Abstract
Antihypertensive drugs of the “calcium channel blocker” or “calcium antagonist” class have been used to establish the physiological role of L-type Ca2+ channels in vascular smooth muscle. In contrast, there has been limited progress on the pharmacology T-type Ca2+ channels. T-type channels play a role in cardiac pacemaking, aldosterone secretion, and renal hemodynamics, leading to the hypothesis that mixed T- and L-type blockers may have therapeutic advantages over selective L-type blockers. The goal of this study was to identify compounds that block the Cav3.2 T-type channel with high affinity, focusing on two classes of compounds: phenylalkylamines (e.g., mibefradil) and dihydropyridines (e.g., efonidipine). Compounds were tested using a validated Ca2+ influx assay into a cell line expressing recombinant Cav3.2 channels. This study identified four clinically approved antihypertensive drugs (efonidipine, felodipine, isradipine, and nitrendipine) as potent T-channel blockers (IC50 < 3 μM). In contrast, other widely prescribed dihydropyridines, such as amlodipine and nifedipine, were 10-fold less potent, making them a more appropriate choice in research studies on the role of L-type currents. In summary, the present results support the notion that many available antihypertensive drugs block a substantial fraction of T-current at therapeutically relevant concentrations, contributing to their mechanism of action.
Calcium influx into cells is a key signal transduction event that leads to a myriad of responses. Calcium enters the cytosol either through plasma membrane ion channels or is released from intracellular pools. Plasma membrane ion channels can be activated by hormones, in the case of receptor-operated channels; depletion of intracellular stores, for so-called store-operated channels; or by membrane depolarization, for voltage-gated channels. Voltage-gated channels can be further classified by their pharmacology. The first class recognized were the L-type channels (Godfraind et al., 1986; Striessnig, 1999; Triggle, 2003), which were identified by their sensitivity to “calcium antagonists.” Molecular biology has further expanded the repertoire of Ca2+ channels, with the cloning of 10 α1 subunits of voltage-activated Ca2+ channels. Alignments of the predicted amino acid sequences of these 10 channels revealed that there are three main subfamilies of α1 subunit and provided the basis for a systematic nomenclature (Ertel et al., 2000). The three subfamilies are called Cav1, Cav2, and Cav3. The Cav1 subfamily contains four members that encode L-type channels: Cav1.1 (α1S), Cav1.2 (α1C), Cav1.3 (α1D), and Cav1.4 (α1F). The third subfamily contains three members that are called T type: Cav3.1 (α1G), Cav3.2 (α1H), and Cav3.3 (α1I).
The ability of clinically relevant drugs to block selectively subclasses of Ca2+ channels suggests that they may all be potential drug targets. The major targets of calcium antagonists or calcium channel blockers (CCBs) are currently Cav1.2 channels. Nondihydropyridine calcium channel blockers of the phenylalkylamine and benzothiazepine classes are useful as antiarrhythmic agents because they slow conduction of action potentials through the heart. Dihydropyridine (DHP) calcium channel blockers are useful as antihypertensive agents because they selectively block Ca2+ influx into vascular smooth muscle because of alternative splicing and state-dependent block. Alternative splicing of the Cav1.2 gene, CACNA1C, produces a variant, Cav1.2b, with increased sensitivity to DHP CCBs, which is preferentially expressed in smooth muscle (Welling et al., 1997). Vascular smooth muscle cells maintain their resting membrane at a relatively depolarized potential (Hirst and Edwards, 1989), leading to more Cav1.2 channels in an inactivated state that has a higher affinity for DHP CCBs (Bean, 1984). Studies with transgenic mice confirm that calcium channel blockers work by blocking Cav1.2 channels (Moosmang et al., 2003), although at micromolar concentrations, they are also capable of blocking other voltage-gated channels, notably T-type Ca2+ channels (Akaike et al., 1989; Cohen et al., 1992). Mibefradil (Posicor) was the first mixed T/L channel blocker to be marketed for its ability to block T-currents (Clozel et al., 1997). It is unfortunate that the drug also blocked major cytochrome P450 enzymes, leading to drug-drug interactions and, ultimately, to its withdrawal from the market (Krayenbühl et al., 1999). Although it is likely that mibefradil's antihypertensive effect was because of its ability to block L-currents (Moosmang et al., 2006), blockade of T-currents may provide other beneficial cardiovascular effects. Evidence is accumulating that T/L blockers, such as efonidipine, differ from traditional CCBs in their effects on renal hemodynamics (Hayashi et al., 2007) and ability to block aldosterone secretion (Okayama et al., 2006), effects that are probably because of T-channel blockade. This is likely to be clinically relevant because dihydropyridine antagonists do not slow progression of nephropathy, requiring the coadministration of a renin-angiotensin aldosterone system blocker (Nathan et al., 2005).
Previous electrophysiological studies have reported that many compounds are capable of blocking T-type currents (for review, see Heady et al., 2001). Nevertheless, because of the low throughput of traditional patch-clamp electrophysiology, these studies have only focused on a limited number of compounds, thereby precluding direct comparisons of potency. The present study circumvents this limitation by using a validated high-throughput fluorescent dye-based assay (Xie et al., 2007) to analyze the block of a recombinant T-type channel. The study focused on the block of Cav3.2 because it is a likely drug target for cardiovascular effects by virtue of its expression in cardiac pacemaker cells, kidney smooth muscle, and adrenal glomerulosa cells (for review, see Perez-Reyes, 2003). The goal of the study was to provide the first large-scale comparison of T-channel block by antihypertensive and antiarrhythmic drugs and their analogs.
