Abstract
Previous reports utilizing pharmacological antagonists implicate kainate receptor (KAR) activation in the development of morphine tolerance, dependence, conditioned place preference (CPP), and locomotor sensitization, but the role of glutamate receptor (GluR) 5-containing KAR in these effects remains unclear because of limited selectivity of the inhibitors employed. Therefore, we examined responses to systemic morphine treatment in mice expressing a constitutive deletion of GluR5 [GluR5 knockout (KO)]. Unlike wild-type (WT) littermates, GluR5 KO mice do not develop tolerance after repeated morphine administration by subcutaneous injection or via subcutaneous pellet implantation. In contrast, GluR5 KO mice do not differ from WT with respect to thermal or mechanical nociceptive thresholds, acute morphine antinociception, morphine disposition in the central nervous system (CNS), morphine physical dependence as revealed by naloxone-precipitated withdrawal or development of place preference and locomotor hyperresponsiveness after chronic morphine administration. It is surprising that continuous subcutaneous infusion of the GluR2/GluR5-preferring antagonist LY293558 [(3S,4aR,6R,8aR)-6-[2-(1(2)H-tetrazole-5-yl)ethyl]decahydroisoquinoline-3-carboxylic acid] decreased the number of naloxone-precipitated jumps to a similar extent in WT and GluR5 KO mice. We observed opioid-induced hypersensitivity in both groups during morphine withdrawal as demonstrated by equivalent reductions in thermal and mechanical thresholds; however, this hypersensitivity was not evident during continuous systemic morphine infusion. These data collectively indicate that KARs containing the GluR5 subunit contribute to the development of morphine tolerance without affecting nociceptive thresholds, morphine analgesia, or disposition in CNS of morphine and its metabolite morphine-3-glucuronide. In addition, constitutive deletion of GluR5 does not alter the morphine-induced increase in locomotor activity or the acquisition of morphine reward as measured by a CPP paradigm.
Excitatory neurotransmission in the central nervous system (CNS) is mediated primarily through glutamatergic signaling via ionotropic glutamate receptors, pharmacologically classified as NMDA, AMPA, and kainate. Of the AMPA and kainate subtypes, GluR1–4 subunits are sensitive to AMPA, whereas GluR5–7 and KA1–2 are preferentially activated by kainate (Dingledine et al., 1999). There is abundant support for the critical involvement of glutamate in transmission of sensory information and in various forms of neuroplasticity that share many common molecular features. Examples of such processes include long-term potentiation, central sensitization, and adaptive responses such as drug tolerance, physical dependence, and reward (Inturrisi, 1997; Vanderschuren and Kalivas, 2000; Ji et al., 2003).
The role of KAR initially remained obscure because of the relatively greater abundance of AMPA receptors (AMPAR) coupled with the lack of suitable pharmacological tools to distinguish between these two subtypes. However, the advent of high-affinity agonists and antagonists exhibiting improved selectivity, some for individual subunits, has since permitted a more detailed assessment of KAR-mediated functions at different synapses (Pinheiro and Mulle, 2006). The utility of these compounds also has prompted the implication of KAR activation in the development and expression of tolerance, physical dependence, and behavioral sensitization to morphine. Continuous subcutaneous administration of the GluR2/GluR5-preferring antagonist LY293558 attenuated the development of tolerance and also reversed established tolerance to repeated subcutaneous injections of morphine in mice, without itself producing analgesia or altering the analgesic ED50 of morphine (Kest et al., 1997). Subcutaneous infusion of LY293558 also blocked acute morphine physical dependence in mice (McLemore et al., 1997). Furthermore, pretreatment with subcutaneous LY293558 dose-dependently inhibited the development, but not the expression, of behavioral sensitization to morphine (Carlezon et al., 1999). Evidence for a role of KAR in the rewarding effects of morphine as measured by conditioned place preference (CPP) is indirect because these studies used the quinoxalinedione antagonists 6-cyano-2,3-dihydroxy-7-nitroquinoxaline or 2,3-dihydroxy-6,7-dinitroquinoxaline, which do not distinguish between AMPAR and KAR. Pretreatment with 2,3-dihydroxy-6,7-dinitroquinoxaline microinjected into the nucleus accumbens inhibited expression of morphine-induced place preference but not locomotor sensitization (Layer et al., 1993). In addition, microinjection of 6-cyano-2,3-dihydroxy-7-nitroquinoxaline into the anterior (but not the posterior) ventral tegmental area blocked the acquisition of morphine CPP without effect on the psychomotor response (Harris et al., 2004; Shabat-Simon et al., 2008).
It is tempting to speculate from these reports that certain neuroadaptive responses to repeated morphine treatment are mediated by KAR, particularly those comprised of GluR5 subunits. However, one must consider that despite the high affinity of LY293558 for GluR5 (Bleakman et al., 1996), it was originally described as an AMPAR antagonist (Ornstein et al., 1993), with 1.5-fold greater binding affinity for GluR2 than for GluR5 subunits (Simmons et al., 1998). The apparent limitations of relying on pharmacological approaches of limited selectivity triggered the generation of knockout (KO) mice deficient in GluR5 or GluR6 (Mulle et al., 2000). The availability of these mutant mice has allowed for more extensive functional evaluations of individual KAR subunits in various regions of the CNS (Pinheiro and Mulle, 2006). Previous studies demonstrating effectiveness of LY293558 in blocking evoked pain (Sang et al., 1998; Simmons et al., 1998) were corroborated by the finding that nociceptive behaviors elicited by capsaicin and formalin were reduced in GluR5 KO mice (Ko et al., 2005). Thus far, evidence implicating GluR5 activation in neuroadaptation to psychostimulants is less substantial because it is based primarily on sensitivity of these responses to inhibition by antagonists that also target AMPAR. Therefore, we sought to determine whether GluR5 is involved in specific behavioral effects of repeated administration of drugs of abuse, particularly in morphine tolerance, physical dependence, and reward. Through the incorporation of parallel studies in WT and GluR5 KO mice, we are able to examine the potential consequences of selective inactivation of GluR5-containing KAR without confounds associated with the use of antagonists.
Materials and Methods
Generation and Backcrossing of GluR5 KO Mice
GluR5 KO mice were produced on a 129/SvEv background at the Salk Institute in the laboratory of Dr. Stephen F. Heinemann as described previously (Mulle et al., 2000). Because the 129/SvEv strain fails to develop tolerance to morphine as a result of a defect in NMDA receptor-mediated signaling (Kolesnikov et al., 1998), both GluR5 KO and their WT littermates were backcrossed with C57BL/6 mice in parallel for over 10 generations to produce congenic C57BL/6 WT and GluR5 KO lines that are suitable for behavioral tests of nociception, morphine antinociception, tolerance, and dependence (Crawley et al., 1997). Mice were backcrossed by one of us (A.C.S.C.) at the University of Colorado at Denver and Health Sciences Center and subsequently transferred to Weill Cornell Medical College, where the mutant allele was reconfirmed by Southern blot analysis of genomic tail DNA (data not shown).
Animals
Male WT and GluR5 KO mice (8–12 weeks old, 25–30 g, n = 8–12 per group) were maintained under climate-controlled conditions on a 12-h light/dark cycle with free access to food and water. As described below, mice were housed individually after implantation with placebo or morphine pellets. The investigator was blinded to the identity and treatment of the mice, and all experiments were conducted according to Institutional Animal Care and Use Committee guidelines. For behavioral experiments, mice were handled and weighed daily and were allowed to acclimate to the testing environment and equipment before measurements. The same set of WT and GluR5 KO mice implanted subcutaneously with a placebo or morphine pellet were used for the measurement of tolerance, nociceptive thermal and mechanical thresholds, and physical dependence. Separate groups of mice were used in: 1) determination of the morphine ED50, 2) examination of the ability of LY293558 to inhibit naloxone-precipitated withdrawal, 3) measurement of brain levels of morphine and morphine-3-glucuronide, and 4) evaluation of locomotor activity and CPP.
