Abstract
The complex regulation of eNOS (endothelial nitric oxide synthase) in cardiovascular physiology occurs at multiple stages. eNOS mRNA levels are controlled both at the transcriptional and post-transcriptional phases [1], and epigenetic mechanisms appear to modulate tissue-specific eNOS expression. The eNOS enzyme reversibly associates with a diverse family of protein partners that regulate eNOS subcellular localization, catalytic function, and biological activity. eNOS enzyme activity and subcellular localization are intimately controlled by post-translational modifications including phosphorylation, nitrosylation, and acylation. The multiple extracellular stimuli affecting eNOS function coordinate their efforts through these key modifications to dynamically control eNOS and NO bioactivity in the vessel wall. This review will focus on the biochemical partners and perturbations of the eNOS protein as this vital enzyme undergoes modulation by diverse signal transduction pathways in the vascular endothelium.
1. INTRODUCTION
Nitric oxide (NO) serves as an endogenous vasodilator, platelet inhibitor, antioxidant, and regulator of vascular endothelium by sustaining its anti-coagulant and anti-thrombogenic properties. Endothelial nitric oxide synthase (eNOS) is expressed not only in endothelial cells, but also in cardiac myocytes and blood platelets, where the enzyme subserves key roles in cardiovascular physiology [2]. eNOS has also been reported to be expressed in diverse tissues including mast cells, renal epithelium, erythrocytes, and leukocytes [3,4,5]. Because NO production must be carefully titrated to respond to diverse physiologic and pathophysiologic stimuli, eNOS is regulated by multiple interdependent control mechanisms and signaling pathways that act throughout the various stages of the enzyme’s life history.
2. Post-Translational Modifications to the eNOS Protein
Endothelial eNOS is subject to several overlapping modes of post-translational regulatory modifications that provide mechanisms for the dynamic stimulation and inhibition of enzymatic activity in response to physiologic and pathophysiologic stimuli.
2.1 Acylation
In quiescent cells, eNOS is specifically targeted to small invaginations of the plasmalemma called caveolae. Caveolae are invaginated membrane microdomains defined by the presence of a scaffolding protein caveolin. The caveolae are enriched in cholesterol and sphingolipids [6], creating a distinct membrane phase with diminished fluidity [7]. This unique fluid phase seems central to facilitating protein-protein and protein-lipid interactions necessary for cellular signaling [8]. Caveolae sequester diverse receptors and signaling proteins from a variety of signal transduction pathways, including G-protein coupled receptors, G proteins, growth factor receptors, calcium regulatory proteins in microdomains that may serve to position eNOS to receive signals from these upstream signaling pathways and facilitate communication with downstream activators [1,9].
Localization of eNOS to caveolae is dependent on irreversible, co-translational myristoylation of its N-terminal glycine (after removal of true N-terminal methionine) [2,9]. Myristoylation initially targets N-Myr-eNOS to the cell membrane in general, where reversible post-translational palmitoylation of the Cys15 and Cys26 residues occurs [10]; however, eNOS palmitoylation appears to be dependent on membrane targeting, and not necessarily the enzyme’s prior myristoylation [11]. Myristoylation and palmitoylation confer on eNOS three acyl anchors that anchor it firmly to the caveolar lipid bilayer. The N-myristoylation of eNOS appears to be catalyzed by an N-myristoyltransferase that recognizes a specific N-terminal consensus sequence that is present on virtually all N-myristoylated proteins. By contrast, no consensus sequence has been identified for thiopalmitoylation, and a large family of palmitoyltransferases has been identified with varying substrate specificities and patterns of expression [12,13]. A recent report has implicated the palmitoyltransferase protein DHHC-21 in the palmitoylation of eNOS [14]. While DHHC-21 co-localizes with eNOS, its specificity for eNOS palmitoylation has not been established. Depalmitoylation of eNOS is catalyzed by acyl protein thioesterase-1 (APT-1) [15], an enzyme that also depalmitoylates the G protein Gαs [16]. In contrast to the irreversible fatty acyl amide that links myristate to eNOS, the protein’s thiopalmitoyl bonds are scissile, and thus the targeting of eNOS to caveolae, and by extension, eNOS enzymatic activity, are subject to dynamic regulation. Prolonged agonist stimulation of eNOS induces depalmitoylation and cytosol translocation [17], likely serving as a mechanism for modulating the activity of the eNOS enzyme following agonist activation [2].
2.2 Intracellular Calcium: Calmodulin Binding
Intracellular calcium level is a critical determinant of eNOS activity because maximal catalytic function of eNOS requires calmodulin binding to a ~50 amino acid domain in order to facilitate transfer of electrons between the enzyme’s reductase and oxygenase domains, [18]. Binding of calmodulin simultaneously disrupts the inhibitory caveolin-eNOS interaction [17]. The ability of calmodulin to activate eNOS is inhibited by CK2 kinase, a mechanism thought to lead to selective disassociation of phosphorylated calmodulin from eNOS without impacting either the total cellular pool of calmodulin (especially since the level of calmodulin relative to total cellular calmodulin binding capacity is low), intracellular calcium levels, or other calmodulin-dependent signals [19,20].
Numerous pathways converge on mobilization of intracellular calcium transients to provide the most rapid mechanisms of eNOS activation via calmodulin. A diverse group of agonists, including bradykinin and acetylcholine [21], activate a G protein-dependent signaling pathway that ultimately releases intracellular calcium stores [22]. In this pathway, phospholipase C (PLC) cleaves membrane component phosphatidylinositol 4,5-triphosphate into diacylglycerol (an activator of protein kinase C) and inositol 1,4,5-triphosphate (IP3), which binds to IP3 receptors that are found in high concentrations in caveolae and regulate intracellular calcium through pleiotropic effects on ion channels [23].
2.3 Phosphorylation
Phosphorylation and dephosphorylation networks complement acylation and calmodulin as major post-translational regulatory influences on eNOS activity. Key serine and threonine residues in eNOS constitute regulatory loci: phosphorylations at Ser 1177 (primary sequence numbering corresponds to human eNOS), Ser 635, and Ser 617 are stimulatory while phosphorylation at Thr 495 and Ser 116 are inhibitory [24]. The activation of eNOS catalytic function by Ser 1177 phosphorylation is due to inhibition of calmodulin dissociation from eNOS and also enhancement of the internal rate of eNOS electron transfer [25,26,27]. Ser 1177 phosphorylation is catalyzed by numerous kinases, including kinase Akt (protein kinase B) as well as the cyclic AMP-dependent protein kinase (PKA), AMP-activated protein kinase (AMPK), PKG, and calcium/calmodulin-dependent protein kinase II (CaM kinase II) (see below) [28,29,30,31]. The relative contributions of these different kinase pathways remains under active investigation, but it is clear that different extracellular stimuli activate distinct kinase pathways leading to eNOS phosphorylation.
Phosphorylation at Ser 617, occurring downstream of either PKA or Akt, appears to sensitize eNOS to calmodulin binding [32] and possibly modulate phosphorylation at other eNOS sites [24]. Phosphorylation at Ser 635 is responsive to PKA and increases eNOS activity in response to PKA dependent agonists as well as basal stimuli like shear stress [32,33]. Phosphorylation at Thr 495, downstream of protein kinase C (PKC) and AMPK [28], attenuates the binding of calmodulin by eNOS; accordingly, agonist mediated dephosphorylation of Thr 495, probably due to protein phosphatase 1, enhances the interaction of eNOS and calmodulin [31]. The phosphorylation state of Thr 495 helps adjust the product mix of eNOS output between superoxide and NO [34]. Phosphorylation of eNOS at Ser 116 inhibits enzyme activity, and dephosphorylation of eNOS at this site is promoted by the eNOS agonist VEGF, but not by several other eNOS agonists [35].
Tyrosine phosphorylation of eNOS Tyr 83 mediated by v-Src was recently identified as another candidate eNOS post-translational modification [36]. The extracellular signals that modulate phosphorylation at this site remain incompletely understood, and it remains unclear whether Tyr 83 phosphorylation is responsible principally for activating eNOS catalytic activity, or whether this site may modulate signaling by serving as a binding site for proteins with Src homology (SH3) domains [36].
2.4 S-Nitrosylation
Reversible S-nitrosylation is now recognized as a significant additional level of in vivo dynamic receptor-mediated post-translational control of the eNOS enzyme in endothelial cells and the vessel wall [37,38]. Quiescent eNOS is inhibited by tonic S-nitrosylation at cysteine residues Cys 94 and Cys 99 that comprise a zinc-tetrathiolate cluster [37,39]. [37]eNOS appears to be the source of the NO required for its own S-nitrosylation, implying a spatial mechanism of specificity in nitrosylation of Cys 94 and Cys 99 given the approximately thirty cysteine residues in eNOS [38]. S-nitrosylation of eNOS leads to enzyme inhibition, whereas denitrosylation is associated with an increase in enzyme activity. Treatment of cultured endothelial cells or intact blood vessels with eNOS agonists promotes rapid and reversible de-nitrosylation of eNOS, temporally associated with enzyme activation. The return to basal eNOS enzyme activity following agonist treatment is associated with the re-nitrosylation of eNOS. These findings might imply some component of temporal selectivity in nitrosylation reactions given that eNOS is denitrosylated during the period of maximal NO production.
Like phosphorylation and acylation, subcellular localization affects eNOS S-nitrosylation and may help generate the apparent temporal selectivity. Membrane targeting is required for S-nitrosylation, as shown by experiments in which the myristoylation-deficient mutant (Myr−) eNOS S-nitrosylation is virtually abolished as compared to the hyper-nitrosylation of the membrane-tethered fusion protein CD8-Myr− eNOS [37]. Conversely, agonist-induced denitrosylation is critically dependent on subcellular localization, as demonstrated by the comparison of denitrosylation in wild type eNOS versus CD8-Myr− eNOS, which is irreversibly anchored to caveolae [11], and remains hyper-nitrosylated despite exposure to eNOS agonists like VEGF [37,38]. The subcellular dependence of nitrosylation may reflect distinct chemical environments that favor or disfavor nitrosylation [40]. For example, it has been hypothesized that the lipid environment of membranes like caveolae support formation of S-nitrosothiols by facilitating the reaction of NO and gaseous oxygen in a hydrophobic milieu [37,41]. In contrast, denitrosylation in the cell’s interior may be facilitated by the cytosolic reducing environment, lack of proximity to membrane-bound enzymes that catalyze nitrosylation, lack of cross-talk with acylation and phosphorylation pathways, and/or presence of multiple trans-nitrosylating enzymes [38].
The mechanisms by which S-nitrosylation inhibits eNOS catalysis remain under investigation. Because zinc can be released from the tetrathiolate cluster upon S-nitrosylation, it was suggested [39] that the mechanism of inhibition might be the dissociation of the eNOS homodimer to form inactive monomers, which are inactivated because the requisite electron transfer from the reductase domain of one monomer to the oxygenase domain of the other monomer cannot occur. In fact, the dissociation of dimeric eNOS enzyme into inactive monomers has been postulated as the mechanism for S-nitrosylation induced inhibition of iNOS [38]. Subsequent experiments with eNOS argued against dimer dissociation as the inhibitory mechanism, instead suggesting that S-nitrosylation of eNOS modifies substrate or cofactor binding, or attenuates electron transfer at the interface between the eNOS monomers [37,42,43].
