Fig. 6A. As demonstrated for novelty (Fig. 5), PKCβ KO mice showed hyperlocomotor activity upon injection stress. As shown in Fig. 6B, PKCβ KO mice in their home cages exhibited significantly more locomotor activity after an acute Sal injection than did WT mice (n = 9-10, Student's t test, p < 0.05); however, WT mice were considerably more responsive to AMPH than PKCβ KO mice. A two-way ANOVA (genotype × dose) revealed a significant main effect of AMPH dose [F(5,87) = 61.49, p < 0.01] and a significant interaction effect of genotype and dose [F(5, 87) = 4.79, p < 0.05]. WT mice showed significant locomotor stimulation to each dose of AMPH compared with Sal; however, PKCβ KO mice did not demonstrate significant locomotor stimulation over saline until the dose of 3 mg/kg AMPH. It is notable that WT mice, given an injection of 3 mg/kg AMPH that did not induce visible stereotyped behavior for both WT and KO mice, exhibited more locomotor activity than the corresponding AMPH-treated group of KO mice (n = 8-9, Student's t test, p < 0.05), which was clearly demonstrated in Fig. 6A. The dose of 5 mg/kg AMPH induced prominent stereotypy in WT mice but not in KO mice, which may explain the trend toward reduced locomotor activity compared with the dose of 3 mg/kg. The dose of 7 mg/kg AMPH induced visible stereotypy in both WT and KO mice.
Discussion
PKCβ Regulates DAT Function. In the present study, we have identified a novel role for PKCβ in the regulation of constitutive and AMPH-induced DAT trafficking and locomotor activity. The impeded responsiveness to AMPH in PKCβ KO mice suggests that PKCβ serves to maintain effective surface levels of DAT and regulates trafficking of DAT to the surface in response to substrate. It is important that the altered AMPH-stimulated trafficking of surface DAT in PKCβ KO mice was mirrored by changes in [3H]DA uptake and the reduced AMPH-stimulated locomotor activity in KO mice compared with WT mice.
PKCβ KO mice exhibit significantly less AMPH-stimulated DA efflux, a finding consistent with our previous report that a specific PKCβ inhibitor blocks AMPH-induced DA efflux from rat striatal slices (Johnson et al., 2005b). Because there was no genotype difference in the total DA content, and there is no compensational expression of other members of PKC isoforms (i.e., PKCα, PKCγ, PKCζ, PKCδ, and PKCε) in PKCβ KO mice compared with WT mice (Standaert et al., 1999; Weeber et al., 2000), the genotype difference in AMPH-induced DA efflux further confirms our hypothesis that PKCβ is a key regulator of AMPH-induced DA efflux, probably through direct or indirect phosphorylation of DAT (Johnson et al., 2005b). Most importantly, we found a novel role of PKCβ in constitutive and AMPH-regulated DAT trafficking.
PKCβ Alters Constitutive DAT Trafficking. DAT undergoes constitutive trafficking (Loder and Melikian, 2003; Holton et al., 2005; Sorkina et al., 2005), which is governed by an endocytic motif in the C terminus of DAT (Holton et al., 2005). The biochemical regulation of constitutive DAT trafficking, however, is unknown. Our data suggest that PKCβ is important in the maintenance of surface DAT under basal conditions, as evidenced by the reduced surface DAT expression level and reduced [3H]DA uptake in PKCβ KO mice compared with WT mice with no genotype difference in the total DAT expression. We are investigating presently whether PKCβ KO mice have an impaired DAT recycling rate, such as a deficiency in exit from the endoplasmic reticulum.
PKCβ Is Critical for Rapid DAT Exocytosis upon Short-Term AMPH Treatment. Our data strongly support the hypothesis that PKC in general, and PKCβ in particular, regulate the rate of AMPH-induced DAT trafficking. The rapid increase in surface DAT in response to AMPH from striata of WT mice is consistent with our previous report on rat striatal synaptosomes and HEK293 cells stably expressing human DAT (Johnson et al., 2005a). Likewise, the reduction in surface DAT in WT mice upon longer exposure to AMPH also has been reported in rat striatum and heterologous expression systems (Saunders et al., 2000; Gulley et al., 2002; Melikian, 2004; Zahniser and Sorkin, 2004). It is notable that upon short exposure to AMPH, there was a rapid decrease in surface DAT in PKCβ KO mice in contrast to a rapid increase in surface DAT in WT mice. It is important that we demonstrated that the rapid trafficking of DAT, in addition to the long-term regulation of surface DAT, has functional consequences for DAT activity. The altered trafficking of DAT in PKCβ KO mice compared with WT was clearly reflected by the changes in [3H]DA uptake.