Materials and Methods
Materials. Mibefradil was provided by Hoffmann-La Roche (Basel, Switzerland). The enantiomers of devapamil were provided by Knoll AG (Ludwigshafen, Germany). Efonidipine and its enantiomers were provided by Nissan Chemical Industries (Tokyo, Japan). Amlodipine was purchased from Penn Bio-Organics (Rensselaer, NY). Nifedipine was purchased from Calbiochem (La Jolla, CA). All other compounds were obtained from either Sigma-Aldrich (St. Louis, MO) or Sigma/RBI, Natick, MA).
Cell Culture. Stable cell lines were created by transfecting human embryonic kidney 293 cells (American Type Culture Collection, Manassas, VA) with human recombinant Cav3.2a, as described previously (Gomora et al., 2002). For measurement of intracellular Ca2+, cells were seeded at 50% confluence into black-walled, clear-bottom 96-well microtiter plates coated with poly-d-lysine (BD Biosciences, San Jose, CA), then grown for 24 h when they approached 80 to 90% confluence.
Calcium Influx Assay. Calcium influx was estimated by measuring the increase in fluorescent signal from Fluo-4-loaded cells in response to an increase in extracellular Ca2+ to 10 mM as described previously (Xie et al., 2007). Test compounds were prepared as 30 mM stocks in dimethyl sulfoxide and stored at -20°C. After thawing, the stock was sonicated for 5 min, then carefully diluted into Hanks' balanced salt solution supplemented with 0.01% Pluronic F127 for testing of the 100 and 30 μM concentrations. Lower concentrations were prepared by serially diluting (0.315-fold) the 30 μM dilution. Twenty microliters of these dilutions was added to the cells, resulting in a 6-fold dilution down to their final concentration. The final concentration of dimethyl sulfoxide in the assay of 100 μM drug was 0.3%. Control experiments without drug indicated that this concentration of dimethyl sulfoxide produced only a minimal inhibition (<15%) of the dye signal; therefore, its effects were disregarded. Cells were incubated with test compound for 30 min at 37°C to allow for equilibrium binding. The cells were loaded into a FLEXStation II (Molecular Devices, Sunnyvale, CA), which was programmed to run in kinetic analysis mode (0.5-Hz sampling rate; excitation, 485; emission, 525; cutoff, 515 nM) and to add 7.5 μl of 160 mM CaCl2 (10 mM final concentration). Calcium influx occurs through T-channels that are open at the resting membrane potential of 293 cells (Chemin et al., 2000). As documented previously, the fluorescence signal is not observed in untransfected cells and is dependent on the membrane potential (Xie et al., 2007).
Data Analysis. Fluorescence signals were acquired for 60 s, then analyzed using SoftMax Pro software (version 4.8; Molecular Devices). The initial 15 s of baseline was used to zero the signal, and the remaining signal was integrated using the area under the curve function. The results were exported to Excel spreadsheets (Microsoft, Redmond, WA). Each row of the 96-well plate contained a buffer control and seven concentrations of test compound. Each compound was screened in triplicate. The results were normalized to the buffer control, averaged, then fit with the following form of the Hill-Langmuir equation: fractional block = (maximal block)/(1 + 10[(log(IC50) - log(drug)) × nH)]. The IC50 and Hill slope (nH) values for each experiment (n) were averaged and are reported as mean ± S.E.M. For illustration purposes, the block at each concentration was averaged across all experiments, and the data were fit using the same equation in Prism software (GraphPad Software Inc., San Diego, CA). Statistical significance was estimated using Prism's built-in algorithms for one-way analysis of variance, Tukey's multiple comparison test, and Student's t test. All chemical structures were drawn using ChemDraw Ultra (CambridgeSoft Corporation, Cambridge, MA).
Results
T-type Ca2+ channels are capable of producing a window current at the resting membrane potential of many cells, including thalamic neurons (Hughes et al., 1999), myoblasts (Bijlenga et al., 2000), and human embryonic kidney 293 cells (Chemin et al., 2000). Increasing extracellular calcium concentrations increases the driving force sufficiently to detect Ca2+ influx via recombinant T-channels using calcium-sensitive fluorescent dyes such as Fura-2 (Chemin et al., 2000). We exploited these properties to develop a high-throughput assay to characterize the pharmacology of human Cav3.2 channels (Xie et al., 2007). The assay relies on stable cell lines that express high levels of channel, cell-permeable Fluo-4-AM dye (which gets trapped inside after cleavage of the AM ester bond), and a fluorometer with integrated fluidics (e.g., fluorometric imaging plate reader or FlexStation; Molecular Devices). This assay has been validated using IonWorks HT electrophysiology (Xie et al., 2007). In brief, cells were loaded with the Fluo-4-AM, free dye was washed off, test compounds were added, and then the response to a 10 mM CaCl2 challenge was measured in the FLEXStation. The FLEXStation was programmed to read basal fluorescence for 15 s to add a bolus of CaCl2, then to continue reading for an additional 45 s. The addition of calcium induced a 2- to 4-fold increase in fluorescence, which typically peaked 30 to 40 s later (Fig. 1A). Preliminary experiments showed that this fluorescent signal slowly decayed back to baseline; however, the assay length was set at 60 s to minimize the contribution of endogenous Ca2+ pumps and transporters. The increase in fluorescent signal was blocked by appropriate concentrations of mibefradil (Fig. 1A). In general, the time course and extent of inhibition were very consistent between triplicate wells and across different experiments as illustrated by the results with mibefradil: average IC50 of 0.25 ± 0.06 μM, a Hill coefficient of 0.9 ± 0.1, and a maximal block of 94 ± 1% (Fig. 1C, n = 15). Mibefradil block of this recombinant Cav3.2 channel has been studied electrophysiologically at both the whole-cell and single-channel level (Martin et al., 2000; Michels et al., 2002). Although block was shown to be state-dependent, its IC50 in the FLEX assay was similar, falling between the values for inactivated and rested channels (0.1 and 0.3 μM). Together with previous studies validating this assay (Xie et al., 2007), we conclude that block of the fluorescent dye signal in this assay is synonymous with block of calcium influx via T-channels.