3. Caveolar Protein Partners
3.1 Caveolin
Caveolins are ~22kDa proteins integrally linked to membrane owing to membrane-spanning domains and irreversible post-translational triple C-terminal palmitoylation [44]. Caveolin-1 and caveolin-2 are ubiquitously expressed and abundant in endothelial cells; caveolin-3 is a muscle-specific isoform expressed in cardiomyocytes and skeletal muscle [45]. Caveolins incorporate into and associate within membranes in a cholesterol-dependent manner, possibly due to the effects of cholesterol on membrane fluidity [46]. Robust protein-protein interactions lead to the binding of caveolar-localized eNOS with caveolin-1, though this protein-protein interaction is apparently not necessary for the localization of eNOS to caveolae [47]. Caveolin tonically inhibits eNOS in quiescent cells both by impeding the signaling of caveolae-targeted receptors that transduce eNOS-stimulatory signals as well as by sterically blocking the calmodulin binding site in eNOS [17]. Caveolins are increasingly recognized as playing dynamic roles in the trafficking of protein cargoes to and from the membrane, in functioning as a “signalosome” [48] by agglomerating and regulating signaling molecules (both transmembrane and cytosolic effectorsincluding G protein coupled receptors, heterotrimeric G proteins, GTPases, PI3-kinases, c-Src kinase, estrogen receptors, VEGF receptors, calcium pumps, TGFβ, MAP kinase, etc) and also functioning in caveolar biogenesis (due to caveolin oligomerization and cholesterol association after intracellular processing) [46,49,50,51]. Disease states in which caveolins have been implicated include atherosclerosis, hypertension, cardiomyopathy, diabetes, and oncogenesis [52,53].
3.2 Endoglin
Endoglin (CD105) is a 180 kDa glycoprotein highly expressed on endothelial cell membranes. Endoglin was first characterized as an accessory TGFβ1 receptor implicated in extra embryonic vasculogenesis, cardiogenesis, and hereditary hemorrhagic telangiectasia (HHT1), a vascular dysplasia characterized by venodilatation, obliteration of capillary network, and arteriovenous malformations wherein the functional and structural distinction between arterioles and venules is lost [54,55,56]. Endoglin is enriched in caveolae and stabilizes eNOS by promoting its association with hsp90 (see below) [55]. In endoglin haplo-insufficient endothelium, the half-life of eNOS protein is diminished, the interaction eNOS/hsp90 is impaired, and the NO output of eNOS is decreased in favor of superoxide production [55,57]. Paradoxically, however, eNOS dependent vasodilatation is enhanced (likely due to reactive oxygen species release from uncoupled eNOS) with a concomitant impairment in the vascular smooth muscle myogenic response (likely due to both superoxide and peroxynitrite, which hyperpolarize vascular smooth muscle) [55]. Endoglin haplo-insufficient mice exhibit vascular malformations similar to HHT patients suggesting a role for eNOS in its pathogenesis [54,56].
4. Signaling Protein Partners
4.1 G Protein–Coupled Receptors
Members of the seven transmembrane guanine nucleotide-binding protein (G-protein)-coupled receptors (GPCR) superfamily transduce multiple diverse signaling pathways into eNOS activity. The cytoplasmic side of the typical GPCR is intimately linked to a heterotrimeric G protein, in which the α, β, and γ subunits may be selected from one of the >20 Gα, 6 Gβ or 12 Gγ known isoforms. Many GPCR subtypes can transmit signals through many of the possible heterotrimeric G protein permutations, implying additional levels of complexity and possible regulatory loci in eNOS control.
Downstream of GPCR and heterotrimeric G proteins, two eNOS-activating mechanisms have been elucidated: mobilization of intracellular calcium and the PI3K (phosphoinositide-3-kinase (PI3K)/Akt cascade (discussed in detail below). GPCR ligands that stimulate intracellular calcium transients include the small molecules bradykinin (B2 receptor), acetylcholine (m2 muscarinic receptor), histamine, adenosine, ADP/ATP, and sphingosine 1-phosphate (S1P), and the protein thrombin [2]. These GPCR pathways are coupled to Gαq proteins that result in activation of phospholipase C and that mobilize intracellular calcium in an IP3 dependent mechanism [21,22]. High resolution microscopy has confirmed the initiation of calcium waves starting initially at caveolae subsequent to GPCR stimulation [58]. Other GPCRs, including the β3 adrenergic receptor, may lead to eNOS activation by pathways involving Gαs activation.
Recent studies have investigated the coupling of GPCR to downstream kinases like PI3K/Akt and have identified specific intermediary roles for low molecular weight GTPases, such as Rac1 in endothelial GPCR-dependent signaling [59]. Rac1 is one member of the Rho GTPase family, signaling proteins that cycle between GTP-bound active form and GDP-bound inactive form due to intrinsic GTPase activity. Members of the Rho GTPase family are controlled both by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs); recruitment of GEFs accelerates the rate-determining step of the GTPase cycle, the dissociation of GDP from the GTPase, while GAPs exert direct control on the rate of GTP hydrolysis. Both GEFs and GAPs are subject to independent measures of extracellular control, and this may represent a method for cell-specific controls of Rac1 function.
Gβγ is a key mediator following stimulation of GPCR, as Rac1 activity is blocked by a Gβγ-specific chelator; the fact that this pathway was not completely attenuated suggests that additional mechanisms may be operational [59]. Gβγ proteins are known to participate in the activation of Rho GTPases by non-receptor Src tyrosine kinases, and Src kinase inhibitors were demonstrated to abrogate both Rac1 and Akt activity, suggesting that Gβγ communicates directly to Src kinases. Rac1 was shown to be upstream of PI3K and Akt by several lines of experimentation, including PI3K kinase inhibitors, dominant negative Rac1, constitutively active Rac1, and siRNA knockdown of Rac1 [59]. Tyrosine kinase inhibitors, but not PI3K kinase inhibitors, blocked Rac1 activation. Overexpression of dominant negative Rac1 or siRNA knockdown of Rac1 attenuated agonist-induced phosphorylation Akt; Rac1 siRNA also impaired basal Akt phosphorylation as well as phosphorylation of eNOS and other Akt targets [59]. Conversely, expression of a constitutively active Rac1 mutant enhanced Akt phosphorylation. Though the specific GEF(s) involved in Rac1 activation in endothelium have not been definitively identified, overexpression of the ubiquitous GEF Tiam1 enhanced the activity of GTPase Rac1; in contrast a dominant negative Tiam1 mutant blocked agonist-induced Rac1 activity. In sum, Rac1 appears to be necessary and sufficient to transmit signal from GPCR activation through to PI3K/Akt. However, there does appear to be some cross-talk of Rac1 and PI3K in other cell types wherein PI3K activation is required at least in part to fully stimulate Rac1 and/or its GEFs [59].
4.1.1 Protein Mediators Activating eNOS in a GPCR Dependent Mechanism
Thrombin is one protein that affects eNOS phosphorylation status of eNOS in a GPCR dependent pathway. Thrombin inhibits Ser 1177 phosphorylation via Rho-dependent Akt inhibition [60,61].
4.1.2 Sphingolipid and Lysophospholipid Mediators Activate eNOS via a GPCR-Dependent Mechanism
A class of platelet-derived lipid mediators including sphingosine 1-phosphate (S1P) and lysophosphatidic acid (LPA) activate eNOS in GPCR-dependent pathways [62]. S1P, a sphingomyelin derivative, is abundant in platelets due to the activity of sphingosine kinase and the absence of sphingosine phosphatase. LPA is a glycerophospholipid product of lysophospholipase D and phospholipase A2, released due to the action of thrombin. These platelet-derived mediators represent an example of platelet-endothelium cross talk in eNOS regulation. Both S1P and LPA activate endothelium through G protein coupled S1P receptors (formerly called EDG receptors) [63,64,65,66]. Activation of PI3Kβ is mediated by Gβγ downstream of the S1P/LPA GPCRs [67,68]. In sustained S1P signaling, PI3K/Akt can directly phosphorylate the S1P receptor. Even in quiescent endothelium half of the cellular complement of S1P receptors are caveolar localized. S1P exposure brings about 90% of S1P receptors to caveolae [63], a mechanism that may help couple S1P signaling to effectors of PI3K pathway and eNOS, although it could have a role in attenuating the response to S1P.
4.2 Hsp90/PI3K/Akt Proteins
4.2.1 PI3K/Akt
Kinase Akt (or protein kinase B) is an important determinant of eNOS phosphorylation at Ser 1177, implying intimate involvement in basal activation of eNOS and agonist-mediated stimulation [24,69]. Kinase Akt is predominantly found in the cytosol in inactive form, and must translocate to the membrane as a prerequisite to both its own activation and the phosphorylation of eNOS [70]. Kinase Akt is itself under direct control of phosphoinositide-3-kinase (PI3K)-dependent phosphorylation pathways, and it appears PI3K recruits Akt to the membrane for phosphorylation [71].
4.2.2 PI3K/Akt Partners
Signals from diverse types of eNOS agonists, including proteins (VEGF, insulin), small molecule hormones (estrogen, platelet-derived lipid mediators), and mechanical forces (shear) can affect eNOS activity through the PI3K/Akt pathway. Though these agonists may activate different isoforms of PI3K, pathways converge on activation of Akt and eNOS Ser 1177 phosphorylation [2].
Part of the endothelial response to shear stress is mediated by calcium-independent PI3K/Akt kinase mechanisms that may serve to maintain basal vascular tone by Ser 1177 phosphorylation [26,69]. Shear stress also stimulates Ser 635 phosphorylation in a PKA dependent manner [33].
Vascular endothelial growth factor (VEGF) exerts pleiotropic effects on eNOS and is one of the more potent stimuli of eNOS activity. VEGF binds to a family of receptor tyrosine kinases including KDR, leading to activation of both PI3Kα and PI3Kβ [35]. Maximal phosphorylation of Ser 1177 occurs within 5 minutes and is accompanied by rapid eNOS denitrosylation [37]. VEGF also induces Ser 116 dephosphorylation via phosphatase calcineurin; in contrast to Ser 1177 phosphorylation, Ser 116 dephosphorylation requires 30 minutes, suggesting distinct roles in the temporal regulation of eNOS activity [35]. VEGF also mobilizes calcium by a KDR receptor tyrosine kinase/c-Src/PLCγ pathway [72]. Further, VEGF sensitizes endothelium to other mediators including S1P (for example by increasing mRNA for S1P receptors); though coordinated control of shared effects like angiogenesis is possible, in vivo roles for synergy between such distinct eNOS signaling pathways has not been proven [73]. Insulin is a vasodilator and activates eNOS via an insulin receptor tyrosine kinase that sits upstream of PI3K, and thus promotes Ser 1177 phosphorylation as well as eNOS denitrosylation [37,74,75,76,77]. Roles of insulin-dependent eNOS signaling in the pathogenesis of diabetic vasculopathy remain poorly understood, although eNOS itself does become non-specifically glycosylated to form O-linked N-acetylglucosamine glycation endproducts that are refractory to eNOS Ser 1177 phosphorylation [78,79].
The cardioprotective effects of estrogen have undergone intense scrutiny; effects of estrogen are observed at the genomic level, where estrogen as a transcription factor gradually modifies a cellular program, but also at the non-genomic level by enhancing eNOS activity [80]. Through non-nuclear signaling pathways both the alpha and beta estrogen receptors activate PI3K/Akt and ERK/MAP kinases [81,82,83]. Estrogen also stimulates calcium transients that regulate eNOS activity and localization [21].
