Abstract
This study was designed to test the hypothesis that the integrity of the globus pallidus (GP) is critical for neurotrophic factor, such as glial-derived neurotrophic factor (GDNF), induced functional changes in rhesus macaques with MPTP-induced parkinsonism, because our previous studies demonstrated that the GP was one of the most affected areas as assessed by the levels of dopamine (DA) and its metabolites. A group of 8 hemiparkinsonian monkeys with pallidal lesions, which positively responsed to intraventricular (ICV) injections of GDNF prior to the lesions, and a group of 8 hemiparkinsonian monkeys without pallidal lesions, were treated with GDNF after a long washout period after the initial ICV infusions of GDNF. Significant behavioral improvements were only seen in the monkeys without pallidal lesions that received GDNF. Monkeys with pallidal lesions failed to exhibit any behavioral improvement even though they had elevated nigral DA levels. The results suggest that the GP is critical for neurotrophic factor induced functional changes in PD monkeys.
Keywords: Globus pallidus, GDNF, lesion, MPTP, ICV, monkey, neurotrophic factor, Parkinson’s disease, substantia nigra, dopamine
INTRODUCTION
Parkinson’s disease (PD) is a common and devastating neurological disorder characterized mainly by impairment of motor function, due largely to a progressive degeneration of the substantia nigra pars compacta dopamine neurons that innervate the striatum (1). Therapeutic strategies for PD include replacing striatal dopamine using the dopamine precursor levodopa or dopamine receptor agonists, or both. As the disease worsens, patients that have become less responsive to pharmacological treatments may choose to undergo surgical treatments, such as deep-brain stimulation (DBS). These treatments provide symptomatic relief, but do not slow or halt the continued degeneration of substantia nigra (SN) dopaminergic neurons. One experimental approach that could potentially slow or reverse the progression of neuronal degeneration in parkinsonian patients involves trophic factor administration. Trophic factors are proteins with enormous therapeutic potential in the treatment of neurodegenerative diseases, including the potential of modifying neuronal dysfunctions. Neurotrophic factors may not only slow the degeneration of nigral dopaminergic neurons due to their neuroprotective properties, but may also enhance the function of residual dopamine neurons or even repair and restore function to injured dopamine neurons. Although there is little evidence that deficiencies of trophic factors are associated with the etiology of PD (2), considerable effort has been devoted to the search for neurotrophic factors (NTF) with survival-promoting activities on midbrain dopaminergic neurons that could potentially be of therapeutic value in the treatment of PD.
Studies from our group have consistently demonstrated that intracerebral administration of GDNF could not only significantly improve overall parkinsonian features, but also increase levels of dopamine (DA) and homovanillic acid (HVA), a metabolite of dopamine, especially in the pallidal regions in which the changes of DA and HVA in the GP were even more robust than what were observed in the caudate and putamen in normal (3), aged (4) and parkinsonian monkeys (5, 6, 7). For example, in the aged animals (4), robust increases of DA were found in the globus pallidus by 390% (P<0.01) on the GDNF-treated hemisphere compared with 50% (P<0.05) in the caudate nucleus, while the increase of DA failed to reach significance in the putamen following GDNF administration. Also, in PD monkeys (5), significant increases of DA levels were seen in the SN, ventral tegmental area (VTA), and globus pallidus (GP) but not in the caudate nucleus and putamen following monthly intraventricular (ICV) injections of GDNF. Thus, the robust effect of GDNF on pallidal DA and DA metabolite levels would suggest that the GP is another area of research that needs to be investigated with regard to the therapeutic effect of trophic factors on PD because the GP receives dopaminergic input from the SN and is involved in regulating motor functions by sending outputs to the motor cortex via the thalamus (8). On the other hand, studies have demonstrated that pallidal lesions can affect the responsiveness to levodopa treatment in MPTP-treated rhesus monkeys (9). Therefore, the objective of the study was designed to specifically address the question of whether or not the GP is a key target for GDNF-induced functional recovery in hemiparkinsonian rhesus monkeys.