Fig. 1.
Characteristics of the dye-based assay of Cav3.2 activity. A, mibefradil block of raw fluorescent signal (r.f.u., relative fluorescence units). A human embryonic kidney 293 cell was loaded with Fluo-4-AM, washed, then incubated with varying micromolar concentrations of mibefradil for 30 min at 37°C. The 96-well plate was loaded into a FLEX-Station, and fluorescence was measured for 15 s before an addition of a bolus of CaCl2 that raised extracellular Ca2+ to 10 mM. Results shown are the mean ± S.E.M. of three replicates. B, structure of mibefradil. C, mibefradil dose-response measurements from 15 separate experiments.
Mibefradil is a benzimidazolyl-substituted tetraline derivative of the phenylalkylamine scaffold (Fig. 1B). Therefore, we tested other phenylalkylamine derivatives, such as verapamil, and the enantiomers of devapamil, (-)-(R)-D888, and (+)-(S)-D888. Verapamil blocked calcium influx, with an apparent IC50 of approximately 30 μM (Table 1). The enantiomers of D888 were ∼10-fold more potent, yet blocked with nearly equal potency. Block by both D888 enantiomers occurred over a wide range of concentrations, resulting in Hill coefficients between 0.6 and 0.7 (Table 1).
TABLE 1.
Block by phenylalkylamines and antiarrhythmics
| IC50 | nH | Maximal Block | n | |
|---|---|---|---|---|
| μM | % | |||
| Mibefradil | 0.25 ± 0.06 | 0.9 ± 0.1 | 93 ± 1 | 15 |
| (-)-(R)-D888 | 2.9 ± 0.6 | 0.6 ± 0.1 | 66 ± 3 | 4 |
| (+)-(S)-D888 | 4.1 ± 0.8 | 0.8 ± 0.1 | 94 ± 2 | 5 |
| Perhexiline | 4.4 ± 1.7 | 0.7 ± 0.1 | 79 ± 7 | 4 |
| Amiodarone | 5.2 ± 1.7 | 0.8 ± 0.1 | 87 ± 7 | 4 |
| Bepridil | 7.1 ± 1.5 | 1.3 ± 0.3 | 91 ± 3 | 5 |
| Verapamil | 32.7 ± 2.4 | 1.0 ± 0.2 | 79 ± 5 | 3 |
| Diltiazem | 96.2 ± 37.8 | 0.7 ± 0.2 | 76 ± 5 | 7 |
Verapamil is useful as both an antihypertensive agent and as an antiarrhythmic agent. Other antiarrhythmic agents that have been shown to block native T-currents include amiodarone and bepridil (Cohen et al., 1992). Although structurally dissimilar from phenylalkylamines, both amiodarone and bepridil and the antianginal drug, perhexiline, blocked recombinant Cav3.2 channels with similar potency (IC50 = 5 μM; Fig. 2). Similar block of recombinant Cav3.2 by amiodarone was measured using electrophysiology (IC50 = 3 μM) (Yamashita et al., 2006).
Fig. 2.
Block by verapamil, its analogs, and antiarrhythmic drugs. A, average dose-response curves for the enantiomers of D888 (devapamil) and verapamil. Data shown represent the mean ± S.E.M. from three to five experiments. The enantiomers of D888 were compared simultaneously in five experiments. Although the Hill coefficient (nH) averaged ∼0.7, this was not statistically different from observed with verapamil (nH = 1.0, data presented in Table 1). Structures and symbol key are given to the right of the graph. The structure for D888 corresponds to the (-)-(R)-enantiomer. Verapamil has an extra methoxy side group on one of its phenyl rings. B, dose-response analysis of perhexiline, amiodarone, and bepridil.
In addition to their distinctive biophysical properties, L-type channels were originally identified by their sensitivity to dihydropyridines. Of particular utility in classifying channels was the ability of Bay K8644 to act as a selective agonist at L-type channels. It is notable that the (-)-(S)-enantiomer of Bay K8644 can act as an agonist, whereas the (+)-(R)-enantiomer acts as an antagonist (Wei et al., 1986). In the T-channel assay, both enantiomers were antagonists and displayed similar potency (IC50 = 23 μM; Fig. 3A; Table 2). Recently, the enantiomers of efonidipine were also found to selectively block L-type channels in cardiac myocytes, with the (+)-(S)-enantiomer being (300-fold more potent than the (-)-(R)-enantiomer. In contrast, the enantiomers showed similar potency to block recombinant Cav3.2 channels (Fig. 3B; Table 2). Consistent with this finding, the racemic mixture showed similar potency. Niguldipine is another dihydropyridine that has been reported to have stereoselective action on both T- and L-type currents in atrial myocytes (Romanin et al., 1992). The (+)-(S)-enantiomer was a very potent inhibitor of the recombinant T-channel, displaying an IC50 of 0.4 μM (Fig. 3C; Table 2). In paired comparisons, (-)-(R)-niguldipine was found to be 3.8-fold less potent (P = 0.02). The (-)-enantiomer also seemed to be less effective, with maximal block saturating around 70% (Table 2). Racemic niguldipine displayed a potency that was intermediate of its two enantiomers (Table 2).
