Abstract
Increased dietary fat consumption is associated with colon cancer development. The exact mechanism by which fat induces colon cancer is not clear, however, increased bile acid excretion in response to high-fat diet may promote colon carcinogenesis. The farnesoid X receptor (FXR) is a member of the nuclear receptor superfamily, and bile acids are endogenous ligands of FXR. FXR is highly expressed in the intestine and liver where FXR is essential for maintaining bile acid homeostasis. The role of FXR in intestine cancer development is not known. The current study evaluated the effects of FXR deficiency in mice on intestinal cell proliferation and cancer development. The results showed that FXR deficiency resulted in increased colon cell proliferation, which was accompanied by an up-regulation in the expression of genes involved in cell cycle progression and inflammation, including cyclin D1 and interleukin-6. Most importantly, FXR deficiency led to an increase in the size of small intestine adenocarcinomas in adenomatous polyposis coli mutant mice. Furthermore, after treatment with a colon carcinogen, azoxymethane, FXR deficiency increased the adenocarcinoma multiplicity and size in colon and rectum of C57BL/6 mice. Loss of FXR function also increased the intestinal lymphoid nodule numbers in the intestine. Taken together, the current study is the first to show that FXR deficiency promotes cell proliferation, inflammation, and tumorigenesis in the intestine, suggesting that activation of FXR by nonbile acid ligands may protect against intestinal carcinogenesis.
Colon cancer is the third most common cancer and is the second major cause of cancer-related death in the United States. Consumption of high-fat diet and increased fecal excretion of bile acids is associated with elevated incidence of colon cancer (Hill, 1974; Stamp, 2002). However, the mechanism by which bile acids contribute to colorectal cancer is not clear. Farnesoid X receptor (FXR), a member of the nuclear receptor superfamily, is critical in maintaining bile acid and lipid homeostasis. The endogenous ligands for FXR are bile acids (Makishima et al., 1999; Parks et al., 1999; Wang et al., 1999). Targeted disruption of the fxr gene in mice clearly demonstrates the importance of this nuclear receptor in regulating bile acid homeostasis (Sinal et al., 2000; Kim et al., 2007a).
FXR is highly expressed in the liver, kidney, and intestines (Forman et al., 1995). In the intestine, FXR plays an important role in regulating bile acid enterohepatic circulation. Activation of FXR induces the expression of ileal bile acid binding protein (IBABP) and ileal bile acid transporters, components essential for bile acid enterohepatic circulation (Hwang et al., 2002; Lee et al., 2006). In addition, activation of FXR induces fibroblast growth factor 15 in the intestine, which is critical in suppressing hepatic bile-acid synthesis (Inagaki et al., 2005). The importance of FXR in intestinal health has been demonstrated further by the ability of FXR to suppress intestinal bacterial growth and colonization (Inagaki et al., 2006).
A role for FXR in suppressing carcinogenesis is emerging. An early study showed that expression of FXR was inversely related to the progression of human colorectal cancers and the degree of malignancy of colon cancer cell lines (De Gottardi et al., 2004). These results indicate that FXR expression levels may serve as an indicator for the degree of malignancy of colon cancer and may indicate a link between FXR and colon carcinogenesis in humans. Recent studies in mice showed that FXR deficiency caused liver hyperproliferation and ultimately leads to spontaneous hepatocarcinomas (Kim et al., 2007b; Yang et al., 2007). In addition, FXR expression has been shown to be elevated in Barrett's esophagus, decreased in esophagus adenoma, further decreased in esophagus adenocarcinoma, and an FXR antagonist has enhanced apoptosis in esophagus-derived cells (De Gottardi et al., 2006). FXR may also be involved in breast carcinogenesis with unclear mechanism (Swales et al., 2006; Journe et al., 2008).
To date, there are no comprehensive studies to evaluate the role of FXR in intestinal cell proliferation and carcinogenesis. In the current study, the effects of FXR deficiency on colon epithelial cell proliferation were determined in mice. In addition, the effect of FXR deficiency on intestinal carcinogenesis was evaluated by using two common murine intestine tumorigenesis models: APCmin mice and azoxymethane (AOM) treatment.
Materials and Methods
Intestinal Cancer Animal Models. To determine the role of FXR in intestinal carcinogenesis, two mouse models were used. APCmin mice, with a mutated APC gene, were used as a model for genetically induced intestinal carcinogenesis. Wild-type (WT) mice treated with AOM, a commonly used colon carcinogen, were used as a model for the chemically induced model. The APCmin mice develop polyps predominantly in the small intestine, whereas the AOM-induced polyps reside mainly in the colon and rectum. These two complimentary models have been widely used for the study of various factors in intestinal carcinogenesis (Nigro, 1985; Moser et al., 1990; Corpet and Pierre, 2003).