MATERIALS AND METHODS
Animals
The project utilized 16 adult female rhesus monkeys obtained from a commercial supplier (HRP, Alice TX). All animals received right intracarotid artery infusion of MPTP (0.4mg/kg) to induce continuously expressed advanced hemi-parkinsonism ((10, 11). Prior to the present study, the animals had also been used in a GDNF dose response study (12). Nine animals received ICV injections of GDNF and had shown significant improvements in motor function. Three monkeys received vehicle as controls. Four monkeys received a sub-threshold dose of GDNF. The doses of GDNF and behavioral responses to the first round of GDNF testing are summarized in Table 1.
Table 1.
Summary of animals responding to initial GDNF or vehicle ICV-injections.
| Initial GDNF Treatments | |||||
|---|---|---|---|---|---|
| Animals | Treatment GDNF | Improved after ICV injections | Animals | Treatment GDNF | Improved after ICV injections |
| (4 doses) | (4 doses) | ||||
| R643 | 100μg | Yes | R343 | 100μg | Yes |
| R189 | 300μg | Yes | R438 | 1000μg | Yes |
| R196 | 300μg | Yes | R665 | 30μg | Yes |
| R333 | 1000μg | Yes | R833 | 1000μg | Yes |
| R728 | 0μg | No | R028 | 0μg | No |
| R406 | 10μg | No | R036 | 10μg | No |
| R653 | 10μg | No | R049 | 10μg | No |
| R943 | 300μg | Yes | R772 | 0μg | No |
After a three-month washout period, the animals were divided into two cohorts: Cohort I (n=8) continued another 6-month washout period with monthly behavioral testing for the first 4 months and the month before the GDNF reinstatement; Cohort II (n=8), immediately received a pallidal lesion and then underwent monthly behavioral testing for 6 months. At the end of the 6-month washout or recovery from pallidal lesions, each cohort was divided into two groups: GDNF-treated or vehicle-treated controls (Table 2).
Table 2.
Summary of animals responding to repeated GDNF or vehicle ICV-injections.
| Repeated GDNF Treatments | |||||
|---|---|---|---|---|---|
| Animals with GPi lesion | Treatment GDNF | Improved after ICV injections | Animals without Gpi lesion | Treatment GDNF | Improved after ICV injections |
| (2 doses) | (1 dose) | ||||
| R643 | 300μg | No | R343 | 300μg | Yes |
| R189 | 300μg | No | R438 | 300μg | Yes |
| R196 | 300μg | No | R665 | 300μg | Yes |
| R333 | 300μg | No | R833 | 300μg | Yes |
| R728 | 300μg | No | R028 | 0μg | No |
| R406 | 300μg | No | R036 | 0μg | No |
| R653 | 0μg | No | R049 | 0μg | No |
| R943 | 0μg | No | R772 | 0μg | No |
MRI-Guided Surgical Procedures
Anatomical MRI procedures
The animal was positioned in an MRI-compatible stereotaxic head-frame and a 8-cm RF surface coil was placed over its head under general anesthesia induced by Ketamine (150 mg per animal, i.m.) followed by pentobarbital (50mg/kg i.v.). Sets of three–dimension anatomical T1-weighted images were collected on a 1.5T Siemens VISION clinical imager. As previously reported (5,7), T1-weighted images were used to identify the site and size of the tissue damage in the brain because images with T1-relaxing times usually provide the best contrast for brain anatomical studies (Fig. 1A&B).
Figure 1.
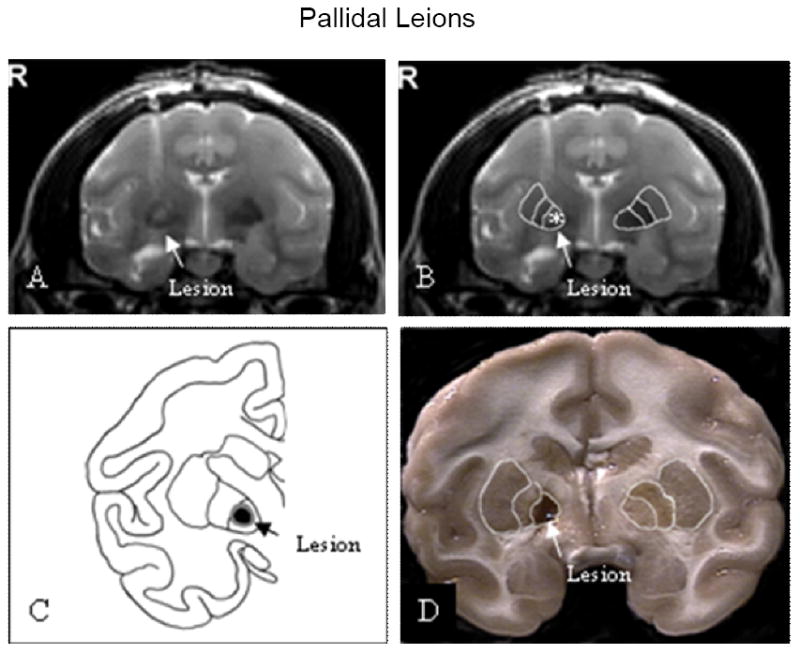
Location of pallidal lesions. Pallidal lesions were shown in coronal post-operative T2-weighted MR images (A & B). A line drawing was used to illustrate the effective lesion and surrounding edema (C). The pallidal lesion was shown in 4-mm thick coronal section through the globus pallidus (D).
