Abstract
Purpose of review
Inflammatory vasculopathies, spanning from atherosclerosis to vasculitides, are driven by innate and adaptive immune responses. Instructed by antigen-presenting cells (APC), T cells have unsurpassed skills to orchestrate protective and pathogenic immunity. Pro- and anti-inflammatory T cells regulate master pathogenic pathways, providing a framework for novel immunotherapeutic strategies.
Recent findings
The multilayered wall of macrovessels creates a unique tissue niche; professional APC, specifically dendritic cells (DC), are superior in triggering and maintaining T-cell responses in this tissue milieu. Plaque-residing DC sense pathogen-derived motifs and edit inflammatory responses. T cells respond to antigen but antigen-nonspecific factors setting cellular response thresholds may be equally important. Dysregulated signal transduction pathways emerge as highly relevant in biasing T cells towards hyperresponsiveness. In the inflamed atheroma and in arteritic lesions, pathogenic T cells coordinate multiple injury pathways. Besides inducing tissue-damaging macrophage functions, they directly inflict cellular injury within the arterial wall. Distinctively, selected T cells induce smooth muscle cell apoptosis, most prominently by upregulating the death-receptor ligand TRAIL.
Summary
Innate sentinels, specifically DC, populate normal arteries, intramural vasculitic lesions, and the inflamed atheroma. They sense microbial motifs and instruct T cells towards pro-inflammatory and tissue destructive effector functions. Microenvironmental factors imposed by the unique structure of the arterial wall appear to be highly conserved across disease entities, modulating inflammation in atherosclerosis and arteritis.
Keywords: vasculitis, atherosclerosis, antigen-presenting cells, cytokines, T cells
Introduction
Blood vessels distribute immune cells throughout the body and are in constant and intimate contact with immune cells. Yet, due to the vital nature of blood vessels, the margin of benefit from vascular inflammation is explicitly small. In humans, medium-sized and large arteries have wall structures substantial enough that the vessel wall itself is targeted by inflammation. Typical inflammatory vasculopathies are the arteritides of macrovessels, including giant cell arteritis, Takayasu arteritis, panarteritis nodosa, and Kawasaki’s disease. These vasculitides are infrequent syndromes in contrast to atherosclerosis, a disease entity now recognized as an immuno-inflammatory process that is one of the major killers in Western societies. In vasculitis, tissue destructive immune responses occur throughout all layers of the arterial wall. In atherosclerosis, immuno-inflammatory infiltrates accumulate in the plaque, a neotissue formed as a result of wall remodeling. Like all other inflammatory diseases, both types of vasculopathies share a leading pathogenic role of adaptive immune responses, mostly mediated by T lymphocytes. Equipped with an enormous spectrum of sensory molecules, T cells are highly cognizant of their microenvironment. This review will summarize how T-cell homeostasis is maintained in the unique microenvironment of the arterial wall and which specific mechanisms the immune system has evolved to deal with the particular challenges encountered in this specialized tissue niche.
1. T cells – a dominant population of wall-infiltrating inflammatory cells in vascular inflammation
Like in most chronic inflammatory syndromes, T cells participate in the vascular infiltrates in atherosclerosis as well as vasculitis [1–3]. Typically, the infiltrates are composed of diverse cell populations, including monocytes/macrophages, lymphocytes, NK cells, mast cells, and dendritic cells (DC). Phenotypic studies have identified CD4 T cells as the predominant lymphocyte population, likely composed of numerous phenotypic and functional subsets. How distinct T-cell subsets promote and suppress inflammatory pathways in both atherosclerosis and vasculitis and which T-cell subpopulations have disease-initiating and disease-sustaining function are two of the most pressing issues in pathogenic studies of inflammatory vasculopathies.
In the atherosclerotic plaque, T cells tend to cluster in the shoulder region, just below the rupture-prone surface area. Often, plaque inflammation is associated with an adventitial infiltrate which has recently been analyzed for participating immune cells [4]. Interestingly, in more than 80% of all samples, adventitial infiltrates were composed of T cells; B cells were infrequently detected and organization into follicular structures was explicitly rare, confirming prior studies showing the absence of B cells in the vascular biopsies of patients with giant cell arteritis (GCA) and chronic lymphocytic leukemia [5]. In essence, the intramural tissue niche selects for T cells and disfavors B cells.
Evidence has surfaced that T-cell positioning in the vessel wall has functional implications. Burke et al. have provided a sophisticated analysis of 52 cases of aortitis [6] assigning large-vessel vasculitis with and without zonal medial laminar necrosis to distinct patient subsets. Whereas aortitis of the Takayasu type was characterized by increased adventitial scarring and more medial destruction, patients with GCA had increased frequencies of media-infiltrating T cells. This study supports the concept that vasculitic T cells display distinct patterns in the aortic wall, associated with differential clinical manifestations. Vasculitic T cells appear to target either the adventitia or the media, suggesting fundamentally different disease pathways.
2. Antigen presenting cells – Transferring information from the innate to the adaptive immune system
Given the enormous power of T cells as regulators and effector cells, pathogenic and autoreactive immunity can only be prevented if their stimulation is tightly controlled. Stimulation requirements of distinct T-cell subsets vary significantly. CD4 and CD8 cells respond to distinct stimulatory conditions; unprimed and primed T-cell populations require specialized signals to enter the activation cascade. What all T cells have in common is the need to be triggered and instructed by antigen-presenting cells (APC), either professional APC or cells that acquire APC function when exposed to an inflammatory milieu. Understanding which cells serve as APC in the vasculitic and plaque microenvironments reveals important clues about the instigators and drivers of chronic vascular inflammation.