4.2.3 hsp90
The role of heat shock protein 90 (hsp90), a chaperone involved in protein trafficking and folding, extends to agonist-dependent eNOS activation. First identified as a partner to eNOS initially termed ENAP-1 (endothelial nitric oxide synthase-associated protein 1) and later identified as hsp90, eNOS becomes robustly associated with hsp90 ashsp90 undergoes reversible tyrosine phosphorylation in response to diverse eNOS agonists [84,85,86]. Hsp90 binding stimulates eNOS activity by cooperatively enhancing the affinity of eNOS for binding calmodulin, balancing output of nitric oxide versus superoxide, and possibly facilitating heme binding [87,88]. Hsp90 also affects eNOS specific activity by means of effects on Akt. Hsp90 can bind both inactive and active Akt and is required for the interaction of Akt with eNOS [86,89]. Hsp90 stimulates eNOS catalysis by increasing the rate of Akt-dependent phosphorylation (and possibly the degree to which Akt phosphorylates the population of eNOS molecules) [90,91,92] The Akt-dependent effects of hsp90 on eNOS may reflect unmasking of phosphorylation sites on eNOS or an effect on the affinity of Akt for eNOS [90]. Furthermore, hsp90 maintains levels of phospho-Akt and thus Akt phosphorylation activity by protecting PI3K from proteasomal degradation [93] as well as inhibiting Akt deactivation by protein phosphatase 2A. Hsp90 and Akt synergistically activate eNOS at both physiologic calcium concentrations and independent of calcium [90,94] indicating that both hsp90-dependent Akt activation and the Akt-independent effects of hsp90 must be simultaneously occurring [90].
Synergistic activation of eNOS can be seen following formation of a ternary complex containing hsp90, Akt, and calmodulin-bound eNOS [90]. The charged middle domain (M domain) of hsp90 contains independent binding sequences for both eNOS and Akt and thus likely provides a scaffold for protein association [91] while allowing for the possibility of binding other regulatory proteins at the N- and C-terminii [85]. One co-chaperone identified so far is cdc37, a 50 kDa phosphoprotein which is found in caveolae and can be bound directly to eNOS or as part of the eNOS-hsp90-Akt complex [95]. In direct complex with eNOS, cdc37 inhibits eNOS activity and may serve to prevent unregulated eNOS activity or dysregulated, uncoupled activity that would produce superoxide. On the other hand, cdc37 binding to the N-terminus of hsp90 blocks its ATPase cycle and locks hsp90 into a conformation that can receive client chaperone proteins [95]. Cdc37 could therefore bind to the eNOS-hsp90-Akt ternary complex and regulate the interactions of both eNOS and hsp90 with Akt [95]. Another co-chaperone CHIP (carboxyl terminus of Hsp70-interacting protein) impairs the association of hsp90 and eNOS, thereby displacing eNOS from normal Golgi trafficking patterns; for example, CHIP redistributes eNOS to the cytoskeleton with concomitant decrease in cellular eNOS activity [96,97].
Pharmacologic interventions and vascular stimuli appear to modify the eNOS-hsp90-Akt complex. Hsp90 mediates the effect of hypoxia on activation of eNOS by increased hsp90-eNOS binding and activation of PI3K-Akt [98]. One of the many effects of statins is to induce eNOS phosphorylation by Akt [99]. At least one Akt-dependent mechanism of statins involves tyrosine phosphorylation of hsp90, which facilitates the ability of hsp90 to bind and activate Akt [100]. PPARγ ligands including the endogenous 15-deoxy- Δ12,14-prostaglandin J2 (15d-PGJ2) and some thiazolidinediones like rosiglitazone act in part by promoting the interaction of hsp90 and eNOS, resulting in Ser 1177 phosphorylation [101].
Finally, NO itself may negatively modulate hsp90 by S-nitrosylation of a cysteine residue that inhibits the hsp90 ATPase function and may disrupt eNOS-hsp90 binding; this mechanism might provide negative feedback to regulate the amount of NO generation and/or facilitate cyclic eNOS activity [102].
4.3 Phosphatases
The relative contribution of protein phosphatases versus phosphoprotein kinases in eNOS regulation remains incompletely understood. However, depending on the site, dephosphorylation of eNOS could either activate the enzyme (e.g. Ser 116 or Thr 495), or attenuate enzyme activity (e.g. Ser 1177), perhaps returning NOS to basal activity after stimulatory phosphorylation. Several phosphatases, including serine-threonine protein phosphatase 1 (PP1), serine-threonine protein phosphatase 2A (PP2A), and calcineurin participate in eNOS regulation.
PP1 primarily dephosphorylates Thr 495 and may activate eNOS [28,31]. A pathway involving the phosphatase calcineurin leads to dephosphorylation of Ser 116 and to the activation of eNOS, while downstream of bradykinin calcineurin also dephosphorylates Thr 495 [103]. The immunosuppressive drug cyclosporine inhibits calcineurin, preventing VEGF-induced Ser 116 dephosphorylation and thus offering a potential mechanism to explain the mechanism of cyclosporine-induced hypertension [35].
PP2A functions as an overall negative regulator of eNOS due to its primary roles in dephosphorylating phospho-Akt and dephosphorylating Ser 1177 despite the fact that it can also dephosphorylate Thr 495 [34,104,105]. Possibly because of its high abundance (comprising ~1% total cellular protein) and robust constitutive activity, the influence of PP2A on eNOS is regulated partly by the proteasome [105]. Proteasomal inhibition causes PP2A ubiquitination and redistribution of PP2A from the cytosol to the membrane, thus leading to the association of PP2A with eNOS, and thereby reducing both phosphorylation of Akt and of eNOS at their respective stimulatory phosphorylation sites [105]. PP2A membrane translocation appears to specifically dephosphorylate eNOS even while other PP2A substrates are not affected. Control of phosphatase proteolysis in vivo may be important given emerging evidence of proteasome dysfunction in diverse diseases including Parkinsonism, Liddle syndrome, and several malignancies [106].
4.3 Partners in Localization and Trafficking
Trafficking and proper subcellular localization of eNOS is crucial for its activity. The actin cytoskeleton in particular seems involved in these functions. The yeast two-hybrid system has identified two actin-dependent eNOS binding proteins that appear to be involved in eNOS translocation and localization [107,108].
4.3.1 Actin Cytoskeleton
Dynamic structural changes in the actin cytoskeleton impact eNOS via links to caveolar membrane domains and caveolar membrane associated proteins [109]. First, shear stress may transduce its effect on eNOS via actin-based cellular architecture [110]. Second, caveolin and caveolae may use the actin network to reversibly translocate between plasmalemma and the Golgi (see NOSIP and NOSTRIN, below) [111]. Third, actin may regulate eNOS-associated proteins like CAT-1, an arginine transporter protein associated with eNOS in caveolae as part of an eNOS-actin-fodrin-CAT-1 complex (where fodrin is the actin binding protein by which actin binds CAT-1) [109]. Association of eNOS with actin and stabilization of actin filaments might help to maintain eNOS-actin-fodrin-CAT-1 in order to directly funnel arginine substrate to eNOS [112,113]. Fourth, the relative abundance of actin monomers versus actin polymers directly affects eNOS protein [114,115,116,117,118,119] (notably, the interaction of eNOS mRNA with G-actin helps connect cell growth and morphology to eNOS mRNA transcript stability). The association of eNOS protein with G-actin resulted in more significant increases in eNOS activity than compared with similar association of eNOS protein with F-actin [109]. Perhaps this finding implies that while eNOS is associated with polymerized F-actin, for example during transport, enzymatic activity is downregulated, though the exact mechanism of interaction and role of actin on control of enzymatic activity remain to be determined. Actin cytoskeletal targeting of eNOS reduces total cellular eNOS activity in proportion to the fraction of cellular eNOS bound to actin [96]. On the other hand, the effect of actin polymerization might imply that eNOS localized to the perinuclear subcellular region might have inherently different activity than plasmalemmal localized eNOS due to the relative predominance of perinuclear G-actin [109]. Connections between plasmalemmal caveolae and the cytoskeleton have been identified in many cellular systems, such as the key role identified for the cytoskeleton-associated GTPase Rac1 in eNOS activation.
4.3.2 NOSIP
One eNOS-associated protein identified using yeast two-hybrid screening is the eNOS Interacting Protein NOSIP, a 34kDa protein that binds the carboxy-terminal of the eNOS oxygenase domain and appears to assist in translocation of eNOS from plasma membrane caveolae to intracellular membranes [107]. Association of eNOS and NOSIP, demonstrated by both in vitro and in vivo co-immunoprecipitation, was inhibited by caveolin-1. In fact, results of a two-hybrid assay suggested that caveolin and NOSIP compete with one another to bind a site on the oxygenase domain of eNOS. Overexpression of NOSIP diminished the NO output of eNOS, possibly by uncoupling eNOS from its caveolar attachments and disrupting interaction with caveolar co-localized effectors of upstream agonists [107]. In addition, NOSIP is homologous to U-box ubiquitin ligases and has been shown to have ubiquitin ligase activity (for example, toward the erythropoietin receptor); how this might effect eNOS localization or activity remains to be determined [120].
Both sub-cellular fractionation and immunofluorescence studies confirm that NOSIP redistributes eNOS from plasma membrane to the intracellular regions, with the actin cytoskeleton as a specifically identified destination [96, 107]. Targeting to the cytoskeleton appears to be cell cycle dependent, occurring during G2 phase; cell-cycle specific NOSIP-dependent eNOS targeting may be due to its tightly controlled nucleocytoplasmic shuttling, where nuclear export outweighs constitutive nuclear import only during G2, resulting in cytoplasmic accumulation of NOSIP during G2 [96]. The mechanisms of NOSIP nuclear export and import remain under investigation, as well as whether nuclear-localized NOSIP facilitates nuclear localization of eNOS at any point during the cell cycle or in response to certain stimuli. Cell cycle-dependent control of eNOS localization and enzymatic activity may be important to apoptosis and proliferation in development and angiogenesis. For example, exogenous NO can cause G2 cell cycle arrest; the NOSIP-mediated cell cycle-dependent regulation of endogenous NO production might limit DNA damage and thus facilitate passage NOSIP may have exert broad roles on eNOS through the G2/M checkpoint [96]. regujlation in non-endothelial cells including epithelial and smooth muscle cells in the pulmonary and gastrointestinal tracts [121,122], as well as regulation of nNOS in the central and peripheral nervous systems [123].
4.3.3 NOSTRIN
The second protein identified by yeast two-hybrid is the eNOS TRafficking INducer protein NOSTRIN, a 58 kDa protein of the PCH family (pombe cdc15 homology) that is robustly expressed in endothelium and highly vascularized tissue [108,124,125]. NOSTRIN shares the characteristic domain structure of PCH proteins, which includes an N-terminal cdc15 domain (consisting of an FCH region [Fes/CIP homology] followed by a coiled-coil structure) and C-terminal coiled coil and SH3 domains [125]. The FCH region is sufficient to direct membrane targeting of NOSTRIN (to plasmalemma and peripheral vesicles), while FCH deletion mutants are found in the cytosol fraction [125]. The NOSTRIN SH3 domain not only binds the oxygenase domain of eNOS, but also the GTPase dynamin and N-WASP (neural Wiskott-Aldrich syndrome protein) [125,126]. The C-terminal coiled-coil motif allows trimerization of NOSTRIN, suggesting that NOSTRIN may serve as a central platform for the association of multiple protein partners in part by simultaneously deploying multiple SH3 binding sites [126]. Co-immunoprecipitation confirms the in vivo interactions of NOSTRIN (via its C-terminus) with eNOS 193 as well as NOSTRIN (via its central domain) with caveolin-1 (at its N-terminus) [124]. Caveolin-1 and NOSTRIN each enhance the binding of the other to eNOS on unique sites; accordingly, a ternary eNOS-NOSTRIN-caveolin-1 complex of unknown stoichiometry has been demonstrated in vivo [124]; this ternary complex may localize at the plasma membrane [108,124]. Overexpression of NOSTRIN can promote the translocation of eNOS from the plasma membrane to intracellular vesicles, with a concomitant reduction in eNOS enzyme activity [108,124]. NOSTRIN-dependent shuttling of eNOS appears to be caveolin-dependent and likely reflects specialized endocytosis of caveolar endosomes that lack protein markers characteristic of other types of endocytosis [124]. Based on the dependence of caveolar endocytosis on the actin cytoskeleton [124], the homology of NOSTRIN to the syndapin family of essential endocytosis effector proteins, and potential SH3 binding partners of NOSTRIN, it was hypothesized that NOSTRIN is the critical adaptor of a multimeric protein complex that binds and regulates dynamin-2 and N-WASP necessary for caveolar endocytosis and eNOS internalization [126].
