Abstract
Spatial bias is an asymmetry of perception and/or representation of spatial information —“where” bias —, or of spatially directed actions — “aiming” bias. A monocular patch may induce contralateral “where” spatial bias (the Sprague effect; Sprague (1966) Science, 153, 1544–1547). However, an ipsilateral patch-induced spatial bias may be observed if visual occlusion results in top-down, compensatory re-allocation of spatial perceptual or representational resources toward the region of visual deprivation. Tactile distraction from a monocular patch may also contribute to an ipsilateral bias. To examine these hypotheses, neurologically normal adults bisected horizontal lines at baseline without a patch, while wearing a monocular patch, and while wearing tactile-only and visual-only monocular occlusion. We fractionated “where” and “aiming” spatial bias components using a video apparatus to reverse visual feedback for half of the test trials. The results support monocular patch-induced ipsilateral “where” spatial errors, which are not consistent with the Sprague effect. Further, the present findings suggested that the induced ipsilateral bias may be primarily induced by visual deprivation, consistent with compensatory “where” resource re-allocation.
Keywords: Spatial neglect, line bisection, representational bias, perceptual bias, spatial attention
Introduction
“Where” and “Aiming” Spatial Systems
Spatial bias is an asymmetry of perception and/or representation of spatial information, or in spatially directed actions. Neurologically normal individuals systematically demonstrate a leftward spatial bias (pseudoneglect, Bowers & Heilman, 1980; McCourt & Jewell, 1999; for review, see Jewell & McCourt, 2000). Spatial neglect, a pathological form of spatial bias, is a condition in which persons after brain injury fail to report, respond, or orient to novel or meaningful stimuli presented to the side opposite a brain lesion, when this failure cannot be attributed to either sensory or motor defects (Heilman, Watson, & Valenstein, 1979), and causes functional disability (Barrett & Burkholder, 2006).
Spatial bias usually is demonstrated in a visuo-motor spatial task, such as line bisection (i.e., marking the midpoint of a horizontal line). These systematic errors may result from one of the following: First, asymmetric function of perceptual-attentional “where” systems may render the viewer relatively unaware of one side of the line, or may increase a propensity to asymmetrically perceive or attend to the other side. Second, an inaccurate or asymmetric internal image of the line in representational “where” systems may produce spatially biased errors. Third, the viewer may make errors due to asymmetry of motor-intentional “aiming” systems (also termed action-intentional, premotor or exploratory bias; Bisiach, Geminiani, Berti, & Rusconi, 1990; Tegnér & Levander, 1991; Coslett, Bowers, Fitzpatrick, Haws, & Heilman, 1990; Mapstone et al., 2003). Frequently, perceptual-attentional and representational “where” bias are considered together, as these constructs can be difficult to separate in task performance. Spatial bias can thus be broadly categorized into two types: “where” (perceptual-attentional-representational) and “aiming” (motor-intentional) spatial errors (Barrett et al., 2006; Garza, Eslinger, & Barrett, in press; Heilman, Watson, & Valenstein, 2003). However, when an individual demonstrates a visuo-motor behavior reflecting spatial bias, it is not always evident whether the bias is primarily from the “where” or “aiming” system. Reversing the left-right direction of visual feedback may dissociate “where” and “aiming” biases by de-coupling the spatial region in which visual feedback appears from the action space of the motor response (Bisiach et al., 1990). In the natural viewing condition where the visual stimuli and the motor response are congruent, “where” and “aiming” errors are additive, contributing to the observed spatial error:
| (1) |
In the left-right reversed viewing condition where the visual stimuli and the motor response are dissociated by 180°, a spatial error contributed by the “where” system is reversed relatively to the direction of bias from the “aiming” system, assuming that the intention of making an aiming response or motor execution is relatively ballistic even when the action is viewed online (Fitts, 1954). Thus, the sign of “where” error is changed in the equation:
| (2) |
The algebraic method of dissociation has been used previously and successfully described the pattern of bias demonstrated in persons with right-hemisphere stroke (Barrett & Burkholder, 2006; Barrett, Crucian, Beversdorf, & Heilman, 2001) as well as in the healthy (Garza, Eslinger, & Barrett, in press). Previous research used various methods to reverse orientation of visual feedback, including online video (Adair, Na, Schwartz, & Heilman, 1998; Coslett et al., 1990; Na et al., 1998; Schwartz, Adair, Na, & Williamson, 1997), a customized pulley (Bisiach et al., 1990; MacLeod & Turnbull, 1999), mirrors (Tegnér & Levander, 1991), or an epidiascope task (Nico, 1996). In the present study, a video apparatus was used.
Spatial Bias Induced by Monocular Patching
Monocular patching has been suggested to induce contralateral spatial bias (Posner & Rafal, 1987). The colliculi, unlike the striate cortex, may receive heavily monocular input from the contralateral eye (Hendrick, Wilson, & Toyne, 1970; Hubel, Levay, & Wiesel, 1975; Pollack & Hickey, 1979; Sylvester, Josephs, Driver, & Rees, 2007). Thus, obstructing visual input of one eye may substantially block visual input from entering the contralateral superior colliculus, decreasing contralateral collicular activity. The ipsilateral superior colliculus, released from intracollicular inhibition, may induce strong contralateral orienting (the Sprague effect (Sprague, 1966). Although the mechanisms of the Sprague effect have been disputed (Wallace, Rosenquist, & Sprague, 1990), a role of intracollicular inhibition in this phenomenon is supported in recent studies in cats (Payne & Rushmore, 2004; Rushmore, Valero-Cabre, Lomber, Hilgetag, & Payne, 2006) and human stroke survivors (Weddell, 2004).