Remarkably, in their native state, human medium-sized and large arteries are populated by professional APC, networks of DC residing within the wall. In medium-sized arteries, such as subclavian, mesenteric, iliac and temporal arteries, DC are positioned at the media-adventitia junction. The aortic and carotid walls harbor, in addition to adventitial DC, a subendothelial network in the intima. It has been proposed that such DC may have a critical role in controlling the process of atherogenesis, but functional studies are lacking [7]. Populated by endogenous DC, human macrovessels have the potential to function as immunosensing and immune-regulatory organs, a dimension of functional activity of particular relevance for mural inflammation.
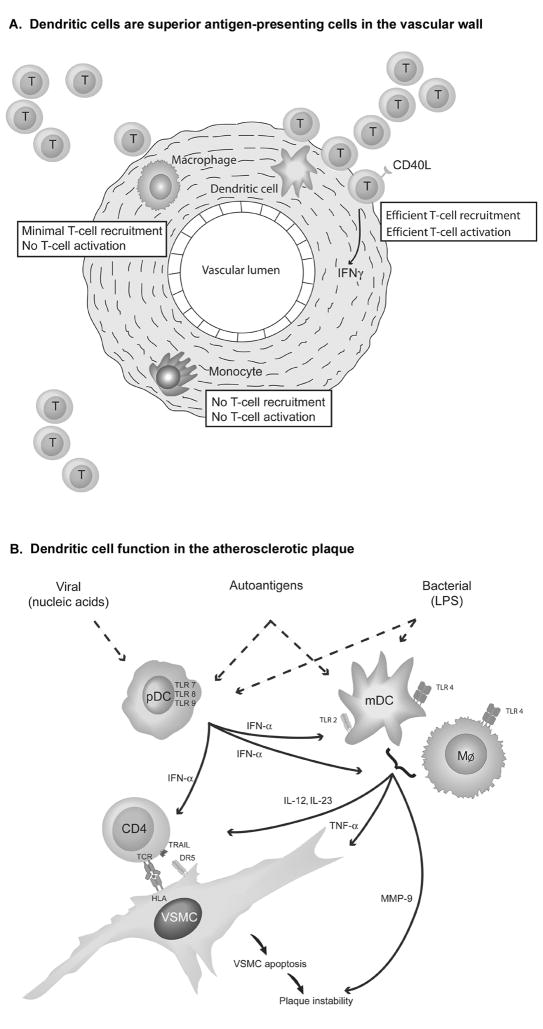
The impact of the specialized environment of the blood vessel wall on APC function of different cell types has recently been investigated in bioengineered human arteries [8]. Bioarteries designed to mimic carotid arteries in diameter and composition were reconstituted with monocytes, macrophages, or myeloid DC (mDC). Interestingly, DC assumed a position at the outer media edge, resembling their physiologic localization at the media-adventitia border, which suggests that they have means of assessing their precise orientation in the 3-D space of the vessel wall. To induce an inflammatory milieu, the bioarteries were stimulated with the bacterial product lipopolysaccharide (LPS), which is recognized by Toll-like receptor (TLR) 4. Autologous T cells were recruited to the vessel wall and stimulated in situ to invade into the media, provided they were given appropriate activation. Wall-embedded monocytes failed to activate T cells. Macrophages, while strongly expressing HLA class II molecules, had minimal APC function. They recruited low numbers of T cells, but failed to activate them. In contrast, wall-integrated DC were highly potent APC. They powerfully attracted autologous T cells and induced upregulation of CD40ligand and interferon-gamma (IFNγ), documenting T-cell instruction towards pro-inflammatory effector pathways. DC-instructed T cells caused media invasion and matrix destruction. In essence, DC in the vessel wall have unique functional capabilities (Fig 1A). Monocytes and macrophages appear to be devoted to alternate functions, not initiating and sustaining adaptive immune responses.
Figure 1. Antigen-presenting Cells as Key Players in Vascular Inflammation.
Figure 1A: Dendritic Cells are Superior Antigen-presenting Cells in the Vessel Wall. To directly compare the capacity of different types of antigen-presenting cells to sense danger signals and initiate adaptive T-cell responses, human bioarteries were engineered to contain monocytes, macrophages, or myeloid dendritic cells, respectively. After exposure to microbial motifs (LPS), recruitment and intra-wall activation of autologous T cells were quantified. Monocytes had no APC function. Macrophages recruited low numbers of T cells but failed to activate them. Dendritic cells were highly efficient in attracting T cells and facilitating their in-situ activation.
Figure 1B: Myeloid and Plasmacytoid Dendritic Cells Regulate Inflammation in the Atherosclerotic Plaque.
Myeloid and plasmacytoid DC populate the inflamed human atheroma. Each type of DC utilizes a distinct profile of pattern recognition receptors and produces a unique cocktail of immunoregulatory cytokines. The signature cytokine of pDC is type I interferon which directly regulates T-cell effector functions by inducing the death ligand TRAIL. Type 1 interferon also amplifies the production of pro-inflammatory cytokines and proteases in mDC and macrophages. (Reproduced from ref. 16, copyright owner Lippincott, Williams & Wilkins, 2007, with permission.)
Overwhelming evidence has been presented that implicates wall-residing DC in both vasculitis as well as atherosclerosis (Fig. 1B). Depletion of CD83+ DC from GCA-affected temporal arteries in a human artery-SCID chimera model essentially abrogated vasculitis, paralyzing both T-cell and macrophage activation [9]. Vice versa, stimulation of vascular DC in normal temporal arteries has emerged as an excellent model to induce vasculitis. Vascular DC express a broad spectrum of pathogen-sensing receptors, implicating macrovessels into infectious surveillance [10]. In vasculitic lesions, vascular DC are highly activated, express the costimulatory molecules CD83 and CD86, and produce T cell-attracting chemokines, identifying them as chief regulators of the inflammatory immune response [11]. DC with a myeloid phenotype also populate the coronary lesions of Kawasaki arteritis where they, again, preferentially sit in the adventitia, assigning unique functions to that wall layer [12].
