Abstract
Salvinorin A is the main active component of the widely available hallucinogenic plant, Salvia divinorum. Salvinorin A is a selective high-efficacy κ-agonist in vitro, with some unique pharmacodynamic properties. Descriptive reports show that salvinorin A-containing products produce robust behavioral effects in humans. However, these effects have not been systematically characterized in human or nonhuman primates to date. Therefore, the present studies focused on the characterization of overt effects of salvinorin A, such as sedation (operationally defined as unresponsiveness to environmental stimuli) and postural relaxation, previously observed with centrally penetrating κ-agonists in nonhuman primates. Salvinorin A was active in these endpoints (dose range, 0.01–0.1 mg/kg i.v.) in nonhuman primates (n = 3–5), similar to the synthetic κ-agonist U69,593 [(+)-(5α,7α,8β)-N-methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro[4.5]-dec-8-yl]-benzeneacetamide], used for comparison herein. Salvinorin A effects could be prevented by a clinically available opioid antagonist, nalmefene (0.1 mg/kg), at doses known to block κ-receptor-mediated effects in nonhuman primates. When injected intravenously, salvinorin A (0.032 mg/kg) could enter the central nervous system (as reflected in cisternal cerebrospinal fluid) within 1 min and reach concentrations that are in the reported range of the affinity (Ki) of this ligand for brain κ-receptors. Consistent with this finding, specific translationally viable behavioral effects (e.g., facial relaxation and ptosis) could also be detected within 1 to 2 min of injection of salvinorin A. These are the first studies documenting rapid unconditioned effects of salvinorin A in a primate species, consistent with descriptive reports of rapid and robust effects of this powerful hallucinogen in humans.
Salvinorin A, a diterpenoid, is the main active component of the hallucinogenic plant Salvia divinorum (Valdes et al., 1984). Salvinorin A-containing products (e.g., leaves, concentrated extracts) have been widely available for the last several years, especially on the internet, and self-administration (mainly by the smoking route) has been reported (Baggott et al., 2004; González et al., 2006; Lange et al., 2008). S. divinorum was originally used as an ethnopharmacological agent by the Mazatec indigenous people of Oaxaca, Mexico; however, its mode of action was only recently discovered. Specifically, salvinorin A is a selective agonist at κ-opioid receptors and has no affinity at 5HT2 sites, common targets of known classic hallucinogens (Roth et al., 2002). Studies indicate that salvinorin A may have relatively unique properties as a ligand at κ-receptors, including ultrahigh efficacy at particular transduction systems and a reduced propensity to promote receptor internalization (Chavkin et al., 2004; Wang et al., 2005).
The behavioral effects of salvinorin A have been studied under several conditions in rodents (Fantegrossi et al., 2005; Zhang et al., 2005; Carlezon et al., 2006), detecting place aversion, motor incoordination, and depressive-like effects. One study in nonhuman primates focused on its operant discriminative effects in subjects trained to discriminate the synthetic κ-agonist U69,593 (Butelman et al., 2004). Studies on the overt behavioral effects of salvinorin A in nonhuman primate models may be especially valuable in view of reported differences between rodent and primate (including human) κ-opioid systems, at the neuroanatomical, neuropharmacological, and gene level (Young et al., 1986; Peckys and Landwehrmeyer, 1999; Liu-Chen, 2004; Butelman et al., 2007). Although there are descriptive reports on the behavioral and subjective effects of salvinorin A-containing products in humans, there is currently a lack of quantitative experimental data available (Siebert, 1994; Baggott et al., 2004; González et al., 2006).
κ-Receptors are located in several brain areas that could mediate sedative-like and postural effects in humans or primates. These potential target regions for the behavioral effects of salvinorin A include cortical areas, including the prefrontal cortex, dorsal and ventral striatal areas, and lateral hypothalamus (Peckys and Landwehrmeyer, 1999).
Pilot studies revealed that a subcutaneous dose of salvinorin A that was active in the κ-agonist (U69,593) drug discrimination assay in primates (0.032 mg/kg) (Butelman et al., 2004) only produced slight or no sedation in rhesus monkeys; this dose is at the practical solubility limit for this compound. In the present studies, therefore, we carried out an evaluation of the overt behavioral effects of intravenous salvinorin A in nonhuman primates (rhesus monkeys), given that this route typically results in greater bioavailability. In addition, intravenous bolus administration may model particular aspects of drug exposure by the smoking route (e.g., in terms of rapid rise in a compound's blood levels). Smoking is thought to be a common route of self-administration of salvinorin A-containing products in humans (González et al., 2006). We used initially observational rating scales for sedation and postural relaxation, previously used to study synthetic κ-opioid ligands. We then used a more recently refined method to quantify translationally viable effects (facial relaxation and ptosis) to characterize the onset and time course of salvinorin A in greater detail. We also carried out initial studies on the ability of a clinically available opioid antagonist (nalmefene) to prevent and/or reverse prominent behavioral effects of this widely available hallucinogen.
Materials and Methods
Subjects
Adult, captive-bred, gonadally intact rhesus monkeys (Macaca mulatta; four male and four female; age range, 8–14 years old approximately; weight range, approximately 7–12.5 kg), were used. Subjects were housed singly in stable colony rooms maintained at 20 to 22°C with controlled humidity, and a 12-h light/dark cycle (lights on at 7:00 AM). They were fed appropriate amounts of primate chow biscuits (PMI Feeds, Richmond, VA) daily, supplemented by appetitive treats. An environmental enrichment plan was in place in the colony rooms. Water was freely available in home cages, via an automatic waterspout. Subjects had complex histories of prior exposure to opioid pharmacological probes but no history of chronic exposure to any agent. Consecutive experiments in the same subjects were typically separated by at least 72 h and were carried out at least 3 h after lights on and 3 h before lights off (e.g., 1000–1600) on each experimental day.
Procedures
Rating Sedation and Posture with Observational Scales. The behavioral effects of salvinorin A were characterized with two rating scales, one measuring sedation and the other measuring posture (Butelman et al., 1999; Ko et al., 1999) (see Table 1). Such scales are particularly responsive to high-efficacy centrally penetrating κ-agonists and to nonopioid compounds known to have sedative effects in humans (Dykstra et al., 1987; Butelman and Woods, 1993). The scales are much less responsive to peripherally selective κ-agonists, consistent with mediation inside the blood-brain barrier for these effects. The sedation scale operationally measures the type of environmental stimulus that is required to obtain an orienting response from a subject, analogously to sedation scales in human studies (Ramsay et al., 1974). The posture scale is based on a global score of facial and whole-body relaxation.
TABLE 1.
