Abstract
The involvement of the endogenous cannabinoid system has been implicated in the rewarding actions of several drugs of abuse. Recent evidence indicates that the transcription factor CREB (cAMP response element binding protein) may be an important biochemical substrate for behavioral plasticity that has been associated with the chronic administration of drugs of abuse and addiction. Increased CREB activity was reported as a chronic effect of drugs of abuse in the neurons of the nucleus accumbens, a brain reward region that expresses high density levels in the CB1 cannabinoid receptors. However, little is known whether a similar change occurs in the hippocampus, a region of the brain that also expresses high density levels of the CB1 cannabinoid receptors and has intimate synaptic connections with the brain’s reward regions. The present study revealed that CREB activities were present in the hippocampal neurons of cultured slice preparations in response to acute and chronic applications of endogenous cannabinoid, anandamide and R(+)-methanandamide (a non-hydrolyzing form of anandamide). When administered acutely at a dose effective for inducing self-administration in vivo, anandamide and R(+)-methanandamide stimulated the expression of pCREB in our hippocampal slice culture. Interestingly, a sub-threshold dose of R(+)-methanandamide, which was not effective in producing acute changes in the CREB activity, was also found to effectively increase pCREB when administered chronically for 10 days. These increases were blocked by the antagonist of the CB1 cannabinoid receptor. Present findings demonstrate: 1) the hippocampus is vulnerable to the direct chemical effect of anandamide and R(+)-methanandamide in isolation of synaptic influences from the midbrain reward neurons, and 2) the effect of R(+)-methanandamide is cumulative as evidenced by the sustained elevation of CREB activities in response to a chronic dosage that is too low and thus fails to exert any acute effect. The ability of hippocampal neurons to integrate a time-dependent effect on the endogenous cannabinoid signaling may be a key function of plasticity as related to the induction and maintenance of maladaptive learning and memory that underlies both cue-induced cravings as well as relapses in drug-seeking.
Keywords: anandamide, cAMP, slice culture, acute exposure, chronic application, plasticity
INTRODUCTION
Repeated exposure to drugs of abuse induces changes in the brain that can persist for months or even years after the use has been discontinued. There are several well-documented changes in the intracellular signaling cascades that are accompanied with long-term consequences of drug-taking [23]. Among these are the up-regulation of cAMP and transcription factor CREB (cAMP response element binding protein), which was first reported in cultured neuroblastoma × glioma cells in response to morphine [34]. In the mammalian nervous system, the striatal reward neurons increase a phosphorylated form of CREB (pCREB)-expression in response to a direct chemical effect of the drugs of abuse and produce drug-associated reward feelings and pleasure [27]. Interestingly, similar changes in the upregulation of pCREB were observed in neurons of the locus coeruleus in response to cocaine [28], in the hippocampus in response to morphine [11] and antidepressant treatment [36], and in the cerebellum in response to exogenous cannabinoid application [4].
Anandamide is an endogenous cannabinoid and produces many behavioral effects similar to those generated by Δ9-tetrahydrocannabinol (THC), the main psychoactive ingredient in marijuana (exogenous cannabinoid). Anandamide was reported to effectively reinforce drug-taking behavior when it was self-administered intravenously by squirrel monkeys [20]. Similarly, R(+)-methanandamide (a synthetic longer-lasting, metabolically-stable anandamide analog) acts as a reinforcer of drug-taking behavior despite their marked pharmacokinetic differences. The reinforcing effects of both anandamide and R(+)-methanandamide appear to be mediated by the CB1 cannabinoid receptor; although some of anandamide’s effects have been attributed to other receptors, such as vanilloid receptors [41] or uncharacterized cannabinoid receptors other than CB1 or CB2 [8, 13, 31]. These reports suggested that the activation of the CB1 cannabinoid receptor by anandamide could be a part of the signaling of natural rewarding events.
The hippocampus is a representative brain region that regulates learning and memory. Memory consolidation requires protein synthesis. The phosphorylation of the transcription factor, CREB, is a key step in this process [1]. However, whether the hippocampus is involved in drug-related reward learning and memory by directly responding to drugs of abuse is not completely understood. The goal of this study is to investigate the effect of anandamide on the hippocampal neuron plasticity by determining its effect on the phosphorylation of hippocampal CREB from the perspective of maladaptive learning.