Drugs
Morphine sulfate and pellet formulations of pharmacologically inert placebo (containing cellulose) and morphine (containing 25-mg morphine base) were obtained from the RTI International (Research Triangle Park, NC) through the National Institute on Drug Abuse (Rockville, MD). Under general isoflurane and local subcutaneous bupivacaine anesthesia, one placebo or one 25-mg morphine pellet wrapped in nylon mesh was implanted subcutaneously on the dorsal surface of each mouse for 3 days before behavioral testing (McLemore et al., 1997). Naloxone hydrochloride was purchased from DuPont Merck Pharmaceutical Co. (Wilmington, DE). The GluR2/GluR5 antagonist LY293558 was generously provided by Dr. Paul L. Ornstein at Eli Lilly & Co. (Indianapolis, IN) (Ornstein et al., 1993). All drugs were dissolved in saline, and the pH was adjusted to 7.0. The doses of morphine sulfate and naloxone hydrochloride were calculated as free base and then injected in a volume of 0.1 ml/10 g b.wt. as follows: morphine ED50, cumulative dose response, described below; morphine locomotor activity and CPP, 10 mg/kg i.p.; and naloxone hydrochloride, 0.2 or 50 mg/kg s.c. LY293558 was administered by continuous subcutaneous infusion via Alzet osmotic pump, model 2001 (Alza, Palo Alto, CA) delivering 60 mg/kg/24 h in 1 μl/h (McLemore et al., 1997).
Motor Function
Motor function was assessed by time spent on a Rotorod (IITC Life Science Inc., Woodland Hills, CA), either operating at 40 rpm with a cutoff of 60 s or accelerating to 40 rpm over a period of 5 min. In addition, we tested reflexes for righting and placing, stepping, and the ability to remain on a wire grid inclined 90° for 30 s (Kest et al., 1997).
Measurement of Thermal Tail Withdrawal Latencies
Before morphine injection, the distal 2 cm of the tail of each WT or GluR5 KO mouse was immersed in a water bath maintained at 55°C. Thermal tail withdrawal (TTW) latencies were measured three times with a cutoff of 10 s to avoid tissue damage and separated by 20-s intervals to avoid sensitization.
Tolerance Paradigms
Repeated Subcutaneous Injections of Morphine followed by ED50 Determination. After baselines were recorded, the day 1 morphine ED50 was estimated using a cumulative dose-response curve as conducted previously in our laboratory (Kest et al., 1997). WT and GluR5 KO mice were injected subcutaneously with 0.625 mg/kg morphine, followed by increasing doses in increments of 0.25 log units every 30 min, with response latencies determined 30 min after administration of each dose (McLemore et al., 1997). An antinociceptive responder was operationally defined as a mouse whose TTW was equal to or greater than double its mean baseline value, which was comprised of the average of three predrug determinations (Kest et al., 1997; McLemore et al., 1997). The day 1 ED50 values, 95% confidence intervals (CIs), and relative potency estimates for morphine analgesia were derived using the BLISS-21 computer program as reported previously (Kest et al., 1997; McLemore et al., 1997). Next, these mice were injected subcutaneously with increasing doses of morphine daily as follows: day 1, 2 × 20 mg/kg (at 1:00 PM and 5:00 PM); day 2, 3 × 40 mg/kg (9:00 AM, 1:00 PM, and 5:00 PM); day 3, 3 × 80 mg/kg (9:00 AM, 1:00 PM, and 5:00 PM). The day 4 morphine ED50 values were then estimated as described above. Tolerance is indicated by a significant increase in ED50 value, indicative of a rightward shift in the dose-response curve.
Subcutaneous Morphine Pellet Implantation followed by a Challenge Dose of Morphine. After determination of baseline TTW latencies, a separate group of WT and GluR5 KO mice were implanted subcutaneously with either one placebo or one 25-mg morphine pellet under isoflurane anesthesia. On day 4 after pellet implantation, baseline TTW latencies were recorded. Each mouse then received 16 mg/kg s.c. morphine, an ED99 dose of morphine for this strain of mice that was estimated from the morphine dose-response curve (see above). TTW latencies were measured again 30 min after the challenge dose of morphine. Tolerance was defined as a significant decrease in the TTW latencies after morphine challenge on day 4 compared with day 1. In addition, we also compared the response to morphine challenge of the WT and GluR5 KO mice on day 4. The pellet remained in place throughout the duration of the tolerance experiment and for the measurement of thermal and mechanical nociceptive thresholds (days 1–4), before its removal on day 5 to assess the development of physical dependence (see below).
Thermal Paw Withdrawal Latencies (Hargreaves' Test). Mice were placed individually in round Plexiglas observation cylinders on a preheated glass surface (Paw Thermal Simulator, University of California, San Diego, CA) maintained at 30°C and allowed to acclimate for 30 min. A radiant thermal stimulus was focused on the midplantar surface of each hindpaw, and the latency to withdrawal of the paw from the heat source was measured using the method of Hargreaves as done previously (Hargreaves et al., 1988). A maximum cutoff of 20 s was employed to prevent tissue damage. Paw withdrawal latencies (PWLs) were recorded on day 1 (baseline) before treatment, on day 4 after subcutaneous implantation of one placebo or one 25-mg morphine pellet, and on day 5 approximately 2 h after withdrawal was precipitated by pellet removal and subcutaneous injection of 0.2 mg/kg naloxone (for dependence paradigms, see below). A significant decrease in response latency was interpreted as thermal hyperalgesia.
Mechanical Stimulus Threshold (von Frey Test). Tactile allodynia was assessed in mice by measuring paw withdrawal thresholds of both hindpaws in response to probing with a series of calibrated von Frey filaments (Stoelting Co., Wood Dale, IL) in logarithmically spaced increments. Mice were placed individually in round Plexiglas observation cylinders with mesh flooring and allowed to acclimate for 30 min. Using a starting force of 1.2 g for mice, each von Frey filament was applied perpendicularly to the midplantar surface of the hindpaw. Paw withdrawal thresholds were determined using the up and down method of Dixon as described previously (Chaplan et al., 1994), in which filaments of sequentially increasing or decreasing forces were presented to each hindpaw. Responses were scored and expressed as the mean 50% g threshold of both paws. Mean 50% g thresholds of right and left paws also were calculated individually, with the same results. Thresholds were recorded on day 1 (baseline) before treatment, on day 4 after subcutaneous implantation of one placebo or one 25-mg morphine pellet, and on day 5 approximately 3 h after withdrawal was precipitated by pellet removal and subcutaneous injection of 0.2 mg/kg naloxone (see below for dependence paradigms). A significant decrease in threshold was interpreted as mechanical allodynia.
