Abstract
Endothelial dysfunction and decreased production of nitric oxide (NO) by endothelial NO synthase (eNOS) are implicated in the pathogenesis of hypertension and insulin resistance. Because the potential influence of increased eNOS expression/activity on these parameters is unclear, the present study examined the effects of eNOS gene therapy on insulin resistance and blood pressure alterations in a fructose-induced hypertension model in rats. As predicted, 2 weeks of fructose consumption in the drinking water resulted in elevated systolic blood pressure and insulin resistance. These and other physiologic alterations were reversed within 2 weeks after a single intravenous injection of a vector containing the human eNOS cDNA (pcDNA3.1-eNOS), whereas injection of an empty vector (pcDNA3.1) was without effect. In support of the beneficial effects of pcDNA3.1-eNOS treatment being because of enhanced eNOS expression and activity, increased eNOS protein levels were documented in aorta, liver, kidney, and heart of fructose-treated rats injected with pcDNA3.1-eNOS, and corresponding elevations in nitrite/nitrate and cGMP concentrations were observed in urine. Furthermore, pcDNA3.1-eNOS treatment prevented fructose-induced decreases in expression levels of insulin receptor substrate-1, the p110 catalytic subunit of phosphatidylinositol 3-kinase, phosphorylated Akt, and phosphorylated AMP-activated protein kinases in liver, aorta, and skeletal muscle. The results of this study cumulatively indicate that gene therapy with human eNOS decreased fructose-induced hypertension and insulin resistance in rats and suggest potential signaling pathways that mediate these effects. These data highlight the potential utility of eNOS gene therapy in the treatment of hypertension and insulin resistance.
Nitric oxide (NO), a potent vasodilator constitutively produced by endothelial nitric-oxide synthase (eNOS), is thought to be the endothelium-derived relaxing factor that mediates relaxation in response to acetylcholine, bradykinin, and substance P in vascular beds (Rees and Moncada, 1989). eNOS is the predominant vascular NO synthase isoform and is responsible for the majority of NO production in the vasculature (Moncada and Higgs, 2006). In addition to its effects on the regulation of blood pressure and regional blood flow, NO influences vascular smooth muscle proliferation and inhibits platelet aggregation and leukocyte adhesion (Moncada and Higgs, 2006).
A number of lines of evidence implicate eNOS-derived NO as a pivotal regulator of blood pressure, vascular tone, and vascular homeostasis. In vivo inhibition of NO synthase activity by nonhydrolyzable analogs of l-arginine results in a dramatic increase in mean arterial blood pressure (Desjardins and Balligand, 2006), whereas mice genetically deficient in eNOS have impaired endothelium-dependent vasodilator responses to acetylcholine and are hypertensive (Huang et al., 1995). Moreover, decreased eNOS activity has been observed in both aortic endothelium and cardiac tissue of fructose-treated rats (Miatello et al., 2001) and gene delivery of naked human eNOS DNA results in improved endothelial function and a prolonged reduction in the systolic blood pressure of spontaneously hypertensive rats (Alexander et al., 2000). It is interesting, however, that human population studies have not demonstrated a link between the variation in the eNOS gene and hypertension (Kato et al., 1999).
Experimental evidence also suggests that NO is involved in the pathogenesis of diabetes and insulin resistance. NADPH oxidases in the vascular wall are activated in diabetes mellitus, leading to enhanced degradation of NO and the production of reactive oxygen species (Guzik et al., 2002). Furthermore, uncoupling of eNOS has been demonstrated in animal models of diabetes (Elrod et al., 2006). Together, these data indicate that diabetes and insulin resistance are characterized, at least in part, by endothelial dysfunction and potentially by altered eNOS expression and NO production. Insulin mediates its effects through binding to insulin receptors and triggering downstream signaling pathways, of which the most important is the phosphatidylinositol 3-kinase (PI3K)-Akt/protein kinase B (PKB) pathway. This pathway is involved in a variety of insulin responses, including transport of glucose through cell membranes, myocardial survival, and antiapoptotic effects in endothelial cells (Okumura et al., 2004; Yang et al., 2007). As such, PI3K-Akt pathway activation by eNOS-derived NO may result in improved endothelial function and rescue of impaired myocardial cells (Kawasaki et al., 2003; Wang et al., 2005).
We hypothesized that overexpression of eNOS and subsequent increase in NO production might be beneficial in attenuating hypertension and insulin resistance. Thus, the present study investigated the effects and underlying mechanisms of eNOS gene therapy on insulin resistance and blood pressure regulation in fructose-induced hypertensive and insulin-resistant rats. Our results indicate that eNOS gene therapy reduced systolic blood pressure and hyperinsulinemia in this model and identified potential signaling pathways involved in mediating these effects.
Materials and Methods
Materials. Materials were obtained from the following suppliers: polyclonal antibodies to β-actin, PI3K, Akt, phosphorylated Akt (p-Akt), AMP-activated protein kinase (AMPK), phosphorylated AMPK (p-AMPK), mitogen-activated protein kinase (MAPK), phosphorylated MAPK (p-MAPK), insulin receptor substrate (IRS)-1, IRS-2, human eNOS, and phosphorylated eNOS were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); mouse anti-rabbit horseradish peroxidase-conjugated secondary antibody was from Sigma-Aldrich (St. Louis, MO); enhanced chemiluminescence substrate (SuperSignal Substrate) was from Pierce Chemical (Rockford, IL); TRIzol reagent was from Promega (Madison, WI); plasmid purification kits were from Invitrogen (Carlsbad, CA); fructose, glucose, triglyceride, and cholesterol reagents were from Ningbo Cicheng Biocompany (Ningbo, China). A full-length cDNA of human eNOS was a generous gift from Dr. James K. Liao (Harvard University, Cambridge, MA).
Preparation of pcDNA3.1-eNOS. The human eNOS cDNA (BC069465) was cloned into the eukaryotic expression vector pcDNA3.1 (Invitrogen) using EcoRI and XhoI restriction sites. Subsequently, the recombinant vector was amplified in DH5α Escherichia coli and plasmid DNA was purified with an endotoxin-free plasmid purification kit (QIAGEN, Valencia, CA) according to the manufacturer's instructions.
Fructose Feeding Protocol. All animal experimental protocols complied with standards stated in the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) and were approved by The Academy of Sciences of China. Male Sprague-Dawley rats weighing 180 to 200 g were obtained from the Experimental Animal Center of Shanghai (Shanghai, People's Republic of China). Animals were housed at 25°C with 12-h light/dark cycles and allowed free access to normal rat chow and water throughout the study period. Rats were randomly assigned to different treatment groups and subjected to a 1-week adaptation period for systolic blood pressure measurements via the tail-cuff method as described below. After this (i.e., beginning at week 0), rats were fed normal rat chow and either normal water (n = 18) or water containing 10% fructose (n = 20) for a total of 5 weeks. Systolic blood pressure was measured weekly until week 5, and gene delivery protocols were undertaken at week 2 as described below and previously (Zhao et al., 2003).
Intravenous Delivery of pcDNA3.1 or pcDNA3.1-eNOS. At week 2 of the study, animals were anesthetized with ethyl ether before injections. The prepared vectors were dissolved in 0.9% NaCl (at 1 mg/ml) and slowly injected via the sublingual vein at a dose of 2.5 mg/kg body weight. Normal-treated rats received injection with empty pcDNA3.1 vector (N + pcDNA3.1), pcDNA3.1-eNOS (N + pcDNA-eNOS), or 0.9% NaCl (normal) (n = 6 per group). Fructose-treated rats either received injection with empty pcDNA3.1 vector (F + pcDNA3.1) or pcDNA3.1-eNOS (F + pcDNA-eNOS) (n = 10 per group).
