Abstract
The high demand for molecular oxygen, the enrichment of polyunsaturated fatty acids in membrane phospholipids and the relatively low abundance of antioxidant defense enzymes are factors rendering cells in the central nervous system (CNS) particularly vulnerable to oxidative stress. Excess production of reactive oxygen species (ROS) in the brain has been implicated as a common underlying factor for the etiology of a number of neurodegenerative diseases, including Alzheimer's disease (AD), Parkinson's disease (PD), and stroke. While ROS are generated by enzymatic and non-enzymatic reactions in the mitochondria and cytoplasm under normal conditions, excessive production under pathological conditions is associated with activation of Ca2+-dependent enzymes including proteases, phospholipases, nucleases, and alterations of signaling pathways which subsequently lead to mitochondrial dysfunction, release of inflammatory factors and apoptosis. In recent years, there is considerable interest to investigate anti-oxidative and anti-inflammatory effects of phenolic compounds from different botanical sources. In this review, we describe oxidative mechanisms associated with AD, PD, and stroke, and evaluate neuroprotective effects of phenolic compounds, such as resveratrol from grape and red wine, curcumin from turmeric, apocynin from Picrorhiza kurroa, and epi-gallocatechin from green tea. The main goal is to provide a better understanding of the mode of action of these compounds and assess their use as therapeutics to ameliorate age-related neurodegenerative diseases.
2. Introduction
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) such as superoxide anion, hydroxyl radicals, hydrogen peroxide, lipid peroxyl radicals, nitric oxide, and peroxynitrite, are generated in different cellular systems through enzymatic and non-enzymatic reactions (Sun & Chen 1998). Many pathological conditions are associated with excessive production of ROS/RNS which can attack key proteins, lipids and DNA, alter signal transduction pathways, destroy membranes and subcellular organelles, and subsequently result in apoptosis and cell death. In the presence of transition metals or redox cycling compounds (including quinones), reactive oxygen species such as superoxide can be converted to the more reactive hydroxy radicals. In some cellular conditions, superoxide anions and nitric oxide can react with each other and form peroxynitrite, a highly toxic anionic compound.
A number of intracellular enzymes are known to produce ROS/RNS, e.g., xanthine/xanthine oxidase, NADPH oxidase, cytochrome P450, nitric oxide synthases, prostaglandin synthases, and enzymes in the electron transport chain in mitochondria. In the cellular/subcellular systems, however, production of ROS/RNS through these oxidative enzymes can be counteracted by intracellular antioxidants, including glutathione, vitamin C and E, Coenzyme Q, and by antioxidant enzymes such as superoxide dismutases (SOD), catalase, and glutathione peroxidase. Recent studies also recognize the role of protein kinases and signaling molecules in regulating transcription factors, such as NFκB and Nrf-2/ARE, and thus genes involved in inflammation and oxidant responses (Lim et al. 2007a, Mattson 2008).
The high demand for molecular oxygen, the high levels of polyunsaturated fatty acids in neural membrane phospholipids, and the high iron content are important factors rendering cells in the central nervous system (CNS) to oxidative stress. Oxidative stress is an important underlying factor for a number of neurodegenerative disesaes (Halliwell 2006). Neurons are particularly at risk to oxidative stress because many major antioxidant defence mechanisms, such as GSH, Nrf-2, and metallothienin, seem to be localized to astrocytes. Excessive ROS production is associated with activation of the Ca2+-dependent enzymes including proteases, phospholipases, and nucleases and alterations of signaling pathways that lead to mitochondrial dysfunction and neuronal apoptosis (Mattson 2007). Increase in oxidative products, such as 4-hydroxynonenal (HNE) for lipid peroxidation, 3-nitrotyrosine (3-NT) for protein carbonyl and protein nitrotyrosine adducts, and 8-hydroxy-deoxyguanosine (8-OHdG) for DNA damage, associated with neurodegenerative diseases support the notion that oxidative stress is a common element in the progression of these diseases (Halliwell 2006, Simonian & Coyle 1996, Sun & Chen 1998).
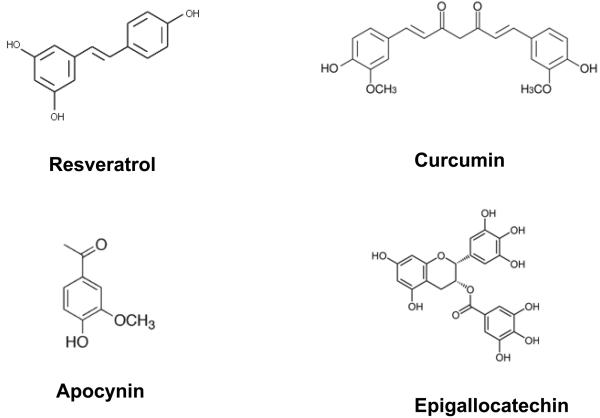
Oxidative stress is also a significant factor associated with the decline of function in the aging brain. With the disproportional increase in aging population (baby boomers) in the next decade, there is increasing attention to develop nutritional therapies to combat these age-related oxidative processes. Considerable attention is focused on botanicals in vegetables, fruits, grains, roots, flowers, seeds, tea and red wine. Other nutritional interventions such as dietary restriction and a Mediterranean diet have also captured considerable attention, in particular among older population and subjects with mild cognitive impairments (Burgener et al. 2008). Compounds such as resveratrol from grape and wine, curcumin from turmeric, and epigallocatechin from green tea, are becoming recognized for their protective effects against inflammatory diseases, cancers, cardiovascular and neurodegenerative diseases. Although the mechanisms whereby these compounds display beneficial effects remain elusive, there is increasing evidence to support their anti-oxidative, anti-inflammatory, anti-apoptotic and metal-chelating properties (Rice-Evans & Miller 1997, Ndiaye et al. 2005). Besides these polyphenolic compounds, there is increasing evidence for NADPH oxidase as an important source of ROS in the central nervous system. Recent studies also place emphasis on ability for apocynin, a phenolic compound derived from Picrorhiza kurroa to inhibit NADPH oxidase (Fig 1). The major goal for this review is to describe oxidative mechanisms underlying neurodegenerative diseases such as AD, PD and stroke and to assess whether these phenolic compounds may offer neuroprotective effects.
Fig 1.
Structure of resveratrol, curcumin, apocynin and epigallocatechin-gallate
3. Oxidative stress and neurodegenerative disorders
3-a. Alzheimer's disease
Alzheimer's disease (AD) is the most common form of dementia affecting more than 4 million people in the U.S. and 15 to 20 million worldwide. With the disproportional increase in the aging population in the next decade, these numbers are projected to triple by 2050. Common pathological hallmarks for AD are accumulation of amyloid plaques and neurofibrillary tangles (McKeel et al. 2004). Besides genetic factors which comprise around 7% of familial AD patients (FAD), epi-genetic and environmental factors are known to play an important role in the onset of sporadic AD. Cardiovascular abnormalities such as hypertension, diabetes, mini-stroke, and atherosclerosis are factors precipitating the increased risk for AD.
Because increase in oxidative stress is associated with early development of AD (Butterfield et al. 2002), there is interest to search for effective therapy to combat the oxidative damage in this disease. There is evidence that at least part of the oxidative mechanism is contributed by the amyloid beta (Abeta) peptides. These peptides (39-43 amino acids) are released from the amyloid precursor protein through beta and gamma secretases and upon release, can be aggregated to oligomeric form. Oligomeric Abeta can confer oxidative insult to neurons and glial cells and initiate changes in synaptic plasticity, events occurring long before their deposition to form the amyloid plaques (Selkoe 2001). Although the mechanism for oligomeric Abeta to confer cytotoxicity that results in synaptic dysfunction is not clearly understood, there is evidence that these peptides can confer specific action on the N-methyl-D-aspartic acid (NMDA) receptors (Snyder et al. 2005). Aside from regulating synaptic plasticity and memory function, activation of NMDA receptor is coupled to ROS production (Kishida & Klann 2007, Kishida et al. 2005). Recent studies further demonstrate that Abeta can induce ROS production in neurons through an NMDA receptor-dependent process (De Felice et al. 2007, Shelat et al. 2008). Thus, NADPH oxidase may be common in NMDA- and Abeta-induced ROS production, and activation of signaling pathways, including PKC and MAPK, which in turn, lead to activation of cytosolic phospholipase A2 (cPLA2) and release of arachidonic acid (AA) (Shelat et al. 2008). Arachidonic acid not only is a precursor for synthesis of prostaglandins, but is also known to serve as a retrograde transmitter in regulating synaptic plasticity (Sang & Chen 2006). Studies by Kriem et al (2005) demonstrated the involvement of cPLA2 in Abeta-induced apoptosis in neurons (Kriem et al. 2005).
Intracellular Abeta may target cytoplasmic signaling pathways and impair mitochondrial function (Wang et al. 2007b). In astrocytes, Abeta treatment was shown to cause the decrease in mitochondrial membrane potential, and this was partly due to activation of phospholipase A2 (Zhu et al. 2006). In most instances, mitochondrial dysfunction is associated with increase production of ROS, release of cytochrome C, which in turn, triggers the apoptotic pathways. Abeta-mediated ROS production is also linked to increased inflammatory responses, including increased production of cytokines, nitric oxide and eicosanoids (Mancuso et al. 2007, Butterfield et al. 2002, Akama & Van Eldik 2000). Other contributions from astrocytes include alterations in the synthesis of ApoE (major risk factor for AD) and D-serine, which is an endogenous activator of NMDA receptors.
NADPH oxidase has been regarded an important source of ROS that mediate the inflammatory responses in astrocytes and microglial cells in the brain. In fact, Abeta-induced ROS from NADPH oxidase in astrocytes is a key factor in mediating neuronal death (Abramov et al. 2004). Therefore, there is strong rationale to develop antioxidant strategy to ameliorate the inflammatory responses associated with the progression of AD. Many recent studies have provided compelling evidence to support dietary supplement of polyphenolic compounds from plant sources to minimize the oxidative events in the AD brain (Anekonda 2006, Chauhan & Sandoval 2007, Ringman et al. 2005). These herbal alternatives may provide greater therapeutic benefit compared to a single-ingredient synthetic pharmaceutical drug which normally has serious side effects (Kotilinek et al. 2008). Table 1 (top) provides a summary of recent studies testing different botanicals on AD models.
Table 1.