Fig. 3.
Stereoselectivity of block by dihydropyridines. A, block by the enantiomers of Bay K8644. Structure of (+)-(R)-Bay K8644 is shown to the right of the dose-response graph. Although the side chains in Bay K8644 are smaller than for most dihydropyridines tested, the (S)-enantiomer is an agonist at L-type channels, whereas the (R)-enantiomer is an antagonist. In contrast, these enantiomers showed a similar potency at blocking Cav3.2. B, enantiomers of efonidipine also showed a similar potency to block Cav3.2, and the block occurred at 10-fold lower concentrations than with Bay K8644. The racemic mixture showed a similar block as either enantiomer. The structure shown corresponds to the (-)-(R)-enantiomer. C, block by the enantiomers of niguldipine. Again, the structure shown corresponds to the (-)-(R)-enantiomer, highlighting that the large hydrophobic side chains of niguldipine occur on the opposite side of the dihydropyridine stereocenter compared with efonidipine. Block by these enantiomers was simultaneously compared with the racemic mixture in five experiments, and in each case, the (S)-enantiomer was ∼4-fold more potent.
TABLE 2.
Block by dihydropyridines
| IC50 | nH | Maximal Block | n | |
|---|---|---|---|---|
| μM | % | |||
| (+)-(S)-Niguldipine | 0.4 ± 0.1 | 0.9 ± 0.1 | 90 ± 3 | 5 |
| Niguldipine | 0.9 ± 0.3 | 0.9 ± 0.2 | 84 ± 1 | 11 |
| (-)-(R)-Niguldipine | 1.5 ± 0.5 | 0.9 ± 0.0 | 70 ± 8 | 5 |
| (-)-(R)-Efonidipine | 2.0 ± 0.3 | 1.2 ± 0.1 | 88 ± 3 | 9 |
| (+)-(S)-Efonidipine | 2.3 ± 0.7 | 0.8 ± 0.1 | 94 ± 3 | 6 |
| Nicardipine | 2.8 ± 1.4 | 1.2 ± 0.1 | 90 ± 9 | 11 |
| Efonidipine | 2.9 ± 1.0 | 1.1 ± 0.1 | 93 ± 3 | 9 |
| Isradipine | 3.1 ± 1.1 | 1.2 ± 0.2 | 97 ± 1 | 4 |
| Nisoldipine | 4.6 ± 1.0 | 1.1 ± 0.2 | 95 ± 1 | 3 |
| Nimodipine | 5.6 ± 0.7 | 1.3 ± 0.3 | 93 ± 2 | 5 |
| Felodipine | 6.8 ± 1.8 | 1.1 ± 0.2 | 95 ± 1 | 5 |
| Nitrendipine | 8.8 ± 2.3 | 0.9 ± 0.4 | 85 ± 5 | 4 |
| Nifedipine | 21.3 ± 1.3 | 0.8 ± 0.2 | 86 ± 3 | 6 |
| (+)-(R)-Bay K8644 | 21.6 ± 4.4 | 1.3 ± 0.2 | 85 ± 6 | 5 |
| (-)-(S)-Bay K8644 | 24.0 ± 6.0 | 1.7 ± 0.2 | 94 ± 4 | 5 |
| Amlodipine | 31.1 ± 8.4 | 0.9 ± 0.3 | 75 ± 6 | 7 |
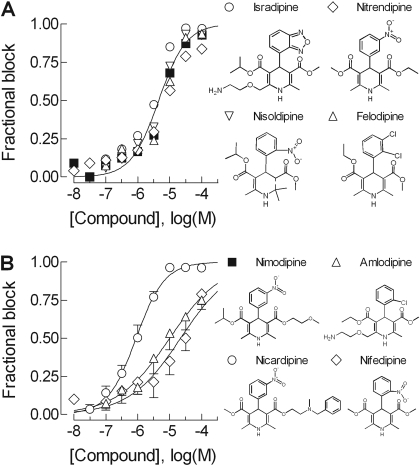
Although both are based on the dihydropyridine scaffold, the large hydrophobic side chains of efonidipine and niguldipine are on the opposite side of the dihydropyridine moiety when in the R-configuration (Fig. 3). To explore this binding pocket in greater detail, we tested a series of dihydropyridines that have been reported previously to block native T-currents (Heady et al., 2001). Most of the dihydropyridines tested blocked Ca2+ influx via the recombinant T-channel with high potency with the following compounds displaying IC50 values in the low micromolar range: niguldipine, nicardipine, isradipine, efonidipine, nisoldipine, felodipine, nimodipine, and nitrendipine (Fig. 4; Table 2). All compounds blocked to 100%, with Hill coefficients of ∼1. It is notable that the two most highly prescribed dihydropyridines in the United States (http://www.rxlist.com), amlodipine and nifedipine, were relatively weak blockers of the T-channel (Fig. 4B; Table 2).
Fig. 4.