Blood Pressure Measurement. Systolic blood pressure was measured weekly in conscious rats with a manometer-tachometer (Rat Tail NIBP System; ADInstruments Pty Ltd., Sydney, Australia) using the tail-cuff method. Rats were placed in a plastic holder mounted on a thermostatically controlled warm plate that was maintained at 35°C during measurements. An average value from five blood pressure readings (that differed by no more than 2 mm Hg) was determined for each animal after they became acclimated to the environment. All blood pressure measurements were made between 9:00 AM and 12:00 PM.
Serum and Urine Analyses. Just before gene delivery at week 2 of the study, approximately 1 ml of blood was drawn from the tail vein of each rat. After coagulation, serum was collected by centrifugation and stored at -80°C. Urine samples were collected over a 24-h period before gene delivery, as described previously (Zhao et al., 2003). Serum glucose was determined by the glucose oxidase/phosphohydrolase method, serum cholesterol by the cholesterol oxidase/phosphohydrolase method, and serum triglyceride by the glycerol phosphate oxidase/phosphohydrolase method as described elsewhere (Zhao et al., 2003). Insulin resistance was calculated using the homeostasis model assessment-insulin resistance (HOMA-IR) method. HOMA-IR significantly correlates with fasting plasma insulin levels and the inverse of the glucose infusion rate (1/glucose infusion rate) in both diabetic and nondiabetic subjects (Ikeda et al., 2001) and has been used previously in rodent models (Thulé et al., 2006). It is a convenient and accurate method for evaluating insulin resistance. Serum insulin levels were measured with a magnetic solid-phase enzyme immunoassay kit from BioChem ImmunoSystems (Rome, Italy). Serum and urine endothelin (ET)-1 concentrations were determined as described previously (Zhao et al., 2003). Urine cGMP and NO concentrations were determined with enzyme immunoassay kits (Cayman Chemical Ann Arbor, MI) according to the manufacturer's instructions. Two weeks after gene delivery (i.e., at week 4 of the study), serum and urine samples were collected and analyzed in a similar manner from six rats from each of the normal, N + pcDNA3.1, N + pcDNA-eNOS, F + pcDNA3.1, and F + pcDNA-eNOS groups. Urine sodium was measured on an AEROSET Clinical Chemistry System (Abbott Laboratories, Abbott Park, IL).
Western Blot Analysis. Two weeks after injection of DNA, empty vector, or vehicle, three rats from each group were anesthetized with pentobarbital (100 mg/kg i.p.), and skeletal muscles, aortas, hearts, kidneys and livers were excised, frozen in liquid nitrogen, and stored at -80°C. Tissue proteins were extracted using TRIzol reagent, and protein concentrations were estimated by the Bradford method. Twenty micrograms of protein per lane and prestained mol. wt. markers (Bio-Rad, Hercules, CA) were separated with 10% SDS-polyacrylamide gel electrophoresis gels and electrophoretically transferred onto polyvinylidene difluoride membranes. Membranes were then incubated at room temperature for 2 h with blocking solution comprised of 5% nonfat dried milk in 10 mM Tris-Cl, pH 7.5, 100 mM NaCl, and 0.1% Tween 20. Membranes were incubated overnight at 4°C with the indicated primary antibodies (eNOS, p-eNOS, Akt, p-Akt, IRS-1, IRS-2, PI3K, MAPK, p-MAPK, AMPK, p-AMPK, and β-actin) and then incubated with a mouse anti-rabbit secondary monoclonal antibody conjugated to horseradish peroxidase at room temperature for 2 h. After each incubation, the membranes were washed four times with 10 mM Tris-Cl, pH 7.5, 100 mM NaCl, and 0.1% Tween 20 at room temperature and developed with enhanced chemiluminescence.
Real-Time PCR Analysis of ET-1, ETA-R, and eNOS mRNA. RNA was extracted from frozen rat aortas using TRIzol reagent. The following oligonucleotide primers were used for amplification of the ET-1, ETA-R, and eNOS cDNAs from reverse-transcribed aortic RNA: ET-1 (forward), 5′-AAGCGTTGCTCCTGCTCCTCC-3′; ET-1 (reverse), 5′-TTCCCTTGGTCTGGTCTTTGTG-3′;ETA-R (forward), 5′-TGCTCAACGCCACGACCAAGT-3′; ETA-R (reverse), 5′-GGTGTTCGCTGAGGGCAATCC-3′; eNOS (forward), 5′-TATTTGATGCTCGGGACTGC-3′; and eNOS (reverse), 5′-AAGATTGCCTCGGTTTGTTG-3. After incubation with Moloney murine leukemia virus reverse transcriptase at 42°C for 15 min, the real-time PCR was carried out in a final volume of 20 μl consisting of 2 μl of cDNA, 0.8 μl of primers, and 1× SBYR green master mix (QIAGEN). Amplification was performed on an ABI7500 real-time PCR system (Applied Biosystems, Darmstadt, Germany). The cycling conditions were 40 cycles with 30 s at 95°C, 20 s at 60°C, and 20 s at 72°C after a preheating step of 5 min at 95°C. The quantities of specific ET-1, ETA-R, and eNOS transcripts were normalized to expression of glyceraldehyde-3-phosphate dehydrogenase.
Statistical Analysis. All data are expressed as mean ± S.E.M. and were analyzed using unpaired Student's t tests or analysis of variance, as appropriate. Statistical significance was defined as P < 0.05.
Results
Fructose Drinking Induces Hypertension and Changes in Plasma Insulin and Urine Osmolarity. All rats in the study were assessed for a variety of physiological parameters 2 weeks after randomization to either control or fructose-containing drinking water administration (Table 1). Compared with control, consumption of fructose-containing water resulted in increased systolic blood pressure, serum insulin and triglyceride levels, and urine volume and a significant decrease in urine osmolarity (all P < 0.05). HOMA-IR was also significantly increased in fructose-treated rats (P < 0.05). Conversely, there were no changes in serum glucose, cholesterol, or body weight. These data indicate that fructose administration induced hypertension that was associated with hyperinsulinemia, insulin resistance, hypertriglyceridemia, and hypo-osmolar diuresis.
TABLE 1.
Physiological parameters determined in rats after 2 weeks of administration of control or fructose-containing drinking water Rats were given normal drinking water (control) or fructose-containing drinking water (10% fructose) for a period of 2 weeks, after which time these parameters were assessed.