Effects of common botanicals on AD, PD and stroke
| Polyphenol/plant name | Model | Effects | References |
|---|---|---|---|
| AD models | |||
| Blueberry | Tg2576 mice | + | (Joseph et al. 2003) |
| EGCG | Tg2576 mice | + | (Rezai-Zadeh et al. 2005) |
| Garlic | Tg2576 mice | + | (Chauhan 2003) (Chauhan 2006) |
| TgCRND8 mice | + | (Chauhan & Sandoval 2007) | |
| Ginkgo biloba | Tg2576 mice | + | (Stackman et al. 2003) |
| TgAPP/PS1 mice | + | (Garcia-Alloza et al. 2006) (Tchantchou et al. 2007) |
|
| Ginseng | Tg2576 mice | + | (Chen et al. 2006) |
| Ginsenoside Rb1 or M1 | Aβ infusion (i.c.v.) mice | + | (Tohda et al. 2004) |
| Pomegranate | Tg2576 mice | + | (Hartman et al. 2006) |
| PD models | |||
| Black tea | 6-OHDA rat | + | (Chaturvedi et al. 2006) |
| Cocoa | 6-OHDA rat | −/+ | (Datla et al. 2007) |
| EGCG | MPTP mice | + | (Choi et al. 2002) (Mandel & Youdim 2004) |
| Ginkgo biloba | 6-OHDA rat | + | (Kim et al. 2004) |
| Grape seed | 6-OHDA rat | − | (Datla et al. 2007) |
| Green tea | MPTP mice | + | (Choi et al. 2002) |
| 6-OHDA rat | + | (Guo et al. 2007) | |
| Quercetin | 6-OHDA rat | − | (Zbarsky et al. 2005) |
| Red clover | 6-OHDA rat | + | (Datla et al. 2007) |
| Tangerine peel extract | 6-OHDA rat | + | (Datla et al. 2007) |
| Stroke models | |||
| Bluberries | rat permanent left CCAO+H | + | (Sweeney et al. 2002) |
| rat transient MCAO | + | (Wang et al. 2005d) | |
| Buckwheat polyphenols | rat repeated ischemia | + | (Pu et al. 2004) |
| Curcuma oil | rat transient MCAO | + | (Rathore et al. 2007) |
| Garlic | rat transient MCAO | + | (Saleem et al. 2006) |
| Ginseng | gerbil transient CCAO | + | (Shen & Zhang 2003) |
| Grape seed extract | neonatal rat H-I | + | (Feng et al. 2005, 2007) |
| Green tea extract | rat transient MCAO | + | (Hong et al. 2000) |
| gerbil transient CCAO | + | (Hong et al. 2001) | |
| Mulberry extract | mouse transient MCAO | + | (Kang et al. 2006) |
| Pomegranate | neonatal mouse H-I | + | (West et al. 2007) |
| Red wine polyphenols | rat transient MCAO | + | (Ritz et al. 2008) |
| Sesame oil | rat transient MCAO | + | (Ahmad et al. 2006) |
| Spinach | rat transient MCAO | + | (Wang et al. 2005d) |
3-b. Parkinson's disease
Parkinson's disease (PD) affects approximately 1% of the population over the age of 50. The clinical manifestations of PD include tremors, bradykinesia, muscle rigidity, and akinesia. The pathological landmarks include a progressive loss of dopaminergic neurons in the substantia nigra (Cardoso et al. 2005). Despite numerous hypotheses and speculations for the etiology of PD, oxidative stress remains the strongest leading theory (Miller et al. 2008).
Increased risk for PD is correlated with exposure to environmental factors including heavy metals and herbicides (Brooks et al. 1999, Liou et al. 1997, Yang & Sun 1998b). MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) is an environmental toxin which can selectively damage the substantia nigra and produces Parkinson-like symptoms in animal models and in humans. Studies with this PD model have provided important information about the possible cause of PD (Adams & Odunze 1991, Langston et al. 1987, Schapira 1996). Besides MPTP, other environmental toxins including rotenone, manganese (Sun et al. 1993), dimethoxyphenylethylamine (DMPEA) (Koshimura et al. 1997) and paraquat (Li & Sun 1999, Yang & Sun 1998a, Yang & Sun 1998b) also target dopamine neurons. These agents can make their way to the substantia nigra and induce apoptotic pathways in dopaminergic neurons (Schober 2004, Lim et al. 2007b).
Dopamine is a neurotransmitter that can undergo metabolism either by monoamine oxidase (MAO) or by autooxidation, producing H2O2, superoxide anion, and hydroxyl radicals. In addition, nitric oxide, which is produced through inflammation-induced microglia activation or excitotoxic insults (Abekawa et al. 1997, Gonzalez-Hernandez et al. 1996), may also play a role in the pathogenesis of PD. Formation of peroxynitrite anions through the combination of ROS with nitric oxide may confer additional toxicity to dopaminergic neurons.
Microglia activation is an important factor contributing to the inflammatory responses in PD (Castano et al. 1998, Gao et al. 2003b). Earlier studies demonstrated higher levels of microglia in the PD brain as compared to the age-matched control brain (McGeer et al. 1988). Activated microglia are present in the substantia nigra in several models of PD, including those induced by exposure to MPTP, rotenone, and 6-OHDA (Block et al. 2006, Gao et al. 2002). Abnormal accumulation of iron in microglia and increased levels of α-synuclein are important pathological features in these models. The ability for microglia to produce high levels of ROS through NADPH oxidase is regarded as an important factor underlying the MPTP-induced dopaminergic neurodegeneration (Gao et al. 2003a, Gao et al. 2003b, Mander et al. 2006, Wu et al. 2003). In our recent study with BV2 microglial cells, paraquat-induced ROS through NADPH oxidase was shown to require protein kinases such as PKCdelta and ERK1/2 (Miller et al. 2007). In microglia-neuron coculture, microglia lacking functional NADPH oxidase failed to produce neurotoxicity in response to paraquat (Wu et al. 2005). The important role of microglia in pathogenesis of PD can be demonstrated by the ability for minocyline, an antibiotic known to inhibit microglial activation to attenuate the neurotoxicity caused by rotenone (Casarejos et al. 2006).
A number of studies have demonstrated the protective effects of plant phenolics against brain damage in PD. These studies have used either a single compound such as resveratrol, curcumin, EGCG, or a complex mixture of extracts from grape, blueberry and green tea (Weinreb et al. 2004, Mercer et al. 2005, Chen et al. 2007, Masuda et al. 2006). Table 1 provides a summary of the studies using different botanicals on PD models. The neuroprotective effects of these phenolic compounds are attributed in part to the free radical scavenging, iron/metal chelating, and their anti-inflammatory properties. There is evidence that these phenolic compounds can target specific signaling pathways and interact with specific proteins, including aggregation of alpha-synuclein (Masuda et al. 2006, Ramassamy 2006, Vafeiadou et al. 2007).
3-c. Stroke
Stroke is the third leading cause of death and the first cause of disability in aging adults. The primary cause of stroke is the interruption of cerebral blood flow either by an arterial or venous obstruction or a cardiac arrest. The pathological manifestations in stroke are diverse, depending on the severity, duration, and localization of the ischemic damage. In the past, many animal models have been developed in which blood flow is focally or globally, permanently or transiently, completely or incompletely interrupted. The most widely established methods of global cerebral ischemia in rodents (rats, mice or gerbils) are the 2- and 4-vessel occlusion. In gerbils, occlusion of both common carotid arteries (CCA) for 5 min can cause delayed neuronal death (DND) of pyramidal neurons in the hippocampal CA1 area after 4 days (Wang et al. 2002). In addition, the DND is accompanied by increased reactive astrocytes and microglial cells in the injured area (Wang et al. 2002).
Focal cerebral ischemia is usually produced by occlusion of the middle cerebral artery (MCA), either through surgical exposure of the artery after craniotomy or by inserting a suture from the CCA to the MCA to block the blood flow (Chan et al. 1990). Cessation of cerebral blood flow is accompanied by rapid metabolic changes including decrease in ATP production, neuronal membrane depolarization and release of excitatory neurotransmitters. Despite obvious limitations with each model, the focal ischemic model appears to reflect the most common form of clinical stroke. In focal ischemia followed by reperfusion (I/R), cerebral infarcts with extensive loss of neurons and activation of glial cells are found within 12 to 24 hours after the insult. The penumbral area surrounding the ischemic core is comprised of a large number of reactive astrocytes and microglial cells. Factors causing activation of glial cells in the penumbral area and their role in preventing the spreading of depression and restoring neuronal function remain to be an important area of study.
Oxidative stress has been regarded as a substantial underlying cause of brain damage and neuronal dysfunction after cerebral I/R (Chan 2001). However, the mechanism(s) underlying ROS production and how neurons and glial cells respond to I/R has not been clearly elucidated. Earlier studies with neurons in culture demonstrated the role of ionotropic glutamate receptors, particularly the NMDA subtype, in triggering massive Ca2+ influx and in turn, the activation of Ca2+-dependent enzymes that trigger mitochondrial dysfunction and apoptotic cell death (Choi 1992). Although mitochondrial dysfunction is known to produce ROS that causes neuronal apoptosis in cerebral ischemia (Chan 2004), recent studies also provided evidence for the involvement of ROS from NADPH oxidase (Wang et al. 2006b, Tang et al. 2007). In order to combat the deleterious effects of oxidative stress associated with I/R, a number of studies have attempted to upregulate antioxidant enzymes, e.g., superoxide dismutases, catalase and glutathione peroxidase. Studies with transgenic mice overexpressing SOD1 or GSH-Px-1 have provided support for an important role of these enzymes to remove superoxide and decrease oxidative injury in both global and transient MCAO ischemic models (Saito et al. 2005).
The underlying role of oxidative stress in neuronal damage after I/R also raises attention to possible beneficial effects of polyphenolic compounds from different plant sources (Bravo 1998, Deschamps et al. 2001, Voko et al. 2003, Youdim & Joseph 2001). Studies suggest that some polyphenols can be preventative as well and may thus can act at multiple levels to influence both the early and late phases in the progression of stroke (Curin et al. 2006, Simonyi et al. 2005). Data in Table 1 provide a summary of recent studies testing different botanicals on stroke models.
4. Botanical phenolics and neurodegenerative disorders
The use of plant-derived supplements for improving health is gaining popularity because most people consider these natural products to be safer and produce less side effects than synthetic drugs (Raskin et al. 2002). Today, one in three Americans use herbal supplements; consumption is generally greater among woman, patients undergoing surgery, and elderly men (Ang-Lee et al. 2001, Morelli & Naquin 2002). There are more than 50 different plant species and over 8000 phenolic compounds identified either in single, pure molecular form or in specific proportions of differing plant extracts. Investigating the health benefits of these natural compounds is an enormous challenge to modern medicine.