Normal human arteries as well as GCA arteries lack plasmacytoid DC (pDC), a specialized subpopulation of DC originating from lymphoid precursors. mDC and pDC express essentially non-overlapping receptor portfolios that sense microbial infections. Typically, motifs from bacterial or viral microorganisms are detected by surface or cytoplasmatic TLR. In a probably oversimplified concept, TLR1, 2, 3, 4, 5 are assigned to mDC whereas pDC are characterized by TLR7 and 9. Both DC types inhabit the inflamed atherosclerotic plaque [13, 14]. Circulating mDC in coronary artery disease patients express an activated phenotype [15]. In carotid and coronary atheromas, CD83+CD86+ mDC produce CCL19 and CCL21 [13] and make physical contact with T cells. Tissue DC chemokines predict production of the T-cell cytokine IFNγ [13]. In contrast, pDC in the inflamed atheroma are potent producers of type I IFN, particularly when driven with TLR9 ligands. Remarkably, IFN-α has powerful T-cell regulatory effects, inducing the cytolytic death receptor ligand TRAIL. IFN-α-stimulated T cells exhibit strong cytotoxic functions, endowing them with tissue-destructive potential. In essence, triggering of plaque-residing pDC by either circulating or locally produced pathogen motifs can drive plaque-destabilizing immune responses.
A recent study demonstrated functional cooperation between plaque pDC and mDC [16]. Stimulation of plaque tissue with IFN-α amplified production of TNF-α, IL-12, IL23 and metalloproteinases, all markers implicated in destabilizing the plaque’s integrity. Cooperation of different pathogens in intensifying plaque inflammation provides a conceptual framework for the association of the global pathogen burden with cardiovascular risk. In summary, the atheroma possesses highly sensitive pathogen sensors, rendering this inflammatory lesion susceptible to local and distal immune-stimulatory effects through host infections. In this process C-reactive protein may play a role. mDC cultured with C-reactive protein undergo activation with enhanced T-cell stimulatory capacity [17]. Still unclear is the contribution of DC replenishment and turn-over in atherosclerosis. However, atherosclerosis-prone mice deficient for the DC growth factor GM-CSF had a dramatic decrease in aortic lesion size, with reduction of lesional T cells and decreased autoantibodies to oxidized lipids [18]. Thus, the overall DC pool may emerge as a risk factor for overshooting inflammatory responses.
3. T-cell stimulatory signals in the vessel wall microenvironment – Going beyond antigen
The core paradigm of adaptive immunity states that recognition of specific antigen controls T-cell function. Indeed, the enormous diversity of clonally distributed antigen receptors is the distinguishing feature of T cells. Coupled with their abilities to generate immunologic memory and to differentiate into highly sophisticated effectors, T cells hold center stage in adaptive immunity. Consequently, any exploration of T-cell function in chronic inflammatory disease has to focus on the search for the antigen. Also, antigen specificity holds the promise to potentially modulate immune responses in a highly selective manner.
Over the last decade, multiple antigens have been implicated in driving inflammation in the atheroma [19]. Experimental support has been provided for candidate antigens, such as heat shock protein 60 [20]. Equally important is the potential contribution from infectious microbes [21] eliciting innate as well as adaptive immune functions. Possibly, different antigens function as disease instigators in different patients, complicating antigen-specific immunotherapy. Cytomegalovirus has attracted particular attention, with support for a potential role coming from different directions. T cells accumulated in human plaque are enriched for those lacking the costimulatory receptor CD28 [22, 23]. CD28 deficiency is a hallmark of senescent CD4 T cells, and accumulation of aged and exhausted CD4 T cells has been associated with chronic cytomegalovirus infection [24]. Krebs et al. have examined whether immune recognition of persistent microbial antigen in the vasculature affects atherogenesis [25] by targeting a transgene to the cardiovascular system. Hypercholesterolemic transgenic mice had dramatically accelerated atherosclerosis, indicating that the site of antigen recognition may be relevant in modulating the intensity of vascular inflammation.
However, antigen-nonspecific mechanisms may be equally important in dictating the outcome of persistent inflammation. Apoprotein C-I accelerates LPS-induced atherosclerosis in mice, probably through a general enhancement in LPS responsiveness [26]. Broad mechanisms determining the availability of immune cells for tissue invasion have been suspected to influence cardiovascular risk. The best examples are mechanisms of leukocyte recruitment, dependent upon the functionality of adhesion molecules and chemokines directing cellular traffic [27, 28]. Doubts have been raised about the role of individual chemokines in tissue inflammation. Also, the redundancy of the chemokine system may limit the therapeutic potential of interfering with cell recruitment. Interestingly, serum samples of patients with unstable angina contain a chemokine signature [29] with CCL5 and CCL18 significantly elevated in patients with refractory ischemic symptoms. Notably, these chemokines have also been associated with vascular inflammation in GCA [30], and tissue expression patterns have distinguished patients with vasculitis versus those with polymyalgia rheumatica [11].