Previous investigators measured the effects of monocular patching on visuo-motor spatial tasks in persons with left-sided spatial neglect after right hemisphere lesions. Butter and Kirsch (1992) reported a patch-induced contralateral bias in twelve of eighteen brain-damaged study participants. However, another study observed the patch-induced contralateral bias in only two of nine participants with spatial neglect (Walker, Young, & Lincoln, 1996). Similar to Walker et al. (1996), Soroker and colleagues showed that one of six participants demonstrated a contralateral bias induced by monocular patching, with two demonstrating an ipsilateral bias (Soroker, Cohen, Baratz, Glicksohn, & Myslobodsky, 1994). If a monocular patch may induce both contralateral and ipsilateral spatial bias (for summary, see Table 1 in Barrett & Burkholder, 2006), this cannot be explained by the Sprague effect alone.
Table 1.
Mean bisection “where” and “aiming” errors under the unpatched condition and while wearing a conventional patch on the left or right eye.
| Bisection error (in mm) | Patching side | ||
|---|---|---|---|
| Bias Type | Left-patched | Unpatched | Right-patched |
| “where” | − 1.0 (1.8) * | − 0.3 (1.2) | 1.5 (1.4) *** |
| “aiming” | − 0.1 (2.0) | 0.3 (1.7) | − 0.6 (2.0) |
Rightward errors are presented in positive value, and leftward in negative. Standard deviations are in the parentheses. Measuring unit is in millimeter.
One-sample two-tailed t-tests compared all errors with zero (the true center of the line) and revealed that with an eye patch, the “where” errors were significantly different from zero: p < 0.05;
p < 0.001.
Monocular patching primarily induced “where” bias ipsilateral to the eye patch.
Studying healthy adults, McCourt and colleagues investigated the influence of monocular patching on spatial bias. Leftward spatial bias was increased by patching the right eye, consistent with the Sprague effect (McCourt, Garlinghouse, & Butler, 2001). However, a leftward spatial bias persisted with right eye patching, although reduced in magnitude. In addition, McCourt et al. did not address whether a monocular patch primarily affects “where” or “aiming” spatial systems. Barrett and colleagues reported that eye patching primarily affects “where” spatial bias after brain injury (Barrett et al., 2001; Barrett, Crucian, & Heilman, 2004; Barrett & Burkholder, 2006), and that eye patching may result in either ipsilateral or contralateral bias. Therefore, the present study not only examined the Sprague effect but also asked whether eye patching primarily affects “where” or “aiming” bias in healthy adults.
Present Study
Because eye patching may induce both contralateral bias (as proposed by Posner and Rafal, 1987) and ipsilateral bias in individual brain-damaged persons (e.g., Barrett et al., 2001), more than one mechanism of patch-induced spatial bias may account for its effect. The current study hypothesized that an eye patch may induce contralateral bias as a result of collicular-cortical disinhibition (i.e., the Sprague effect), but visual loss, resulting from monocular visual deprivation, may also induce top-down, compensatory re-allocation of spatial perceptual or representational resources toward the region of lost input (Doricchi & Angelelli, 1999; Doricchi, Onida, & Guariglia, 2002b). Spatial bias ipsilateral to a monocular patch might also be induced by tactile distraction that results from an eye patch’s physical contact on the skin (see Barrett et al., 2001). In the current study, the direction of errors that a group of healthy adults made in performing the line bisection task with and without a monocular patch was used to evaluate for the presence of the above effects. In order to test whether tactile distraction alone, or visual loss alone, could account for patch-induced bias, the patch components of tactile stimulation and visual occlusion were separated.
Previously, Barrett and colleagues reported that eye patching may primarily induce “where” spatial bias in stroke survivors (Barrett et al., 2001, 2004; Barrett & Burkholder, 2006). Furthermore, visual input may be critical in misperception of a horizontal line in persons with spatial neglect (Doricchi, Galati, DeLuca, Nico, & D’Olimpio, 2002a), and visual representation may be critical to spatial misrepresentation in persons with hemianopia (Doricchi et al., 2002b). Therefore, the present study hypothesized that perceptual-attentional or representational “where” bias, rather than motor-intentional “aiming” bias, would be primarily affected by visual occlusion resulting from eye patching. Since effects of eye patching may change in near versus far space (Barrett, Schwartz, Crucian, Kim, & Heilman, 2000), both near and far spatial regions were assessed.
Experiment
Methods
Participants
Sixteen neurologically healthy right-handed individuals were tested. They were between the ages of 18 and 40 (mean age 28.1 yr; eight females) and had 20/40 vision or better in both eyes.
