Abstract
Pannexin (Panx) 1 is a widely expressed protein that shares structural, but not amino acid, homology with gap junction proteins, the connexins. Panx1 does not form gap junctions in mammalian cells, but it may function as a plasma membrane hemichannel. Little is known of the pharmacological properties of panx1 expression in mammalian cells. Here, we identify three variants in the human PANX1 gene. We expressed these variants and mouse Panx1 in mammalian cells and compared Panx1-induced currents. All human Panx1 variants and the mouse Panx1 showed identical protein expression levels, localization patterns, and functional properties, although the frequency of functional expression was species-dependent. Panx1 currents were independent of changes in extracellular or intracellular calcium or phospholipase C transduction. We found compounds that inhibited Panx1 currents with a rank order of potency: carbenoxolone > disodium 4,4′-diisothiocyanatostilbene-2,2′-disulfonate (DIDS) ≈ disodium 4-acetamido-4′-isothiocyanato-stilben-2,2′-disulfonate ≈ 5-nitro-2-(3-phenylpropylamino)benzoic acid > indanyloxyacetic acid 94 >> probenecid >> flufenamic acid = niflumic acid. Triphosphate nucleotides (ATP, GTP, and UTP) rapidly and reversibly inhibited Panx1 currents via mechanism(s) independent of purine receptors. When Panx1 was coexpressed with purinergic P2X7 receptor (P2X7R), DIDS was found to act as a P2X7R antagonist to inhibit ATP-evoked currents, but none of the other compounds inhibited P2X7R currents. This is the first detailed pharmacological characterization of Panx1-mediated currents in mammalian cells and sheds new, although contradictory, light on the hypothesis that Panx1 acts as a hemichannel to allow passage of large molecules in response to P2X7R activation.
Pannexins are a three-membered family of membrane proteins (Panx1–3) that bear topological similarity, but minimal amino acid homology, to the large family of gap junction channels, the connexins (Panchin, 2005; Barbe et al., 2006). Unlike connexins, pannexins do not form gap junctions when expressed in mammalian cells (Huang et al., 2007a) but do lead to the appearance of ionic currents whose properties resemble “undocked” gap junction hemichannels (Scemes et al., 2007). Panx1 has distinct but generally ubiquitous expression in excitable and nonexcitable cells and has generated increasing interest as a likely hemichannel conduit for nonvesicular release of ATP from erythrocytes and taste receptor cells (Locovei et al., 2006a; Huang et al., 2007b). Panx1 has also been shown to form protein-protein association with the purinergic P2X7 receptor (P2X7R), whose activation by extracellular ATP opens a typical cationic channel within milliseconds followed seconds later by an opening, or activation, of a large pore permeable to molecules up to 900 Da (Pelegrin and Surprenant, 2006). The initial phase of this P2X7R-activated dye-permeable pore is inhibited by blockade of Panx1 using siRNA knockdown techniques, by a Panx1-mimetic inhibitory peptide, and by the relatively nonselective gap junction channel blocker, carbenoxolone (CBX) (Pelegrin and Surprenant, 2006, 2007). These results have provided the evidence for the hypothesis that Panx1 hemichannels open in response to conformational changes of the P2X7R protein complex upon its activation, thus allowing passage of the larger molecules through its open hemichannel (Pelegrin and Surprenant, 2006; Scemes et al., 2007). Mechanical and osmotic stimuli and changes in intracellular calcium have also been suggested to open Panx1 hemichannels in the absence of P2X7R presence or activation (Locovei et al., 2006b; Vanden Abeele et al., 2006).
Because the molecular identification of pannexins has occurred relatively recently (Bruzzone et al., 2003; Baranova et al., 2004), there have been few pharmacological characterizations of ectopically expressed Panx1 currents, and all have been carried out using the oocyte expression system (Bruzzone et al., 2005; Locovei et al., 2006b; Silverman et al., 2008), with the exception of our previous study of human Panx1 currents expressed in HEK293 cells (Pelegrin and Surprenant, 2006). Expression of Panx1 in either oocytes or mammalian cells results in ionic currents that are in general agreement; that is, currents activate upon depolarizations greater than ∼10 mV, show minimal time dependence of activation, are rapidly and reversibly inhibited by CBX at concentrations (EC50 ∼ 5 μM) that are 5- to 20-fold less than required to inhibit connexin-mediated currents, are independent of external calcium, and are only weakly inhibited by flufenamic acid, a commonly used inhibitor of both chloride channels and gap junction channels (Bruzzone et al., 2005). Few other pharmacological properties of Panx1-mediated currents are known, although probenecid, a compound long used clinically for the treatment of gout and thought to act by inhibiting specific plasma membrane anionic transporters, has been shown recently to block Panx1 currents in oocytes without inhibiting connexin (46 and 32) currents (Silverman et al., 2008). In contrast, there seem to be striking discrepancies in results obtained from oocyte expression and mammalian cell expression when P2X7R is coexpressed with Panx1. In the oocyte expression system, currents in response to ATP activation of ectopically expressed P2X7R is inhibited by CBX and mefloquine (Iglesias et al., 2008), but in mammalian expression systems, no inhibition of P2X7R-mediated currents by these compounds is observed (Pelegrin and Surprenant, 2006).
The present study was undertaken to expand the currently limited repertoire of compounds that may act on Panx1, particularly in mammalian cell expression systems. We carried out quantitative comparisons of currents induced by expression of human and mouse Panx1 in HEK293 cells (in the absence of P2X7R). We found that ATP and other nucleotides directly inhibited both human and mouse Panx1 currents in a purine receptor-independent manner, that Panx1 currents were independent of intracellular calcium concentrations, and that some nonselective chloride channel inhibitors (NPPB, DIDS, IAA-94) blocked Panx1 currents, whereas others (FFA, NFA, 9-AC, MFQ, DPC) had little or no effect. DIDS, but none of the other compounds, inhibited P2X7R-mediated currents in cells expressing only P2X7R or in cells coexpressing both P2X7R and Panx1. These results expand the previously limited pharmacological profile of panx1 currents and show that inhibition of panx1 currents is independent of inhibition of P2X7 receptor currents.