T cells require a second signal to complement antigen-derived activation signals, usually provided by membrane contact between the T cell and the APC. T-cell subsets differ in their costimulatory requirements, and a broad spectrum of molecules has costimulatory potential. CD28 and CD80/86 interactions, critically involved in priming naive T cells, may be less relevant in the atherosclerotic plaque as T cells accumulated in human atheroma often lack CD28 [22]. A recent study describes CD137 expressed in human atherosclerotic lesions, and treatment of arthrosclerosis-prone mice with CD137 agonists markedly increased inflammation [31]. CD137 is a member of the TNF receptor superfamily typically expressed on activated CD4+ and CD8+ T cells. Agonistic antibodies against CD137 that function as immune stimulators boost anti-tumor and anti-viral immune responses in mice. Counterintuitively, CD137 agonists also suppress murine autoimmune disease, emphasizing the complexity of reconditioning immune responses in vivo [32]. Another avenue of immunotherapy may lie in exploiting physiologic immunosuppressive signals provided by co-inhibitory pathways that counterbalance costimulation. In mice, the PD-1/PD-L pathway, which inhibits T-cell activation, has a role in regulating proatherogenic immune responses [33].
An exciting new prospect of modulating cell activation in vascular inflammation has come from work identifying disease risk genes in Kawasaki disease. A genome search led to inositol 1,4,5-triphosphate 3-kinase C (ITPKC) [34], a negative regulator of T-cell activation targeting the Ca/NFAT signaling cascade. The ITPKC polymorphism, altering the threshold setting in T-cell activation overall, was associated with higher risk for coronary artery lesions in both Japanese and American children. Along the same line, Fougerat and colleagues have recently demonstrated that genetic and pharmacologic targeting of phosphoinositide 3-kinase-gamma (PI3Kγ) attenuates early and advanced lesions in atherosclerosis-prone mice [35].
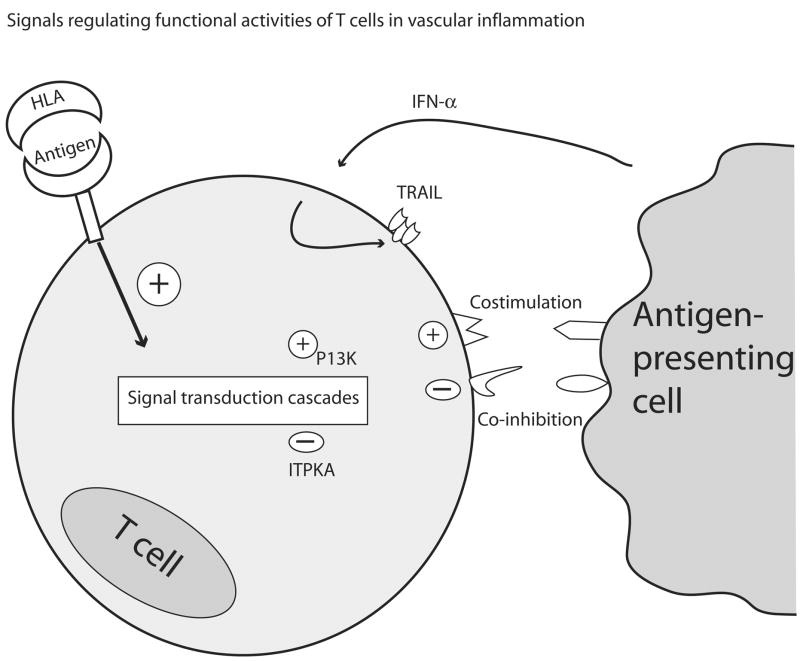
T-cell dependent, antigen-nonspecific disease mechanisms may involve novel cellular mechanisms, such as the expulsion of vesicles, so-called exosomes, from T cells [36]. Through such exosomes, T cells may edit systemic and tissue-residing immune responses. Finally, posttranslational modifications of proteins, influenced by conditions in the local tissue environment, could have profound implications on the distinction between self and non-self. In a recent study analyzing 1,000 subjects, Wang et al. have provided strong evidence for a potential role of protein carbamylation in a series of clinical scenarios, all linked with cardiovascular risk [37]. Carbamylation alters protein structure; studies are needed to explore the impact of carbamylation on protein immunogenicity. Posttranslationally modified proteins are now recognized as important targets of autoimmunity in rheumatoid arthritis [38]. In summary, not specific antigen but cellular thresholds regulating cellular responsiveness to antigen may be driving forces biasing immune responses towards persistence versus resolution (Fig. 2). Understanding the immunological impact of post-translational protein modifications, reflecting exposure to environmental stressors, is a necessary step that has the potential to widen the scope of dysfunctional immune responses in atherosclerosis and vasculitis.
Figure 2. Antigen-nonspecific signals determine T-cell response thresholds.
Recognition of specific antigen is a critical factor in initiating T-cell stimulation. However, the intensity of the activation cascade is modulated by numerous additional signals, including positive and negative amplification loops functioning in the cytoplasm. Such signals may be as important as antigen in determining whether, when, and where T cells in the microenvironment of the vascular wall are activated.
4. Vessel wall destructive – T cells Extending the armamentarium beyond cytokines
Ideally, immunotherapy of atherosclerosis or vasculitis would abrogate pathogenic immune responses, stopping them before they take hold in the artery’s wall. Most elegantly, identification of disease-inducing antigens would open the door to a vaccination approach. Less ideal, yet potentially highly efficacious, would be immunomodulation interfering with distal tissue injury pathways. Such a strategy requires detailed understanding of the molecular networks directing cellular damage. Injury pathways at work in the vessel wall in arteritis and in the atheroma are insufficiently understood. Emphasis has been placed on tissue-injurious macrophages, which undoubtedly are highly effective in releasing enzymes and reactive oxygen intermediates [39–44]. They are equally important in supplying growth factors, such as PDGF and VEGF that maintain maladaptive repair mechanisms leading to wall remodeling and excessive tissue growth.