Animals. WT and APCmin mice, in the C57BL/6 genetic background, were obtained from The Jackson Laboratory (Bar Harbor, ME). FXR KO mice, with 10 generations backcrossed into the C57BL/6 genetic background, were described in detail previously (Sinal et al., 2000; Guo et al., 2006). APCmin/FXR KO mice, with both FXR deficiency and APC gene mutation, were generated by cross-breeding FXR KO mice with APCmin mice, and the double KO mice were selected by an established polymerase chain reaction genotyping method (Supplemental Fig. 1). One-year-old male and female APCmin and APCmin/FXR KO mice were used to evaluate the effect of FXR deficiency on polyp formation (n = 15/group for male and female APCmin mice and n = 20/group for male and female APCmin/FXR KO mice). All animals were housed in the Association for Assessment and Accreditation of Laboratory Animal Care-accredited facilities at the University of Kansas Medical Center under a standard 12-h light/dark cycle with access to chow and water ad libitum. All protocols and procedures were approved by the Institutional Animal Care and Use Committee at the University of Kansas Medical Center.
AOM Treatment. AOM was purchased from Sigma-Aldrich (St. Louis, MO). Both male and female WT and FXR KO mice (8–10 weeks old) were intraperitoneally injected with sterile saline with or without AOM (10 mg/kg body weight) once a week for 6 weeks. There were eight groups with n = 15 to 20 mice per group. The mice were euthanized 6 months after the last injection, and tissues were harvested for histopathological analysis and immunohistochemistry.
Morphometric Analysis of Ileum and Colon. Male WT and FXR KO mice, n = 6 mice per genotype, at 2 and 12 months of age, were used. Ileum and colon were collected and processed for standard H&E staining. In 10 random 20× (ileum) or 40× (colon) microscopic fields per mouse, the height of ileal villi and colon crypts was measured by using an ocular meter calibrated with a stage micrometer. In addition, the number of colon goblet cells was counted.
Polyp Histopathologic Analysis. Immediately after euthanasia, entire small intestine, colon, and rectum were removed and flushed gently with ice-cold PBS to clear the undigested and fecal materials. The intestines were then opened longitudinally and placed flat onto filter paper, grossly evaluated, and fixed overnight in 10% PBS-buffered formaldehyde. Polyps in the small intestine, colon, and rectum were examined using a dissecting microscope. The location, number, and diameter size of the polyps were recorded. Polyp size was measured by using an ocular micrometer. Polyps were then cut into longitudinal sections, embedded in paraffin, and sliced into 5-μm sections. For histopathologic diagnosis, the sections were H&E stained and evaluated independently by two pathologists (Dr. Xin Gao, Department of Pathology, Emory University; and Dr. David Pinson, Department of Pathology, University of Kansas Medical Center). The prevalence of polyps (percentage of animals with polyps), average polyp size, and polyp multiplicity (number of polyps/mouse) were calculated.
Immunohistochemistry Staining of Bromodeoxyuridine, TUNEL, FXR, and β-Catenin. To assess cell proliferation in the colon, both male and female WT and FXR KO mice (n = 6/group), at 2 or 12 months of age, were intraperitoneally injected with bromodeoxyuridine (BrdU) at 20 mg/kg body weight. Two hours later, the mice were euthanized, and the colon was dissected and fixed in 10% PBS-buffered formalin before embedding in paraffin. The BrdU immunostaining was carried out using a BrdU Detection Kit (BD Biosciences, San Diego, CA). The BrdU labeling index (percentage) was determined by the number of BrdU-positive nuclei in a total of 500 cells in 10 random 40× microscopic fields.
The colons of male WT and FXR KO mice at 2 and 12 months of age were used for TUNEL and β-catenin staining. FXR immunostaining was conducted in the colons of 2-month-old WT male mice. Apoptosis was detected by TUNEL staining by the In Situ Cell Death Detection kit obtained from Roche Applied Science (Indianapolis, IN). FXR and β-catenin protein were determined by immunostaining using an AEC kit for FXR detection (Zymed Laboratories, South San Francisco, CA) and an ABC kit for β-catenin detection (Vector Laboratories, Burlingame, CA). The primary antibodies for FXR and β-catenin were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) and Abcam Inc. (Cambridge, MA), respectively.
Real-Time Quantitative Polymerase Chain Reaction Assay. Male 2- and 12-month-old WT or FXR KO mice were euthanized, and colons were collected (n = 6 mice per genotype per age). Colons were opened longitudinally, quickly rinsed with ice-cold PBS, and snap frozen in liquid nitrogen. Total RNA was isolated with TRIzol reagent according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). The mRNA levels of genes involved in cell cycle progression, tumor suppression, and inflammation were quantified using the SYBR green chemistry with a standard protocol and an ABI Prism 7900 Detection system (Applied Biosystems, Foster City, CA). The mRNA levels of these genes were normalized to 18s mRNA levels. The primer sequences used for real-time quantitative polymerase chain reaction are listed in Supplemental Table 1.
