Abstract
Polyethylene glycol (PEG), a high-molecular-weight colloid present in new organ preservation solutions, protects against cold ischemia injuries leading to better graft function of transplanted organs. This protective effect cannot be totally explained by immuno-camouflaging property or signaling-pathway modifications. Therefore, we sought for an alternative mechanism dependent on membrane fluidity. Using the Langmuir–Pockles technique, we show here that PEGs interacted with lipid monolayers of defined composition or constituted by a renal cell lipid extract. High-molecular-weight PEGs stabilized the lipid monolayer at low surface pressure. Paradoxically, at high surface pressure, PEGs destabilized the monolayers. Hypothermia reduced the destabilization of saturated monolayer whereas unsaturated monolayer remained unaffected. Modification of ionic strength and pH induced a stronger stabilizing effect of PEG 35,000 Da which could explain its reported higher effectiveness on cold-induced injuries during organ transplantation. This study sheds a new light on PEG protective effects during organ preservation different from all classical hypotheses.
Keywords: Organ transplantation, Polyethylene Glycol, Hypothermia, Membrane Fluidity, Glycerophospholipid
Introduction
Transplanted organs are subjected to a sequence of cold ischemia-reperfusions that can affect graft function and survival. Preservation solutions, developed in order to prevent cold ischemia injury, contain agents such as colloids that limit cell swelling. The inclusion of hydroxyethyl starch (HES) in the so-called University of Wisconsin (UW) solution, the standard preservation solution used in transplantation, has become increasingly controversial, and it has been suggested that HES could be efficiently replaced by polyethylene glycols (PEGs) [1, 2]. Recent reports have shown that substituting HES by a 35,000 Da PEG (PEG35000) in an extracellular UW-like solution (IGL-1®) improved rat liver viability or pig kidney function [3, 4]. In addition, our group has previously demonstrated in an auto-transplanted pig kidney model the beneficial effect on early and long-term cold ischemia-reperfusion injury of a saline solution with a composition mimicking an extracellular milieu and containing a 20,000 Da PEG (PEG20000) [5].
PEG, a linear polymer of ethylene oxide with hydroxyl terminal groups [H–(O–CH2–CH2)n–OH], is a neutral, water soluble compound. In transplantation, PEG has been suggested to act as an immuno-camouflaging molecule that limits cellular infiltration of the graft [6]. Indeed, the size of high-molecular-weight PEG could be of paramount importance to avoid cell–cell interaction via immunological synapses and therefore to impair the T-cell activation which participates in the initial inflammatory response and contributes to the cold ischemia–reperfusion injuries of the transplanted organ [7–9]. However, the immuno-camouflaging theory cannot totally explain the PEG protective effect observed during organ transplantation. Indeed, tissue injury occurs during allograft as well as during auto-graft without any allergenic stimulation [10].
We previously demonstrated that PEG present in an extracellular type solution protected, to some extent, cultured renal epithelial cells against damages caused by hypothermia and that the different PEGs tested from 400 to 20,000 Da reduced the cold-induced cell death by 50% [11]. Only PEG35000 was able to protect totally against necrosis. However, the mechanism by which PEG prevents cell swelling and thus maintains cell viability is not clear yet. The effects of PEG appeared to be related to preservation of cell shape. Indeed, it has been shown that PEG can preserve actin filament and microtubule morphology after prolonged cold storage [12]. No membrane receptor for PEG is known, and, therefore, the mechanism of PEG’s action remains poorly understood. Fluorescence polarization studies have suggested that PEG interacts with and stabilizes lipids in the plasma membrane [13].
The present study was undertaken to further explore the interaction between PEG and glycerophospholipids in monolayers with the Langmuir–Pockles technique in order to better characterize a possible interaction between phospholipid membranes and PEGs of different molecular weights.
Materials and methods
Lipids and subphase
Synthetic 1,2-dipalmitoyl phosphatidylcholine (DPPC), 1,2-dipalmytoyl-phosphatidylserine (DPPS), 1,2-dipalmitoyl phosphatidylethanolamine (DPPE), 1-stearoyl-2-oleoyl phosphatidylcholine (SOPC), and 1-stearoyl-2-docosahexenoyl phosphatidylserine (SDPS) were purchased from Avanti Polar Lipids Inc (Birmingham, AL, USA). The lipids were dissolved at 1 mg/mL in chloroform (Merck, VWR International, Oslo, Norway) and stored at −20°C in the dark.
For all the experiments, ultra-pure water (‘Milli-Q water’, resistivity = 18.2 MΩ/cm at 25°C), produced by a Milli-Q Academic instrument (Millipore, France), was used as the subphase or for buffer preparation. Some experiments were conducted with an extracellular buffer subphase, the EC solution: 118 mM NaCl, 5 mM KCl, 25 mM NaHCO3, 1.75 mM CaCl2, 1.2 mM MgCl2, 11 mM glucose, pH 7.4. Ethylene glycol (EG; Sigma-Aldrich, Oslo, Norway) or PEG from 400 to 35,000 Da (Fluka, Oslo, Norway) was added in water or EC at the concentration of 1 or 30 g/L.
Langmuir–Pockles technique
Langmuir monolayer isotherms
The outer leaflet of the membrane was mimicked by a lipid monolayer system with the Langmuir–Pockles technique as described previously [14, 15]. Briefly, the instrument used is based on a trough where the area of the lipid monolayer at the air–water interface can be modulated. A KSV Minitrough (KSV Instruments Inc, Helsinki, Finland; 75 mm × 364 mm × 5 mm) is coated with polytetrafluorethylene to prevent any leakage of the subphase over the edges. The trough was filled with ~170 mL Milli-Q water or EC with or without PEG. The system was thermostatted at 37°C or 4°C by circulating water in channels underneath the trough. Fifteen microliters of glycerophospholipid at 1 mg/mL in chloroform were carefully spread drop wise on the surface and the chloroform was allowed to evaporate for 5 min before starting measurement of the surface pressure (mN/m) versus area per lipid molecule (Å2). The film area was modulated with two identical mobile barriers on each side of the trough, which are coated with Delrin to prevent the monolayer from sliding under the barrier. The barriers cause compression or expansion of the monolayer spread at the air–water interface, thereby increasing or reducing the surface pressure, respectively. The barriers were driven at 5 mm/min while an electrobalance recorded the surface tension exerted on a platinum Wilhelmy plate immersed through the monolayer. The entire apparatus was set on a vibration isolation table and the surface pressure and mean molecular area (mma) were recorded by the KSV software. For each condition, two surface pressure/molecular area isotherms were recorded. Results were presented as percentages of variation in mma or in surface pressure induced by the presence of PEG in the subphase compared to the control isotherm without PEG.