Dependence Paradigms
Naloxone-Precipitated Withdrawal in Mice Made Morphine-Dependent by Subcutaneous Pellet Implantation. Dependence was assessed after induction of naloxone-precipitated withdrawal in mice that had received subcutaneous infusion from either one placebo or one morphine pellet. On day 5 after implantation (1 day after assessing morphine tolerance and thermal and mechanical thresholds), the pellet was removed under anesthesia. Three hours later, withdrawal was precipitated by subcutaneous injection of 0.2 mg/kg naloxone, and mice were placed individually into round Plexiglas observation cylinders. Naloxone-precipitated withdrawal was measured as the mean number of jumps and wet dog shakes in the first 15 min and the amount of weight loss 2 h after naloxone administration. We have used 50 mg/kg previously to precipitate withdrawal (Kest et al., 1997) but found there was no significant difference in the number of jumps measured in the 15-min period that occurred after subcutaneous injection of naloxone at doses of 0.2 or 50 mg/kg (0.2 mg/kg, 136 ± 18; 50 mg/kg, 111 ± 16; p > 0.05). Therefore, in this report, we use 0.2 mg/kg naloxone.
Morphine Dependence in Mice Receiving the GluR2/GluR5 Antagonist LY293558 and Implanted with a Morphine Pellet. LY293558 was delivered via osmotic pump implanted subcutaneously 16 h before implantation of the morphine 25-mg pellet. On day 4 after pellet implantation, withdrawal was precipitated 3 h after removal of the pellet and pump by the subcutaneous injection of 50 mg/kg naloxone. The mean number of jumps occurring in the first 15 min was counted (McLemore et al., 1997).
Brain Levels of Morphine and Morphine-3-Glucuronide. Mice were implanted with one placebo or one 25-mg morphine pellet under general isoflurane and local subcutaneous bupivacaine anesthesia. On day 4 after pellet implantation, the whole brain minus cerebellum was removed, homogenized in 1× phosphate buffer, and stored at -20°C. Brain levels of morphine and morphine-3-glucuronide (M3G), its major metabolite found in rodents, were determined under blinded conditions using high-pressure liquid chromatography with electrospray ionization and tandem mass spectrometry at the Center for Human Toxicology, University of Utah (Salt Lake City, UT) as described previously (Zelcer et al., 2005). The lower limit of quantitation for this assay is 1.00 ng/ml for morphine and 0.25 ng/ml for M3G.
CPP and Locomotor Activity. A three-chamber place preference apparatus (MED Associates, St. Albans, VT) was used. This apparatus was made of two equally sized (16.8 × 12 cm) preference chambers (one white with a mesh floor and one black with a bar floor) connected by a central chamber (7.2 × 12 cm), which was gray with a smooth floor. The chambers were separated from one another by sliding doors and fitted with photobeams that were wired to a computer to record animal location and activity. Morphine preference and locomotor activity was measured as described previously (Walters et al., 2005), with minor modifications.
Day 1 (Preconditioning). Mice were placed in the central chamber for a 1-min (60 s) period of habituation with the sliding doors closed, followed by a 20-min (1200 s) preconditioning period of free exploration throughout the whole apparatus. Time spent in each chamber was recorded, and then mice were returned to their cages. Any mouse that spent more than 50% of the preconditioning period in the central gray chamber was excluded.
Days 2 to 9 (Conditioning). WT and GluR5 KO mice (n = 12) were given one intraperitoneal injection of morphine at a dose of 10 mg/kg every other day (days 2, 4, 6, 8) or saline (days 3, 5, 7, 9) and confined to either the black or the white chamber for 20 min. As a zero drug control, separate groups of WT and GluR5 KO mice (n = 6) received vehicle only (saline) once per day in both chambers. Distance traveled (centimeters) was recorded for both control (saline) and morphine groups.
Day 10 (Test). In the absence of drug treatment, mice were again placed in the central chamber with the doors closed. After a 1-min habituation period, the doors were raised and the mice were allowed to walk freely about the chamber. Time spent in each chamber was recorded. Preference was defined as the time spent in the morphine-paired chamber on the test day minus time spent in the morphine-paired side on the preconditioning day. Initial experiments showed that the preference obtained by pairing in the black or pairing in the white was indistinguishable, so all data were collapsed across paired chamber (data not shown).
Statistical Analysis. Data were expressed as the mean ± S.E.M. with the exception of ED50 values, which were depicted with 95% CI and graphed using GraphPad Prism 4 software (GraphPad Software Inc., San Diego, CA). p values were determined in Statview (Adept Scientific Inc., Bethesda, MD) as follows: two-group comparisons by Student's t test and multiple group comparisons by one-, two-, or three-way ANOVA as appropriate. Bonferroni/Dunn post hoc analysis was used in conjunction with ANOVA. A value of p < 0.05 was considered significant.
Results
Development of Tolerance after Repeated Subcutaneous Injection of Morphine in WT but Not GluR5 KO Mice. GluR5 KO mice do not differ from WT littermates in breeding or general health. In addition, GluR5 KO mice do not exhibit impairment of motor function compared with WT as assessed by time spent on a Rotorod at 40 rpm (WT, 56.8 ± 2.3 s; GluR5 KO, 49.2 ± 3.0 s; p > 0.05) and maximum speed level on a Rotorod for 30 s (WT, 8.0 ± 0.7 s; GluR5 KO, 8.8 ± 1.7 s; p > 0.05) (data not shown), similar to the GluR5(Q636R) editing mutant mice (Sailer et al., 1999). First, we examined the development of antinociceptive tolerance in WT and GluR5 KO mice after repeated subcutaneous injections of morphine. We derived the morphine ED50 on day 1 (baseline) and again on day 4, after chronic administration (Table 1) from the cumulative dose-response curves for TTW at 55°C. WT mice exhibited a significant increase in morphine ED50 on day 4 (4-fold; *, p < 0.05) compared with day 1 baseline, indicating the development of tolerance. GluR5 KO mice did not differ from WT mice (p > 0.05) with respect to baseline morphine ED50 derived on day 1. However, the marked decrease in morphine potency that occurred after repeated administration in WT mice was not observed in GluR5 KO mice. That is, compared with day 1 baseline, there was no difference (p > 0.05) in morphine ED50 on day 4. These data indicate that GluR5 KO mice do not differ from WT littermates with respect to acute morphine antinociception. However, unlike WT, GluR5 KO mice do not develop antinociceptive tolerance after repeated daily subcutaneous injections of morphine.
TABLE 1.
Tolerance to the analgesic effect of morphine does not develop in GluR5 KO mice On day 1, ED50 values (milligrams per kilogram) for morphine with the 95% CI were determined in WT and GluR5 KO mice (n = 8) via a cumulative dose-response assessment after measurement of TTW latencies at 55°C. Next, morphine was injected subcutaneously 20 mg/kg b.i.d. on day 1, 40 mg/kg t.i.d. on day 2, and 80 mg/kg t.i.d. on day 3. On day 4, the ED50 determination for morphine was repeated.