Apparatus and stimuli
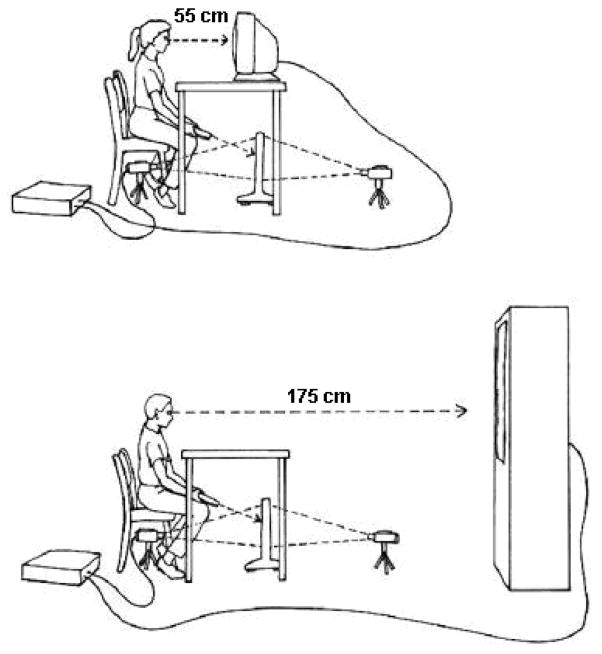
Participants performed line bisections on a video apparatus in the General Clinical Research Center, Penn State College of Medicine. The apparatus consisted of a non-glare, transparent acrylic workscreen placed on the floor, holding a white paper with a horizontal 22.4 × 0.3 cm black line printed on it. The 22.4cm-long line was positioned 15.0 cm above the floor and 55.0 cm in front of the participants. A Sony (DCR-TRV730) Digital 8 camera was on the floor beneath the participant’s chair and projected the image of the workscreen onto the near and far Sony television screens (viewscreens). The near viewscreen was located 55.0 cm away from the participant and measured 40.0 × 30.0 cm. The far viewscreen was located 175.0 cm away from the participant and measured 123.0 × 92.5 cm. The projected line subtended a visual angle of 38.1° on both the near and far viewscreens appearing at eye level. The participant bisected the projected line using a Laserlyte laser pointer. Wooden dowels were attached to the back of the participant’s chair and rested on either side of the participant’s head during testing to ensure that the participant’s head was centered during each trial. See Figure 1 for the schematic illustration of the apparatus setup.
Figure 1.
Top diagram demonstrates near space apparatus. Bottom diagram demonstrates far space apparatus.
In order to test whether a monocular patch would induce primarily “where” rather than “aiming” spatial bias, line bisection errors were measured when participants performed the tasks in a Natural versus left-right Reversed visual feedback condition. In the Natural condition, left and right as participants saw them on the viewscreen corresponded with the left and right sides of the actual workspace where they bisected lines. In the Reversed condition, a 180° change in camera perspective reversed the image on the viewscreen. Therefore, in the Reversed condition, when participants moved their hands rightward in the actual workspace, the laser point would appear to move leftward on the viewscreen, and vice versa.
Performance was recorded with the image of a metric ruler affixed to the back of the workscreen (not visible to the participant). A second camera was placed on the back side of the workscreen recorded the line, laser pointer, and the metric ruler. In this setting, it was the magnitude (in mm) of hand movements that was recorded, which was independent of the viewing distance (i.e., near vs. far) or the visual feedback conditions (Natural vs. Reversed). An error was defined as mm deviation from the true center of the line. Rightward errors were coded positive and leftward negative. Derived from Equations 1 and 2, two dependent variables —“where” and “aiming” errors — were calculated from the Equations 3 and 4:
| (3) |
| (4) |
Participants bisected lines under three different patching conditions: wearing a conventional eye patch, wearing a tactile-stimulation-only device around the eye, and blocking vision in one eye with a visual-occlusion-only device that did not contact the face. All three “patch types” were applied to one eye or the other in separate trial blocks. In another trial block, participants performed the task without any object between their face and the viewscreen, namely, the unpatched condition. The conventional eye patch was a commercial product, Flents® Eye Patch, 7.0 cm in diameter. The tactile-stimulation-only device (named the “tactile-only patch” for convenience) was made by cutting out a conventional patch to leave only a 1-cm rim. It was placed on the participant’s face in the same fashion as a conventional patch. The visual-occlusion-only device (named “visual-only patch” for convenience) was a white plastic typing page holder measuring approximately 8 ½ × 11 inches (21.6 × 28.0 cm) and mounted on a hospital bedside table. The visual-only patch was positioned approximately 2.0 cm in front of the participant’s face with the edge near the midline of the face.
Design and procedure
To begin each trial, participants were instructed to move the pointer to either the left or right upper corner of the viewscreen. They then moved the pointer to bisect the line, and verbally indicated when they were satisfied with the pointer placement. For the following trials, the starting corners were alternated. Each participant performed a total of 224 trials, in 28 trial blocks of 8 trials per block. Two variables — viewing distance (near vs. far) and visual feedback condition (Natural vs. Reversed) — formed four subsets, which were pseudorandomized across participants. Within each subset, conditions of three patch types —conventional, tactile-only, and visual-only — and the unpatched condition were also pseudorandomized. The order in which each eye was patched was randomly selected and blocked within a trial block.
Results
Prior to the planned analyses, two overall analyses of variance (ANOVAs) were conducted on the two dependent variables (“where” error and “aiming” error). Each overall ANOVA consisted of three within-subjects variables: viewing distance (near vs. far), patch type (no-patch, conventional, tactile-only, and visual-only), patching side (left vs. right), and one between-subjects variable: gender (male vs. female). For “where” errors, two main effects were found from patch type [F(3, 42) = 2.98, p = 0.042 ] and patching side [ F(1, 14) = 65.82, p < 0.001], and two two-way interactions were shown: patch type × patching side [F(3, 42) = 26.63, p < 0.001] and distance × gender [F(1, 14) = 6.06, p = 0.027]. For “aiming” errors, the ANOVA yielded a main effect from patch type [F(1, 14) = 3.25, p = 0.031] and an interaction of patch type × patching side [F(3, 42) = 3.45, p = 0.025]. Although the ANOVA on “where” errors revealed an interaction between distance and gender, these two factors showed no main effect or interaction with any other independent variables that were investigated in this study. Therefore, these data were collapsed in the following analyses. Analyses were conducted to examine whether 1) monocular patching primarily induces “where” spatial bias rather than “aiming” spatial bias, and whether 2) a patch-induced contralateral spatial bias was consistent with the Sprague effect, or whether 3) tactile distraction or compensatory orienting more appropriately account for observed ipsilateral induced spatial bias.