Western Blot Analysis. Male 2- and 12-month-old WT or FXR KO mice were euthanized, and colons were collected (n = 6 mice per genotype per age). Pooled colon from 6 mice/group were homogenized in radioimmunoprecipitation assay lysis buffer (50 mM Tris HCl, pH 8.0, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 2 mM EDTA, and protease inhibitors). The protein concentration was determined by the BCA protein assay kit (Pierce Chemical, Rockford, IL). One hundred micrograms of protein from pooled tissue extracts was electrophoresed on SDS-polyacrylamide gel electrophoresis gel, transferred to nitrocellulose membranes, and blocked with 5% nonfat milk for 30 min at room temperature. Cyclin D1, c-Myc, β-catenin, and APC were detected by incubating membranes overnight at 4°C with primary antibodies. Membranes were then incubated with specific horseradish peroxidase-conjugated secondary antibodies for 2 h at room temperature. The bands corresponding to the correct molecular weight of each protein were identified by chemiluminescence detection system. The level of β-actin labeling was used as a loading control. The primary antibodies for cyclin D1, c-Myc, or β-actin were from Santa Cruz Biotechnology, Inc. and for APC were from Millipore Bioscience Research Reagents (Temecula, CA).
Statistical Analysis. The data were expressed as means ± S.E.M. The significance of cancer prevalence (number of polyps per mouse) between WT and FXR KO mice treated with AOM was analyzed by Fisher's exact test. For multiple group comparison, the data were analyzed by one-way analysis of variance followed by Student-Newman-Keuls test. For comparison between two groups, Student's t test was used. The level of significance was set at P < 0.05.
Results
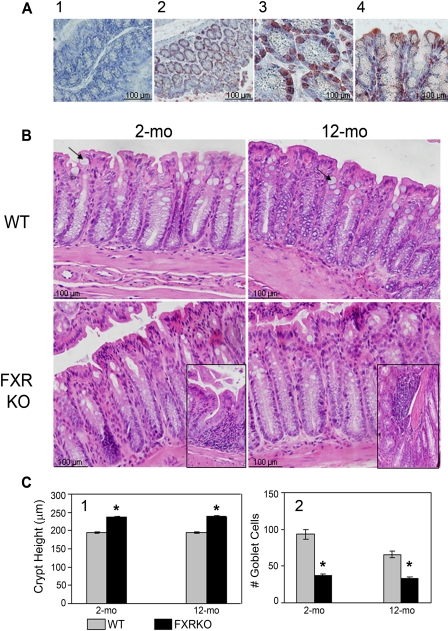
FXR Protein Expression in Colon and Effect of FXR Deficiency on Ileum and Colon Morphology. Previous studies have shown that FXR is highly expressed in the colon at the mRNA levels (Forman et al., 1995). The exact location of FXR in the colon is not clear. Therefore, the current study determined the location of FXR protein in the colon of mice, and the result showed that FXR was located predominantly in the epithelial cell nucleus and sparsely in the cytoplasm and the apical region of the crypts (Fig. 1A). FXR deficiency affected murine ileum and colon morphology examined at 2 and 12 months of age. FXR KO mice had similar ileum morphology compared with WT mice at both 2 and 12 months of age, except the height of villi was slightly higher in FXR deficiency mice (Supplemental Fig. 2). Two-month-old FXR KO mice had similar colon morphology compared with those of 2-month-old WT mice. However, 12-month-old FXR KO colon showed tall and almost villi-form papillary folds (Fig. 1B). In addition, FXR KO mice, regardless of age, had moderately increased colon crypt height (Fig. 1, B and C1). Furthermore, loci of lymphoid cells were detected with loss of FXR function (Fig. 1B, inserts). It is interesting that FXR deficiency seemed to significantly decrease the number of goblet cells (Fig. 1, B and C2).
Fig. 1.
Localization of FXR protein in the colon and effect of FXR deficiency on colon histomorphology. A, localization of FXR protein in the colon of WT mice (1, negative control; 2, cross-section of crypts, 20×; 3, cross-section of crypts, 40×; and 4, longitudinal section of crypts, 40×). B, H&E staining of colons from 2- and 12-month-old male WT and FXR KO mice (40×). Arrows, goblet cells and minimized pictures at the bottom show loci of lymphoid cells in the colon of FXR KO mice. C-1, quantification of average crypt height in the colons of these mice. C-2, quantification of the number of colon goblet cells per mouse per 40× macroscopic field gathered from 10 random fields. n = 6 per genotype per age. *, P < 0.05.
FXR Deficiency on Colon Cell Proliferation and Apoptosis. To directly determine the effect of FXR deficiency on colon cell proliferation, the BrdU labeling index (LI) was quantified in the colons of male and female WT and FXR KO mice at both 2 and 12 months of age. The 2-month-old male FXR KO mice showed increased BrdU labeling in the colon compared with WT mice. The BrdU LI decreased with age; however, FXR deficiency resulted in more reduction (Fig. 2, A and C, for male mice and Supplemental Fig. 3, A and B, for female mice). Paradoxically, FXR deficiency also increased the number of cells undergoing apoptosis, evaluated by the TUNEL staining (Fig. 2, B and D). Although the cell identity was not precisely determined, it seemed that more goblet cells were undergoing apoptosis than other cell types.
Fig. 2.
Colon cell proliferation determined by BrdU labeling index and apoptosis evaluated by TUNEL staining. All pictures are in 40× magnification. A, BrdU staining in the colon, a BrdU-positive cell is a cell with nuclei stained brown. B, TUNEL staining of the colon. C, quantification of the percentage of BrdU positively stained cell (LI). D, quantification of the percentage of TUNEL positively stained cells. *, P < 0.05.