Sedation and postural relaxation rating scales From Ko et al. (1999) and Butelman et al. (1999), based on Dykstra et al. (1987).
| Score | Operational Description of Stimulus Required to Elicit a Behavioral Responsea |
|---|---|
| Sedation scale (home cage or chaired subjects) | |
| 0 | No observable sedation, subject is alert to environmentb |
| 1 | Subject is attentive to ordinary movements of observerc |
| 2 | Subject responds to loud noise in room (one hand clap) |
| 3 | Subject responds only to opening of cage latch (home cage studies) or knocking on chair (chaired studies) |
| 4 | Subject responds only to loud noise 3 feet away from its head |
| 5 | Subject responds only to touch |
| 6 | Subject does not respond to touch |
| Postural relaxation score (home cage only) | |
| 0 | No observable facial or whole-body relaxation |
| 1 | Slight facial relaxation (including jaw slackening) or shoulder droop |
| 2 | Pronounced facial relaxation (including jaw slackening) or shoulder droop |
| 3 | Subject braces itself to sit up |
| 4 | Subject does not sit up |
Stimuli are presented in sequential order, until a behavioral response (e.g., an orienting response) is elicited
Observer is stationary in room; lack of subject alertness is defined by apparent staring for >15 s
“Ordinary” movement by observer is walking within room
Studies in chaired animals only utilized the sedation scale (with minor modifications from the scale used in the home cage) (Butelman et al., 1999; Ko et al., 1999) because this scale cannot robustly quantify posture in chaired subjects. Studies in chaired subjects were the initial dose-ranging studies and were used in a limited manner for evaluation of high-dose salvinorin A.
All rating was carried out by an observer experienced with the individual subjects and blind to the pharmacological conditions under study (e.g., drug or vehicle administration or antagonist pretreatment status) after repeated training. All studies were carried out with a time course design, with salvinorin A or U69,593 administered as a single bolus dose, followed by rating at standard time points (e.g., 5, 15, 30, 60, and, if applicable, 90 min). In antagonist pretreatment studies, a single dose of antagonist (e.g., nalmefene) was administered as pretreatment before salvinorin A, compared with vehicle pretreatment. Studies were reviewed by the Rockefeller University Animal Care and Use Committee, in accordance with the Guide for Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Facial Relaxation and Ptosis: Detailed Time Course Analysis. In follow-up studies, the cumulative duration of two specific behaviors were videotaped in chaired subjects in 1-min windows: facial relaxation and ptosis (partial or complete). Very brief events (e.g., <1 s in duration) were not scored to avoid baseline behaviors such as blinking. Facial relaxation and ptosis have been observed previously with κ-agonists in nonhuman primates (including other experiments herein) but have never been quantified specifically in themselves over detailed time windows.
All time windows were rated blind by a main trained rater, using the Observer XT System (Noldus, Wageningen, The Netherlands). After initial training, the main rater for the data reported herein exhibited a coefficient of variation of approximately 5% when rating a time window of peak pharmacological activity for salvinorin A (e.g., one with a mean of 28.9 s of ptosis in a 1-min period in five independent determinations). For comparison, a second trained rater independently scored the same time period repeatedly and obtained a mean of 26.3 s of ptosis. Thus, the average of the mean scores for the two raters differed by approximately 9%. Toward the end of all the reported studies (collected over several months), the same time window was again rated repeatedly by the main rater. The mean of these latter redeterminations for the main rater deviated from the mean of the original determinations by less than 3%.
Design. All observational studies were carried out with a repeated measures design (n = 3–6, depending on study and available subjects). The order of session within each experiment (e.g., vehicle or drug condition) was determined randomly. The design of each study is described below.
Studies with Sedation and Posture Observational Rating Scales
Intravenous Dose-Ranging Experiment in Chaired Subjects. Salvinorin A (0.032 and 0.1 mg/kg i.v.) was compared with the synthetic κ-agonist U69,593 (0.01, 0.032, and 0.056 mg/kg i.v.) in three male subjects. Each compound was compared with its vehicle.
Sedative and Postural Effects in the Home Cage. Further experiments were carried out in the home cage (to evaluate effects on posture in addition to sedation), with a maximal salvinorin A bolus of 0.032 mg/kg i.v. The effects of salvinorin A (0.01 or 0.032 mg/kg i.v.) were studied in six subjects (three males and three females) versus vehicle.
Dose-Dependent Nalmefene Prevention of the Effects of Salvinorin A in the Home Cage. This study determined the effect of salvinorin A (0.032 mg/kg) after 30-min pretreatment with nalmefene (two doses, 0.01 or 0.1 mg/kg s.c., or vehicle). It is known that the smaller nalmefene dose (0.01 mg/kg) is sufficient to cause robust antagonism of μ-receptor- but not κ-receptor-mediated effects in this species (France and Gerak, 1994; Butelman et al., 2007). In contrast, nalmefene (0.1 mg/kg) is sufficient to cause antagonism of κ-receptor-mediated effects in this species.
Potential Effects of the Cannabinoid CB1 Antagonist Rimonabant on Salvinorin A-Induced Behavior in the Home Cage. Because of recent reports on apparent modulation of salvinorin A effects by CB1 receptors in rats (Braida et al., 2008), this study determined the effects of salvinorin A (0.032 mg/kg i.v.) after 30-min pretreatment with the CB1 antagonist rimonabant (1 mg/kg i.v.) or vehicle. This rimonabant dose has produced antagonism of CB1 receptor-mediated effects in this species in prior studies (McMahon et al., 2005).
Detailed Analysis of Onset of Salvinorin A on Facial Relaxation and Ptosis
Time Course of Salvinorin A Effects on Facial Relaxation and Ptosis. Descriptive reports in humans mention ultrafast onset effects of salvinorin A-containing products, and this is consistent with the onset of detection of salvinorin A in CSF after intravenous administration herein (see below).
These separate studies therefore compared the effects of intravenous vehicle or intravenous salvinorin A (0.01 and 0.032 mg/kg) on videotaped sessions in chaired animals on facial relaxation and ptosis. The following 1-min time windows were used: preinjection baseline and 0 to 1, 1 to 2, 4 to 5, 14 to 15, 29 to 30, and 59 to 60 min after salvinorin A injection. These time windows are consistent with other studies reported herein and were selected a priori.
This was followed by studies on the ability of nalmefene (0.1 mg/kg) to either prevent or reverse salvinorin A (0.032 mg/kg i.v.)-induced facial relaxation or ptosis. Lastly, the serotonergic antagonist ketanserin (0.1 mg/kg i.m.) was studied for its potential ability to prevent similar effects of intravenous salvinorin A in these endpoints. Similar ketanserin doses were sufficient to block serotonergic effects in this species in prior studies (Li et al., 2008).