Materials and Methods
Materials. All salts and compounds used in this study were from Sigma Chemical (Poole, Dorset, UK), with the exception of DPC from Merck Chemicals (Nottingham, UK) and U73122 and U73343 from Calbiochem (San Diego, CA). Panx1 antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), Millipore Corporation (Billerica, MA), or DIATHEVA s.r.l. (Fano, Italy) or generously supplied by S. Scherer (University of Pennsylvania, Philadelphia, PA). Secondary Abs were from Dako UK Ltd. (Ely, Cambridgeshire, UK), and all cell culture media, Optimem, and Lipofectamine 2000 were from Invitrogen (Paisley, UK).
Cells, Transfections, Cloning, and siRNA. HEK293 cells were originally obtained from the American Type Culture Collection (Manassas, VA) and maintained as described previously (Pelegrin and Surprenant, 2006). Transient transfection was carried out similarly for all cell lines using Lipofectamine 2000 (Invitrogen, Carlsbad, CA), as described in detail previously (Roger et al., 2008). Unless otherwise stated, Panx1 cDNA (1 μg) was cotransfected with eGFP (0.1 μg), and electrophysiological recordings were made 24 to 48 h post-transfection. siRNA for human Panx1 has been described previously (Pelegrin and Surprenant, 2006). Human Panx1 expression vectors have been described previously (Pelegrin and Surprenant, 2006); the full-length sequence encoding mouse Panx1 was amplified from total mouse C57 BL/6 brain mRNA using reverse transcriptase-PCR and cloned into pcDNA3.1(-) vector (Invitrogen). c-myc (EQKLISEEDL) and eGFP tags were introduced in-frame at the 3′ end of the coding sequence of mouse Panx1 using overlapping PCR and Accuzyme proof-reading DNA polymerase (Bioline); DsRed tag was introduced in-frame at the 3′ end of the coding sequence of human Panx1; FLAG tag (DYKDDDDK) was introduced in the second extracellular loop of human Panx1-myc at position 162 to 168, hence incorporating a c-myc, eGFP, or DsRed fusion tag at the C terminus or a FLAG tag at the second extracellular loop of the expressed protein. The different human Panx1 splice variants and the deletion of V377 were generated from the hPanx1-ee construct using the PCR overlap extension method (Young et al., 2007) and Accuzyme DNA polymerase. Cloned products were confirmed by sequencing (Beckman Coulter CEQ 2000 Dye Terminator; Beckman Coulter, Fullerton, CA), and protein expression was verified by Western blotting.
Immunohistochemistry. For immunohistochemistry, cells were transfected as described above on coverslips or in 35-mm Petri dishes and plated onto coverslips 4 h post-transfection; cells were examined 24 to 48 h later. Cells were washed twice with PBS followed by fixation [4% (v/v) formaldehyde in PBS] for 30 min at room temperature, then permeabilized with blocking solution (0.1% Triton X-100 and 0.3% bovine serum albumin in PBS) for 40 min. Cells were then incubated with primary Ab against Myc-tag (1:1000 in the blocking solution) for 1 h at room temperature. After washing three times with PBS, anti-mouse IgG Alexa Fluor 594 conjugated secondary Ab (1:200; Invitrogen) was applied for 30 min. 4,6-Diamidino-2-phenylindole (Invitrogen) was used for nuclear staining. After brief rinse in H2O, coverslips were mounted on slides with Gold antifade reagent (Invitrogen). Cells were photographed using Zeiss Plan-Neofluar 100× oil and AxioVision software (Zeiss, Welwyn Garden City, UK), Nikon Eclipse TE300 confocal 60× objective and EZ-C1 software (Nikon, Tokyo, Japan), or Olympus IX71 100× objective with SoftWoRx 3.5 [DeltaVision; Image Solutions (UK) Ltd, Preston, UK], as indicated. We generated several epitope-tagged Panx1 proteins; there were no differences between human and mouse Panx1 protein localization when expressed in HEK293 cells (Supplemental Fig. 1). C-terminal fusion of large epitopes (eGFP or DsRed) resulted in Panx1 protein remaining intracellular in vacuole-like compartments, whereas smaller epitopes (c-myc or ee-tags) showed intense, plasma membrane-delimited expression (Supplemental Fig. 1, A and B).
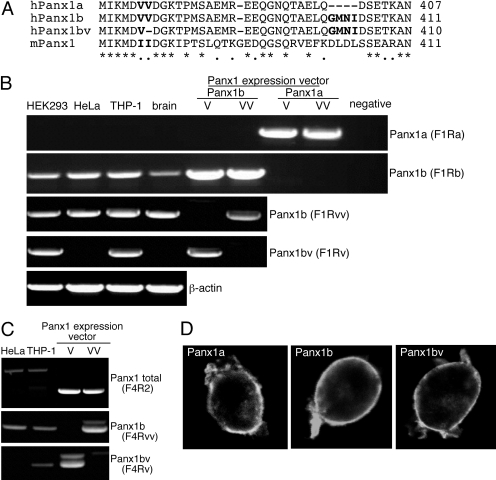
Reverse Transcription and PCR Analysis. Human brain RNA (Ambion, Austin, TX) and total RNA from other cells were isolated using the RNeasy Mini kit (QIAGEN, Valencia, CA), followed by reverse transcription using SUPERSCRIPT III (Invitrogen) RNase H-reverse transcriptase with oligo(dT). Genomic DNA was isolated by digesting 106 cells in a buffer containing 23 mg/ml proteinase K and 0.5% Triton X-100 at 56°C for 60 min. Panx1 was amplified by standard PCR protocols from cDNA using specific oligonucleotides that differentiate between the alternative splice variant on exon 5 (F1/Ra or F1/Rb; Supplemental Table 1) or the deletion of a single Val at position 377 in exon 4 (F1/Rvv or F1/Rv; Supplemental Table 1); oligonucleotides annealing at both sides of intron 4 (F4/R2, Table 1) were used to amplify human Panx1 from genomic PCR, and this fragment contains V377 position in the exon 4; β-actin primers have been reported previously (Pelegrin and Surprenant, 2006). The PCR conditions to distinguish between the different Panx1 variants were 20 cycles with a stringent annealing temperature of 65°C for the a/b splicing variant and 70°C for the V377 deletion. The obtained product sizes for all PCR products were as expected from their mRNA sequence or from the genomic DNA and were for F1/Ra, 675 bp (Panx1a); for F1/Rb, 690 bp (Panx1b) or 687 bp (Panx1bv); for F1/Rvv, 610 bp (Panx1b); for F1/Rv, 607 bp (Panx1bv); for F4/Rvv, 445 bp (Panx1b); for F4/Rv, 442 bp (Panx1bv); for F4/R2, 1110 (Panx1b), 1098 (Panx1a), and 1107 (Panx1bv) bp; and 1129 bp for β-actin.