Block of Cav3.2 by a series of dihydropyridines. A, dose-response curves of dihydropyridines that are capable of blocking with IC50 values in the 3 to 6 μM range, which included felodipine, isradipine, nimodipine, nisoldipine, and nitrendipine. Because there were no statistical differences in the estimated potencies to block Cav3.2, only one fit to the data is shown, and the error bars were not plotted. Structures are shown to the right, except nimodipine, which is shown next to B. B, dose-response curves of dihydropyridines that block with IC50 values in the 20 to 30 μM range (nifedipine and amlodipine) compared with a higher potency blocker (nicardipine). The lines represent fits to the average data, which are usually close to the average of fits to individual experiments. However, this was not the case for nifedipine and amlodipine, where the fit to the average produces a shallower slope (nH = 0.5) than observed in individual experiments (Table 2).
Discussion
Calcium channel blockers played a fundamental role in studies that led to understanding of the molecular diversity and physiological functions of voltage-gated Ca2+ channels. The first generation of these drugs has been available since the early 1960s, and CCBs continue to be widely used for the treatment of hypertension, angina, and arrhythmia (Abernethy and Schwartz, 1999). The binding site of these drugs has been extensively characterized, beginning with radioligand binding studies, biochemical purification of the drug-channel complexes, and finally through mutagenesis studies on recombinant channels. The lack of selective T-type channel blockers has severely impaired progress into their function. The present study describes the application of a high-throughput assay for T-channel blockers to evaluate block by a wide range of compounds. The key findings are: 1) that T-channels can be differentially blocked by the enantiomers of some DHPs, confirming the presence of a specific binding site distinct from that found on L-type channels; 2) that many compounds block with high affinity, which should be useful for future studies on native channels; 3) that established drug scaffolds provide a useful starting point for the development of novel T-channel blockers; and 4) that many drugs block the channel at clinically relevant concentrations, implying that T/L antagonists may have a unique therapeutic profile.
Block of native T-currents can be difficult to measure for many reasons, including their small amplitude and variable contamination of other channel types, notably low voltage-activated L-type channels (Lipscombe et al., 2004). Comparison between studies is further complicated by the finding that the apparent potency of many compounds is dependent on assay variables such as holding potential, test potential, pulse frequency, divalent cation concentration, temperature, and choice of preparation. Electrophysiological studies of mibefradil block provide excellent examples of these confounding variables, leading to a wide range (0.07–3 μM) in IC50 estimates (McDonough and Bean, 1998; Martin et al., 2000). The goal of the present study was to compare block under identical conditions using a cell line expressing human recombinant Cav3.2 channels. Although HEK-293 cells can be induced to express voltage-gated Ca2+ channels (Berjukow et al., 1996), these currents were never observed under our growth conditions, and there was no dye signal in our untransfected HEK-293 cells (Xie et al., 2007). Therefore, the Ca2+ influx measured using the Fluo-4 dye occurs via recombinant T-channels that are open at the resting membrane potential of HEK-293 cells (Chemin et al., 2000). The use of these “window currents” for high-throughput screening of recombinant T-channels has been validated recently (Xie et al., 2007). Because of their expression in cardiac pacemaker tissue, vascular smooth muscle, and adrenal glomerulosa cells, the present study examined the block of Cav3.2 channels by a series of antihypertensive and antiarrhythmic drugs and their analogs, focusing on compounds of the dihydropyridine and phenylalkylamine class.
The dihydropyridine scaffold has proven extremely useful for the development of novel antihypertensive drugs (Triggle, 2003). Many DHPs have stereoenantiomers that differ in their ability to block L-type channels, with the most extreme example being Bay K8644, where the (-)-(S)-enantiomer is an agonist that slows channel closing, whereas the (+)-(R)-enantiomer is an antagonist that stabilizes inactivated states. The present results show that both enantiomers are weak antagonists, consistent with previous electrophysiological studies that showed little or no block at concentrations below 1 μM (Michels et al., 2002).
Another example of stereoselective enantiomers is efonidipine, whose (+)-(S)-enantiomer is 400-fold more potent than the (-)-(R)-enantiomer at blocking L-type currents in guinea pig myocytes (Tanaka et al., 2004). Similar results were obtained using recombinant L-type channels generated by Cav1.2 (Furukawa et al., 2004). The only published study using recombinant T channels (rat Cav3.1) provided equivocal results on their selectivity (Furukawa et al., 2004). Using the Xenopus laevis oocyte system, there was no difference between the efonidipine enantiomers. In contrast, using mammalian cells, (-)-(R)-efonidipine was more potent at a holding potential of -60 mV but less potent at a holding potential of -100 mV (Furukawa et al., 2004). In the present study, the enantiomers were equipotent. This result combined with previous studies on L-type channels indicates that (-)-(R)-efonidipine can be used to selectively block T-currents, with little effect on L-type currents (Furukawa et al., 2004; Tanaka et al., 2004).
Niguldipine is another DHP whose enantiomers are commercially available and have been characterized for their selectivity. Binding studies using tritiated analogs of the two enantiomers of niguldipine revealed at 40-fold higher affinity for the (+)-(S)-enantiomer for L-type channels in skeletal muscle and heart (Handrock and Herzig, 1996). It is notable that its selectivity was reduced to ∼4-fold when measured by patch-clamp electrophysiology. The authors examined this discrepancy in greater detail and concluded that the data were more consistent with the guarded receptor model rather than the more widely accepted modulated receptor model (Ertel and Cohen, 1994). T-currents have also been reported to be more potently blocked by (+)-(S)-niguldipine than its (-)-(R)-enantiomer (Romanin et al., 1992). Based on the block at a single concentration (1 μM), this early study estimated that (+)-(S)-niguldipine was 6-fold more potent. L-type currents were blocked by a similar extent as T-type, indicating that this DHP has little selectivity. In good agreement with these results, we find that the (+)-(S)-enantiomer is ∼4-fold more potent. In addition, it was one of the most potent compounds tested, just as potent as mibefradil. In summary, many DHP enantiomers block T-channels almost equally well, whereas their block of L-type channels typically shows large selectivity.