Lesion of the internal globus pallidus (GPi)
Stereotaxic coordinates of the target were determined by MRI. The inner cavities of the ear bars on the frame were filled with mineral oil that was easily visible on T1-weighted scans, and was used as the zero point reference. Lateral measurements were determined from coronal images by measuring the distance from the sagittal sinus/third ventricle to the target site. Vertical measurements were determined from the surface of the brain to the target at the lateral coordinate. The lesion site should be in the mid-part of the internal globus pallidus. Animals were anesthetized with isoflurane (1-3%) and placed in the MRI-compatible stereotaxic frame. A Radionics Lesion Generator (RFG-3B and TCZ probe with a 1.0mm × 1.0mm tip, David Kopf Instruments, Tujunga, CA) was used to make the lesion. After the probe was stereotaxically inserted into the target, a RF lesion (83°C and 60 sec) was carried out after an electrical stimulation at the level of 6Hz/10 mA and 60 Hz/5mA and did not induce any undesired responses. The lesion site and size were verified by MRI taken 4 to 6 hours post surgery.
Intracerebrooventricular injection
ICV injection(s) of either GDNF or vehicle were resumed about 11 months after the last ICV injection of the initial GDNF or vehicle treatments. At least a 6-month recovery period was given to the animals that received pallidal lesions. The surgical procedure for the GDNF reinstatement was similar to the one used in the initial study (5,12). It was important to note that the animals with pallidal lesions received two ICV injections of GDNF with a 3-week interval because no significant behavioral changes were observed after the first ICV injection. For assurance that the lack of behavioral responses was not due to other reasons other than the pallidal lesion, an additional dose of GDNF was given to those pallidotomized animals. For a summary of behavioral changes, see Table 2.
Behavioral Evaluation
Motor dysfunctions were assessed from the weekly videotapes using a nonhuman primate Parkinsonian Rating Scale (PRS) for MPTP-lesioned parkinsonian monkeys that was patterned after the human Unified Parkinson’s Disease Rating Scale (11,13). Briefly, the videotapes were evaluated blindly and scored by two independent experienced raters in the following categories: rigidity, bradykinesia, posture, balance, tremor, and hand dexterity.
Histology and Neurochemistry
Intact 4 mm thick coronal sections through the striatum and pallidum were post-fixed in a 4% paraformaldehyde solution then cut into 40-μm-thick sections on a sliding knife microtome for immunohistochemical staining for tyrosine hydroxylase (TH, monoclonal antibody, 1:1000; Chemicon International, Temecula, CA, USA) to assess dopaminergic fibers in the pallidal areas. Procedures were described previously (3,7). For the nigral DA levels in the animals with pallidal lesins, 10 tissue punches from the right SN (ipsilateral to the MPTP administration and the pallidal lesion) from each monkey were obtained from the 4 mm thick coronal section throught the SN as previous described (14). The punches were transferredto storage tubes, weighted, frozen on dry ice snow and stored at -70° unitl they could be assayed. Tissue levels of DA were determined using the methods of Hall et al.