TABLE 1.
IC50 values (micromolar) for inhibition of Panx1 currents and comparison with actions of these compounds at P2X7R Numbers in parentheses are n values.
|
IC50 Values
|
|||
|---|---|---|---|
| Mouse Panx1 | Human Panx1 | Effect on P2X7R Current | |
| μM | |||
| CBX | 4 ± 0.6 (29) | 2 ± 1 (18) | 5–10% Increase (14) |
| DIDS | 9 ± 2 (6) | 11 ± 2 (4) | Receptor antagonist* |
| NPPB | 15 ± 2 (12) | 21 ± 4 (4) | 15% Increase (6) |
| SITS | 11 ± 3 (6) | 13 ± 3 (4) | Not examined |
| IAA-94 | 95 ± 6 (5) | Not examined | Not examined |
| FFA | >1000 (6) | >1000 (8) | No effect (5) |
| NFA | >1000 (5) | >1000 (4) | No effect (5) |
| Probenecid | 352 ± 31 (5) | 360 ± 21 (3) | No effect (3) |
| MFQ | No effect** (12) | No effect** (5) | No effect* (12) |
| ATP | 752 ± 42 (8) | 825 ± 56 (12) | Receptor agonist*** |
| UTP | 1256 ± 56 (7) | 1350 ± 60 (4) | No effect (4) |
| GTP | 1290 ± 87 (6) | 1420 ± 108 (3) | No effect (4) |
IC50 for DIDS at rat, mouse, and human P2X7R = 90, 130, and 195 μM, respectively (see Fig. 8)
Concentrations from 1 nM to 10 μM
EC50 for activation at rat, mouse, and human P2X7R = 640 ± 22, 985 ± 35, and 952 ± 120 μM, respectively. Numbers of cells tested are indicated in parentheses
Electrophysiology. Whole-cell patch-clamp recordings were made at room temperature using an EPC9 amplifier, Pulse acquisition software (HEKA, Lambrecht/Pfalz, Germany), and AxographX analysis software (AxoGraph Scientific, Sydney, Australia) as detailed recently (Roger et al., 2008). Membrane potential was held at -60 mV unless otherwise indicated. Compounds were applied by the RSC fast flow system (Biologique, Grenoble, France); drug equilibration time ranged from 20 ms to 1 s, depending on the distance of the fast-flow tube from the recorded cell. We also superfused compounds for 3 to 6 min in addition to fast-flow application for those compounds that showed little or no effect to ensure complete drug access and equilibration. Recordings were made only from single cells to rule out possible contributions because of electrical coupling between cells, and in the majority of recordings, the cell was lifted up from the coverslip after obtaining whole-cell configuration to further ensure complete drug delivery and confirm absence of coupled cells. Cells were chosen of approximately similar size and shape throughout this study; in accordance, whole-cell membrane capacitance showed minimal variability (range, 8–12 pF; mean ± S.E.M., 9.6 ± 0.2 pF); therefore, all results are shown as absolute current values. Standard intracellular/extracellular solutions were: 147 mM NaCl, 10 mM HEPES, 10 mM EGTA/147 NaCl, 2 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 10 mM HEPES, and 13 mM glucose; intracellular calcium concentrations were calculated using WebMaxC software (http://www.stanford.edu/~cpatton/webmaxcS.html). All solutions were at pH 7.3 and 300 to 315 mOs/mol. In most experiments, voltage ramps (-120 to 80 mV) were applied at 2- to 10-s intervals after steady-state dialysis of internal solutions had been attained, usually within 2 to 3 min after obtaining whole-cell conditions. Voltage steps (20-mV increments at 1-s intervals from -120 to 80 mV) were also applied before, during, and after application of compounds. Concentration-response inhibition curves were determined by least-squares fit to the Hill equation: I/Imax = 1/[1 + (B/IC50)nH], where I is the current as a fraction of control (Imax at 70 mV), and B is the concentration of inhibitor. Figures show curves fitted to the mean of all experiments; Kaleidagraph software (Abelbeck/Synergy, Reading, PA) was used for curve fitting and statistical analysis via Student's t test.
Fura-2 Calcium Assay. Cells were plated in a 96-well black assay plate with clear bottom (Corning Inc., Corning, NY) and cultured overnight to reach 90 to approximately 100% confluence. Cells were preincubated with 4 μM Fura-2 AM (Invitrogen) at 37°C for 30 min. Before recording, Fura-2 AM was removed and replaced with the standard extracellular solution. Fluorescence was recorded by an automatic fluorescence plate reader, FlexStation 3 (Molecular Devices, Sunnyvale, CA) over 5 to 10 min at 4-s intervals. The dual excitation for Fura-2 was 340 nm/380 nm, and the emission was 510 nm. Agonists were added into the wells automatically by the machine at designated time points, whereas any antagonists were added to the wells 5 min before recording started. Data were acquired by the SoftMax Pro 5 software, and the intracellular calcium level was expressed as the ratio of the emission intensities for 340 and 380 nm.
Results
Splice Variants and Single-Nucleotide Polymorphism in Human Panx1. It has been reported that human Panx1 presents an alternatively spliced exon 5 leading to the synthesis of Panx1 with two intracellular C terminus lengths: Panx1a and Panx1b, containing a four-amino acid insert (GMNI) at position 400 (Fig. 1A) (Baranova et al., 2004). We examined the expression of these two variants in different human cell lines (HEK293, HeLa, and THP-1) and in total human brain by using oligonucleotides to specifically amplify by PCR both splicing variants (Supplemental Table 1) and confirmed the selectivity of both oligonucleotide pairs by amplifying an expression vector carrying either human Panx1a or Panx1b coding sequence (Fig. 1B). It is surprising that there was no expression of Panx1a in any cell line or tissue examined (Fig. 1B), although Panx1b was strongly expressed in all preparations (Fig. 1C). Both variants similarly trafficked to the plasma membrane when tagged with c-myc or ee-epitopes as revealed by immunocytochemistry in HEK293 cells expressing either Panx1a or b (Fig. 1D). We initially cloned human Panx1 from the THP-1 macrophage cell line (Pelegrin and Surprenant, 2006) and noted that some clones contained a Panx1 coding sequence carrying a deletion of a single valine in the intracellular C terminus domain at position 377 (Panx1bv; Fig. 1A). By using specific oligonucleotides (Supplemental Fig. 2), we were able to differentially amplify both Panx1 variants from cDNA or genomic DNA. We found that HEK293 and THP-1 cells express both versions V377/V378 (Panx1b) and single V377 (Panx1bv) mRNAs, whereas HeLa cells or human brain express only Panx1b mRNA (Fig. 1B). Genomic differential amplification for V377 deletion revealed that THP-1 cells carry both versions in their genome, and, as expected, HeLa cells only presented with the Panx1b version (Fig. 1C). Localization of human Panx1bv was found in the plasma membrane, similar to Panx1b (Fig. 1D), indicating no major trafficking defects in Panx1 by the deletion of V377.
