Abstract
Background
Tumor necrosis factor α (TNFα), an inflammatory cytokine, was reported to be elevated in trials of heart failure (HF) with reduced ejection fraction (EF) and associated with mortality. Whether this is true for HF with preserved EF is unknown and community data are lacking. We evaluated the distribution of TNFα, its association with baseline characteristics and mortality, and its benefit to assess risk in community HF patients.
Methods and Results
Olmsted County residents with active HF from July 2004 to March 2007 (n=486, mean age 76.7 years, 55% EF ≥50%) were prospectively recruited. Clinical characteristics and TNFα were measured. Elevated TNFα (> assay limit of normal of 2.8pg/mL) was present in 143 (29%). Higher TNFα was associated with decreased creatinine clearance, non-smoking status, anemia, and greater comorbidity (ptrend<0.05 for all). Mortality increased with increasing TNFα (p=0.016), with 1-year mortality estimates of 16%, 18%, 23%, and 32% from lowest to highest quartile, respectively. After adjustment for age, sex, and EF, the hazard ratios for death were 1.24, 1.37, and 1.90 from second to highest TNFα quartile, respectively (ptrend=0.007). TNFα contributed to risk assessment as indicated by the increases in the area under the receiver operating characteristics curves in all models examined (p<0.05 for all). Results did not differ by EF (p=0.60 interaction term of TNFα and EF).
Conclusions
TNFα was elevated in a large portion of community HF patients, was associated with a large decrease in survival, and provided a significant incremental increase in risk assessment above established indicators. TNFα is useful for risk assessment in HF patients with preserved and reduced EF.
Keywords: heart failure, inflammation, epidemiology, risk factors
Introduction
Inflammation plays a key role in the pathogenesis of heart failure (HF)1. Tumor necrosis factor alpha (TNFα), an inflammatory cytokine, has been implicated in HF progression as a mediator of myocardial dysfunction and adverse remodeling2. Studies to date have shown that some patients with HF have elevated circulating levels of TNFα compared with controls3,4 and have suggested that increased circulating levels are associated with increased mortality5,6. However, attempts to utilize TNFα as a therapeutic target in patients with severe systolic dysfunction has resulted in an increase in all-cause mortality7–9. To date, the role that TNFα plays in HF has not been fully elucidated and warrants further investigation.
Most published studies on TNFα are not generalizeable to the entire HF population for several reasons. The vast majority of reports pertained to small number of patients (<150) and focused on clinical trial populations. In addition, they have only included patients with severe systolic dysfunction (ejection fraction (EF)<30–35%), although heart failure with preserved EF accounts for approximately half of all HF cases10. The substantial selection biases hinder the use of TNFα for risk prediction among all patients with HF.
In the present study, we aim to address these gaps in knowledge by examining the distribution of TNFα and its association with baseline characteristics among all HF patients in a population-based setting. Next, we determined whether an association between serum TNFα level and mortality exists and whether this association differs by EF. Finally, we evaluated whether TNFα confers an incremental benefit in predicting risk above recognized predictors in community HF patients.
Methods
Study design
This study is a population-based study conducted in Olmsted County which is located in Southeastern Minnesota and has a population of 124,277 (90% Caucasian, 51% female)11. This type of research is feasible in Olmsted County because nearly all medical care in virtually every specialty is provided by relatively few providers including Mayo Clinic, Olmsted Medical Center, and a few private practitioners. The records from each institution are easily retrievable because Mayo Clinic maintains extensive indices which, through the Rochester Epidemiology Project, are extended to the records of other care providers to county residents. The result is the linkage of all medical records from all sources of care through a centralized system12.
Identification of patients
To identify potential HF cases, natural language processing of the text of the electronic medical record is utilized13. The vast majority of patient visits to the system are transcribed and appear in the record within 24 hours, making prompt ascertainment of newly diagnosed HF cases possible. Next, the complete records of potential cases are reviewed by trained abstractors to collect additional data and verify HF cases using Framingham criteria14. Patients are then contacted directly to obtain consent for participation in the study, which involves Doppler echocardiography and obtaining venous blood samples to measure biomarkers. Recruitment for the cohort is ongoing, allowing assessment of multiple factors in community HF patients over time. Hospitalized patients are contacted while in the hospital, and patients recruited from a clinical setting are contacted at their next clinic visit for consent, enrollment and collection of data. All patients provided written authorization to participate in the study, which was approved by the Mayo Clinic Institutional Review Board.
Data Collection
Echocardiography
All echocardiograms were obtained and analyzed at Mayo Clinic Echocardiography laboratory according to the guidelines of the American Society of Echocardiography15. Left ventricular ejection fraction was measured using M-mode, quantitative, and semi-quantitative methods as previously described and validated16 with excellent correlation between the methods. Ejection fraction values were averaged when multiple measurements were performed. Though EF was dichotomized (<50%, ≥50%)10 for descriptive purposes, it was examined as a continuous variable in all analyses. Diastolic function was assessed by an approach which integrates Doppler measurements of the mitral inflow and Doppler tissue imaging of the mitral annulus using the medial annulus velocity, a method which has been previously validated17. Diastolic function was then classified based on these results into four categories including normal diastolic function, mild diastolic dysfunction (impaired relaxation without increased filling pressures), moderate diastolic dysfunction (impaired relaxation or pseudonormal with moderate elevation of filling pressures) and severe diastolic dysfunction (severely reduced compliance). In the case of severe mitral stenosis, mitral valve prosthesis, or missing data, diastolic function was classified as indeterminate.