Polyphenols such as resveratrol were initially identified as the plant's defensive response against stress from ultraviolet radiation, pathogens, and physical damage (Ferguson 2001). For this and other reasons, the polyphenol content in a specific plant source may vary, and differences in procedures for extraction, processing, and storage may also affect purity of the product and inconsistency in the package product.
Polyphenols are divided into different groups depending on the number of phenol rings and the chemical groups attached to the rings. Flavonoids make up the largest and the most important single group of polyphenols and can be divided into subgroups such as flavanols (catechin, epicatechin), flavonols (quercetin, myricetin, kaempferol), flavanons (hesperetin, naringenin), flavons (apigenin, luteolin), isoflavonoids (genistein, daidzein) and anthocyanins (cyaniding, malvidin). The capacity of flavonoids to act as an antioxidant is dependent upon their molecular structure, the position of hydroxyl groups and other substitutions in the chemical structure of these polyphenols. A number of excellent reviews dealing with their structure, absorption, metabolism, and pharmacokinetics have been published (Bravo 1998, Ross & Kasum 2002, Manach & Donovan 2004). Besides scavenging free radicals, many phenolics also exhibit multiple biological properties, e.g. anti-inflammatory, anticancer, antiviral, antimicrobial, vasorelaxant, and anticlotting activities (Rahman et al. 2007). In general, these phenolic compounds are rapidly converted to their glucuronide derivatives upon ingestion and are transported to the circulatory system and different body organs including the brain. In recent years, a number of reviews have reported on neuroprotective effects of polyphenols in cell and animal models,(Wang et al. 2001, Dajas et al. 2003, Mandel & Youdim 2004, Simonyi et al. 2005). This review is limited to neuroprotective effects of resveratrol from grape and wine, curcumin from turmeric, apocynin from Picrorhiza kurroa, and epigallocatechin-3-gallate from green tea (Fig 1).
There is evidence that some phenolic compounds exert their mode of action and target different intracellular pathways on a concentration-dependent manner. For example, low dose of red wine polyphenols was shown to promote angiogenesis via activation of the Akt/PI3K/eNOS, p38MAPK pathway but not the NF-κB pathway. However, at high dose, they can be anti-angiogenic through inhibition of the Akt/PI3K/eNOS pathway and enhancing the NF-κB pathway (Baron-Menguy et al. 2007). Another example is epicatechin, which not only exerts antioxidant activity but also can modulate protein kinase signaling pathways, depending on the concentration of the compound administered. In the study by Schroeter et al. (Schroeter et al. 2007), epicatechin stimulated ERK- and PI3K-dependent CREB phosphorylation at low 100 – 300 nmol/L but this effect was no longer apparent at the higher concentration of 30 mumol/L. These dose effects may be important to explain the anti- versus pro-oxidant actions of the phenolics and differences in experimental outcomes from different laboratories. It is also important to recognize that results from studies of phenolic compounds in cell culture system may not correspond to their action in vivo (Halliwell 2008).
4-a. Resveratrol
Epidemiological studies have reported that despite consuming a fatty diet, the population with moderate wine consumption has a lower incidence of cardiovascular diseases. A widely held theory for the cardioprotective effects of the “French paradox” is the anti-platelet aggregation properties of compounds in red wine in preventing the development of atherosclerotic plaques. In recent years, studies further indicated that red wine and grape polyphenols may also offer protective effects against neurodegenerative diseases (Esposito et al. 2002, Simonyi et al. 2002, Sun et al. 1999a, Sun et al. 1999b). Studies from our laboratory provided evidence that dietary supplement of polyphenols extracted from grape skin and seeds could ameliorate oxidative damage in synaptic membranes in the brain induced by chronic alcohol consumption (Sun et al. 1999a, Sun et al. 1999b). Grape polyphenols also prevented chronic ethanol-induced increase in COX-2 mRNA expression in the rat brain (Simonyi et al. 2002).
Although grape also contains other types of polyphenols, trans-resveratrol (3,4′,5-trihydroxystilbene) is considered the most effective compound in producing beneficial health effects. In addition to grapes, resveratrol is found in a variety of plant species including peanuts and berries (Baur & Sinclair 2006). Resveratrol is also highly concentrated in some oriental herbal plants, such as kojo-kan, polygonum caspidatum, which is used to treat fevers, hyperlipidemia, atherosclerosis, and inflammation (Chung et al. 1992). In our studies with PC-12 cells, resveratrol was more effective in protecting against oxidative damage than vitamins E and C combined (Chanvitayapongs et al. 1997). A number of studies using cell models have provided information for the underlying mechanisms for neuroprotective effects of resveratrol (Gao et al. 2006a, Gao et al. 2006b, Lu et al. 2006, Raval et al. 2006, Cho et al. 2008, Tsai et al. 2007). Studies with cell culture models of Parkinson's disease also demonstrated neuroprotective effects of resveratrol in alleviating oxidative damage induced by neurotoxins (Gelinas & Martinoli 2002, Alvira et al. 2007).
Studies from our laboratory demonstrated the ability for resveratrol to protect against ischemia-induced DND in the gerbil global ischemia model (Wang et al. 2002) and neuronal excitotoxicity in rats induced by kainic acid (Wang et al. 2005c). The neuroprotective effects of resveratrol can be demonstrated by different mode of administration, e.g., by i.p. injection and by supplementing as grape powder formulation (Wang et al. 2005a).
Studies to examine bioavailability of resveratrol indicated that this compound is rapidly conjugated to its glucuronide derivative which is probably the vehicle for transportation to the circulatory system. Apparently, this form of resveratrol can readily cross the blood brain barrier albeit at lower levels when compared to that in the liver (Wang et al. 2002).
Besides excellent free radical scavenger properties, resveratrol can offer other effects to the cell, e.g., increasing the lifespan in yeast (Howitz et al. 2003). This effect is explained by its ability to activate sirtuins, which belong to a conserved family of NAD+-dependent deacetylases (class III histone deacetylases) (Baur & Sinclair 2006). In the lower organisms including yeast, C elegans, and flies, increase in sirtuins is associated with extended lifespan. The multiple roles of resveratrol as an antioxidant and as a life-promoting agent make it an attractive candidate for treatment of neurodegenerative diseases (Anekonda 2006, Mancuso et al. 2007, Baur & Sinclair 2006).
Several studies demonstrated the ability for resveratrol to protect neurons against Abeta-induced toxicity in vitro (Chen et al. 2005, Han et al. 2004, Jang & Surh 2003). In fact, resveratrol combined with other polyphenolic compounds, such as catechin from green tea, can produce synergism in the protective effects (Conte et al. 2003a, Conte et al. 2003b). In a rat model of sporadic AD, chronic administration of resveratrol ameliorated the cognitive impairment and oxidative damage induced by intracerebroventricular injection of streptozotocin (Sharma & Gupta 2002). Red wine consumption also significantly attenuated AD-type deterioration of spatial memory function and Abeta neuropathology in Tg2576 mice (Wang et al. 2006a). There is evidence that resveratrol can inhibit formation and extension of Abeta fibrils and destabilize the fibrilized Abeta (Ono et al. 2006, Ono & Yamada 2006). Another study demonstrated its ability to reduce Abeta secretion in several cell lines via a mechanism that involves the proteasome (Marambaud et al. 2005).
A number of studies have demonstrated the ability of resveratrol to suppress neuroinflammatory responses, e.g., attenuating iNOS and COX-2 expression (Bi et al. 2005, Kim et al. 2007, Kim et al. 2006). However, it is not clear whether this action is related to the ability of resveratrol to minimize ROS production from NADPH oxidase. Consequently, despite strong evidence for therapeutic potential of resveratrol for treatment of cancer, angiogenesis, myocardial infarction as well as different neurodegenerative diseases (Baur & Sinclair 2006), more investigations are needed to understand proper usage of this polyphenol and its mechanism of action on different cell types.
4-b. Curcumin
Curcumin (diferuloylmethane) is derived from turmeric, the powdered rhizome of the medicinal plant Curcuma longa Linn. It has been used for centuries throughout Asia as a food additive and a traditional herbal medicine. Recent studies demonstrated that besides potent antioxidative and anti-inflammatory properties of curcumin, it also exhibits anti-amyloidogenic effects (Ono et al. 2004). Curcumin can bind amyloid directly and inhibit Abeta aggregation as well as prevent fibril and oligomer formation (Yang et al. 2005). These anti-fibril effects of curcumin were also evidenced in studies with alpha synuclein, the protein involved in PD (Ono & Yamada 2006).
Curcumin supplementation has been recently considered as an alternative, nutritional approach to reduce oxidative, inflammatory damage and amyloid pathology associated with AD (Wu et al. 2006). However, because curcumin is common in many curry spices and is widely consumed by different populations, it is difficult for well designed studies to evaluate health effects of this polyphenol. When conventional NSAID, ibuprofen, and curcumin were compared for their ability to protect against Abeta-induced damage, dietary curcumin, not ibuprofen, was shown to suppress oxidative damage and reduced synaptophysin loss (Frautschy et al. 2001). Dietary curcumin also prevented Abeta-induced spatial memory deficits in the Morris water maze and post-synaptic density loss and reduced Abeta deposits (Frautschy et al. 2001). To evaluate whether curcumin could affect Alzheimer-like pathology in Tg2576 mice, both low and high doses of curcumin significantly lowered oxidized proteins and interleukin-1β, a proinflammatory cytokine elevated in the brains of these mice (Lim et al. 2001). Beside its anti-amyloid properties, curcumin can also offer antioxidant, anti-inflammatory and cholesterol lowering properties, all are important on ameliorating the deleterious consequences of AD (Ringman et al. 2005). Several clinical trials are in progress to address safety, tolerability, and bioavailability of this compound (Ringman et al. 2005, Fiala et al. 2007).
Besides AD, there is in vitro and in vivo data suggesting that curcumin exerts a protective effect against neurodegeneration in cerebral ischemia and Parkinson's disease. In a study in which curcumin was administered through i.v. injection (1 and 2 mg/kg) after focal ischemia, the neuroprotective effects were attributed to a protection of blood-brain barrier integrity (Jiang et al. 2007). In our laboratory, curcumin administered either through i.p. injection (30 mg/kg) or through a dietary supplementation (2.0 g/kg diet) for 2 months indicated significantly attenuated ischemia-induced DND as well as glial cell activation in the gerbil model (Wang et al. 2005b). Most interestingly, curcumin administration not only reduced ischemia-induced lipid peroxidation and mitochondrial dysfunction, it also ameliorated the increase in locomotor activity observed at 24 hour after ischemic insult, thus correlating behavioral deficits with the extent of neuronal damage (Wang et al. 2005b). Consistent with other studies, bioavailability study indicated a rapid increase in curcumin in plasma and other body organs including the brain within 1 hour after i.p. injection (Ringman et al. 2005, Goel et al. 2008).