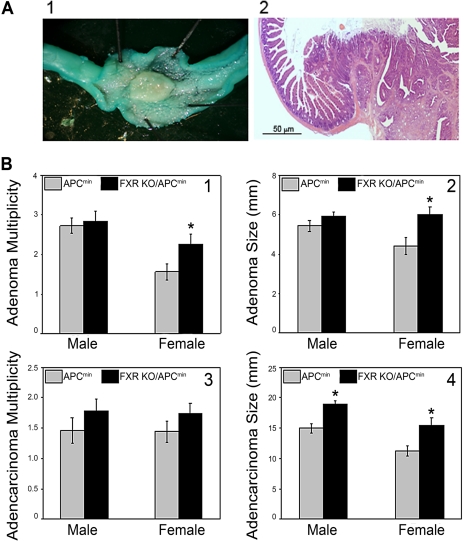
FXR Deficiency on Intestinal Carcinogenesis in APCmin Mice. In this study, all mice with the APC gene mutation developed both adenomas and adenocarcinomas in the small intestine. In particular, all the mice had a polyp with 10 to 20 mm in diameter between the stomach and duodenum (Fig. 3A and Supplemental Fig. 3C). These large polyps were diagnosed independently by two pathologists as noninvasive, papillary adenocarcinomas with minimal cystic mucosal degeneration. FXR deficiency in female, but not male, APCmin mice increased the adenoma multiplicity and average size (Fig. 3, B1 and B2). FXR deficiency did not increase the multiplicity of adenocarcinomas in either gender (Fig. 3B3); however, the size of adenocarcinomas moderately increased in both male and female FXR KO mice in the APCmin background (Fig. 3B4).
Fig. 3.
The effects of FXR deficiency on intestinal carcinogenesis in APCmin mice. A, small intestinal polyp stained with 0.5% methylene blue for 2 min (left) and a cross-section view of a small intestinal polyp (right; 10×). B1, adenoma multiplicity expressed as number of adenoma polyps per mouse. B2, average size of adenoma polyps. B3, adenocarcinoma multiplicity. B4, average size of adenocarcinoma polyps. n = 15 (male and female APCmin mice) and n = 20 (male and female APCmin/FXR KO mice). *, P < 0.05.
FXR Deficiency and AOM-Induced Colon Carcinogenesis. AOM treatment results in colon carcinogenesis; however, mice from the C57BL/6 genetic background have been shown to be relatively resistant to AOM-induced colon carcinogenesis (Nambiar et al., 2003). In agreement with this, the current study showed that a standard AOM treatment of WT mice in the C57BL/6 genetic background only resulted in 29% prevalence of adenocarcinoma in males and 7% in females (Fig. 4B1). The adenocarcinomas in these mice were diagnosed as noninvasive and papillary adenocarcinomas (Fig. 4A). FXR deficiency significantly increased adenocarcinoma prevalence, with 57% in male mice and 43% in female mice (Fig. 4B1). Furthermore, the average adenocarcinoma size was larger in FXR KO mice (Fig. 4B2). Adenocarcinoma multiplicity remained the same regardless of gender or FXR deficiency (data not shown). Consistent with a previous report showing AOM treatment producing polyps mainly in the colorectal region (Nigro et al., 1975), AOM treatment in the current study did not result in any small intestine adenocarcinomas.
Fig. 4.
Effects of FXR deficiency on adenocarcinoma development in AOM-treated mice. A, left, picture of a colon polyp stained with 0.5% methylene blue for 2 min. Right, cross-section view of a colon adenocarcinoma (10×). B1, adenocarcinoma prevalence (percentage of animals with adenocarcinomas) in male and female WT and FXR KO mice after AOM treatment. B2, average size of adenocarcinomas in male and female WT and FXR KO mice with AOM treatment. *, P < 0.05.
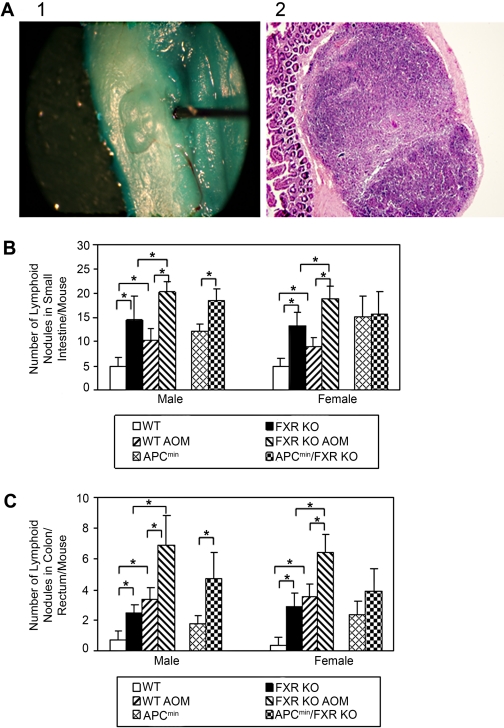
FXR Deficiency Increased Lymphoid Nodules in Small Intestines and Colon. In both male and female mice, the number of lymphoid nodules/mouse in small intestine and colon was significantly higher in FXR KO than in WT mice. These numbers further increased after APC gene mutation and AOM treatment in male mice and AOM treatment in female mice (Fig. 5). In APCmin female mice, FXR deficiency did not increase the number of lymphoid nodules (Fig. 5).