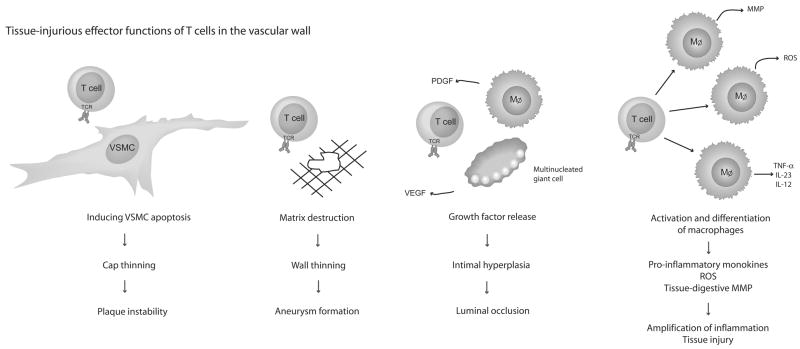
Recent studies have suggested that T cells have alternate means of promoting tissue injury, either by directly harming cells or by guiding other effector cells (Fig. 3). GCA is a typical TH1 disease with tissue IFNγ levels correlating with clinical patterning of the syndrome [45, 46]. The current model proposes that IFNγ-producing T cells promote numerous maladaptive macrophage functions, eventually leading to loss of medial smooth muscle cells and lumen-occlusive intimal hyperplasia. In autoantibody-associated vasculitis, T cells have also been implicated in directly activating polymorphonuclear neutrophils, inducing an array of cellular damage [47].
Figure 3. Tissue-injurious effector functions of T cells in the vascular wall.
T cells cause tissue injury in the vascular wall through numerous pathways. They orchestrate the function of other inflammatory cells, leading to the release of proteases, reactive oxygen intermediates, cytokines, and growth factors. Alternatively, T cells function as effector cells directly damaging wall-residing cells and matrices. Of particular interest are cytotoxic CD4 T cells that kill VSMC by triggering the death pathway, weakening the tissue scaffold.
Excessive production of TH1 cytokines has also been considered the major pathway through which T cells exhibit pro-atherosclerotic functions. Human carotid and coronary plaque contain high levels of tissue IFNγ [13]. In organ culture, human atherosclerotic coronary arteries secrete IFNγ with T cells representing the principal source [48]. Notably, this inflammatory response is elicited with IL-12 and IL-18 and does not require TCR signaling, suggesting antigen-independent amplification loops in plaque inflammation. Possibilities include a weakness in the TH2 arm of the immune system. Support for this notion derives from a recent study demonstrating that IL-33 suppresses atherosclerosis development in mice [49]. IL-33’s protective effect on plaque size was counteracted by anti-IL-5 antibodies. IL-33 is a cytokine of the IL-1 family implicated in TH2 polarization [50]. It functions as a chemoattractant for human TH2 cells [51], induces IL-4, IL-9 and IL-13, and participates in protective immunity towards intestinal nematodes [52].
A critical component of the blood vessel wall is vascular smooth muscle cells (VSMC), responsible for stability, contractility, and remodeling. In the atherosclerotic plaque, VSMC loss jeopardizes the protective cap’s intactness. Consequences of VSMC apoptosis in vivo were studied in an elegant transgenic mouse model in which the human diphtheria toxin receptor was expressed by a minimal smooth muscle cell 22 alpha promoter [53]. Normal murine arteries were functionally unaffected by VSMC loss. However, atherosclerosis-prone mice developed thinning of the fibrous cap, depletion of plaque matrix, accumulation of cellular debris, and marked intimal inflammation. Thus, VSMC loss in the plaque induces plaque vulnerability. In a chimera model in which human carotid atheroma was engrafted into SCID mice, the adaptive transfer of plaque-derived T cells resulted in massive VSMC apoptosis [54]. Underlying molecular mechanisms included the induction of TRAIL, a member of the tumor necrosis factor superfamily, on the surface of CD4 T cells. TRAIL is one of the most potent cytotoxic molecules. Notably, T cell TRAIL expression is regulated by IFN-α, a cytokine abundantly expressed in human atherosclerotic plaque [14]. TRAIL may have pathogenic function extending beyond cytotoxicity [55], such as enhancing endothelial-cell and VSMC proliferation. The balance among the different functional activities of this TNF-like cytokine may critically determine the stability of the atherosclerotic lesion. TRAIL-mediated cell death, however, may only be one of multiple processes through which T cells threaten the survival of plaque-stabilizing cells. Patients with acute coronary syndromes carry high frequencies of CD4 T cells that rapidly form stable immunologic synapses with VSMC, inducing apoptotic death [56, 57]. Based on the synapse morphology, granule-dependent death mechanisms participate in apoptosis induction. More importantly, it is unknown why acute coronary syndrome patients have such a profound disturbance of the functional T-cell repertoire. Up to 10% of their circulating CD4 T cells display cytolytic function towards VSMC. Besides vulnerability of plaque-residing cells, the functional composition of the patient’s T-cell repertoire may thus determine whether the chronic process of atherosclerosis is complicated by plaque rupture and acute atherothrombosis. In that concept, mechanisms shaping and remodeling an individual’s T-cell pool gain relevance in the immune defects underlying atherosclerosis as well as vasculitis.
Conclusion
Innate and adaptive immune responses are the driving forces in inflammatory vasculopathies. T cells represent the adaptive arm; they excel in discriminatory recognition, memorize antigenic encounters, and have unsurpassed skills to orchestrate and fine-tune protective and pathogenic immune responses. They require instruction by innate cells, specifically APC that sense danger signals and regulate T-cell function through both chemokine/cytokine secretion and crosslinking of cell surface receptors. Plasmacytoid dendritic cells emerge as critical sentinels in the atherosclerotic plaque where they recognize microbial motifs and determine the direction and intensity of inflammation. In bioengineered arteries mimicking human macrovessels, monocytes fail to function as APC and macrophages have minimal ability to activate T-cell responses. Instead, dendritic cells are highly proficient to trigger autologous T cells, biasing them towards tissue destructive effector functions. Dendritic cells are specialized in sensing pathogen-derived motifs, reviving the interest in how microbes interfere with inflammatory pathways in the vessel wall in both atherosclerosis and arteritides.