The different compression phases
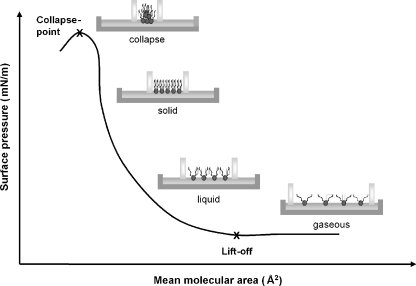
A typical surface pressure/molecular area isotherm, showing the different compression phases of a glycerophospholipid monolayer and how the glycerophospholipid molecules are arranged in four phases, is depicted in Fig. 1. In the first phase, the lipids are in the gaseous phase with the acyl groups apart in the air (bottom right in Fig. 1). Compression with the barriers forces the lipid monolayer molecules into the liquid state that causes a slight elevation of the surface pressure, starting with the so-called “lift-off point”. Further compression squeezes the lipid molecules into a solid state that gives a steep rise in the surface tension. By even more compression, at the so-called collapse-point, the lipids cannot be forced further together and the layer collapses into a many-layered structure.
Fig. 1.
Schematic representation of the Langmuir trough and barriers showing the different phases of a surface pressure/molecular area isotherm obtained during compression of a glycerophospholipid monolayer
Cell culture and total cell lipid extraction
Porcine proximal tubular epithelial cell line (LLC-PK1; CL-101) was obtained from American Type Culture Collection (ATCC, LGC-Promochem, Molsheim, France). Cell confluence was reached by incubating cells in a humidified atmosphere (95% air, 5% CO2) at 37°C in M199 (GIBCO®, Invitrogen Life Technologies, Cergy-Pontoise, France) supplemented with 3% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin (Sigma-Aldrich). Then, cells were washed with phosphate-buffered saline solution (GIBCO®) and scraped into 20 mM Tris–HCl pH 7.4, 0.5 M NaCl. After four cycles of freezing at −20°C and thawing at room temperature, total lipid fraction was extracted from cell homogenate by adding four volumes of ice-cold methanol/chloroform solution (2:1, v/v) followed by adding one volume each of ice-cold chloroform and water. After centrifugation (10 min, 1,000 rpm), the organic layer was collected and evaporated to dryness under a stream of dry nitrogen and dissolved at 1 mg/mL with chloroform. Total cell lipid extract was spread at the air–water interface for monolayer study.
Statistical analyses
Data are expressed as means ± SD and compared for statistical significance by variance analysis and Turkey–Kramer’s test for multiple comparison tests. A p value ≤0.05 was considered significant.
Results
Lateral compression isotherms
To form the basis for our observations, lateral compression isotherm measurements were first performed at 37°C on pure water subphase with lipids containing saturated acyl chains (16:0; Fig. 2, black isotherms). Figure 2a displays a representative isotherm of DPPC monolayer. Lift-off areas occurred around 141 Å2 per molecule indicating the formation of a liquid phase (Fig. 2d). Further compression forces the lipids into a solid phase and the surface pressure of biological membranes, which is about 30 mN/m, was reached for a mma of 121 Å2. As the surface area for each molecule was even more reduced, the collapse-point occurred at a surface pressure of 43 mN/m. Compression of DPPE (Fig. 2b) and DPPS (Fig. 2c) monolayers on pure water produced similar isotherms but with different mmas at lift-off and 30 mN/m and surface pressure at collapse, as shown in Fig. 2d.
Fig. 2.
Representative surface pressure/molecular area isotherm of DPPC (a), DPPS (b), and DPPE (c) monolayer obtained at 37°C in pure Milli-Q water in absence (dark isotherms) or in the presence (gray isotherms) of PEG. Three particular isotherm points were studied: the lift-off (i), 30 mN/m (ii) which corresponds to the surface pressure of biological membrane and the collapse-point (iii). Apparent mean molecular area (mma) measured at the lift-off and 30 mN/m and apparent surface pressure (Π) measured at the collapse-point for DPPC, DPPS and DPPE monolayers obtained at 37°C in pure Milli-Q water and in presence of 1 g/L of PEG 35,000 Da is shown in (d)
Addition of PEG35000 to the subphase (1 g/L) changed the isotherms (Figs. 2a–c, gray isotherms). For all three glycerophospholipids PEG35000 lowered the surface pressure at collapse, and for DPPC (Fig. 2a) and DPPS (Fig. 2c) the lift-off took place at lower mmas with than without PEG35000 (Fig. 2d). PEG35000 did not change the mma at lift-off in the DPPE monolayer. At 30 mN/m, PEG35000 caused no significant changes in the isotherms (Fig. 2).