|
Group
|
Morphine ED50 (95% CI)
|
|
|---|---|---|
| Day 1 | Day 4 | |
| WT | 1.3(0.7–2.1) | 5.2(3.2–8.5)a |
| GluR5 KO | 0.9(0.4–1.5) | 1.1(0.6–2.0) |
Significantly different (*, p < 0.05) from day 1 WT
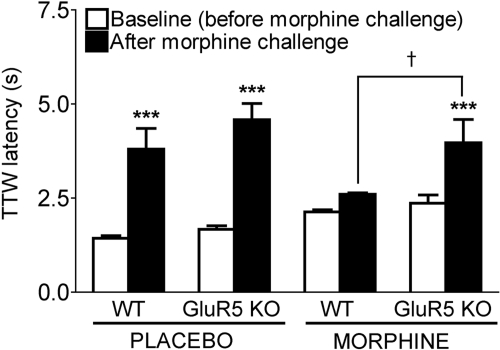
Development of Tolerance after Continuous Subcutaneous Infusion of Morphine in WT but Not GluR5 KO Mice. Next, we assessed the development of tolerance in WT and GluR5 KO mice by measuring TTW latencies at 55°C before and after a 16 mg/kg s.c. challenge dose of morphine on day 4 of subcutaneous infusion from one placebo or one 25-mg morphine pellet (Fig. 1). Three-way ANOVA revealed a main effect of genotype (WT versus GluR5 KO, F1,39 = 7.751; **, p < 0.01) and of morphine challenge (baseline versus challenge, F1,39 = 58.532; ***, p < 0.001) but not of pretreatment (placebo pellet versus morphine pellet, F1,39 = 0.146, p > 0.05). Bonferroni/Dunn post hoc analysis showed that WT and GluR5 KO mice did not differ with respect to their baseline TTW latencies on day 4 after subcutaneous pellet implantation with placebo (WT, 1.4 ± 0.1 s; GluR5 KO, 1.7 ± 0.1 s; F1,9 = 4.310, p > 0.05) or morphine (WT, 2.1 ± 0.1 s; GluR5 KO, 2.4 ± 0.1 s; F1,9 = 1.611, p > 0.05). After morphine challenge, there was no difference in mean latencies between placebo pellet-implanted WT and GluR5 KO mice (WT, 3.8 ± 0.6 s; GluR5 KO, 4.6 ± 0.4 s; F1,9 = 1.309, p > 0.05), indicating that constitutive deletion of GluR5 does not affect acute morphine antinociception. WT mice implanted with a 25-mg morphine pellet failed to experience a significant increase in TTW latency after morphine challenge (2.6 ± 0.1 s, p > 0.05), demonstrating the development of antinociceptive tolerance. In contrast, chronic morphine-treated GluR5 KO mice continued to display a significant increase in TTW latency after morphine challenge on day 4 (4.0 ± 0.6 s; ***, p < 0.001), which was significantly greater than that of morphine-implanted WT mice postchallenge (†, p < 0.05). These results illustrate that in contrast to WT, GluR5 KO mice fail to develop antinociceptive tolerance with continuous subcutaneous infusion of morphine from a 25-mg pellet.
Fig. 1.
GluR5 KO mice fail to develop tolerance to systemic morphine administration by subcutaneous pellet implantation. Male WT and GluR5 KO mice (n = 8) were implanted with one placebo or one 25-mg morphine pellet for 3 days, and mean TTW latencies at 55°C were measured on day 4 before (baseline) and 30 min after subcutaneous administration of an ED99 challenge dose of 16 mg/kg morphine. WT and GluR5 KO mice exhibit similar baseline mean TTW latencies (p > 0.05) after placebo or morphine pellet implantation. After morphine challenge, placebo-treated WT (***, p < 0.001) and GluR5 KO (***, p < 0.001) mice display a significant increase in mean TTW latency compared with baseline. However, morphine pellet-implanted WT mice fail to exhibit an increase in TTW latency after morphine challenge, indicating the development of antinociceptive tolerance. In contrast, GluR5 KO mice implanted with a morphine pellet display a significant increase in mean TTW latency after morphine challenge (*, p < 0.05), which is not different from that of placebo-treated GluR5 KO mice postchallenge (p > 0.05) but is greater than morphine pellet-implanted WT mice postchallenge (†, p < 0.05).
Development of Morphine Dependence in Both WT and GluR5 KO Mice. The development of morphine dependence after continuous systemic infusion in WT and GluR5 KO mice was assessed by the measurement of stereotypic behaviors associated with morphine withdrawal, which was precipitated on day 5 by pellet removal followed 3 h later by the subcutaneous injection of naloxone 0.2 mg/kg (Table 2). Two-way ANOVA revealed a main effect of pretreatment before naloxone (placebo pellet, morphine pellet, morphine pellet + saline, morphine pellet + LY293558, jumps, F1,56 = 53.55, ****, p < 0.0001; wet dog shakes, F1,28 = 544.5, ****, p < 0.0001; weight loss, F1,28 = 437.2, ****, p < 0.0001) but not of genotype (WT versus GluR5 KO, jumps, F1,28 = 0.3074, p > 0.05; wet dog shakes, F1,28 = 0.500, p > 0.05; weight loss, F1,28 = 0.8265, p > 0.05). There were no apparent signs of withdrawal (naloxone-precipitated jumps, wet dog shakes, or marked weight loss) in either WT or GluR5 KO mice that had been implanted with a placebo pellet. Morphine-treated WT mice exhibited an increase in the number of jumps after the induction of withdrawal (placebo pellet versus morphine pellet, ***, p < 0.001), which was partially blocked by subcutaneous administration of the GluR2/GluR5-preferring antagonist LY293558 (morphine pellet + saline versus morphine pellet + LY293558, *, p < 0.05), suggesting that dependence may be mediated in part by GluR5. We explored this possibility further in the GluR5 KO mice, reasoning that if morphine dependence is mediated by GluR5, it should be absent or decreased in the mutant mice. However, GluR5 KO mice treated with continuous morphine also experienced a significant increase in withdrawal behaviors measured (placebo pellet versus morphine pellet, ***, p < 0.001). It is interesting that subcutaneous administration of LY293558 to GluR5 KO morphine-treated mice also resulted in partial prevention of the increase in the number of jumps precipitated by naloxone (morphine pellet + saline versus morphine pellet + LY293558, ***, p < 0.001). Moreover, the ability of LY293558 to reduce withdrawal-induced jumping behavior did not differ between WT and GluR5 KO mice (p > 0.05). Thus, although GluR5 KO mice failed to develop antinociceptive tolerance to morphine, they still became physically dependent, as demonstrated by the expression of naloxone-precipitated withdrawal symptoms to a degree equivalent to their WT littermates. In addition, withdrawal signs indicative of morphine dependence were sensitive to inhibition by LY293558 in both WT and GluR5 KO mice.
TABLE 2.
Naloxone-precipitated withdrawal signs do not differ in morphine-dependent WT and GluR5 KO mice On day 4 after placebo (PLAC) or 25-mg morphine (MOR) pellet implantation, withdrawal was precipitated in WT or GluR5 KO mice (n = 8) by subcutaneous injection of naloxone (0.2 mg/kg) 3 h after removal of the pellet. We measured the number of jumps and wet dog shakes occurring within the first 15 min and the amount of weight loss in grams at 2 h after naloxone injection. In separate groups of WT and GluR5 KO mice, osmotic pumps delivering saline (SAL) or LY293558 at 60 mg/kg/24 h (LY) at 1 μl/h were implanted subcutaneously 16 h before insertion of the morphine pellet and then removed along with the pellet before naloxone injection.