Fig. 1.
Characterization of human Panx1 variants. A, multiple alignment of the different human Panx1 variants (Panx1a AAK91713, Panx1b NP_056183, Panx1bv FM201789) with mouse Panx1 (NP_062355) corresponding to the residues 372 to 407 of the intracellular C terminus in human Panx1a. B, expression profile of Panx1 variants by reverse transcriptase-PCR in different human cell lines and in the brain. Note the lack of Panx1a expression. C, detection of Panx1b and Panx1bv by PCR in the genomic DNA of HeLa and THP-1 cells. Expression vectors for the different Panx1 variants were used as a positive control to confirm the specific amplification by the different oligonucleotide combinations (B and C). D, cellular localization of the different Panx1-ee-tagged variants expressed in HEK293 by confocal microscopy. Note similar membrane localization for all variants.
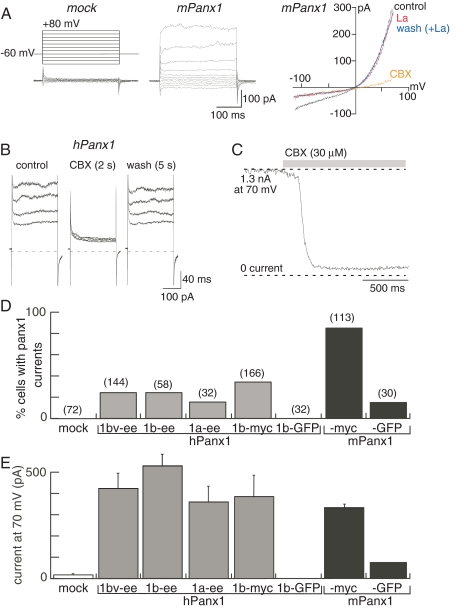
Functional Expression of Human and Mouse Panx1. Transient transfection of mPanx1 (untagged, ee, or c-myc tagged) in HEK293 cells resulted in the appearance of a distinct current whose properties were virtually identical to those described previously for heterologous expression of hPanx1 in HEK293 cells (Pelegrin and Surprenant, 2006). The Panx1-associated current, activated at membrane potentials >10 mV, showed no desensitization during maintained depolarizations for up to 1 min and was rapidly (within 10 ms) and reversibly (within 1 s) blocked by CBX (30 or 50 μM) (Fig. 2A). In approximately 50% Panx1 or vector-transfected cells and 20% of untransfected cells, a lanthanum-sensitive inward current was apparent (Fig. 2A). In the absence of lanthanum, this current was increased by up to 10-fold by CBX (data not shown); therefore, we used lanthanum (2 mM) in all solutions when this Panx1-independent current was observed. Lanthanum neither had any effect on the CBX-inhibited outward current recorded in Panx1-transfected cells (Fig. 2A), nor did it alter the actions of any of the compounds we examined in the present study. Therefore, we defined the difference between the current in the presence of 2 mM lanthanum and the current in the presence of 2 mM lanthanum plus 50 μM CBX as the Panx1 current. Although both human and mouse Panx1 protein showed intense plasma membrane localization in >95% of all transfected cells (e.g., Supplemental Fig. 1), the frequency of recording hPanx1-associated currents was very low (8–35% cells), compared with mPanx1-transfected cells (80–85% cells; Fig. 2D). No hPanx1-mediated currents were recorded from hPanx1-eGFP transfections, and only two of 30 mPanx1-GFP cells exhibited currents (Fig. 2D), thus confirming the minimal to absent functional expression of these epitope-tagged proteins. However, when all other data were considered (i.e., excluding GFP and DsRed-tagged cDNA), there were no significant differences in average or maximal current amplitudes of Panx1-associated currents in cells transfected with any of the human or mouse Panx1 constructs (Fig. 2E).
Fig. 2.
Functional expression of mouse and human Panx1. A, currents in response to voltage steps from -120 to 80 mV and voltage ramps from -120 to 80 mV recorded from HEK293 cells expressing mPanx1. Lanthanum (2 mM) blocks the inward but not the outward current, whereas CBX (30 μM) blocks only the outward current. We have defined the Panx1 current as the CBX-sensitive component. B, CBX inhibition of human Panx1 currents; C, rapid onset of CBX inhibition. D, frequency of expression of Panx1 currents from cells transfected with the indicated constructs; in all transfections, equivalent percentage of cells (> 80%) expressed Panx1 protein as determined by immunohistochemistry (as per Supplemental Fig. 1). E, mean current at 70 mV recorded from cells expressing Panx1 currents; there were no significant differences among the human variants or between mouse and human Panx1 currents.