Effects of PEG molecular weight on surface pressure/molecular area isotherms of the DPPC saturated monolayer
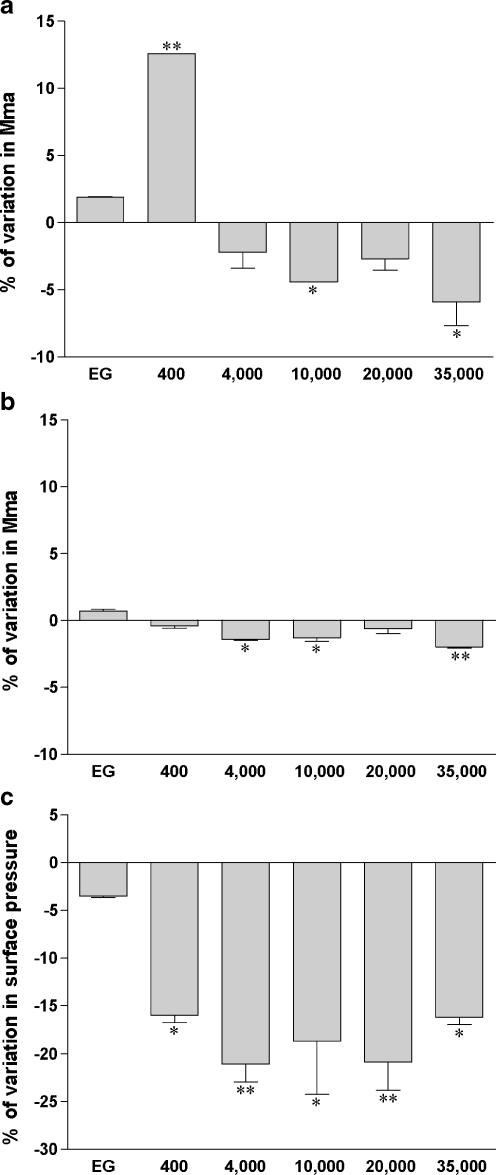
The EG monomer did not modify the DPPC isotherm significantly (Fig. 3). However, in the presence of PEG400, the lift-off occurred at a 12.6 ± 0.1% higher surface area compared to that obtained without PEG400. At 30 mN/m, no more interaction of PEG400 with the glycerophospholipid monolayer took place (Fig. 3b).
Fig. 3.
PEG modifies DPPC monolayer isotherms. DPPC was applied on Milli-Q water subphase and monolayer compression was performed at 37°C without (zero reference) or with 30 g/L of PEG of different molecular weights (ethylene glycol (EG), PEG 400 Da, PEG 4,000 Da, PEG 10,000 Da, PEG 20,000 Da, PEG 35,000 Da). Surface pressure/mean molecular area isotherms were obtained as described in the “Materials and methods” section. Relative modification from pure Milli-Q water subphase was expressed as the percentage of variation in mean molecular area (mma) measured at the lift-off point (a) or at 30 mN/m (b) or as the percentage of variation in surface pressure (Π) measured at the collapse-point (c). Results are means ± SD of two independent experiments. *p < 0.05, **p < 0.001 PEG-containing subphase versus pure Milli-Q water subphase
In contrast to PEG400, the higher molecular weight PEG stabilized the DPPC monolayer in the gaseous phase (Fig. 3a). The PEGs from 4,000 through 35,000 Da caused lift-off at a molecular areas at 2% to 6% lower than in pure water. At 30 mN/m, all PEG polymers did not produce any changes in mma compared to isotherms obtained in the absence of PEG.
The most conspicuous PEG effects were at the collapse-point which occurred at a surface pressure 16% to 21% lower than seen without PEG for each PEG polymer from 400 to 35,000 Da (Fig. 3c). This suggests that the presence of PEG destabilized the monolayer at high surface pressures.
Effects of glycerophospholipid headgroups on the interaction of PEGs with different molecular weights with saturated monolayers
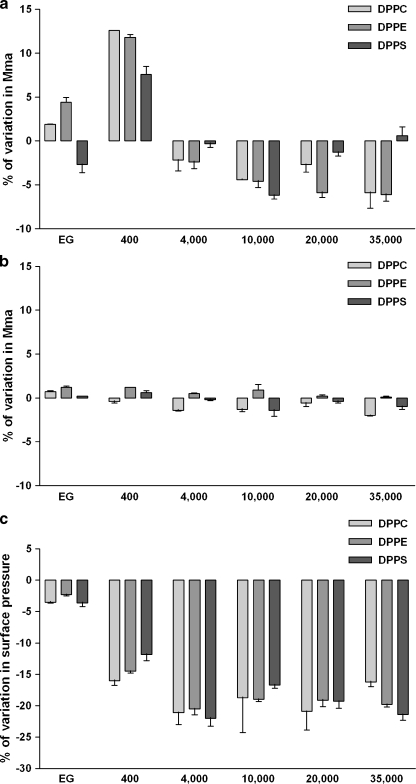
In order to test whether the PEG effects on monolayers are dependent on the nature of the glycerophospholipid headgroup, we kept the two acyl groups constant (both 16:0), and altered the headgroup. DPPE and DPPC are both electroneutral but DPPE has a smaller headgroup than DPPC, and DPPS has a net negative charge. PEG reduced mma at lift-off and at 30 mN/m of DPPC and DPPE monolayers, and decreased the surface pressure in the collapse-point for all three lipids. Neither the size nor the charge of the glycerophospholipids influenced the effects of the PEGs on the isotherms for the three lipids (Fig. 4). As we observed with DPPC monolayers, the DPPE and DPPS isotherms were only altered to a small extent by EG in the subphase while 30 g/L of PEG400 increased the molecular area at the lift-off (Fig. 4a). The PEGs of higher molecular weights reduced the mma of the glycerophospholipids significantly in the gaseous phase at lift-off, although this effect was markedly less with DPPS than with DPPC and DPPE. At 30 mN/m, whatever the glycerophospholipid headgroup and the PEG polymer tested, PEG caused insignificant modifications (Fig. 4b). However, the collapse-point appeared at a lower surface pressure in presence of PEG polymers with each of the saturated monolayer types (Fig. 4c). This monolayer destabilization induced by the presence of PEG was higher with the polymer size from 4,000 to 35,000 Da than with PEG400. In addition, PEGs with molecular weights higher than 4,000 Da gave practically the same lowering of the surface pressure at collapse for all three glycerophospholipids.
Fig. 4.