Fig. 5.
Effects of FXR deficiency on average number of intestinal lymphoid nodules per mouse. A, left, picture of a lymphoid nodule in the small intestine with 0.5% methylene blue staining for 2 min. Right, cross-section view of the same lymphoid nodule (10×). B, average number of small intestine lymphoid nodules per mouse in male and female WT, FXR KO, with treatment of vehicle or AOM, and in APCmin and FXR KO/APCmin mice. C, average number of colon/rectum lymphoid nodules per mouse in male and female WT, FXR KO, with treatment of vehicle or AOM, and in APCmin and FXR KO/APCmin mice. n = 15 per genotype per treatment per gender except for n = 20 for FXR KO/APCmin mice. *, P < 0.05.
FXR Deficiency and Expression of Genes Involved in Cell Proliferation, Tumor Suppression, and Inflammation in Colon. To understand the mechanism by which FXR deficiency enhances colon epithelial cell proliferation and carcinogenesis, mRNA levels of several genes widely known to be critical for cell proliferation, tumor suppression, or inflammation were quantified in the colons of 2- and 12-month-old male WT and FXR KO mice. This study analyzed mRNA levels of β-catenin, K-ras, c-myc, cyclin D1, cyclin E1, cyclin A2, CDKN1A, and mdm2 genes, genes known to be involved in cell cycle progression. For tumor suppression, this study quantified the mRNA expression of APC, Gadd45a, phosphatase and tensin homolog (PTEN), and p53. For inflammation, NFκB, ICAM, IL-1β, IL-6, and TNFα mRNA levels were quantified. Differences in mRNA levels were selectively presented with more than 50% reduction or 1.5-fold induction. In the colons of 2-month-old mice, FXR deficiency did not affect mRNA levels of β-catenin, c-myc, cyclin E1, cyclin A2, CDKN1A, mdm2, APC, Gadd45a, NFκB, IL-1β, or TNFα (data not shown) but did increase mRNA expression of cyclin D1 (1.6-fold), K-ras (2.1-fold), p53 (1.6-fold), ICAM (1.5-fold), and IL-6 (2.0-fold) (Fig. 6A). In the colons of 12-month-old mice, FXR deficiency did not alter mRNA levels of cyclins D1, E1, and A2, mdm2, p53, NFκB, ICAM, IL-1β, or TNFα (data not shown) but increased mRNA expression of CDKN1A (1.5-fold) and reduced mRNA levels of APC, Gadd45a, PTEN, and IL-6 to 40, 40, 40, and 30% of the levels in WT mouse colon, respectively (Fig. 6B).
Fig. 6.
Expression of genes involved in cell proliferation, tumor suppression, and inflammation in the colons of 2- and 12-month-old male WT and FXR KO mice. A, mRNA levels of genes in 2-month-old male mice. B, mRNA levels of genes in 12-month-old male mice. n = 6/genotype/time point. *, P < 0.05. C, Western blot analysis of cyclin D1, c-Myc, β-catenin, and APC protein in the colon of 2- and 12-month-old WT and FXR KO male mice, with n = 6 per group. D, immunohistochemistry staining of β-catenin in the colon of 2- and 12-month-old WT and FXR KO male mice.
We further evaluated protein levels of several genes critical for cell proliferation (Fig. 6C). In agreement with increased mRNA levels of cyclin D1 in 2-month-old FXR KO mice, the protein levels of cyclin D1 were also elevated. It is interesting that although mRNA levels of β-catenin and c-Myc were not altered in the colons of 2-month-old FXR KO mice, their protein levels were increased. Furthermore, although mRNA levels of APC showed no change at 2 months of age and moderately decreased at 12 months of age in the colons of FXR KO mice compared with WT mice, protein levels were increased at both ages. The immunoblot density of APC protein was quantified with the results shown as WT colon at 2 months old, 1.0; FXR KO colon at 2 months old, 3.2; WT colon at 12 months old, 5.5; and FXR KO colon at 12 months old, 8.4 (data not shown). Because the β-catenin-mediated pathway is critical for intestine proliferation, the effect of FXR deficiency on β-catenin immunostaining in the colon was evaluated, and the results showed an increased apical cell membrane/cytosolic intensity of β-catenin with FXR deficiency regardless of the age (Fig. 6D).
Discussion
The present study showed that FXR deficiency in mice increased intestinal cell proliferation and promoted intestinal carcinogenesis. These data suggest that activation of FXR by nonbile acid ligands may suppress intestinal carcinogenesis.