TNFα measurement
Venous samples were obtained as soon as possible after recruitment of patients in both the inpatient and outpatient setting. All venous samples were stored in EDTA tubes, processed and stored at −70C until TNFα measurement took place (samples were not thawed in the interim). TNFα level was quantified by enzyme-linked immunoassay (TNFSF1A Immunoassay, RND systems, Minneapolis, MN). Samples were incubated in microtitre plate wells with a monoclonal antibody specific for TNFα. Following a wash, an enzyme linked polyclonal antibody specific for TNFα was added. After incubation and second wash, an amplifier solution was added and the color developed in proportion to the amount of TNFα bound in the first step. The optical density of each well was measured to determine the concentration of TNFα present in each sample. The reference range for the assay is 0.550 pg/mL to 2.816 pg/mL. All of the assays were performed in the Mayo Clinic Immunochemical Core Laboratory and technicians were blinded to the clinical characteristics and outcomes of the patients.
Additional patient data
Data pertaining to baseline patient characteristics were obtained by review of the medical record by trained nurse abstractors. Prior myocardial infarction (MI) occurring in Olmsted County was defined through use of standardized MI criteria, which have been previously described and validated18. Physician’s diagnosis was used to document history of coronary artery disease (CAD), history of chronic obstructive pulmonary disease (COPD), and history of atrial fibrillation/flutter. Hypertension was defined as systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg or the use of anti-hypertensive medications. Smoking status was classified as ‘current’, ‘former’ (quit at least 6 months ago), or ‘never’ based on documentation. Hyperlipidemia was defined using National Cholesterol Education Program guidelines19 or use of hyperlipidemia medications. Diabetes mellitus was defined using the American Diabetes Association criteria20. The height and weight documented at the time of HF diagnosis were utilized to calculate body mass index (BMI). Creatinine clearance was calculated based on the last outpatient creatinine value using the Cockcroft-Gault equation21. Severe renal disease was defined as a Creatinine clearance <30ml/min. The hemoglobin value nearest the date of HF diagnosis was used to diagnose anemia using World Health Organization criteria (hemoglobin <13 mg/dL in men or 12 mg/dL in women)22. Charlson Index, a comorbidity score reflecting the cumulative increased likelihood of one-year mortality, was also collected23. New York Heart Association functional class was also collected.
Mortality Follow-up
Follow-up took place through passive surveillance of the community medical records. The ascertainment of death included death certificates filed in Olmsted County, obituary notices and electronic files of death certificates obtained from the State of Minnesota Department of Vital and Health Statistics12. All-cause mortality was used as the endpoint in all analyses.
Statistical Analysis
Subjects were divided into quartiles based on TNFα level (<1.5, 1.5≤TNFα<2.1, 2.1≤TNFα<3.1, ≥3.1pg/mL). Because the TNFα distribution was skewed, log TNFα was utilized for all analyses of TNFα as a continuous variable. Baseline characteristics are presented as frequencies or means with standard deviations. Trends in baseline characteristics across quartiles were analyzed using generalized linear models for continuous variables and Mantel Haenszel χ2 for categorical variables. In addition, log TNFα was analyzed as a continuous variable with all baseline characteristics to ensure that a significant association was not missed by quartile analysis. All patients had TNFα measurements and echocardiography performed. Data was complete in all other areas with the exception of smoking status (n=1 missing), prior history of MI (n=11 missing, also used to calculate Charlson index), hemoglobin (n=1 missing) and creatinine clearance (n=14 missing).
Mortality was assessed using the Kaplan-Meier method with censoring at the time of last follow-up. Cox proportional hazard regression analysis was utilized to estimate both unadjusted and adjusted hazard ratios for mortality by TNFα group using the lowest quartile as the reference. Baseline clinical characteristics were adjusted for in the multivariable models using forced entry. Individual comorbidities were adjusted for rather than Charlson index score. In an ancillary analysis, to investigate whether the association between TNFα and mortality differed among those with incident or prevalent HF, we restricted the sample to those with incident HF and performed Cox proportional hazard regression analysis. Finally, logistic regression analysis was performed to assess the incremental value of TNFα in determining risk of death in HF patients. Models before and after the addition of TNFα were compared using the area under the receiver operating characteristic (ROC) curve with methods previously described24 and were one-tailed. A p value <0.05 was used as the level of significance. Analyses were performed using SAS 9.1 (SAS Institute Inc., Cary, NC) and JMP Version 6.0.0. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Patient Identification and Baseline Characteristics
Four hundred eighty-six consecutive patients with active HF were enrolled in the study between July 29, 2004 and March 7, 2007 and underwent echocardiography and TNFα assay. The consent rate for the study during this time period was 71%. The mean age of participants was 76.7±13.0 years, 236 (48.6%) were male, and 266 (54.8%) had preserved EF (≥50%) (Table 1). The population had a high index of comorbidity with 316 (66.5%) with Charlson Index ≥3 and overall poor functional status with 355 (73.0%) having NYHA class III/IV HF symptoms.