NOSTRIN recruits dynamin to caveolae and drives endocytosis by dynamin GTPase-mediated vesicle fission [126]. N-WASP and NOSTRIN co-localize with eNOS along the actin cytoskeleton. Because disruption of actin filaments traps NOSTRIN-eNOS at the peripheral membrane and perinuclear region, NOSTRIN-N-WASP is thought to promote actin polymerization to help shuttle vesicular cargoes [124,125,126]. The homotrimeric NOSTRIN complex with multiple SH3 sites is thus well suited to connect the control of actin polymerization to vesicle fission as a critical step in caveolar endocytosis and downregulation of total cell eNOS output [127]. It appears that endocytosed eNOS is not subject to proteolysis, but is instead recycled back to the caveolae through the Golgi in a process that might depend on the acylation status of eNOS [124]. In a model of hypoxia, anterograde transport is blocked, trapping co-localized eNOS, caveolin, and NOSTRIN in dysfunctional endoplasmic reticulum and Golgi compartments thus preventing relocalization to caveolae [128]. The inhibitory influence of NOSTRIN on eNOS, independent of translocation, might help to prevent undesired activation of eNOS during its transit cycle [124]. Whether or not clathrin endocytosis is involved with NOSTRIN mediated endocytosis remains unclear, but the mouse NOSTRIN ortholog binds to Disabled-2 (dab2), which helps link transported vesicles and clathrin [124,126,129].
One condition in which NOSTRIN may be implicated is pre-eclampsia, where higher placental levels of NOSTRIN protein were detected [130]. Though eNOS protein levels were not significantly different, the elevated expression of NOSTRIN was correlated with diminished eNOS activity and NO output [130].
5. DYNAMIC REGULATION OF eNOS: COORDINATED MODEL OF eNOS ACTIVATION AND LOCALIZATION INVOLVING POST-TRANSLATIONAL MODIFICATION AND PROTEIN PARTNERS
eNOS regulation requires multiple effectors that exert exquisite temporal control in concert with distinct spatial subcellular localization. The best-characterized target for eNOS-derived NO in the vascular wall is soluble guanylate cyclase in vascular smooth muscle cells and platelets. The recognition of endogenous endothelial S-nitrosoproteins [2] has led to a renewed focus on intracellular as well as extracellular targets for eNOS-derived NO, and has provided a new perspective on the signaling consequences of eNOS translocation. The recent identification of eNOS itself as a target for eNOS-derived NO in endothelial cells [37]— and the recognition that eNOS localization fundamentally regulates eNOS S-nitrosylation [38] — necessarily broadens the range of possible roles for eNOS as the enzyme undergoes intracellular translocation in response to extracellular signals.
Our understanding of the fine points of eNOS regulation remains limited; for example, there are difficulties in extrapolating findings from a particular experimental in vitro system or assay to the in vivo setting, and signaling pathways may vary among different mammals and in cells from different vascular beds (e.g. BAEC v. HUVEC). This section will connect extracellular signals to the regulation of co- and post-translational modifications of acylation and phosphorylation, calcium transients, and eNOS protein partners in order to develop a robustly dynamic model of eNOS activation/deactivation cycles and localization.
The eNOS protein life cycle begins with its co-translational myristoylation and subsequent dual palmitoylation, which together establish the membrane attachment of eNOS [6] [Figure 3]. A significant majority of the total cellular eNOS pool remains sequestered in intracellular membranes, though caveolar localization is required for agonist-mediated stimulation. The triggers and mechanisms of the reversible traffic of eNOS from peripheral to internal membranes are incompletely understood. Recent discoveries of new eNOS-associated proteins and of the dynamic reversible targeting of these proteins raise the possibility that a vesicular pathway is used and that NOSIP and/or the ternary complex of NOSTRIN-eNOS-caveolin-1 might function in achieving some dynamic equilibrium of anterograde and retrograde transport of eNOS and caveolin-1.
In quiescent endothelium and cardiomyocytes, the myristoylated and palmitoylated eNOS is caveolae bound and becomes tonically inhibited both by virtue of its binding to caveolin-1 (to the exclusion of calmodulin), as well as by eNOS S-nitrosylation [Figure 3]. Low amounts of hsp90 appear to be associated with caveolar-localized eNOS in the quiescent state. MAPK and PKC may also exert basal inhibitory effects. A basal level of NO production is maintained via shear stress (via PI3K/Akt) and agonist-mediated calcium transients.
Despite the inhibitory association with caveolin, caveolar targeting appears to be important for eNOS activation by clustering receptors and effector proteins downstream of numerous eNOS agonists, and this localization seems to be required for efficient agonist-mediated NO release. Dynamic activation of eNOS occurs with a rapid phase of increased enzymatic catalysis that is followed by relatively slower mechanisms of inactivation and eNOS internalization. This kinetic pattern presumably helps the endothelial cell quickly respond to stimuli and then eventually return NO output to baseline. Over a longer period of time, eNOS is returned to the caveolar membrane and becomes primed for subsequent agonist stimuli [Figure 3].
Agonist-stimulated intracellular calcium transients that lead to eNOS-calmodulin binding might represent some of the initial rapid steps in eNOS activation, and may be required for further activation of eNOS by other agonist mediated pathways like phosphorylation. Calcium transients are released from endoplasmic reticulum in an IP3-dependent mechanism as part of PLC signaling. Association of eNOS and calmodulin may be facilitated by dephosphorylation of eNOS at Thr 495 [31]. Calcium and calmodulin work together with hsp90 to displace caveolin from eNOS to release its tonic inhibition [Figure 3]. Certain agonists like VEGF and S1P simultaneously stimulate both calcium dependent mechanisms as well as PI3K/Akt dependent signaling [67], while other agonists like insulin, insulin-like growth factors, estrogen, and shear stress work in both calcium-dependent and calcium-independent mechanisms and may only stimulate a particular PI3K isoform [26,37,69,90]. eNOS-calmodulin/hsp90 appears to recruit PI3K-stimulated Akt to an oligomeric protein complex that phosphorylates Ser 1177; together with calmodulin binding, phosphorylation of Ser 1177 enhances the eNOS catalytic rate as a result of increased electron flux [91] [Figure 3]. Although agonists like VEGF promote both calcium and phosphorylation-dependent activation of eNOS, there is a definitive temporal sequence where calcium-dependent activation precedes phosphorylation-dependent activation; this switch is affected in part by the recruitment of hsp90 to eNOS [94]. In general, it appears that simultaneous phosphorylation of Ser 1177 and dephosphorylation of Thr 495 help coordinate maximal early activation of eNOS, although the roles of complementary phosphorylation pathways such as MAPK/ERK remain to be defined [131]. The time course of agonist-induced eNOS denitrosylation parallels that of Ser 1177 phosphorylation, though these modifications do not appear to be interdependent [37].
After a period of agonist stimulation and eNOS activation, eNOS inactivation is partly regulated by retrograde redistribution of eNOS from caveolae to subcellular membranes [6 [Figure 3].]. Depalmitoylation seems to facilitate cytosolic translocation of eNOS from caveolae to intracellular compartments including the Golgi, perinuclear region, mitochondria, and the cytoskeleton [6,132]. Calmodulin binding by active eNOS increases the suitability of eNOS as a substrate for acyl-protein thioesterase-1 mediated depalmitoylation [15]. However, if retrograde transport is an endocytic mechanism, for example a process mediated by NOSTRIN and/or NOSIP, eNOS would remain membrane bound during transport and it is thus unclear what role depalmitoylation would play. Once caveolin-1 is displaced by calmodulin, NOSIP is free to bind and may exert some inhibitory role on eNOS. The trimeric NOSTRIN complex could recruit the key mediators of vesicle fission and actin polymerization to drive caveolar endocytosis. It is possible that different types of agonists and antagonists of eNOS could drive different translocation patterns: for example in ECV-304 endothelial cells acetylcholine promoted translocation to the Golgi while platelet-activating factor, which seems to counteractsvasculoprotective effects, mediated translocation to the cytosol [133].
During inward translocation, eNOS is inactivated through multiple mechanisms. eNOS becomes distant from calcium waves at the sarcolemmal membrane and therefore there is insufficient calcium-calmodulin stimulation [134]. On the other hand, inhibitory phosphorylation (e.g. Ser 116) and dephosphorylation at stimulatory sites [35, 135] may be calcium-calmodulin dependent [Figure 3]. It is also possible that translocation is required for enzyme denitrosylation, possibly related to the reducing environment of the cell interior [38]. There is however a report of caveolar internalization causing activation of eNOS [136]; since denitrosylation leads to eNOS activation, and since eNOS denitrosylation is enhanced in the cell interior, it is plausible that translocation of the enzyme to internal membranes may still support NO generation, possibly to modulate intracellular targets such as N-ethylmaleimide-sensitive factor and other nitrosoproteins involved in regulation of endothelial cell secretion [137,138]. Indeed, if oxidative stress attenuates the highly reducing intracellular environment — manifest as decreases in the ratio of reduced to oxidized glutathione or thioredoxin — then this could lead to a suppression of eNOS denitrosylation pathways and lead ultimately to decreases in enzyme activity.
The endpoint of retrograde transport appears to deposit eNOS primarily to subplasmalemmal vesicles in the perinuclear region of the cell, where a significant fraction of eNOS resides. The ongoing cycling of eNOS between plasmalemmal and internal membranes appears to be rather rapid, and quite plausibly is required for optimal eNOS bioactivity. On one hand, translocation of eNOS from the plasmalemma may exert a major regulatory function by decoupling eNOS from upstream signaling pathways and activating molecules [139]. However, fluorescent resonance energy transfer (FRET) imaging methods have revealed that eNOS agonists appear to contemporaneously activate eNOS localized at both intracellular and peripheral membranes [139]. Moreover, some studies have suggested that caveolar localized eNOS is continuously active due to constitutive Ser 1177 phosphorylation, and postulate that translocation is the major method of eNOS activity regulation (instead of solely functioning in the deactivation cycle) [134]. The fact that eNOS is differentially phosphorylated depending on its subcellular targeting [70] may provide another indication that eNOS translocation may have more subtle consequences than merely the activation and deactivation of the enzyme— and may in fact lead to the selective S-nitrosylation of endothelial proteins [131] and thereby alter endothelial function.