The current study showed that FXR deficiency enhanced colon cell proliferation in vivo, indicating that activation of FXR may suppress colon cell proliferation. Although the underlying mechanism is not clear, FXR may be involved in regulating cell proliferation by multiple mechanisms. An increase in bile acid levels has been shown to cause cell proliferation (Mahmoud et al., 1999; Pai et al., 2004). FXR is an essential link in regulating bile acid homeostasis. Loss of FXR function in mice leads to increased biliary bile acid secretion (Lambert et al., 2003). In addition, intestinal FXR determines the expression of bile acid binding protein, IBABP, which binds to bile acids and is thought to be critical for trafficking bile acids from the apical to basal side of intestinal epithelial cells. FXR deficiency results in markedly decreased IBABP expression (Sinal et al., 2000). However, simply sequestering bile acids by a bile acid binding resin proved to not be very useful because coadministering cholestyramine, a well known bile acid binding resin, has been shown to increase tumor burden (Campbell et al., 1975). Therefore, loss of FXR function may mainly affect the free intracellular bile acids in the intestine, which may promote cell proliferation and increase carcinogenesis.
In addition, intestine cancer development is also associated with increased inflammation. A previous study has shown that FXR deficiency increased intestinal bacterial, and activation of FXR reduces intestinal inflammation (Inagaki et al., 2006). The current study also showed increased lymphoid nodules with FXR deficiency, and the colons of FXR KO mice expressed higher mRNA levels of IL-6, indicating an up-regulated immune response. In addition, FXR KO mice had reduced goblet cells that are critical for native immune response in the intestine. A loss of goblet cell function has been associated with intestinal carcinogenesis (Whiteley et al., 1996). Indeed, previous studies have suggested a role of FXR in regulating inflammation showing livers of FXR KO mice express higher levels of proinflammatory genes, including IL-1β, interferon γ, TNFα, and IL-6 (Kim et al., 2007b; Yang et al., 2007).
Furthermore, FXR may regulate cell proliferation and/or apoptosis through the function of its target genes. A FXR target gene, small heterodimer partner (SHP), has been shown to suppress cell proliferation and promote apoptosis (Dawson et al., 2007; Farhana et al., 2007). A tumor-suppressive role of SHP also has been demonstrated that in human hepatocarcinomas where SHP expression was down-regulated, and mice with SHP deficiency had spontaneous liver tumors (He et al., 2008; Zhang et al., 2008). In correlation, SHP inhibits the liver receptor homolog 1, which has been shown to enhance cell proliferation by promoting G1-to-S phase transition (Botrugno et al., 2004). This current study showed that the protein levels of β-catenin, c-Myc, and cyclin D1 were increased in colons of FXR KO mice. In addition, a higher intensity of cytosolic immunostaining of β-catenin was shown in the colons of FXR KO mice. These results indicate that the β-catenin-dependent signaling pathway may be more activated in FXR KO mice.
Paradoxically, APC mRNA levels were not altered at 2 months of age and moderately decreased at 12 months of age in the colon of FXR KO mice compared with those of WT mice. However, APC protein levels were increased in both age groups. APC serves as a tumor suppressor by promoting proteolysis of β-catenin, thus reducing β-catenin signaling. Inactivation of APC leads to cytosolic accumulation of β-catenin and enhanced β-catenin-dependent transcriptional activation of c-myc and cyclin D1 (Gumbiner, 1997). The mechanism by which FXR deficiency increases APC protein levels is not clear. However, it seems that this is a post-translational rather than transcriptional phenomenon. Post-translational modification of APC has been reported in other studies (Jaiswal and Narayan, 2004; Umar et al., 2005). The increase in APC protein levels may result from a compensational response of cells to reach a balance between proliferation and cell cycle arrest. This compensational response has been reported, showing increased epithelial cell proliferation led to increased APC protein levels despite reduced APC mRNA levels (Umar et al., 2005). Indeed, FXR KO mouse colon showed increased APC protein levels from 2 to 12 months of age, further supporting the hypothesis that increased APC protein levels may result from a homeostatic response after increased cell proliferation. FXR gene expression in the small intestine was decreased in the colon of APCmin mice (unpublished data), further indicating a link between FXR function and intestine tumorigenesis.
FXR may regulate overlapping but distinct pathways in male and female regarding intestinal homeostasis. The current study has shown that FXR deficiency resulted in increased colon epithelial cell proliferation in both male and female mice at a younger age. However, in the older mice, FXR deficiency seemed to have less of an effect on colon epithelial cell proliferation in males, where as female mice still showed increased cell proliferation, but to less of an extent. In correlation to this, the female APCmin/FXR KO mice had higher adenoma multiplicity and larger adenomas compared with APCmin mice, indicating that female mice may be more sensitive to FXR deficiency in early stages of intestinal tumorigenesis.
In summary, the present study provides direct evidence that FXR deficiency promotes intestinal carcinogenesis. A previous study reported that FXR expression was decreased in human colon cancer, and there is a reciprocal relationship between the expression levels of FXR and the degree of malignancy of colon cancer cell lines (De Gottardi et al., 2004). However, direct evidence is missing for the in vivo effect of FXR deficiency in colon cancer progression. The present study showed FXR deficiency resulted in increased adenocarcinoma size in the small intestine of the APCmin mice and increased prevalence and size of AOM-induced adenocarcinomas in the colon, thereby providing clear evidence that the loss of normal FXR function may increase the susceptibility of the intestine to proliferation and carcinogenesis. Therefore, FXR-regulated pathway(s) may participate in tumor suppression and decreased FXR expression, and function may serve as a marker to indicate intestinal tumor malignancy. Activation of FXR by non–bile-acid ligands may be a novel target to prevent or reduce colon carcinogenesis.