The first antihypertensive drug to be marketed as a T-channel blocker was mibefradil (Ro 40-5967), which was introduced to the market in 1997, then abruptly withdrawn because of drug-drug interactions. Depending on the cell type, mibefradil blocks T-type Ca2+ channels 10 to 30 times more potently than L-type Ca2+ channels (Martin et al., 2000; Perchenet et al., 2000). Many studies indicate that mibefradil is also capable of blocking various other ion channels at micromolar concentrations (Heady et al., 2001). In the present study, mibefradil was found to be one of the most potent blockers of Cav3.2-mediated Ca2+ influx, displaying an IC50 of 0.25 μM. Mibefradil block is state-dependent, showing ∼20-fold higher affinity for inactivated states than rested states (70 versus 1400 nM, respectively; McDonough and Bean, 1998; Martin et al., 2000; Perchenet et al., 2000). Therefore, the intermediate affinity observed in our study suggests that a fraction of the channels were in the inactivated state. This conclusion is supported by the relatively depolarized membrane potential of HEK-293 cells and measurements of Cav3.2 channel availability at this potential (Xia et al., 2003). Despite being synthesized from the same alkylamine scaffold, verapamil and devapamil were considerably less potent at blocking Cav3.2.
In addition to antihypertensive drugs, block of T-type currents might contribute to the antiarrhythmic activity of amiodarone and bepridil (Cohen et al., 1992). Recent studies using the same recombinant Cav3.2 channel as this study found that long-term incubation (72 h) with amiodarone down-regulated channel expression and shifted its voltage dependence ∼6 mV (Yamashita et al., 2006). The mechanism of these long-term effects remains unknown. As observed for many state-dependent blockers that preferentially bind to inactivated states, short-term exposure to amiodarone blocked currents and shifted the steady-state inactivation to more negative voltages. Its apparent IC50 was 2.4 μM. In the present study, we obtained a similar estimate of its potency. Therefore, the potency of block determined for amiodarone, mibefradil, and efonidipine by this dye-based assay matches estimates of potency determined by the electrophysiology.
The present study provides the first comprehensive survey of T-channel block by a large series of DHPs, many of which are in clinical use. Their potency could be grouped into three classes: high-affinity blockers with IC50 values of 1 to 3 μM, efonidipine (Landel, only available in Japan), isradipine (DynaCirc), nicardipine (Cardene), and niguldipine; medium-affinity blockers with IC50 values of 5 to 10 μM, felodipine (Plendil), nimodipine (Nimotop), nisoldipine (Sular), and nitrendipine; and low-affinity blockers with IC50 values in the 20 to 30 μM range, amlodipine (Norvasc), Bay K8644, and nifedipine (Procardia). From a research perspective, these results identify nifedipine as the best choice to implicate L-type channels in physiological events. From a clinical perspective, these results identify DHPs that can block both T- and L-type channels at clinically relevant concentrations. For example, pharmacokinetics studies have found serum concentrations of 100 ng/ml for nifedipine (Brown et al., 1986), 20 ng/ml for isradipine and nicardipine (Zhou et al., 1995; Inotsume et al., 1997), and 14 ng/ml for efonidipine (Saito et al., 1996). As noted by Narahashi (2000), partial block of T-channels is relevant because of “pharmacological amplification” because they depolarize the membrane to the point where other channels open. T-currents play an important role in the kidney, mediating efferent arteriole tone (Hayashi et al., 2007). Because of this, mixed T- and L-type blockers may have a therapeutic advantage over selective L-type blockers by providing renoprotection via reduced glomerular hypertension. Contributing to this advantage is that T-type channels play a major role in aldosterone secretion (Chen et al., 1999), and lowering aldosterone levels would be beneficial in the treatment of hypertensive patients with kidney disease (Nathan et al., 2005). It is notable that efonidipine has been shown to reduce both proteinuria in hypertensive patients (Hayashi et al., 2003) and aldosterone secretion in healthy volunteers (Okayama et al., 2006). If this advantage could be proven clinically, then many patients would benefit from a switch to mixed T/L blockers such as those identified in this study.
Acknowledgments
We thank Winny Huang for technical support, Drs. K. Lynch and M. Johnson for help with the FLEXStation, Dr. J.-P. Clozel (formerly of Hoffmann-La Roche) for providing mibefradil, Drs. Gruenhagen and Wernet (Knoll, AG) for providing the enantiomers of D888, and Dr. A. Yamamoto (Nissan Chemical Industries) for providing efonidipine and enantiomers.
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant NS038691].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.145672.
ABBREVIATIONS: CCB, calcium channel blocker; DHP, dihydropyridine; AM, acetoxymethyl ester; Bay K8644, S-(-)-1,4-dihydro-2,6-dimethyl-5-nitro-4-(2-[trifluoromethyl]phenyl)-3-pyridine carboxylic acid methyl ester; HEK, human embryonic kidney.