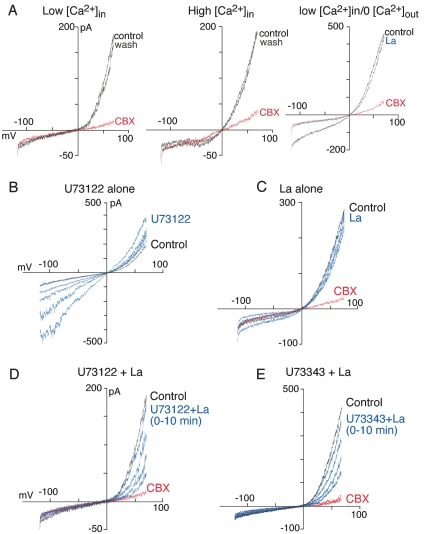
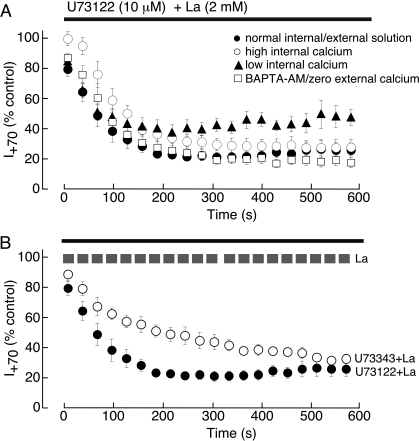
Panx1 Currents Are Calcium-Independent. Changing extracellular calcium concentration from control (2 mM) to 10 mM or to calcium-free/1 mM EGTA solution did not alter hPanx1 or mPanx1 currents (n = 9–15 for each; Fig. 3A). We also did not observe any differences in average or maximal current amplitudes of Panx1 currents from cells dialyzed with low intracellular calcium (calculated <1 nM) or with high intracellular calcium (calculated 720 nM; Fig. 3A). However, it has been reported that activation of PLC-coupled metabotropic P2Y receptors leads to activation of human Panx1 when expressed in oocytes (Locovei et al., 2006b). Therefore, we examined in more detail possible modulation of Panx1 currents by altered intracellular calcium concentrations by using the PLC inhibitor U73122. Application of U73122 to untransfected, vector-transfected, or Panx1-transfected HEK293 cells resulted in a time-dependent increase in inward and outward currents; lanthanum (2 mM) completely blocked these U73122-induced currents (Fig. 3B) and, therefore, were not attributed to panx1 activation. After blockade of the Trp-like currents with lanthanum (Fig. 3C), U73122 now produced a time-dependent inhibition of the Panx1-associated current; the maximal inhibition by U73122 (10 μM) was 80% of the inhibition produced by CBX (Fig. 3D). The time constant of inhibition was 1.3 min (Fig. 4). Neither the kinetics of U73122 inhibition nor the maximal inhibition were altered by dialyzing cells with low (<1 nM) or high (720 nM) intracellular calcium. Likewise, no changes occurred when external calcium was removed in addition to treating cells with BAPTA-AM (Fig. 4A). Moreover, U73343 (10 μM), an inactive analog of U73122 in terms of PLC inhibition, was equally effective to inhibit Panx1 currents, although with slower kinetics (time constant of inhibition, 2.5 min; Fig. 4B). We confirmed the selective effectiveness of U73122 in blocking P2Y-mediated calcium transients from untransfected, vector-transfected, and Panx1-transfected HEK cells and found that a 5-min exposure to a 10 μM concentration of U73122 blocked 95 to 100% of the ATP-evoked calcium responses, whereas the same concentration of the PLC-inactive analog, U73343, had no effect (Supplemental Fig. 3).
Fig. 3.
Panx1 currents are not modulated by calcium. A, mPanx1 currents recorded with low internal calcium (<1 nM), high internal calcium (720 nM) with 2 mM external calcium, or low internal calcium and zero external calcium. B, activation of Trp-like currents in response to U73122 (10 μM, blue traces recorded at 1-min intervals). C, currents in absence (black trace) or presence of 2 mM lanthanum (blue traces at 1-min intervals), which show no change in CBX-sensitive Panx1 current over this time course. D, currents in U73122 + lanthanum (blue traces at 1-min intervals); Panx1 currents are now inhibited. E, PLC-inactive analog, U73343 (10 μM), also inhibited Panx1 currents.
Fig. 4.
Kinetics of calcium-independent inhibition of Panx1 currents by U73122 and U73343. Summary of all experiments as illustrated in Fig. 3. A, inhibition by U73122 as a function of time in different internal/external calcium concentrations; there were no significant differences. B, kinetics of inhibition by U73122 and U73343; inhibition by U73343 was slower than U73122, but the same maximal inhibition occurred with either compound.
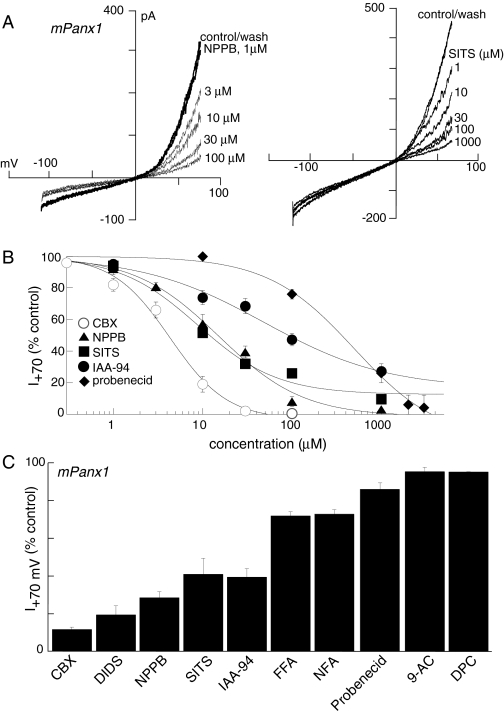
Inhibition of Panx1 Currents by Nonselective Chloride Channel/Anion Transporter Blockers. We examined a number of compounds that are known to inhibit gap junctions, cation and anion transporters, Trp channels, and chloride channels, all of which are relatively nonselective but may provide a “Panx1-specific repertoire.” Examples of two compounds, NPPB and SITS, which were effective inhibitors of mouse and human Panx1 currents, are shown in Fig. 5A, concentration-inhibition curves for compounds that inhibited Panx1 currents are shown in Fig. 5B, and inhibitor potencies are shown in Fig. 5C and Table 1. The most potent inhibitor was CBX (IC50, 5 μM) and the weakest probenecid (IC50, 350 μM). Lanthanum (2 mM; Figs. 2 and 3), gadolinium (300 μM), 2-APB, 9-AC, and DPC (all at 100 μM) were without effect (data not shown, n = 4–6).
Fig. 5.
Inhibition of Panx1 currents by some chloride channel/anion transporter inhibitors. A, two examples of concentration-dependent inhibition of mPanx1 currents by NPPB (left) and SITS (right); note NPPB inhibited inward and outward current; lanthanum (2 mM) present throughout. B, concentration-inhibition curves for inhibitory compounds; each point is mean ± S.E.M. of five to 12 points. C, histogram, mean currents in the presence of 100 μM of each of the compounds, except for CBX, which was 50 μM.