Statistical Analysis
The initial repeated measures analysis of variance (ANOVA) was used to assess the variance among all pallidotomized animals and then followed by Dunnett analysis: compared the data of post pallidotomy followed by ICV treatment with pre-pallidotomy baseline to determine when the behavioral changes became significant. Paired t-tests were used to compare the difference between the initial and reinstatement of GDNF treatments in the same group of animals for each week time point. Unpaired t-tests were used to analyze the difference in dopamine levels between GDNF- and vehicle-treated groups in animals having a prior pallidotomy. P values <0.05 were considered statistically significant.
RESULTS
Pallidal lesions
Anatomical MRIs taken 4-6 hours after the surgery from the 8 animals that received the lesion on the MPTP lesioned side demonstrated that the size of the lesions were about 3 mm (mean 2.99±0.008) in diameter and the majority of the lesions were located in the globus pallidus interna with a small proportion of the lesions in some animals extending into the globus pallidus externa (Fig. 1A-C). A typical pallidal lesion is shown in a 4mm coronal section (Fig. 1D). Immunocytohistology revealed that unilateral MPTP administration produced extensive loss of TH-positive staining in the lesioned side putamen and caudate (Fig. 2 A&B). However, TH-positive fibers were relatively spared in the intact pallidum on the side ipsilateral to the MPTP administration (Fig. 2 D). As seen in Fig 2C, the pallidal lesions destroyed the majority of the TH-positive fibers in the lesioned and adjacent areas.
Figure 2.
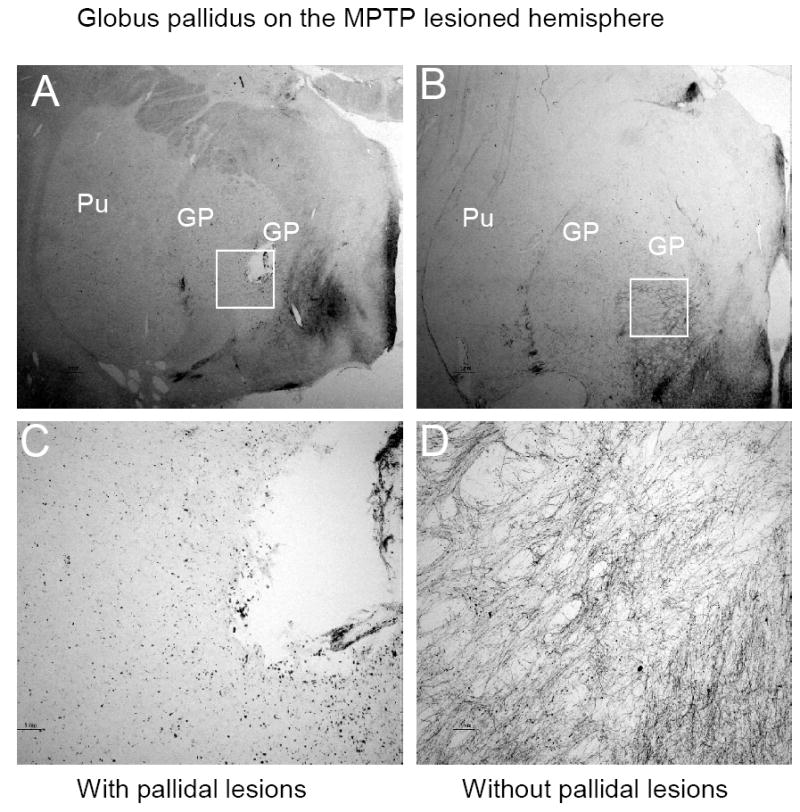
TH staining in coronal section through the GP on the side ipsilateral to MPTP administration. A typical location of the pallidal lesion was demonstrated in panel A, which was primarily in the internal segment of the GP. The same structure without the lesion from another animal is shown in panel B. High magnification of regions surrounding the lesion revealed a massive loss of the TH positive fiber in the lesioned internal segment of the GP(C), while abundant TH positive fibers are visible in the same areas without a pallidal lesion(D).
Behavioral changes GDNF reinstatement (s)
For those animals with the pallidal lesions, an instant improvement in PD features was seen in all animals, which lasted less than 2 months. However, the behavioral changes after the pallidal lesions gradually faded away and returned to pre-surgery levels (Fig. 3A). The PD features were not improved by both ICV injections of GDNF (Fig. 3A). By contrast, behavioral improvements were observed in those animals without pallidal lesions after receiving a single dose of GDNF through the ICV injection, while little changes were seen in the vehicle recipients (Fig. 3B). Interesting, the nigral level of dopamine was significantly (P=0.038) elevated in those animals with pallidal lesions, although the reinstatement of GDNF did not trigger the behavioral improvement (Fig. 4).