The difference in AMPH-induced DAT trafficking between PKCβ WT and KO mice is not due to compensatory changes that occurred during development in PKCβ KO mice because AMPH-induced rapid DAT trafficking in WT mice was delayed in the presence of the specific PKCβ inhibitor (100 nM LY379196), similar to the DAT trafficking pattern in PKCβ KO mice. The concentration of DAT on the plasmalemmal surface is determined by rate constants of processes transporting DAT to the surface (exocytosis) and those transporting DAT away from the surface (endocytosis). The fact that AMPH rapidly elicited a decrease, as opposed to an increase, in surface DAT in KO mice suggests that PKCβ is intrinsically important for rapid transport of DAT to the surface. Because transport of DAT to the surface was slowed, but not stopped, it would appear that PKCβ is not absolutely required for substrate-induced DAT exocytosis but plays a major role in regulating the rate of exocytosis (rapid exocytosis). This is consistent with the finding of Johnson et al. (2005a) that the ability of AMPH to rapidly increase surface DAT reflected an increase in exocytosis of DAT, not a slowing of endocytosis. Boudanova et al. (2008) reported that 5 μM AMPH (10 min at 37°C) increased endocytic rates of DAT in PC12 cells; this process seems to be intact and potentially accelerated in PKCβ KO mice (Fig. 3B). In the knockout, the decrease in efficiency of exocytosis ultimately leads to facilitation of endocytosis such that there is no hindrance to processes promoting endocytosis. In addition, the source of the DAT and PKCβ that rapidly traffic to the surface is unknown, although we have shown that both PKCβI and PKCβII can coimmunoprecipitate with DAT under unstimulated conditions (Johnson et al., 2005b). It is possible that the cytoskeleton could be a site of interaction of PKCβ with DAT because PKCβ has profound effects on the actin cytoskeleton (Larsson, 2006), and PKCβII specifically binds to F-actin (Blobe et al., 1996).
Although PKC activation can clearly result in internalization of DAT, it is not entirely clear whether AMPH-stimulated DAT internalization is mediated by PKC. Exposure of LLC-PK1 cells to methamphetamine for 10 min induces DAT internalization, which is blocked by 10 μM BIM I (Cervinski et al., 2005). However, 1 μM BIM I did not block DAT internalization induced by 30 min of AMPH exposure in PC12 cells (Boudanova et al., 2008). In addition, the structural determinants in the C terminus of DAT required for PKC-mediated internalization were not required for AMPH-stimulated DAT internalization (Boudanova et al., 2008). The discrepancy could be attributed to different doses of the PKC inhibitor and DAT substrates, different cell lines, duration of drug incubation, different levels of DAT expression, or different species of DAT used in these studies (rDAT versus hDAT). We do not yet know whether PKCβ binds directly to DAT, although DAT coimmunoprecipitates with PKCβ in rat (Johnson et al., 2005b) and mouse striatum (unpublished observation). DAT trafficking and function are regulated by interactions with many other proteins, such as receptor for activated C kinase 1 (RACK1) (Lee et al., 2004), syntaxin 1 (Lee et al., 2004), protein interacting with C kinase-1 (PICK1) (Torres et al., 2001), dopamine D2 receptor (Bolan et al., 2007; Lee et al., 2007; Zapata et al., 2007), and DAT oligomerization (Torres et al., 2003; Chen and Reith, 2008). PKCβ may associate with a DAT adaptor protein to alter DAT recycling.
PKCβ Regulates Basal and AMPH-Induced Locomotor Behavior. A notable finding of our study is that alterations in both basal and AMPH-stimulated locomotor activity in PKCβ KO mice compared with WT were reflective of the altered trafficking of surface DAT. The higher basal locomotor activity in response to novelty observed in PKCβ KO mice compared with WT mice is consistent with the lower surface DAT expression level, which would result in higher levels of synaptic DA in KO mice. Increased basal locomotor activity is reported in mice with DAT deletion or DAT knockdown (Gainetdinov et al., 1999; Zhuang et al., 2001).