Detection of Intravenously Administered Salvinorin A in Cerebrospinal Fluid from Cisterna Magna. Subjects (n = 3) were fasted overnight and were anesthetized with Telazol (3 mg/kg i.m.). The area around the occiput and upper dorsal neck was clipped, and the skin was disinfected with isopropanol and iodine swabs. The subject was placed on a heating pad (37°C). A spinal needle (22 g, 1.5 inches; BD Biosciences, San Jose, CA) was percutaneously inserted in the cisterna magna using a previously described technique (Lipman et al., 1988). Flow of clear CSF was confirmed. The needle's sterile stylet was replaced into the needle before the study and between samples (the stylet completely occludes the lumen of the needle up to its tip, thus minimizing sample cross-contamination). Samples (approximately 300 μl each) were collected in chilled Eppendorf tubes and then immediately placed on dry ice. CSF samples (one per subject) were collected preinjection and at the following times after the end of salvinorin A injection (0.032 mg/kg i.v. injected over approximately 20 s): 0, 1, 2, 5, 15, and 30 min after administration and 180 (±5) min in a separate determination. The first drop of CSF at each time point (approximately 50 μl) was discarded to further minimize the sample to sample cross-contamination. After the experiment, the subjects were allowed to recover under observation and were returned to the home cage. Blood samples were not analyzed herein because prior studies under identical dosing conditions have already shown that salvinorin A blood concentrations decay very rapidly over the 1st min after administration and then gradually decline further over 60 min (Schmidt et al., 2005a; see also Hooker et al., 2008).
LC-MS Analysis of CSF Samples. Concentrations of salvinorin A were determined with an LC-MS technique at the Rockefeller University Proteomics Resource Center, based on a previously reported method (Schmidt et al., 2005b). The samples were spiked with deuterated salvinorin A as internal standard and purified using Oasis HLB solid phase extraction cartridges (Waters, Milford, MA). The analytes eluted from solid phase extraction were dried under nitrogen and reconstituted with 10% methanol/water. The samples were analyzed using nano-LC-tandem mass spectrometry. Each sample was separated by gradient elution with the Dionex capillary/nanohigh-performance liquid chromatography system at a flow rate of 250 nl/min and analyzed by a QSTAR XL mass spectrometer using information-dependent, automated acquisition. Data are presented as mean (±S.E.M.) in nanograms per milliliter.
Data Analysis for Behavioral Studies. Data from sedation and postural observational rating scale experiments were analyzed non-parametrically (e.g., Friedman's ANOVA, followed by Dunn's tests when appropriate; GraphPad Prism; GraphPad Software Inc., San Diego, CA). Data from the facial relaxation and ptosis experiments were analyzed parametrically (e.g., repeated measures ANOVA followed by post hoc Newman-Keuls tests; SigmaStat 3.1; Systat Software, Inc., San Jose, CA). The α level was set at p < 0.05 throughout; therefore, values denoted as significant used this p value as a cutoff.
Pharmacological Agents. Salvinorin A (extracted in the Laboratory of Dr. T.E. Prisinzano, University of Iowa, College of Pharmacy, Iowa City, Iowa) was dissolved daily in a vehicle composed of ethanol/Tween 80/sterile water [1:1:8 (v/v)]. U69,593 (Pharmacia and Upjohn, Kalamazoo, MI) was dissolved in sterile water, acidified with lactic acid (1 drop/5 ml). Nalmefene HCl (Baker Norton, Miami, FL) was dissolved in sterile water. Rimonabant-free base (RTI International, Research Triangle Park, NC; kindly provided through the National Institute on Drug Abuse Drug Supply System), was dissolved daily in a vehicle composed of dimethyl sulfoxide/Tween 80/sterile water (1:1:8, by volume). All injections were made in a volume of 0.05 to 0.2 ml/kg. Ketanserin tartrate (Sigma-Aldrich, St. Louis MO) was dissolved daily in 5% dimethyl sulfoxide/95% sterile water. Ketanserin dose is expressed as the base. For all other agents, doses are expressed in the forms described above. In relevant conditions, appropriate vehicle injections and volumes thereof were used for comparisons in behavioral studies.
Results
Effects of Salvinorin A on Sedation and Posture Scores with Observational Rating Scales
Baseline (Preinjection) Scores and Effects of Vehicle Administration on Sedation and Posture Scales. Unless otherwise stated, sedation and postural scores obtained preinjection were “0”; that, is subjects did not display signs of sedation or postural relaxation preinjection. In addition, unless otherwise stated, administration of vehicle (e.g., ethanol/Tween 80/sterile water, 1:1:8; 0.16 ml/kg) resulted in homogeneous scores of 0 for each individual session of the experiments described below (e.g., standard time points 5–90 min after administration). That is, with the present scales, there was a very low frequency of “false-positive” scores under these rating conditions.
Effects of Intravenous Salvinorin A in Chaired Subjects (Dose-Ranging Study). Intravenous salvinorin A doses were compared (0.032 and 0.1 mg/kg i.v. versus vehicle) in three chaired male subjects. Salvinorin A (0.032–0.1 mg/kg i.v.) resulted in dose-dependent increases in sedation scores, in each of the subjects (maximal scores of 4–6 were encountered in this group) (Fig. 1). A fast onset and short duration of action of intravenous salvinorin A was observed in all subjects (peak effects by the first time point, 5 min postinjection, with a partial decline by 30 min postinjection). Friedman's ANOVA at 5, 15, and 30 min postinjection revealed significant effects of salvinorin A dose [F(3) = 6, 6 and 5.6, respectively; p < 0.03], no significance was obtained at 60 min. Post hoc Dunn's tests at 5 and 15 min postinjection revealed that the larger salvinorin A dose (0.1 mg/kg) was significantly different from vehicle (p < 0.05). One subject in these initial determinations exhibited tremors within 30 s of salvinorin A injection (0.1 mg/kg). These were immediately treated with naltrexone (0.32 mg/kg); the tremors rapidly declined, and the subject recovered uneventfully. This subject was not studied further with this intravenous salvinorin A dose. Therefore, remaining studies reported below used a smaller maximal dose of salvinorin A (0.032 mg/kg) to minimize such undesirable effects.
Fig. 1.
Sedative effects of intravenous salvinorin A (0.032 and 0.1 mg/kg) in chaired subjects (n = 3; dose ranging study) (left). Sedative effects of U69,593 (0.01, 0.032, and 0.056 mg/kg i.v.) (right). Abscissae, time from intravenous injection (minutes); ordinates, median sedation score. Points above “BL” represent median baseline preinjection values.
In a direct comparison, U69,593 (0.01, 0.032, and 0.056 mg/kg i.v.) was compared with vehicle. U69,593 also produced robust dose-dependent sedation scores (maximal scores of 6 were obtained at the largest U69,593 dose in each of the subjects), with a fast onset and longer duration of action than salvinorin A. Friedman's ANOVA at 5, 15, 30, and 60 min revealed a significant effect of U69,593 dose [F(4) = 7.8, 8.8, 8, and 8, respectively; p < 0.02].