| Group | Treatment | Number of Jumps | Number of Wet Dog Shakes | Weight Loss |
|---|---|---|---|---|
| mean ± S.E.M. | g ± S.E.M. | |||
| WT | PLAC | 0 ± 0 | 0 ± 0 | 0.2 ± 0.1 |
| MOR | 102 ± 9a | 16 ± 1a | 1.0 ± 0.1a | |
| MOR + SAL | 110 ± 14a | N.D. | N.D. | |
| MOR + LY | 68 ± 7a,c | N.D. | N.D. | |
| GluR5 KO | PLAC | 0 ± 0 | 0 ± 0 | 0.1 ± 0.1 |
| MOR | 108 ± 15b | 17 ± 1b | 1.0 ± 0.1b | |
| MOR + SAL | 99 ± 10b | N.D. | N.D. | |
| MOR + LY | 58 ± 9b,d | N.D. | N.D. | |
N.D., not determined
Significantly different (***, p < 0.001) from WT placebo
Significantly different (***, p < 0.001) from GluR5 KO placebo
Significantly different (*, p < 0.05) from WT morphine
Significantly different (*, p < 0.05) from GluR5 KO morphine
Equivalent Thermal and Mechanical Nociceptive Thresholds in WT and GluR5 KO Mice. We then evaluated the presence of thermal hyperalgesia and tactile allodynia in WT and GluR5 KO mice by measuring thermal PWLs and mechanical paw withdrawal 50% g threshold (PWT) on day 1 before (baseline), on day 4 after morphine pellet implantation, and on day 5 immediately after the assessment of withdrawal signs induced by subcutaneous injection of naloxone (0.2 mg/kg) 3 h after pellet removal (Table 3). Two-way ANOVA revealed a main effect of day (day 1 baseline or day 4 versus day 5 withdrawal) but no main effect of pretreatment (placebo pellet or morphine pellet, thermal PWL, F1,20 = 0.038, p > 0.05; mechanical PWT, F1,20 = 1.580, p > 0.05) or of genotype (WT versus GluR5 KO, thermal PWL, F1,20 = 1.666, p > 0.05; mechanical PWT, F1,20 = 0.422, p > 0.05). On day 4 of continuous morphine treatment in WT mice, there was no difference compared with baseline (p > 0.05) in thermal PWL or in mechanical PWT. Likewise, after continuous morphine administration in GluR5 KO mice, there was no significant change (p > 0.05) from baseline in thermal PWL or in mechanical threshold. Furthermore, GluR5 KO mice were not different from WT mice (p > 0.05) with respect to thermal or mechanical thresholds measured at baseline and on day 4. In this study, opioid-induced hypersensitivity was not observed in either WT or GluR5 KO mice during continuous morphine infusion. However, thermal hyperalgesia and mechanical allodynia were evident in both WT and GluR5 KO mice during withdrawal precipitated by morphine pellet removal and naloxone injection on day 5, as demonstrated by a significant reduction compared with day 1 in mean thermal PWL (F2,40 = 18.500; ****, p < 0.0001) and 50% g threshold (F2,40 = 15.117; ****, p < 0.0001). These data indicated that acute thermal or mechanical nociceptive thresholds are not affected by the absence of GluR5. In addition, hypersensitivity is observed to a similar extent in both WT and GluR5 KO mice during naloxone-precipitated withdrawal but not during continuous morphine administration.
TABLE 3.
WT and GluR5 KO mice do not differ with respect to thermal or mechanical nociception or hypersensitivity after naloxone-precipitated morphine withdrawal Thermal PWLs in seconds and 50% g mechanical paw withdrawal thresholds (mean ± S.E.M.) were measured in WT and GluR5 KO mice (n = 8) before (baseline), on day 4 of continuous infusion via subcutaneous implantation of a 25-mg morphine (MOR) pellet, and on day 5 during withdrawal, approximately 24 h after pellet removal and subcutaneous injection of 0.2 mg/kg naloxone (NAL). There was no significant effect of genotype or of sustained morphine treatment (p > 0.05) with respect to mean PWL or 50% g threshold. Hypersensitivity associated with naloxone-precipitated withdrawal was observed in WT and GluR5 KO mice, as demonstrated by a marked reduction (p < 0.05) in mean thermal PWL and mean 50% g threshold that is statistically similar in both groups.
| Group | Day/Treatment | PWL | 50% g Threshold |
|---|---|---|---|
| (s) (mean ± S.E.M.) | mean ± S.E.M. | ||
| WT | Day 1 Baseline | 7.8 ± 0.5 | 1.0 ± 0.1 |
| Day 4 MOR | 8.5 ± 0.7 | 1.0 ± 0.2 | |
| Day 5 MOR + NAL | 4.8 ± 0.4a,c | 0.3 ± 0.1a,c | |
| GluR5 KO | Day 1 Baseline | 7.7 ± 0.4 | 1.0 ± 0.1 |
| Day 4 MOR | 8.4 ± 0.9 | 1.0 ± 0.1 | |
| Day 5 MOR + NAL | 5.3 ± 0.7b,d | 0.5 ± 0.1b,d |
Significantly different (****, p < 0.0001) from WT baseline
Significantly different (****, p < 0.0001) from GluR5 KO baseline
Significantly different (**, p < 0.01) from WT morphine day 4
Significantly different (**, p < 0.01) from GluR5 KO morphine day 4
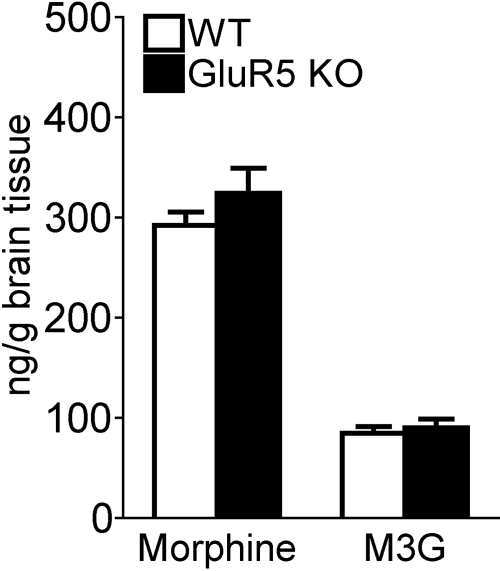
Equivalent Disposition of Morphine and Morphine-3-Glucuronide in CNS of WT and GluR5 KO Mice. Brain levels of morphine and its major metabolite in rodents, M3G, were measured in mice that had received 3 days of either placebo or morphine pellet implantation (Fig. 2). As expected, neither morphine nor M3G was detected in brains of placebo-treated WT or GluR5 KO mice (data not shown). There was no difference between WT and GluR5 KO mice in brain levels of morphine (WT, 291.9 ± 13.6 ng/g; GluR5 KO, 324.0 ± 25.2 ng/g; t1,20 = 1.12; p > 0.05) or of M3G (WT, 84.4 ± 6.70 ng/g; GluR5 KO, 90.0 ± 8.6 ng/g; t1,20 = 0.51; p > 0.05) on day 4 of continuous morphine treatment. These data indicated that the disposition of morphine in the CNS is unchanged in GluR5 KO mice compared with their WT littermates.
Fig. 2.
CNS disposition of morphine does not differ between WT and GluR5 KO mice. WT and GluR5 KO mice (n = 8) were implanted with one placebo or one 25-mg morphine pellet for 3 days. Whole brain minus cerebellum was harvested on day 4, and levels of morphine and M3G were measured. No morphine or M3G was detected in brains of placebo-treated WT or GluR5 KO mice (data not shown). There is no significant difference (p > 0.05) between WT and GluR5 KO mice in brain levels of morphine or M3G after continuous morphine treatment.