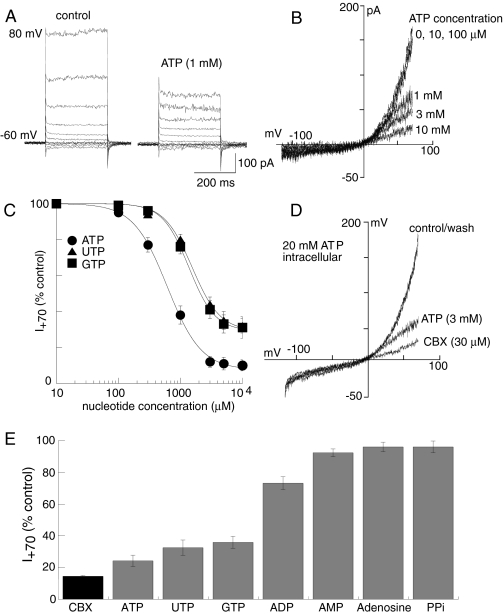
Inhibition of Panx1 Currents by Nucleoside Triphosphates. We examined the actions of ATP on Panx1 currents with the hypothesis that it was likely to directly activate Panx1 currents. To our surprise, ATP, UTP, and GTP, but not ADP, AMP, adenosine, pyrophosphates, or monophosphates, rapidly and reversibly inhibited Panx1 currents with kinetics similar to CBX (Fig. 6). Maximal inhibition by ATP was 90% CBX inhibition, whereas maximal inhibition by GTP or UTP was 70% CBX inhibition (Fig. 6, C and E). BzATP at 300 μM and 1 mM inhibited panx1 currents by 14 ± 4 and 36 ± 6% (n = 3), respectively; these concentrations of ATPγS inhibited panx1 currents by 24 ± 5 and 46 ± 6% (n = 3), and αβ-meATP (0.5 mM) also inhibited panx1 currents by 34 ± 3% (n = 3). It was not economically feasible to examine higher concentrations of these analogs. There were no significant differences in ATP concentration-inhibition curve or kinetics of ATP inhibition when high concentrations of ATP were included in the recording pipette (10–20 mM, n = 17 for mPanx1 and 14 for hPanx1; Fig. 6D); intracellular ATP also did not alter the average or maximal Panx1 currents. The simultaneous inclusion of nonselective purine receptor antagonists, suramin (200 μM) and pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid (100 μM), did not alter the inhibition by ATP, GTP, or UTP (n = 2 for each nucleotide).
Fig. 6.
Extracellular nucleoside triphosphates inhibit Panx1 currents. Voltage steps (A) and ramps (B) in control and presence of ATP as indicated. C, concentration inhibition curves for ATP, UTP, and GTP inhibition of mPanx1 as indicated; n = 5 to 14 for each point. D, currents recorded with high intracellular ATP (20 mM); no differences in peak current or inhibition by extracellular ATP or CBX were observed. E, Panx1 currents in presence of maximal concentrations of nucleotides, nucleosides, and phosphates as indicated.
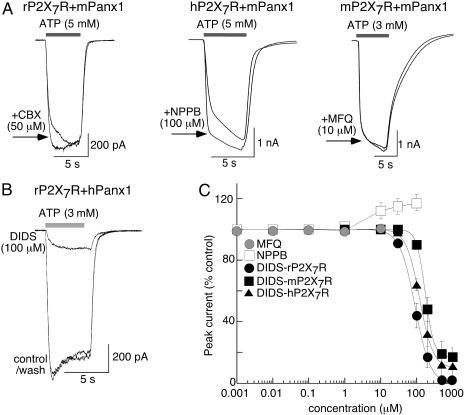
Actions of Panx1 Inhibitors on P2X7R-Mediated Currents. We examined the actions of compounds that inhibited Panx1-currents on ATP-evoked currents in cells coexpressing rat P2X7R + mPanx1, human P2X7R + mPanx1, mouse P2X7R + mPanx1, and rat P2X7R + hPanx1. In all these cells, a CBX-sensitive Panx1 current was recorded in the absence of ATP (data not shown). ATP evoked typical P2X7R-mediated currents (Fig. 7A). CBX, NPPB, FFA, MFQ, NFA, and probenecid had no effect or increased by 10 to 20% the peak current in response to ATP (Fig. 7A; Table 1). In contrast, DIDS readily inhibited P2X7R-mediated currents (Fig. 7, B and C) with IC50 values that were 10- to 20-fold higher than IC50 values for inhibition of Panx1 currents (Fig. 7C; Table 1).
Fig. 7.
Actions of Panx1 inhibitors on ATP-evoked currents in cells coexpressing Panx1 and P2X7 receptor. A, examples of ATP-evoked currents in the presence and absence of CBX, NPPB, and MFQ as indicated; currents were slightly increased or unaltered. B, example of ATP-evoked currents in the absence and presence of DIDS. C, summary of all experiments as illustrated in A and B; results for NPPB and MFQ at each of the three P2X7Rs are pooled because there was minimal or no effect, whereas inhibition by DIDS is shown for each species, n = 5 to 18 for each point. DIDS IC50 values were 90 ± 4, 130 ± 12, and 195 ± 21 μM for rat, mouse, and human P2X7R, respectively.
Discussion
This study provides the first detailed pharmacological characterization of Panx1-associated currents in mammalian cells and presents results that may call for revision of current hypotheses regarding Panx1 function.
Protein and Functional Expression of Panx1 Variants. Human Panx1 presents an alternatively spliced exon 5, which leads to a four-amino acid insert (GMNI) within the intracellular C terminus (Panx1b; Fig. 1A). This splicing variant was initially characterized by expressed sequences tag database searches (Baranova et al., 2004); however, no protein or functional expression studies of this Panx1b variant have been carried out previously. In the present study, we examined several different human cell lines and brain tissue and found that Panx1b is the only Panx1 present. Although the initial report by Baranova et al. (2004) suggested the Panx1a splice variant is the more common form, our study reveals it is the Panx1b variant that should be considered to be most prominent. We also identified a novel difference in the intracellular C terminus of human Panx1, a specific deletion of valine at position 377 (Panx1bv). The V377 coding sequence is present in exon 4, and the lack of this amino acid cannot be explained by an alternative spliced exon. However, the two consecutive valines at positions 377 and 378 present in the human Panx1 sequence (accession numbers AAK91713 and NP_056183) are encoded by a duplication of the same codon GTT at position 1131 in the coding sequence; therefore, there are four different possibilities for a trinucleotide deletion to occur in that position to produce a single valine at position 377 (GTT1131, GTT1134, TTG1132, or TGT1133). Both forms of Panx1, b and bv, were expressed in HEK293 and THP-1 human cell lines. Coding sequences for both variants were found in THP-1 genomic DNA, indicating that these cells carry the V377 deletion only in one allele of the PANX1 gene. We were unable to detect expression of Panx1bv from HeLa cells or from the brain, and further studies will be needed to explore whether Panx1bv is present in the human population. In any case, all Panx1 variants (Panx1a, Panx1b, and Panx1bv) were found to be equally trafficked to the plasma membrane (unless fused with GFP or DsRed), and they all showed the same electrophysiological profile, indicating that their functional properties remain the same irrespective of the Panx1 splice variant expressed in a specific tissue or the presence of Panx1bv in the genome.