In the present study, an in vitro slice culture model was used. Changes in the expression of pCREB were assayed immunohistochemically in response to the administration of anandamide and R(+)-methanandamide. The hippocampal slice culture was selected because: 1) the chemical effect of anandamide and R(+)-methanandamide could be assessed directly on the expression of pCREB in the hippocampal neurons; 2) the hippocampal slice culture can eliminate potential neuron-circuit activities produced by synapses made by extrahippocampal neurons and other brain regions which can cause changes in CREB activities independent of anandamide and R(+)-methanandamide; and 3) a transient elevation of pCREB was reported as a possible result of decapitation and cardiac perfusion [29]. Thus, culturing slices for at least several days before continuing the experiments can rule out the possibility of some method-related changes in the expression of pCREB.
MATERIALS AND METHODS
Hippocampal slice culture was prepared from postnatal 6 day-old rat pups according to the method of Stoppini [35]. Adequate measures were taken to minimize pain or discomfort. Experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23). All protocols were approved by the University of Texas at Brownsville Institutional Animal Care and Use Committee.
The slice culture was maintained in vitro for 7–10 days before experiments. For the application of anandamide (Tocris) or R(+)-methanandamide (a non-hydrolyzing form of anandamide, Sigma Aldrich Chemical), stock solutions were prepared in the vehicle, soya oil (Tocrisolve) for anandamide and 50% ethanol for R(+)-methanandamide, respectively. They were then dissolved in the culture media for the final concentrations of 50, 100, and 200 nM. Twenty-five to thirty slices were used for each experimental condition and incubated in a given concentration for either 5 hours (for an acute application) or 10 days (for a chronic application) at 35°C with 5% CO2. In the case of chronic application, only R(+)-methanandamide was used in order to minimize degradation, and the culture media that contained R(+)-methanandamide was periodically changed (refreshed 100 %) every two days. Working solution was made fresh each time that the culture media was changed during the long-term application of R(+)-anandamide for 10 days. There was no difference in the pCREB expression between cultures that were raised in the plain culture media and in the 0.0007% ethanol-containing culture media (the matching ethanol concentration used for 100 nM R(+)-methanandamide application for 10 days). There was no difference between cultures raised in the 0.0014% ethanol-containing culture media (the matching ethanol concentration used for 200 nM R(+)-methanandamide application for 5 hours and plain culture media. Similarly, there was no difference in the pCREB expression between 0.0014% soya oil-containing culture media (the highest matching soya oil concentration used in the experiment) and plain culture media for a five hour incubation period.
In some experiments, the antagonist of the CB1 cannabinoid receptor, AM251 (3 μM, Tocris) was added to the culture media. At the end of the experiments, the hippocampal slices were immersion-fixed overnight with 4% paraformaldehyde in the sodium phosphate buffered solution (PBS) and processed for immunocytochemistry using an anti-pCREB antibody (Cell Signaling, 1:50 in dilution). A fluorescence-tagged secondary antibody (Alexa 488, Invitrogen) was used for visualization. A control for immunohistochemistry consisted of several slices from each experimental condition that were incubated with a blocking peptide for pCREB (Cell Signalling, 1:50 in dilution) for several hours before the application of the primary antibody. Localization of pCREB was analyzed with a confocal microscope using Fluoview software (Olympus) and quantified by measuring the intensity of fluorescence with IPLab imaging software (BD Science). Data were summarized as mean ± SEM (standard error of the mean) and tested for significance with a student t-test.