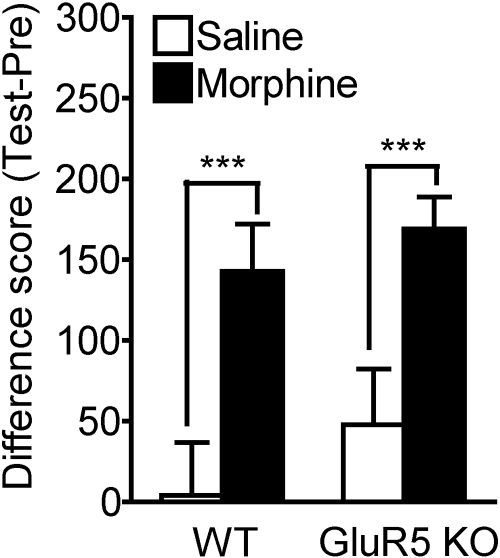
Equivalent Morphine-Induced Place Preference and Locomotor Hyperresponsiveness in WT and GluR5 KO Mice. We evaluated the acquisition of morphine-mediated CPP in WT and GluR5 KO mice (Fig. 3). Two-way ANOVA revealed a main effect of treatment (morphine versus saline, F1,32 = 13.302; ***, p < 0.001) but not of genotype (WT versus GluR5 KO, F1,32 = 1.044; p > 0.05). Both WT and GluR5 KO mice exhibited place preference to morphine at a dose of 10 mg/kg i.p., as demonstrated by a significant increase in the difference scores (time spent on drug-paired side on test day minus preconditioning day) for morphine-treated versus saline zero drug controls (WT saline, 3.98 ± 46.47 s; WT morphine, 142.64 ± 29.43 s; *, p < 0.05; GluR5 KO saline, 47.48 ± 48.48 s; GluR5 KO morphine, 168.93 ± 19.89 s; *, p < 0.05). However, the difference scores do not vary between morphine-treated WT and GluR5 KO mice; that is, WT and GluR5 KO mice do not differ with respect to the degree of morphine preference at this dose.
Fig. 3.
Conditioned place preference to morphine does not differ between WT and GluR5 KO mice. CPP to morphine was examined in WT and GluR5 KO mice. After a preconditioning session on day 1, mice were subjected to an 8-day conditioning period followed by a test session on day 10. Morphine was administered at a dose of 10 mg/kg i.p. every other day on days 2, 4, 6, and 8 to the morphine group (n = 12) and paired with either the black or the white chamber. Intraperitoneal saline was given on alternate days 3, 5, 7, and 9 and paired with the opposite side. For the zero drug control (saline group), another set of WT and GluR5 KO mice (n = 6) received saline every day in either chamber, alternating sides each day. The difference score was obtained by subtracting the amount of time (seconds) spent in the drug-paired chamber on test day (Test) minus the amount of time (seconds) spent in the drug-paired chamber on the preconditioning day (Pre). Although both WT and GluR5 KO mice exhibit significant morphine CPP at this dose compared with saline (***, p < 0.001), there is no difference between WT and GluR5 KO mice with respect to the degree of morphine preference (p > 0.05).
In addition, we assessed the effect of morphine on locomotor activity in WT and GluR5 KO mice by measuring distance traveled in the chamber during the 20-min period after intraperitoneal injection of saline or 10 mg/kg morphine (data not shown). Similar to our findings with CPP, we observed a main effect of treatment (morphine versus saline, F1,33 = 100.746; ****, p < 0.0001) but not of genotype (WT versus GluR5 KO, F1,32 = 0.069; p > 0.05) on locomotor activity. On day 2, both WT and GluR5 KO mice displayed an increase in distance traveled after the first administration of morphine versus that of saline (WT saline, 1119 ± 124 cm; WT morphine, 2019 ± 69 cm; ****, p < 0.0001; GluR5 KO saline, 1051 ± 80 cm; GluR5 KO morphine, 1984 ± 63 cm; ****, p < 0.0001). Likewise, locomotor activity was enhanced in both WT and GluR5 KO mice on day 8 after the last administration of morphine versus that of saline (WT saline, 1185 ± 91 cm; WT morphine, 2635 ± 112 cm; ****, p < 0.0001; GluR5 KO saline, 1160 ± 120 cm; GluR5 KO morphine, 2530 ± 114 cm; ****, p < 0.0001). We also found a main effect of treatment day, such that distance traveled by WT and GluR5 KO mice after the last injection of morphine on day 8 was greater than that recorded after the first exposure on day 2 (WT morphine day 8 versus day 2, F1,22 = 21.97; ****, p = 0.0001; GluR5 KO morphine day 8 versus day 2, F1,22 = 17.205; ***, p < 0.001). These data collectively indicate that WT and GluR5 KO mice both develop CPP and increased locomotor activity after repeated intermittent administration of morphine, yet there is no difference between genotypes with respect to either phenomenon. We elected not to examine the expression of behavioral sensitization after morphine withdrawal because we did not observe a significant effect of GluR5 deletion on its acquisition.
Discussion
In this report, we describe behavioral phenotypes of GluR5 KO mice with respect to the development of tolerance, physical dependence, and conditioned reward after repeated systemic administration of morphine. Although developmental compensation is a potential confound of constitutive gene deletion, there is no evidence in the GluR5 KO mice of corresponding changes in the levels of mRNA encoding other KAR subunits (Contractor et al., 2000). Furthermore, the WT and GluR5 KO mice used in this study were fully backcrossed to generate congenic C57BL/6 lines, which are suitable for behavioral tests of nociception, morphine antinociception, tolerance, and dependence, unlike the 129/SvEv strain, which fails to develop tolerance to morphine (Kolesnikov et al., 1998). Therefore, the behavioral phenotypes we observe in GluR5 mutant mice are probably attributable specifically to the absence of GluR5 subunit, not to the background strain.
We find that GluR5 deletion does not affect baseline thermal or mechanical thresholds, indicating that acute nociception is not mediated by GluR5 (Table 3). These data are in agreement with other studies conducted in GluR5 KO maintained on a mixed 129 × C57BL/6 background (Ko et al., 2005) or in GluR5(Q636R) editing mutants (Sailer et al., 1999), which found no difference from WT mice with respect to mechanical or thermal thresholds. The lack of involvement of GluR5 in baseline nociceptive sensitivity is further supported by the finding that acute morphine antinociception (ED50 value, Table 1) is not different between WT and GluR5 KO.
GluR5 KO mice develop morphine physical dependence in equal measure to WT, and naloxone-precipitated withdrawal signs in both groups are sensitive to inhibition by the GluR2/GluR5 antagonist LY293558 (Table 2). The effectiveness of LY293558 in reducing the intensity of morphine withdrawal in GluR5 KO mice indicates that this antagonist is likely to be operating through another mechanism to block physical dependence, perhaps via inhibition of GluR2-containing AMPAR (Simmons et al., 1998). This interpretation is supported by a previous report demonstrating that although LY293558 was effective in blocking morphine dependence, the more selective GluR5 antagonist LY382884, which exhibits no measurable affinity for AMPAR subunits (Simmons et al., 1998), failed to suppress the intensity and occurrence of naltrexone-precipitated morphine withdrawal symptoms (Rasmussen and Vandergriff, 2003).
Although it has been demonstrated that pretreatment with LY293558 blocked the development of behavioral sensitization to morphine (Carlezon et al., 1999), we report that constitutive deletion of GluR5 does not affect morphine-mediated conditioned reward or locomotor hyperactivity (Fig. 3; Results). These findings are not altogether surprising because GluR5 expression is negligible in both the nucleus accumbens (Bettler et al., 1990; Casassus and Mulle, 2002) and in the ventral tegmental area (Bischoff et al., 1997), two brain regions classically associated with drug reward (Vanderschuren and Kalivas, 2000; Kauer and Malenka, 2007). It is possible that in the previous study, the ability of LY293558 to prevent the acquisition of behavioral sensitization to morphine instead reflected the inhibition of GluR2-containing AMPAR, as postulated above with regard to physical dependence.