Inhibition of Panx1 Currents by Chloride Channel/Transporter/Gap Junction Channel Blockers. We identified a number of compounds that reversibly inhibited Panx1 currents (Table 1); all are known to inhibit rather nonselectively gap junction channels, chloride channels, and/or anion transporters. The rank order of potency for inhibition of either human or mouse Panx1 currents was CBX > DIDS ≈ SITS ≈ NPPB > IAA-94 >> probenecid >> FFA = NFA. However, not all gap junction/chloride channel blockers were effective because heptanol, 9-AC, MFQ, and DPC did not alter human or mouse Panx1 currents, nor did the nonselective Trp-channel/voltage-gated calcium channel blockers, lanthanum and gadolinium. Therefore, the profile presented by the above repertoire of inhibitors provides a useful pharmacological fingerprint to identify and distinguish native Panx1 currents. Although we have not examined directly mechanisms of inhibition by any of these compounds, the millisecond on and off rates of inhibition by CBX, NPPB, and DIDS suggest direct channel block. Probenecid, recently shown to block Panx1-induced but not connexin-mediated currents when expressed in oocytes (Silverman et al., 2008), showed much slower kinetics with on and off rates in the minute domain, suggesting a distinct mechanism of inhibition. Because probenecid had been considered previously to act solely through anion transporter inhibition, Silverman et al. (2008) have raised the possibility that Panx1 may function as an essential component in a transport protein complex rather than as a separate permeation pathway. Our results add weight to this possibility because all the compounds (except CBX) we found to inhibit Panx1 currents are also known to inhibit various anion transporters and other members of the ATP-binding cassette family of transporters (Gelband et al., 1996; Strange et al., 1996; Kidd and Thorn, 2000). It is not known whether CBX alters transporter functions because no studies examining actions of CBX on anion or other types of transporters have been carried out to date.
Calcium Independence. Panx1 currents were unaltered by increasing or removing extracellular calcium, by markedly reducing levels of intracellular calcium (to <1 nM) via dialysis with high concentrations of BAPTA, or by loading cells with BAPTA-AM, or by greatly increasing intracellular calcium levels via dialysis of μM concentrations of calcium. Although studies to date on Panx1 function are in agreement that Panx1 currents are not modulated by extracellular calcium (Bruzzone et al., 2005; Pelegrin and Surprenant, 2006), it has been reported that Panx1 currents in oocytes can be activated by increased intracellular calcium, particularly by activation of the PLC pathway by purinergic P2Y receptors (Locovei et al., 2006b). In the present study, we further examined the actions of the PLC inhibitor, U73122, and found it did inhibit Panx1 currents, but the inhibition was not via PLC transduction because the PLC-inactive analog, U73343, was equally effective in blocking Panx1 currents, and the U73122-induced inhibition of currents did not depend on levels of intracellular calcium. Thus, the inhibition of Panx1 currents by U73122 and U73343 is independent of modulation of intracellular calcium and probably reflects direct modulation of the Panx1 protein or protein complex itself.
Nucleotide Inhibition of Panx1 Currents and Involvement of Panx1 in P2X7R Function. In cells expressing Panx1 (but lacking P2X7R), trinucleotides all rapidly and reversibly inhibited Panx1 currents in a manner similar to that observed for CBX. Although HEK293 cells are well known to endogenously express several P2Y receptors (but not P2X receptors), the nucleotide inhibition of Panx1 was clearly not mediated via activation of any metabotropic P2Y receptor because inhibitory concentrations of ATP, GTP, and UTP were 50- to 100-fold greater than required to activate P2Y receptors because GTP does not activate P2Y (or P2X) receptors and because the combined application of suramin and pyridoxal phosphate-6-azophenyl-2′,4′-disulfonic acid did not alter the inhibitory actions of these trinucleotides. The rapid kinetics of the nucleotide inhibition, the high concentrations required, and the lack of effect of intracellular ATP are in keeping with direct channel block as the most likely mechanism of action. There are several examples of similar blockade of channel activity by ATP, particularly at chloride channels (e.g., Jackson and Strange, 1995).
ATP rapidly and reversibly inhibited Panx1 currents over the same concentration range (IC50 = 750 μM) that activates P2X7R channels. This result was unexpected because our previous results had led us to hypothesize that activation of P2X7R by extracellular ATP induced the opening of Panx1 channels, which allowed passage of higher mol. wt. molecules (up to 900 Da). The evidence for this hypothesis was that Panx1 and P2X7R showed protein-protein interaction and that CBX, siRNA directed against Panx1, and a Panx1-mimetic peptide all inhibited the early dye uptake associated with P2X7R activation without altering P2X7R currents or calcium flux (Pelegrin and Surprenant, 2006). In the present study, we again found that CBX produced a small increase in peak ATP-evoked currents in cells cotransfected with any combination of mouse, rat, and human P2X7R with mouse or human Panx1, although the Panx1 currents recorded (in the absence of ATP) in the same cells showed the same CBX-induced inhibition as was observed in cells expressing only Panx1. Similar small increases in ATP-evoked currents occurred in the presence of NPPB, FFA, and probenecid, although DIDS inhibited P2X7R-mediated currents in a concentration-dependent manner, thus demonstrating that DIDS is an effective P2X7R antagonist. Irrespective of the small increase in ATP-gated currents in P2X7R-expressing cells by panx1 blockers, these results show clearly that, with the exception of DIDS, blockers of panx1 currents do not inhibit P2X7R. Is it possible to reconcile these results with the hypothesis that activation of Panx1 hemichannels in response to P2X7R stimulation provides the conduit for dye uptake? That is, ATP potently inhibits Panx1 currents in cells lacking P2X7R at the same concentrations that activate the P2X7R and its subsequent downstream signaling to dye uptake. We believe these results are not compatible with Panx1 acting as a dye-permeable hemichannel, although it is possible to imagine a situation where two functionally distinct populations of Panx1 hemichannels exist: one population associating within the P2X7R protein complex whose conformation does not allow direct ATP binding (so it opens in response to P2X7R activation) but retains inhibitory actions of CBX/NPPB/probenecid and a second population that does not associate with P2X7R and is available for direct ATP inhibition by these panx1 blockers. However, we think it more likely that there remain one (or more) yet-to-be-identified molecule(s) that link Panx1 to P2X7R-mediated dye uptake and other downstream signaling cascades initiated by P2X7R. We predict at least one of these postulated Panx1-P2X7R-interacting proteins will be a type of anion transporter. Mutagenesis approaches designed to delineate residues in the Panx1 protein that are sites of interaction for ATP and other inhibitory compounds and to determine regions that can be directly associated with ion flux should provide important information concerning how Panx1 may (or may not) function as an ion channel. Proteomic approaches aimed at identifying proteins associated within a P2X7R-Panx1 cocomplex should provide new candidates that may clarify how Panx1 is modulated by, or itself modulates, the P2X7R. Most pertinently, continued pharmacological investigations aimed at identifying further, and more selective, agonists and antagonists of Panx1 will be key to furthering our understanding of Panx1 function.