Glycerophospholipid headgroups do not alter PEG-induced isotherm variations. DPPC, DPPE and DPPS were applied on Milli-Q water subphase and monolayer compression was performed at 37°C without (zero reference) or with 30 g/L of PEG of different molecular weights (ethylene glycol (EG), PEG 400 Da, PEG 4,000 Da, PEG 10,000 Da, PEG 20,000 Da, PEG 35,000 Da). Surface pressure/mean molecular area isotherms were obtained as described in the “Materials and methods” section. Relative modification from pure Milli-Q water subphase was expressed as the percentage of variation in mean molecular area (mma) measured at the lift-off point (a) or at 30 mN/m (b) or as the percentage of variation in surface pressure (Π) measured at the collapse-point (c). Results are means ± SD of two independent experiments
Since the PEG of 4,000 Da and higher molecular weights induced the same pattern of isotherm modifications for DPPC, DPPE, and DPPS, the next experiments were performed only with DPPC. Phosphatidylcholine (PC) is mainly present in the outer leaflet of the plasma membrane, with which PEG is in contact during organ preservation, while phosphatidylethanolamine (PE) is predominantly located in the inner leaflet and phosphatidylserine (PS) are almost exclusively in the cytoplasmic leaflet [16–18].
Effects of PEG concentration on surface pressure/molecular area isotherms of saturated monolayer
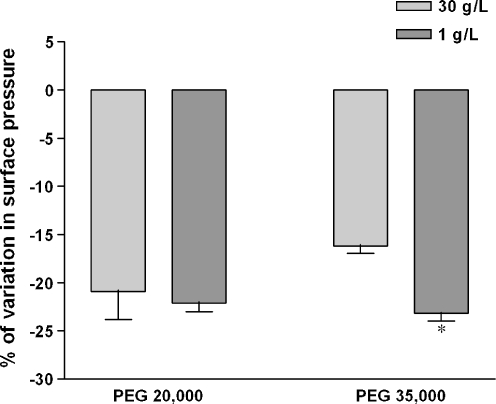
We have previously shown in our in vitro model of cold-preservation that the storage of LLC-PK1 cells during 24 h at 4°C in EC was very deleterious inducing more than 80% of cell death [11]. Adding only 1 g/L of PEG35000 in the preservation solution protected cells against cold-induced injury, decreasing the LDH release by around 20%. Other PEG polymers, particularly PEG20000, which is present like PEG35000 in a new commercial preservation solution, respectively, the SCOT and IGL-1® solutions, did not bring the same effectiveness. Therefore, one should expect that if the polymer protective effect against cold-induced injury were related to different interactions between PEG20000 and PEG35000 with plasma membrane phospholipids, it would be most noticeable at 1 g/L. Presence of 1 g/L of PEG20000 in the subphase lowered the surface pressure at the collapse-point in the same way as did 30 g/L of the same polymer or 1 g/L of PEG35000 (Fig. 5). Furthermore, and surprisingly, a different decrease in the surface pressure appeared with 1 and 30 g/L of PEG35000. However, it seemed not to be related to the cold-protective effect of PEG35000 since the surface pressure at the collapse-point did not differ between 1 g/L of both polymers (Fig. 5).
Fig. 5.
PEG concentration does not alter PEG-induced isotherm variations. DPPC was applied on Milli-Q water subphase and monolayer compression was performed at 37°C without (zero reference) or with 1 or 30 g/L of PEG 20,000 Da or PEG 35,000 Da. Surface pressure/mean molecular area isotherms were obtained as described in the “Materials and methods” section. Relative modification from pure Milli-Q water subphase was expressed as the percentage of variation in surface pressure (Π) measured at the collapse-point. Results are means ± SD of two independent experiments. *p < 0.05, 1 g/L versus 30 g/L of PEG
Effect of ionic strength and pH modifications on the interaction of PEG with saturated monolayer
In order to determine if the ionic strength or pH could alter the interactions of PEG with saturated monolayers, the next experiments were performed with the EC preservation solution used in the in vitro model [17] as subphase in the Langmuir trough. The surface pressure/molecular area isotherms obtained in EC subphase were different from those obtained with pure Milli-Q water as subphase in that the lift-off occurred at higher mma (data not shown). Such differences between pure water and salt-containing solutions have previously been described [19]. However, the PEG added in the ionic subphase caused a tighter packing of the glycerophospholipids, thus stabilizing the DPPC lipid monolayer in the gaseous phase (Fig. 6a). The lift-off occurred at a lower surface area with 1 g/L of both polymers in the salt-containing solution than in the pure Millli-Q water. Moreover, the decrease in the surface area induced by PEG35000 appeared to be a little more pronounced than the decrease induced by PEG20000 highlighting a more stabilizing effect by PEG35000 (−7.8 ± 0.6% for PEG20000 versus −10.0 ± 0.1% for PEG35000). At 30 mN/m, these differences induced by ions and pH modifications were reduced but still detectable (Fig. 6b). One should notice that the ionic subphase also reduced the destabilization induced by PEG at the collapse-point (Fig. 6c). A slight decrease in the variation of surface pressure occurred with PEG in ionic subphase compared with the polymer in the water subphase. Surprisingly, under these conditions, the different stabilizations, observed at the lift-off and at 30 mN/m between both polymers in EC, were not detected.
Fig. 6.