Table 1.
Baseline Patient Characteristics
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |||
|---|---|---|---|---|---|---|
| Characteristics | Overall (n=486) | TNFα<1.5 pg/mL (n=118) | 1.5 TNFα<2.1 pg/mL (n=130) | 2.1 TNFα<3.1 pg/mL (n=120) | TNFα 3.1 pg/mL (n=118) | Ptrend |
| Age (yrs), mean (SD) | 76.7 (13.0) | 74.4 (12.5) | 77.0 (14.0) | 78.2 (11.8) | 77.1 (13.2) | 0.09 |
| Male | 236 (48.6) | 52 (44.1) | 65 (50.0) | 64 (53.3) | 55 (46.6) | 0.60 |
| EF | 49.2 (16.4) | 47.9 (17.4) | 47.7 (16.4) | 50.3 (16.2) | 51.1 (15.6) | 0.07 |
| EF≥50% | 266 (54.8) | 61 (51.7) | 66 (50.8) | 69 (58.0) | 70 (59.3) | 0.14 |
| Risk Factors | ||||||
| Hypertension | 391 (80.4) | 86 (72.9) | 109 (83.8) | 98 (81.7) | 98 (83.1) | 0.09 |
| Current smoker | 40 (8.2) | 20 (17.1) | 5 (3.8) | 10 (8.3) | 5 (4.2) | 0.003 |
| Hyperlipidemia | 326 (67.1) | 78 (66.1) | 89 (68.5) | 79 (65.8) | 80 (67.8) | 0.91 |
| Diabetes | 148 (30.5) | 30 (25.4) | 42 (32.3) | 42 (35.0) | 34 (28.8) | 0.50 |
| BMI (kg/m2), mean (SD) | 29.2 (7.9) | 29.5 (8.3) | 29.3 (7.9) | 28.8 (7.7) | 29.2 (7.8) | 0.64 |
| Comorbidities | ||||||
| Prior MI | 118 (24.8) | 26 (22.0) | 36 (28.3) | 23 (19.3) | 33 (29.7) | 0.46 |
| Prior CAD | 261 (53.7) | 58 (49.2) | 66 (50.8) | 66 (55.0) | 71 (60.2) | 0.07 |
| COPD | 140 (28.8) | 42 (35.6) | 29 (22.3) | 39 (32.5) | 30 (25.4) | 0.30 |
| Anemia | 258 (53.2) | 42 (35.6) | 63 (48.8) | 74 (61.7) | 79 (66.9) | <0.001 |
| Severe renal disease | 81 (17.2) | 4 (3.5) | 16 (12.6) | 25 (21.6) | 36 (31.3) | <0.001 |
| Creatinine clearance (ml/min), mean (SD) | 58.8 (33.9) | 70.2 (33.2) | 61.0 (33.4) | 56.2 (35.0) | 47.7 (30.6) | <0.001 |
| C- Reactive Protein (mg/dL) | 4.34 (6.61) | 2.41 (4.39) | 3.64 (5.48) | 4.39 (6.17) | 7.01 (8.91) | <0.001 |
| Atrial fibrillation/Flutter | 155 (31.9) | 44 (37.3) | 43 (33.1) | 37 (30.8) | 31 (26.3) | 0.07 |
| NYHA class 3 or 4 | 355 (73.0) | 89 (75.4) | 101 (77.7) | 81 (67.5) | 84 (71.2) | 0.20 |
| Charlson Index ≥3 | 316 (66.5) | 64 (54.2) | 83 (65.4) | 87 (73.1) | 82 (73.9) | 0.001 |
| Moderate or severe diastolic dysfunction | 369 (75.9) | 84 (71.2) | 98 (75.4) | 95 (79.2) | 92 (78.0) | 0.17 |
All values are reported as n(%) unless otherwise noted
BMI = body mass index, CAD= coronary artery disease, COPD= chronic obstructive pulmonary disease, EF= ejection fraction, MI= myocardial infarction, NYHA= New York Heart Association
TNFα Levels
TNFα levels ranged from 0.3 to 28.0 pg/mL with a mean value of 3.1± 3.4 pg/mL and median value of 2.1 pg/mL (25th–75th percentile 1.5–3.1 pg/mL). 143 (29%) of the cohort had TNFα levels above the reference range for the test (>2.8 pg/mL). The distribution of TNFα did not differ significantly between patients recruited in an inpatient and outpatient setting (p=0.31). Higher TNFα was associated with current non-smoking status, increased prevalence of anemia, and a higher level of comorbidity (Table 1). In addition, higher TNFα was associated with decreasing creatinine clearance (Figure 1a) and decreasing hemoglobin (Figure 1b). The graded association is suggestive of a “dose response” effect. There was no association between TNFα and BMI or NYHA functional class. The distribution of TNFα [median (25th–75th percentile)] was 2.2 (1.5–3.2) and 1.9 (1.4–2.9 pg/mL) among those with preserved and reduced EF, respectively. Though a non-significant trend toward increasing age and EF with higher TNFα quartile were noted, both relationships remained non-significant when TNFα was examined as a continuous variable (p=0.13 age vs. log TNFα, p=0.15 EF vs. log TNFα, data not shown), indicating there was no significant association between age and TNFα or EF and TNFα. No gender-based differences were present. Finally, there was a trend toward increased frequency of CAD among those with higher TNFα, but it was not significant (p=0.069).