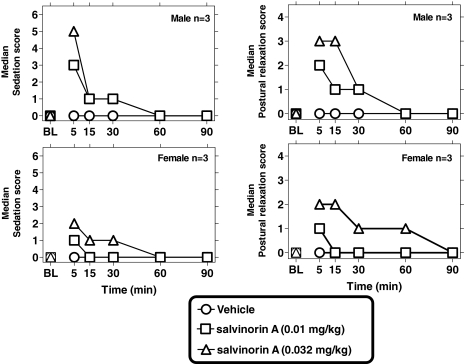
Intravenous Salvinorin A in the Home Cage: Effects on Sedation and Posture. Salvinorin A (0.01–0.032 mg/kg i.v.) caused dose-dependent sedation and postural changes in the subjects, with peak scores observed 5 min after administration (Fig. 2). Dose-dependent sedation was detected by Friedman's ANOVA (vehicle and two salvinorin A doses) at 5 and 15 min after salvinorin A [F(3) = 10.6 and 9.6, respectively; p < 0.002] but not at later time points. Postural effects were detected by Friedman's ANOVA (vehicle and two salvinorin A doses) at 5, 15, and 30 min postsalvinorin A [F(3) = 11.6, 9.0 and 6.6, respectively; p < 0.001, 0.01, and 0.03, respectively] but not at later time points. The present postural scale is scored from either facial or whole-body relaxation, and both were typically observed in the subjects.
Fig. 2.
Sedative (left) and postural effects of intravenous salvinorin A in the home cage (0.01 and 0.032 mg/kg; n = 6; three males and three females). Other details as in Fig. 1.
Dose-Dependent Nalmefene Prevention of Salvinorin A Sedative and Postural Effects in the Home Cage. The largest dose of salvinorin A studied in the home cage (0.032 mg/kg i.v.) was studied after subcutaneous pretreatment with nalmefene (0.01 or 0.1 mg/kg, versus vehicle pretreatment; n = 5) (Fig. 3). Subjects pretreated with vehicle before salvinorin A exhibited sedation and postural scores similar to those observed above. In contrast, nalmefene (0.01 or 0.1 mg/kg) caused dose-dependent prevention of these salvinorin A-induced effects. The smaller dose of nalmefene did not cause substantial changes in the sedative or postural effects of salvinorin A (0.032 mg/kg i.v.). In contrast, the larger dose of nalmefene (0.1 mg/kg) resulted in near complete prevention of sedative and postural effects in all subjects. Thus, Friedman's ANOVA for sedation scores detected a dose-dependent effect of nalmefene pretreatment at 5 and 15 min postsalvinorin A [F(3) = 6.71 and 8.6, respectively; p < 0.03 and 0.001, respectively]. Post hoc Dunn's tests on these sedation scores showed that the larger, but not the smaller, nalmefene pretreatment dose (0.1 and 0.01 mg/kg, respectively) was different from vehicle pretreatment (p < 0.05). Likewise, Friedman's ANOVA for postural scores detected a dose-dependent effect of nalmefene pretreatment at 5 and 15 min postsalvinorin A [F(3) = 9.6 and 9.3, respectively, p < 0.001]. Post hoc Dunn's tests on these postural scores showed that the larger, but not the smaller, nalmefene pretreatment dose (i.e., 0.1 and 0.01 mg/kg) was different from vehicle pretreatment (p < 0.01).
Fig. 3.
Nalmefene prevention of the sedative (left) and postural (right) effects of intravenous salvinorin A (0.032 mg/kg; n = 5). Nalmefene (0.01 or 0.1 mg/kg) was administered as a pretreatment (PT) and compared with vehicle pretreatment. Other details as in Fig. 1.
CB1 Antagonist Pretreatment to Intravenous Salvinorin A in the Home Cage. The effects of salvinorin A (0.032 mg/kg, n = 5) were also studied separately after pretreatment with the CB1 antagonist rimonabant (1 mg/kg i.v.) or its vehicle. Median scores after rimonabant pretreatment to salvinorin A (in either sedation or posture scales) were essentially superimposable to scores after vehicle pretreatment when tested 5 to 90 min after salvinorin A administration (data not shown).
Detailed Analysis of Salvinorin A-Induced Effects on Facial Relaxation and Ptosis
Preinjection Values and Effects of Vehicle. Preinjection scores for facial relaxation and ptosis were typically 0 s (in a 60-s window) for the reported experiments. As mentioned above, brief events (<1 s) were not scored to avoid detecting baseline behaviors such as blinking. Intravenous vehicle administration (scored for standard windows 0–60 min after administration) was also typically without effect on these scores (see Fig. 4).
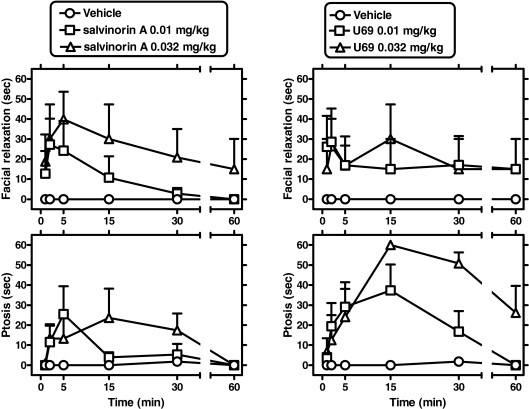
Fig. 4.
Effects of intravenous salvinorin A on facial relaxation and ptosis (top left and bottom left, respectively; n = 5). Effects of intravenous U69,593 on facial relaxation and ptosis (top right and bottom right, respectively; n = 4). Abscissae, time from the end of intravenous injection (note axis break). Ordinates, mean (±S.E.M.) cumulative time for each behavior, within a 60-s time window.
Salvinorin A Time Course in Facial Relaxation and Ptosis. Salvinorin A doses (0.01 and 0.032 mg/kg i.v.) were compared with vehicle in separate studies in chaired subjects (n = 5). Dose- and time-dependent increases in facial relaxation and ptosis were observed in all subjects, with very rapid onsets (typically by 1–2 min from the end of i.v. injection) (Fig. 4). Two-way (dose × time) repeated measures ANOVA were carried out for either dependent variable (see below).
Facial Relaxation. Significant main effects of dose [F(2,8) = 5.63; p < 0.03], time [F(4,5) = 4.88; p < 0.004] were detected. Newman-Keuls comparisons revealed a significant effect of salvinorin A (0.01 mg/kg) versus vehicle for the time windows occurring 1 to 2 and 4 to 5 min after intravenous administration, q = 4.24 and 5.52, respectively (p < 0.05). A similar profile was observed with salvinorin A (0.032 mg/kg) versus vehicle at 1 to 2 and 4 to 5 min, q = 3.64 and 3.94, respectively (p < 0.05).