| Variable | Control Group (n = 18) | Fructose-Treated Group (n = 20) |
|---|---|---|
| Systolic blood pressure (mm Hg) | 119.4 ± 1.1 | 134.3 ± 1.2* |
| Glucose (mM) | 4.36 ± 0.20 | 4.57 ± 0.28 |
| Triglyceride (mM) | 0.47 ± 0.23 | 0.98 ± 0.51* |
| Cholesterol (mM) | 1.35 ± 0.17 | 1.49 ± 0.25 |
| Insulin (mIU/l) | 9.63 ± 0.21 | 12.33 ± 0.32* |
| HOMA-IR | 1.87 ± 0.24 | 2.78 ± 0.17* |
| Body weight (g) | 216 ± 3 | 219 ± 15 |
| Urine volume (ml/day/100g) | 4.1 ± 0.2 | 10.9 ± 0.7** |
| Urine osmolarity (mOsml/kg H2O) | 826 ± 110 | 499 ± 34* |
P < 0.05 vs. control group
P < 0.001 vs. control group
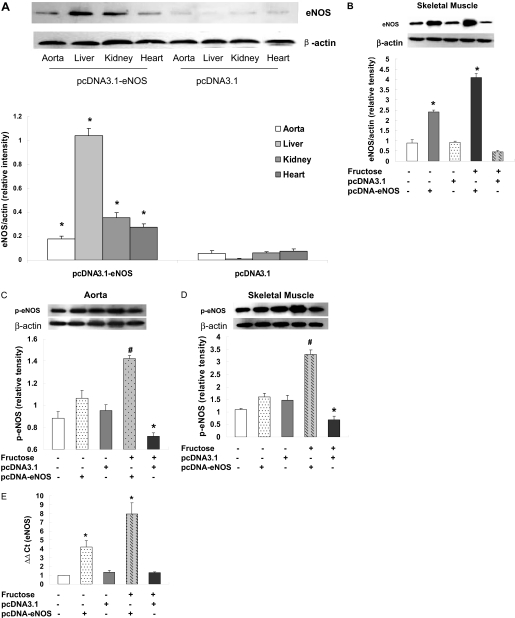
Human eNOS Gene Delivery Reduces Hypertension in Fructose-Treated Rats. Expression of human eNOS protein in fructose-treated rats was assessed by Western blotting 2 weeks after gene delivery (i.e., at week 4 of the study). Levels of eNOS protein were increased in the liver, kidney, heart, and aorta of F + pcDNA-eNOS rats compared with F + pcDNA3.1 rats at this time (Fig. 1A), indicating successful delivery of the vector to these tissues. The faint bands observed in the control group samples probably represent cross-reactivity of the antibody to endogenous rat eNOS. Likewise, eNOS expression was increased in skeletal muscle of pcDNA-eNOS rats compared with pcDNA3.1 rats in both fructose- and water-treated control conditions (Fig. 1B). Furthermore, phosphorylated-eNOS proteins were increased in aorta (Fig. 1C) and skeletal muscle (Fig. 1D) in rats receiving pcDNA-eNOS compared with pcDNA3.1 rats in fructose-treated animals, and eNOS mRNA levels quantitated by real-time PCR were also significantly increased in aorta of both fructose- and water-treated rats receiving eNOS (Fig. 1E).
Fig. 1.
eNOS protein levels in tissues of fructose-treated rats. Levels of eNOS protein were quantified by Western blotting and densitometry in aorta, liver, kidney, and heart tissue of fructose-treated rats 2 weeks after injection of pcDNA3.1-eNOS or empty pcDNA3.1 vector (A). Levels of eNOS protein were also quantified by Western blotting in skeletal muscle after injection of pcDNA3.1-eNOS or empty pcDNA3.1 vector and in the presence or absence of fructose in drinking water as indicated (B). p-eNOS (1177) protein was quantified by Western blotting in aorta (C) and skeletal muscle (D). eNOS mRNA levels were quantitated by real-time PCR in aorta (E). Blots are representative of individual rat samples from at least three independent experiments, which are quantified in the bar graphs (*, P < 0.05 from controls).
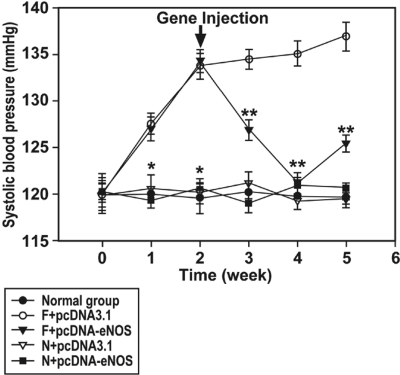
Administration of the pcDNA3.1-eNOS vector decreased systolic blood pressure at 1, 2, and 3 weeks after injection (i.e., at weeks 3, 4, and 5 of the study) in fructose-treated rats, whereas administration of the control pcDNA3.1 vector had no effect (Fig. 2). The maximum reduction in blood pressure in F + pcDNA-eNOS rats was observed 2 weeks after injection (i.e., at week 4 of the study) when levels reached 120.3 ± 0.5 mm Hg, similar to those observed in all three control water-treated groups (Fig. 2). Neither administration of pcDNA3.1 nor pcDNA3.1-eNOS vectors altered systolic blood pressure in control water-treated rats compared with rats injected with saline alone (Fig. 2). These data indicate that gene delivery of human eNOS reduces hypertension in fructose-treated animals but has no effect on blood pressure in control animals.
Fig. 2.
Systolic blood pressure is increased in fructose-treated rats and reduced by eNOS gene delivery. Systolic blood pressure was increased in fructose-treated rats compared with control (water-treated) rats for the duration of the study. Delivery of the human eNOS gene 2 weeks after initiation of fructose administration reduced systolic blood pressure but did not alter blood pressure in control (water-treated) animals. *, P < 0.01 versus fructose-treated groups; **, P < 0.01 versus F + pcDNA3.1 group; n = 6 to 10 per group for weeks 1–4; n = 3 to 7 per group for week 5.
Effects of eNOS Gene Delivery on Physiological and Biochemical Parameters. Physiological and biochemical parameters related to hypertension and hyperinsulinemia were assessed in animals in each experimental group 2 weeks after gene delivery (i.e., at week 4 of the study) (Table 2). Compared with the three water-treated control groups, serum insulin, insulin resistance (HOMA-IR), water consumption, and urine volume were all significantly greater, whereas urine osmolarity was significantly lower in the F + pcDNA3.1 group (all P < 0.05). Conversely, serum insulin and insulin resistance (HOMA-IR) were not altered in F + pcDNA-eNOS rats compared with the three water-treated control groups of rats (Table 2), whereas urine volume, water consumption, and osmolarity changes were partially restored to normal levels in F + pcDNA-eNOS rats. It is interesting that urine osmolarity was also significantly increased in N + pcDNA-eNOS rats compared with N + pcDNA3.1 rats (P < 0.05). Drinking fructose water significantly increased urinary sodium excretion; however, eNOS overexpression attenuated the change in urinary sodium (Table 2).
TABLE 2.
Physiological parameters determined in control and fructose-treated rats 2 weeks after injection of empty pcDNA3.1 vector or pcDNA3.1-eNOS N + pcDNA3.1, N + pcDNA-eNOS, and normal groups were given control water, and F + pcDNA3.1 and F + pcDNA-eNOS groups were given water containing 10% fructose. N + pcDNA3.1 and F + pcDNA3.1 groups were injected with empty pcDNA3.1 vector, N + pcDNA-eNOS and F + pcDNA-eNOS groups were injected with pcDNA3.1-eNOS, and the normal group was injected with saline. All parameters were measured at week 4 of the study (i.e., after 4 weeks on the respective water treatments and 2 weeks after injections).