The neuroprotective effects of curcumin relavant to PD are likely to be associated with its antioxidant and anti-inflammatory properties (Chen & Le 2006, Jagatha et al. 2008, Zbarsky et al. 2005). As found for Abeta, curcumin also can inhibit aggregation of alpha-synuclein (Pandey et al. 2008). Recent studies have identified other molecular targets of curcumin, including its action on transcription factors, growth factors, antioxidant enzymes, cell-survival kinases and signaling molecules (Ramassamy 2006, Salvioli et al. 2007, Goel et al. 2008). On the other hand, it is worth noting that excessive application of curcumin may produce pro-oxidative effects (Ahsan et al. 1999). Therefore, more studies are needed to understand the different modes of action of curcumin on specific enzymes and pathways prior to recommendation for its use as a therapeutic agent.
4-c. Apocynin
Apocynin (4-hydroxy-3-methoxy-acetophenone) was discovered during activity-guided isolation of immunomodulatory constituents from Picrorhiza kurroa, a creeping plant native to the mountains of India, Nepal, Tibet and Pakistan (Picrorhiza kurroa, Monograph, 2001). Picrorhiza kurroa has been used as an herbal medicine for centuries for treatment of a number of inflammatory diseases. Apocynin may also be obtained from other sources, e.g. from the rhizome of Canadian hemp (Apocymum cannabinum), other Apocynum species (e.g. A. androsaemifolium) or from the rhizomes of Iris species. This compound has been regarded as a powerful anti-oxidant and anti-inflammatory agent, specifically, for blocking the activity of NADPH oxidase through interfering with the assembly of the cytosolic NADPH oxidase components with its membrane components (Stolk et al. 1994).
NADPH oxidase is increasingly recognized for its dual-edge roles in health and disease and has been implicated in the pathogenesis of many diseases, including cardiovascular and neurodegenerative diseases (Bedard & Krause 2007). In recent years, it has become apparent that brain cells constitutively express a superoxide-generating enzyme analogous to the NADPH oxidase in phagocytes (Infanger et al. 2006). The prototypic NADPH oxidase comprises a membrane-associated cytochrome b558 with one p22 phox and one gp91 phox subunit and several regulatory cytosolic subunits (p47 phox, p40 phox, p67 phox and the small G protein Rac1 or Rac2). Upon phosphorylation, the cytosolic subunits are translocated to bind with the membrane subunits. Consequently, a number of receptor-signaling pathways are linked to activation of NADPH oxidase leading to rapid production of superoxide anions (Bedard & Krause 2007).
Altered NADPH oxidase function has been linked to neurological disorders such as stroke, Alzheimer's and Parkinson's diseases (Lambeth 2007). Several reports of human studies (on AD, PD and stroke) demonstrated upregulation of different subunits expression in microglial cells (Wu et al. 2003). Genetic deletion of gp91phox mitigates neuronal loss in a variety of animal models of neurodegeneration, including the MPTP model of PD and cerebral ischemia (Zhang et al. 2004). Apocynin has been effective in ameliorating neuropathological damages in both in vivo and in vitro models of PD (Anantharam et al. 2007, Gao et al. 2003a, Gao et al. 2003c, Gao et al. 2003b). Apocynin also retarded disease progression and extended survival in a mouse ALS model (Boillee & Cleveland 2008). Immunohistochemical studies demonstrated that the increase in NADPH oxidase subunits expression after transient focal cerebral ischemia is mainly derived from activated microglial cells. Apocynin was effective in preventing ischemic damage and blood-brain barrier disruption in different animal models of experimental stroke (Wang et al. 2006b, Tang et al. 2007, Kahles et al. 2007). In our study using the gerbil global cerebral ischemia model, apocynin inhibited ischemia/reperfusion-induced increase in lipid peroxidation, oxidative DNA damage, and glial cell activation in the hippocampus (Wang et al. 2006b).
NADPH oxidase-dependent production of superoxide radicals has been identified as a major contributor to oxidative and inflammatory responses in the brain under different injury conditions. Activation of NADPH oxidase in glial cells is linked to increased secretion of cytokines and other inflammatory factors (Dringen 2005). Superoxide produced from NADPH oxidase may interact with nitric oxide from iNOS to form the toxic peroxynitrite, which is considered an important factor associated with neuronal death (Brown 2007). Aside from suppressing NF-κB pathway and preventing COX-2 expression in activated monocytes (Barbieri et al. 2004), apocynin is also effective against Abeta-induced microglial proliferation and lipopolysaccharide (LPS) and interferon γ-induced neuronal death (Li et al. 2004, Jekabsone et al. 2006, Shibata et al. 2006).
An apparent limitation for therapeutic use of apocynin is the high concentrations needed for exerting beneficial effects. Furthermore, most studies have used acute treatment and few studies employed a preventative, dietary approach. In vitro studies suggest that apocynin may be converted to diapocynin through chemical catalysis using ferrous sulfate and sodium persulfate or through peroxidases such as myeloperoxidase. However, our recent study failed to detect diapocynin in rat plasma and tissues after systemic injection of apocynin (Wang et al. 2007a). However, our study on bioavailability showed that similar to other polyphenols, apocynin is rapidly converted to its glucuronide derivative and transported to the circulation system and other body organs, including the brain. More studies are necessary for considering the potential therapeutic use of apocynin for treatment of neurodegenerative disorders.
4-d. Other natural phenolics
Many other phenolic compounds in fruits and vegetables are good candidates for consideration as therapeutics in combating aging and neurodegenerative diseases (Vauzour et al. 2007). Among these, there is special interest regarding the neuroprotective actions of (−)-epigallocatechin-3- gallate (EGCG) from green tea (Sutherland et al. 2006). Besides its free radical scavenging, iron chelating, and anti-inflammatory properties, EGCG can exert its action on different sites of the apoptotic pathways, including altering the expression of anti- and pro-apoptotic genes. These studies further implicate that green tea extract may also exert protection through controlling calcium homeostasis, activation of MAPK, PKC, antioxidant enzymes, survival genes and modulating enzymes for processing of the amyloid precursor protein (Mandel et al. 2004, Mandel & Youdim 2004, Weinreb et al. 2008). EGCG was shown to inhibit 6-OHDA-induced NF-kB-mediated expression of cell death and cell cycle genes (Levites et al. 2002a, Levites et al. 2002b).
In light of the neuroprotective effects from different polyphenols and plant extracts, a summary of recent studies describing neuroprotective effects of different botanical compounds in different animal models for AD, PD, and stroke is provided in Table 1. Amentoflavonoid, a naturally occurring bioflavonoid, was able to rescue neurons from hypoxic-ischemic injury (Shin et al. 2006, Yi et al. 2006). This compound seems to implement multiple mechanisms including direct blockade of cell death cascades and anti-inflammatory inhibition of microglial. Coenzyme Q (CoQ) is enriched in a number of diets and is a potent antioxidant. This redox active compound has been implicated to play an important role in improving mitochondrial function. However, whether CoQ(10) can be used as a therapeutic agent for treatment of PD remains to be investigated (Storch et al. 2007).
5. Botanical phenolics on intracellular signaling pathways
It is becoming recognized that besides their anti-oxidative and anti-inflammatory properties, many phenolics may also have specific action on intracellular signaling pathways (Fig 2). These signaling pathways are interrelated and are evolved form ROS from NADPH oxidase and mitochondria. In particular, these signaling pathways are downstream of ROS produced from NADPH oxidase upon injury due to cerebral ischemia, Abeta and excitotoxicity. In our studies, we further link these kinases to activation of cPLA2 and release of arachidonic acid. There is also evidence that ROS produced from NADPH oxidase is linked to transcriptional pathways, such as the NF-κB pathway and the Nrf/ARE pathway for induction of antioxidant and inflammatory genes (Santangelo et al. 2007) and subsequently, triggering the apoptotic pathway (Zhu et al. 2006). Successful identification of these compounds and their action on intracellular signaling pathways will be important for effective use to combat neurodegenerative diseases.
Fig 2.
Signaling pathways associated with oxidative stress
6. Concluding Remarks
Despite complex and diverse genetic and epi-genetic factors underlying manifestations of different neurodegenerative diseases, there are strong reasons to believe that oxidative stress is a common factor playing a central role in the pathogenesis of these diseases. While many pathological conditions are associated to ROS production from mitochondria, more recent studies have unveiled an important role of ROS from NADPH oxidase. Studies here indicate that phenolic compounds such as resveratrol from grape and wine, curcumin from turmeric, epigallocatechin from green tea, and apocynin from Picrorhiza kurroa, not only exhibit potent antioxidative properties for scavenging free radicals, but may also act on specific signaling pathways for regulating inflammatory responses. These studies support the use of plant-derived phenolic supplements in promoting general health and prevent against age-related diseases in humans.
Acknowledgement
Supported by grants (P02 AG018357 and 1R21AT003859) from NIH.