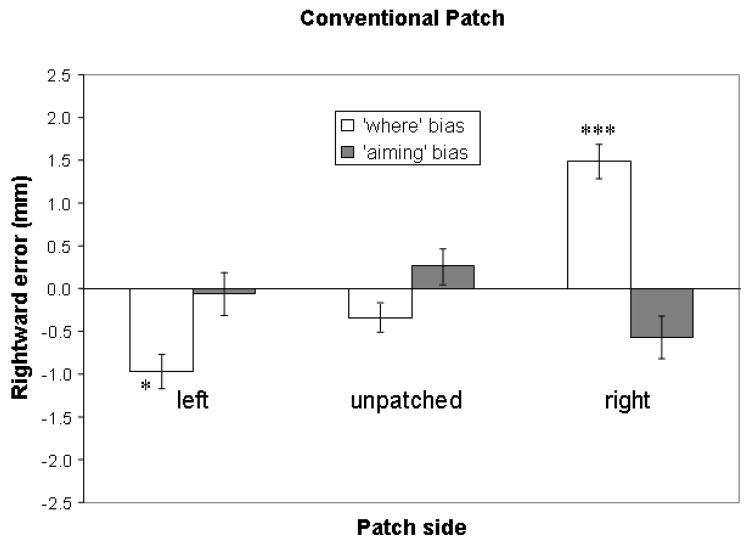
Data from trials in the unpatched and conventional patch conditions were used to test the first hypothesis. A 2 × 3 within-subjects analysis of variance (ANOVA) with bias type (“where” vs. “aiming”), and patching side (left, right, and unpatched) as variables was conducted. This revealed a main effect of patching side [F(2, 30) = 7.77, p = 0.002] and an interaction of bias type and patching side [F(2, 30) = 24.84, p < .001]. Paired-samples two-tailed t-tests revealed that the interaction primarily reflected from differences in “where” errors between the unpatched condition and the right patch, and between the unpatched condition and the left patch (see Figure 2). The right eye patch, comparing to the unpatched condition, induced a more rightward “where” error [t(15) = −5.48, p < 0.001] but a more leftward “aiming” error [t(15) = 2.59, p = 0.021], whereas the left eye patch tended to induce a more leftward “where” error than the unpatched condition [t(15) = 2.07, p = 0.056] without significant difference in “aiming” errors compared to the unpatched condition [t(15) = 0.92, p = 0.372]. Performances with monocular patching did differ from performance with binocular viewing (i.e., unpatched condition). To assess whether line bisection performance was biased compared with accurate performance, a series of one-sample two-tailed t-tests were conducted: in the unpatched condition, bisection performance was leftward, but these errors did not reach significance compared with perfect performance (“where” error = −0.3 mm, SD = 1.2, ns; “aiming” error = 0.3 mm, SD = 1.7, ns). The right eye patch induced rightward “where” errors (1.5 mm, SD = 1.4, t(15) = 4.16, p < 0.001) without significant leftward “aiming” errors (−0.6mm, SD = 2.0, ns). The left eye patch induced leftward “where” errors (−1.0mm, SD = 2.0, t(15) = −2.16, p = 0.047) without significant leftward “aiming” errors (−0.1 mm, SD = 2.0, ns). Only “where” errors with monocular patching were significantly different from zero (i.e., the true center of the line) while the other errors were not distinguishable from accurate performance. These results of one-sample two-tailed t-tests are indicated as numbers of asterisks in Table 1 and Figure 2. Overall, patch-induced spatial errors appeared consistent with a primary “where” rather than “aiming” bias.
Figure 2.
Mean line bisection errors with standard error bars in the unpatched condition and conditions while wearing a conventional patch on the left or right eye. Rightward errors are plotted positive and upward. One-sample two-tailed t-tests revealed that with an eye patch, the “where” errors were significantly different from zero (the true center of the line): * p < 0.05; *** p < 0.001. No “aiming” error was statistically different from zero. Patch-induced errors may be primarily dependent on “where” systems and ipsilateral to the patching side.
The Sprague effect predicted that an eye patch would induce contralateral bias. However as revealed in Figure 2, confirmed with the results of the t-tests described previously, direction of induced spatial bias was primarily ipsilateral to eye patching. Specifically, we found “where” ipsilateral bias to either eye when patched. These results were not consistent with the Sprague effect.