Acknowledgments
The authors thank Tamela Yeargin for manuscript editing.
Sources of Support: This work was funded in part by grants from the National Institutes of Health (lRO1 AR42527, RO1 AR41974, RO1 AI44142, RO1 AI57266, RO1 EY11916, and R01 AG15043) and a grant from the Dana Foundation.
References
- 1.Abdulahad WH, Stegeman CA, Limburg PC, Kallenberg CG. CD4-positive effector memory T cells participate in disease expression in ANCA-associated vasculitis. Ann N Y Acad Sci. 2007;1107:22–31. doi: 10.1196/annals.1381.003. [DOI] [PubMed] [Google Scholar]
- 2.Robertson AK, Hansson GK. T cells in atherogenesis: for better or for worse? Arterioscler Thromb Vasc Biol. 2006;26:2421–32. doi: 10.1161/01.ATV.0000245830.29764.84. [DOI] [PubMed] [Google Scholar]
- 3.Weyand CM, Goronzy JJ. Medium- and large-vessel vasculitis. N Engl J Med. 2003;349:160–9. doi: 10.1056/NEJMra022694. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe M, Sangawa A, Sasaki Y, et al. Distribution of inflammatory cells in adventitia changed with advancing atherosclerosis of human coronary artery. J Atheroscler Thromb. 2007;14:325–31. doi: 10.5551/jat.e489. * Study of inflammatory cells in adventitial infiltrates showing that T cells are the dominant cell type, with B cells rarely contributing. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Taboada V, Brack A, Hunder GG, et al. The inflammatory infiltrate in giant cell arteritis selects against B lymphocytes. J Rheumatol. 1996;23:1011–4. [PubMed] [Google Scholar]
- 6.Burke AP, Tavora F, Narula N, et al. Aortitis and ascending aortic aneurysm: description of 52 cases and proposal of a histologic classification. Hum Pathol. 2008;39:514–26. doi: 10.1016/j.humpath.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Wick G, Perschinka H, Millonig G. Atherosclerosis as an autoimmune disease: an update. Trends Immunol. 2001;22:665–9. doi: 10.1016/s1471-4906(01)02089-0. [DOI] [PubMed] [Google Scholar]
- 8.Han JW, Shimada K, Ma-Krupa W, et al. Vessel wall-embedded dendritic cells induce T-cell autoreactivity and initiate vascular inflammation. Circ Res. 2008;102:546–53. doi: 10.1161/CIRCRESAHA.107.161653. ** Functional comparison of different types of antigen-presenting cells initiating T-cell responses in the wall of bioengineered human arteries. Monocytes fail to function as APC, macrophages have minimal T-cell stimulatory capacity. Only dendritic cells effectively activate autologous T cells in this unique microenvironment. [DOI] [PubMed] [Google Scholar]
- 9.Ma-Krupa W, Jeon MS, Spoerl S, et al. Activation of arterial wall dendritic cells and breakdown of self-tolerance in giant cell arteritis. J Exp Med. 2004;199:173–83. doi: 10.1084/jem.20030850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma-Krupa W, Kwan M, Goronzy JJ, Weyand CM. Toll-like receptors in giant cell arteritis. Clin Immunol. 2005;115:38–46. doi: 10.1016/j.clim.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Krupa WM, Dewan M, Jeon MS, et al. Trapping of misdirected dendritic cells in the granulomatous lesions of giant cell arteritis. Am J Pathol. 2002;161:1815–23. doi: 10.1016/S0002-9440(10)64458-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yilmaz A, Rowley A, Schulte DJ, et al. Activated myeloid dendritic cells accumulate and co-localize with CD3+ T cells in coronary artery lesions in patients with Kawasaki disease. Exp Mol Pathol. 2007;83:93–103. doi: 10.1016/j.yexmp.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 13.Erbel C, Sato K, Meyer FB, et al. Functional profile of activated dendritic cells in unstable atherosclerotic plaque. Basic Res Cardiol. 2007;102:123–32. doi: 10.1007/s00395-006-0636-x. [DOI] [PubMed] [Google Scholar]
- 14.Niessner A, Sato K, Chaikof EL, et al. Pathogen-sensing plasmacytoid dendritic cells stimulate cytotoxic T-cell function in the atherosclerotic plaque through interferon-alpha. Circulation. 2006;114:2482–9. doi: 10.1161/CIRCULATIONAHA.106.642801. [DOI] [PubMed] [Google Scholar]
- 15.Dopheide JF, Sester U, Schlitt A, et al. Monocyte-derived dendritic cells of patients with coronary artery disease show an increased expression of costimulatory molecules CD40, CD80 and CD86 in vitro. Coron Artery Dis. 2007;18:523–31. doi: 10.1097/MCA.0b013e3282eff1ad. [DOI] [PubMed] [Google Scholar]
- 16.Niessner A, Shin MS, Pryshchep O, et al. Synergistic proinflammatory effects of the antiviral cytokine interferon-alpha and Toll-like receptor 4 ligands in the atherosclerotic plaque. Circulation. 2007;116:2043–52. doi: 10.1161/CIRCULATIONAHA.107.697789. ** Plasmacytoid and myeloid dendritic cells in the inflamed atherosclerotic plaque sense microbial products and cooperate to determine the direction and intensity of inflammation. [DOI] [PubMed] [Google Scholar]
- 17.Van Vre EA, Bult H, Hoymans VY, et al. Human C-reactive protein activates monocyte-derived dendritic cells and induces dendritic cell-mediated T-cell activation. Arterioscler Thromb Vasc Biol. 2008;28:511–8. doi: 10.1161/ATVBAHA.107.157016. [DOI] [PubMed] [Google Scholar]