The eNOS localized to subplasmalemmal vesicles, while not only primed for anterograde transport back to the plasmalemma, has been also considered a reservoir of intracellular eNOS production. The subplasmalemmal population of eNOS has been shown to be activated by calcium/calmodulin and Akt [134,139], and this arrangement has been confirmed in living cells by FRET analysis of eNOS and calmodulin fluorophores FRET [140]. Roles of intracellular NO generated by the subplasmalemmal pool of eNOS remain speculative. Because FRET imaging studies show that the kinetics of association of calmodulin and eNOS are similar regardless of whether occurring at the plasmalemma or subplasmalemmal locations [140], targets of intracellular NO like guanylate cyclase might be involved in dynamic downstream regulation [1,2]. Alternatively, intracellular NO might be more suited to longer term regulation strategies, for example by modification of targets such as mitochondrial iron-sulfur cluster enzymes that participate in oxidative phosphorylation, or by (trans)nitrosylation-mediated regulation of signalling proteins like NSF, transcription factors (fos, jun), cholesterol metabolism (HMG-CoA synthase), enzymes that affect cellular redox state (glutathione, catalase), enzymes that affect energy transduction (malate dehydrogenase, creatine kinase, glyceraldehyde-3-phosphaste dehydrogenase), and cytoskeletal proteins (tubulin, actin) [2,138]. One might speculate whether NOSIP has a special role in regulating the intracellular eNOS pool given both the nucleocytoplasmic dependence of NOSIP and the theorized role of NOSIP in preventing ROS mediated DNA damage in G2. Multiple pathways of regulation might impact intracellular regulation, as for example shear stress has been demonstrated to not only increase total cellular eNOS mRNA, but also the relative fraction of eNOS protein bound to the subplasmalemmal vesicles as compared to plasmalemma [141].
The enzymatic steps facilitating re-palmitoylation of subplasmalemmal vesicular-bound eNOS remain incompletely characterized, although palmitoylation is an intriguing mechanism for the control of anterograde vesicular transport to the plasmalemma, and also consistent with experimental observations that document caveolar relocalization after cytoplasmic translocation following agonist stimulation. Caveolar retargeting is required for reassociation with caveolin and S-nitrosylation (showing again the subcellular dependence of nitrosylation and denitrosylation), which resets the system [Figure 3]. The fact that re-palmitoylation and renitrosylation occur gradually (and both on a slower scale than depalmitoylation and denitrosylation, respectively [142]) and only once eNOS is returned to the caveolar membrane and docked with caveolin suggest that it may be one of the final steps in return of eNOS to its basal plasmalemmal state [2,37].
Further genetic, biochemical, pharmacologic, and proteomic studies will help to unify these diverse models for eNOS regulationby post-translational modifications, subcellular localization, and dynamic transport directed by the concert of eNOS agonists. Our current knowledge of eNOS partners and pathways is largely based on analyses performed in endothelial cells, and the modulation of eNOS in cardiac myocytes and other non-endothelial cells remains less completely characterized. Moreover, analyses performed in cultured cell systems must be validated in in vivo models in order to more fully delineate the roles of eNOS partners and pathways in normal physiology and in disease states. Such studies may permit the synthesis of a comprehensive model for the cellular regulation of eNOS, will enhance our understanding of the perturbations in eNOS signaling that are seen in cardiovascular diseasestates, and may lead to the identification of novel targets for pharmacological intervention.
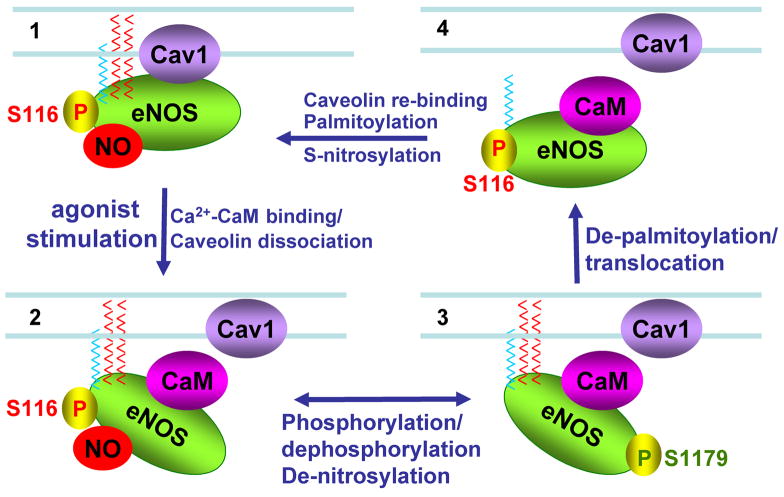
Dynamic relationship between key eNOS post-translational modifications.
This figure demonstrates some of the interelationships of caveolin binding, calmodulin binding, acylation, and phosphorylation on eNOS activity. Panel 1 shows quiescent eNOS anchored to caveolae by myristoylation and palmitoylation (blue and red chains respectively); in this state eNOS is inhibited by caveolin binding, inhibitory phosphorylation at serine 116, and nitrosylation at cysteines 96 and 101. Panel 2 shows displacement of caveolin binding by calcium-calmodulin. Denitrosylation plus stimulatory phosphorylation at serine 1179 enhances enzyme activity in panel 3, accompanied by hsp90 binding. After prolonged agonist stimulation, eNOS becomes depalmitoylated and translocates from peripheral to internal membranes. eNOS is inactivated by calmodulin dissociation allowing re-binding of caveolin, accompanied by re-nitrosylation of the enzyme and dephosphorylation of eNOS at its stimulatory phosphorylation sites. See text for details.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
David Dudzinski, Department of Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA.
Thomas Michel, Department of Medicine, Cardiovascular Division, Brigham and Women’s Hospital, Harvard Medical School, 75 Francis Street, Boston, MA, 02115, p: (617) 732-7376, f: (617) 732-5132, e: tmichel@rics.bwh.harvard.edu.
References
- 1.Dudzinski DM, Michel T. The Vascular Biology of Nitric Oxide and Nitric Oxide Synthases. In: Colman RW, George JN, Goldhaber SZ, Marder VJ, Clowes AW, editors. Hemostasis and Thrombosis: Basic Principles and Clinical Practice. 5. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 653–66. [Google Scholar]
- 2.Dudzinski DM, Igarashi J, Greif DM, Michel T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu Rev Pharmacol Toxicol. 2006;46:235–76. doi: 10.1146/annurev.pharmtox.44.101802.121844. [DOI] [PubMed] [Google Scholar]
- 3.Lowenstein CJ, Michel T. What’s in a name? eNOS and anaphylactic shock. J Clin Invest. 2006;116:2075–8. doi: 10.1172/JCI29406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleinbongard P, Schulz R, Rassaf T, Lauer T, Dejam A, Jax T, et al. Red blood cells express a functional endothelial nitric oxide synthase. Blood. 2006;107:2943–51. doi: 10.1182/blood-2005-10-3992. [DOI] [PubMed] [Google Scholar]
- 5.de Frutos T, Sánchez de Miguel L, Farré J, Gómez J, Romero J, Marcos-Alberca P, et al. Expression of an endothelial-type nitric oxide synthase isoform in human neutrophils: modification by tumor necrosis factor-alpha and during myocardial infarction. J Am Coll Cardiol. 2001;37:800–7. doi: 10.1016/s0735-1097(00)01185-2. [DOI] [PubMed] [Google Scholar]
- 6.Govers R, Rabelink TJ. Cellular regulation of endothelial nitric oxide synthase. Am J Physiol Renal Physiol. 2001;280:F193–206. doi: 10.1152/ajprenal.2001.280.2.F193. [DOI] [PubMed] [Google Scholar]
- 7.Brown DA, London E. Structure of detergent-resistant membrane domains: does phase separation occur in biological membranes? Biochem Biophys Res Commun. 1997;240:1–7. doi: 10.1006/bbrc.1997.7575. [DOI] [PubMed] [Google Scholar]
- 8.Shaul PW, Anderson RG. Role of plasmalemmal caveolae in signal transduction. Am J Physiol Lung Cell Mol Physiol. 1998;275:L843–51. doi: 10.1152/ajplung.1998.275.5.L843. [DOI] [PubMed] [Google Scholar]
- 9.Shaul PW, Smart EJ, Robinson LJ, German Z, Yuhanna IS, Ying Y, et al. Acylation targets endothelial nitric-oxide synthase to plasmalemmal caveolae. J Biol Chem. 1996;271:6518–22. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- 10.Shaul PW. Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol. 2002;64:749–74. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- 11.Prabhakar P, Cheng V, Michel T. A chimeric transmembrane domain directs endothelial nitric-oxide synthase palmitoylation and targeting to plasmalemmal caveolae. J Biol Chem. 2000;275:19416–21. doi: 10.1074/jbc.M001952200. [DOI] [PubMed] [Google Scholar]
- 12.Fukata M, Fukata Y, Adesnik H, Nicoll RA, Bredt DS. Identification of PSD-95 palmitoylating enzymes. Neuron. 2004;44:987–96. doi: 10.1016/j.neuron.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 13.El-Husseini Ael-D, Bredt DS. Protein palmitoylation: a regulator of neuronal development and function. Nat Rev Neurosci. 2002;3:791–802. doi: 10.1038/nrn940. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Hernando C, Fukata M, Bernachtez PN, Fukata Y, Lin MI, Bredt DS, et al. Identification of Golgi-localized acyl transferases that palmitoylate and regulate endothelial nitric oxide synthase. J Cell Biol. 2006;174:369–77. doi: 10.1083/jcb.200601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeh DC, Duncan JA, Yamashita S, Michel T. Depalmitoylation of endothelial nitric-oxide synthase by acyl-protein thioesterase 1 is potentiated by Ca2+-calmodulin. J Biol Chem. 1999;274:33148–33154. doi: 10.1074/jbc.274.46.33148. [DOI] [PubMed] [Google Scholar]
- 16.Duncan JA, Gilman AG. Characterization of Saccharomyces cerevisiae acyl-protein thioesterase 1, the enzyme responsible for G protein αsubunit deacylation in vivo. J Biol Chem. 2002;277:31740–52. doi: 10.1074/jbc.M202505200. [DOI] [PubMed] [Google Scholar]
- 17.Michel JB, Feron O, Sase K, Prabhakar P, Michel T. Caveolin versus calmodulin. J Biol Chem. 1997;272:25907–12. doi: 10.1074/jbc.272.41.25907. [DOI] [PubMed] [Google Scholar]
- 18.Chen PF, Wu KK. Characterization of the roles of the 594–645 region in human endothelial nitric-oxide synthase in regulating calmoduling binding and electron transfer. J Biol Chem. 2000;275:13155–63. doi: 10.1074/jbc.275.17.13155. [DOI] [PubMed] [Google Scholar]
- 19.Greif DM, Sacks DB, Michel T. Calmodulin phosphorylation and modulation of endothelial nitric oxide synthase catalysis. Proc Natl Acad Sci USA. 2004;101:1165–70. doi: 10.1073/pnas.0306377101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tran Q-K, Black DJ, Persechini A. Intracellular coupling via limiting calmodulin. J Biol Chem. 2003;278:24247–50. doi: 10.1074/jbc.C300165200. [DOI] [PubMed] [Google Scholar]