| Variable | N + pcDNA3.1 (n = 6) | N + pcDNA-eNOS (n = 6) | Normal (n = 6) | F + pcDNA3.1 (n = 10) | F + pcDNA-eNOS (n = 10) |
|---|---|---|---|---|---|
| Glucose (mM) | 4.59 ± 0.25 | 4.78 ± 0.47 | 4.31 ± 0.33 | 4.81 ± 0.61 | 4.62 ± 0.63 |
| Triglyceride (mM) | 0.49 ± 0.11 | 0.44 ± 0.14 | 0.48 ± 0.13 | 0.58 ± 0.22 | 0.57 ± 0.13 |
| Cholesterol (mM) | 1.38 ± 0.09 | 1.42 ± 0.11 | 1.41 ± 0.12 | 1.44 ± 0.24 | 1.40 ± 0.21 |
| Insulin (mIU/l) | 9.43 ± 0.78 | 9.55 ± 1.09 | 9.40 ± 0.95 | 13.10 ± 0.95* | 9.25 ± 1.12# |
| HOMA-IR | 1.87 ± 0.12 | 1.78 ± 0.15 | 1.82 ± 0.13 | 2.88 ± 0.29* | 1.88 ± 0.18# |
| Body weight (g) | 250 ± 11 | 254 ± 9 | 249 ± 16 | 250 ± 7 | 246 ± 12 |
| Urine volume (ml/day/100 g) | 4.5 ± 0.6 | 4.2 ± 0.8 | 4.2 ± 0.4 | 20.9 ± 3.6* | 14.8 ± 4.0 |
| Urine osmolarity (mOsml/kg H2O) | 834 ± 109 | 1,381 ± 157* | 903 ± 32 | 169 ± 18* | 387 ± 77# |
| Water consumption volume (ml/day/100 g) | 5.95 ± 1.63 | 4.78 ± 0.51 | 5.40 ± 2.98 | 40.1 ± 1.74* | 34.29 ± 2.16*# |
| Urine sodium (mmol/24 h) | 719.8 ± 241.7 | 666.9 ± 120.3 | 700.3 ± 169 | 1475.1 ± 329.8* | 1150 ± 337.9 |
P < 0.05 vs. N + pcDNA3.1 group
P < 0.05 vs. F + pcDNA3.1 group
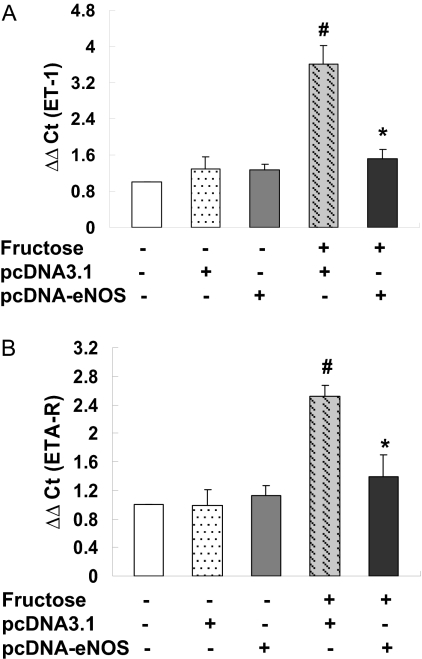
Aortic ET-1 and ETA-R mRNA Expression and ET-1 Levels in Serum and Urine. The expression of ET-1 and ETA-R mRNA transcripts in rat aortas was determined by real-time PCR 2 weeks after gene delivery (i.e., at week 4 of the study) to examine the effects of fructose feeding and human eNOS gene delivery on the endothelin pathway, which has been shown to play a role in blood pressure homeostasis. Fructose administration resulted in a significant increase in aortic ET-1 (Fig. 3A) and ETA-R (Fig. 3B) mRNA levels in rats injected with the empty pcDNA3.1 vector; these changes were attenuated in F + pcDNA-eNOS rats (Fig. 3). Specifically, aortic ET-1 and ETA-R transcript levels were 2.38- and 1.82-fold lower, respectively, in F + pcDNA-eNOS rats compared with F + pcDNA3.1 rats (P < 0.05).
Fig. 3.
Real-time PCR analysis of ET-1, ETA-R, and eNOS mRNA expression in aortic tissue. Levels of ET-1, ETA-R, and eNOS transcripts were assessed by real-time PCR using SYBR green in aortic tissue samples from three rats from each treatment group at week 4 of the study. Both ET-1 (A) and ETA-R (B) transcripts were significantly increased in fructose-treated rats injected with empty vector (F + pcDNA3.1 group) compared with all other groups. eNOS mRNA (C) was increased in both fructose-treated rats injected with pcDNA3.1-eNOS (F + pcDNA-eNOS group) and control (Normal) rats injected with pcDNA3.1-eNOS (N + pcDNA-eNOS). #, P < 0.05 versus all other groups; *, P < 0.05 versus F + pcDNA3.1 group; n = 3 per group.
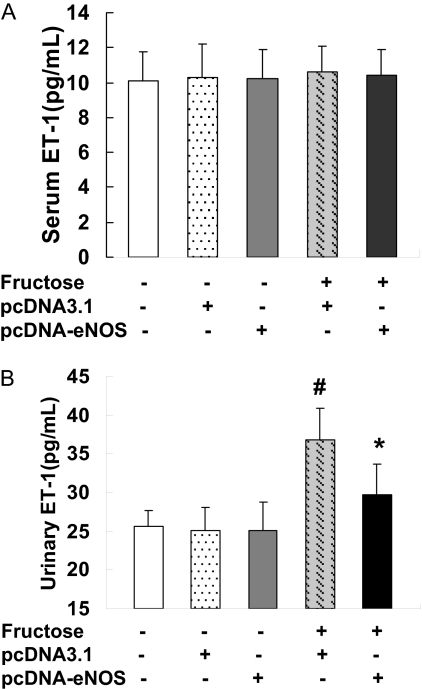
There were no significant differences in serum ET-1 concentrations between the groups (Fig. 4A). However, fructose administration resulted in increased urinary ET-1 levels in rats injected with pcDNA3.1, an effect that was not observed in F + pcDNA-eNOS rats (P < 0.05) (Fig. 4B).
Fig. 4.
Serum and urine ET-1 levels. Serum (A) and urine (B) ET-1 concentrations were quantified by enzyme-linked immunosorbent assay in samples collected from six rats per treatment group at week 4 of the study. Serum ET-A levels did not differ among the groups, whereas urine levels were elevated in fructose-treated rats injected with empty pcDNA3.1 vector (F + pcDNA3.1) but not in fructose-treated rats injected with pcDNA3.1-eNOS (F + pcDNA-eNOS). *, P < 0.05 versus normal group; #, P < 0.05 versus F + pcDNA3.1 group; n = 6 per group.
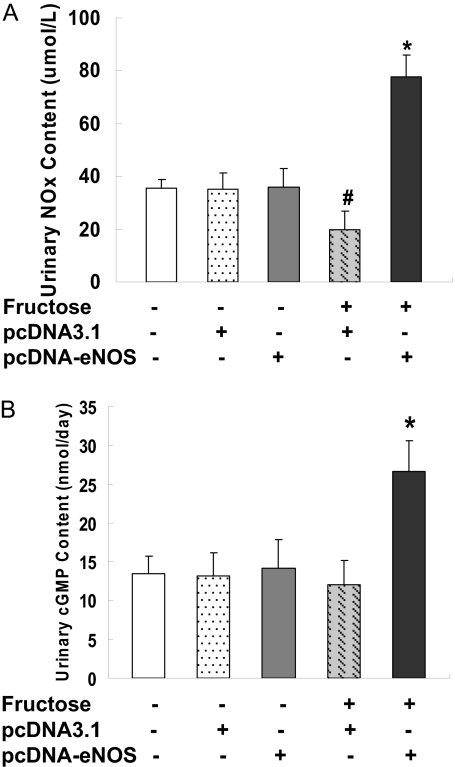
Increased Urinary cGMP and NO Levels after eNOS Gene Delivery. Urine samples collected 2 weeks after gene delivery (i.e., at week 4 of the study) were also assessed for cGMP and NO content. Both cGMP and NO [measured as nitrite + nitrate (NOx)] levels in urine were increased 2-fold in F + pcDNA-eNOS rats compared with F + pcDNA3.1 rats (both P < 0.05) (Fig. 5, A and B), suggestive of functional enzyme activity resulting from delivery of the eNOS cDNA via vector injection.
Fig. 5.
Urinary NOx content and cGMP levels. Urinary NOx content (A) and cGMP levels (B) were determined at week 4 of the study in six animals per treatment group. Both cGMP and NOx contents (measured as nitrite + nitrate) were increased in fructose-treated rats injected with pcDNA3.1-eNOS (F + pcDNA-eNOS group) compared with fructose-treated rats injected with empty pcDNA3.1 vector (F + pcDNA3.1 group) and control (Normal) rats. *, P < 0.05 versus normal group; #, P < 0.05 versus F + pcDNA3.1 group; n = 6 per group.