However, unlike WT, GluR5 KO mice fail to develop tolerance after repeated systemic morphine treatment (Table 1; Fig. 1). These observations indicate that morphine tolerance, but not dependence, is mediated in part by GluR5 activation. Given the lack of opioid-induced hypersensitivity during sustained subcutaneous morphine infusion in the absence of withdrawal, the development of antinociceptive tolerance in WT mice with continuous systemic morphine does not seem to be associated with an abnormal pain state or with increased pain sensitivity. However, hypersensitivity is observed during morphine withdrawal to a similar extent in both WT and GluR5 KO mice (Table 3), in agreement with previous documentation of hypersensitivity after intermittent administration or abrupt termination of the opioid, but not with continuous infusion (Li et al., 2001). It is also unlikely that the lack of tolerance development in GluR5 KO mice with continuous morphine infusion is because of pharmacokinetic differences in morphine disposition in the CNS because WT and GluR5 KO have equivalent brain levels of morphine and M3G on day 4 (Fig. 2), when tolerance is evident in WT but not GluR5 KO mice. Unlike humans, mice do not biotransform morphine to the active metabolite morphine-6-glucuronide (Zelcer et al., 2005), so we measured the major biotransformation product of morphine in this study. Furthermore, because there is no difference in acute morphine antinociception between WT and GluR5 KO, the mutant phenotype cannot be explained by enhanced morphine analgesia.
The notion that morphine tolerance and dependence are dissociable phenomena has been suggested previously. Similar to our observations with GluR5 KO mice, β-arrestin-2 KO mice fail to develop antinociceptive tolerance with repeated systemic morphine administration while retaining susceptibility to physical dependence (Bohn et al., 2000). Unlike in GluR5 KO mice, however, morphine analgesia is enhanced in the β-arrestin-2 KO mice relative to WT (Bohn et al., 2000). In addition, it has been reported that PKCγ KO mice exhibit less tolerance to development than WT after sustained morphine treatment without changes in acute morphine antinociception (Zeitz et al., 2001). However, naloxone administration elicits differential effects in WT and PKCγ KO mice with regard to some withdrawal signs associated with dependence (Zeitz et al., 2001). In contrast, all withdrawal signs measured in GluR5 KO mice are equivalent in occurrence and intensity to those observed in WT mice. The mechanisms responsible for the failure of GluR5 KO mice to develop tolerance with repeated systemic morphine are currently under investigation. We did not observe changes in β-arrestin-2 levels in the spinal cord (SC) of GluR5 KO relative to WT mice (unpublished data). Therefore, cellular adaptations in the SC of GluR5 KO mice may involve altered expression and function of other mediators previously associated with morphine tolerance including (but not limited to) components of the NMDA receptor/nitric oxide cascade (Pasternak et al., 1995), PKC isoforms α, γ, or ε (Smith et al., 2007), other protein kinases, such as protein kinase A, extracellular signal-regulated kinase, P38 mitogen-activated protein kinase, calcium/calmodulin-dependent kinase II, and calcium/calmodulin-dependent kinase IV (Liu and Anand, 2001), and a variety of neuropeptides like substance P, calcitonin gene-related peptide, and dynorphin (King et al., 2005). It has been postulated that GluR5-mediated stimulation of spontaneous GABA release in SC substantia gelatinosa may lead to a net increase in excitability, a response that reportedly is absent in GluR5 KO mice (Xu et al., 2006). This phenomenon may contribute to the lack of morphine tolerance development in these mutant mice. Alternatively, deletion of GluR5 could alter either excitatory or inhibitory neurotransmission at other GluR5 KAR-expressing synapses such as the anterior cingulate cortex, basolateral amygdala, bed nucleus of the stria terminalis, lateral septum, hypothalamus, hippocampus, and nucleus of the solitary tract (Bettler et al., 1990; Pinheiro and Mulle, 2006), regions known to be modulated by chronic morphine treatment.
In summary, the current findings suggest specific involvement of GluR5 in morphine tolerance but not in acute morphine antinociception, physical dependence, or reward. Future studies with GluR5 KO mice may lend valuable insights into the mechanisms of morphine tolerance. In addition, antagonists exhibiting greater selectivity in targeting GluR5 may offer significant clinical utility in the management of antinociceptive tolerance induced by chronic systemic morphine treatment.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA001457, DA000198, Training Grant DA007274, and Center Grant DA005130].
J.J.B. and A.M.G. contributed equally to this work.
High-performance liquid chromatography and mass spectrometry of morphine and M3G in WT and GluR5 KO mice were conducted by Drs. Rodger Foltz and David Andrenyak at the Center for Human Toxicology of the University of Utah (Salt Lake City, UT) under a services contract to NIDA and Dr. H. Singh of the Division of Neuroscience and Behavioral Research at NIDA. Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.144121.
ABBREVIATIONS: CNS, central nervous system; NMDA, N-methyl-d-aspartate; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid; GluR, glutamate receptor; KAR, kainate receptor; AMPAR, AMPA receptor; LY293558, (3S,4aR,6R,8aR)-6-[2-(1(2)H-tetrazole-5-yl)ethyl]decahydroisoquinoline-3-carboxylic acid; CPP, conditioned place preference; KO, knockout; WT, wild type; TTW, thermal tail withdrawal; CI, confidence interval; PWL, paw withdrawal latency; M3G, morphine-3-glucuronide; ANOVA, analysis of variance; LY382884, 3S,4aR,6S,8aR-6-((4-carboxyphenyl)methyl)-1,2,3,4,4a,5,6,7,8,8a-decahydroisoquinoline-3-carboxylic acid; PKC, protein kinase C; SC, spinal cord.