- 21.Goetz RM, Thatte HS, Prabhakar P, Cho MR, Michel T, Golan DE. Estradiol induces the calcium dependent translocation of endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 1999;96:2788–93. doi: 10.1073/pnas.96.6.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loscalzo J, Welch G. Nitric oxide and its role in the cardiovascular system. Prog Cardiovasc Dis. 1995;38:87–104. doi: 10.1016/s0033-0620(05)80001-5. [DOI] [PubMed] [Google Scholar]
- 23.Fujimoto T, Nakade S, Miyawaki A, Mikoshiba K, Ogawa K. Localization of inositol 1,4,5-triphosphate receptor-like protein in plasmalemmal caveolae. J Cell Biol. 1992;119:1507–13. doi: 10.1083/jcb.119.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauer PM, Fulton D, Boo YC, Sorescu GP, Kemp BE, Jo H, et al. Compensatory phosphorylation and protein-protein interactions revealed by loss of function and gain of function mutants of multiple serine phosphorylation sites in endothelial nitric oxide synthase. J Biol Chem. 2003;278:14841–9. doi: 10.1074/jbc.M211926200. [DOI] [PubMed] [Google Scholar]
- 25.McCabe TJ, Fulton D, Roman LJ, Sessa W. Enhanced electron flux and reduced calmodulin dissociation may explain “calcium-independent” eNOS activation by phosphorylation. J Biol Chem. 2000;275:6123–8. doi: 10.1074/jbc.275.9.6123. [DOI] [PubMed] [Google Scholar]
- 26.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–5. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 27.Gallis B, Corthals GL, Goodlett DR, Ueba H, Kim F, Presnell SR, et al. Identification of flow-dependent endothelial nitric-oxide synthase phosphorylation sites by mass spectrometry and regulation of phosphorylation and nitric oxide production by the phosphatidylinositol 3-kinase inhibitor LY294002. J Biol Chem. 1999;274:30101–8. doi: 10.1074/jbc.274.42.30101. [DOI] [PubMed] [Google Scholar]
- 28.Fulton D, Gratton J-P, Sessa WC. Post-translational control of endothelial nitric oxide synthase: Why isn’t calcium/calmodulin enough? J Pharmacol Exp Ther. 2001;299:818–24. [PubMed] [Google Scholar]
- 29.Michell BJ, Chen ZP, Tiganis T, Stapleton D, Katsis F, Power DA, et al. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem. 2001;276:17625–8. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- 30.Chen ZP, Mitchellhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, et al. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–9. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- 31.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr495 regulates Ca+2/calmodulindependent endothelial nitric oxide synthase activity. Circ Res. 2001;88:E68–75. doi: 10.1161/hh1101.092677. [DOI] [PubMed] [Google Scholar]
- 32.Michell BJ, Harris MB, Chen ZP, Ju H, Venema VJ, Blackstone MA, et al. Identification of regulatory sites of phosphorylation of the bovine endothelial nitric-oxide synthase at serine 617 and serine 635. J Biol Chem. 2002;277:42344–51. doi: 10.1074/jbc.M205144200. [DOI] [PubMed] [Google Scholar]
- 33.Boo YC, Hwang J, Sykes M, Michell BJ, Kemp BE, Lum H, et al. Shear stress stimulates phosphorylation of eNOS at Ser635 by a protein kinase A-dependent mechanism. Am J Physiol Heart Circ Physiol. 2002;283:H1819–28. doi: 10.1152/ajpheart.00214.2002. [DOI] [PubMed] [Google Scholar]
- 34.Lin MI, Fulton D, Babbitt R, Fleming I, Busse R, Pritchard KA, Jr, et al. Phosphorylation of threonine 495 in endothelial nitric-oxide synthase coordinates the coupling of L-arginine metabolism to efficient nitric oxide production. J Biol Chem. 2003;278:44719–26. doi: 10.1074/jbc.M302836200. [DOI] [PubMed] [Google Scholar]
- 35.Kou R, Greif D, Michel T. Dephosphorylation of endothelial nitric-oxide synthase by vascular endothelial growth factor. Implications for the vascular responses to cyclosporin A. J Biol Chem. 2002;277:29669–73. doi: 10.1074/jbc.M204519200. [DOI] [PubMed] [Google Scholar]
- 36.Fulton D, Church JE, Ruan L, Li C, Sood SG, Kemp BE, et al. Src kinase activates endothelial nitric-oxide synthase by phosphorylating Tyr 83. J Biol Chem. 2005;43:35943–52. doi: 10.1074/jbc.M504606200. [DOI] [PubMed] [Google Scholar]
- 37.Erwin PA, Lin AJ, Golan DE, Michel T. Receptor-regulated dynamic S-nitrosylation of endothelial nitric-oxide synthase in vascular endothelial cells. J Biol Chem. 2005;280:19888–94. doi: 10.1074/jbc.M413058200. [DOI] [PubMed] [Google Scholar]
- 38.Erwin PA, Mitchell DA, Sartoretto J, Marletta MA, Michel T. Subcellular targeting and differential S-nitrosylation of endothelial nitric-oxide synthase. J Biol Chem. 2006;281:151–7. doi: 10.1074/jbc.M510421200. [DOI] [PubMed] [Google Scholar]
- 39.Ravi K, Brennan LA, Levic S, Ross PA, Black SM. S-nitrosylation of endothelial nitric oxide synthase is associated with monomerization and decreased enzyme activity. Proc Natl Acad Sci USA. 2004;101:2619–24. doi: 10.1073/pnas.0300464101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–66. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 41.Liu X, Miller MJ, Joshi MS, Thomas DD, Lancaster JR., Jr Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of membranes. Proc Natl Acad Sci USA. 1998;95:2175–9. doi: 10.1073/pnas.95.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H, Raman CS, Glaser CB, Blasko E, Young TA, Parkinson JF, et al. Crystal structures of zinc-free and -bound heme domain of human inducible nitric-oxide synthase. Implications for dimer stability and comparison with endothelial nitric-oxide synthase. J Biol Chem. 1999;274:21276–84. doi: 10.1074/jbc.274.30.21276. [DOI] [PubMed] [Google Scholar]
- 43.Xie QW, Leung M, Fuortes M, Sassa S, Nathan C. Complementation analysis of mutants of nitric oxide synthase reveals that the active site requires two hemes. Proc Natl Acad Sci USA. 1996;93:4891–6. doi: 10.1073/pnas.93.10.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parat M-L, Fox PL. Palmitoylation of caveolin-1 in endothelial cells is post-translational but irreversible. J Biol Chem. 2001;276:15776–82. doi: 10.1074/jbc.M006722200. [DOI] [PubMed] [Google Scholar]
- 45.Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–8. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 46.Li S, Song KS, Lisanti MP. Expression and characterization of recombinant caveolin. J Biol Chem. 1996;271:568–73. [PubMed] [Google Scholar]
- 47.Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, et al. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science. 2001;293:2449–52. doi: 10.1126/science.1062688. [DOI] [PubMed] [Google Scholar]
- 48.Feron O, Balligand JL. Caveolins and the regulation of endothelial nitric oxide synthase in the heart. Cardiovasc Res. 2006;69:788–97. doi: 10.1016/j.cardiores.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 49.Head BP, Insel PA. Do caveolins regulate cells by actions outside of caveolae? Trends Cell Biol. 2007;17:51–7. doi: 10.1016/j.tcb.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 50.Parton RG, Hanzal-Bayer M, Hancock JF. Biogenesis of caveolae: a structural model for caveolin-induced domain formation. J Cell Sci. 2006;119:787–96. doi: 10.1242/jcs.02853. [DOI] [PubMed] [Google Scholar]
- 51.Schwartz EA, Reaven E, Topper JN, Tsao PS. Transforming growth factor-β receptors localize to caveolae and regulate endothelial nitric oxide synthase in normal human endothelial cells. Biochem J. 2005;390:199–206. doi: 10.1042/BJ20041182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams TM, Lisanti MP. The caveolin genes: from cell biology to medicine. Ann Med. 2004;36:584–95. doi: 10.1080/07853890410018899. [DOI] [PubMed] [Google Scholar]
- 53.Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol. 2005;288:C494–506. doi: 10.1152/ajpcell.00458.2004. [DOI] [PubMed] [Google Scholar]
- 54.Arthur HM, Ure J, Smith AJ, Renforth G, Wilson DI, Torsney E, et al. Endoglin, an ancillary TGFβ receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev Biol. 2000;217:42–53. doi: 10.1006/dbio.1999.9534. [DOI] [PubMed] [Google Scholar]
- 55.Toporsian M, Gros R, Kabir MG, Vera S, Govindaraju K, Eidelman DH, et al. A role for endoglin in coupling eNOS activity and regulating vascular tone revealed in hereditary hemorrhagic telangiectasia. Circ Res. 2005;96:684–92. doi: 10.1161/01.RES.0000159936.38601.22. [DOI] [PubMed] [Google Scholar]
- 56.Sorensen LK, Brooke BS, Li DY, Urness LD. Loss of distinct arterial and venous boundaries in mice lacking endoglin, a vascular-specific TGFβ coreceptor. Dev Biol. 2003;261:235–50. doi: 10.1016/s0012-1606(03)00158-1. [DOI] [PubMed] [Google Scholar]
- 57.Jerkic M, Rivas-Elena JV, Prieto M, Carron R, Sanz-Rodriguez F, Perez-Barriocanal F, et al. Endoglin regulates nitric oxide-dependent vasodilatation. FASEB J. 2004;18:609–11. doi: 10.1096/fj.03-0197fje. [DOI] [PubMed] [Google Scholar]
- 58.Isshiki M, Ando J, Korenaga R, Kogo H, Fujimoto T, Fujita T, et al. Endothelial Ca2+ waves preferentially originate at specific loci in caveolin-rich cell edges. Proc Natl Acad Sci USA. 1998;95:5009–14. doi: 10.1073/pnas.95.9.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gonzalez E, Kou R, Michel T. Rac1 modulates sphingosine 1-phosphate-mediated activation of phosphoinositides 3-kinase/Akt signaling pathways in vascular endothelial cells. J Biol Chem. 2006;281:3210–6. doi: 10.1074/jbc.M510434200. [DOI] [PubMed] [Google Scholar]
- 60.Eto M, Barandier C, Rathgeb L, Kozai T, Joch H, Yang Z, et al. Thrombin suppresses endothelial nitric oxide synthase and upregulates endothelin-converting enzyme-1 expression by distinct pathways: role of Rho/ROCK and mitogen-activated protein kinase. Circ Res. 2001;89:583–90. doi: 10.1161/hh1901.097084. [DOI] [PubMed] [Google Scholar]
- 61.Ming XF, Viswambharan H, Barandier C, Ruffieux J, Kaibuchi K, Rusconi S, et al. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol. 2002;22:8467–77. doi: 10.1128/MCB.22.24.8467-8477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li H, Junk P, Huwiler A, Burkhardt C, Wallerath T, Pfeilschifter J, et al. Dual effect of ceramide on human endothelial cells: induction of oxidative stress and transcriptional upregulation of endothelial nitric oxide synthase. Circulation. 2002;106:2250–66. doi: 10.1161/01.cir.0000035650.05921.50. [DOI] [PubMed] [Google Scholar]
- 63.Ishii I, Fukushima N, Ye X, Chun J. Lysophospholipid receptors: Signaling and biology. Annu Rev Biochem. 2004;73:321–54. doi: 10.1146/annurev.biochem.73.011303.073731. [DOI] [PubMed] [Google Scholar]