RESULTS
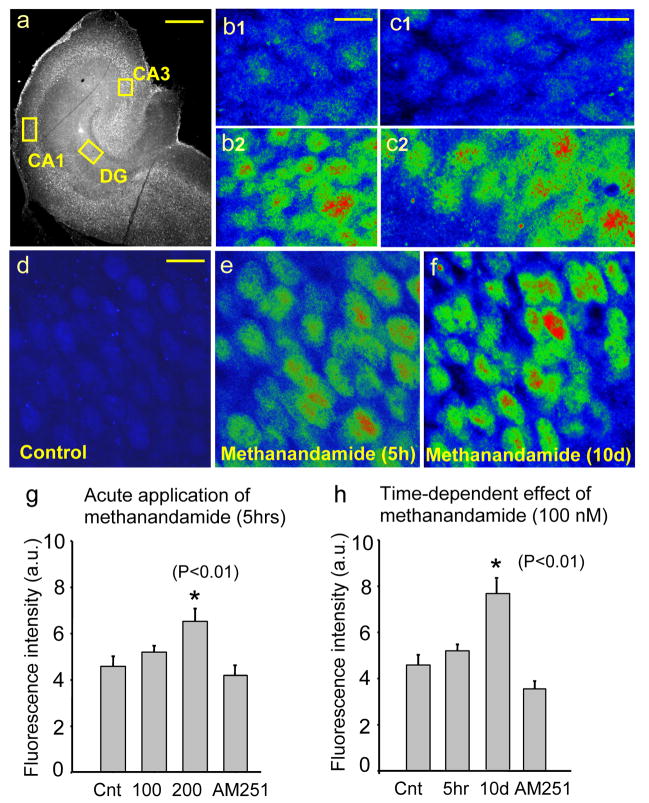
A basal level of pCREB expression was detected in the principal neurons of all hippocampal subfields in the control hippocampus, which received no anandamide or R(+)-methanandamide (Fig. 1a). There were no detectable differences in the pattern of basal pCREB expression between microwave-treated rat brain [29] and our hippocampal slice culture. These results suggest that the cultured hippocampal slice is an excellent preparation to study the expression and activities of pCREB. The application of anandamide to the culture media with a concentration that was reported effective for inducing a self-administration in the monkey [20], increased the magnitude and pattern of pCREB expression in the dentate granule cells (Fig. 1b2), CA3 pyramidal cells (Fig. 1c2) and CA1 pyramidal cells (Fig. 1e) in our slice culture.
Figure 1.
a. A basal expression of pCREB in the hippocampus. Areas enclosed with yellow boxes are shown with higher magnification in b–f. b. pCREB immunoreactivity in the dentate gyrus granule cells (DG) in control (b1) and after the application of anandamide for 5 hours (b2). c. pCREB immunoreactivity in the CA3 pyramidal cells in control (c1) and after the application of anandamide for 5 hours (c2). d. pCREB immunoreactivity in the CA1 pyramidal cells in the control medium for 10 days. e. An acute application (5 hours) of R(+)-methanandamide in the concentration of 200 nM increased pCREB immunoreactivity in the CA1 pyramidal cells. f. A chronic application (10 days) of R(+)-methanandamide in the concentration of 100 nM increased pCREB immunoreactivity in the CA1 pyramidal cells. g. A summary of the acute application of R(+)-methanandamide with two different doses (in nM). h. A summary of the chronic application of R(+)-methanandamide in the concentration of 100 nM. AM 251 is the antagonist of the CB1 cannabinoid receptor. Calibration: 1000 μm for a; 15 μm for b, c, d, e and f.
The dose and duration of the application of anandamide and R(+)-methanandamide determined the intensity of pCREB immunoreactivity. In the CA1 pyramidal cells, a short term application of 100 nM R(+)-methanandamide for 5 hours did not increase the expression of pCREB when compared with the basal level (Fig. 1g). A similar result was obtained with the application of 100 nM anandamide (5.12 ± 0.35 SEM in fluorescence intensity, n=26 slices, p<0.102 when compared with the control). However, 200 nM of R(+)-methanandamide for the identical duration of application (5 hours) increased pCREB (p<0.01)(Fig. 1g). A significant increase in pCREB was also observed as a result of the application of anandamide (200 nM for 5 hours) in 30 slices (7.35 ± 0.83 SEM in fluorescence intensity, p<0.02 when compared with control). There were no significant differences in the magnitude of pCREB between anandamide vs. R(+)-methanandamide application with either concentration.
Although a short term application of R(+)-methanandamide in 100 nM did not increase pCREB, a chronic application of the identical concentration of 100 nM for 10 days increased pCREB significantly (p<0.01)(Fig. 1h). In this chronic application, only R(+)-methanandamide was used because R(+)-methanandamide is a non-hydrolyzing form of anandamide, and thus considered to be both stable and less vulnerable to degradation over time. During chronic application, culture media that contained R(+)-methanandamide was periodically changed (refreshed 100 %) every two days.