As might be expected from our data indicating that there is less AMPH-stimulated striatal DA efflux from PKCβ KO mice in vitro, PKCβ KO mice had a reduced behavioral response to lower doses of AMPH. These behavioral responses again mirror the biochemical data of surface DAT trafficking. The reduced surface DAT, in combination with the inability of AMPH to elicit a rapid trafficking of DAT to the membrane, could explain the hyperactivity of PKCβ KO mice and the fact that they were unresponsive to relatively low doses of AMPH (1 and 2 mg/kg) after the AMPH treatment. The delayed AMPH-stimulated increase in surface DAT in PKCβ KO mice was reflected by an increase in [3H]DA uptake 1 h after AMPH compared with the Veh treatment. The fact that both WT and KO mice achieved similar locomotor activity in response to high doses of AMPH suggests that the lack of PKCβ elicits a shift to the right in the dose-response curve for AMPH. However, an action of AMPH at other transporters, such as the serotonin transporter, could also contribute to AMPH-induced increases in locomotor behavior in PKCβ KO mice. The reduction in locomotor activity in response to AMPH in PKCβ KO mice compared with WT is in agreement with our previous report demonstrating that infusion of the PKC inhibitor (Ro31-8220) into the nucleus accumbens attenuated AMPH-stimulated locomotor behavior (Browman et al., 1998). Therefore, the genotype difference in acute AMPH-induced locomotor stimulation could be explained by less surface DAT expression, the slower AMPH-induced DAT trafficking, and/or less AMPH-induced DA efflux from PKCβ KO mice than from WT mice.
Our data also support the notion that the basal DAT expression level and locomotor activity dictate the strength of responsiveness to psychostimulant-induced locomotor activation (Zahniser and Sorkin, 2004). For example, DAT KO and DAT knockdown mice exhibiting high basal locomotor activity do not respond to AMPH stimulation behaviorally (Gainetdinov et al., 1999; Zhuang et al., 2001). PKCβ KO mice display higher basal locomotor activity and less AMPH-stimulated locomotor activity compared with WT, similar to the observation in DAT KO and DAT knockdown mice.
To summarize, PKCβ KO mice showed delayed DAT trafficking upon AMPH treatment in vitro and reduced responsiveness to AMPH treatment in vitro and in vivo. Our data suggest that PKCβ probably regulates the rate of response to AMPH through DAT trafficking and is important to the maintenance of surface levels of DAT. This study provides a new insight into the role of PKCβ as an important regulator of dopaminergic homeostasis.
Acknowledgments
We thank Conor Daining for assistance with animal husbandry, genotyping, and surgery.
This study has been supported by the National Institutes of Health National Institute on Drug Abuse [Grant DA11697]; and by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant NS48602].
doi:10.1124/jpet.108.147959.
ABBREVIATIONS: DAT, dopamine transporter; DA, dopamine; AMPH, amphetamine; PKC, protein kinase C; BIM I, bisindoylmaleimide I; KO, knockout; WT, wild type; KRB, Krebs-Ringer bicarbonate; WIN 35,428, 2β-carbomethoxy-3β-(4-fluorophenyl)tropane; Veh, vehicle; ANOVA, analysis of variance; LY, LY379196; Sal, saline; V, Veh; A, AMPH; Ro31-8220, bisindolylmaleimide IX.