References
- Abernethy DR and Schwartz JB (1999) Drug therapy: calcium-antagonist drugs. N Engl J Med 341 1447-1457. [DOI] [PubMed] [Google Scholar]
- Akaike N, Kostyuk PG, and Osipchuk YV (1989) Dihydropyridine-sensitive low-threshold calcium channels in isolated rat hypothalamic neurones. J Physiol (Lond) 412 181-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean BP (1984) Nitrendipine block of cardiac calcium channels: high-affinity binding to the inactivated state. Proc Natl Acad Sci U S A 81 6388-6392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berjukow S, Döring F, Froschmayr M, Grabner M, Glossmann H, and Hering S (1996) Endogenous calcium channels in human embryonic kidney (HEK293) cells. Br J Pharmacol 118 748-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijlenga P, Liu JH, Espinos E, Haenggeli CA, Fischer-Lougheed J, Bader CR, and Bernheim L (2000) T-type α1H Ca2+ channels are involved in Ca2+ signaling during terminal differentiation (fusion) of human myoblasts. Proc Natl Acad Sci U S A 97 7627-7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GR, Fraser DG, Castile JA, Gaudreault P, Platt DR, and Friedman PA (1986) Nifedipine serum concentrations following sublingual and oral doses. Int J Clin Pharmacol Ther Toxicol 24 283-286. [PubMed] [Google Scholar]
- Chemin J, Monteil A, Briquaire C, Richard S, Perez-Reyes E, Nargeot J, and Lory P (2000) Overexpression of T-type calcium channels in HEK-293 cells increases intracellular calcium without affecting cellular proliferation. FEBS Lett 478 166-172. [DOI] [PubMed] [Google Scholar]
- Chen XL, Bayliss DA, Fern RJ, and Barrett PQ (1999) A role for T-type Ca2+ channels in the synergistic control of aldosterone production by ANG II and K+. Am J Physiol Renal Physiol 276 F674-F683. [DOI] [PubMed] [Google Scholar]
- Clozel JP, Ertel EA, and Ertel SI (1997) Discovery and main pharmacological properties of mibefradil (Ro 40-5967), the first selective T-type calcium channel blocker. J Hypertens Suppl 15 S17-S25. [DOI] [PubMed] [Google Scholar]
- Cohen CJ, Spires S, and Van Skiver D (1992) Block of T-type Ca channels in guinea pig atrial cells by antiarrhythmic agents and Ca channel antagonists. J Gen Physiol 100 703-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, Tsien RW, and Catterall WA (2000) Nomenclature of voltage-gated calcium channels. Neuron 25 533-535. [DOI] [PubMed] [Google Scholar]
- Ertel EA and Cohen CJ (1994) Voltage-dependent interactions: the influence and significance of membrane potential on drug-receptor interactions. Drug Dev Res 33 204-213. [Google Scholar]
- Furukawa T, Miura R, Honda M, Kamiya N, Mori Y, Takeshita S, Isshiki T, and Nukada T (2004) Identification of R(-)-isomer of efonidipine as a selective blocker of T-type Ca2+ channels. Br J Pharmacol 143 1050-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfraind T, Miller R, and Wibo M (1986) Calcium antagonism and calcium entry blockade. Pharmacol Rev 38 321-416. [PubMed] [Google Scholar]
- Gomora JC, Murbartián J, Arias JM, Lee JH, and Perez-Reyes E (2002) Cloning and expression of the human T-type channel Cav3.3: insights into prepulse facilitation. Biophys J 83 229-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handrock R and Herzig S (1996) Stereoselectivity of Ca2+ channel block by dihydropyridines: no modulation by the voltage protocol. Eur J Pharmacol 309 317-321. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Kumagai H, and Saruta T (2003) Effect of efonidipine and ACE inhibitors on proteinuria in human hypertension with renal impairment. Am J Hypertens 16 116-122. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Wakino S, Sugano N, Ozawa Y, Homma K, and Saruta T (2007) Ca2+ channel subtypes and pharmacology in the kidney. Circ Res 100 342-353. [DOI] [PubMed] [Google Scholar]
- Heady TN, Gomora JC, Macdonald TL, and Perez-Reyes E (2001) Molecular pharmacology of T-type Ca2+ channels. Jpn J Pharmacol 85 339-350. [DOI] [PubMed] [Google Scholar]
- Hirst GD and Edwards FR (1989) Sympathetic neuroeffector transmission in arteries and arterioles. Physiol Rev 69 546-604. [DOI] [PubMed] [Google Scholar]
- Hughes SW, Cope DW, Toth TI, Williams SR, and Crunelli V (1999) All thalamocortical neurones possess a T-type Ca2+ “window” current that enables the expression of bistability-mediated activities. J Physiol (Lond) 517 805-815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inotsume N, Iwaoka T, Honda M, Nakano M, Okamoto Y, Naomi S, Tomita K, Teramura T, and Higuchi S (1997) Pharmacokinetics of nicardipine enantiomers in healthy young volunteers. Eur J Clin Pharmacol 52 289-292. [DOI] [PubMed] [Google Scholar]
- Krayenbühl JC, Vozeh S, Kondo-Oestreicher M, and Dayer P (1999) Drug-drug interactions of new active substances: mibefradil example. Eur J Clin Pharmacol 55 559-565. [DOI] [PubMed] [Google Scholar]
- Lipscombe D, Helton TD, and Xu W (2004) L-type calcium channels: the low down. J Neurophysiol 92 2633-2641. [DOI] [PubMed] [Google Scholar]