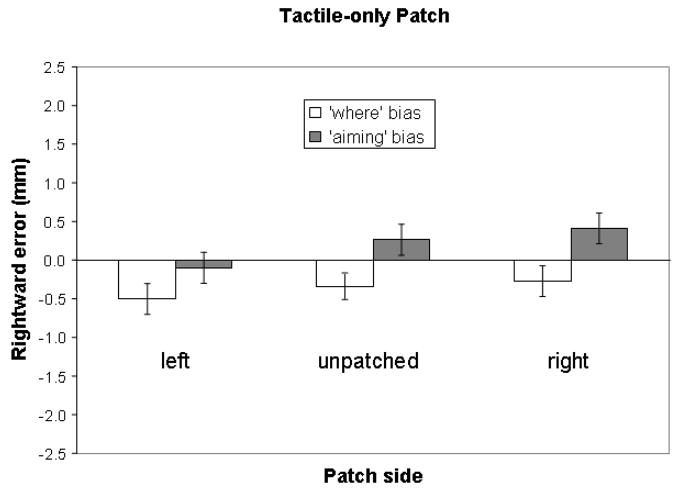
We next addressed whether an ipsilateral “where” bias induced by a monocular patch could be better explained by tactile distraction, or by compensatory ipsilateral re-allocation of perceptual attention. To test the tactile distraction hypothesis, the analysis was a one-way within-subjects ANOVA with patching side (unpatched, tactile-only right patch, and tactile-only left patch) as variables. No main effect was found. Consistently, paired-samples two-tailed t-tests supported no differences between the tactile-only left (−0.5 mm, SD = 1.8, ns), or right patch (−0.3 mm, SD = 1.7, ns) and the unpatched (−0.3 mm, SD = 1.2, ns) conditions. This indicates that tactile stimulation alone did not provide observable distraction to induce a significant error distinguishable from the performance demonstrated in the unpatched condition (Figure 3). One-sample two-tailed t-tests also showed no difference between zero and errors made in any tactile-only patch condition. This is inconsistent with the tactile distraction hypothesis that tactile stimulation would induce ipsilateral patch-induced spatial bias.
Figure 3.
Mean line bisection errors with standard error bars in the unpatched condition and the condition with the tactile-only patch – a tactile device around the eye without occluding vision. Rightward errors are plotted positive and upward. In this figure, no error was statistically different from zero, which indicates that a tactile-only patch did not induce spatial bias.
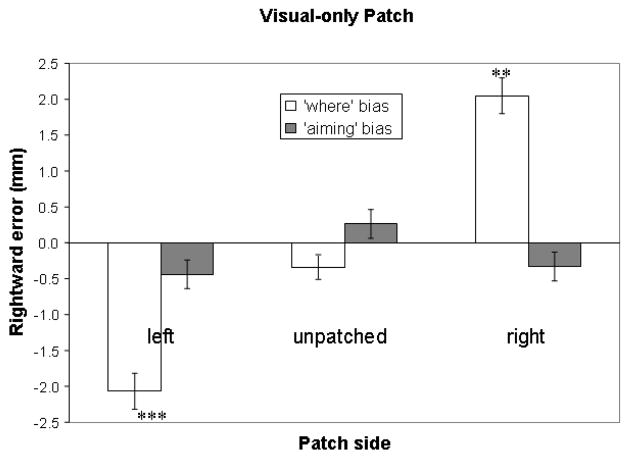
Compensatory ipsilateral orienting may result from re-allocation of ipsilateral “where” resources after visual deprivation due to monocular visual occlusion. This hypothesis was tested on “where” errors with a one-way within-subjects ANOVA with patching side (unpatched, visual-only right patch, and visual-only left patch) as variables. The analysis revealed a main effect of patching side [F (1,15) = 42.76, p < 0.001]. Following up, paired-samples two-tailed t-tests showed that the visual-only right patch induced more rightward “where” errors than the unpatched [t(15) = −6.54, p < .001], and the visual-only left patch induced more leftward “where” errors than the unpatched [t(15) = 4.21, p = 0.001]. Consistent with induced “where” errors with the conventional patch, the visual-only patch induced a significantly different performance than when both eyes were not occluded. Also consistent with findings with the conventional patch, the visual-only patch produced ipsilateral “where” errors, shown by one-sample two-tailed t-tests that revealed significant differences between zero and visual-only left (−2.1 mm, SD = 2.0, t(15) = −4.16, p < 0.001), and visual-only right patches (2.0 mm, SD = 2.0, t(15) = 4.07, p = 0.001). These results of t-tests are indicated as numbers of asterisks in Table 2 and Figure 4. Therefore, visual occlusion may account for patch-induced spatial bias, as a result of compensatory ipsilateral re-allocation of perceptual attention.
Table 2.
Mean bisection “where” and “aiming” errors under conditions of tactile-only patch and visual-only patch.
| Bisection error (in mm) | Patch type | |||
|---|---|---|---|---|
| Tactile-only | Visual-only | |||
| Bias Type | Left-patched | Right-patched | Left-patched | Right-patched |
| “where” | − 0.5 (1.8) | − 0.3 (1.7) | − 2.1 (2.0) *** | 2.0 (2.0) ** |
| “aiming” | − 0.1 (1.7) | − 0.4 (1.8) | − 0.4 (1.6) | − 0.3 (1.6) |
Rightward errors are presented in positive value, and leftward in negative. Standard deviations are in the parentheses. Measuring unit is in millimeter.
One-sample two-tailed t-tests compared all errors with zero (the true center of the line) and revealed that with one eye blocked from view, the “where” errors were significantly different from zero (the true center of the line): p < 0.01;
p < 0.001.
Visual occlusion, rather than tactile stimulation, induced ipsilateral “where” bias.
Figure 4.
Mean line bisection errors with standard error bars in the unpatched condition and the condition with the visual-only patch – a visual occlusion device in front of the eye without touching the face. Rightward errors are plotted positive and upward. One-sample two-tailed t-tests showed that with one eye blocked from view, the “where” errors were significantly different from zero (the true center of the line): ** p < 0.01; *** p < 0.001. No “aiming” error was statistically different from zero. This result indicates that visual loss may induce “where” bias, ipsilateral to the side of visual occlusion.