Figure 1.
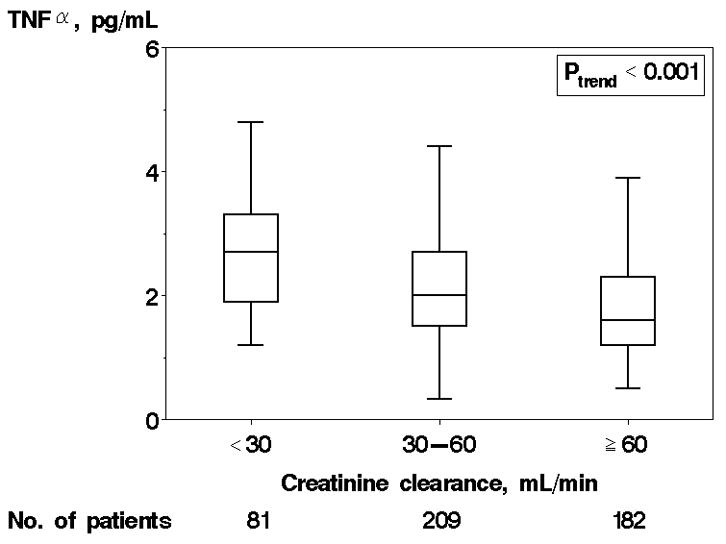
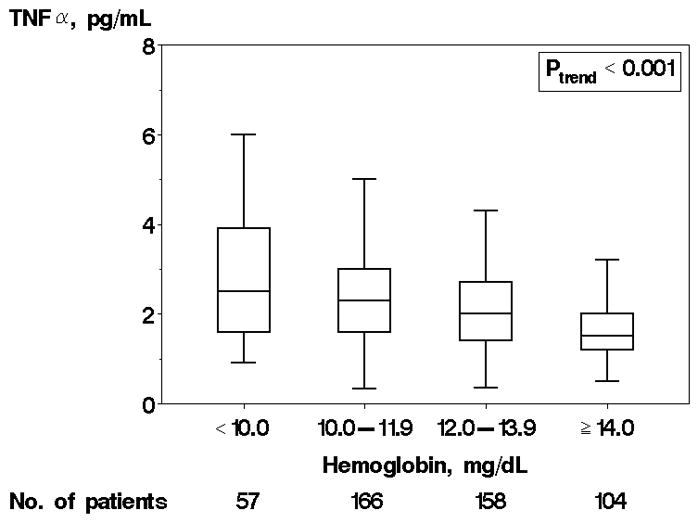
Tumor Necrosis Factor α (TNFα) levels by Creatinine Clearance and Hemoglobin. Caption: TNFα levels by creatinine clearance (A) and hemoglobin (B) are shown. Each box displays the median, 25th percentile, and 75th percentile values; horizontal bars represent 1.5 times the interquartile range.
No.= number
TNFα Level and Association with Mortality
After a mean follow-up of 17 ± 10 months, 147 (30.2%) patients were dead. The one year Kaplan-Meier mortality estimate was 22% (18%–26%) overall, and increased from lowest to highest quartile [16%(9%–22%), 18%(11%–25%), 23%(15%–29%), and 32%(23%–40%)] respectively p=0.016, Figure 2). The unadjusted hazard ratios (HR) (95% CI) for death were 1.34 (0.82–2.21), 1.47 (0.89– 2.44), 2.10 (1.30–3.38) from lowest to highest quartile, respectively, using the lowest quartile as the referent (ptrend=0.002, Table 2). After adjustment for age, sex, EF, and comorbidities, this relationship held with a hazard ratio for death of 1.88 (1.09–3.25) in the highest vs. lowest quartile (ptrend across quartiles= 0.028). The largest impact on the increase in hazard ratios observed from Model 1 to 2 was due to adjustment for smoking status, while adjustment for creatinine clearance and anemia attenuated the association between TNFα and mortality. Adding C-reactive protein (CRP) to the model did not appreciably change the association between TNFα and mortality. The relationship between TNFα and mortality was similar among those with incident HF compared with the overall population. No interaction between TNFα and EF was noted (p=0.60 for interaction term), indicating that associations between TNFα and death did not differ significantly for patients with preserved vs. reduced EF.
Figure 2.