References
- Blobe GC, Stribling DS, Fabbro D, Stabel S, and Hannun YA (1996) Protein kinase C beta II specifically binds to and is activated by F-actin. J Biol Chem 271 15823-15830. [DOI] [PubMed] [Google Scholar]
- Bolan EA, Kivell B, Jaligam V, Oz M, Jayanthi LD, Han Y, Sen N, Urizar E, Gomes I, Devi LA, et al. (2007) D2 receptors regulate dopamine transporter function via an extracellular signal-regulated kinases 1 and 2-dependent and phosphoinositide 3 kinase-independent mechanism. Mol Pharmacol 71 1222-1232. [DOI] [PubMed] [Google Scholar]
- Boudanova E, Navaroli DM, and Melikian HE (2008) Amphetamine-induced decreases in dopamine transporter surface expression are protein kinase C-independent. Neuropharmacology 54 605-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browman KE, Kantor L, Richardson S, Badiani A, Robinson TE, and Gnegy ME (1998) Injection of the protein kinase C inhibitor Ro31-8220 into the nucleus accumbens attenuates the acute response to amphetamine: tissue and behavioral studies. Brain Res 814 112-119. [DOI] [PubMed] [Google Scholar]
- Cervinski MA, Foster JD, and Vaughan RA (2005) Psychoactive substrates stimulate dopamine transporter phosphorylation and down-regulation by cocaine-sensitive and protein kinase C-dependent mechanisms. J Biol Chem 280 40442-40449. [DOI] [PubMed] [Google Scholar]
- Chang MY, Lee SH, Kim JH, Lee KH, Kim YS, Son H, and Lee YS (2001) Protein kinase C-mediated functional regulation of dopamine transporter is not achieved by direct phosphorylation of the dopamine transporter protein. J Neurochem 77 754-761. [DOI] [PubMed] [Google Scholar]
- Chen N and Reith ME (2008) Substrates dissociate dopamine transporter oligomers. J Neurochem 105 910-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Zhang M, Park S, and Gnegy ME (2007) C57BL/6J mice show greater amphetamine-induced locomotor activation and dopamine efflux in the striatum than 129S2/SvHsd mice. Pharmacol Biochem Behav 87 158-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi L and Reith ME (2003) Substrate-induced trafficking of the dopamine transporter in heterologously expressing cells and in rat striatal synaptosomal preparations. J Pharmacol Exp Ther 307 729-736. [DOI] [PubMed] [Google Scholar]
- Cowell RM, Kantor L, Hewlett GH, Frey KA, and Gnegy ME (2000) Dopamine transporter antagonists block phorbol ester-induced dopamine release and dopamine transporter phosphorylation in striatal synaptosomes. Eur J Pharmacol 389 59-65. [DOI] [PubMed] [Google Scholar]
- Daniels GM and Amara SG (1999) Regulated trafficking of the human dopamine transporter: clathrin-mediated internalization and lysosomal degradation in response to phorbol esters. J Biol Chem 274 35794-35801. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Wetsel WC, Jones SR, Levin ED, Jaber M, and Caron MG (1999) Role of serotonin in the paradoxical calming effect of psychostimulants on hyperactivity. Science 283 397-401. [DOI] [PubMed] [Google Scholar]
- Gorentla BK and Vaughan RA (2005) Differential effects of dopamine and psychoactive drugs on dopamine transporter phosphorylation and regulation. Neuropharmacology 49 759-768. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Doolen S, and Zahniser NR (2002) Brief, repeated exposure to substrates down-regulates dopamine transporter function in Xenopus oocytes in vitro and rat dorsal striatum in vivo. J Neurochem 83 400-411. [DOI] [PubMed] [Google Scholar]
- Holton KL, Loder MK, and Melikian HE (2005) Nonclassical, distinct endocytic signals dictate constitutive and PKC-regulated neurotransmitter transporter internalization. Nat Neurosci 8 881-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC.
- Jirousek MR, Gillig JR, Gonzalez CM, Heath WF, McDonald JH 3rd, Neel DA, Rito CJ, Singh U, Stramm LE, Melikian-Badalian A, et al. (1996) (S)-13-[(dimethylamino)methyl]-10,11,14,15-tetrahydro-4,9:16,21-dimetheno-1H,13H-dibenzo[e,k]-pyrrolo[3,4-h][1,4,13]oxadiazacyclohexadecene-1,3(2H)-dione (LY333531) and related analogues: isozyme selective inhibitors of protein kinase C beta. J Med Chem 39 2664-2671. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Furman CA, Zhang M, Guptaroy B, and Gnegy ME (2005a) Rapid delivery of the dopamine transporter to the plasmalemmal membrane upon amphetamine stimulation. Neuropharmacology 49 750-758. [DOI] [PubMed] [Google Scholar]
- Johnson LA, Guptaroy B, Lund D, Shamban S, and Gnegy ME (2005b) Regulation of amphetamine-stimulated dopamine efflux by protein kinase C beta. J Biol Chem 280 10914-10919. [DOI] [PubMed] [Google Scholar]