The mean spatial error, in the unpatched condition, made in near space (−0.30 mm, SD = 3.04) was to the left of that in far space (−0.13 mm, SD = 2.67). This directional difference was consistent with previous research that found a distance effect on spatial bias, but this paired-samples comparison did not reach significance [t(15) = −0.63, p = 0.538] (cf. Garza et al., in press; Varnava, McCarthy, & Beaumont, 2002; Wilkinson & Halligan, 2003). Previous research suggested that viewing distance may affect patch-induced spatial bias, especially in persons with spatial neglect (Barrett et al., 2000). However, no main effect or interaction was found with viewing distances used in the present study. It is possible that larger follow-up studies including more participants will replicate a near-far difference.
Discussion
This study presented evidence of “where” spatial bias induced by visual occlusion from an eye patch in healthy adults. Induced “where” bias was ipsilateral to the side of the eye patch. This finding is not consistent with the Sprague effect (Sprague, 1966).
Ipsilateral spatial bias induced by eye patching might be the result of bottom-up distraction by a novel tactile stimulus (i.e., the patch on the face), or top-down re-allocation of spatial perceptual or representational resources based on loss of visual input. The current results indicate that visual occlusion, rather than tactile distraction, may account for the ipsilaterally induced “where” bias. This finding that visual occlusion induced ipsilateral spatial bias may thus be explained by a compensatory mechanism of “where” attentional-representational resource allocation.
Compensatory orienting was reported in persons with hemianopia, a visual field defect caused by visual cortical lesions (Barton & Black, 1998; Doricchi & Angelelli, 1999; Doricchi et al., 2002b; Kerkhoff, 1993). Visual occlusion by monocular patching and hemianopia may both trigger asymmetrical perceptual-attentional re-allocation — altered criteria for stimulus detection in the area of decreased visual input —, or asymmetric spatial representation — increased representational resources devoted to the region of visual loss. Thus, compensatory resource reallocation may account for the present findings.
McCourt et al. (2001) also studied effects of monocular patching on spatial bias in healthy adults. Judging from their methodology (i.e., brief stimulus presentation, usage of chinrest, and no actual line bisection movement), they might have isolated “where” bias. Thus, it is possible to compare the findings of McCourt et al.’s study with the current findings on “where” bias. Inconsistent with the present study, however, they found that leftward midline perception of a line (when viewing binocularly) was increased as the right eye was occluded (more leftward) and reduced as the left eye was occluded (leftward, but lesser in magnitude). That is, the induced misperception of the midline was biased toward the opposite side of a right, but not a left eye patch. The discrepancy between the two studies may result from the differences in experimental tasks. In the present study, participants were allowed to view a line for an unlimited time period as they moved to mark the midpoint. In McCourt et al.’s study, a line was presented for 150 ms. This short period of time may facilitate bottom-up rather than top-down processing, and thus inhibit compensatory resource re-allocation, which may require time to be initiated and to produce effects. Whether the cortical-colliculus system may be involved at the beginning of visual occlusion, before top-down compensatory effects may overcome the Sprague effect, requires further investigation.
Acknowledgments
We thank participants who took part in the experiment, and John Garza, Daymond Wagner, Scott Pekrul, and Erin Zimmerman who assisted with data collection, and Paul Eslinger and Kenneth Heilman for theoretical advice. This work was supported by the National Institute on Disability and Rehabilitation Research (H133P020012, PI: DeLuca), the National Institute of Neurological Disorders and Stroke (K02 NS47099, K08 NS02085, PI: Barrett), the Henry H. Kessler Foundation and the Penn State College of Medicine General Clinical Research Center (NIH/NCRR C06 RR016499 and M01 RR010732). Dr. Barrett has no financial conflicts of interest related to this research to disclose. These data were presented in preliminary form at the 37th annual meeting of the Society for Neuroscience, November 2007, San Diego, CA; Program No. 423.3. 2007 Abstract Viewer/Itinerary Planner. Online (www.sfn.org).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adair JC, Na DL, Schwartz RL, Heilman KM. Analysis of primary and secondary influences on spatial neglect. Brain And Cognition. 1998;37(3):351–367. doi: 10.1006/brcg.1998.1002. [DOI] [PubMed] [Google Scholar]
- Barrett AM, Burkholder S. Monocular patching in subjects with right-hemisphere stroke affects perceptual-attentional bias. Journal of Rehabilitation Research and Development. 2006;43(3):337–345. doi: 10.1682/jrrd.2005.01.0015. [DOI] [PubMed] [Google Scholar]