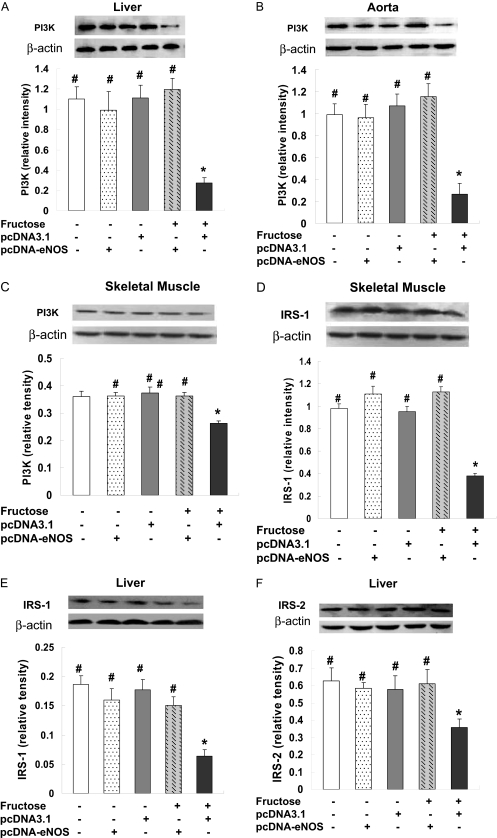
Effects of eNOS Gene Delivery on the PI3-Kinase/Akt Signaling Pathways. PI3K is recruited to IRS signaling complexes through binding of Src homology 2 domains in its 85-kDa regulatory subunit to specific phosphotyrosine residues in insulin receptor substrate (Virkamäki et al., 1999), a process that leads to activation of the PI3K p110 catalytic subunit. To investigate the signaling mechanisms through which eNOS attenuates fructose-induced insulin resistance, we evaluated the expression of PI3K and Akt associated with the insulin signaling cascade in several tissues. PI3K p110 catalytic subunit protein expression was significantly decreased in livers from F + pcDNA3.1-treated rats compared with normal controls, whereas pcDNA3.1-eNOS treatment increased p110 expression to normal levels (Fig. 6A). A similar expression pattern was observed in aorta (Fig. 6B) and skeletal muscle (Fig. 6C). IRS-1 protein expression was significantly decreased in skeletal muscle from F + pcDNA3.1-treated rats compared with normal rats, whereas pcDNA3.1-eNOS treatment restored IRS-1 expression to normal levels (Fig. 6D). Likewise, IRS-1 and IRS-2 protein levels were significantly decreased in livers from F + pcDNA3.1-treated rats, whereas pcDNA3.1-eNOS treatment restored IRS-1 (Fig. 6E) and IRS-2 (Fig. 6F) expression to normal levels.
Fig. 6.
eNOS gene delivery prevents the fructose-induced decrease in PI3K, IRS-1, and IRS-2 protein expression in liver, aorta, and skeletal muscle. PI3K (A–C), IRS-1 (D and E), and IRS-2 (F) protein levels were assessed by Western blotting in liver (A, E, and F), aorta (B), and skeletal muscle (C and D) from three rats per treatment group at week 4 of the study. Representative Western blots from one rat per group and densitometric analyses of three rats per group are shown. In all three tissues, PI3K protein levels were decreased in fructose-treated rats injected with empty pcDNA3.1 vector (F + pcDNA3.1 group) but not in fructose-treated rats injected with pcDNA3.1-eNOS (F + pcDNA-eNOS group). *, P < 0.05 versus normal group; #, P < 0.05 versus F + pcDNA3.1 group; n = 3 per group.
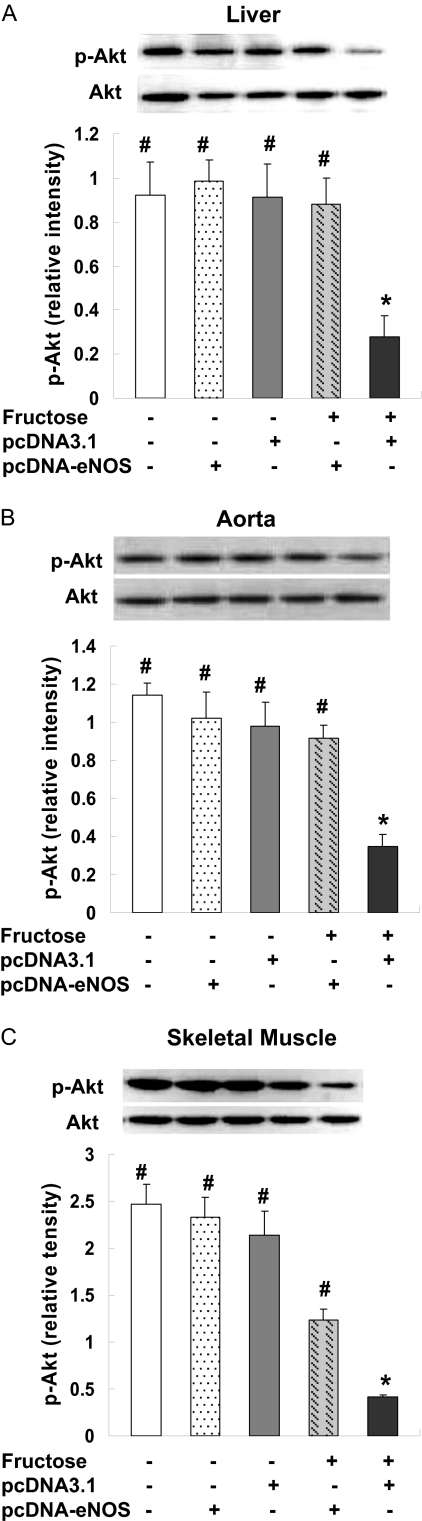
The expression of Akt, a serine/threonine kinase that lies downstream of PI3K, was also assessed in liver, aorta, and skeletal muscle. Because phosphorylation of Akt is required for its activation (Alessi and Cohen, 1998), levels of p-Akt at position Thr308 and of total Akt were determined by immunoblotting. A significant decrease of p-Akt was noted in livers of F + pcDNA3.1 rats compared with control animals, whereas the level of p-Akt in F + pcDNA-eNOS was comparable with that observed in control animals (Fig. 7A). There were no changes in total Akt levels between the groups. F + pcDNA-eNOS rats also had preserved p-Akt levels in aorta (Fig. 7B) and skeletal muscle (Fig. 7C) compared with pcDNA3.1 rats. Together, these data indicate that reduced insulin resistance in F + pcDNA-eNOS rats was associated with partial activation of the PI3K/Akt signaling pathway.
Fig. 7.
eNOS gene delivery prevents the fructose-induced decrease in p-Akt detection in liver, aorta and skeletal muscle. p-Akt levels were assessed by Western blotting in liver (A), aorta (B), and skeletal muscle (C) samples from three rats per treatment group at week 4 of the study. Representative Western blots from one rat per group and densitometric analyses of three rats per group are shown. In all tissues, p-AKT protein levels were decreased in fructose-treated rats injected with empty pcDNA3.1 vector (F + pcDNA3.1 group) but not in fructose-treated rats injected with pcDNA3.1-eNOS (F + pcDNA-eNOS group). Levels of Akt (unphosphorylated) did not differ among the treatment groups. *, P < 0.05 versus normal group; #, P < 0.05 versus F + pcDNA3.1 group; n = 3 per group.