Ptosis. Significant main effects of dose [F(2,8) = 9.08; p < 0.01] and a dose × time interaction [F(10,40) = 2.12; p < 0.05] were observed. Newman-Keuls comparisons revealed a significant effect of salvinorin A (0.01 mg/kg) versus vehicle for the time windows occurring 1 to 2 and 4 to 5 min after administration, q = 3.43 and 5.05, respectively (p < 0.05). Significant effects of the larger salvinorin A dose (0.032 mg/kg) versus vehicle were detected at 4 to 5 and 14 to 15 min after administration, q = 3.87 and 5.00, respectively (p < 0.05).
U69,593 Time Course in Facial Relaxation and Ptosis. U69,593 doses (0.01 and 0.032 mg/kg i.v.) were compared with vehicle, in separate studies in chaired subjects (n = 4), under identical conditions. U69,593 induced increases in facial relaxation, but its time course was less well defined than that observed with salvinorin A (above) (see Fig. 4). The effects of U69,593 on ptosis displayed a slower offset than those observed with salvinorin A. Two-way (dose × time) repeated measures ANOVA were carried out for either dependent variable (see below).
Facial Relaxation. No significant effects or interactions for the effects of U69,593 on facial relaxation were detected, probably because of relatively large intersubject variability with this ligand's effects on this endpoint.
Ptosis. Significant main effects of dose [F(2,6) = 18.63; p < 0.003], time (F[5,15] = 6.19; p < 0.003), and a dose × time interaction [F(10,30) = 2.97; p < 0.01] were observed. Newman-Keuls comparisons revealed a significant effect of U69,593 (0.01 mg/kg) versus vehicle for the time windows occurring 4 to 5 and 14 to 15 min after administration, q = 3.89 and 4.98, respectively (p < 0.05). Significant effects of the larger U69,593 dose (0.032 mg/kg) versus vehicle were detected at 4 to 5, 14 to 15, 29 to 30, and 59 to 60 min after administration, q = 3.20, 8.00, 6.53, and 3.48, respectively (p < 0.05).
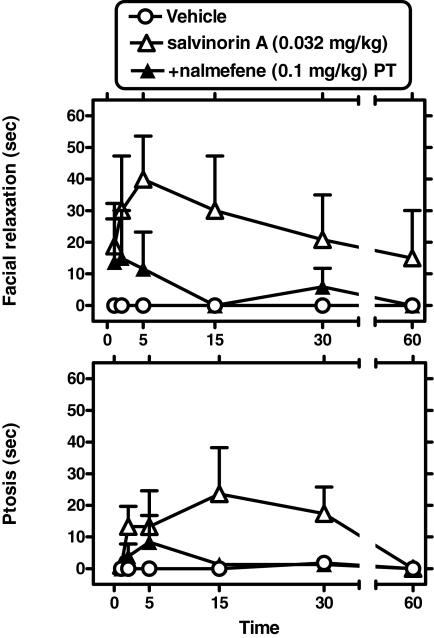
Nalmefene Prevention of Salvinorin A-Induced Facial Relaxation and Ptosis. Nalmefene (0.1 mg/kg s.c.) was administered as a 30-min pretreatment to salvinorin A (0.032 mg/kg; n = 5), followed by scoring as described above. Nalmefene alone, measured 19 to 20 min after administration, did not cause ptosis or facial relaxation (data not shown). However, nalmefene prevented the effects of salvinorin A on both facial relaxation and ptosis. Two-way (time × pretreatment condition) repeated measures ANOVA were carried out for either dependent variable (see below) (Fig. 5).
Fig. 5.
Nalmefene prevention of salvinorin A-induced facial relaxation (top) and ptosis (bottom) (n = 5). Nalmefene (0.1 mg/kg s.c.) was administered as a 30-min pretreatment (PT) to salvinorin A (0.032 mg/kg). Other details as in Fig. 4.
Facial Relaxation. A significant main effect of pretreatment condition was detected [F(1,4) = 14.88; p < 0.02].
Ptosis. A significant main effect of pretreatment condition was detected [F(1,4) = 14.46; p < 0.02].
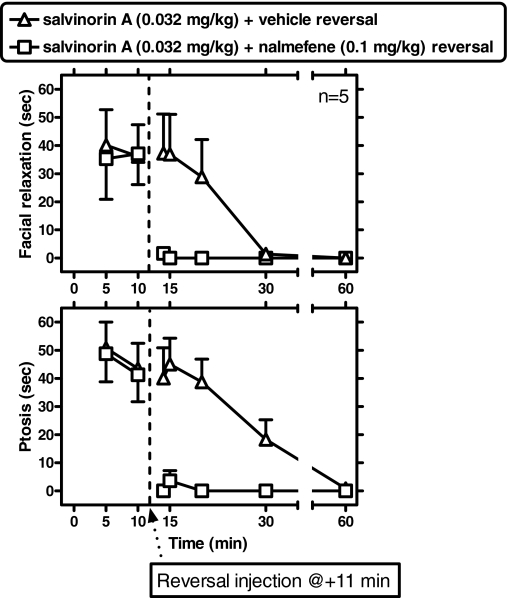
Nalmefene Reversal of Ongoing Salvinorin A-Induced Facial Relaxation and Ptosis. In a “reversal” experiment, nalmefene (0.1 mg/kg i.v.) was administered 11 min after salvinorin A (0.032 mg/kg i.v., i.e., after the onset of its effects) (Fig. 6). This was compared with a vehicle intravenous injection administered 11 min after salvinorin A. Modified postreversal time windows for analysis and presentation were selected a priori to capture potentially rapid reversal effects of nalmefene in the minutes after its administration. These time windows (with time 0 considered as the time of salvinorin A administration) were: 13 to 14, 14 to 15, 19 to 20, 29 to 30, and 59 to 60 min. Nalmefene caused rapid and robust reversal of ongoing salvinorin A-induced facial relaxation or ptosis (compared with a vehicle reversal injection) (see below).
Fig. 6.
Nalmefene reversal of ongoing salvinorin A-induced facial relaxation (top) and ptosis (bottom) (n = 5). Nalmefene (0.1 mg/kg i.v.) or vehicle were administered 11 min after salvinorin A (0.032 mg/kg). Therefore, observations after this nalmefene or vehicle treatment are used to evaluate the effectiveness of reversal. Note modified time windows to focus on postreversal period. Other details as in Fig. 4.
Facial Relaxation. There was a significant interaction between reversal condition and time [F(4,16) = 5.13; p < 0.01]; Newman-Keuls tests detected a significant difference because of nalmefene reversal for the 13- to 14-, 14- to 15-, and 19- to 20-min time windows (q = 4.76, 4.92, and 3.84, respectively) but not at later time windows (p < 0.05).
Ptosis. There was a significant main effect of reversal condition [F(1,4) = 28.34] and an interaction between reversal condition and time [F(4,16) = 7.40; p < 0.001]. Newman-Keuls tests detected a significant difference because of nalmefene reversal for the 13- to 14-, 14- to 15-, 19- to 20-, and 29- to 30-min time windows (q = 7.22, 7.44, 6.97, and 3.30, respectively) but not at later time windows (p < 0.05).