References
- Bettler B, Boulter J, Hermans-Borgmeyer I, O'Shea-Greenfield A, Deneris ES, Moll C, Borgmeyer U, Hollmann M, and Heinemann S (1990) Cloning of a novel glutamate receptor subunit, GluR5: expression in the nervous system during development. Neuron 5 583-595. [DOI] [PubMed] [Google Scholar]
- Bischoff S, Barhanin J, Bettler B, Mulle C, and Heinemann S (1997) Spatial distribution of kainate receptor subunit mRNA in the mouse basal ganglia and ventral mesencephalon. J Comp Neurol 379 541-562. [DOI] [PubMed] [Google Scholar]
- Bleakman R, Schoepp DD, Ballyk B, Bufton H, Sharpe EF, Thomas K, Ornstein PL, and Kamboj RK (1996) Pharmacological discrimination of GluR5 and GluR6 kainate receptor subtypes by (3S,4aR,6R,8aR)-6-[2-(1(2)H-tetrazole-5-yl)ethyl]decahyd roisdoquinoline-3 carboxylic-acid. Mol Pharmacol 49 581-585. [PubMed] [Google Scholar]
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, and Caron MG (2000) Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature 408 720-723. [DOI] [PubMed] [Google Scholar]
- Carlezon WA Jr, Rasmussen K, and Nestler EJ (1999) AMPA antagonist LY293558 blocks the development, without blocking the expression, of behavioral sensitization to morphine. Synapse 31 256-262. [DOI] [PubMed] [Google Scholar]
- Casassus G and Mulle C (2002) Functional characterization of kainate receptors in the mouse nucleus accumbens. Neuropharmacology 42 603-611. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, and Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53 55-63. [DOI] [PubMed] [Google Scholar]
- Contractor A, Swanson GT, Sailer A, O'Gorman S, and Heinemann SF (2000) Identification of the kainate receptor subunits underlying modulation of excitatory synaptic transmission in the CA3 region of the hippocampus. J Neurosci 20 8269-8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN, Belknap JK, Collins A, Crabbe JC, Frankel W, Henderson N, Hitzemann RJ, Maxson SC, Miner LL, Silva AJ, et al. (1997) Behavioral phenotypes of inbred mouse strains: implications and recommendations for molecular studies. Psychopharmacology (Berl) 132 107-124. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, and Traynelis SF (1999) The glutamate receptor ion channels. Pharmacol Rev 51 7-61. [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, and Joris J (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32 77-88. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Byrne R, and Aston-Jones G (2004) Glutamate-associated plasticity in the ventral tegmental area is necessary for conditioning environmental stimuli with morphine. Neuroscience 129 841-847. [DOI] [PubMed] [Google Scholar]
- Inturrisi C (1997) Preclinical evidence for a role of glutamatergic systems in opioid tolerance and dependence. Semin Neurosci 9 110-119. [Google Scholar]
- Ji RR, Kohno T, Moore KA, and Woolf CJ (2003) Central sensitization and LTP: do pain and memory share similar mechanisms? Trends Neurosci 26 696-705. [DOI] [PubMed] [Google Scholar]
- Kauer JA and Malenka RC (2007) Synaptic plasticity and addiction. Nat Rev Neurosci 8 844-858. [DOI] [PubMed] [Google Scholar]
- Kest B, McLemore G, Kao B, and Inturrisi CE (1997) The competitive alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate receptor antagonist LY293558 attenuates and reverses analgesic tolerance to morphine but not to delta or kappa opioids. J Pharmacol Exp Ther 283 1249-1255. [PubMed] [Google Scholar]
- King T, Ossipov MH, Vanderah TW, Porreca F, and Lai J (2005) Is paradoxical pain induced by sustained opioid exposure an underlying mechanism of opioid antinociceptive tolerance? Neurosignals 14 194-205. [DOI] [PubMed] [Google Scholar]
- Ko S, Zhao MG, Toyoda H, Qiu CS, and Zhuo M (2005) Altered behavioral responses to noxious stimuli and fear in glutamate receptor 5 (GluR5)- or GluR6-deficient mice. J Neurosci 25 977-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnikov Y, Jain S, Wilson R, and Pasternak GW (1998) Lack of morphine and enkephalin tolerance in 129/SvEv mice: evidence for a NMDA receptor defect. J Pharmacol Exp Ther 284 455-459. [PubMed] [Google Scholar]
- Layer RT, Uretsky NJ, and Wallace LJ (1993) Effects of the AMPA/kainate receptor antagonist DNQX in the nucleus accumbens on drug-induced conditioned place preference. Brain Res 617 267-273. [DOI] [PubMed] [Google Scholar]
- Li X, Angst MS, and Clark JD (2001) A murine model of opioid-induced hyperalgesia. Molecular Brain Research 86 56-62. [DOI] [PubMed] [Google Scholar]
- Liu JG and Anand KJ (2001) Protein kinases modulate the cellular adaptations associated with opioid tolerance and dependence. Brain Res Brain Res Rev 38 1-19. [DOI] [PubMed] [Google Scholar]
- McLemore GL, Kest B, and Inturrisi CE (1997) The effects of LY293558, an AMPA receptor antagonist, on acute and chronic morphine dependence. Brain Res 778 120-126. [DOI] [PubMed] [Google Scholar]
- Mulle C, Sailer A, Swanson GT, Brana C, O'Gorman S, Bettler B, and Heinemann SF (2000) Subunit composition of kainate receptors in hippocampal interneurons. Neuron 28 475-484. [DOI] [PubMed] [Google Scholar]
- Ornstein PL, Arnold MB, Augenstein NK, Lodge D, Leander JD, and Schoepp DD (1993) (3SR,4aRS,6RS,8aRS)-6-[2-(1H-tetrazol-5-yl)ethyl]decahydroisoquinoline-3-carboxylic acid: a structurally novel, systemically active, competitive AMPA receptor antagonist. J Med Chem 36 2046-2048. [DOI] [PubMed] [Google Scholar]
- Pasternak GW, Kolesnikov YA, and Babey AM (1995) Perspectives on the N-methyl-d-aspartate/nitric oxide cascade and opioid tolerance. Neuropsychopharmacology 13 309-313. [DOI] [PubMed] [Google Scholar]
- Pinheiro P and Mulle C (2006) Kainate receptors. Cell Tissue Res 326 457-482. [DOI] [PubMed] [Google Scholar]
- Rasmussen K and Vandergriff J (2003) The selective iGluR1–4 (AMPA) antagonist LY300168 attenuates morphine-withdrawal-induced activation of locus coeruleus neurons and behavioural signs of morphine withdrawal. Neuropharmacology 44 88-92. [DOI] [PubMed] [Google Scholar]
- Sailer A, Swanson GT, Pérez-Otaño I, O'Leary L, Malkmus SA, Dyck RH, Dickinson-Anson H, Schiffer HH, Maron C, Yaksh TL, Gage FH, O'Gorman S, and Heinemann SF (1999) Generation and analysis of GluR5(Q636R) kainate receptor mutant mice. J Neurosci 19 8757-8764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang CN, Hostetter MP, Gracely RH, Chappell AS, Schoepp DD, Lee G, Whitcup S, Caruso R, and Max MB (1998) AMPA/kainate antagonist LY293558 reduces capsaicin-evoked hyperalgesia but not pain in normal skin in humans. Anesthesiology 89 1060-1067. [DOI] [PubMed] [Google Scholar]
- Shabat-Simon M, Levy D, Amir A, Rehavi M, and Zangen A (2008) Dissociation between rewarding and psychomotor effects of opiates: differential roles for glutamate receptors within anterior and posterior portions of the ventral tegmental area. J Neurosci 28 8406-8416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons RM, Li DL, Hoo KH, Deverill M, Ornstein PL, and Iyengar S (1998) Kainate GluR5 receptor subtype mediates the nociceptive response to formalin in the rat. Neuropharmacology 37 25-36. [DOI] [PubMed] [Google Scholar]
- Smith FL, Gabra BH, Smith PA, Redwood MC, and Dewey WL (2007) Determination of the role of conventional, novel and atypical PKC isoforms in the expression of morphine tolerance in mice. Pain 127 129-139. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ and Kalivas PW (2000) Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 151 99-120. [DOI] [PubMed] [Google Scholar]
- Walters CL, Godfrey M, Li X, and Blendy JA (2005) Alterations in morphine-induced reward, locomotor activity, and thermoregulation in CREB-deficient mice. Brain Res 1032 193-199. [DOI] [PubMed] [Google Scholar]
- Xu H, Wu LJ, Zhao MG, Toyoda H, Vadakkan KI, Jia Y, Pinaud R, and Zhuo M (2006) Presynaptic regulation of the inhibitory transmission by GluR5-containing kainate receptors in spinal substantia gelatinosa. Mol Pain 2 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitz KP, Malmberg AB, Gilbert H, and Basbaum AI (2001) Reduced development of tolerance to the analgesic effects of morphine and clonidine in PKC gamma mutant mice. Pain 94 245-253. [DOI] [PubMed] [Google Scholar]
- Zelcer N, van de Wetering K, Hillebrand M, Sarton E, Kuil A, Wielinga PR, Tephly T, Dahan A, Beijnen JH, and Borst P (2005) Mice lacking multidrug resistance protein 3 show altered morphine pharmacokinetics and morphine-6-glucuronide antinociception. Proc Natl Acad Sci U S A 102 7274-7279. [DOI] [PMC free article] [PubMed] [Google Scholar]