References
- Abekawa T, Ohmori T, Koyama T. Effect of no synthesis inhibition on striatal dopamine release and stereotyped behavior induced by a single administration of methamphetamine. Prog Neuropsychopharmacol Biol Psychiatry. 1997;21:831–838. doi: 10.1016/s0278-5846(97)00083-3. [DOI] [PubMed] [Google Scholar]
- Abramov AY, Canevari L, Duchen MR. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J Neurosci. 2004;24:565–575. doi: 10.1523/JNEUROSCI.4042-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JD, Jr., Odunze IN. Biochemical mechanisms of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity. Could oxidative stress be involved in the brain? Biochemical pharmacology. 1991;41:1099–1105. doi: 10.1016/0006-2952(91)90646-m. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Yousuf S, Ishrat T, Khan MB, Bhatia K, Fazli IS, Khan JS, Ansari NH, Islam F. Effect of dietary sesame oil as antioxidant on brain hippocampus of rat in focal cerebral ischemia. Life sciences. 2006;79:1921–1928. doi: 10.1016/j.lfs.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Ahsan H, Parveen N, Khan NU, Hadi SM. Pro-oxidant, anti-oxidant and cleavage activities on DNA of curcumin and its derivatives demethoxycurcumin and bisdemethoxycurcumin. Chem Biol Interact. 1999;121:161–175. doi: 10.1016/s0009-2797(99)00096-4. [DOI] [PubMed] [Google Scholar]
- Akama KT, Van Eldik LJ. Beta-amyloid stimulation of inducible nitric-oxide synthase in astrocytes is interleukin-1beta- and tumor necrosis factor-alpha (TNFalpha)-dependent, and involves a TNFalpha receptor-associated factor- and NFkappaB-inducing kinase-dependent signaling mechanism. J Biol Chem. 2000;275:7918–7924. doi: 10.1074/jbc.275.11.7918. [DOI] [PubMed] [Google Scholar]
- Alvira D, Yeste-Velasco M, Folch J, Verdaguer E, Canudas AM, Pallas M, Camins A. Comparative analysis of the effects of resveratrol in two apoptotic models: inhibition of complex I and potassium deprivation in cerebellar neurons. Neuroscience. 2007;147:746–756. doi: 10.1016/j.neuroscience.2007.04.029. [DOI] [PubMed] [Google Scholar]
- Anantharam V, Kaul S, Song C, Kanthasamy A, Kanthasamy AG. Pharmacological inhibition of neuronal NADPH oxidase protects against 1-methyl-4-phenylpyridinium (MPP+)-induced oxidative stress and apoptosis in mesencephalic dopaminergic neuronal cells. Neurotoxicology. 2007;28:988–997. doi: 10.1016/j.neuro.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anekonda TS. Resveratrol--a boon for treating Alzheimer's disease? Brain Res Rev. 2006;52:316–326. doi: 10.1016/j.brainresrev.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Ang-Lee MK, Moss J, Yuan CS. Herbal medicines and perioperative care. Jama. 2001;286:208–216. doi: 10.1001/jama.286.2.208. [DOI] [PubMed] [Google Scholar]
- Barbieri SS, Cavalca V, Eligini S, Brambilla M, Caiani A, Tremoli E, Colli S. Apocynin prevents cyclooxygenase 2 expression in human monocytes through NADPH oxidase and glutathione redox-dependent mechanisms. Free radical biology & medicine. 2004;37:156–165. doi: 10.1016/j.freeradbiomed.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Baron-Menguy C, Bocquet A, Guihot AL, Chappard D, Amiot MJ, Andriantsitohaina R, Loufrani L, Henrion D. Effects of red wine polyphenols on postischemic neovascularization model in rats: low doses are proangiogenic, high doses anti-angiogenic. Faseb J. 2007;21:3511–3521. doi: 10.1096/fj.06-7782com. [DOI] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiological reviews. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Bi XL, Yang JY, Dong YX, Wang JM, Cui YH, Ikeshima T, Zhao YQ, Wu CF. Resveratrol inhibits nitric oxide and TNF-alpha production by lipopolysaccharide-activated microglia. Int Immunopharmacol. 2005;5:185–193. doi: 10.1016/j.intimp.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Block ML, Li G, Qin L, Wu X, Pei Z, Wang T, Wilson B, Yang J, Hong JS. Potent regulation of microglia-derived oxidative stress and dopaminergic neuron survival: substance P vs. dynorphin. Faseb J. 2006;20:251–258. doi: 10.1096/fj.05-4553com. [DOI] [PubMed] [Google Scholar]
- Boillee S, Cleveland DW. Revisiting oxidative damage in ALS: microglia, Nox, and mutant SOD1. J Clin Invest. 2008;118:474–478. doi: 10.1172/JCI34613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Brooks AI, Chadwick CA, Gelbard HA, Cory-Slechta DA, Federoff HJ. Paraquat elicited neurobehavioral syndrome caused by dopaminergic neuron loss. Brain Research. 1999;823:1–10. doi: 10.1016/s0006-8993(98)01192-5. [DOI] [PubMed] [Google Scholar]
- Brown GC. Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochem Soc Trans. 2007;35:1119–1121. doi: 10.1042/BST0351119. [DOI] [PubMed] [Google Scholar]
- Burgener SC, Buettner L, Coen Buckwalter K, et al. Evidence supporting nutritional interventions for persons in early stage Alzheimer's disease (AD) J Nutr Health Aging. 2008;12:18–21. doi: 10.1007/BF02982159. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Griffin S, Munch G, Pasinetti GM. Amyloid beta-peptide and amyloid pathology are central to the oxidative stress and inflammatory cascades under which Alzheimer's disease brain exists. J Alzheimers Dis. 2002;4:193–201. doi: 10.3233/jad-2002-4309. [DOI] [PubMed] [Google Scholar]
- Cardoso SM, Moreira PI, Agostinho P, Pereira C, Oliveira CR. Neurodegenerative pathways in Parkinson's disease: therapeutic strategies. Curr Drug Targets CNS Neurol Disord. 2005;4:405–419. doi: 10.2174/1568007054546072. [DOI] [PubMed] [Google Scholar]
- Casarejos MJ, Menendez J, Solano RM, Rodriguez-Navarro JA, Garcia de Yebenes J, Mena MA. Susceptibility to rotenone is increased in neurons from parkin null mice and is reduced by minocycline. Journal of neurochemistry. 2006;97:934–946. doi: 10.1111/j.1471-4159.2006.03777.x. [DOI] [PubMed] [Google Scholar]
- Castano A, Herrera AJ, Cano J, Machado A. Lipopolysaccharide intranigral injection induces inflammatory reaction and damage in nigrostriatal dopaminergic system. Journal of neurochemistry. 1998;70:1584–1592. doi: 10.1046/j.1471-4159.1998.70041584.x. [DOI] [PubMed] [Google Scholar]
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]
- Chan PH. Mitochondria and neuronal death/survival signaling pathways in cerebral ischemia. Neurochemical research. 2004;29:1943–1949. doi: 10.1007/s11064-004-6869-x. [DOI] [PubMed] [Google Scholar]
- Chan PH, Fishman RA, Wesley MA, Longar S. Pathogenesis of vasogenic edema in focal cerebral ischemia. Role of superoxide radicals. Adv Neurol. 1990;52:177–183. [PubMed] [Google Scholar]
- Chanvitayapongs S, Draczynska-Lusiak B, Sun AY. Amelioration of oxidative stress by antioxidants and resveratrol in PC12 cells. Neuroreport. 1997;8:1499–1502. doi: 10.1097/00001756-199704140-00035. [DOI] [PubMed] [Google Scholar]
- Chaturvedi RK, Shukla S, Seth K, Chauhan S, Sinha C, Shukla Y, Agrawal AK. Neuroprotective and neurorescue effect of black tea extract in 6-hydroxydopamine-lesioned rat model of Parkinson's disease. Neurobiology of disease. 2006;22:421–434. doi: 10.1016/j.nbd.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Chauhan NB. Anti-amyloidogenic effect of Allium sativum in Alzheimer's transgenic model Tg2576. Journal of herbal pharmacotherapy. 2003;3:95–107. [PubMed] [Google Scholar]
- Chauhan NB. Effect of aged garlic extract on APP processing and tau phosphorylation in Alzheimer's transgenic model Tg2576. Journal of ethnopharmacology. 2006;108:385–394. doi: 10.1016/j.jep.2006.05.030. [DOI] [PubMed] [Google Scholar]
- Chauhan NB, Sandoval J. Amelioration of early cognitive deficits by aged garlic extract in Alzheimer's transgenic mice. Phytother Res. 2007;21:629–640. doi: 10.1002/ptr.2122. [DOI] [PubMed] [Google Scholar]
- Chen F, Eckman EA, Eckman CB. Reductions in levels of the Alzheimer's amyloid beta peptide after oral administration of ginsenosides. Faseb J. 2006;20:1269–1271. doi: 10.1096/fj.05-5530fje. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhang M, Qu Z, Xie B. Compositional analysis and preliminary toxicological evaluation of a tea polysaccharide conjugate. J Agric Food Chem. 2007;55:2256–2260. doi: 10.1021/jf0632740. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L. SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem. 2005;280:40364–40374. doi: 10.1074/jbc.M509329200. [DOI] [PubMed] [Google Scholar]
- Chen S, Le W. Neuroprotective therapy in Parkinson disease. Am J Ther. 2006;13:445–457. doi: 10.1097/01.mjt.0000174353.28012.a7. [DOI] [PubMed] [Google Scholar]
- Cho IJ, Ahn JY, Kim S, Choi MS, Ha TY. Resveratrol attenuates the expression of HMG-CoA reductase mRNA in hamsters. Biochem Biophys Res Commun. 2008;367:190–194. doi: 10.1016/j.bbrc.2007.12.140. [DOI] [PubMed] [Google Scholar]
- Choi DW. Excitotoxic cell death. J Neurobiol. 1992;23:1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Choi JY, Park CS, Kim DJ, Cho MH, Jin BK, Pie JE, Chung WG. Prevention of nitric oxide-mediated 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson's disease in mice by tea phenolic epigallocatechin 3-gallate. Neurotoxicology. 2002;23:367–374. doi: 10.1016/s0161-813x(02)00079-7. [DOI] [PubMed] [Google Scholar]
- Chung MI, Teng CM, Cheng KL, Ko FN, Lin CN. An antiplatelet principle of Veratrum formosanum. Planta Med. 1992;58:274–276. doi: 10.1055/s-2006-961453. [DOI] [PubMed] [Google Scholar]