Ionic strength and pH solution modifications increase PEG-induced isotherm variations at low surface area and decrease PEG-induced isotherm variations at the collapse-point on DPPC monolayer. DPPC was applied on Milli-Q water subphase or on EC solution subphase and monolayer compression was performed at 37°C without (zero reference) or with 1 g/L of PEG 20,000 Da or PEG 35,000 Da. Surface pressure/mean molecular area isotherms were obtained as described in the “Materials and methods” section. Relative modification from pure Milli-Q water subphase was expressed as the percentage of variation in mean molecular area (mma) measured at the lift-off point (a) or at 30 mN/m (b) or as the percentage of variation in surface pressure (Π) measured at the collapse-point (c). Results are means ± SD of two independent experiments. *p < 0.05, **p < 0.001, EC solution subphase versus pure Milli-Q water subphase or PEG 20,000 Da versus PEG 35,000 Da
Effect of ionic strength and pH modifications on the interaction of PEG with total cell lipid extract monolayer
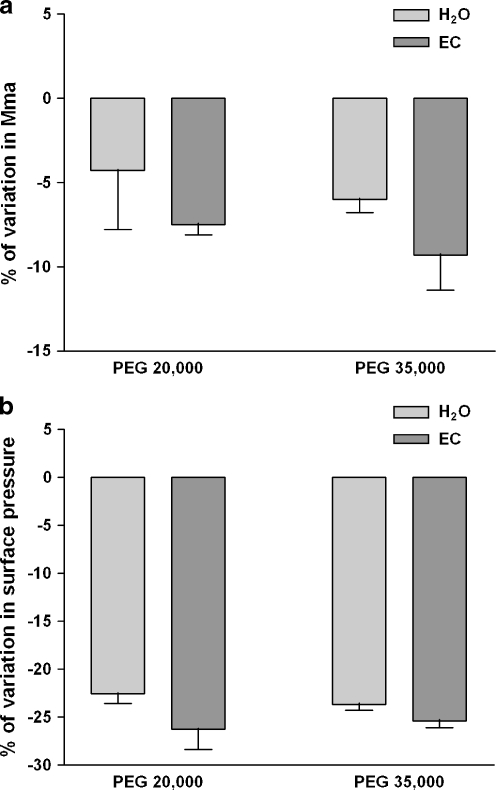
The monolayer experiments were then performed with a total lipid extract from the LLC-PK1 cells. Surface pressure/molecular area isotherms showed that the PEG, added in the water subphase, interacted with cell lipid extract in a same pattern as with monolayers of the pure lipids, the lift-off occurring at a smaller surface area and the collapse-point appearing at a lower surface pressure (Fig. 7a–b). Surprisingly, and contrary to DPPC monolayers, the variations in the surface area at the lift-off and in the surface pressure at the collapse-point induced by the presence of PEG in EC were not significantly modified even if the tendency at the lift-off seemed to underline an increase in the surface area (Fig. 7a–b). Furthermore, the different stabilization observed on DPPC monolayers between PEG20000 and PEG35000 in EC was not detected with the total LLC-PK1 cell lipid extract.
Fig. 7.
Ionic strength and pH solution modifications do not alter PEG-induced isotherm variations on total cell lipid extract monolayer. Total LLC-PK1 cell lipid extract was applied on Milli-Q water subphase or on EC solution subphase and monolayer compression was performed at 37°C without (zero reference) or with 1 g/L of PEG 20,000 Da or PEG 35,000 Da. Surface pressure/mean molecular area isotherms were obtained as described in the “Materials and methods” section. Relative modification from pure Milli-Q water subphase was expressed as the percentage of variation in surface pressure measured at the collapse-point. Relative modification from pure Milli-Q water subphase was expressed as the percentage of variation in mean molecular area (mma) measured at the lift-off point (a) or as the percentage of variation in surface pressure (Π) measured at the collapse-point (b). Results are means ± SD of two independent experiments
Effect of hypothermia on the interaction of PEG with saturated and unsaturated monolayers
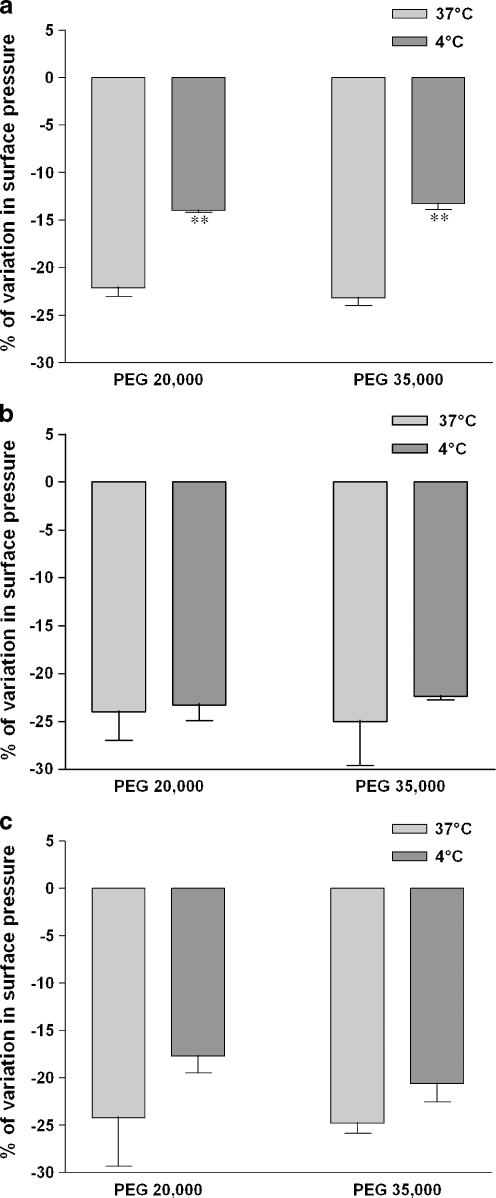
The surface pressure/molecular area isotherms obtained at 4°C with DPPC were different from those obtained at 37°C in that the lift-off occurred at lower mma with the saturated monolayer at 4°C (data not shown). This difference is most likely related to the phase-transition temperature that influences the conformation and the regularity in packing of the glycerophospholipids. The PEG added in the water subphase still induced a reduction in the surface pressure at the collapse-point on DPPC monolayer at 4°C. However, the destabilization induced by the polymer was lowered at 4°C and the collapse-point occurred at an identical surface pressure in presence of both PEG polymers (Fig. 8a).
Fig. 8.