Supplementary Material
Acknowledgments
We thank Elizabeth Martin for molecular and cell biology support and Austen Sitko for cell culture and transfections.
This work was supported by the Biotechnology and Biological Sciences Research Council and by AstraZeneca Charnwood UK.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.146365.
ABBREVIATIONS: P2X7R, purinergic P2X7 receptor; panx, pannexin; siRNA, small interfering RNA; CBX, carbenoxolone; HEK, human embryonic kidney; NPPB, 5-nitro-2-(3-phenylpropylamino)benzoic acid; DIDS, disodium 4,4′-diisothiocyanatostilbene-2,2′-disulfonate; IAA-94, indanyloxyacetic acid 94; FFA, flufenamic acid; NFA, niflumic acid; 9-AC, anthracene-9-carboxylic acid; MFQ, mefloquine; DPC, diphenylamine-2-carboxylate; U73122, 1-[6-((17β-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl]-1H-pyrrole-2,5-dione; U73343, 1-[6-((17β-3-methoxyestra-1,3,5(10)-trien-17-yl)amino)hexyl]-2,5-pyrrolidinedione; Ab, antibody; eGFP, enhanced green fluorescent protein; PCR, polymerase chain reaction; PBS, phosphate-buffered saline; AM, acetoxymethyl ester; PLC, phospholipase C; BAPTA, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid; SITS, disodium 4-acetamido-4′-isothiocyanato-stilben-2,2′-disulfonate.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
References
- Baranova A, Ivanov D, Petrash N, Pestova A, Skoblov M, Kelmanson I, Shagin D, Nazarenko S, Geraymovych E, Litvin O, et al. (2004) The mammalian pannexin family is homologous to the invertebrate pannexin gap junction proteins. Genomics 83 706-716. [DOI] [PubMed] [Google Scholar]
- Barbe MT, Monyer H, and Bruzzone R (2006) Cell-cell communication beyond connexins: the pannexin channels. Physiology 21 103-114. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Barbe MT, Jakob NJ, and Monyer H (2005) Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem 92 1033-1043. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, and Monyer H (2003) Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A 100 13644-13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelband CH, Greco PG, and Martens JR (1996) Voltage-dependent chloride channels: invertebrates to man. J Exp Zool 275 277-282. [DOI] [PubMed] [Google Scholar]
- Huang Y, Grinspan JB, Abrams CK, and Scherer SS (2007a) Pannexin1 is expressed in neurons and glia but does not form functional gap junctions. Glia 55 46-56. [DOI] [PubMed] [Google Scholar]
- Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, and Roper SD (2007b) The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A 104 6436-6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, and Scemes E (2008) P2X7 receptor-pannexin1 complex: pharmacology and signalling. Am J Physiol Cell Physiol 295 C752-C760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PS and Strange K (1995) Characterization of the voltage-dependent properties of a volume-sensitive anion conductance. J Gen Physiol 105 661-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd JF and Thorn P (2000) Intracellular Ca2+ and Cl- channel activation in secretory cells. Annu Rev Physiol 62 493-513. [DOI] [PubMed] [Google Scholar]
- Locovei S, Bao L, and Dahl G (2006a) Pannexin 1 in erythrocytes: function without a gap. Proc Natl Acad Sci U S A 103 7655-7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locovei S, Wang J, and Dahl G (2006b) Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett 580 239-244. [DOI] [PubMed] [Google Scholar]
- Panchin YV (2005) Evolution of gap junction proteins: the pannexin alternative. J Exp Biol 208 1415-1419. [DOI] [PubMed] [Google Scholar]
- Pelegrin P and Surprenant A (2006) Pannexin 1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J 25 5071-5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelegrin P and Surprenant A (2007) Pannexin 1 couples to maitotoxin- and nigericin-induced interleukin-1beta release through a dye uptake-independent pathway. J Biol Chem 282 2386-2394. [DOI] [PubMed] [Google Scholar]
- Roger S, Pelegrin P, and Surprenant A (2008) Facilitation of P2X7 receptor currents and membrane blebbing via constitutive and dynamic calmodulin binding. J Neurosci 28 6393-6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scemes E, Suadicani SO, Dahl G, and Spray DC (2007) Connexin and pannexin mediated cell-cell communication. Neuron Glia Biol 3 199-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman W, Locovei S, and Dahl G (2008) Probenecid, a gout remedy, inhibits pannexin-1 channels. Am J Physiol Cell Physiol 295 C761-C767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange K, Emma F, and Jackson PS (1996) Cellular and molecular physiology of voltage-sensitive anion channels. Am J Physiol 270 C711-C730. [DOI] [PubMed] [Google Scholar]
- Vanden Abeele F, Bidaux G, Gordienko D, Beck B, Panchin YV, Baranova AV, Ivanov DV, Skryma R, and Prevarskaya N (2006) Functional implications of calcium permeability of the channel formed by pannexin 1. J Cell Biol 174 535-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MT, Pelegrin P, and Surprenant A (2007) Amino acid residues in the P2X7 receptor that mediate differential sensitivity to ATP and BzATP. Mol Pharmacol 71 92-100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.