- 64.Igarashi J, Michel T. Agonist-modulated targeting of the EDG-1 receptor to plasmalemmal caveolae. J Biol Chem. 2000;275:32363–70. doi: 10.1074/jbc.M003075200. [DOI] [PubMed] [Google Scholar]
- 65.Hla T, Lee MJ, Ancellin N, Paik JH, Kluk MJ. Lysophospholipids—receptor revelations. Science. 2001;294:1875–8. doi: 10.1126/science.1065323. [DOI] [PubMed] [Google Scholar]
- 66.Kou R, Igarashi J, Michel T. Lysophosphatidic acid and receptor-mediated activation of endothelial nitric-oxide synthase. Biochemistry. 2002;41:4982–8. doi: 10.1021/bi016017r. [DOI] [PubMed] [Google Scholar]
- 67.Igarashi J, Michel T. Sphingosine-1-phosphate and isoform-specific activation of phosphoinositide 3-kinase β. J Biol Chem. 2001;276:36281–88. doi: 10.1074/jbc.M105628200. [DOI] [PubMed] [Google Scholar]
- 68.Murga C, Fukuhara S, Gutkind JS. A novel role for phosphatidylinositol 3-kinase β in signaling from G protein coupled receptors to Akt. J Biol Chem. 2000;275:12069–73. doi: 10.1074/jbc.275.16.12069. [DOI] [PubMed] [Google Scholar]
- 69.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gonzalez E, Kou R, Lin AJ, Golan DE, Michel T. Subcellular targeting and agonist-induced site-specific phosphorylation of endothelial nitric-oxide synthase. J Biol Chem. 2002;277:39554–60. doi: 10.1074/jbc.M207299200. [DOI] [PubMed] [Google Scholar]
- 71.Vanhaesebroeck B, Leevers SJ, Panayotou G, Waterfield MD. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci. 1997;22:267–72. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- 72.He H, Venema VJ, Gu X, Venema RC, Marrero MB, Caldwell RB. Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. J Biol Chem. 1999;274:25130–5. doi: 10.1074/jbc.274.35.25130. [DOI] [PubMed] [Google Scholar]
- 73.Igarashi J, Erwin PA, Dantas APV, Chen H, Michel T. VEGF induces S1P receptors in endothelial cells: implications for cross-talk between sphingolipid and growth factor receptors. Proc Natl Acad Sci USA. 2003;100:10664–9. doi: 10.1073/pnas.1934494100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Steinberg HO, Brechtel G, Johnson A, Fineberg N, Baron AD. Insulin mediated skeletal muscle vasodilation is nitric oxide dependent. A novel action of insulin to increase nitric oxide release. J Clin Invest. 1994;94:1172–9. doi: 10.1172/JCI117433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zeng G, Quon MJ. Insulin stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J Clin Invest. 1996;98:894–8. doi: 10.1172/JCI118871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zeng G, Nystrom FH, Ravichandran LV, Cong LN, Kirby M, Mostowski M, et al. Roles for insulin receptor, PI3-kinase, and Akt in insulin-signaling pathways related to production of nitric oxide in human vascular endothelial cells. Circulation. 2000;101:1539–45. doi: 10.1161/01.cir.101.13.1539. [DOI] [PubMed] [Google Scholar]
- 77.Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS in independent of Ca2+ but requires phosphorylation by Akt at Ser1179. J Biol Chem. 2001;276:30392–98. doi: 10.1074/jbc.M103702200. [DOI] [PubMed] [Google Scholar]
- 78.Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Invest. 2001;108:1341–8. doi: 10.1172/JCI11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salt IP, Morrow VA, Brandie FM, Connell JM, Petrie JR. High glucose inhibits insulin-stimulated nitric oxide production without reducing endothelial nitric oxide synthase Ser1177 phosphorylation in human aortic endothelial cells. J Biol Chem. 2003;278:18791–7. doi: 10.1074/jbc.M210618200. [DOI] [PubMed] [Google Scholar]
- 80.Caulin-Glaser T, Garcia-Cardena G, Sarrel P, Sessa WC, Bender JR. 17β-estradiol regulation of human endothelial cell basal nitric oxide release, independent of cytosolic Ca2+ mobilization. Circ Res. 1997;81:885–92. doi: 10.1161/01.res.81.5.885. [DOI] [PubMed] [Google Scholar]
- 81.Guo X, Razandi M, Pedram A, Kassab G, Levin ER. Estrogen induces vascular wall dilation: mediation through kinase signaling to nitric oxide and estrogen receptors α and β. J Biol Chem. 2005;280:19704–10. doi: 10.1074/jbc.M501244200. [DOI] [PubMed] [Google Scholar]
- 82.Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH-kinase. Nature. 2000;407:538–41. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. Estrogen receptor α mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J Clin Invest. 1999;103:401–6. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Venema VJ, Marrero MB, Venema RC. Bradykinin-stimulated protein tyrosine phosphorylation promotes endothelial nitric oxide synthase translocation to the cytoskeleton. Biochem Biophys Res Commun. 1996;226:703–10. doi: 10.1006/bbrc.1996.1417. [DOI] [PubMed] [Google Scholar]
- 85.Harris MB, Ju H, Venema VJ, Blackstone M, Venema RC. Role of heat shock protein 90 in bradykinin-stimulated endothelial nitric oxide release. Gen Pharmacol. 2000;35:165–70. doi: 10.1016/s0306-3623(01)00104-5. [DOI] [PubMed] [Google Scholar]
- 86.Garcia-Cardena G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, et al. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature. 1998;392:821–4. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- 87.Pritchard KA, Jr, Ackerman AW, Gross ER, Stepp DW, Shi Y, Fontana JT, et al. Heat shock protein 90 mediates the balance of nitric oxide and superoxide anion from endothelial nitric-oxide synthase. J Biol Chem. 2001;276:17621–4. doi: 10.1074/jbc.C100084200. [DOI] [PubMed] [Google Scholar]
- 88.Bender AT, Silverstein AM, Demady DR, Kanelakis KC, Noguchi S, Pratt WB, Osawa Y. Neuronal nitric-oxide synthase is regulated by the Hsp90-based chaperone system in vivo. J Biol Chem. 1999;274:1472–8. doi: 10.1074/jbc.274.3.1472. [DOI] [PubMed] [Google Scholar]
- 89.Takahashi S, Mendelsohn ME. Calmodulin-dependent and -independent activation of endothelial nitric-oxide synthase by heat shock protein 90. J Biol Chem. 2003;278:9339–44. doi: 10.1074/jbc.M212651200. [DOI] [PubMed] [Google Scholar]
- 90.Takahashi S, Mendelsohn ME. Synergistic activation of endothelial nitric-oxide synthase (eNOS) by HSP90 and Akt: calcium-independent eNOS activation involves formation of an HSP90-Akt-CaM-bound eNOS complex. J Biol Chem. 2003;278:30821–7. doi: 10.1074/jbc.M304471200. [DOI] [PubMed] [Google Scholar]
- 91.Fontana J, Fulton D, Chen Y, Fairchild TA, McCabe TJ, Fujita N, et al. Domain mapping studies reveal that the M domain of hsp90 serves as a molecular scaffold to regulate Akt-dependent phosphorylation of endothelial nitric oxide synthase and NO release. Circ Res. 2002;90:866–73. doi: 10.1161/01.res.0000016837.26733.be. [DOI] [PubMed] [Google Scholar]
- 92.Sato S, Fujita N, Tsuruo T. Modulation of Akt kinase activity by binding to Hsp90. Proc Natl Acad Sci USA. 2000;97:10832–7. doi: 10.1073/pnas.170276797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wei Q, Xia Y. Roles of 3-phosphoinositide-dependent kinase 1 in the regulation of endothelial nitric-oxide synthase phosphorylation and function by heat shock protein 90. J Biol Chem. 2005;280:18081–6. doi: 10.1074/jbc.M413607200. [DOI] [PubMed] [Google Scholar]
- 94.Brouet A, Sonveaux P, Dessy C, Balligand J-L, Feron O. Hsp90 ensures the transition from the early Ca2+-dependent to the late phosphorylation-dependent activation of the endothelial nitric-oxide synthase in vascular endothelial growth factor-exposed endothelial cells. J Biol Chem. 2001;275:32663–9. doi: 10.1074/jbc.M101371200. [DOI] [PubMed] [Google Scholar]
- 95.Harris MB, Bartoli M, Sood SG, Matts RL, Venema RC. Direct interaction of the cell division cycle 37 homolog inhibits endothelial nitric oxide synthase activity. Circ Res. 2006;98:335–41. doi: 10.1161/01.RES.0000203564.54250.0b. [DOI] [PubMed] [Google Scholar]
- 96.Schleicher M, Brundin F, Gross S, Müller-Esterl W, Oess S. Cell cycle-regulated inactivation of endothelial NO synthase through NOSIP-dependent targeting to the cytoskeleton. Mol Cell Biol. 2005;25:8251–8. doi: 10.1128/MCB.25.18.8251-8258.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jiang J, Cyr D, Babbitt RW, Sessa WC, Patterson C. Chaperone-dependent regulation of endothelial nitric-oxide synthase intracellular trafficking by the co-chaperone/ubiquitin ligase CHIP. J Biol Chem. 2003;278:49332–41. doi: 10.1074/jbc.M304738200. [DOI] [PubMed] [Google Scholar]
- 98.Chen JX, Meyrick B. Hypoxia increases Hsp90 binding to eNOS via PI3K-Akt in porcine coronary artery endothelium. Lab Invest. 2004;84:182–90. doi: 10.1038/labinvest.3700027. [DOI] [PubMed] [Google Scholar]
- 99.Kureishi Y, Luo Z, Shiojima I, Bialik A, Fulton D, Lefer DJ, et al. The HMG-CoA reductase inhibitor simvastatin activates the protein kinase Akt and promotes angiogenesis in normocholesterolemic animals. Nat Med. 2000;6:1004–10. doi: 10.1038/79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brouet A, Sonveuax P, Dessy C, Moniotte S, Balligand JL, Feron O. Hsp90 and caveolin are key targets for the proangiogenic nitric oxide-mediated effects of statins. Circ Res. 2001;89:866–73. doi: 10.1161/hh2201.100319. [DOI] [PubMed] [Google Scholar]
- 101.Polikandriotis JA, Mazzella LJ, Rupnow HL, Hart CM. Peroxisome proliferator-activated receptor γ ligands stimulate endothelial nitric oxide production through distinct peroxisome proliferator-activated receptor γdependent mechanisms. Arterioscler Thromb Vasc Biol. 2005;25:1810–6. doi: 10.1161/01.ATV.0000177805.65864.d4. [DOI] [PubMed] [Google Scholar]
- 102.Martínez-Ruiz A, Villanueva L, González de Orduña C, López-Ferrer D, Ángeles Higeuras M, Tarín C, et al. S-nitrosylation of Hsp90 promotes the inhibition of its ATPase and endothelial nitric oxide synthase regulatory activities. Proc Natl Acad Sci USA. 2005;102:8525–30. doi: 10.1073/pnas.0407294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Harris MB, Ju H, Venema VJ, Liang H, Zou R, Michell BJ, et al. Reciprocal phosphorylation and regulation of endothelial nitric-oxide synthase in response to bradykinin stimulation. J Biol Chem. 2001;276:16587–91. doi: 10.1074/jbc.M100229200. [DOI] [PubMed] [Google Scholar]