- Barrett AM, Buxbaum LJ, Coslett HB, Edwards E, Heilman KM, Hillis AE, et al. Cognitive rehabilitation interventions for neglect and related disorders: Moving from bench to bedside in stroke patients. Journal Of Cognitive Neuroscience. 2006;18(7):1223–1236. doi: 10.1162/jocn.2006.18.7.1223. [DOI] [PubMed] [Google Scholar]
- Barrett AM, Crucian GP, Beversdorf DQ, Heilman KM. Monocular patching may worsen sensory-attentional neglect: A case report. Archives of Physical Medicine and Rehabilitation. 2001;82(4):516–518. doi: 10.1053/apmr.2001.21973. [DOI] [PubMed] [Google Scholar]
- Barrett AM, Schwartz RL, Crucian GP, Kim M, Heilman KM. Attentional grasp in far extrapersonal space after thalamic infarction. Neuropsychologia. 2000;38(6):778–784. doi: 10.1016/s0028-3932(99)00144-x. [DOI] [PubMed] [Google Scholar]
- Barrett AM, Crucian GP, Heilman KM. Eye patching biases spatial attention after thalamic hemorrhage in a patient without spatial neglect. Archives of Physical Medicine and Rehabilitation. 2004;85(6):1017–1020. doi: 10.1016/j.apmr.2003.08.076. [DOI] [PubMed] [Google Scholar]
- Barton JJS, Black SE. Line bisection in hemianopia. Journal of Neurology Neurosurgery and Psychiatry. 1998;64(5):660–662. doi: 10.1136/jnnp.64.5.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beis JM, Andre JM, Baumgarten A, Challier B. Eye patching in unilateral spatial neglect: Efficacy of two methods. Archives of Physical Medicine and Rehabilitation. 1999;80(1):71–76. doi: 10.1016/s0003-9993(99)90310-6. [DOI] [PubMed] [Google Scholar]
- Bisiach E, Geminiani G, Berti A, Rusconi ML. Perceptual and premotor factors of unilateral neglect. Neurology. 1990;40(8):1278–1281. doi: 10.1212/wnl.40.8.1278. [DOI] [PubMed] [Google Scholar]
- Bowers D, Heilman KM. Pseudoneglect - effects of hemispace on a tactile line bisection task. Neuropsychologia. 1980;18(4–5):491–498. doi: 10.1016/0028-3932(80)90151-7. [DOI] [PubMed] [Google Scholar]
- Butter CM, Kirsch N. Combined and separate effects of eye patching and visual stimulation on unilateral neglect following stroke. Archives of Physical Medicine and Rehabilitation. 1992;73(12):1133–1139. [PubMed] [Google Scholar]
- Coslett HB, Bowers D, Fitzpatrick E, Haws B, Heilman KM. Directional hypokinesia and hemispatial inattention in neglect. Brain. 1990;113:475–486. doi: 10.1093/brain/113.2.475. [DOI] [PubMed] [Google Scholar]
- Doricchi F, Angelelli P. Misrepresentation of horizontal space in left unilateral neglect - Role of hemianopia. Neurology. 1999;52(9):1845–1852. doi: 10.1212/wnl.52.9.1845. [DOI] [PubMed] [Google Scholar]
- Doricchi F, Galati G, DeLuca L, Nico D, D’Olimpio F. Horizontal space misrepresentation in unilateral brain damage I. Visual and proprioceptive-motor influences in left unilateral neglect. Neuropsychologia. 2002a;40(8):1107–1117. doi: 10.1016/s0028-3932(02)00010-6. [DOI] [PubMed] [Google Scholar]
- Doricchi F, Onida A, Guariglia P. Horizontal space misrepresentation in unilateral brain damage II. Eye-head centered modulation of visual misrepresentation in hemianopia without neglect. Neuropsychologia. 2002b;40(8):1118–1128. doi: 10.1016/s0028-3932(02)00011-8. [DOI] [PubMed] [Google Scholar]
- Fitts PM. The information capacity of the human motor system in controlling the amplitude of movement. Journal of Experimental Psychology. 1954;47:381. [PubMed] [Google Scholar]
- Garza JP, Eslinger PJ, Barrett AM. Perceptual-attentional and motor-intentional bias in near and far space. Brain And Cognition. doi: 10.1016/j.bandc.2008.02.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilman KM, Watson RT, Valenstein E. Neglect and related disorders. In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology. New York: Oxford University Press; 1979. pp. 268–307. [Google Scholar]
- Heilman KM, Watson RT, Valenstein E. Neglect and related disorders. In: Heilman KM, Valenstein E, editors. Clinical Neuropsychology. New York: Oxford University Press; 2003. pp. 296–346. [Google Scholar]
- HendrickA Wilson ME, Toyne MJ. Distribution of optic nerve fibers in macaca-mulatta. Brain Research. 1970;23(3):425–427. doi: 10.1016/0006-8993(70)90068-5. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Levay S, Wiesel TN. Mode of termination of retinotectal fibers in macaque monkey - autoradiographic study. Brain Research. 1975;96(1):25–40. doi: 10.1016/0006-8993(75)90567-3. [DOI] [PubMed] [Google Scholar]
- Husain M, Mattingley JB, Rorden C, Kennard C, Driver J. Distinguishing sensory and motor biases in parietal and frontal neglect. Brain. 2000;123:1643–1659. doi: 10.1093/brain/123.8.1643. [DOI] [PubMed] [Google Scholar]
- Jewell G, McCourt ME. Pseudoneglect: A review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia. 2000;38(1):93–110. doi: 10.1016/s0028-3932(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Kerkhoff G. Displacement of the egocentric visual midline in altitudinal postchiasmatic scotomas. Neuropsychologia. 1993;31(3):261–265. doi: 10.1016/0028-3932(93)90090-m. [DOI] [PubMed] [Google Scholar]