PEG-induced isotherm variations under hypothermia depend on lipid saturation. DPPC (a), SOPC (b), and SDPS (c) were applied on Milli-Q water subphase and monolayer compression was performed at 4°C or 37°C without (zero reference) or with 1 g/L of PEG 20,000 Da or PEG 35,000 Da. Surface pressure/mean molecular area isotherms were obtained as described in the “Materials and methods” section. Relative modification from pure Milli-Q water subphase was expressed as the percentage of variation in surface pressure (Π) measured at the collapse-point. Results are means ± SD of two independent experiments. **p < 0.001, 4°C versus 37°C
The degree of unsaturation of the hydrophobic fatty acid chain influences the transition temperature of the glycerophospholipids and unsaturated phospholipids generally have a lower-phase-transition temperature than saturated phospholipids. In order to determine the role of the phase-transition temperature on the PEG interaction during hypothermia with lipids, we used glycerophospholipids with stearoyl in the sn-1 position and oleoyl (SOPC) or docosahexenoyl (SDPS) in the sn-2 position. The hypothermia-induced decrease in the surface pressure observed with saturated monolayers did not occur with SOPC and SDPS monolayers although a non-significant decrease with SDPS monolayers was seen (Fig. 8b–c). Furthermore, no difference between PEG20000 and PEG35000 was detected with monounsaturated monolayers. Therefore, the PEG interaction with phospholipids at low temperature was dependent of the unsaturation of the acyl chains.
Discussion
This study demonstrates that PEG interacts with glycerophospholipids in monolayers and perturbs the surface pressure/surface area isotherms. Although a precise interpretation of our observations is difficult, some generalizations can be made.
First, we observed that the polymer-induced changes in the isotherms differed with the size of the molecule. The monomer EG did not affect the packing of saturated glycerophospholipids in pure Milli-Q water while a 400 Da PEG increased the surface area at the lift-off point. This apparent increase in molecular area does not mean that the individual phospholipid molecules increased their area. The instrument recorded an increase in the area for each molecule, which could result from a modification of the orientation of the lipid molecule induced by the PEG or from an insertion of the PEG400 in the monolayer between the lipid molecules. This effect diminished with the compression of the monolayer as if the PEG was squeezed out of the monolayer.
Secondly, and in sharp contrast to PEG400, the PEG with higher molecular weights lowered the mma at lift-off. This apparent stabilization of the lipid monolayer in the gaseous state, already described by Maggio et al. [20], occurred only when intermolecular interactions between the acyl chains were weak (at low surface pressures).
Thirdly, at 30 mN/m, the surface pressure of biological membranes, none of the PEGs showed significant interaction with the monolayers, and fourthly, all PEG polymers lowered the surface pressure at the collapse-point. This indicated that all PEGs, irrespective of molecular weight and chain length, caused destabilization of the lipid monolayer, thus in apparent contradiction with the stabilization observed at low surface pressures for PEG with high molecular weights. However, studies by others have suggested that the dehydrating ability of PEGs would reduce the lipid molecular motion which may cause denser packing of the lipids and a decrease in the membrane fluidity [13, 21].
Biological membranes have an asymmetric distribution of phospholipids between the two leaflets [22, 23]. PC molecules are, together with the choline-containing sphingomyelin, most abundant in the outer leaflet of the plasma membrane while molecular species of PE are predominantly located in the inner leaflet and while molecular species of PS and PI are present almost exclusively in the cytoplasmic leaflet [17, 18]. The substitution of the three CH3 groups in PC by hydrogen atoms in PE results in a decrease in area per lipid in PE bilayers, making it possible for inter- or intra-molecular hydrogen bonds to occur [24]. The PC and PE contain zwitterionic headgroups; the negative and positive charges balance each other resulting in a distance narrow enough to acquire maximum van der Waals interaction. With the negatively charged serine as the polar head group, very little changes (decrease in mma) occurred at lift-off with PEGs with molecular weight of 4,000 Da and above. However, we showed that the bulkiness of the headgroup, i.e., choline versus ethanolamine, did not modify the PEG-induced alterations of saturated lipid monolayer isotherms. Therefore, most experiments of the study were performed with PC glycerophospholipids, the main headgroup of the outer leaflet with which the preservation solution and therefore the PEG would be in contact with during organ preservation.
With a buffered saline solution with a composition mimicking the extracellular milieu as the subphase, the glycerophospholipid isotherms showed a less stable lipid packing. It has been assumed that the presence of salt and the pH modification induced alterations on glycerophospholipid packing principally due to electrostatic screening [19, 25, 26]. However, despite its neutral nature, the PEG interaction with the glycerophospholipids was increased under physiological ionic strength and pH both at the lift-off and at the collapse-point. In addition, for the first time, we obtained a different interaction with lipid monolayers between PEG20000 and PEG35000 at low surface pressures. The presence of 1 g/L of PEG35000 stabilized the DPPC monolayer in the gaseous phase more strongly than PEG20000. This could be in agreement with the higher effectiveness of PEG35000 against cold-induced injuries observed in our cellular preservation model and also with the higher protection of PEG35000 observed during organ transplantation [3, 4, 11].
Hypothermia lowered the destabilization induced by the PEGs on the DPPC monolayer as well as with other saturated glycerophospholipid, DPPS and DPPE, monolayers (data not shown). However, this stabilizing effect of hypothermia did not occur with phospholipids containing unsaturated acyl chains. This different response to PEG between saturated and unsaturated glycerophospholipids under hypothermia may be dependent on the phase-transition temperature of the lipids and therefore, on the fluidity of the monolayer. Each lipid has its specific transition temperature at which the acyl chains undergo dramatic conformational change [27]. So, in bilayers at neutral pH, DPPC, DPPS, and DPPE have shown a phase-transition temperature of, respectively, 41.4°C, 53°C, and 63°C while SOPC monounsaturated glycerophospholipids have shown a transition temperature of 5.6°C [28–31]. In addition, it has been demonstrated that PEG increases the phase-transition temperature of glycerophospholipids, possibly by changing the solvation property of water [32–34].