- 104.Greif DM, Kou R, Michel T. Site-specific dephosphorylation of endothelial nitric oxide synthase by protein phosphatase 2A: evidence for crosstalk between phosphorylation sites. Biochemistry. 2002;41:15845–53. doi: 10.1021/bi026732g. [DOI] [PubMed] [Google Scholar]
- 105.Wei Q, Xia Y. Proteasome inhibition down-regulates endothelial nitric-oxide synthase phosphorylation and function. J Biol Chem. 2006;281:21652–9. doi: 10.1074/jbc.M602105200. [DOI] [PubMed] [Google Scholar]
- 106.Reinstein E, Ciechanover A. Narrative review: protein degradation and human diseases: the ubiquitin connection. Ann Intern Med. 2006;145:676–84. doi: 10.7326/0003-4819-145-9-200611070-00010. [DOI] [PubMed] [Google Scholar]
- 107.Dedio J, Konig P, Wohlfart P, Schroeder C, Kummer W, Müller-Esterl W. NOSIP, a novel modulator of endothelial nitric oxide synthase activity. FASEB J. 2001;15:79–89. doi: 10.1096/fj.00-0078com. [DOI] [PubMed] [Google Scholar]
- 108.Zimmermann K, Opitz N, Dedio J, Müller-Esterl W, Oess S. NOSTRIN: A protein modulating nitric oxide release and subcellular distribution of endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 2002;99:17167–72. doi: 10.1073/pnas.252345399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Su Y, Edwards-Bennett S, Bubb MR, Block ER. Regulation of endothelial nitric oxide synthase by the actin cytoskeleton. Am J Physiol Cell Physiol. 2003;284:C1542–9. doi: 10.1152/ajpcell.00248.2002. [DOI] [PubMed] [Google Scholar]
- 110.Hutcheson IR, Griffith TM. Mechanotransduction through the endothelial cytoskeleton: mediation of flow- but not agonist-induced EDRF release. Br J Pharmacol. 1996;118:720–6. doi: 10.1111/j.1476-5381.1996.tb15459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Conrad PA, Smart EJ, Ying YS, Anderson RG, Bloom GS. Caveolin cycles between plasma membrane caveolae and the Golgi complex by microtubule-dependent and microtubule-independent steps. J Cell Biol. 1995;131:1421–33. doi: 10.1083/jcb.131.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zharikov SI, Sigova AA, Chen S, Bubb MR, Block ER. Cytoskeletal regulation of the L-arginine/NO pathway in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L465–73. doi: 10.1152/ajplung.2001.280.3.L465. [DOI] [PubMed] [Google Scholar]
- 113.Skidgel RA. Proliferation of regulatory mechanisms for eNOS: an emerging role for the cytoskeleton. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1179–82. doi: 10.1152/ajplung.00045.2002. [DOI] [PubMed] [Google Scholar]
- 114.Searles CD, Miwa Y, Harrison DG, Ramasamy S. Posttranscriptional regulation of endothelial nitric oxide synthase during cell growth. Circ Res. 1999;85:588–95. doi: 10.1161/01.res.85.7.588. [DOI] [PubMed] [Google Scholar]
- 115.Laufs U, Endres M, Stagliano N, Amin-Hanjani S, Dao-Shan C, Yang S-X, et al. Neuroprotection mediated by changes in the endothelial actin cytoskeleton. J Clin Invest. 2000;106:15–24. doi: 10.1172/JCI9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Arnal J-F, Yamin J, Dockery S, Harrison DG. Regulation of endothelial nitric oxide synthase mRNA, protein and activity during cell growth. Am J Physiol. 1994;267:C1381–8. doi: 10.1152/ajpcell.1994.267.5.C1381. [DOI] [PubMed] [Google Scholar]
- 117.Searles CD, Ide L, Davis ME, Cai H, Weber M. Actin cytoskeleton organization and posttranscriptional regulation of endothelial nitric oxide synthase during cell growth. Circ Res. 2004;95:488–95. doi: 10.1161/01.RES.0000138953.21377.80. [DOI] [PubMed] [Google Scholar]
- 118.Brandes RP. Statin-mediated inhibition of rho: Only to get more NO? Circ Res. 2005;96:927–9. doi: 10.1161/01.RES.0000168040.70096.2a. [DOI] [PubMed] [Google Scholar]
- 119.Flowers MA, Wang Y, Stewart RJ, Patel B, Marsden PA. Reciprocal regulation of ET-1 and endothelial constitutive NOS in proliferating endothelial cells. Am J Physiol Heart Circ Physiol. 1995;260:H1988–97. doi: 10.1152/ajpheart.1995.269.6.H1988. [DOI] [PubMed] [Google Scholar]
- 120.Friedman AD, Nimbalkar D, Quelle FW. Erythropoietin receptors associate with ubiquitin ligase, p33RUL, and require its activity for erythropoietin-induced proliferation. J Biol Chem. 2003;278:26851–61. doi: 10.1074/jbc.M210039200. [DOI] [PubMed] [Google Scholar]
- 121.Konig P, Dedio J, Oess S, Papadakis T, Fischer A, Müller-Esterl W, et al. NOSIP and its interacting protein, eNOS, in the rat trachea and lung. J Histochem Cytochem. 2005;53:155–64. doi: 10.1369/jhc.4A6453.2005. [DOI] [PubMed] [Google Scholar]
- 122.Konig P, Dedio J, Müller-Esterl W, Kummer W. Distribution of novel eNOS-interacting protein NOSIP in the liver, pancreas, and gastrointestinal tract of the rat. Gastroenterology. 2002;123:314–24. doi: 10.1053/gast.2002.34212. [DOI] [PubMed] [Google Scholar]
- 123.Dreyer J, Schleicher M, Tappe A, Schilling K, Kuner T, Kusumawidijaja G, et al. Nitric oxide synthase (NOS)-interacting protein interacts with neuronal NOS and regulates its distribution and activity. J Neurosci. 2004;24:10454–65. doi: 10.1523/JNEUROSCI.2265-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schilling K, Opitz N, Wiesenthal A, Oess S, Tikkanen R, Müller-Esterl W, et al. Translocation of endothelial nitric-oxide synthase involves a ternary complex with caveolin-1 and NOSTRIN. Mol Biol Cell. 2006;17:3870–80. doi: 10.1091/mbc.E05-08-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Icking A, Schilling A, Wiesenthal A, Opitz N, Müller-Esterl W. FCH/Cdc15 domain determines distinct subcellular localization of NOSTRIN. FEBS Lett. 2006;580:223–8. doi: 10.1016/j.febslet.2005.11.078. [DOI] [PubMed] [Google Scholar]
- 126.Icking A, Matt S, Opitz N, Wiesenthal A, Müller-Esterl W, Schilling K. NOSTRIN functions as a homotrimeric adaptor protein facilitating internalization of eNOS. J Cell Sci. 2005;118:5059–69. doi: 10.1242/jcs.02620. [DOI] [PubMed] [Google Scholar]
- 127.Tsujita K, Suetsugu S, Sasaki N, Furutani M, Oikawa T, Takenawa T. Coordination between the actin cytoskeleton and membrane deformation by a novel membrane tubulation domain of PCH proteins is involved in endocytosis. J Cell Biol. 2006;172:269–79. doi: 10.1083/jcb.200508091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mukhopadhyay S, Xu F, Sehgal PB. Aberrant cytoplasmic sequestration of eNOS in endothelial cells after monocrotaline, hypoxia and senescence: subcellular eNOS localization and live-cell caveolar and cytoplasmic NO imaging studies. Am J Physiol Heart Circ Physiol. 2007;292:H1373–89. doi: 10.1152/ajpheart.00990.2006. [DOI] [PubMed] [Google Scholar]
- 129.Choi YJ, Cho SY, Kim HW, Kim JA, Bae SH, Park SS. Cloning and characterization of mouse disabled 2 interacting protein 2, a mouse orthologue of human NOSTRIN. Biochem Biophys Res Commun. 2005;326:594–9. doi: 10.1016/j.bbrc.2004.11.079. [DOI] [PubMed] [Google Scholar]
- 130.Xiang W, Chen H, Xu X, Zhang M, Jiang R. Expression of endothelial nitric oxide synthase traffic inducer in the placentas of women with pre-eclampsia. Int J Gynaecol Obstet. 2005;89:103–7. doi: 10.1016/j.ijgo.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 131.Bernier SG, Haldar S, Michel T. Bradykinin-regulated interactions of the mitogen-activates protein kinase pathway with the endothelial nitric-oxide synthase. J Biol Chem. 2000;276:30707–15. doi: 10.1074/jbc.M005116200. [DOI] [PubMed] [Google Scholar]
- 132.Prabhakar P, Thatte HS, Goetz RM, Cho MR, Golan DE, Michel T. Receptor-regulated translocation of endothelial nitric-oxide synthase. J Biol Chem. 1998;273:27383–8. doi: 10.1074/jbc.273.42.27383. [DOI] [PubMed] [Google Scholar]
- 133.Sanchez FB, Savalia NB, Duran RG, Lal BK, Boric MP, Duran WN. Functional significance of differential eNOS translocation. Am J Physiol Heart Circ Physiol. 2006;291:H1058–64. doi: 10.1152/ajpheart.00370.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jagnandan D, Sessa WC, Fulton D. Intracellular location regulates calcium-calmodulin-dependent activation of organelle-restricted eNOS. Am J Physiol Cell Physiol. 2005;289:C1024–33. doi: 10.1152/ajpcell.00162.2005. [DOI] [PubMed] [Google Scholar]
- 135.Church JE, Fulton D. Differences in eNOS activity because of subcellular localization are dictated by phosphorylation state rather than the local calcium environment. J Biol Chem. 2006;281:1477–88. doi: 10.1074/jbc.M505968200. [DOI] [PubMed] [Google Scholar]
- 136.Maniatis NA, Brovkovych V, Allen SE, John TA, Shajahan AN, Tiruppathi C, et al. Novel Mechanism of Endothelial Nitric Oxide Synthase Activation Mediated by Caveolae Internalization in Endothelial Cells. Circ Res. 2006;99:870–7. doi: 10.1161/01.RES.0000245187.08026.47. [DOI] [PubMed] [Google Scholar]
- 137.Toporsian M, Gros R, Kabir MG, Vera S, Govindaraju K, Eidelman DH, et al. A role for endoglin in coupling eNOS activity and regulating vascular tone revealed in hereditary hemorrhagic telangiectasia. Circ Res. 2005;96:684–92. doi: 10.1161/01.RES.0000159936.38601.22. [DOI] [PubMed] [Google Scholar]
- 138.Lowenstein CJ, Tsuda H. N-ethylmaleimide-sensitive factor: a redox sensor in exocytosis. Biol Chem. 2006;387:1377–83. doi: 10.1515/BC.2006.173. [DOI] [PubMed] [Google Scholar]
- 139.Zhang Q, Church JE, Jagnandan D, Catravas JD, Sessa WC, Fulton D. Functional relevance of Golgi- and plasma membrane-localized endothelial NO synthase in reconstituted endothelial cells. Arterioscler Thromb Vasc Biol. 2006;26:1015–21. doi: 10.1161/01.ATV.0000216044.49494.c4. [DOI] [PubMed] [Google Scholar]
- 140.Jobin CM, Chen H, Lin AJ, Yacono PW, Igarashi J, Michel T, et al. Receptor-regulated dynamic interaction between endothelial nitric oxide synthase and calmodulin revealed by fluorescence resonance energy transfer in living cells. Biochemistry. 2003;42:11716–25. doi: 10.1021/bi035066w. [DOI] [PubMed] [Google Scholar]
- 141.Cheng C, van Haperen R, de Waard M, van Damme CA, Tempel D, Hanemaaijer L, et al. Shear stress affects the intracellular distribution of eNOS: direct demonstration by a novel in vivo technique. Blood. 2005;106:3691–8. doi: 10.1182/blood-2005-06-2326. [DOI] [PubMed] [Google Scholar]
- 142.Feron O, Saldana F, Michel JB, Michel T. The endothelial nitric-oxide synthase-caveolin regulatory cycle. J Biol Chem. 1998;273:3125–8. doi: 10.1074/jbc.273.6.3125. [DOI] [PubMed] [Google Scholar]