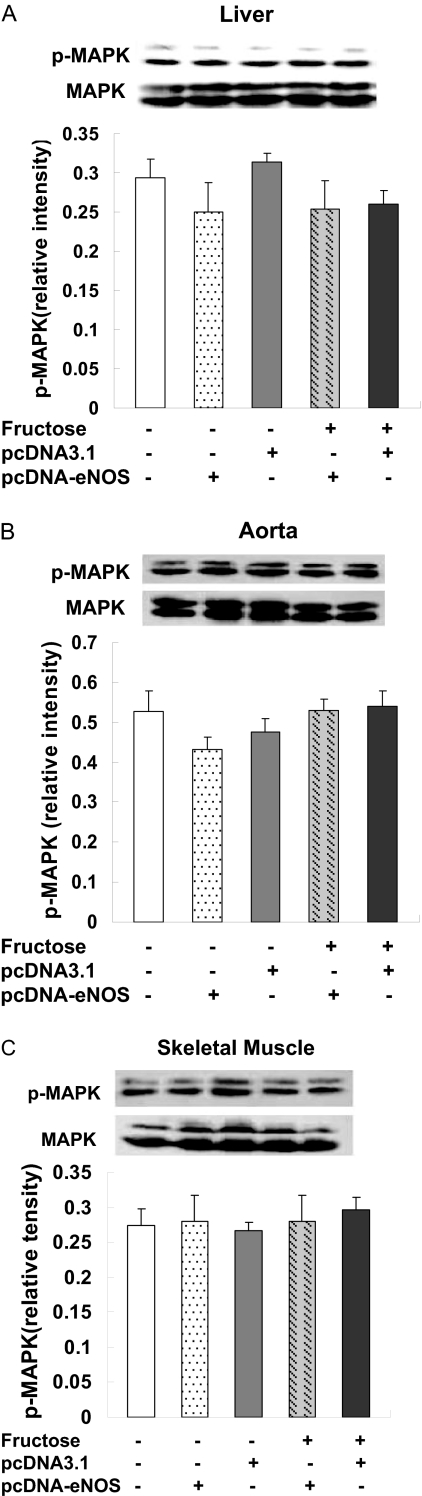
Effects of eNOS Gene Delivery on Activity of p42/44 MAPK and AMPK. Activation of the p42/44 MAPK signaling cascade is an important response to injury in multiple cell types and tissues. We found that p-MAPK levels, indicative of activation of this cascade, were largely unchanged in livers, aortas, and skeletal muscles of F + pcDNA3.1 rats compared with control rats. Likewise, p-MAPK levels were not different in the F + pcDNA-eNOS group, indicative of normal activation of the p42/p44 MAPK pathway by eNOS gene delivery (Fig. 8, A–C). Total MAPK levels did not differ between the treatment groups.
Fig. 8.
eNOS gene delivery does not effect p-MAPK and MAPK protein expression in liver, aorta, and skeletal muscle. p-MAPK levels were assessed by Western blotting in liver (A), aorta (B), and skeletal muscle (C) samples from three rats per treatment group at week 4 of the study. Representative Western blots from one rat per group and densitometric analyses of three rats per group are shown. In these tissues, MAPK phosphorylation was not significantly changed between the groups.
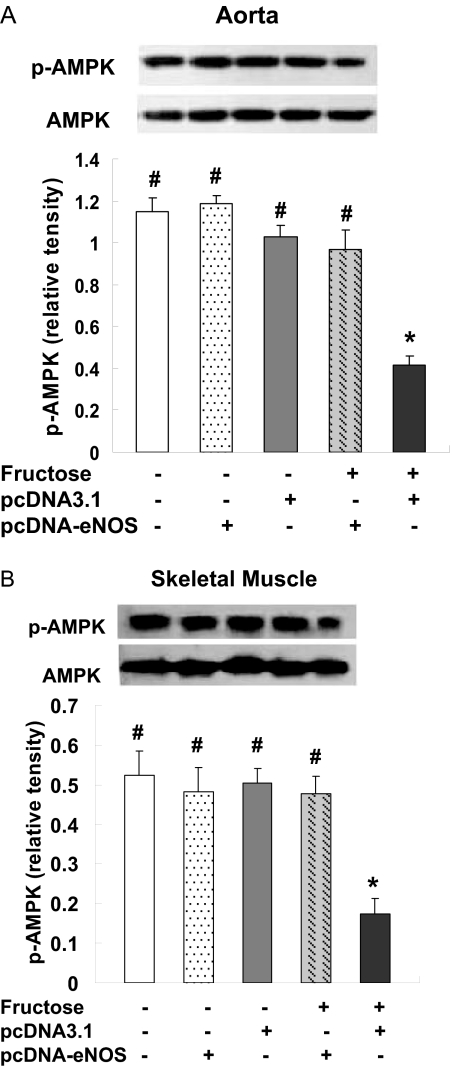
AMPK is a key sensor and regulator of intracellular and whole-body energy metabolism and is activated by phosphorylation. Compared with the control group, AMPK phosphorylation was down-regulated in aorta (Fig. 9A) and skeletal muscle (Fig. 9B) of F + pcDNA3.1 rats but not in F + pcDNA-eNOS rats, whereas total AMPK did not differ among the treatment groups. These results suggest that reversal of insulin resistance in pcDNA3.1-eNOS-treated rats was associated with activation of AMPK.
Fig. 9.
eNOS gene delivery prevents the fructose-induced decrease in p-AMPK in aorta and skeletal muscle. p-AMPK levels were assessed by Western blotting in aorta (A) and skeletal muscle (B) samples from three rats per treatment group at week 4 of the study. Representative Western blots from one rat per group and densitometric analyses of three rats per group are shown. In both aorta and skeletal muscle, p-AMPK protein levels were decreased in fructose-treated rats injected with empty pcDNA3.1 vector (F + pcDNA3.1 group) but not in fructose-treated rats injected with pcDNA3.1-eNOS (F + pcDNA-eNOS group). Levels of AMPK (unphosphorylated) did not differ among the treatment groups. *, P < 0.05 versus normal group; #, P < 0.05 versus F + pcDNA3.1 group; n = 3 per group.
Discussion
This study was undertaken to examine the effects of eNOS gene delivery in a fructose-induced hypertension and hyperinsulinemia model in rats. Our data indicate that systemic delivery of the human eNOS cDNA via a single i.v. injection of the eukaryotic expression vector pcDNA3.1 resulted in a reduction of blood pressure and improved sensitivity to insulin in fructose-treated rats. The expression of human eNOS protein was detected in the kidney, liver, heart, aorta, and skeletal muscle, and the beneficial effect of eNOS gene delivery on blood pressure lasted for at least 3 weeks. The ability of eNOS to attenuate insulin resistance in this study suggests that pathophysiological changes resulting from reduced NO production contribute to the development of hypertension, hyperinsulinemia, and insulin resistance and raises the potential for future therapeutic applications in treating insulin resistance-related hypertension via eNOS gene delivery.
Previous studies have demonstrated that rats treated with high-fructose drinking water develop systemic hypertension, hyperinsulinemia, and hypertriglyceridemia (Hwang et al., 1987; Thorburn et al., 1989) and that the fructose-induced increase in blood pressure can be mitigated by preventing hyperinsulinemia (Reaven et al., 1989). Although the pathophysiological mechanisms responsible for elevated blood pressure and hyperinsulinemia in fructose-treated animals are not completely understood, elevated sympathetic nervous system activity (Verma et al., 1999), impaired endothelium-dependent dilation (Richey et al., 1998), reduction of capillary permeability (Chakir et al., 1998), and elevated vascular expression of ET-1 and ETA receptor genes (Juan et al., 1998) have all been implicated. A direct relationship between hypertension and hyperinsulinemia has not yet been demonstrated. Although our results are not conclusive, they are consistent with these previous findings in that eNOS overexpression attenuated changes in fructose-induced ET-1 and ETA-R expression, blood pressure, and insulin sensitivity.
Angiotensin-converting enzyme inhibitors decrease blood pressure and improve insulin sensitivity in fructose-induced hypertensive and hyperinsulinemic rats, effects that have been shown to be dependent on NO production (Erlich and Rosenthal, 1996). We observed that human eNOS gene delivery resulted in increased tissue eNOS protein expression and increased urinary levels of NOx and cGMP, indicative of overexpression of functional eNOS protein. Thus, the beneficial effects of eNOS gene delivery on blood pressure in the present study may be because of, in part, vascular smooth muscle relaxation resulting from increased NO production and intracellular cGMP production. Moreover, the significant increase in urine volume observed in fructose-treated rats may be related to the hyperfiltration and hyperplasia of mesangial cells in glomeruli that have been observed by others (Manitius et al., 1995). Our observation of a reduction in urine volume in fructose-treated rats injected with pcDNA3.1-eNOS suggests a potential ameliorative effect of this treatment against hypertension-related end organ damage.