Figure 3.
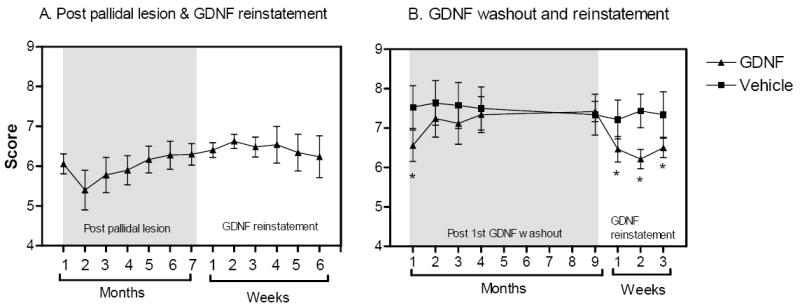
A. Behavioral changes after the pallidal lesions and GDNF reinstatement. A transient behavioral improvement was observed after the pallidal lesioning and faded away 3 months post-surgery. The GDNF reinstatement did not induce any behavioral changes in any lesioned animal. B. Behavioral improvements were found immediately after the reinstatement of GDNF in animals without any pallidal lesion and were sustained throughout the study.
Figure 4.
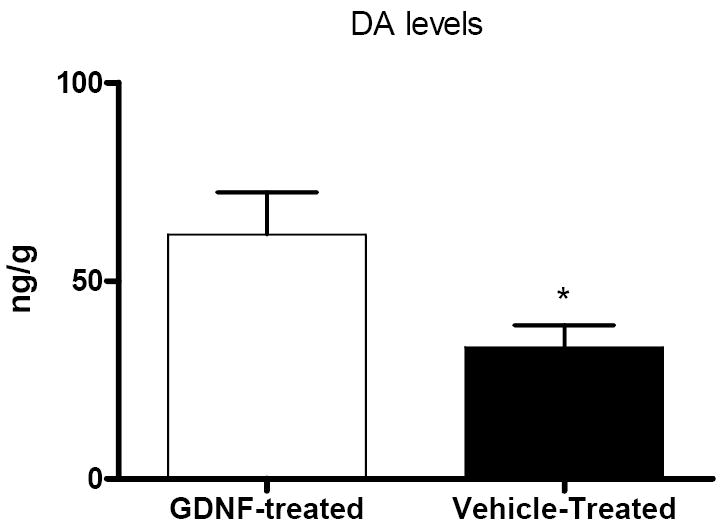
Nigral dopamine levels in the animals with pallidal lesions. Dopamine levels were significantly higher in the animals with a pallidal lesion after the GDNF treatment than in the animals without a pallidal lesion but with vehicle treatment. *P= 0.038.
DISCUSSION
This study suggests that the GP, particularly the internal segment, plays a critical role in GDNF-induced behavioral improvements in hemiparkinsonian monkeys. The results demonstrated that a single intraventricular injection of GDNF can ameliorate parkinsonian features, at least for a short period of time, in those monkeys with MPTP-induced parkinsonism. However, the behavioral improvements induced by GDNF treatment were blunted by the pallidal lesion, although they did show improvements during the initial GDNF treatment and and had elevated nigral DA levels after the GDNF reinstatement. In contrast, the GDNF reinstatement did trigger significant behavioral improvements in those animals without pallidal lesions, which were similar to those observed during the initial GDNF treatments. The results strongly suggest that the integrity of the GP could directly affect the anti-parkinsonian effects of GDNF.
This assumption that the GP plays a critical role in neurotrophic factor induced behavioral improvement was further supported by a recent double blinded study in hemiparkinsonian monkeys. In the study, the neurorestorative effects of another exogenous trophic factor, neurturin (NTN), was tested. The results showed that the NTN recipients showed a significant and sustained behavioral improvement in their parkinsonian features during the treatment period, an effect not seen in the vehicle-treated animals. At study termination, locomotor activity levels were increased by 50% in the NTN vs. vehicle recipients. Also, DOPAC levels were significantly increased by 150% in the GP ipsilateral (right) to NTN infusion, while HVA levels were elevated bilaterally in the NTN-treated animals by 10% on the left and 67% on the right hemisphere. No significant changes in DA function were seen in the putamen. These data support the hypothesis that the effects of neurotrophic factors on pallidal DA function are of importance, particularly when considering that the globus pallidus receives dopaminergic input from the substantia nigra and is involved in regulating motor functions by sending outputs to the motor cortex via the thalamus (8).