In both acute and long-term applications, the effect of R(+)-methanadamide was blocked by the CB1 cannabinoid receptor antagonist, AM251 (3 μM) (Fig. 1g and h). The effect of an acute application of anandamide was also inhibited by AM251 (4.01± 0.08 SEM in fluorescence intensity, n=27 slices, p<0.01 when compared with the anandamide application without AM251). However, the application of AM251 alone did not produce any detectable change in the expression of pCREB when compared with the control (p<0.361 for 5 h application of AM251 alone in 20 slices, and p<0.600 for 10 day application of AM251 alone in 18 slices). This result suggested that the effects of R(+)-methanandamide and anandamide on the enhanced expression of pCREB were mediated through the activation of the CB1 cannabinoid receptor. Finally, the results of increased pCREB expression, described above in the CA1, were consistent with the results obtained from the remaining hippocampal subfields of DG and CA3.
In conclusion: 1) anandamide enhanced the phosphorylation of CREB in the cultured hippocampal slices that were prepared from an immature hippocampus, which demonstrates the greatest levels of synaptogenesis and plasticity; and 2) R(+)-methanandamide could cause a cumulative effect of pCREB expression in the hippocampal neurons in response to a chronic dosage typically considered sub-threshold for producing any acute effect. This cumulative effect on the sustained elevation of CREB phosphorylation might have occurred at either the level of CB1 cannabinoid receptor or its downstream cascade of cAMP signaling pathways.
DISCUSSION
The present study demonstrates that anandamide has both dose-dependent and time-dependent effects on the expression of pCREB in the hippocampal neurons. This finding suggests that anandamide can modulate hippocampal neuron plasticity and learning by stimulating CREB activities. Furthermore, the changes in the activity of the transcription factor might act as a molecular messenger for the induction and the maintenance of maladaptive learning in the hippocampus.
Learning the predictive cues for reward and connecting that information with appropriate responses require the storage of specific patterns of information in the brain. Such information-rich data are likely stored using mechanisms similar to those underlying all other forms of associative long-term memory [19]. The best-characterized candidate mechanisms for changing synaptic strength are long-term potentiation (LTP) and long-term depression (LTD); both have been hypothesized to play critical roles in many forms of experience-dependent plasticity including various forms of learning and memory. The hippocampus is the foremost studied and best characterized region of the brain for this type of synaptic plasticity. There is evidence to show that theta burst stimulation, effective for inducing LTP, also effectively elicits relapse to cocaine-seeking [37]. This suggests that hippocampal neuron activities that are capable of inducing LTP may also be involved in drug-related maladaptive learning. It has been reported that the blockade of the CB1 cannabinoid receptor was effective in reducing cue-induced reinstatement of drug seeking, an animal analogue of cue-induced relapse in human addicts [5]. As reported previously, the hippocampus is one of the regions of the brain that expresses a high concentration of the CB1 cannabinoid receptors [15]. Thus, the present findings of: 1) anandamide and R(+)-methanandamide stimulated pCREB activities in the hippocampal neurons, and 2) the anandamide-mediated expression of pCREB was sensitive to the antagonist of the CB1 cannabinoid receptor, suggest the possibility that the hippocampus may be involved in conditioning processes for relapse-like behavior in laboratory animals in concert with the drug-induced activity of midbrain reward neurons.