Endothelins are activated in deoxycorticosterone acetate salt-induced and -sensitive hypertension models, and a similar role for endothelins seems to be present in humans with the metabolic syndrome, type 2 diabetes mellitus, and obesity-related insulin resistance (Wollesen et al., 1999; el-Mesallamy et al., 2007; Feldstein and Romero, 2007). We found that vascular levels of ET-1 and ETA-R transcripts were higher in fructose-treated rats than in controls. However, serum levels of ET-1 and ETA-R transcripts and urinary ET-1 levels were significantly lower in fructose-treated rats injected with pcDNA3.1-eNOS than in fructose-treated rats injected with the empty pcDNA3.1 vector. This suggests that decreased activation of the endothelin pathway may have mediated some of the beneficial effects of human eNOS gene delivery on blood pressure and insulin resistance in our study.
The precise molecular mechanisms for attenuation of insulin resistance in fructose-treated rats remain to be elucidated. Recent studies indicate that the ability of insulin to vasodilate skeletal muscle vasculature is mediated by endothelium-derived NO (Steinberg et al., 1994). These effects may explain, at least in part, our observation of improved insulin sensitivity after eNOS gene delivery, although direct evidence is lacking. In addition, we probed the signaling molecules and pathways downstream from insulin/insulin receptors, including PI3K, AMPK, and MAPK pathways in skeletal muscle. We showed that PI3K p110, p-AKT, and p-AMPK protein expression were significantly decreased in skeletal muscle from F + pcDNA3.1-treated rats compared with normal controls, whereas pcDNA3.1-eNOS treatment increased their expression to normal levels. These results indicate that eNOS overexpression activates the insulin/insulin receptor-related signaling pathways and suggest that eNOS may potentiate insulin receptor signaling in muscles and thus improve insulin sensitivity.
Other data suggest that defects in insulin signaling, including PI3K and Akt pathways, contribute to insulin resistance in diabetic rodents and humans (Kohn et al., 1996; Kim et al., 1999). As such, we attempted to identify whether these and other signaling pathways were involved in mediating the beneficial effects of eNOS gene delivery on blood pressure and insulin resistance observed in this study. We found that the levels of the PI3K p110 catalytic subunit were significantly decreased in liver, aorta, and muscle of fructose-treated rats. eNOS treatment corrected these defects, indicating potential involvement of PI3K in mediating the effects of eNOS. Recent evidence indicates that the altered serine/threonine PKB (Akt) and PI3K signaling can affect events downstream from the insulin receptor (Shao et al., 2002). Sustained PI3K activation after insulin receptor activation in models such as gestational diabetes mellitus can be counter-productive because it enhances serine phosphorylation, reduces tyrosine phosphorylation, and increases degradation of IRS-1 (Shao et al., 2002; Ropelle et al., 2006). Down-regulation of IRS-1 protein and signaling leads to reduced glucose transporter 4 translocation to the plasma membrane. In contrast, the present study shows that eNOS overexpression maintains normal expression levels of both PI3K p110 and IRS-1 and maintains insulin sensitivity in fructose-fed rats. Akt is also involved in eliciting antiapoptotic effects of growth factors and metabolic effects of insulin (Cross et al., 1995; Bevan, 2001). More specifically, Akt isoforms are phosphorylated at the T-loop (Thr308 in PKB) by 3-phosphoinositide-dependent protein kinase 1, and this phosphorylation seems to be crucial for Akt activation (Burén et al., 2003). High glucose levels combined with high insulin have been shown to result in impaired sensitivity to insulin stimulation of Akt activity (Ferri et al., 1997). We evaluated Akt phosphorylation on Thr308 in skeletal muscle, liver, and aorta of rats and found that p-Akt was decreased in F + pcDNA3.1 rats but not in F + pcDNA-eNOS rats. In addition, we evaluated the phosphorylation status of AMPK because small molecule-mediated activation of AMPK represents a promising approach for the treatment of type 2 diabetes and the metabolic syndrome (Cool et al., 2006). We observed that AMPK phosphorylation was decreased in the liver of fructose-treated rats, but this was prevented by eNOS gene delivery. Combined, these data suggest that the improvement of insulin resistance after eNOS gene delivery was because of, at least in part, increased activation of PI3K, Akt, and AMPK signaling pathways.
In conclusion, we have demonstrated beneficial effects of gene therapy with human eNOS in a rat model of fructose-induced hypertension and hyperinsulinemia. These beneficial effects were associated with changes in systemic endothelin pathway activation and the activation of the PI3K/Akt and AMPK signaling pathways in skeletal muscle, liver, and aorta. The ability of eNOS gene delivery to exert a broad spectrum of beneficial effects in this model warrants further investigation of this approach to the treatment of hypertension associated with insulin resistance.
This research was supported in part by the Intramural Research Program of the National Institutes of Health National Institute of Environmental Health Sciences [Grant Z01 ES025034]; by the National 863 Plan Project [Grant 2004AA217121]; the National Education Ministration Project and Nature Science Foundation Committee Project [Grants 30540087, 30700377]; and the 973 program [Grant 2007CB512004].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.143396.
ABBREVIATIONS: NO, nitric oxide; eNOS, endothelial nitric-oxide synthase; PI3K, phosphatidylinositol 3-kinase; Akt, cellular homolog of the v-alt oncogene, an S/T protein kinase; PKB, protein kinase B; p, phosphorylated; AMPK, AMP-activated protein kinase; MAPK, mitogen-activated protein kinase; IRS, insulin receptor substrate; N, normal; F, fructose; HOMA-IR, homeostasis model assessment-insulin resistance; ET, endothelin; PCR, polymerase chain reaction; ETA-R, endothelin receptor A; NOx, nitrite + nitrate.
References
- Alessi DR and Cohen P (1998) Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev 8 55-62. [DOI] [PubMed] [Google Scholar]
- Alexander MY, Brosnan MJ, Hamilton CA, Fennell JP, Beattie EC, Jardine E, Heistad DD, and Dominiczak AF (2000) Gene transfer of endothelial nitric oxide synthase but not Cu/Zn superoxide dismutase restores nitric oxide availability in the SHRSP. Cardiovasc Res 47 609-617. [DOI] [PubMed] [Google Scholar]
- Bevan P (2001) Insulin signalling. J Cell Sci 114 1429-1430. [DOI] [PubMed] [Google Scholar]
- Burén J, Liu HX, Lauritz J, and Eriksson JW (2003) High glucose and insulin in combination cause insulin receptor substrate-1 and -2 depletion and protein kinase B desensitisation in primary cultured rat adipocytes: possible implications for insulin resistance in type 2 diabetes. Eur J Endocrinol 148 157-167. [DOI] [PubMed] [Google Scholar]
- Chakir M, Plante GE, and Maheux P (1998) Reduction of capillary permeability in the fructose-induced hypertensive rat. Am J Hypertens 11 563-569. [DOI] [PubMed] [Google Scholar]
- Cool B, Zinker B, Chiou W, Kifle L, Cao N, Perham M, Dickinson R, Adler A, Gagne G, Iyengar R, et al. (2006) Identification and characterization of a small molecule AMPK activator that treats key components of type 2 diabetes and the metabolic syndrome. Cell Metab 3 403-416. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, and Hemmings BA (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378: 785-789. [DOI] [PubMed] [Google Scholar]
- Desjardins F and Balligand JL (2006) Nitric oxide-dependent endothelial function and cardiovascular disease. Acta Clin Belg 61 326-334. [DOI] [PubMed] [Google Scholar]
- el-Mesallamy H, Suwailem S, and Hamdy N (2007) Evaluation of C-reactive protein, endothelin-1, adhesion molecule (s), and lipids as inflammatory markers in type 2 diabetes mellitus patients. Mediators Inflamm 2007: 73635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod JW, Duranski MR, Langston W, Greer JJ, Tao L, Dugas TR, Kevil CG, Champion HC, and Lefer DJ (2006) eNOS gene therapy exacerbates hepatic ischemia-reperfusion injury in diabetes: a role for eNOS uncoupling. Circ Res 99 78-85. [DOI] [PubMed] [Google Scholar]
- Erlich Y and Rosenthal T (1996) Contribution of nitric oxide to the beneficial effects of enalapril in the fructose-induced hyperinsulinemic rat. Hypertension 28 754-757. [DOI] [PubMed] [Google Scholar]
- Feldstein C and Romero C (2007) Role of endothelins in hypertension. Am J Ther 14 147-153. [DOI] [PubMed] [Google Scholar]
- Ferri C, Bellini C, Desideri G, Baldoncini R, Properzi G, Santucci A, and De Mattia G (1997) Circulating endothelin-1 levels in obese patients with the metabolic syndrome. Exp Clin Endocrinol Diabetes 105 (Suppl 2): 38-40. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, and Channon KM (2002) Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation 105 1656-1662. [DOI] [PubMed] [Google Scholar]
- Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, and Fishman MC (1995) Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature 377 239-242. [DOI] [PubMed] [Google Scholar]
- Hwang IS, Ho H, Hoffman BB, and Reaven GM (1987) Fructose-induced insulin resistance and hypertension in rats. Hypertension 10 512-516. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Suehiro T, Nakamura T, Kumon Y, and Hashimoto K (2001) Clinical significance of the insulin resistance index as assessed by homeostasis model assessment. Endocr J 48 81-86. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC.