- MacLeod MS, Turnbull OH. Motor and perceptual factors in pseudoneglect. Neuropsychologia. 1999;37(6):707–713. doi: 10.1016/s0028-3932(98)00115-8. [DOI] [PubMed] [Google Scholar]
- Mapstone M, Weintraub S, Nowinski C, Kaptanoglu G, Gitelman DR, Mesulam MM. Cerebral hemispheric specialization for spatial attention: spatial distribution of search-related eye fixations in the absence of neglect. Neuropsychologia. 2003;41(10):1396–1409. doi: 10.1016/s0028-3932(03)00043-5. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Jewell G. Visuospatial attention in line bisection: stimulus modulation of pseudoneglect. Neuropsychologia. 1999;37(7):843–855. doi: 10.1016/s0028-3932(98)00140-7. [DOI] [PubMed] [Google Scholar]
- McCourt ME, Garlinghouse M, Butler J. The influence of viewing eye on pseudoneglect magnitude. Journal of The International Neuropsychological Society. 2001;7(3):391–395. doi: 10.1017/s1355617701003137. [DOI] [PubMed] [Google Scholar]
- Na DL, Adair JC, Kang Y, Chung CS, Lee KH, Heilman KM. Motor perseverative behavior on a line cancellation task. Neurology. 1999;52(8):1569–1576. doi: 10.1212/wnl.52.8.1569. [DOI] [PubMed] [Google Scholar]
- Na DL, Adair JC, Williamson DJG, Schwartz RL, Haws B, Heilman KM. Dissociation of sensory-attentional from motor-intentional neglect. Journal of Neurology Neurosurgery and Psychiatry. 1998;64(3):331–338. doi: 10.1136/jnnp.64.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nico D. Detecting directional hypokinesia: The epidiascope technique. Neuropsychologia. 1996;34(5):471–474. doi: 10.1016/0028-3932(95)00123-9. [DOI] [PubMed] [Google Scholar]
- Payne BR, Rushmore RJ. Functional circuitry underlying natural and interventional cancellation of visual neglect. Experimental Brain Research. 2004;154(2):127–153. doi: 10.1007/s00221-003-1660-9. [DOI] [PubMed] [Google Scholar]
- Pollack JG, Hickey TL. Distribution of retino-collicular axon terminals in rhesus-monkey. Journal of Comparative Neurology. 1979;185(4):587–602. doi: 10.1002/cne.901850402. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rafal RD. Cognitive theories of attention and the rehabilitation of attentional deficits. In: Meir MJ, Benton A, Diller L, editors. Neuropsychological Rehabilitation. New York: Guildford Press; 1987. pp. 182–201. [Google Scholar]
- Rushmore RJ, Valero-Cabre A, Lomber SG, Hilgetag CC, Payne BR. Functional circuitry underlying visual neglect. Brain. 2006;129:1803–1821. doi: 10.1093/brain/awl140. [DOI] [PubMed] [Google Scholar]
- Schwartz RL, Adair JC, Na D, Williamson DJG, Heilman KM. Spatial bias: Attentional and intentional influence in normal subjects. Neurology. 1997;48(1):234–242. doi: 10.1212/wnl.48.1.234. [DOI] [PubMed] [Google Scholar]
- Serfaty C, Soroker N, Glicksohn J, Sepkuti J, Myslobodsky MS. Does monocular viewing improve target detection in hemispatial neglect? Restorative Neurology and Neuroscience. 1995;9(2):77–83. doi: 10.3233/RNN-1995-9202. [DOI] [PubMed] [Google Scholar]
- Soroker N, Cohen T, Baratz C, Glicksohn J, Myslobodsky MS. Is there a place for ipsilateral eye patching in neglect rehabilitation? Behavioural Neurology. 1994;7(3–4):59–164. doi: 10.3233/BEN-1994-73-408. [DOI] [PubMed] [Google Scholar]
- Sprague JM. Interaction of cortex and superior colliculus in mediation of visually guided behavior in cat. Science. 1966;153(3743):1544–1547. doi: 10.1126/science.153.3743.1544. [DOI] [PubMed] [Google Scholar]
- Sylvester R, Josephs O, Driver J, Rees G. Visual fMRI responses in human superior colliculus show a temporal-nasal asymmetry that is absent in lateral geniculate and visual cortex. Journal of Neurophysiology. 2007;97(2):1495–1502. doi: 10.1152/jn.00835.2006. [DOI] [PubMed] [Google Scholar]
- Tegnér R, Levander M. Through a looking-glass - a new technique to demonstrate directional hypokinesia in unilateral neglect. Brain. 1991;114:1943–1951. doi: 10.1093/brain/114.4.1943. [DOI] [PubMed] [Google Scholar]
- Varnava A, McCarthy M, Beaumont JG. Line bisection in normal adults: direction of attentional bias for near and far space. Neuropsychologia. 2002;40(8):1372–1378. doi: 10.1016/s0028-3932(01)00204-4. [DOI] [PubMed] [Google Scholar]
- Walker R, Young AW, Lincoln NB. Eye patching and the rehabilitation of visual neglect. Neuropsychological Rehabilitation. 1996;6(3):219–231. [Google Scholar]
- Wallace SF, Rosenquist AC, Sprague JM. Ibotenic acid lesions of the lateral substantia nigra restore visual orientation behavior in the hemianopic cat. Journal of Comparative Neurology. 1990;296(2):22–252. doi: 10.1002/cne.902960204. [DOI] [PubMed] [Google Scholar]
- Weddell RA. Subcortical modulation of spatial attention including evidence that the Sprague effect extends to man. Brain and Cognition. 2004;55(3):497–506. doi: 10.1016/j.bandc.2004.02.075. [DOI] [PubMed] [Google Scholar]
- Wilkinson D& Halligan P. The effects of stimulus size on bisection judgements in near and far space. Visual Cognition. 2003;10(3):319–340. [Google Scholar]