The ionic strength and pH of the solution and a high lipid-phase-transition temperature modulated the PEG interaction with the lipids and stabilized the monolayer. However, the experiments performed on total lipid extract of LLC-PK1 cells showed that the stabilization effect of PEG in physiological ionic strength and pH conditions was not reproduced and the isotherm compression was not modified during hypothermia (data not shown). Some studies have suggested that the composition of the lipid component may change in response to environmental stress such as temperature [35]. Indeed, Hazel and Landrey have demonstrated changes in headgroup composition and in molecular species composition in kidney plasma membranes of trout during cold acclimation [36, 37]. It would be of particular interest to discriminate whether the membrane fluidity could be altered with the lipid composition of the plasma membrane of renal epithelial cells, subjected or not to hypothermia and whether a different interaction on lipid monolayer can be observed between PEGs polymers.
Acknowledgments
This work was supported by a Marie Curie Training Site grant from the European Commission (agreement QLK5-CT-2001-60076) and a sponsorship from Banque Tarneaud (Poitiers, France).
Abbreviations
- DPPC
1,2-dipalmitoyl phosphatidylcholine
- DPPE
1,2-dipalmitoyl phosphatidylethanolamine
- DPPS
1,2-dipalmitoyl phosphatidylserine
- EC
extracellular
- EG
ethylene glycol
- HES
hydroxyethyl starch
- LDH
lactate dehydrogenase
- mma
mean molecular area in Å2
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PS
phosphatidylserine
- PEG
polyethylene glycol
- SDPS
1-stearoyl-2-docosahexenoyl phosphatidylserine
- SOPC
1-stearoyl-2-oleoyl phosphatidylcholine
- UW
University of Wisconsin
- Π
Greek Pi, surface pressure
References
- 1.Wicomb WN, Collins GM. 24-hour rabbit heart storage with UW solution. Effects of low-flow perfusion, colloid, and shelf storage. Transplantation. 1989;48:6–9. doi: 10.1097/00007890-198907000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Mosbah IB, Franco-Gou R, Abdennebi HB, et al. Effects of polyethylene glycol and hydroxyethyl starch in University of Wisconsin preservation solution on human red blood cell aggregation and viscosity. Transplant Proc. 2006;38:1229–1235. doi: 10.1016/j.transproceed.2006.02.068. [DOI] [PubMed] [Google Scholar]
- 3.Ben Abdennebi H, Steghens JP, Hadj-Aissa A, et al. A preservation solution with polyethylene glycol and calcium: a possible multiorgan liquid. Transpl Int. 2002;15:348–354. doi: 10.1111/j.1432-2277.2002.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 4.Badet L, Ben Abdennebi H, Petruzzo P, et al. Effect of IGL-1, a new preservation solution, on kidney grafts (a pre-clinical study) Transpl Int. 2005;17:815–821. doi: 10.1007/s00147-004-0789-1. [DOI] [PubMed] [Google Scholar]
- 5.Doucet C, Dutheil D, Petit I, et al. Influence of colloid, preservation medium and trimetazidine on renal medulla injury. Biochim Biophys Acta. 2004;1673:105–114. doi: 10.1016/j.bbagen.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 6.Eugene M. Polyethyleneglycols and immunocamouflage of the cells tissues and organs for transplantation. Cell Mol Biol (Noisy-le-grand) 2004;50:209–215. [PubMed] [Google Scholar]
- 7.Lee WY, Sehon AH. Suppression of reaginic antibodies with modified allergens. I. Reduction in allergenicity of protein allergens by conjugation to polyethylene glycol. Int Arch Allergy Appl Immunol. 1978;56:159–170. doi: 10.1159/000232019. [DOI] [PubMed] [Google Scholar]
- 8.Scott MD, Murad KL. Cellular camouflage: fooling the immune system with polymers. Curr Pharm Des. 1998;4:423–438. [PubMed] [Google Scholar]
- 9.Hauet T, Goujon JM, Baumert H, et al. Polyethylene glycol reduces the inflammatory injury due to cold ischemia/reperfusion in autotransplanted pig kidneys. Kidney Int. 2002;62:654–667. doi: 10.1046/j.1523-1755.2002.00473.x. [DOI] [PubMed] [Google Scholar]
- 10.Wanders A, Akyurek ML, Waltenberger J, et al. Ischemia-induced transplant arteriosclerosis in the rat. Arterioscler Thromb Vasc Biol. 1995;15:145–155. [PubMed] [Google Scholar]
- 11.Dutheil D, Rioja-Pastor I, Tallineau C, et al. Protective effect of PEG 35,000 Da on renal cells: paradoxical activation of JNK signaling pathway during cold storage. Am J Transplant. 2006;6:1529–1540. doi: 10.1111/j.1600-6143.2006.01343.x. [DOI] [PubMed] [Google Scholar]
- 12.Stefanovich P, Ezzell RM, Sheehan SJ, et al. Effects of hypothermia on the function, membrane integrity, and cytoskeletal structure of hepatocytes. Cryobiology. 1995;32:389–403. doi: 10.1006/cryo.1995.1039. [DOI] [PubMed] [Google Scholar]
- 13.Ohno H, Sakai T, Tsuchida E, et al. Interaction of human erythrocyte ghosts or liposomes with polyethylene glycol detected by fluorescence polarization. Biochem Biophys Res Commun. 1981;102:426–431. doi: 10.1016/0006-291X(81)91538-2. [DOI] [PubMed] [Google Scholar]
- 14.Blois A, Holmsen H, Martino G, et al. Interactions of chromogranin A-derived vasostatins and monolayers of phosphatidylserine, phosphatidylcholine and phosphatidylethanolamine. Regul Pept. 2006;134:30–37. doi: 10.1016/j.regpep.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Broniec A, Gjerde AU, Olmheim AB, et al. Trifluoperazine causes a disturbance in glycerophospholipid monolayers containing phosphatidylserine (PS): effects of pH, acyl unsaturation, and proportion of PS. Langmuir. 2007;23:694–699. doi: 10.1021/la061628b. [DOI] [PubMed] [Google Scholar]