Supplementary Material
This research was supported in part by the Intramural Research Program of the National Institutes of Health National Cancer Institute; by the National Institutes of Health [Grants R01-DK81343, K12-HD052027, P20-RR015563, P20-RR021940]; and by the Kansas Masonic Cancer Research Institute.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.145409.
ABBREVIATIONS: FXR, farnesoid X receptor; IBABP, ileal bile acid binding protein; APCmin, adenomatous polypsosis coli mutant; AOM, azoxymethane; WT, wild type; KO, knockout; H&E, hematoxylin and eosin; PBS, phosphate-buffered saline; TUNEL, terminal deoxynucleotidyl transferase dUTP nick-end labeling; BrdU, bromodeoxyuridine; LI, labeling index; NF, nuclear factor; ICAM, intercellular adhesion molecule; IL, interleukin; TNF, tumor necrosis factor; SHP, small heterodimer partner.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
References
- Botrugno OA, Fayard E, Annicotte JS, Haby C, Brennan T, Wendling O, Tanaka T, Kodama T, Thomas W, Auwerx J, et al. (2004) Synergy between LRH-1 and [beta]-catenin induces G1 cyclin-mediated cell proliferation. Mol Cell 15 499-509. [DOI] [PubMed] [Google Scholar]
- Campbell RL, Singh DV, and Nigro ND (1975) Importance of the fecal stream on the induction of colon tumors by azoxymethane in rats. Cancer Res 35 1369-1371. [PubMed] [Google Scholar]
- Corpet DE and Pierre F (2003) Point: From animal models to prevention of colon cancer: systematic review of chemoprevention in min mice and choice of the model system. Cancer Epidemiol Biomarkers Prev 12 391-400. [PMC free article] [PubMed] [Google Scholar]
- Dawson MI, Xia Z, Liu G, Ye M, Fontana JA, Farhana L, Patel BB, Arumugarajah S, Bhuiyan M, Zhang XK, et al. (2007) An adamantyl-substituted retinoid-derived molecule that inhibits cancer cell growth and angiogenesis by inducing apoptosis and binds to small heterodimer partner nuclear receptor: effects of modifying its carboxylate group on apoptosis, proliferation, and protein-tyrosine phosphatase activity. J Med Chem 50 2622-2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gottardi A, Dumonceau JM, Bruttin F, Vonlaufen A, Morard I, Spahr L, Rubbia-Brandt L, Frossard JL, Dinjens WN, Rabinovitch PS, et al. (2006) Expression of the bile acid receptor FXR in Barrett's esophagus and enhancement of apoptosis by guggulsterone in vitro. Mol Cancer 5 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gottardi A, Touri F, Maurer CA, Perez A, Maurhofer O, Ventre G, Bentzen CL, Niesor EJ, and Dufour JF (2004) The bile acid nuclear receptor FXR and the bile acid binding protein IBABP are differently expressed in colon cancer. Dig Dis Sci 49 982-989. [DOI] [PubMed] [Google Scholar]
- Farhana L, Dawson MI, Leid M, Wang L, Moore DD, Liu G, Xia Z, and Fontana JA (2007) Adamantyl-substituted retinoid-related molecules bind small heterodimer partner and modulate the Sin3A repressor. Cancer Res 67 318-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW, et al. (1995) Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 81 687-693. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM (1997) Carcinogenesis: a balance between beta-catenin and APC. Curr Biol 7 R443-R446. [DOI] [PubMed] [Google Scholar]
- Guo GL, Santamarina-Fojo S, Akiyama TE, Amar MJ, Paigen BJ, Brewer B Jr, and Gonzalez FJ (2006) Effects of FXR in foam-cell formation and atherosclerosis development. Biochim Biophys Acta 1761 1401-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He N, Park K, Zhang Y, Huang J, Lu S, and Wang L (2008) Epigenetic inhibition of nuclear receptor small heterodimer partner is associated with and regulates hepatocellular carcinoma growth. Gastroenterology 134 793-802. [DOI] [PubMed] [Google Scholar]
- Hill MJ (1974) Colon cancer: a disease of fibre depletion or of dietary excess? Digestion 11 289-306. [DOI] [PubMed] [Google Scholar]
- Hwang ST, Urizar NL, Moore DD, and Henning SJ (2002) Bile acids regulate the ontogenic expression of ileal bile acid binding protein in the rat via the farnesoid X receptor. Gastroenterology 122 1483-1492. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, McDonald JG, Luo G, Jones SA, Goodwin B, Richardson JA, et al. (2005) Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab 2 217-225. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Moschetta A, Lee YK, Peng L, Zhao G, Downes M, Yu RT, Shelton JM, Richardson JA, Repa JJ, et al. (2006) Regulation of antibacterial defense in the small intestine by the nuclear bile acid receptor. Proc Natl Acad Sci U S A 103 3920-3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal AS and Narayan S (2004) Zinc stabilizes adenomatous polyposis coli (APC) protein levels and induces cell cycle arrest in colon cancer cells. J Cell Biochem 93 345-357. [DOI] [PubMed] [Google Scholar]