- Juan CC, Fang VS, Hsu YP, Huang YJ, Hsia DB, Yu PC, Kwok CF, and Ho LT (1998) Overexpression of vascular endothelin-1 and endothelin-A receptors in a fructose-induced hypertensive rat model. J Hypertens 16 1775-1782. [DOI] [PubMed] [Google Scholar]
- Kato N, Sugiyama T, Morita H, Nabika T, Kurihara H, Yamori Y, and Yazaki Y (1999) Lack of evidence for association between the endothelial nitric oxide synthase gene and hypertension. Hypertension 33 933-936. [DOI] [PubMed] [Google Scholar]
- Kawasaki K, Smith RS Jr, Hsieh CM, Sun J, Chao J, and Liao JK (2003) Activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates nitric oxide-induced endothelial cell migration and angiogenesis. Mol Cell Biol 23 5726-5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YB, Nikoulina SE, Ciaraldi TP, Henry RR, and Kahn BB (1999) Normal insulin-dependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3-kinase, in muscle in type 2 diabetes. J Clin Invest 104 733-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn AD, Summers SA, Birnbaum MJ, and Roth RA (1996) Expression of a constitutively active Akt Ser/Thr kinase in 3T3–L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem 271 31372-31378. [DOI] [PubMed] [Google Scholar]
- Manitius J, Baines AD, and Roszkiewicz A (1995) The effect of high fructose intake on renal morphology and renal function in rats. J Physiol Pharmacol 46 179-183. [PubMed] [Google Scholar]
- Miatello R, Risler N, Castro C, González S, Rüttler M, and Cruzado M (2001) Aortic smooth muscle cell proliferation and endothelial nitric oxide synthase activity in fructose-fed rats. Am J Hypertens 14 1135-1141. [DOI] [PubMed] [Google Scholar]
- Moncada S and Higgs EA (2006) Nitric oxide and the vascular endothelium. Handb Exp Pharmacol 213-254. [DOI] [PubMed]
- Okumura H, Nagaya N, Itoh T, Okano I, Hino J, Mori K, Tsukamoto Y, Ishibashi-Ueda H, Miwa S, Tambara K, et al. (2004) Adrenomedullin infusion attenuates myocardial ischemia/reperfusion injury through the phosphatidylinositol 3-kinase/Akt-dependent pathway. Circulation 109 242-248. [DOI] [PubMed] [Google Scholar]
- Reaven GM, Ho H, and Hoffmann BB (1989) Somatostatin inhibition of fructose-induced hypertension. Hypertension 14 117-120. [DOI] [PubMed] [Google Scholar]
- Rees DD, Palmer RM, and Moncada S (1989) Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A 86 3375-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richey JM, Si X, Halter JB, and Webb RC (1998) Fructose perfusion in rat mesenteric arteries impairs endothelium-dependent vasodilation. Life Sci 62 PL55-62. [DOI] [PubMed] [Google Scholar]
- Ropelle ER, Pauli JR, Prada PO, de Souza CT, Picardi PK, Faria MC, Cintra DE, Fernandes MF, Flores MB, Velloso LA, et al. (2006) Reversal of diet-induced insulin resistance with a single bout of exercise in the rat: the role of PTP1B and IRS-1 serine phosphorylation. J Physiol 577 997-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J, Yamashita H, Qiao L, Draznin B, and Friedman JE (2002) Phosphatidylinositol 3-kinase redistribution is associated with skeletal muscle insulin resistance in gestational diabetes mellitus. Diabetes 51 19-29. [DOI] [PubMed] [Google Scholar]
- Steinberg HO, Brechtel G, Johnson A, Fineberg N, and Baron AD (1994) Insulin-mediated skeletal muscle vasodilation is nitric oxide dependent: a novel action of insulin to increase nitric oxide release. J Clin Invest 94 1172-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn AW, Storlien LH, Jenkins AB, Khouri S, and Kraegen EW (1989) Fructose-induced in vivo insulin resistance and elevated plasma triglyceride levels in rats. Am J Clin Nutr 49 1155-1163. [DOI] [PubMed] [Google Scholar]
- Thulé PM, Campbell AG, Kleinhenz DJ, Olson DE, Boutwell JJ, Sutliff RL, and Hart CM (2006) Hepatic insulin gene therapy prevents deterioration of vascular function and improves adipocytokine profile in STZ-diabetic rats. Am J Physiol Endocrinol Metab 290 E114-E122. [DOI] [PubMed] [Google Scholar]
- Verma S, Bhanot S, and McNeill JH (1999) Sympathectomy prevents fructose-induced hyperinsulinemia and hypertension. Eur J Pharmacol 373 R1-4. [DOI] [PubMed] [Google Scholar]
- Virkamäki A, Ueki K, and Kahn CR (1999) Protein-protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J Clin Invest 103 931-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wei X, Xiao X, Hui R, Card JW, Carey MA, Wang DW, and Zeldin DC (2005) Arachidonic acid epoxygenase metabolites stimulate endothelial cell growth and angiogenesis via mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling pathways. J Pharmacol Exp Ther 314 522-532. [DOI] [PubMed] [Google Scholar]
- Wollesen F, Berglund L, and Berne C (1999) Plasma endothelin-1 and total insulin exposure in diabetes mellitus. Clin Sci (Lond) 97 149-156. [DOI] [PubMed] [Google Scholar]
- Yang S, Lin L, Chen JX, Lee CR, Seubert JM, Wang Y, Wang H, Chao ZR, Tao DD, Gong JP, et al. (2007) Cytochrome P-450 epoxygenases protect endothelial cells from apoptosis induced by tumor necrosis factor-alpha via MAPK and PI3K/Akt signaling pathways. Am J Physiol Heart Circ Physiol 293 H142-H151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Wang P, Xiao X, Chao J, Chao L, Wang DW, and Zeldin DC (2003) Gene therapy with human tissue kallikrein reduces hypertension and hyperinsulinemia in fructose-induced hypertensive rats. Hypertension 42 1026-1033. [DOI] [PubMed] [Google Scholar]