Although there is evidence to support the cannabinoid receptor-mediated stimulation of cyclic AMP production [17 for review], there are also reports to show that the activation of the cannabinoid receptor inhibited cyclic AMP production. In N18TG2 neuroblastoma cells that expressed endogenous CB1 receptors, the activation of these receptors inhibited adenylyl cyclase [18] and the cAMP/PKA pathway [33, 40]. The inhibition was sensitive to pertussis toxin indicating that Gi/o proteins mediated the inhibition. Current understanding of the two opposing effects of the CB1 receptor on the cAMP production may be explained by the following statements. 1) The CB1 receptor may stimulate the cellular production of an endogenous stimulator of adenylyl cyclase (such as prostaglandins) that could stimulate the production of cAMP [3, 16]. 2) The type of isoform of adenylyl cyclase in a given target cell and the way in which the particular isoform responds to Gi/o-mediated regulation may determine the production of cAMP [32]. 3) Gβγ released from Gi as a result of the CB1 receptor activation can augment Gs-mediated response via certain isoforms of adenylyl cyclase [32]. 4) Direct interaction between the CB1 cannabinoid receptor and Gs protein may occur through a cross talk between the CB1 receptor and other neurotransmitter receptors such as D2 dopamine receptor [2, 10]. 5) The type of cannabinoid agonist and receptor occupancy can change a preferential coupling of the CB1 receptor to Gq/11 [24] or Gs [2]. 6) Lastly, the activation of the CB1 cannabinoid receptor directly stimulates ERK1/2 [6] and MAP kinases [7] for the expression of pCREB.
The CB1 receptors are preferentially expressed in the axon terminals of a subpopulation of GABAergic neurons [21] and glutamatergic neurons in the hippocampus [22]. Although the precise cellular mechanism of action of anandamide and R(+)-methanandamide in the present study is unknown, there may be a possibility that pCREB expression in the hippocampal pyramidal neurons could be mediated trans-synaptically by the inhibition of GABAergic inputs to the pyramidal cells that was induced by the activation of the CB1 cannabinoid receptor [38]. Current understanding is that the CB1 receptor-mediated inhibition of GABA release is likely mediated by 2-AG (2-arachidonoylglycerol) and not by anandamide [39]. To date, there is no report to show that the exogenous application of anandamide inhibited GABA-mediated IPSCs, although such inhibition was reported with WIN 55,212 and CP55,940, which are structurally different types of the CB1 receptor agonists [12]. In addition, anandamide is reported to inhibit metabolism and physiological actions of 2-AG [26]. Nevertheless, we cannot rule out the possibility that the trans-synaptic activation of pCREB by the CB1 receptor-mediated disinhibition might have occurred in the present study and amplified excitatory inputs to the pyramidal cell. Indeed, disinhibition can increase the pyramidal cell excitation and stimulate the expression of pCREB by amplifying the activities of the Group I metabotropic glutamate receptor [25], the L-type Ca2+ channel [9], the NMDA receptor [14], and the calcium-permeable AMPA receptors [30].
In summary, the present study suggests that hippocampal synaptic plasticity and CREB activities are sensitive to the endogenous cannabinoid and may be vulnerable to cannabinoid-mediated maladaptive modulation. A long-term enhancement of pCREB produced by a chronic low-dose application of R(+)-methanandamide further suggests the possibility that a persistent stimulation of pCREB may be a molecular substratum for cellular memory in the hippocampus for the cue-elicited relapse and craving of drug-taking, which plays a key role in the induction and maintenance of the reinforcing effects of psychoactive drugs. Further investigations are needed to identify the cellular signaling cascades involved in the stimulation of pCREB resulting from activation of the CB1 receptor in the hippocampal neuron.