- 18.Shaposhnik Z, Wang X, Weinstein M, et al. Granulocyte macrophage colony-stimulating factor regulates dendritic cell content of atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2007;27:621–7. doi: 10.1161/01.ATV.0000254673.55431.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu Rev Pathol. 2006;1:297–329. doi: 10.1146/annurev.pathol.1.110304.100100. [DOI] [PubMed] [Google Scholar]
- 20.Rossmann A, Henderson B, Heidecker B, et al. T-cells from advanced atherosclerotic lesions recognize hHSP60 and have a restricted T-cell receptor repertoire. Exp Gerontol. 2008;43:229–37. doi: 10.1016/j.exger.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Ayada K, Yokota K, Kobayashi K, et al. Chronic infections and atherosclerosis. Ann N Y Acad Sci. 2007;1108:594–602. doi: 10.1196/annals.1422.062. [DOI] [PubMed] [Google Scholar]
- 22.Liuzzo G, Goronzy JJ, Yang H, et al. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation. 2000;101:2883–8. doi: 10.1161/01.cir.101.25.2883. [DOI] [PubMed] [Google Scholar]
- 23.Liuzzo G, Kopecky SL, Frye RL, et al. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation. 1999;100:2135–9. doi: 10.1161/01.cir.100.21.2135. [DOI] [PubMed] [Google Scholar]
- 24.Pourgheysari B, Khan N, Best D, et al. The cytomegalovirus-specific CD4+ T-cell response expands with age and markedly alters the CD4+ T-cell repertoire. J Virol. 2007;81:7759–65. doi: 10.1128/JVI.01262-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krebs P, Scandella E, Bolinger B, et al. Chronic immune reactivity against persisting microbial antigen in the vasculature exacerbates atherosclerotic lesion formation. Arterioscler Thromb Vasc Biol. 2007;27:2206–13. doi: 10.1161/ATVBAHA.107.141846. [DOI] [PubMed] [Google Scholar]
- 26.Westerterp M, Berbee JF, Pires NM, et al. Apolipoprotein C-I is crucially involved in lipopolysaccharide-induced atherosclerosis development in apolipoprotein E-knockout mice. Circulation. 2007;116:2173–81. doi: 10.1161/CIRCULATIONAHA.107.693382. [DOI] [PubMed] [Google Scholar]
- 27.Rao RM, Yang L, Garcia-Cardena G, Luscinskas FW. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ Res. 2007;101:234–47. doi: 10.1161/CIRCRESAHA.107.151860b. [DOI] [PubMed] [Google Scholar]
- 28.Sainz J, Sata M. Open sesame! CXCR4 blockade recruits neutrophils into the plaque. Circ Res. 2008;102:154–6. doi: 10.1161/CIRCRESAHA.107.170241. [DOI] [PubMed] [Google Scholar]
- 29.Kraaijeveld AO, de Jager SC, de Jager WJ, et al. CC chemokine ligand-5 (CCL5/RANTES) and CC chemokine ligand-18 (CCL18/PARC) are specific markers of refractory unstable angina pectoris and are transiently raised during severe ischemic symptoms. Circulation. 2007;116:1931–41. doi: 10.1161/CIRCULATIONAHA.107.706986. [DOI] [PubMed] [Google Scholar]
- 30.Bruhl H, Vielhauer V, Weiss M, et al. Expression of DARC, CXCR3 and CCR5 in giant cell arteritis. Rheumatology (Oxford) 2005;44:309–13. doi: 10.1093/rheumatology/keh485. [DOI] [PubMed] [Google Scholar]
- 31.Olofsson PS, Soderstrom LA, Wagsater D, et al. CD137 is expressed in human atherosclerosis and promotes development of plaque inflammation in hypercholesterolemic mice. Circulation. 2008;117:1292–301. doi: 10.1161/CIRCULATIONAHA.107.699173. ** This study implicates the costimulatory receptor CD137, predominantly expressed on activated CD8 T cells, in regulating plaque inflammation. [DOI] [PubMed] [Google Scholar]
- 32.Sun Y, Chen JH, Fu Y. Immunotherapy with agonistic anti-CD137: two sides of a coin. Cell Mol Immunol. 2004;1:31–6. [PubMed] [Google Scholar]
- 33.Gotsman I, Grabie N, Dacosta R, et al. Proatherogenic immune responses are regulated by the PD-1/PD-L pathway in mice. J Clin Invest. 2007;117:2974–82. doi: 10.1172/JCI31344. ** This study demonstrates that deficiency for the co-inhibitory ligand PD-L1/2 enhances T-cell activity and increases the atherosclerotic burden of hypercholesterolemic mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Onouchi Y, Gunji T, Burns JC, et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat Genet. 2008;40:35–42. doi: 10.1038/ng.2007.59. ** This study suggests that inositol 1,4,5-trisphosphate 3-kinase C (ITPKC) is a disease risk gene in the vasculitic syndrome Kawasaki disease. As a negative regulator of T-cell activation, ITPKC may induce T-cell hyperreactivity in patients with this vasculitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fougerat A, Gayral S, Gourdy P, et al. Genetic and pharmacological targeting of phosphoinositide 3-kinase-gamma reduces atherosclerosis and favors plaque stability by modulating inflammatory processes. Circulation. 2008;117:1310–7. doi: 10.1161/CIRCULATIONAHA.107.720466. * This study identifies phosphoinositide 3-kinase-gamma, an enzyme that generates lipid-based second messengers and amplifies intracellular signaling pathways in leukocytes, as a suitable target for immunomodulatory interventions in atherosclerosis. [DOI] [PubMed] [Google Scholar]
