Abstract
There is an urgent need to develop novel therapies for controlling recurrent virus infections in the immune suppressed. Disease associated with persistent gammaherpesvirus infection (EBV, HHV-8) is a significant problem in AIDS patients and transplant recipients, and clinical management of these conditions is difficult. Disease occurs because of a failure in immune surveillance to control the persistent infection, which arises in AIDS patients principally because of an erosion of the CD4+ T cell compartment. Immune surveillance failure followed by gammaherpesvirus recrudescence can be modeled using murine gammaherpesvirus in CD4 T cell depleted mice. We show that enhancement of IL-2 signaling using IL-2/anti-IL-2 immune complexes substantially improves immune surveillance in the context of suppressed immunity, and enhances control of the infection. This effect was not due solely to increased numbers of virus-specific CD8 T cells, but rather to enhanced cytotoxicity, mediated by the perforin-granzyme pathway.
Keywords: T cell help, Memory, Viral
Introduction
Herpesviruses such as Epstein-Barr virus and human cytomegalovirus infect the majority of the human population. Persistent infection of B lymphocytes with Epstein Barr virus is believed to be controlled mainly by CD8+ T cells and patients are generally asymptomatic into advanced age (1). However, immune suppressed patients, such as those undergoing transplantation, can develop EBV-associated lymphoproliferative disease. Additionally, the progression of HIV infection to AIDS can be accompanied by γ-herpesvirus-associated lymphomas (2). The development of AIDS is concomitant with declining CD4+ T cell numbers and both human and mouse model data support the hypothesis that CD4+ T cell help plays a critical role in the control of persistent viral infections (3, 4). It is possible to dissect the interaction between CD4+ and CD8+ T cells in gammaherpesvirus infection through the use of the murine γ-herpesvirus model, MHV-68. When MHC class II-/- mice are intranasally infected with MHV-68, they clear the initial viral burden with comparable kinetics to wild-type mice. However, control ultimately breaks down and the virus reactivates by day 40 post-infection, while no detectable virus can be found in the lungs of wild-type animals (5).
Reactivation in class II-/- mice is due to a failure in immune surveillance by CD8 T cells, a process that, like the differentiation of memory CD8 T cells, is dependent upon CD4 T cell help. In the MHV-68 model, therapeutic vaccination, which massively expands the size of the memory CD8 T cell pool, even in CD4-deficient mice, does not prevent reactivation indicating that qualitative, rather than quantitative, changes are necessary to restore control over chronic virus reactivation (6).
Several factors, including CD40 and TRAIL expression, have been associated with CD4+ T cell help (7, 8). Recent studies suggest that IL-2 produced by CD4+ T cells is essential for primary T cell responses indicating an additional mechanism of CD4 help (9). Moreover, IL-2 signaling during the initial priming phase of a CD8 T cell response was shown to be necessary for formation of optimal memory cells against LCMV Armstrong (10, 11). Interestingly, administration of the IL-2 antibody clone S4B6 complexed to IL-2 (IL-2 complex) has been shown to cause homeostatic proliferation of naïve CD8+ T cells that subsequently develop protective capabilities (12). Additionally, IL-2 alone given therapeutically has been shown to enhance anti-viral immune responses (13). We hypothesized that administration of IL-2 complex may restore the ability of “helpless” CD8+ T cells to control a persistent virus infection. We report here that the administration of IL-2 complex rapidly reduced persistent viral burden in the lungs of CD4+ T cell depleted mice infected with MHV-68. IL-2 complex administration caused a massive upregulation in granzyme B production and the therapeutic reduction in viral load was dependent upon the granzyme/perforin effector pathway.
Materials and Methods
Mice and virus
MHV-68 virus (clone G2.4) was originally obtained from Dr. A.A. Nash (University of Edinburgh, U.K.). Mice were infected intranasally (i.n.) with 400 PFU under anesthesia. C57BL/6 mice were purchased from the National Cancer Institute (Bethesda, MD). Perforin transgenic mice (C57BL/6-Prf1tm1Sdz/J) were purchased from The Jackson Laboratory (Bar Harbor, ME). All experiments were performed according to Institutional Animal Care and Use Committee approved protocols at Dartmouth Hitchcock Medical Center Animal Facility (Lebanon, NH).
Depletion of CD4+ and CD8+ Lymphocytes
For CD4 depletions, mice were given 500 μg of the GK1.5 monoclonal antibody one day prior to infection, 500 μg at the time of infection and 200 μg of GK1.5 every three days for the duration of the experiment. For CD8 Depletions, mice were given 500 μg of the TIB-210 monoclonal antibody on day 0, 1 and 3 relative to IL-2 complex administration.