Acknowledgments
This work is supported by NIH grants R15DA021683 and SC1GM081179.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abel T, Kandel E. Positive and negative regulatory mechanisms that mediate long-term memory storage. Brain Res Brain Res Rev. 1998;26:360–78. doi: 10.1016/s0165-0173(97)00050-7. [DOI] [PubMed] [Google Scholar]
- 2.Bonhaus DW, Chang IK, Kwan J, Martin GR. Dual activation and inhibition of adenylyl cyclase by cannabinoid receptor agonists: evidence for agonist-specific trafficking of intracellular responses. J Pharmacol Exp Ther. 1998;287:884–888. [PubMed] [Google Scholar]
- 3.Burstein S, Budrow J, Debatis M, Hunter SA, Subramanian A. Phospholipase participation in cannabinoid-induced release of free arachidonic acid. Biochem Pharmacol. 1994;48:1253–1264. doi: 10.1016/0006-2952(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 4.Casu MA, Pisu C, Sanna A, Tambaro S, Spada GP, Mongeau R, Pani L. Effect of delta 9 tetrahydrocannabibol on phosphorylated CREB in rat cerebellum: an immunohistochemical study. Brain Res. 2005;1048:41–47. doi: 10.1016/j.brainres.2005.04.053. [DOI] [PubMed] [Google Scholar]
- 5.De Vries T, Schoffelmeer ANM. Cannabinoid CB1 receptors control conditioned drug seeking. Trends in Pharmacol Sci. 2005;26:420–426. doi: 10.1016/j.tips.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Derkinderen P, Ledent C, Parmentier M, Girault JA. Cannabinoids activate p38 mitogen-activated protein kinases through CB1 receptors in hippocampus. J Neurochem. 2001;77:957–960. doi: 10.1046/j.1471-4159.2001.00333.x. [DOI] [PubMed] [Google Scholar]
- 7.Derkinderen P, Valjent E, Toutant M, Corvol JC, Enslen H, Ledent, Trzaskos J, Caboche J, Girault JA. Regulation of extracellular signal-regulated kinase by cannabinoids in hippocampus. J Neurosci. 2003;23:2371–82. doi: 10.1523/JNEUROSCI.23-06-02371.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Marzo V, De Petrocellis L, Fezza F, Ligresti A, Bisogno T. Anandamide receptors. Prostaglandins Leukot Essent Fatty Acids. 2002;66:377–391. doi: 10.1054/plef.2001.0349. [DOI] [PubMed] [Google Scholar]
- 9.Dolmetsch RF, Pajvani U, Spotts FK, Greenberg ME. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science. 2001;294:333–339. doi: 10.1126/science.1063395. [DOI] [PubMed] [Google Scholar]
- 10.Felder CC, Joyce KE, Briley EM, Glass M, Mackie KP, Fahey K, Cullinan GJ, Hunden DC, Johnson DW, Chaney MO, Koppel GA, Brownstein M. LY320135, a novel cannabinoid CB1 receptor antagonist, unmasks coupling of the CB1 receptor to stimulation of cAMP accumulation. J Pharmacol Exp Ther. 1998;284:291–297. [PubMed] [Google Scholar]
- 11.Gao C, Chen L, Tao Y, Chen J, Xu X, Zhang G, Chi Z. Colocalization of phosphorylated CREB with calcium/calmodulin-dependent protein kinase IV in hippocampal neurons induced by ohmfentanyl stereoisomers. Brain Res. 2004;1024:25–33. doi: 10.1016/j.brainres.2004.06.084. [DOI] [PubMed] [Google Scholar]
- 12.Hájos N, Katona I, Naiem SS, MacKie K, Ledent C, Mody I, Freund TF. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–49. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- 13.Hajos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- 14.Hardingham GE, Arnold FJL, Bading H. A calcium microdomain near NMDA receptors: on switch for ERK-dependent synapse-to-nucleus communication. Nat Neurosci. 2001:565–566. doi: 10.1038/88380. [DOI] [PubMed] [Google Scholar]
- 15.Herkenham M. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hillard CJ, Bloom AS. Possible role of prostaglandins in the effects of the cannabinoids on adenylate cyclase activity. Bur J Pharmacol. 1983;91:21–27. doi: 10.1016/0014-2999(83)90357-6. [DOI] [PubMed] [Google Scholar]
- 17.Howlett AC. Cannabinoid receptor signaling, Handbook of Experimental Pharmacology. Vol. 168. Springer-Verlag; New York: 2005. pp. 54–69. [DOI] [PubMed] [Google Scholar]
- 18.Howlett AC, Johnson MR, Melvin LS, Milne GM. Nonclassical cannabinoid analgetics inhibit adenylate cyclase: development of a cannabinoid receptor model. Mol Pharmacol. 1988;33:297–302. [PubMed] [Google Scholar]