- 16.Chap HJ, Zwaal ZR, Deenen LL. Action of highly purified phospholipases on blood platelets. Evidence for an asymmetric distribution of phospholipids in the surface membrane. Biochim Biophys Acta. 1977;467:146–164. doi: 10.1016/0005-2736(77)90192-4. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JA, Dawson RM. Asymmetry of the phospholipid bilayer of rat liver endoplasmic reticulum. Biochim Biophys Acta. 1977;470:342–356. doi: 10.1016/0005-2736(77)90126-2. [DOI] [PubMed] [Google Scholar]
- 18.Wang CT, Shiao YJ, Chen JC, et al. Estimation of the phospholipid distribution in the human platelet plasma membrane based on the effect of phospholipase A2 from Naja nigricollis. Biochim Biophys Acta. 1986;856:244–258. doi: 10.1016/0005-2736(86)90034-9. [DOI] [PubMed] [Google Scholar]
- 19.Agasosler AV, Tungodden LM, Cejka D, et al. Chlorpromazine-induced increase in dipalmitoylphosphatidylserine surface area in monolayers at room temperature. Biochem Pharmacol. 2001;61:817–825. doi: 10.1016/S0006-2952(01)00542-1. [DOI] [PubMed] [Google Scholar]
- 20.Maggio B, Ahkong QF, Lucy JA. Poly(ethylene glycol), surface potential and cell fusion. Biochem J. 1976;158:647–650. doi: 10.1042/bj1580647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vijayalakshmi A, KrishnaKumari VV, Madhusudhana Rao N. Probing polyethylene glycol-phospholipid membrane interactions using enzymes. J Colloid Interface Sci. 1999;219:190–194. doi: 10.1006/jcis.1999.6471. [DOI] [PubMed] [Google Scholar]
- 22.Rothman JE, Lenard J. Membrane asymmetry. Science. 1977;195:743–753. doi: 10.1126/science.402030. [DOI] [PubMed] [Google Scholar]
- 23.Balasubramanian K, Schroit AJ. Aminophospholipid asymmetry: a matter of life and death. Annu Rev Physiol. 2003;65:701–734. doi: 10.1146/annurev.physiol.65.092101.142459. [DOI] [PubMed] [Google Scholar]
- 24.Tieleman DP, Marrink SJ, Berendsen HJ. A computer perspective of membranes: molecular dynamics studies of lipid bilayer systems. Biochim Biophys Acta. 1997;1331:235–270. doi: 10.1016/s0304-4157(97)00008-7. [DOI] [PubMed] [Google Scholar]
- 25.Cevc G, Watts A, Marsh D. Non-electrostatic contribution to the titration of the ordered-fluid phase transition of phosphatidylglycerol bilayers. FEBS Lett. 1980;120:267–270. doi: 10.1016/0014-5793(80)80313-9. [DOI] [PubMed] [Google Scholar]
- 26.Kruijff B, Cullis PR. The influence of poly(l-lysine) on phospholipid polymorphism. Evidence that electrostatic polypeptide–phospholipid interactions can modulate bilayer/non-bilayer transitions. Biochim Biophys Acta. 1980;601:235–240. doi: 10.1016/0005-2736(80)90528-3. [DOI] [PubMed] [Google Scholar]
- 27.Huang C, Li S. Calorimetric and molecular mechanics studies of the thermotropic phase behavior of membrane phospholipids. Biochim Biophys Acta. 1999;1422:273–307. doi: 10.1016/s0005-2736(99)00099-1. [DOI] [PubMed] [Google Scholar]
- 28.Xu H, Huang CH. Scanning calorimetric study of fully hydrated asymmetric phosphatidylcholines with one acyl chain twice as long as the other. Biochemistry. 1987;26:1036–1043. doi: 10.1021/bi00378a009. [DOI] [PubMed] [Google Scholar]
- 29.Browning JL, Seelig J. Bilayers of phosphatidylserine: a deuterium and phosphorus nuclear magnetic resonance study. Biochemistry. 1980;19:1262–1270. doi: 10.1021/bi00547a034. [DOI] [PubMed] [Google Scholar]
- 30.Huang C, Wang ZQ, Lin HN, et al. Interconversion of bilayer phase transition temperatures between phosphatidylethanolamines and phosphatidylcholines. Biochim Biophys Acta. 1994;1189:7–12. doi: 10.1016/0005-2736(94)90273-9. [DOI] [PubMed] [Google Scholar]
- 31.Wang G, Lin HN, Li S, et al. Phosphatidylcholines with sn-1 saturated and sn-2 cis-monounsaturated acyl chains. Their melting behavior and structures. J Biol Chem. 1995;270:22738–22746. doi: 10.1074/jbc.270.39.22738. [DOI] [PubMed] [Google Scholar]
- 32.Maggio B, Lucy JA. Interactions of water-soluble fusogens with phospholipids in monolayers. FEBS Lett. 1978;94:301–304. doi: 10.1016/0014-5793(78)80962-4. [DOI] [PubMed] [Google Scholar]
- 33.Tilcock CP, Fisher D. Interaction of phospholipid membranes with poly(ethylene glycol)s. Biochim Biophys Acta. 1979;557:53–61. doi: 10.1016/0005-2736(79)90089-0. [DOI] [PubMed] [Google Scholar]
- 34.Winterhalter M, Burner H, Marzinka S, et al. Interaction of poly(ethylene-glycols) with air–water interfaces and lipid monolayers: investigations on surface pressure and surface potential. Biophys J. 1995;69:1372–1381. doi: 10.1016/S0006-3495(95)80006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quinn PJ. Effects of temperature on cell membranes. Symp Soc Exp Biol. 1988;42:237–258. [PubMed] [Google Scholar]
- 36.Hazel JR, Landrey SR. Time course of thermal adaptation in plasma membranes of trout kidney. I. Headgroup composition. Am J Physiol. 1988;255:R622–R627. doi: 10.1152/ajpregu.1988.255.4.R622. [DOI] [PubMed] [Google Scholar]
- 37.Hazel JR, Landrey SR. Time course of thermal adaptation in plasma membranes of trout kidney. II. Molecular species composition. Am J Physiol. 1988;255:R628–R634. doi: 10.1152/ajpregu.1988.255.4.R628. [DOI] [PubMed] [Google Scholar]