Until recently little was known about how pallidal lesions affect neurophysiological properties of other structures in the basal ganglia, especially the subthalamus nucleus (STN). It has been more than a decade since the ablative surgical procedure to reduce excess pallidal inhibitory output to the thalamus and brainstem was reintroduced for treatment of intractable PD. Ondo and coworkers (2006) reported that STN DBS patients with prior unilateral pallidotomy displayed less improvement in Unified Parkinson’s Disease Rating Scale (UPDRS) motor off score than patients without the ablative surgery; suggesting the earlier lesion may severely affect neurophysiological properties of the STN on the ipsilateral side to the surgical procedure (15). This hypothesis has also been previously investigated by Mogilner and coworkers (2002). They found in PD patients that pallidotomy could significantly decrease the mean firing frequency in the STN on the ipsilateral side to the pallidal lesions as compared with the contralateral, intact side (16). These findings were not significantly different from the data collected from patients without prior pallidotomy, although these findings were challenged by a later study (17). The authors of the later study attributed the difference to the sample size of their study.
Although a lot of controversy surrounds the current model of the basal ganglia, it is apparent that hyperactivity of the STN and internal segment of the GP (GPi)/substantia nigra reticlata (SNr) is the hall mark for PD (For a review see 18). The STN and GPi play crucial roles in both pathophysiological changes of PD and therapy-induced functional recovery. Many studies in both humans and nonhuman primates have revealed dopaminergic input from the substantia nigra to both the GPi and STN, although those dopaminergic inputs are much less than in the striatum (8,19,20). The extrastriatal dopaminergic innervations allow SNc DA neurons to directly affect the neuronal activity of both the GPi and the STN. It is important to point out that those extrastriatal dopaminergic pathways are partly spared in idiopathic PD and parkinsonian monkeys induced by MPTP administration (21,22).
STN neurons project to both the GPi and SNr using glutamate as their neurotransmitter (23), and thus have an excitatory effect on their pallidal and nigral targets (24). Lesioning of the GPi may indirectly lead remaining axon terminals afferent from the STN to undergo retrograde degeneration, resulting in a relative deactivation of the STN. The direct destruction of the GPi and the possible degeneration of STN neurons would be further reducing dopaminergic input from the SNc. The cumulative effects of ablating the GPi could be a major attributive factor for blocking GDNF-induced behavioral improvement in parkinsonian monkeys, even though the trophic factor evoked more dopamine release in the SN.
Behavioral changes are induced by net effects rather than actions from individual nuclei in the basal ganglia. Anatomical findings obtained from monkeys with anterograde tract-tracing methods reveal a number of pathways (projections) that connect to the GPi and to the STN. For example, the STN receives major input from the primary motor cortex (cortico-subthalamic projection) and premotor cortex (Parent and Hazrati 1995). These major projections of the STN afferent from the cortex involve controlling behavior in response to somatosensory stimulation (25,26,27). Activities of cortico-subthalamic pathways are eventually regulated by GPi-thalamus-cortex pathways (28). Clearly, directly ablating the GPi plus secondary effects on the STN could compromise GDNF-induced behavioral improvement in parkinsonian monkeys.
In summary, the current study demonstrated that GDNF-induced behavioral improvements in parkinsonian rhesus monkeys were waning during a several month long washout period; and were restored when GDNF treatment was reinstituted. Perhaps more importantly, the results from this study strongly indicate that pallidal lesions could block GDNF-induced behavioral improvements because the functional changes were not found in the animals with pallidal lesions, even though significant increases of dopamine levels were found in the SN and behavioral improvements were seen during the initial GDNF treatments in those animals. Immunohistological data revealed that pallidotomy caused a massive loss of dopaminergic fibers in the GPi and surrounding areas. Thus, clinical implications from the present study combined with evidence from STN DBS clinical trials suggest that carefully selecting patients would be the key for successful surgical treatments of PD at least for STN DBS and intracerebral infusion of neurotrophic factors such as GDNF.