- Kantor L and Gnegy ME (1998) Protein kinase C inhibitors block amphetamine-mediated dopamine release in rat striatal slices. J Pharmacol Exp Ther 284 592-598. [PubMed] [Google Scholar]
- Kantor L, Hewlett GH, Park YH, Richardson-Burns SM, Mellon MJ, and Gnegy ME (2001) Protein kinase C and intracellular calcium are required for amphetamine-mediated dopamine release via the norepinephrine transporter in undifferentiated PC12 cells. J Pharmacol Exp Ther 297 1016-1024. [PubMed] [Google Scholar]
- Larsson C (2006) Protein kinase C and the regulation of the actin cytoskeleton. Cell Signal 18 276-284. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Pei L, Moszczynska A, Vukusic B, Fletcher PJ, and Liu F (2007) Dopamine transporter cell surface localization facilitated by a direct interaction with the dopamine D2 receptor. EMBO J 26 2127-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Kim MY, Kim DH, and Lee YS (2004) Syntaxin 1A and receptor for activated C kinase interact with the N-terminal region of human dopamine transporter. Neurochem Res 29 1405-1409. [DOI] [PubMed] [Google Scholar]
- Leitges M, Schmedt C, Guinamard R, Davoust J, Schaal S, Stabel S, and Tarakhovsky A (1996) Immunodeficiency in protein kinase Cbeta-deficient mice. Science 273 788-791. [DOI] [PubMed] [Google Scholar]
- Loder MK and Melikian HE (2003) The dopamine transporter constitutively internalizes and recycles in a protein kinase C-regulated manner in stably transfected PC12 cell lines. J Biol Chem 278 22168-22174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melikian HE (2004) Neurotransmitter transporter trafficking: endocytosis, recycling, and regulation. Pharmacol Ther 104 17-27. [DOI] [PubMed] [Google Scholar]
- Melikian HE and Buckley KM (1999) Membrane trafficking regulates the activity of the human dopamine transporter. J Neurosci 19 7699-7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith ME and Coffey LL (1994) [3H]WIN 35,428 binding to the dopamine uptake carrier. II. Effect of membrane fractionation procedure and freezing. J Neurosci Methods 51 31-38. [DOI] [PubMed] [Google Scholar]
- Saunders C, Ferrer JV, Shi L, Chen J, Merrill G, Lamb ME, Leeb-Lundberg LM, Carvelli L, Javitch JA, and Galli A (2000) Amphetamine-induced loss of human dopamine transporter activity: an internalization-dependent and cocaine-sensitive mechanism. Proc Natl Acad Sci U S A 97 6850-6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkina T, Hoover BR, Zahniser NR, and Sorkin A (2005) Constitutive and protein kinase C-induced internalization of the dopamine transporter is mediated by a clathrin-dependent mechanism. Traffic 6 157-170. [DOI] [PubMed] [Google Scholar]
- Standaert ML, Bandyopadhyay G, Galloway L, Soto J, Ono Y, Kikkawa U, Farese RV, and Leitges M (1999) Effects of knockout of the protein kinase c beta gene on glucose transport and glucose homeostasis. Endocrinology 140 4470-4477. [DOI] [PubMed] [Google Scholar]
- Sulzer D and Galli A (2003) Dopamine transport currents are promoted from curiosity to physiology. Trends Neurosci 26 173-176. [DOI] [PubMed] [Google Scholar]
- Torres GE (2006) The dopamine transporter proteome. J Neurochem 97 (Suppl 1): 3-10. [DOI] [PubMed] [Google Scholar]
- Torres GE, Carneiro A, Seamans K, Fiorentini C, Sweeney A, Yao WD, and Caron MG (2003) Oligomerization and trafficking of the human dopamine transporter. Mutational analysis identifies critical domains important for the functional expression of the transporter. J Biol Chem 278 2731-2739. [DOI] [PubMed] [Google Scholar]
- Torres GE, Yao WD, Mohn AR, Quan H, Kim KM, Levey AI, Staudinger J, and Caron MG (2001) Functional interaction between monoamine plasma membrane transporters and the synaptic PDZ domain-containing protein PICK1. Neuron 30 121-134. [DOI] [PubMed] [Google Scholar]
- Weeber EJ, Atkins CM, Selcher JC, Varga AW, Mirnikjoo B, Paylor R, Leitges M, and Sweatt JD (2000) A role for the beta isoform of protein kinase C in fear conditioning. J Neurosci 20 5906-5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahniser NR and Sorkin A (2004) Rapid regulation of the dopamine transporter: role in stimulant addiction? Neuropharmacology 47 (Suppl 1): 80-91. [DOI] [PubMed] [Google Scholar]
- Zapata A, Kivell B, Han Y, Javitch JA, Bolan EA, Kuraguntla D, Jaligam V, Oz M, Jayanthi LD, Samuvel DJ, et al. (2007) Regulation of dopamine transporter function and cell surface expression by D3 dopamine receptors. J Biol Chem 282 35842-35854. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, and Hen R (2001) Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci U S A 98 1982-1987. [DOI] [PMC free article] [PubMed] [Google Scholar]