- Conte A, Pellegrini S, Tagliazucchi D. Effect of resveratrol and catechin on PC12 tyrosine kinase activities and their synergistic protection from beta-amyloid toxicity. Drugs Exp Clin Res. 2003a;29:243–255. [PubMed] [Google Scholar]
- Conte A, Pellegrini S, Tagliazucchi D. Synergistic protection of PC12 cells from beta-amyloid toxicity by resveratrol and catechin. Brain research bulletin. 2003b;62:29–38. doi: 10.1016/j.brainresbull.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Curin Y, Ritz MF, Andriantsitohaina R. Cellular mechanisms of the protective effect of polyphenols on the neurovascular unit in strokes. Cardiovasc Hematol Agents Med Chem. 2006;4:277–288. doi: 10.2174/187152506778520691. [DOI] [PubMed] [Google Scholar]
- Dajas F, Rivera F, Blasina F, Arredondo F, Echeverry C, Lafon L, Morquio A, Heizen H. Cell culture protection and in vivo neuroprotective capacity of flavonoids. Neurotox Res. 2003;5:425–432. doi: 10.1007/BF03033172. [DOI] [PubMed] [Google Scholar]
- Datla KP, Zbarsky V, Rai D, Parkar S, Osakabe N, Aruoma OI, Dexter DT. Short-term supplementation with plant extracts rich in flavonoids protect nigrostriatal dopaminergic neurons in a rat model of Parkinson's disease. Journal of the American College of Nutrition. 2007;26:341–349. doi: 10.1080/07315724.2007.10719621. [DOI] [PubMed] [Google Scholar]
- De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- Deschamps V, Barberger-Gateau P, Peuchant E, Orgogozo JM. Nutritional factors in cerebral aging and dementia: epidemiological arguments for a role of oxidative stress. Neuroepidemiology. 2001;20:7–15. doi: 10.1159/000054752. [DOI] [PubMed] [Google Scholar]
- Dringen R. Oxidative and antioxidative potential of brain microglial cells. Antioxidants & redox signaling. 2005;7:1223–1233. doi: 10.1089/ars.2005.7.1223. [DOI] [PubMed] [Google Scholar]
- Esposito E, Rotilio D, Di Matteo V, Di Giulio C, Cacchio M, Algeri S. A review of specific dietary antioxidants and the effects on biochemical mechanisms related to neurodegenerative processes. Neurobiol Aging. 2002;23:719–735. doi: 10.1016/s0197-4580(02)00078-7. [DOI] [PubMed] [Google Scholar]
- Feng Y, Liu YM, Fratkins JD, LeBlanc MH. Grape seed extract suppresses lipid peroxidation and reduces hypoxic ischemic brain injury in neonatal rats. Brain research bulletin. 2005;66:120–127. doi: 10.1016/j.brainresbull.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Feng Y, Liu YM, Leblanc MH, Bhatt AJ, Rhodes PG. Grape seed extract given three hours after injury suppresses lipid peroxidation and reduces hypoxic-ischemic brain injury in neonatal rats. Pediatric research. 2007;61:295–300. doi: 10.1203/pdr.0b013e318030c92d. [DOI] [PubMed] [Google Scholar]
- Ferguson LR. Role of plant polyphenols in genomic stability. Mutat Res. 2001;475:89–111. doi: 10.1016/s0027-5107(01)00073-2. [DOI] [PubMed] [Google Scholar]
- Fiala M, Cribbs DH, Rosenthal M, Bernard G. Phagocytosis of amyloid-beta and inflammation: two faces of innate immunity in Alzheimer's disease. J Alzheimers Dis. 2007;11:457–463. doi: 10.3233/jad-2007-11406. [DOI] [PubMed] [Google Scholar]
- Frautschy SA, Hu W, Kim P, Miller SA, Chu T, Harris-White ME, Cole GM. Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol Aging. 2001;22:993–1005. doi: 10.1016/s0197-4580(01)00300-1. [DOI] [PubMed] [Google Scholar]
- Gao D, Zhang X, Jiang X, Peng Y, Huang W, Cheng G, Song L. Resveratrol reduces the elevated level of MMP-9 induced by cerebral ischemia-reperfusion in mice. Life sciences. 2006a;78:2564–2570. doi: 10.1016/j.lfs.2005.10.030. [DOI] [PubMed] [Google Scholar]
- Gao HM, Hong JS, Zhang W, Liu B. Distinct role for microglia in rotenone-induced degeneration of dopaminergic neurons. J Neurosci. 2002;22:782–790. doi: 10.1523/JNEUROSCI.22-03-00782.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Hong JS, Zhang W, Liu B. Synergistic dopaminergic neurotoxicity of the pesticide rotenone and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson's disease. J Neurosci. 2003a;23:1228–1236. doi: 10.1523/JNEUROSCI.23-04-01228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Liu B, Zhang W, Hong JS. Critical role of microglial NADPH oxidase-derived free radicals in the in vitro MPTP model of Parkinson's disease. Faseb J. 2003b;17:1954–1956. doi: 10.1096/fj.03-0109fje. [DOI] [PubMed] [Google Scholar]
- Gao HM, Liu B, Zhang W, Hong JS. Synergistic dopaminergic neurotoxicity of MPTP and inflammogen lipopolysaccharide: relevance to the etiology of Parkinson's disease. Faseb J. 2003c;17:1957–1959. doi: 10.1096/fj.03-0203fje. [DOI] [PubMed] [Google Scholar]
- Gao ZB, Chen XQ, Hu GY. Inhibition of excitatory synaptic transmission by trans-resveratrol in rat hippocampus. Brain research. 2006b;1111:41–47. doi: 10.1016/j.brainres.2006.06.096. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Dodwell SA, Meyer-Luehmann M, Hyman BT, Bacskai BJ. Plaque-derived oxidative stress mediates distorted neurite trajectories in the Alzheimer mouse model. Journal of neuropathology and experimental neurology. 2006;65:1082–1089. doi: 10.1097/01.jnen.0000240468.12543.af. [DOI] [PubMed] [Google Scholar]
- Gelinas S, Martinoli MG. Neuroprotective effect of estradiol and phytoestrogens on MPP+-induced cytotoxicity in neuronal PC12 cells. J Neurosci Res. 2002;70:90–96. doi: 10.1002/jnr.10315. [DOI] [PubMed] [Google Scholar]
- Goel A, Kunnumakkara AB, Aggarwal BB. Curcumin as “Curecumin”: From kitchen to clinic. Biochemical pharmacology. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Hernandez T, Perez de la Cruz MA, Mantolan-Sarmiento B. Histochemical and immunohistochemical detection of neurons that produce nitric oxide: effect of different fixative parameters and immunoreactivity against non-neuronal NOS antisera. J Histochem Cytochem. 1996;44:1399–1413. doi: 10.1177/44.12.8985132. [DOI] [PubMed] [Google Scholar]
- Guo S, Yan J, Yang T, Yang X, Bezard E, Zhao B. Protective effects of green tea polyphenols in the 6-OHDA rat model of Parkinson's disease through inhibition of ROS-NO pathway. Biological psychiatry. 2007;62:1353–1362. doi: 10.1016/j.biopsych.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Oxidative stress and neurodegeneration: where are we now? Journal of neurochemistry. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Archives of biochemistry and biophysics. 2008;476:107–112. doi: 10.1016/j.abb.2008.01.028. [DOI] [PubMed] [Google Scholar]
- Han YS, Zheng WH, Bastianetto S, Chabot JG, Quirion R. Neuroprotective effects of resveratrol against beta-amyloid-induced neurotoxicity in rat hippocampal neurons: involvement of protein kinase C. British journal of pharmacology. 2004;141:997–1005. doi: 10.1038/sj.bjp.0705688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman RE, Shah A, Fagan AM, Schwetye KE, Parsadanian M, Schulman RN, Finn MB, Holtzman DM. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer's disease. Neurobiology of disease. 2006;24:506–515. doi: 10.1016/j.nbd.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Hong JT, Ryu SR, Kim HJ, et al. Neuroprotective effect of green tea extract in experimental ischemia-reperfusion brain injury. Brain research bulletin. 2000;53:743–749. doi: 10.1016/s0361-9230(00)00348-8. [DOI] [PubMed] [Google Scholar]
- Hong JT, Ryu SR, Kim HJ, Lee JK, Lee SH, Yun YP, Lee BM, Kim PY. Protective effect of green tea extract on ischemia/reperfusion-induced brain injury in Mongolian gerbils. Brain research. 2001;888:11–18. doi: 10.1016/s0006-8993(00)02935-8. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: distribution, regulation, and function. Antioxidants & redox signaling. 2006;8:1583–1596. doi: 10.1089/ars.2006.8.1583. [DOI] [PubMed] [Google Scholar]
- Jagatha B, Mythri RB, Vali S, Bharath MM. Curcumin treatment alleviates the effects of glutathione depletion in vitro and in vivo: therapeutic implications for Parkinson's disease explained via in silico studies. Free radical biology & medicine. 2008;44:907–917. doi: 10.1016/j.freeradbiomed.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Jang JH, Surh YJ. Protective effect of resveratrol on beta-amyloid-induced oxidative PC12 cell death. Free radical biology & medicine. 2003;34:1100–1110. doi: 10.1016/s0891-5849(03)00062-5. [DOI] [PubMed] [Google Scholar]
- Jekabsone A, Mander PK, Tickler A, Sharpe M, Brown GC. Fibrillar beta-amyloid peptide Abeta1-40 activates microglial proliferation via stimulating TNF-alpha release and H2O2 derived from NADPH oxidase: a cell culture study. J Neuroinflammation. 2006;3:24. doi: 10.1186/1742-2094-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Wang W, Sun YJ, Hu M, Li F, Zhu DY. Neuroprotective effect of curcumin on focal cerebral ischemic rats by preventing blood-brain barrier damage. Eur J Pharmacol. 2007;561:54–62. doi: 10.1016/j.ejphar.2006.12.028. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Denisova NA, Arendash G, Gordon M, Diamond D, Shukitt-Hale B, Morgan D. Blueberry supplementation enhances signaling and prevents behavioral deficits in an Alzheimer disease model. Nutritional neuroscience. 2003;6:153–162. doi: 10.1080/1028415031000111282. [DOI] [PubMed] [Google Scholar]
- Kahles T, Luedike P, Endres M, Galla HJ, Steinmetz H, Busse R, Neumann-Haefelin T, Brandes RP. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke. 2007;38:3000–3006. doi: 10.1161/STROKEAHA.107.489765. [DOI] [PubMed] [Google Scholar]