IL-2 immune complex treatment
MHV-68 infected intact or CD4 depleted mice were injected with either 50 μg of anti-IL-2 mAb (clone S4B6) mixed with 1.5 μg of murine IL-2 (mIL-2; eBioscience) or 50 μg of control rat IgG (R-IgG; Jackson ImmunoResearch Laboratories) i.p. daily for 4 days. Treatment began 42 days after i.n. infection for all experiments except in Figure 1B. For this experiment, the 4 day regimen began on day 81 post infection.
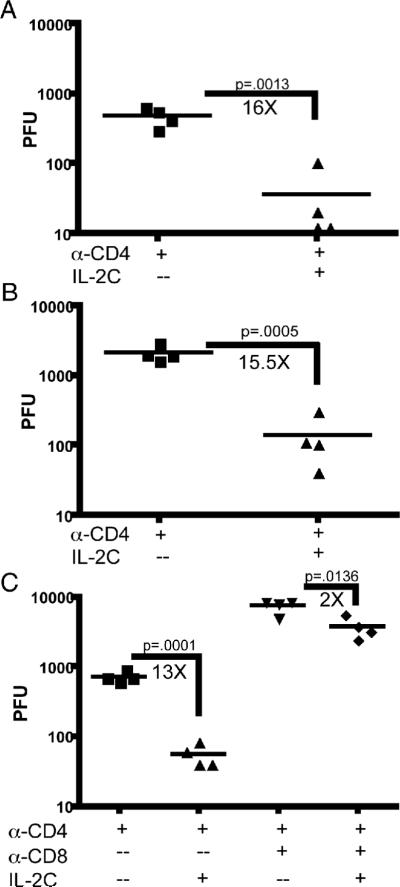
Figure 1. IL-2 complex reduces the viral load of MHV-68 in the lung of CD4 depleted mice.
Mice were depleted of CD4 T cells by antibody 1 day prior to i.n. infection with MHV-68. After 42 days, mice were given 4 daily injections of IL-2 complex or Rat-Ig. The virus titer in the lung of CD4 depleted mice was determined by standard plaque assay at day 46 (A) and day 85 (B). Mice were depleted of CD8+ T cells at the time of IL-2 complex therapy and viral titer was determined (C).
IFN-γ blockade
Mice were given i.p. injections of the IFN-γ blocking antibody R4-6A2 (1mg/mouse) on the first day of IL-2 complex administration. A second injection of equal concentration was given on the 3rd day of the therapy.
Intracellular cytokine staining
Splenocytes or lung lymphocytes were isolated and processed as previously described before being stained with the appropriate cell surface and cytokine antibodies (14).
Determination of viral titer
The titration of virus on 3T3 monolayers was performed as previously described (15).
Statistical Analysis
Comparisons between IL-2 complex treated intact and CD4 depleted mice with Rat-IgG treated controls were performed using the unpaired Student's t test. Viral titer data for CD8 depletion, perforin deficient mice and IFN-γ was analyzed using a one-way analysis of variance with a Bonferroni post-test to compare individual groups.
Results and Discussion
Mice lacking CD4 T cells control acute respiratory infection with MHV-68, and no virus replication can be detected in the lungs about 2 weeks post-infection (5). However, approximately 40 days post-infection the virus spontaneously reactivates in the lungs. As IL-2/anti-IL-2 immune complexes have been shown to potently stimulate memory CD8+ T cells, we tested whether this therapy enhanced control of virus reactivation. Mice were depleted of CD4+ T cells one day prior to i.n. infection with MHV-68, continuing for the duration of all experiments, while a control group was left intact. 42 days after infection, intact and CD4 depleted mice were injected with IL-2 complex or RatIg for 4 days i.p. (Supplemental Fig. 1). Intact mice had no viral replication in the lungs (data not shown), while CD4 depleted mice had high levels of reactivating virus as previously reported (5). Mice treated with IL-2 complex had significantly lower levels of virus in the lungs (Fig. 1A). Nearly identical results were seen if MHC Class II deficient mice were treated with IL-2 complex (data not shown). A similar reduction of viral load was observed if treatment was delayed to 85 days after infection (Fig. 1B). To determine whether CD8+ T cells were responsible for the therapeutic effect of IL-2 complex, we administered a CD8 depleting antibody during IL-2 treatment. Mice retaining CD8+ T cells had a 13-fold reduction in virus load upon IL-2 complex administration, while mice depleted of CD8 T cells only experienced a 2-fold reduction indicating that therapy is largely dependent upon CD8+ cells (Fig. 1C).