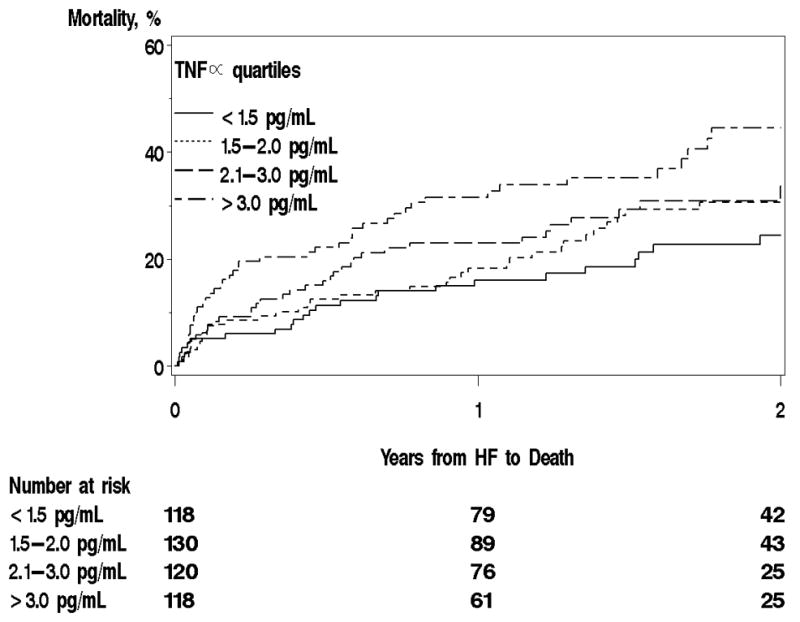
Kaplan-Meier Mortality Curves by TNFα Quartile.
Caption: Mortality during follow-up was analyzed by TNFα quartile.
HF= heart failure
Table 2.
Hazard Ratios (95% CI) for Death Associated with TNFα Quartile
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P value (trend) | |
|---|---|---|---|---|---|
| TNFα<1.5 pg/mL | 1.5 TNFα<2.1 pg/mL | 2.1 TNFα<3.1 pg/mL | TNFα 3.1 pg/mL | ||
| Unadjusted | 1 (reference) | 1.34 (0.82, 2.19) | 1.47 (0.89, 2.44) | 2..10 (1.30, 3.38)† | 0.002 |
| Model 1 | 1 | 1.24 (0.76, 2.04) | 1.37 (0.83, 2.28) | 1.90(1.18, 3.08)† | 0.007 |
| Model 2 | 1 | 1.46 (0.87, 2.43) | 1.57 (0.92, 2.64) | 2.37 (1.43, 3.93)† | <0.001 |
| Model 3 | 1 | 1.27 (0.75, 2.14) | 1.26 (0.73, 2.16) | 1.88(1.09, 3.25)* | 0.028 |
Model 1 adjusted for age, sex, and EF
Model 2 adjusted for above plus hypertension, hyperlipidemia, smoking status, body mass index, diabetes mellitus, coronary artery disease, chronic obstructive pulmonary disease, New York Heart Association functional class, atrial fibrillation/flutter
Model 3 adjusted for above plus creatinine clearance, anemia
p<0.05;
p<0.01
Addition of TNFα Level to Mortality Risk Assessment
Inclusion of TNFα in the model resulted in a notable increase in predictive value of mortality in patients with heart failure (Table 3). For instance, the area under the receiver operating characteristic curve increased from 0.67 to 0.70 in a model that included age and sex (p=0.035), and from 0.74 to 0.78 in a model that include age, sex, EF, NYHA functional class, and traditional risk factors (p=0.018).
Table 3.
Area under receiver operating characteristic curves predicting 6-month mortality
| Area under the curve Overall (n=486) | P value | ||
|---|---|---|---|
| Model without TNFα | Model with TNFα | ||
| Unadjusted | 0.500 | 0.600 (0.534,0.666) | <0.01 |
| Model 1* | 0.671 (0.610,0.732) | 0.700 (0.642,0.758) | 0.035 |
| Model 2† | 0.687 (0.627,0.747) | 0.723 (0.667,0.779) | 0.030 |
| Model 3‡ | 0.742 (0.689, 0.795) | 0.777 (0.728,0.826) | 0.018 |
Model 1- Age, sex
Model 2- Above + risk factors (hypertension, hyperlipidemia, diabetes mellitus, tobacco use, prior coronary artery disease)
Model 3- Above + New York Heart Association functional class, ejection fraction
Discussion
In the present study, we demonstrated that serum TNFα is elevated in a large proportion of community HF patients with a wide range of EF, and that elevated circulating TNFα was strongly associated with decreased creatinine clearance, anemia, and a high degree of comorbidity. Second, we identified a strong independent association between elevated TNFα and mortality in HF patients, irrespective of EF. Finally, we have demonstrated that TNFα improved risk prediction in HF above traditional risk indicators.
HF is characterized by an inflammatory state with reports of elevation of several inflammatory cytokines, including TNFα in selected patients with HF1,25. TNFα is an inflammatory cytokine produced mainly by monocytes and macrophages with pleiotropic effects. It binds to cell surface receptors preventing the usual rise in intracellular calcium concentrations, resulting in activation of multiple signal transduction pathways, kinases, and transcription factors26,27. TNFα was first recognized as a possible mediator of cardiac dysfunction in sepsis after exposure to endotoxin28,29. Since that time, elevated TNFα has been demonstrated in many cardiac conditions, including myocarditis, hypertrophic cardiomyopathy30, MI31, following cardiopulmonary bypass32, and HF.
TNFα has been implicated in HF disease progression. Animal studies have demonstrated increased cardiac expression of TNFα in failing myocardium33 and noted that overexpression of myocardial TNFα resulted in left ventricular dysfunction and dilatation34–37. TNFα has also been shown to be a mediator of hypertrophy38 and to contribute to adverse left ventricular remodeling39. Despite its many deleterious effects, TNFα was demonstrated to have some protective effects, namely against acute ischemic injury40,41. Overall, TNFα has a complex role in the inflammatory processes that is still incompletely elucidated.