- Kang TH, Hur JY, Kim HB, Ryu JH, Kim SY. Neuroprotective effects of the cyanidin-3-O-beta-d-glucopyranoside isolated from mulberry fruit against cerebral ischemia. Neuroscience letters. 2006;391:122–126. doi: 10.1016/j.neulet.2005.08.053. [DOI] [PubMed] [Google Scholar]
- Kim MS, Lee JI, Lee WY, Kim SE. Neuroprotective effect of Ginkgo biloba L. extract in a rat model of Parkinson's disease. Phytother Res. 2004;18:663–666. doi: 10.1002/ptr.1486. [DOI] [PubMed] [Google Scholar]
- Kim YA, Kim GY, Park KY, Choi YH. Resveratrol inhibits nitric oxide and prostaglandin E2 production by lipopolysaccharide-activated C6 microglia. Journal of medicinal food. 2007;10:218–224. doi: 10.1089/jmf.2006.143. [DOI] [PubMed] [Google Scholar]
- Kim YA, Lim SY, Rhee SH, Park KY, Kim CH, Choi BT, Lee SJ, Park YM, Choi YH. Resveratrol inhibits inducible nitric oxide synthase and cyclooxygenase-2 expression in beta-amyloid-treated C6 glioma cells. Int J Mol Med. 2006;17:1069–1075. [PubMed] [Google Scholar]
- Kishida KT, Klann E. Sources and targets of reactive oxygen species in synaptic plasticity and memory. Antioxidants & redox signaling. 2007;9:233–244. doi: 10.1089/ars.2007.9.ft-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida KT, Pao M, Holland SM, Klann E. NADPH oxidase is required for NMDA receptor-dependent activation of ERK in hippocampal area CA1. Journal of neurochemistry. 2005;94:299–306. doi: 10.1111/j.1471-4159.2005.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshimura I, Imai H, Hidano T, Endo K, Mochizuki H, Kondo T, Mizuno Y. Dimethoxyphenylethylamine and tetrahydropapaverine are toxic to the nigrostriatal system. Brain research. 1997;773:108–116. doi: 10.1016/s0006-8993(97)00922-0. [DOI] [PubMed] [Google Scholar]
- Kotilinek LA, Westerman MA, Wang Q, et al. Cyclooxygenase-2 inhibition improves amyloid-beta-mediated suppression of memory and synaptic plasticity. Brain. 2008;131:651–664. doi: 10.1093/brain/awn008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriem B, Sponne I, Fifre A, et al. Cytosolic phospholipase A2 mediates neuronal apoptosis induced by soluble oligomers of the amyloid-beta peptide. Faseb J. 2005;19:85–87. doi: 10.1096/fj.04-1807fje. [DOI] [PubMed] [Google Scholar]
- Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free radical biology & medicine. 2007;43:332–347. doi: 10.1016/j.freeradbiomed.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston JW, Irwin I, Ricaurte GA. Neurotoxins, parkinsonism and Parkinson's disease. Pharmacol Ther. 1987;32:19–49. doi: 10.1016/0163-7258(87)90062-3. [DOI] [PubMed] [Google Scholar]
- Levites Y, Amit T, Youdim MB, Mandel S. Involvement of protein kinase C activation and cell survival/ cell cycle genes in green tea polyphenol (−)-epigallocatechin 3-gallate neuroprotective action. J Biol Chem. 2002a;277:30574–30580. doi: 10.1074/jbc.M202832200. [DOI] [PubMed] [Google Scholar]
- Levites Y, Youdim MB, Maor G, Mandel S. Attenuation of 6-hydroxydopamine (6-OHDA)-induced nuclear factor-kappaB (NF-kappaB) activation and cell death by tea extracts in neuronal cultures. Biochemical pharmacology. 2002b;63:21–29. doi: 10.1016/s0006-2952(01)00813-9. [DOI] [PubMed] [Google Scholar]
- Li M, Pisalyaput K, Galvan M, Tenner AJ. Macrophage colony stimulatory factor and interferon-gamma trigger distinct mechanisms for augmentation of beta-amyloid-induced microglia-mediated neurotoxicity. Journal of neurochemistry. 2004;91:623–633. doi: 10.1111/j.1471-4159.2004.02765.x. [DOI] [PubMed] [Google Scholar]
- Li X, Sun AY. Paraquat induced activation of transcription factor AP-1 and apoptosis in PC12 cells. J Neural Transm. 1999;106:1–21. doi: 10.1007/s007020050137. [DOI] [PubMed] [Google Scholar]
- Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HJ, Lee KS, Lee S, Park JH, Choi HE, Go SH, Kwak HJ, Park HY. 15d-PGJ2 stimulates HO-1 expression through p38 MAP kinase and Nrf-2 pathway in rat vascular smooth muscle cells. Toxicology and applied pharmacology. 2007a;223:20–27. doi: 10.1016/j.taap.2007.04.019. [DOI] [PubMed] [Google Scholar]
- Lim ML, Mercer LD, Nagley P, Beart PM. Rotenone and MPP+ preferentially redistribute apoptosis-inducing factor in apoptotic dopamine neurons. Neuroreport. 2007b;18:307–312. doi: 10.1097/WNR.0b013e32801b3ca6. [DOI] [PubMed] [Google Scholar]
- Liou HH, Tsai MC, Chen CJ, Jeng JS, Chang YC, Chen SY, Chen RC. Environmental risk factors and Parkinson's disease: a case-control study in Taiwan. Neurology. 1997;48:1583–1588. doi: 10.1212/wnl.48.6.1583. [DOI] [PubMed] [Google Scholar]
- Lu KT, Chiou RY, Chen LG, Chen MH, Tseng WT, Hsieh HT, Yang YL. Neuroprotective effects of resveratrol on cerebral ischemia-induced neuron loss mediated by free radical scavenging and cerebral blood flow elevation. J Agric Food Chem. 2006;54:3126–3131. doi: 10.1021/jf053011q. [DOI] [PubMed] [Google Scholar]
- Manach C, Donovan JL. Pharmacokinetics and metabolism of dietary flavonoids in humans. Free radical research. 2004;38:771–785. doi: 10.1080/10715760410001727858. [DOI] [PubMed] [Google Scholar]
- Mancuso C, Scapagini G, Curro D, Giuffrida Stella AM, De Marco C, Butterfield DA, Calabrese V. Mitochondrial dysfunction, free radical generation and cellular stress response in neurodegenerative disorders. Front Biosci. 2007;12:1107–1123. doi: 10.2741/2130. [DOI] [PubMed] [Google Scholar]
- Mandel S, Weinreb O, Amit T, Youdim MB. Cell signaling pathways in the neuroprotective actions of the green tea polyphenol (−)-epigallocatechin-3-gallate: implications for neurodegenerative diseases. Journal of neurochemistry. 2004;88:1555–1569. doi: 10.1046/j.1471-4159.2003.02291.x. [DOI] [PubMed] [Google Scholar]
- Mandel S, Youdim MB. Catechin polyphenols: neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radic Biol Med. 2004;37:304–317. doi: 10.1016/j.freeradbiomed.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Mander PK, Jekabsone A, Brown GC. Microglia proliferation is regulated by hydrogen peroxide from NADPH oxidase. J Immunol. 2006;176:1046–1052. doi: 10.4049/jimmunol.176.2.1046. [DOI] [PubMed] [Google Scholar]
- Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer's disease amyloid-beta peptides. J Biol Chem. 2005;280:37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- Masuda M, Suzuki N, Taniguchi S, Oikawa T, Nonaka T, Iwatsubo T, Hisanaga S, Goedert M, Hasegawa M. Small molecule inhibitors of alpha-synuclein filament assembly. Biochemistry. 2006;45:6085–6094. doi: 10.1021/bi0600749. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Calcium and neurodegeneration. Aging Cell. 2007;6:337–350. doi: 10.1111/j.1474-9726.2007.00275.x. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Dietary factors, hormesis and health. Ageing research reviews. 2008;7:43–48. doi: 10.1016/j.arr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, Itagaki S, Akiyama H, McGeer EG. Rate of cell death in parkinsonism indicates active neuropathological process. Ann Neurol. 1988;24:574–576. doi: 10.1002/ana.410240415. [DOI] [PubMed] [Google Scholar]
- McKeel DW, Jr., Price JL, Miller JP, Grant EA, Xiong C, Berg L, Morris JC. Neuropathologic criteria for diagnosing Alzheimer disease in persons with pure dementia of Alzheimer type. Journal of neuropathology and experimental neurology. 2004;63:1028–1037. doi: 10.1093/jnen/63.10.1028. [DOI] [PubMed] [Google Scholar]
- Mercer LD, Kelly BL, Horne MK, Beart PM. Dietary polyphenols protect dopamine neurons from oxidative insults and apoptosis: investigations in primary rat mesencephalic cultures. Biochemical pharmacology. 2005;69:339–345. doi: 10.1016/j.bcp.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Miller RL, James-Kracke M, Sun GY, Sun AY. Oxidative and Inflammatory Pathways in Parkinson's Disease. Neurochemical research. 2008 doi: 10.1007/s11064-008-9656-2. [DOI] [PubMed] [Google Scholar]
- Miller RL, Sun GY, Sun AY. Cytotoxicity of paraquat in microglial cells: Involvement of PKCdelta- and ERK1/2-dependent NADPH oxidase. Brain research. 2007;1167:129–139. doi: 10.1016/j.brainres.2007.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli V, Naquin C. Alternative therapies for traditional disease states: menopause. Am Fam Physician. 2002;66:129–134. [PubMed] [Google Scholar]
- Ndiaye M, Chataigneau M, Lobysheva I, Chataigneau T, Schini-Kerth VB. Red wine polyphenol-induced, endothelium-dependent NO-mediated relaxation is due to the redox-sensitive PI3-kinase/Akt-dependent phosphorylation of endothelial NO-synthase in the isolated porcine coronary artery. Faseb J. 2005;19:455–457. doi: 10.1096/fj.04-2146fje. [DOI] [PubMed] [Google Scholar]
- Ono K, Hasegawa K, Naiki H, Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer's beta-amyloid fibrils in vitro. J Neurosci Res. 2004;75:742–750. doi: 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]
- Ono K, Naiki H, Yamada M. The development of preventives and therapeutics for Alzheimer's disease that inhibit the formation of beta-amyloid fibrils (fAbeta), as well as destabilize preformed fAbeta. Curr Pharm Des. 2006;12:4357–4375. doi: 10.2174/138161206778793010. [DOI] [PubMed] [Google Scholar]
- Ono K, Yamada M. Antioxidant compounds have potent anti-fibrillogenic and fibril-destabilizing effects for alpha-synuclein fibrils in vitro. Journal of neurochemistry. 2006;97:105–115. doi: 10.1111/j.1471-4159.2006.03707.x. [DOI] [PubMed] [Google Scholar]