Next we examined what changes had occurred in the CD8 T cell compartment as a result of IL-2 complex treatment. We analyzed the lymphocyte populations of the lung and spleen, as these are major sites of viral persistence. Infected mice treated with IL-2 complex had significantly higher total numbers and percentages of CD8+ T cells when compared to controls, leading to an increase in the total number of splenocytes (Supplementary Fig. 2A,C,E). An increase in the CD8+ T cell population was also observed in the lungs of IL-2 complex treated mice (Supplementary Fig. 2B,D,F). The expansion of CD8+ T cells, as well as total splenocytes and lymphocytes, was independent of CD4+ T cells.
Depletion of CD4+ T cells resulted in an increased percentage of CD8 T cells specific for the two dominant viral epitopes, ORF61524-531/Kb and ORF6487-495/Db, as previously reported (16). IL-2 complex treatment had no effect on the percentage of virus specific cells in intact mice (Supplementary Fig. 3A,C,E). However, IL-2 complex administration lowered the percentage of CD8+ T cells that were specific for ORF6 in the spleen as well as for ORF61 in the lung of CD4 depleted mice (Supplementary Fig. 3C,E). Despite the decrease in the percentage of virus-specific cells, there was a dramatic increase in the total number of splenic ORF6 and ORF61 specific cells of CD4 depleted mice due to the large increase in total CD8+ T cells (Supplementary Fig. 3B,D). However, IL-2 complex treatment caused no significant difference in the total number of ORF61 specific cells in the lung regardless of CD4 depletion (Supplementary Fig. 3F). Although the number of virus-specific T cells increased in the spleen as a result of IL-2 complex therapy, we believed it was unlikely that elevated numbers were the sole reason for decreased viral load, as therapeutic vaccination, which causes a massive increase in virus-specific cells, had no effect upon viral burden in CD4-deficient mice in previous studies (6).
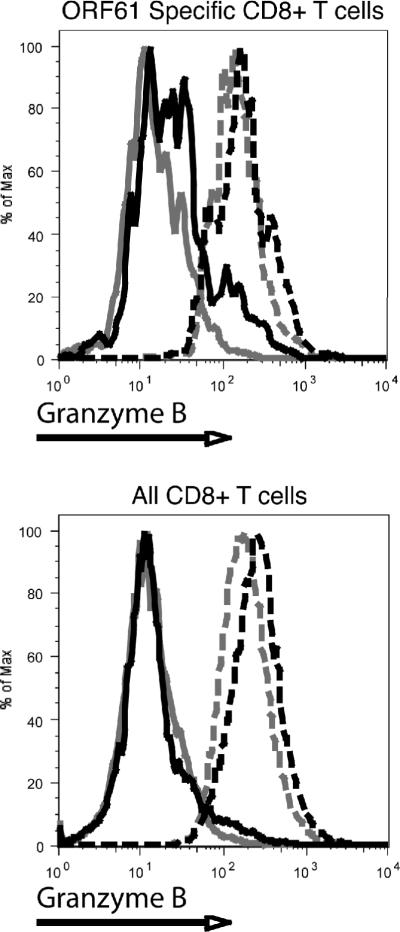
To determine if T cell effector functions were altered by IL-2 complex treatment, we analyzed the ability of MHV-68 specific CD8+ T cells to produce IFN-γ and granzyme B, both described as necessary for optimal control of MHV-68 (17, 18). As one of the few reported phenotypic changes for MHV-68 specific “helpless” CD8+ T cells is a decreased ability to produce TNF-α, we also analyzed the production of this cytokine (19). IL-2 complex administration resulted in decreased production of IFN-γ by ORF61 specific T cells in the spleen and lung in a CD4 independent manner, as determined by mean fluorescence intensity (Supplemental Fig 4A). Unlike previous studies (19), decreased production of TNF-α by “helpless” cells was not observed, however, the administration of IL-2 complex resulted in a decreased percentage of TNF-α, IFN-γ double positive cells. (Supplemental Fig. 4B). Both intact and CD4 depleted mice had decreased percentages of double positive cells upon treatment, but only in the CD4 deficient animals did it reach statistical significance. Most notable among changes to effector function was the greater than 8-fold upregulation of granzyme B. This increase was not restricted to any subset of CD8+ T cells, as all CD8+ cells in both the lung and spleen expressed high levels of granzyme B and this effect was independent of CD4 help (Fig. 2 and Supplemental figure 4C,D).
Figure 2. IL-2 Complex causes an increase in the production of granzyme B by both specific and non-specific CD8 T cells.