Acknowledgments
This work was supported by grants from AG13494 to Don Gash and NS050242 to Zhiming Zhang. This work would not have been possible without the RhGDNF that was supplied by Amgen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lang AE, Lozano AM. Parkinson’s disease. First of two parts. N Engl J Med. 1998;339(15):1044–53. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- 2.Hornykiewicz O. Parkinson’s disease and the adaptive capacity of the nigrostriatal dopamine system: possible neurochemical mechanisms. Adv Neurol. 1993;60:140–7. [PubMed] [Google Scholar]
- 3.Gash DM, Zhang Z, Cass WA, Ovadia A, Simmerman L, Martin D, Russell D, Collins F, Hoffer BJ, Gerhardt GA. Morphological and functional effects of intranigrally administered GDNF in normal rhesus monkeys. J Comp Neurol. 1995;363:345–58. doi: 10.1002/cne.903630302. [DOI] [PubMed] [Google Scholar]
- 4.Maswood N, Grondin R, Zhang Z, Stanford JA, Surgener SP, Gash DM, Gerhardt GA. Effects of chronic intraputamenal infusion of glial cell line-derived neurotrophic factor (GDNF) in aged Rhesus monkeys. Neurobiol Aging. 2002;23:881–9. doi: 10.1016/s0197-4580(02)00022-2. [DOI] [PubMed] [Google Scholar]
- 5.Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, Russell D, Martin D, Lapchak PA, Collins F, Hoffer BJ, Gerhardt GA. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380:252–5. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- 6.Gash DM, Zhang Z, Ai Y, Grondin R, Coffey R, Gerhardt GA. Trophic factor distribution predicts functional recovery in parkinsonian monkeys. Ann Neurol. 2005;58:224–33. doi: 10.1002/ana.20549. [DOI] [PubMed] [Google Scholar]
- 7.Grondin R, Zhang Z, Yi A, Cass WA, Maswood N, Andersen AH, Elsberry DD, Klein MC, Gerhardt GA, Gash DM. Chronic, controlled GDNF infusion promotes structural and functional recovery in advanced parkinsonian monkeys. Brain. 2002;125:2191–201. doi: 10.1093/brain/awf234. [DOI] [PubMed] [Google Scholar]
- 8.Smith Y, Kieval JZ. Anatomy of the dopamine system in the basal ganglia. Trends Neurosci. 2000;23(10 Suppl):S28–33. doi: 10.1016/s1471-1931(00)00023-9. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z, Zhang M, Ai Y, Avison C, Gash DM. MPTP-Induced pallidal lesions in rhesus monkeys. Exp Neurol. 1999;155:140–9. doi: 10.1006/exnr.1998.6976. [DOI] [PubMed] [Google Scholar]
- 10.Smith RD, Zhang Z, Kurlan R, McDermott M, Gash DM. Developing a stable bilateral model of parkinsonism in rhesus monkeys. Neuroscience. 1993;52:7–16. doi: 10.1016/0306-4522(93)90176-g. [DOI] [PubMed] [Google Scholar]
- 11.Ovadia A, Zhang Z, Gash DM. Increased susceptibility to MPTP toxicity in middle-aged rhesus monkeys. Neurobiol Aging. 1995;16(6):931–7. doi: 10.1016/0197-4580(95)02012-8. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Miyoshi Y, Lapchak PA, Collins F, Hilt D, Lebel C, Kryscio R, Gash DM. Dose response to intraventricular glial cell line-derived neurotrophic factor administration in parkinsonian monkeys. J Pharmacol Exp Ther. 1997;282:1396–401. [PubMed] [Google Scholar]
- 13.Kurlan R, Kim MH, Gash DM. Oral levodopa dose-response study in MPTP-induced hemiparkinsonian monkeys: assessment with a new rating scale for monkey parkinsonism. Mov Disord. 1991;6(2):111–8. doi: 10.1002/mds.870060205. [DOI] [PubMed] [Google Scholar]