Lack of Influence of the 5T2 Antagonist Ketanserin on Salvinorin A-Induced Facial Relaxation and Ptosis. Ketanserin (0.1 mg/kg i.m.) was administered 30 min before salvinorin A (0.032 mg/kg). Ketanserin alone was without effect when observed 19 to 20 min after administration; ketanserin was also ineffective in modulating the effects of salvinorin A under these conditions (studied 5–60 min after salvinorin A; data not shown).
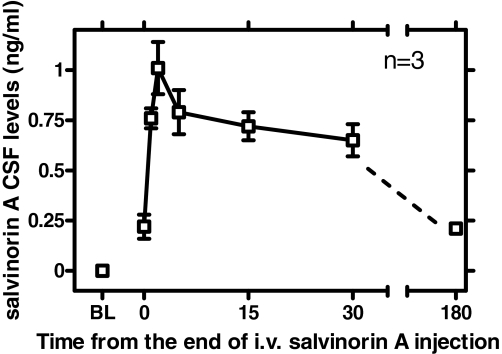
Detection of Salvinorin A in CSF after Intravenous Administration. Preinjection baseline samples for each subject (n = 3) demonstrated no MS signal peak for salvinorin A (mol. wt. = 432.5). Immediately after the end of intravenous bolus injection of salvinorin A (0.032 mg/kg, injected over approximately 20 s), CSF samples from each of the subjects displayed a signal for salvinorin A [mean 0.22 ng/ml (S.E.M. = 0.06)], termed time 0 (Fig. 7). The peak salvinorin A value was observed at 2 min after the end of injection [i.e., Cmax was 1.01 ng/ml (S.E.M.) = 0.01)]. This value then declined gradually, up to 30 min. A sample taken from the same subjects in a separate session at 180 (±5) min after injection revealed a greater time-dependent decrease [mean = 0.21 ng/ml (S.E.M. = 0.02)].
Fig. 7.
Time course of salvinorin A levels detected in CSF after intravenous injection (0.032 mg/kg) (n = 3). Abscissa, time from the end of intravenous injection (minutes). Ordinate, salvinorin A CSF levels quantified by LC-MS (mean ± S.E.M.).
Discussion
When injected by the intravenous route, salvinorin A (0.032–0.1 mg/kg) caused robust and dose-dependent sedative effects, with a fast onset and relatively short duration of action (e.g., 5 and 15 min, respectively). This maximal intravenous dose of salvinorin A was limited by practical and safety considerations; therefore, subsequent studies reported herein used a smaller maximal dose (0.032 mg/kg i.v.). There are no available data on the safety of relatively high-dose salvinorin A administration in humans, and this is of interest, in view of the reported high-potency salvinorin A preparations (e.g., concentrated extracts) available for sale (Hoover et al., 2008). These are the first studies to characterize the unconditioned behavioral effects of salvinorin A in primates. The sedative effects of salvinorin A were of similar magnitude as those observed with a selective high-efficacy κ-agonist, U69,593, and demonstrated fast onset and relatively shorter duration of action (see also below).
Centrally penetrating κ-agonists (but not peripherally selective κ-agonists) also have prominent postural effects in primates (Butelman et al., 1999), suggesting that this effect is also mediated by central nervous system κ-receptors. Salvinorin A, when studied in the home cage, also produced such postural effects, with peak effects of short duration, observed at similar times as sedative effects (e.g., 5–15 min after intravenous administration).
The opioid antagonist nalmefene produced a dose-dependent prevention of salvinorin A-induced sedation and postural effects. The smaller nalmefene dose (0.01 mg/kg) was ineffective, whereas the larger dose (0.1 mg/kg) almost fully prevented the effects of salvinorin A. Prior studies in this species show that the smaller nalmefene dose is sufficient to block μ-receptor-mediated effects, whereas the larger dose can also block κ-receptor-mediated effects (France and Gerak, 1994; Butelman et al., 2007). Taken together, these data support the conclusion that salvinorin A's sedative and postural effects are mediated by κ-receptors. From a translational perspective, it is of interest that nalmefene doses in this approximate range have been studied clinically and experimentally in humans (Kaplan et al., 1999; Bart et al., 2005), suggesting that pharmacological tools to block salvinorin A effects in humans are currently available. It is interesting that nalmefene has partial agonist effects at human κ-receptors under certain conditions (Bart et al., 2005); nalmefene's ability to block the actions of a high-efficacy κ-agonist such as salvinorin A is highly consistent with receptor theory (Roth et al., 2002; Chavkin et al., 2004).
A recent publication suggested CB1 receptor involvement in the behavioral effects of salvinorin A in rats (Braida et al., 2008). Salvinorin A does not have affinity at CB1 receptors (Roth et al., 2002; Capasso et al., 2008); therefore, this observed effect could be potentially because of a downstream interaction between κ-opioid and CB1 systems (Mason et al., 1999). However, in contrast to the full blockade observed with nalmefene herein, the CB1 antagonist rimonabant (1 mg/kg i.v.) was ineffective in modulating the sedative or postural effects of salvinorin A. In previous studies (Wiley et al., 1995; Vivian et al., 1998; McMahon et al., 2005), similar rimonabant doses were sufficient to block the effects of CB1 agonists in rhesus monkeys. Therefore, these data do not support downstream effects of salvinorin A at CB1 receptors, possibly suggesting primate versus rodent differences in the interaction between κ-receptor and CB1 systems.
Descriptive reports in humans may indicate ultrafast onset (e.g., within 1–2 min of self-administration) and short duration of action of salvinorin A effects (Siebert, 1994; Baggott et al., 2004; González et al., 2006), although quantitative laboratory-based data in humans are not available at this time. It is also known that fast onset exacerbates the abuse potential of compounds from different pharmacological classes (Winger et al., 2002; Spencer et al., 2006; Yano and Steiner, 2007); it is unknown whether this is of relevance to the broad experimentation with salvinorin A-containing products in humans. Therefore, we carried out follow-up studies using two specific endpoints, facial relaxation and ptosis, because these endpoints have several advantages: 1) they are more easily quantifiable that rating scale data (above); 2) they are more amenable to the study of ultrafast onset and detailed time course analysis, compared with rating scale data; 3) they are translationally viable (i.e., easily quantifiable in humans); and 4) they are putatively mediated by more defined neuroanatomical/cranial nerve pathways, thus aiding mechanistic interpretation of behavioral and neuroimaging studies.