Lung lymphocytes were stained intracellularly for granzyme B. Representative flow cytometric plots for the granzyme B staining from intact mice treated with Rat-Ig (grey solid) and IL-2 complex (grey-dashed) and CD4 depleted mice treated with Rat-Ig (black solid) and IL-2 complex (black dashed) in lung.
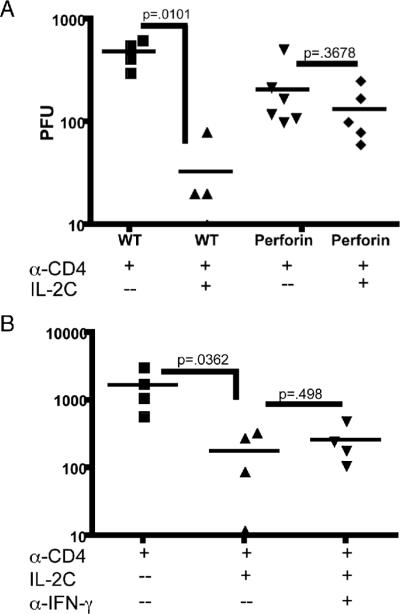
As granzyme B production was dramatically increased by administration of IL-2 complex, we determined whether this effector pathway was responsible for the reduction in viral load. Granzyme B relies upon perforin for lysis of target cells, therefore we tested whether therapy was still observed in mice lacking perforin. Perforin deficient and WT mice were CD4 depleted, infected and given IL-2 complex after 42 days. IL-2 complex therapy of CD4 depleted wild type mice resulted in a 14.7 fold reduction in viral load, consistent with previous experiments (Fig. 3A). However, only a non-significant 2-fold reduction in virus titer was observed after IL-2 complex administration to CD4 depleted perforin deficient mice (Fig. 3A). This indicated the perforin/granzyme pathway was essential for the function of IL-2 complex in this system. During these experiments we noted CD4 depleted perforin deficient mice consistently had reduced viral titers when compared with wild-type mice, potentially due to compensation by other antiviral effector mechanisms. Although we observed a decrease in IFN-γ production upon IL-2 complex administration, we wanted to determine whether it played any role in the therapeutic value of IL-2 complex. However, blockade of IFN-γ with a neutralizing antibody did not reduce the effectiveness of IL-2 complex therapy (Fig. 3B).
Figure 3. IL-2 complex administration is not effective at reducing viral loads in the absence of perforin, but is effective when IFN-γ is blocked.
(A) Wild-type and perforin deficient mice were depleted of CD4 T cells and treated with IL-2 complex as in Figure 1A. (B) An IFN-γ blocking antibody was given at the same time as IL-2 complex administration. Viral loads were determined via the standard plaque assay.
Our data show that an effective way to overcome defective immune surveillance in mice lacking T cell help is to treat animals with IL-2 immune complexes, which greatly enhances the quality of the CD8 T cell response, rapidly leading to improved control of virus reactivation. One key question that remains is how long this effect endures. Our initial experiments (data not shown) indicate that two weeks after the termination of IL-2 complex treatment, the virus titers were equivalent in both treated and untreated groups. This likely indicates that for therapeutic effect, IL-2 complex needs to be present, and that it may not permanently 'reprogram' the CD8 T cell response. While IL-2 alone has been shown to have relative success at treating several metastatic tumors, toxicity concerns, such as vascular leakage syndrome, necessitate careful optimization of the dose administered (20). IL-2/IL-2 antibody complexes represent a potential avenue for improving therapy while minimizing side effects, as previous studies have not revealed any overt signs of toxicity in mice, though closer examinations are necessary (21). Such treatment could prove useful for immune suppressed patients, such as transplant recipients, or for patients where T cell function has been dampened by inhibitory signals, as seen with various tumors and persistent viral infections. In addition to our results, another study showed IL-2 complex was more protective against a mouse model of lung tumor metastasis than using IL-2 alone (22). It is worth noting that the anti-human IL-2 antibody MAB602 expanded memory CD8+ T cells and NK cells in a similar manner to the murine antibody used in our experiments (12). While safety and efficacy must be tested in humans, the use of enhanced signaling through antibody/cytokine complexes provides an exciting potential avenue of therapeutic intervention.
Supplementary Material
Acknowledgements
The authors would like to thank Rameeza Allie and Ching-Yi Tsai for technical assistance and helpful discussions.
Funding was provided in part by the NIH grants AI069943, CA103642 and T32AI07363.
Footnotes
The authors have no conflicting financial interests.