- Journe F, Laurent G, Chaboteaux C, Nonclercq D, Durbecq V, Larsimont D, and Body JJ (2008) Farnesol, a mevalonate pathway intermediate, stimulates MCF-7 breast cancer cell growth through farnesoid-X-receptor-mediated estrogen receptor activation. Breast Cancer Res Treat 107 49-61. [DOI] [PubMed] [Google Scholar]
- Kim I, Ahn SH, Inagaki T, Choi M, Ito S, Guo GL, Kliewer SA, and Gonzalez FJ (2007a) Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res 48 2664-2672. [DOI] [PubMed] [Google Scholar]
- Kim I, Morimura K, Shah Y, Yang Q, Ward JM, and Gonzalez FJ (2007b) Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis 28 940-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert G, Amar MJ, Guo G, Brewer HB Jr, Gonzalez FJ, and Sinal CJ (2003) The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J Biol Chem 278 2563-2570. [DOI] [PubMed] [Google Scholar]
- Lee H, Zhang Y, Lee FY, Nelson SF, Gonzalez FJ, and Edwards PA (2006) FXR regulates organic solute transporters alpha and beta in the adrenal gland, kidney, and intestine. J Lipid Res 47 201-214. [DOI] [PubMed] [Google Scholar]
- Mahmoud NN, Dannenberg AJ, Bilinski RT, Mestre JR, Chadburn A, Churchill M, Martucci C, and Bertagnolli MM (1999) Administration of an unconjugated bile acid increases duodenal tumors in a murine model of familial adenomatous polyposis. Carcinogenesis 20 299-303. [DOI] [PubMed] [Google Scholar]
- Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, and Shan B (1999) Identification of a nuclear receptor for bile acids. Science 284 1362-1365. [DOI] [PubMed] [Google Scholar]
- Moser AR, Pitot HC, and Dove WF (1990) A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 247 322-324. [DOI] [PubMed] [Google Scholar]
- Nambiar PR, Girnun G, Lillo NA, Guda K, Whiteley HE, and Rosenberg DW (2003) Preliminary analysis of azoxymethane induced colon tumors in inbred mice commonly used as transgenic/knockout progenitors. Int J Oncol 22 145-150. [PubMed] [Google Scholar]
- Nigro ND (1985) Animal model for colorectal cancer. Prog Clin Biol Res 186 161-173. [PubMed] [Google Scholar]
- Nigro ND, Singh DV, Campbell RL, and Sook M (1975) Effect of dietary beef fat on intestinal tumor formation by azoxymethane in rats. J Natl Cancer Inst 54 439-442. [PubMed] [Google Scholar]
- Pai R, Tarnawski AS, and Tran T (2004) Deoxycholic acid activates {beta}-catenin signaling pathway and increases colon cell cancer growth and invasiveness. Mol Biol Cell 15 2156-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks DJ, Blanchard SG, Bledsoe RK, Chandra G, Consler TG, Kliewer SA, Stimmel JB, Willson TM, Zavacki AM, Moore DD, et al. (1999) Bile acids: natural ligands for an orphan nuclear receptor. Science 284 1365-1368. [DOI] [PubMed] [Google Scholar]
- Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, and Gonzalez FJ (2000) Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell 102 731-744. [DOI] [PubMed] [Google Scholar]
- Stamp DH (2002) Three hypotheses linking bile to carcinogenesis in the gastrointestinal tract: certain bile salts have properties that may be used to complement chemotherapy. Med Hypotheses 59 398-405. [DOI] [PubMed] [Google Scholar]
- Swales KE, Korbonits M, Carpenter R, Walsh DT, Warner TD, and Bishop-Bailey D (2006) The farnesoid X receptor is expressed in breast cancer and regulates apoptosis and aromatase expression. Cancer Res 66 10120-10126. [DOI] [PubMed] [Google Scholar]
- Umar S, Wang Y, and Sellin JH (2005) Epithelial proliferation induces novel changes in APC expression. Oncogene 24 6709-6718. [DOI] [PubMed] [Google Scholar]
- Wang H, Chen J, Hollister K, Sowers LC, and Forman BM (1999) Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell 3 543-553. [DOI] [PubMed] [Google Scholar]
- Whiteley LO, Hudson L Jr, and Pretlow TP (1996) Aberrant crypt foci in the colonic mucosa of rats treated with a genotoxic and nongenotoxic colon carcinogen. Toxicol Pathol 24 681-689. [DOI] [PubMed] [Google Scholar]
- Yang F, Huang X, Yi T, Yen Y, Moore DD, and Huang W (2007) Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res 67 863-867. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu P, Park K, Choi Y, Moore DD, and Wang L (2008) Orphan receptor small heterodimer partner suppresses tumorigenesis by modulating cyclin D1 expression and cellular proliferation. Hepatology 48 289-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.