Salvinorin A (0.01–0.032 mg/kg; without probing larger doses because of the limitations alluded to above) caused rapid and dose-dependent effects on facial relaxation and ptosis. Effects in these endpoints could be detected within 1 to 2 min from the end of intravenous administration, peaking soon thereafter, and declined substantially for time points after 15 min. U69,593 produced longer lasting effects, under similar conditions. Nalmefene (0.1 mg/kg) robustly prevented these effects of salvinorin A and was also able to reverse ongoing effects of this powerful hallucinogen. This suggests that these effects of salvinorin A are both initiated and maintained by opioid (κ) receptors. These are the first studies, to our knowledge, on the reversal of an ongoing in vivo effect of salvinorin A by an opioid antagonist.
The 5HT2 antagonist ketanserin, in contrast, was ineffective in preventing these salvinorin A-induced effects (5HT2-like receptors, particularly 5HT2A, are thought to mediate the major effects of classic hallucinogens, like d-lysergic acid diethylamide) (Roth et al., 2002). The selected dose of ketanserin is sufficient to block serotonergic effects in this species (Li et al., 2008). Therefore, these data are consistent with a mode of action of salvinorin A not depending on agonist effects at 5HT2 receptors.
These are also the first studies to document the entry of intact salvinorin A into central tissues as reflected by CSF, after administration of a behaviorally active intravenous dose (0.032 mg/kg). Salvinorin A was detectable by LC-MS within 1 min of the end of intravenous bolus administration, and maximal concentrations were observed 2 min after administration. This ultrafast entry of salvinorin A into central tissues is consistent with descriptive reports in humans, with a recent neuroimaging study (Hooker et al., 2008), and with the onset of effects on facial relaxation and ptosis, observed after this salvinorin A dose (reported above).
It is intriguing that the maximal salvinorin A concentration observed in CSF (≈1 ng/ml) is approximately equivalent to 2 nM, which is very similar to the reported Ki for salvinorin A at κ-receptors in brain tissue (Roth et al., 2002). Therefore, the present CSF detection data are consistent with brain κ-receptor occupancy by salvinorin A, at times when its robust behavioral and neuroendocrine effects are observed (Butelman et al., 2007). It has been pointed out that CSF concentrations of a particular ligand are not necessarily identical to brain extracellular concentrations under all conditions; therefore, this extrapolation should be interpreted with caution (Shen et al., 2004).
Overall, these are the first studies to characterize the overt behavioral effects of the widely available hallucinogen, salvinorin A, in a primate species with considerable κ-receptor gene homology to humans (Butelman et al., 2007). Consistent with descriptive information from humans, the effects of intravenous salvinorin A are robust, of ultrafast onset, and of relatively short duration. The behavioral effects of salvinorin A can both be prevented and reversed by a clinically available opioid antagonist, nalmefene, at a dose sufficient to cause κ-receptor blockade in this species. This confirms that salvinorin A is a unique hallucinogen whose actions are initiated and maintained by κ-receptors.
Acknowledgments
We thank Marek Mandau for technical assistance.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA017369, DA018151, DA05130].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.145342.
ABBREVIATIONS: 5HT2, 5-hydroxytryptamine 2; U69, U69,593, (+)-(5α,7α,8β)-N-methyl-N-[7-(1-pyrrolidinyl)-1-oxaspiro[4.5]dec-8-yl]-benzene-acetamide; CSF, cerebrospinal fluid; LC, liquid chromatography; MS, mass spectrometry; ANOVA, analysis of variance.
References
- Baggott MJ, Erowid E, Erowid F, and Mendelson JE (2004) Use of Salvia divinorum, an unscheduled hallucinogenic plant: a web-based survey of 500 users, in College on Problems of Drug Dependence Abstract (2004 Annual Meeting); 12–17 June 2004; San Juan, Puerto Rico. College on Problems of Drug Dependence, Philadelphia.
- Bart G, Schluger JH, Borg L, Ho A, Bidlack JM, and Kreek MJ (2005) Nalmefene induced elevation in serum prolactin in normal human volunteers: partial kappa-opioid agonist activity? Neuropsychopharmacology 30 2254-2262. [DOI] [PubMed] [Google Scholar]
- Braida D, Limonta V, Capurro V, Fadda P, Rubino T, Mascia P, Zani A, Gori E, Fratta W, Parolaro D, and Sala M (2008) Involvement of kappa-opioid and endocannabinoid system on salvinorin A-induced reward. Biol Psychiatry 63 286-292. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Harris TJ, and Kreek MJ (1999) Effects of E-2078, a stable dynorphin A(1–8) analog, on sedation and serum prolactin levels in rhesus monkeys. Psychopharmacology (Berl) 147 73-80. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Harris TJ, and Kreek MJ (2004) The plant-derived hallucinogen, salvinorin A, produces kappa-opioid agonist-like discriminative effects in rhesus monkeys. Psychopharmacology 172 220-224. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Mandau M, Tidgewell K, Prisinzano TE, Yuferov V, and Kreek MJ (2007) Effects of salvinorin A, a kappa-opioid hallucinogen, on a neuroendocrine biomarker assay in non-human primates with high kappa-receptor homology to humans. J Pharmacol Exp Ther 320 300-306. [DOI] [PubMed] [Google Scholar]
- Butelman ER and Woods JH (1993) Effects of clonidine, dexmedetomidine and xylazine on thermal antinociception in rhesus monkeys. J Pharmacol Exp Ther 264 762-769. [PubMed] [Google Scholar]
- Capasso R, Borrelli F, Cascio MG, Aviello G, Huben K, Zjawiony JK, Marini P, Romano B, Di Marzo V, Capasso F, and Izzo AA (2008) Inhibitory effect of salvinorin A, from Salvia divinorum, on ileitis-induced hypermotility: cross-talk between kappa-opioid and cannabinoid CB(1) receptors. Br J Pharmacol 155 681-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA Jr, Béguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, and Cohen BM (2006) Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther 316 440-447. [DOI] [PubMed] [Google Scholar]
- Chavkin C, Sud S, Jin W, Stewart J, Zjawiony JK, Siebert DJ, Toth BA, Hufeisen SJ, and Roth BL (2004) Salvinorin A, an active component of the hallucinogenic sage Salvia divinorum, is a highly efficacious kappa opioid receptor agonist: structural and functional considerations. J Pharmacol Exp Ther 308 1197-1203. [DOI] [PubMed] [Google Scholar]
- Dykstra LA, Gmerek DE, Winger G, and Woods JH (1987) Kappa opioids in rhesus monkeys: I. Diuresis, sedation, analgesia and discriminative stimulus effects. J Pharmacol Exp Ther 242 413-420. [PubMed] [Google Scholar]
- Fantegrossi WE, Kugle KM, Valdes LJ 3rd, Koreeda M, and Woods JH (2005) Kappa-opioid receptor-mediated effects of the plant-derived hallucinogen, salvinorin A, on inverted screen performance in the mouse. Behav Pharmacol 16 627-633. [DOI] [PubMed] [Google Scholar]
- France CP and Gerak LR (1994) Behavioral effects of 6-methylene naltrexone (nalmefene) in rhesus monkeys. J Pharmacol Exp Ther 270 992-999. [PubMed] [Google Scholar]
- González D, Riba J, Bouso JC, Gómez-Jarabo G, and Barbanoj MJ (2006) Pattern of use and subjective effects of Salvia divinorum among recreational users. Drug Alcohol Depend 85 157-162. [DOI] [PubMed] [Google Scholar]
- Hooker JM, Xu Y, Schiffer W, Shea C, Carter P, and Fowler JS (2008) Pharmacokinetics of the potent hallucinogen, salvinorin A in primates parallels the rapid onset, short duration of effects in humans. Neuroimage 41 1044-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover V, Marlowe DB, Patapis NS, Festinger DS, and Forman RF (2008) Internet access to Salvia divinorum: implications for policy, prevention, and treatment. J Subst Abuse Treat 35 22-27. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC.