Human studies of TNFα were performed in specific populations, such as those enrolled in a randomized controlled trial of the inotropic drug vesnarinone5, and are thus not generalizeable to the majority of HF patients. Specifically, clinical studies evaluating serum TNFα and clinical trials targeting TNFα were restricted to patients with severe systolic dysfunction (EF ≤30–35%). Thus, although more than half of all HF patients have preserved EF10, there have been no clinical studies examining the role of TNFα in these patients. Further, while prior studies reported that TNFα was elevated in the setting of systolic dysfunction3,4,42, its distribution and determinants remain incompletely defined. Indeed, there are conflicting reports on the association of TNFα level with HF severity with some studies demonstrating increased levels with worsening NYHA functional class6,43, and other studies demonstrating no association5,42. Similarly, studies noted that higher TNFα may be associated with older age5, decreased renal function3,6, and decreased body weight3, while other failed to do so43. Finally, the need for community studies examining the role of TNFα was recognized2 but up to now not addressed.
The present study contributes to address these gaps in knowledge as it demonstrates that elevated circulating TNFα is present in approximately one third of community HF patients with a wide range of EF and is associated with decreasing hemoglobin and creatinine clearance, greater comorbidity, and current non-smoking status. While we identified a marginal trend towards higher TNFα levels with older age, we however, found no association between TNFα and NYHA functional class. In addition, the present data challenges the prior notion that TNFα may be a marker of “cardiac cachexia”3 given the lack of association with BMI.
In clinical trials of HF with severe systolic dysfunction, elevated TNFα was associated with increased mortality5,6. However, data from HF patients in the community and with preserved EF were lacking. The present study demonstrated that elevated TNFα is independently associated with mortality in patients with both preserved and reduced EF, thereby underscoring the relevance of this biomarker for risk prediction in all categories of the HF syndrome.
Further, the data presented herein indicated that TNFα makes a substantial a contribution to risk prediction. Indeed, as recently underscored, hazard ratios and p values are useful in determining statistical significance, but in order to demonstrate clinical utility in risk prediction, further measures must be reported44. One established way of examining incremental changes in predictive ability involves measuring the area under the ROC curve. By using ROC analysis, we demonstrated that TNFα provides an improved risk prediction over established indicators.
Strengths and Limitations
Though Olmsted County is becoming more diverse, the population studied was primarily Caucasian, so these data should be replicated in other racial and ethnic groups. The participation rate for the study was 71%, which is similar to rates reported in other community studies of cardiovascular disease45,46. Further, it should be noted that blood specimens are stored for future research as a part of the present study, and assessment of other factors, including biomarkers, is likely in future investigations. The present study has several notable strengths. First, we examined TNFα in a large community HF population, which has not been previously reported. Second, patients with the full spectrum of EF were examined, and complete EF ascertainment was present. This enabled evaluating the role of TNFα in patients with preserved EF for the first time.
Conclusions
Elevated levels of the inflammatory cytokine TNFα are present in a large subset of community patients with HF, both with preserved and reduced EF. TNFα is independently associated with increased mortality. Furthermore, TNFα improves risk prediction above other established indicators, and can be useful for risk stratification in patients with HF.
Acknowledgments
We thank the following individuals for their support with study coordination, data collection, and data entry and analysis: Ellen Koepsell, RN, Kay A. Traverse, RN, and Susan Stotz, RN.
Funding Sources- This study was supported by grants from the National Institute of Health (RO1 HL 59205, RO1 HL 72435) and by an American Heart Association Postdoctoral Greater Midwest Fellowship Award to Dr. Dunlay. Dr. Roger is an established investigator of the American Heart Association.
Footnotes
“This is an un-copyedited author manuscript that was accepted for publication in Circulation, copyright The American Heart Association. This may not be duplicated or reproduced, other than for personal use or within the “Fair Use of Copyrighted Materials” (section 107, title 17, U.S. Code) without prior permission of the copyright owner, The American Heart Association. The final copyedited article, which is the version of record, can be found at http://circ.ahajournals.org. The American Heart Association disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.”