References
- 1.Rickinson AB, Moss DJ. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu Rev Immunol. 1997;15:405–431. doi: 10.1146/annurev.immunol.15.1.405. [DOI] [PubMed] [Google Scholar]
- 2.Grogg KL, Miller RF, Dogan A. HIV infection and lymphoma. J Clin Pathol. 2007;60:1365–1372. doi: 10.1136/jcp.2007.051953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Far M, Halwani R, Said E, Trautmann L, Doroudchi M, Janbazian L, Fonseca S, van Grevenynghe J, Yassine-Diab B, Sekaly RP, Haddad EK. T-cell exhaustion in HIV infection. Curr HIV/AIDS Rep. 2008;5:13–19. doi: 10.1007/s11904-008-0003-7. [DOI] [PubMed] [Google Scholar]
- 4.Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 5.Cardin RD, Brooks JW, Sarawar SR, Doherty PC. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belz GT, Stevenson PG, Castrucci MR, Altman JD, Doherty PC. Postexposure vaccination massively increases the prevalence of gammaherpesvirus-specific CD8+ T cells but confers minimal survival advantage on CD4-deficient mice. Proc Natl Acad Sci U S A. 2000;97:2725–2730. doi: 10.1073/pnas.040575197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 8.Sarawar SR, Lee BJ, Reiter SK, Schoenberger SP. Stimulation via CD40 can substitute for CD4 T cell function in preventing reactivation of a latent herpesvirus. Proc Natl Acad Sci U S A. 2001;98:6325–6329. doi: 10.1073/pnas.101136898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson EB, Livingstone AM. Cutting edge: CD4+ T cell-derived IL-2 is essential for help-dependent primary CD8+ T cell responses. J Immunol. 2008;181:7445–7448. doi: 10.4049/jimmunol.181.11.7445. [DOI] [PubMed] [Google Scholar]
- 10.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bachmann MF, Wolint P, Walton S, Schwarz K, Oxenius A. Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur J Immunol. 2007;37:1502–1512. doi: 10.1002/eji.200637023. [DOI] [PubMed] [Google Scholar]
- 12.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 13.Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 14.Fuse S, Bellfy S, Yagita H, Usherwood EJ. CD8+ T cell dysfunction and increase in murine gammaherpesvirus latent viral burden in the absence of 4-1BB ligand. J Immunol. 2007;178:5227–5236. doi: 10.4049/jimmunol.178.8.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sunil-Chandra NP, Efstathiou S, Arno J, Nash AA. Virological and pathological features of mice infected with murine gamma-herpesvirus 68. J Gen Virol. 1992;73(Pt 9):2347–2356. doi: 10.1099/0022-1317-73-9-2347. [DOI] [PubMed] [Google Scholar]
- 16.Stevenson PG, Belz GT, Altman JD, Doherty PC. Virus-specific CD8(+) T cell numbers are maintained during gamma-herpesvirus reactivation in CD4-deficient mice. Proc Natl Acad Sci U S A. 1998;95:15565–15570. doi: 10.1073/pnas.95.26.15565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doherty PC, Topham DJ, Tripp RA, Cardin RD, Brooks JW, Stevenson PG. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol Rev. 1997;159:105–117. doi: 10.1111/j.1600-065x.1997.tb01010.x. [DOI] [PubMed] [Google Scholar]
- 18.Virgin HW. Immune regulation of viral infection and vice versa. Immunol Res. 2005;32:293–315. doi: 10.1385/IR:32:1-3:293. [DOI] [PubMed] [Google Scholar]
- 19.Liu H, Andreansky S, Diaz G, Hogg T, Doherty PC. Reduced functional capacity of CD8+ T cells expanded by post-exposure vaccination of gamma-herpesvirus-infected CD4-deficient mice. J Immunol. 2002;168:3477–3483. doi: 10.4049/jimmunol.168.7.3477. [DOI] [PubMed] [Google Scholar]
- 20.Schwartzentruber DJ. Guidelines for the safe administration of high-dose interleukin-2. J Immunother. 2001;24:287–293. doi: 10.1097/00002371-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Mostbock S, Lutsiak ME, Milenic DE, Baidoo K, Schlom J, Sabzevari H. IL-2/anti-IL-2 antibody complex enhances vaccine-mediated antigen-specific CD8(+) T cell responses and increases the ratio of effector/memory CD8(+) T cells to regulatory T cells. J Immunol. 2008;180:5118–5129. doi: 10.4049/jimmunol.180.7.5118. [DOI] [PubMed] [Google Scholar]
- 22.Kamimura D, Sawa Y, Sato M, Agung E, Hirano T, Murakami M. IL-2 in vivo activities and antitumor efficacy enhanced by an anti-IL-2 mAb. J Immunol. 2006;177:306–314. doi: 10.4049/jimmunol.177.1.306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.