- 36.Zakharova L, Svetlova M, Fomina AF. T cell exosomes induce cholesterol accumulation in human monocytes via phosphatidylserine receptor. J Cell Physiol. 2007;212:174–81. doi: 10.1002/jcp.21013. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z, Nicholls SJ, Rodriguez ER, et al. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med. 2007;13:1176–84. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 38.Vossenaar ER, van Venrooij WJ. Citrullinated proteins: sparks that may ignite the fire in rheumatoid arthritis. Arthritis Res Ther. 2004;6:107–11. doi: 10.1186/ar1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Borkowski A, Younge BR, Szweda L, et al. Reactive nitrogen intermediates in giant cell arteritis: selective nitration of neocapillaries. Am J Pathol. 2002;161:115–23. doi: 10.1016/S0002-9440(10)64163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaiser M, Weyand CM, Bjornsson J, Goronzy JJ. Platelet-derived growth factor, intimal hyperplasia, and ischemic complications in giant cell arteritis. Arthritis Rheum. 1998;41:623–33. doi: 10.1002/1529-0131(199804)41:4<623::AID-ART9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 41.Kaiser M, Younge B, Bjornsson J, et al. Formation of new vasa vasorum in vasculitis. Production of angiogenic cytokines by multinucleated giant cells. Am J Pathol. 1999;155:765–74. doi: 10.1016/S0002-9440(10)65175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rittner HL, Hafner V, Klimiuk PA, et al. Aldose reductase functions as a detoxification system for lipid peroxidation products in vasculitis. J Clin Invest. 1999;103:1007–13. doi: 10.1172/JCI4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rittner HL, Kaiser M, Brack A, et al. Tissue-destructive macrophages in giant cell arteritis. Circ Res. 1999;84:1050–8. doi: 10.1161/01.res.84.9.1050. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez-Pla A, Bosch-Gil JA, Rossello-Urgell J, et al. Metalloproteinase-2 and -9 in giant cell arteritis: involvement in vascular remodeling. Circulation. 2005;112:264–9. doi: 10.1161/CIRCULATIONAHA.104.520114. [DOI] [PubMed] [Google Scholar]
- 45.Weyand CM, Ma-Krupa W, Goronzy JJ. Immunopathways in giant cell arteritis and polymyalgia rheumatica. Autoimmun Rev. 2004;3:46–53. doi: 10.1016/S1568-9972(03)00064-8. [DOI] [PubMed] [Google Scholar]
- 46.Weyand CM, Tetzlaff N, Bjornsson J, et al. Disease patterns and tissue cytokine profiles in giant cell arteritis. Arthritis Rheum. 1997;40:19–26. doi: 10.1002/art.1780400105. [DOI] [PubMed] [Google Scholar]
- 47.Iking-Konert C, Vogl T, Prior B, et al. T lymphocytes in patients with primary vasculitis: expansion of CD8 + T cells with the propensity to activate polymorphonuclear neutrophils. Rheumatology (Oxford) 2008;47:609–16. doi: 10.1093/rheumatology/ken028. [DOI] [PubMed] [Google Scholar]
- 48.Ranjbaran H, Sokol SI, Gallo A, et al. An inflammatory pathway of IFN-gamma production in coronary atherosclerosis. J Immunol. 2007;178:592–604. doi: 10.4049/jimmunol.178.1.592. * A study of plasma cytokines induced in patients undergoing cardiac catheterization. Describes induction of the IL-12/IFN-gamma axis independent from IL-6 or CRP. [DOI] [PubMed] [Google Scholar]
- 49.Miller AM, Xu D, Asquith DL, et al. IL-33 reduces the development of atherosclerosis. J Exp Med. 2008;205:339–46. doi: 10.1084/jem.20071868. ** First description that Interleukin-33, a cytokine of the IL-1 family that has TH2 immunoregulatory activity, can inhibit the development of atherosclerosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martinon F, Gaide O, Petrilli V, et al. NALP inflammasomes: a central role in innate immunity. Semin Immunopathol. 2007;29:213–29. doi: 10.1007/s00281-007-0079-y. [DOI] [PubMed] [Google Scholar]
- 51.Komai-Koma M, Xu D, Li Y, et al. IL-33 is a chemoattractant for human Th2 cells. Eur J Immunol. 2007;37:2779–86. doi: 10.1002/eji.200737547. [DOI] [PubMed] [Google Scholar]
- 52.Humphreys NE, Xu D, Hepworth MR, et al. IL-33, a potent inducer of adaptive immunity to intestinal nematodes. J Immunol. 2008;180:2443–9. doi: 10.4049/jimmunol.180.4.2443. [DOI] [PubMed] [Google Scholar]
- 53.Clarke MC, Figg N, Maguire JJ, et al. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12:1075–80. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- 54.Sato K, Niessner A, Kopecky SL, et al. TRAIL-expressing T cells induce apoptosis of vascular smooth muscle cells in the atherosclerotic plaque. J Exp Med. 2006;203:239–50. doi: 10.1084/jem.20051062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kavurma MM, Bennett MR. Expression, regulation and function of trail in atherosclerosis. Biochem Pharmacol. 2008;75:1441–50. doi: 10.1016/j.bcp.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 56.Flavahan NA. A farewell kiss triggers a broken heart? Circ Res. 2006;98:1117–9. doi: 10.1161/01.RES.0000223519.26857.5d. [DOI] [PubMed] [Google Scholar]
- 57.Pryshchep S, Sato K, Goronzy JJ, Weyand CM. T cell recognition and killing of vascular smooth muscle cells in acute coronary syndrome. Circ Res. 2006;98:1168–76. doi: 10.1161/01.RES.0000220649.10013.5c. [DOI] [PubMed] [Google Scholar]