Disclosures- none
References
- 1.Torre-Amione G. Immune activation in chronic heart failure. American Journal of Cardiology. 2005;95(suppl):3C–8C. doi: 10.1016/j.amjcard.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong EJ, Morrow DA, Sabatine MS. Inflammatory biomarkers in acute coronary syndromes: part I: introduction and cytokines. Circulation. 2006;113:e72–e75. doi: 10.1161/CIRCULATIONAHA.105.595520. [DOI] [PubMed] [Google Scholar]
- 3.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. New England Journal of Medicine. 1990;323:236–41. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 4.Dutka DP, Elbown JS, Delamere F, Shale DJ, Morris GK. Tumour necoris factor alpha in severe congestive heart failure. British Heart Journal. 1993;70:141–3. doi: 10.1136/hrt.70.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deswal A, Peterson NJ, Feldman AM, Young JB, White BG, Mann DL. Cytokines and cytokine receptors in advanced heart failure: An analysis of the cytokine database from the vesnarinone trial (VEST) Circulation. 2001;103:2055–9. doi: 10.1161/01.cir.103.16.2055. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Reyna TS, Arrieta O, Castillo-Martinez L, Guevara P, Rebollar V, Granados J. Tumour necrosis factor alpha and troponin T as predictors of poor prognosis in patients with stable heart failure. Clinical Investigation in Medicine. 2005;28:23–9. [PubMed] [Google Scholar]
- 7.Chung ES, Packer M, Lo KH, Fasanmade AA, Wilkerson JT. Randomized, double-blind, placebo-controlled, pilot trial of Infliximab, a chimeric monoclonal antibody to tumor necrosis factor-α, in patients with moderate-to-severe heart failure. Circulation. 2003;107:3133–40. doi: 10.1161/01.CIR.0000077913.60364.D2. [DOI] [PubMed] [Google Scholar]
- 8.Mann DL, McMurray JJ, Packer M, Swedberg K, Borer JS, Colucci WS, Djian J, Drexler H, Feldman A, Kober L, Krum H, Liu P, Nieminen M, Tavazzi L, van Veldhuizzen DJ, Waldenstrom A, Warren M, Westheim A, Zannad F, Fleming T. Targeted anticytokine therapy in patients with chronic heart failure: results of the randomized Etanercept worldwide evaluation (RENEWAL) Circulation. 2004;109:1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 9.Anker SD, Coats AJ. How to RECOVER from RENAISSANCE? The significance of the results from RECOVER, RENAISSANCE, RENEWAL, and ATTACH. International Journal of Cardiology. 2002;86:123–30. doi: 10.1016/s0167-5273(02)00470-9. [DOI] [PubMed] [Google Scholar]
- 10.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. Journal of the American Medical Association. 2006;296:2209–16. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Census Bureau State and County QuickFacts. [Accessed September 27, 2007]; Available at: http://quickfacts.census.gov/qfd/states/27/27109.html.
- 12.Melton LJ. History of the Rochester Epidemiology Project. Mayo Clinic Proceedings. 1996;71(3):266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 13.Pakhomov SV, Buntrock J, Chute CG. Prospective recruitment of patients with congestive heart failure using an ad-hoc binary classifier. Journal of Biomedical Informatics. 2005;38:145–53. doi: 10.1016/j.jbi.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–15. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Biereg M, Devereux RB. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Echocardiography. Journal of the American Society of Echocardiography. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Quinones MA, Waggoner AD, Reduto LA, Nelson JD, Young JB, Winters WL, Ribeiro LG, Miller RR. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981;64:744–53. doi: 10.1161/01.cir.64.4.744. [DOI] [PubMed] [Google Scholar]
- 17.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik MM. Clinical utility of doppler echocardiography and tissue doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous doppler- catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 18.Roger VL, Killian J, Henkel M, Weston SA, Goraya TY, Yawn BP, Kottke TE, Frye RL, Jacobsen SJ. Coronary disease surveillance in Olmsted County objectives and methodology. Journal of Clinical Epidemiology. 2002;55:593–601. doi: 10.1016/s0895-4356(02)00390-6. [DOI] [PubMed] [Google Scholar]
- 19.Grundy SM, Becker D, Clark LT, et al. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III) Circulation. 2002;106:3143–57. [PubMed] [Google Scholar]
- 20.Genuth S, Albert KG, Bennett P. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–71. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 21.Cockroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Nutritional Anemias: Report of a WHO scientific group. WHO Technical Report Series. 1968;405:1. [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, McKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–43. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 25.Mann DL. Targeted anticytokine therapy and the failing heart. American Journal of Cardiology. 2005;95(suppl):9C–16C. doi: 10.1016/j.amjcard.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Khanna D, McMahon M, Furst DE. Anti-tumor necrosis factor α therapy and heart failure. Arthritis & Rheumatism. 2004;50:1040–50. doi: 10.1002/art.20164. [DOI] [PubMed] [Google Scholar]
- 27.Vilcek J, Lee TH. Tumor necrosis factor: new insights into the molecular mechanisms of its multiple actions. Journal of Biological Chemistry. 1991;266:7313–6. [PubMed] [Google Scholar]