- Martin RL, Lee JH, Cribbs LL, Perez-Reyes E, and Hanck DA (2000) Mibefradil block of cloned T-type calcium channels. J Pharmacol Exp Ther 295 302-308. [PubMed] [Google Scholar]
- McDonough SI and Bean BP (1998) Mibefradil inhibition of T-type calcium channels in cerebellar Purkinje neurons. Mol Pharmacol 54 1080-1087. [DOI] [PubMed] [Google Scholar]
- Michels G, Matthes J, Handrock R, Kuchinke U, Groner F, Cribbs LL, Pereverzev A, Schneider T, Perez-Reyes E, and Herzig S (2002) Single-channel pharmacology of mibefradil in human native T-type and recombinant Cav3.2 calcium channels. Mol Pharmacol 61 682-694. [DOI] [PubMed] [Google Scholar]
- Moosmang S, Haider N, Brüderl B, Welling A, and Hofmann F (2006) Antihypertensive effects of the putative T-type calcium channel antagonist mibefradil are mediated by the L-type calcium channel Cav1.2. Circ Res 98 105-110. [DOI] [PubMed] [Google Scholar]
- Moosmang S, Schulla V, Welling A, Feil R, Feil S, Wegener JW, Hofmann F, and Klugbauer N (2003) Dominant role of smooth muscle L-type calcium channel Cav1.2 for blood pressure regulation. EMBO J 22 6027-6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T (2000) Neuroreceptors and ion channels as the basis for drug action: past, present, and future. J Pharmacol Exp Ther 294 1-26. [PubMed] [Google Scholar]
- Nathan S, Pepine CJ, and Bakris GL (2005) Calcium antagonists: effects on cardiorenal risk in hypertensive patients. Hypertension 46 637-642. [DOI] [PubMed] [Google Scholar]
- Okayama S, Imagawa K, Naya N, Iwama H, Somekawa S, Kawata H, Horii M, Nakajima T, Uemura S, and Saito Y (2006) Blocking T-type Ca2+ channels with efonidipine decreased plasma aldosterone concentration in healthy volunteers. Hypertens Res 29 493-497. [DOI] [PubMed] [Google Scholar]
- Perchenet L, Bénardeau A, and Ertel EA (2000) Pharmacological properties of Cav3.2, a low voltage-activated Ca2+ channel cloned from human heart. Naunyn Schmiedeberg Arch Pharmacol 361 590-599. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E (2003) Molecular physiology of low-voltage-activated T-type calcium channels. Physiol Rev 83 117-161. [DOI] [PubMed] [Google Scholar]
- Romanin C, Seydl K, Glossmann H, and Schindler H (1992) The dihydropyridine niguldipine inhibits T-type Ca2+ currents in atrial myocytes. Pflugers Arch 420 410-412. [DOI] [PubMed] [Google Scholar]
- Saito T, Fujii K, Takizawa T, Toyosaki T, Kuwabara Y, Kobayashi S, Ichikawa H, Karaki A, Yamazaki Y, Iwata J, Yamada K, Tomiya H, Takeda K, and Inagaki Y (1996) Effects of the new calcium antagonist efonidipine hydrochloride on resting and exercise hemodynamics in patients with stable effort angina. Arzneimittelforschung 46 861-867. [PubMed] [Google Scholar]
- Striessnig J (1999) Pharmacology, structure and function of cardiac L-type Ca2+ channels. Cell Physiol Biochem 9 242-269. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Komikado C, Shimada H, Takeda K, Namekata I, Kawanishi T, and Shigenobu K (2004) The R(-)-enantiomer of efonidipine blocks T-type but not L-type calcium current in guinea pig ventricular myocardium. J Pharmacol Sci 96 499-501. [DOI] [PubMed] [Google Scholar]
- Triggle DJ (2003) 1,4-Dihydropyridines as calcium channel ligands and privileged structures. Cell Mol Neurobiol 23 293-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei XY, Luchowski EM, Rutledge A, Su CM, and Triggle DJ (1986) Pharmacologic and radioligand binding analysis of the actions of 1,4-dihydropyridine activator-antagonist pairs in smooth muscle. J Pharmacol Exp Ther 239 144-153. [PubMed] [Google Scholar]
- Welling A, Ludwig A, Zimmer S, Klugbauer N, Flockerzi V, and Hofmann F (1997) Alternatively spliced IS6 segments of the α1C gene determine the tissue-specific dihydropyridine sensitivity of cardiac and vascular smooth muscle L-type Ca2+ channels. Circ Res 81 526-532. [DOI] [PubMed] [Google Scholar]
- Xia M, Imredy JP, Santarelli VP, Liang HA, Condra CL, Bennett P, Koblan KS, and Connolly TM (2003) Generation and characterization of a cell line with inducible expression of Cav3.2 (T-type) channels. Assay Drug Dev Technol 1 637-645. [DOI] [PubMed] [Google Scholar]
- Xie X, Van Deusen AL, Vitko I, Babu DA, Davies LA, Huynh N, Cheng H, Yang N, Barrett PQ, and Perez-Reyes E (2007) Validation of high throughput screening assays against three subtypes of Cav3 T-type channels using molecular and pharmacologic approaches. Assay Drug Dev Technol 5 191-203. [DOI] [PubMed] [Google Scholar]
- Yamashita N, Kaku T, Uchino T, Isomoto S, Yoshimatsu H, and Ono K (2006) Short- and long-term amiodarone treatments regulate Cav3.2 low-voltage-activated T-type Ca2+ channel through distinct mechanisms. Mol Pharmacol 69 1684-1691. [DOI] [PubMed] [Google Scholar]
- Zhou LX, Finley DK, Hassell AE, and Holtzman JL (1995) Pharmacokinetic interaction between isradipine and lovastatin in normal, female and male volunteers. J Pharmacol Exp Ther 273 121-127. [PubMed] [Google Scholar]