- 14.Gerhardt GA, Cass WA, Huettl P, Brock S, Zhang Z, Gash DM. GDNF improves dopamine function in the substantia nigra but not the putamen of unilateral MPTP-lesioned rhesus monkeys. Brain Res. 1999;817:163–71. doi: 10.1016/s0006-8993(98)01244-x. [DOI] [PubMed] [Google Scholar]
- 15.Ondo WG, Silay YS. Intravenous flumazenil for Parkinson’s disease: a single dose, double blind, placebo controlled, cross-over trial. Mov Disord. 2006;21(10):1614–7. doi: 10.1002/mds.21022. [DOI] [PubMed] [Google Scholar]
- 16.Mogilner AY, Sterio D, Rezai AR, Zonenshayn M, Kelly PJ, Beric A. Subthalamic nucleus stimulation in patients with a prior pallidotomy. J Neurosurg. 2002;96:660–5. doi: 10.3171/jns.2002.96.4.0660. [DOI] [PubMed] [Google Scholar]
- 17.Kleiner-Fisman G, et al. Subthalamic nucleus deep brain stimulation for parkinson’s disease after successful pallidotomy: clinical and electrophysiological observations. Mov Disord. 2004;19(10):1209–14. doi: 10.1002/mds.20151. [DOI] [PubMed] [Google Scholar]
- 18.Yelnik J. Functional anatomy of the basal ganglia. Mov Disord. 2002;17(Suppl 3):S15–21. doi: 10.1002/mds.10138. [DOI] [PubMed] [Google Scholar]
- 19.Presa P, Pardo BG, Martinez P, Bernatchez L. Phylogeographic congruence between mtDNA and rDNA ITS markers in brown trout. Mol Biol Evol. 2002;19:2161–75. doi: 10.1093/oxfordjournals.molbev.a004041. [DOI] [PubMed] [Google Scholar]
- 20.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Brain Res Rev. 1995;20:128–54. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- 21.Parent A, Lavoie B, Smith Y, Bedard P. The dopaminergic nigropallidal projection in primates: distinct cellular origin and relative sparing in MPTP-treated monkeys. Adv Neurol. 1990;53:111–6. [PubMed] [Google Scholar]
- 22.Schneider JS, Dacko S. Relative sparing of the dopaminergic innervation of the globus pallidus in monkeys made hemi-parkinsonian by intracarotid MPTP infusion. Brain Res. 1991;556(2):292–6. doi: 10.1016/0006-8993(91)90318-p. [DOI] [PubMed] [Google Scholar]
- 23.Rinvik E, Ottersen OP. Terminals of subthalamonigral fibres are enriched with glutamate-like immunoreactivity: an electron microscopic, immunogold analysis in the cat. J Chem Neuroanat. 1993;6(1):19–30. doi: 10.1016/0891-0618(93)90004-n. [DOI] [PubMed] [Google Scholar]
- 24.Hammond C, Deniau JM, Rizk A, Feger J. Electrophysiological demonstration of an excitatory subthalamonigral pathway in the rat. Brain Res. 1978;151:235–44. doi: 10.1016/0006-8993(78)90881-8. [DOI] [PubMed] [Google Scholar]
- 25.DeLong MR, Crutcher MD, Georgopoulos AP. Primate globus pallidus and subthalamic nucleus: functional organization. J Neurophysiol. 1985;53(2):530–43. doi: 10.1152/jn.1985.53.2.530. [DOI] [PubMed] [Google Scholar]
- 26.Fink-Jensen A, Mikkelsen JD. A direct neuronal projection from the entopeduncular nucleus to the globus pallidus. A PHA-L anterograde tracing study in the rat. Brain Res. 1991;542(1):175–9. doi: 10.1016/0006-8993(91)91016-t. [DOI] [PubMed] [Google Scholar]
- 27.Matsumura M, Sawaguchi T, Kubota K. GABAergic inhibition of neuronal activity in the primate motor and premotor cortex during voluntary movement. J Neurophysiol. 1992;68:692–702. doi: 10.1152/jn.1992.68.3.692. [DOI] [PubMed] [Google Scholar]
- 28.Obeso JA, Rodriguez-Oroz MC, Rodriguez M, Macias R, Alvarez L, Guridi J, Vitek J, DeLong MR. Pathophysiologic basis of surgery for Parkinson’s disease. Neurology. 2000;55:S7–12. [PubMed] [Google Scholar]