- Kaplan JL, Marx JA, Calabro JJ, Gin-Shaw SL, Spiller JD, Spivey WL, Gaddis GM, Zhao N, and Harchelroad FP Jr (1999) Double-blind, randomized study of nalmefene and naloxone in emergency department patients with suspected narcotic overdose. Ann Emerg Med 34 42-50. [DOI] [PubMed] [Google Scholar]
- Ko MC, Johnson MD, Butelman ER, Willmont KJ, Mosberg HI, and Woods JH (1999) Intracisternal nor-binaltorphimine distinguishes central and peripheral kappa-opioid antinociception in rhesus monkeys. J Pharmacol Exp Ther 291 1113-1120. [PMC free article] [PubMed] [Google Scholar]
- Lange JE, Reed MB, Croff JM, and Clapp JD (2008) College student use of Salvia divinorum. Drug Alcohol Depend 94 263-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Rice KC, and France CP (2008) Discriminative stimulus effects of 1-(2,5-dimethoxy-4-methylphenyl)-2-aminopropane (DOM) in rhesus monkeys. J Pharmacol Exp Ther 324 827-833. [DOI] [PubMed] [Google Scholar]
- Lipman B, Palmer D, Noble J, Haughton V, and Collier D (1988) Effects of lumbar puncture on flow of cerebrospinal fluid. Invest Radiol 23 359-360. [DOI] [PubMed] [Google Scholar]
- Liu-Chen LY (2004) Agonist-induced regulation and trafficking of kappa opioid receptors. Life Sci 75 511-536. [DOI] [PubMed] [Google Scholar]
- Mason DJ Jr, Lowe J, and Welch SP (1999) Cannabinoid modulation of dynorphin A: correlation to cannabinoid-induced antinociception. Eur J Pharmacol 378 237-248. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Amin MR, and France CP (2005) SR141716A differentially attenuates the behavioral effects of delta9-THC in rhesus monkeys. Behav Pharmacol 16 363-372. [DOI] [PubMed] [Google Scholar]
- Peckys D and Landwehrmeyer GB (1999) Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: a 33P in situ hybridization study. Neuroscience 88 1093-1135. [DOI] [PubMed] [Google Scholar]
- Ramsay MA, Sevege TM, Simpson BR, and Goodwin R (1974) Controlled sedation with alphaxalone-alphadolone. Br Med J 2 656-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, and Rothman RB (2002) Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist. Proc Natl Acad Sci U S A 99 11934-11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MD, Schmidt MS, Butelman ER, Harding WW, Tidgewell K, Murry DJ, Kreek MJ, and Prisinzano TE (2005a) Pharmacokinetics of the plant-derived hallucinogen salvinorin A in nonhuman primates. Synapse 58 208-210. [DOI] [PubMed] [Google Scholar]
- Schmidt MS, Prisinzano TE, Tidgewell K, Harding W, Butelman ER, Kreek MJ, and Murry DJ (2005b) Determination of salvinorin A in body fluids by high performance liquid chromatography-atmospheric pressure chemical ionization. J Chromatogr B Analyt Technol Biomed Life Sci 818 221-225. [DOI] [PubMed] [Google Scholar]
- Shen DD, Artru AA, and Adkison KK (2004) Principles and applicability of CSF sampling for the assessment of CNS drug delivery and pharmacodynamics. Adv Drug Deliv Rev 56 1825-1857. [DOI] [PubMed] [Google Scholar]
- Siebert DJ (1994) Salvia divinorum and salvinorin A: new pharmacologic findings. J Ethnopharmacol 43 53-56. [DOI] [PubMed] [Google Scholar]
- Valdes LJ, Butler WM, Hatfield GM, Paul AG, and Koreeda M (1984) Divinorin A, a psychotropic terpenoid, and divinorin B from the hallucinogenic Mexican mint, Salvia divinorum. J Org Chem 49 4716-4720. [Google Scholar]
- Vivian JA, Kishioka S, Butelman ER, Broadbear J, Lee KO, and Woods JH (1998) Analgesic, respiratory and heart rate effects of cannabinoid and opioid agonists in rhesus monkeys: antagonist effects of SR 141716A. J Pharmacol Exp Ther 286 697-703. [PubMed] [Google Scholar]
- Volkow ND (2006) PET study examining pharmacokinetics, detection and likeability, and dopamine transporter receptor occupancy of short- and long-acting oral methylphenidate. Am J Psychiatry 163 359-361. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tang K, Inan S, Siebert D, Holzgrabe U, Lee DY, Huang P, Li JG, Cowan A, and Liu-Chen LY (2005) Comparison of pharmacological activities of three distinct k-ligands (salvinorin A, TRK-820 and 3FLB) on kappa opioid receptors in vitro and their antipruritic and antinociceptive activities in vivo. J Pharmacol Exp Ther 312 220-230. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Lowe JA, Balster RL, and Martin BR (1995) Antagonism of the discriminative stimulus effects of delta-9-tetrahydrocannabinol in rats and rhesus monkeys. J Pharmacol Exp Ther 275 1-6. [PubMed] [Google Scholar]
- Winger G, Hursh SR, Casey KL, and Woods JH (2002) Relative reinforcing strength of three N-methyl-d-aspartate antagonists with different onsets of action. J Pharmacol Exp Ther 301 690-697. [DOI] [PubMed] [Google Scholar]
- Yano M and Steiner H (2007) Methylphenidate and cocaine: the same effects on gene regulation? Trends Pharmacol Sci 28 588-596. [DOI] [PubMed] [Google Scholar]
- Young EA, Walker JM, Lewis ME, Houghten RA, Woods JH, and Akil H (1986) [3H]dynorphin A binding and kappa selectivity of prodynorphin peptides in rat, guinea-pig and monkey brain. Eur J Pharmacol 121 355-365. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, and Kreek MJ (2005) Effects of the plant-derived hallucinogen salvinorin A on basal dopamine levels in the caudate putamen and in a conditioned place aversion assay in mice: agonist actions at kappa opioid receptors. Psychopharmacology 179 551-558. [DOI] [PubMed] [Google Scholar]