- 19.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 20.Justinova Z, Solinas M, Gianluigi T, Redhi GH, Goldberg SR. The endogenous cannabinoid anandamide and its synthetic analog R(+)-methanandamide are intravenously self-administered by squirrel monkeys. J Neurosci. 2005;25:5645–5650. doi: 10.1523/JNEUROSCI.0951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-shosaku T, Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelley AE, Schiltz CA. Accessories to addiction: G protein regulators play a key role in cocaine seeking and neuroplasticity. Neuron. 2004;42:181–183. doi: 10.1016/s0896-6273(04)00223-5. [DOI] [PubMed] [Google Scholar]
- 24.Lauckner JE, Hille B, Mackie K. The cannabinoid agonist WIN 55.212-2 increases intracellular calcium via CB1 receptor coupling to Gq/11 G proteins. Proc Nat Acad Sci. 2005;102:19144–19149. doi: 10.1073/pnas.0509588102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mao L, Wang JQ. Glutamate cascade to cAMP response element-binding protein phosphorylation in cultured striatal neurons through calcium-coupled group I metabotropic glutamate receptors. Mol Pharmacol. 2002;62:473–484. doi: 10.1124/mol.62.3.473. [DOI] [PubMed] [Google Scholar]
- 26.Maccarrone M, Rossi S, Bari M, De Chiara V, Fezza F, Musella A, Gasperi V, Prosperetti C, Bernardi G, Finazzi-Agro A, Cravatt BF, Centonze D. Anandamide inhibits metabolism and physiological actions of 2-arachidonoylglycerol in the striatum. Nat Neurosci. 2008;11:152–159. doi: 10.1038/nn2042. [DOI] [PubMed] [Google Scholar]
- 27.Nestler EJ. Molecular mechanisms of drug addiction. Neuropharmacol. 2004;47:24–32. doi: 10.1016/j.neuropharm.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 28.Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- 29.O’Callaghan LP, Sriram K. Focused microwave irradiation of the brain preserves in vivo protein phosphorylation: comparison with other methods of sacrifice and analysis of multiple phosphoproteins. J Neurosci Methods. 2004;135:159–68. doi: 10.1016/j.jneumeth.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Perkinton MS, Sihra TS, Williams RJ. Calcium-permeable AMPA receptors induce phosphorylation of cAMP response element-binding protein through a phosphotidylinositol 3-kinase-dependent stimulation of the mitogen-activated protein kinase signaling cascade in neurons. J Neurosci. 1999;19:5861–5874. doi: 10.1523/JNEUROSCI.19-14-05861.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pertwee RG. Novel pharmacological targets for cannabinoids. Curr Neuropharmacol. 2004;2:9–29. [Google Scholar]
- 32.Rhee MH, Bayewitch M, Avidor-Reiss T, Levy R, Vogel Z. Cannabinoid receptor activation differentially regulates the various adenylyl cyclase isozymes. J Neuroschem. 1998;77:1525–1534. doi: 10.1046/j.1471-4159.1998.71041525.x. [DOI] [PubMed] [Google Scholar]
- 33.Rueda D, Navarro B, Martinez-Serrano A, Guzman M, Galve-Roperh I. The endocannabinoid anandamide inhibits neuronal progenitor cell differentiation through attenuation of the Rap1/B-Raf/ERK pathway. J Biol Chem. 2002;277:46645–46650. doi: 10.1074/jbc.M206590200. [DOI] [PubMed] [Google Scholar]
- 34.Sharma SK, Klee WA, Nirenberg M. Dual regulation of adenylate cyclase accounts for narcotic dependence and tolerance. PNAS USA. 1975;72:3092–3096. doi: 10.1073/pnas.72.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Meth. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 36.Thome J, Saki N, Shin KH, Steffen C, Zhang YJ, Impey S, Storm D, Duman RS. cAMP response element-mediated gene transcription is upregulated by chronic antidepressant treatment. J Neurosci. 2000;20:4030–4036. doi: 10.1523/JNEUROSCI.20-11-04030.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vorel SR. Relapse to cocaine-seeking after hippocampal theta burst stimulation. Science. 2001;292:1175–1178. doi: 10.1126/science.1058043. [DOI] [PubMed] [Google Scholar]
- 38.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signaling at hippocampal synapses. Nature. 2001;410:588–595. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, Watanabe M. Localization of diacylglycerol lipase-a around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci. 2006;26:4740–4751. doi: 10.1523/JNEUROSCI.0054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou D, Song ZH. CB1 cannabinoid receptor-mediated neurite remodeling in mouse neuroblastoma NIE-115 cells. J Neurosci Res. 2001;65:346–353. doi: 10.1002/jnr.1160. [DOI] [PubMed] [Google Scholar]
- 41.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]