- 28.Suffredini AF, Fromm RE, Parker MM, Brenner M, Kovacs JA, Wesley RA, Parillo JE. The cardiovascular response of normal humans to the administration of endotoxin. New England Journal of Medicine. 1989;321:280–7. doi: 10.1056/NEJM198908033210503. [DOI] [PubMed] [Google Scholar]
- 29.Kapadia S, Lee J, Torre-Amione G, Birdsall HH, Ma TS, Mann DL. Tumor necrosis factor-α gene and protein expression in adult feline myocardium after endotoxin administration. Journal of Clinical Investigation. 1995;96:1042–52. doi: 10.1172/JCI118090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumori A, Yamada T, Suzuki H, Matoba Y, Sasayama S. Increased circulating cytokines in patients with myocarditis and cardiomyopathy. British Heart Journal. 1994;72:561–6. doi: 10.1136/hrt.72.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Latini R, Bianchi M, Correale E, Dinarello CA, Fantuzzi G, Fresco C, Maggioni A, Mengozzi M, Romano S, Shapiro L, Zironi M, Tognoni G, Turato R, Ghezzi P. Cytokines in acute myocardial infarction: selective increase in circulating tumor necrosis factor, its soluble receptor and interleukin-1 receptor antagonist. Journal of Cardiovascular Pharmacology. 1994;23:1–6. [PubMed] [Google Scholar]
- 32.Hattler BG, Zeevi A, Oddis CV, Finkel MS. Cytokine induction during cardiac surgery: analysis of TNF-alpha expression pre and post-cardiopulmonary bypass. Journal of Cardiac Surgery. 1995;10:418–22. doi: 10.1111/j.1540-8191.1995.tb00671.x. [DOI] [PubMed] [Google Scholar]
- 33.Torre-Amione G, Kapadia S, Lee J, Bias RD, Liebowitz R, Mann DL. Expression and functional significance of tumor necrosis factor receptors in human myocardium. Circulation. 1995;92:1487–93. doi: 10.1161/01.cir.92.6.1487. [DOI] [PubMed] [Google Scholar]
- 34.Bryant D, Becker L, Richardson J, Shelton-1 J, Franco F, Peshock R, Thompson M, Biroir B. Cardiac failure in transgenic mice with myocardial expression of tumor necrosis factor-α. Circulation. 1998;97:1375–81. doi: 10.1161/01.cir.97.14.1375. [DOI] [PubMed] [Google Scholar]
- 35.Kubota T, McTiernan CF, Frye CS, Slawson SE, Lemster BH, Koretsky AP, Demetris AJ, Feldman AM. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-α. Circulation Research. 1997;81:627–35. doi: 10.1161/01.res.81.4.627. [DOI] [PubMed] [Google Scholar]
- 36.Sun M, Dawood F, Wen W, Chen M, Dixon I, Krishenbaum LA, Liu PP. Excessive tumor necrosis factor activation after infarction contributes to susceptibility of myocardial rupture and left ventricular dysfunction. Circulation. 2004;110:3221–8. doi: 10.1161/01.CIR.0000147233.10318.23. [DOI] [PubMed] [Google Scholar]
- 37.Bozkurt B, Kribbs SB, Clubb FJ, Michael LH, Didenko VV, Hornsby PJ, Seta Y, Oral H, Spinale FG, Mann DL. Pathophysiologically relevant concentrations of tumor necrosis factor-α promote left ventricular dysfunction and remodeling in rats. Circulation. 1998;97:1382–91. doi: 10.1161/01.cir.97.14.1382. [DOI] [PubMed] [Google Scholar]
- 38.Yokoyama T, Nakano M, Bednarczyk JL, McIntyre BW, Entman M, Mann DL. Tumor necrosis factor α provodes a hypertrophic growth response in adult cardiac myocytes. Circulation. 1997;95:1247–52. doi: 10.1161/01.cir.95.5.1247. [DOI] [PubMed] [Google Scholar]
- 39.Sun M, Chen M, Dawood F, Zurawska U, Li JY, Parker T, Kassiri Z, Kirshenbaum LA, Arnold M, Khokha R, Liu PP. Tumor necrosis factor-α mediates cardiac remodeling and ventricular dysfunction after pressure overload state. Circulation. 2007;115:1398–1407. doi: 10.1161/CIRCULATIONAHA.106.643585. [DOI] [PubMed] [Google Scholar]
- 40.Nakano M, Knowlton AA, Dibbs Z, Mann DL. Tumor necrosis factor α confers resistance to hypoxic injury in the adult mammalian cardiac myocyte. Circulation. 1998;97:1392–1400. doi: 10.1161/01.cir.97.14.1392. [DOI] [PubMed] [Google Scholar]
- 41.Lecour S, Smith RM, Woodward B, Opie LH, Rochette L, Sack MN. Identification of a novel role for sphingolipid signaling in TNFα and ischemic preconditioning mediated cardioprotection. Journal of Mollecular and Cellular Cardiology. 2002;34:509–18. doi: 10.1006/jmcc.2002.1533. [DOI] [PubMed] [Google Scholar]
- 42.Aukrust P, Ueland T, Lien E, Bendtzen K, Muller F, Andreassen AK, Norday I, Aass H, Espevik T, Simonsen S, Froland SS, Gullestad L. Cytokine network in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. American Journal of Cardiology. 1999;83:376–82. doi: 10.1016/s0002-9149(98)00872-8. [DOI] [PubMed] [Google Scholar]
- 43.Torre-Amione G, Kapadia S, Benedict C, Oral H, Young JB, Mann DL. Proinflammatory cytokine levels in patients with depressed left ventricular ejection fraction: A report from the studies of left ventricular dysfunction (SOLVD) Journal of the American College of Cardiology. 1996;27:1201–6. doi: 10.1016/0735-1097(95)00589-7. [DOI] [PubMed] [Google Scholar]
- 44.Greenland P. When is a new prediction marker useful? Arch Intern Med. 2005;165:2454–2456. doi: 10.1001/archinte.165.21.2454. [DOI] [PubMed] [Google Scholar]
- 45.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. American Journal of Epidemiology. 1979;110:281–90. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 46.Luepker RV, Jacobs DR, Gillum RF, Folsom AR, Prineas RJ, Blackburn H. Population risk of cardiovascular disease: the Minnesota Heart Survey. Journal of Chronic Disease. 1985;38:671–82. doi: 10.1016/0021-9681(85)90021-9. [DOI] [PubMed] [Google Scholar]