- Pandey N, Strider J, Nolan WC, Yan SX, Galvin JE. Curcumin inhibits aggregation of alpha-synuclein. Acta neuropathologica. 2008;115:479–489. doi: 10.1007/s00401-007-0332-4. [DOI] [PubMed] [Google Scholar]
- Pu F, Mishima K, Egashira N, et al. Protective effect of buckwheat polyphenols against long-lasting impairment of spatial memory associated with hippocampal neuronal damage in rats subjected to repeated cerebral ischemia. Journal of pharmacological sciences. 2004;94:393–402. doi: 10.1254/jphs.94.393. [DOI] [PubMed] [Google Scholar]
- Rahman M, Riaz M, Desai UR. Synthesis of biologically relevant biflavanoids--a review. Chem Biodivers. 2007;4:2495–2527. doi: 10.1002/cbdv.200790205. [DOI] [PubMed] [Google Scholar]
- Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. Eur J Pharmacol. 2006;545:51–64. doi: 10.1016/j.ejphar.2006.06.025. [DOI] [PubMed] [Google Scholar]
- Raskin I, Ribnicky DM, Komarnytsky S, et al. Plants and human health in the twenty-first century. Trends Biotechnol. 2002;20:522–531. doi: 10.1016/s0167-7799(02)02080-2. [DOI] [PubMed] [Google Scholar]
- Rathore P, Dohare P, Varma S, Ray A, Sharma U, Jaganathanan NR, Ray M. Curcuma Oil: Reduces Early Accumulation of Oxidative Product and is Anti-apoptogenic in Transient Focal Ischemia in Rat Brain. Neurochem Res. 2007 doi: 10.1007/s11064-007-9515-6. [DOI] [PubMed] [Google Scholar]
- Raval AP, Dave KR, Perez-Pinzon MA. Resveratrol mimics ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2006;26:1141–1147. doi: 10.1038/sj.jcbfm.9600262. [DOI] [PubMed] [Google Scholar]
- Rezai-Zadeh K, Shytle D, Sun N, et al. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci. 2005;25:8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice-Evans C, Miller N. Measurement of the antioxidant status of dietary constituents, low density lipoproteins and plasma. Prostaglandins Leukot Essent Fatty Acids. 1997;57:499–505. doi: 10.1016/s0952-3278(97)90435-x. [DOI] [PubMed] [Google Scholar]
- Ringman JM, Frautschy SA, Cole GM, Masterman DL, Cummings JL. A potential role of the curry spice curcumin in Alzheimer's disease. Curr Alzheimer Res. 2005;2:131–136. doi: 10.2174/1567205053585882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz MF, Ratajczak P, Curin Y, Cam E, Mendelowitsch A, Pinet F, Andriantsitohaina R. Chronic treatment with red wine polyphenol compounds mediates neuroprotection in a rat model of ischemic cerebral stroke. The Journal of nutrition. 2008;138:519–525. doi: 10.1093/jn/138.3.519. [DOI] [PubMed] [Google Scholar]
- Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- Saito A, Maier CM, Narasimhan P, et al. Oxidative stress and neuronal death/survival signaling in cerebral ischemia. Mol Neurobiol. 2005;31:105–116. doi: 10.1385/MN:31:1-3:105. [DOI] [PubMed] [Google Scholar]
- Saleem S, Ahmad M, Ahmad AS, Yousuf S, Ansari MA, Khan MB, Ishrat T, Islam F. Behavioral and histologic neuroprotection of aqueous garlic extract after reversible focal cerebral ischemia. Journal of medicinal food. 2006;9:537–544. doi: 10.1089/jmf.2006.9.537. [DOI] [PubMed] [Google Scholar]
- Salvioli S, Sikora E, Cooper EL, Franceschi C. Curcumin in Cell Death Processes: A Challenge for CAM of Age-Related Pathologies. Evid Based Complement Alternat Med. 2007;4:181–190. doi: 10.1093/ecam/nem043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang N, Chen C. Lipid signaling and synaptic plasticity. Neuroscientist. 2006;12:425–434. doi: 10.1177/1073858406290794. [DOI] [PubMed] [Google Scholar]
- Santangelo C, Vari R, Scazzocchio B, Di Benedetto R, Filesi C, Masella R. Polyphenols, intracellular signalling and inflammation. Ann Ist Super Sanita. 2007;43:394–405. [PubMed] [Google Scholar]
- Schapira AH. Neurotoxicity and the mechanisms of cell death in Parkinson's disease. Adv Neurol. 1996;69:161–165. [PubMed] [Google Scholar]
- Schober A. Classic toxin-induced animal models of Parkinson's disease: 6-OHDA and MPTP. Cell Tissue Res. 2004;318:215–224. doi: 10.1007/s00441-004-0938-y. [DOI] [PubMed] [Google Scholar]
- Schroeter H, Bahia P, Spencer JP, Sheppard O, Rattray M, Cadenas E, Rice-Evans C, Williams RJ. (−)Epicatechin stimulates ERK-dependent cyclic AMP response element activity and up-regulates GluR2 in cortical neurons. Journal of neurochemistry. 2007;101:1596–1606. doi: 10.1111/j.1471-4159.2006.04434.x. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis. 2001;3:75–80. doi: 10.3233/jad-2001-3111. [DOI] [PubMed] [Google Scholar]
- Sharma M, Gupta YK. Chronic treatment with trans resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life sciences. 2002;71:2489–2498. doi: 10.1016/s0024-3205(02)02083-0. [DOI] [PubMed] [Google Scholar]
- Shelat PB, Chalimoniuk M, Wang JH, Strosznajder JB, Lee JC, Sun AY, Simonyi A, Sun GY. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A(2) in cortical neurons. Journal of neurochemistry. 2008;106:45–55. doi: 10.1111/j.1471-4159.2008.05347.x. [DOI] [PubMed] [Google Scholar]
- Shen L, Zhang J. Ginsenoside Rg1 increases ischemia-induced cell proliferation and survival in the dentate gyrus of the adult gerbils. Neurosci. Letters. 2003;344:1–4. doi: 10.1016/s0304-3940(03)00318-5. [DOI] [PubMed] [Google Scholar]
- Shibata H, Katsuki H, Okawara M, Kume T, Akaike A. c-Jun N-terminal kinase inhibition and alpha-tocopherol protect midbrain dopaminergic neurons from interferon-gamma/lipopolysaccharide-induced injury without affecting nitric oxide production. J Neurosci Res. 2006;83:102–109. doi: 10.1002/jnr.20700. [DOI] [PubMed] [Google Scholar]
- Shin DH, Bae YC, Kim-Han JS, Lee JH, Choi IY, Son KH, Kang SS, Kim WK, Han BH. Polyphenol amentoflavone affords neuroprotection against neonatal hypoxic-ischemic brain damage via multiple mechanisms. Journal of neurochemistry. 2006;96:561–572. doi: 10.1111/j.1471-4159.2005.03582.x. [DOI] [PubMed] [Google Scholar]
- Simonian NA, Coyle JT. Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 1996;36:83–106. doi: 10.1146/annurev.pa.36.040196.000503. [DOI] [PubMed] [Google Scholar]
- Simonyi A, Wang Q, Miller RL, Yusof M, Shelat PB, Sun AY, Sun GY. Polyphenols in cerebral ischemia: novel targets for neuroprotection. Mol Neurobiol. 2005;31:135–147. doi: 10.1385/MN:31:1-3:135. [DOI] [PubMed] [Google Scholar]
- Simonyi A, Woods D, Sun AY, Sun GY. Grape polyphenols inhibit chronic ethanol-induced COX-2 mRNA expression in rat brain. Alcohol Clin Exp Res. 2002;26:352–357. [PubMed] [Google Scholar]
- Snyder EM, Nong Y, Almeida CG, et al. Regulation of NMDA receptor trafficking by amyloid-beta. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Eckenstein F, Frei B, Kulhanek D, Nowlin J, Quinn JF. Prevention of age-related spatial memory deficits in a transgenic mouse model of Alzheimer's disease by chronic Ginkgo biloba treatment. Experimental neurology. 2003;184:510–520. doi: 10.1016/s0014-4886(03)00399-6. [DOI] [PubMed] [Google Scholar]
- Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. American journal of respiratory cell and molecular biology. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- Storch A, Jost WH, Vieregge P, et al. Randomized, double-blind, placebo-controlled trial on symptomatic effects of coenzyme Q(10) in Parkinson disease. Archives of neurology. 2007;64:938–944. doi: 10.1001/archneur.64.7.nct60005. [DOI] [PubMed] [Google Scholar]
- Sun AY, Chen YM. Oxidative stress and neurodegenerative disorders. J Biomed Sci. 1998;5:401–414. doi: 10.1007/BF02255928. [DOI] [PubMed] [Google Scholar]
- Sun AY, Yang WL, Kim HD. Free radical and lipid peroxidation in manganese-induced neuronal cell injury. Ann N Y Acad Sci. 1993;679:358–363. doi: 10.1111/j.1749-6632.1993.tb18322.x. [DOI] [PubMed] [Google Scholar]
- Sun GY, Xia J, Draczynska-Lusiak B, Simonyi A, Sun AY. Grape polyphenols protect neurodegenerative changes induced by chronic ethanol administration. Neuroreport. 1999a;10:93–96. doi: 10.1097/00001756-199901180-00018. [DOI] [PubMed] [Google Scholar]
- Sun GY, Xia J, Xu J, Allenbrand B, Simonyi A, Rudeen PK, Sun AY. Dietary supplementation of grape polyphenols to rats ameliorates chronic ethanol-induced changes in hepatic morphology without altering changes in hepatic lipids. The Journal of nutrition. 1999b;129:1814–1819. doi: 10.1093/jn/129.10.1814. [DOI] [PubMed] [Google Scholar]
- Sutherland BA, Rahman RM, Appleton I. Mechanisms of action of green tea catechins, with a focus on ischemia-induced neurodegeneration. J Nutr Biochem. 2006;17:291–306. doi: 10.1016/j.jnutbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Sweeney MI, Kalt W, MacKinnon SL, Ashby J, Gottschall-Pass KT. Feeding rats diets enriched in lowbush blueberries for six weeks decreases ischemia-induced brain damage. Nutritional neuroscience. 2002;5:427–431. doi: 10.1080/1028415021000055970. [DOI] [PubMed] [Google Scholar]
- Tang LL, Ye K, Yang XF, Zheng JS. Apocynin attenuates cerebral infarction after transient focal ischaemia in rats. J Int Med Res. 2007;35:517–522. doi: 10.1177/147323000703500411. [DOI] [PubMed] [Google Scholar]
- Tchantchou F, Xu Y, Wu Y, Christen Y, Luo Y. EGb 761 enhances adult hippocampal neurogenesis and phosphorylation of CREB in transgenic mouse model of Alzheimer's disease. Faseb J. 2007;21:2400–2408. doi: 10.1096/fj.06-7649com. [DOI] [PubMed] [Google Scholar]
- Tohda C, Matsumoto N, Zou K, Meselhy MR, Komatsu K. Abeta(25-35)-induced memory impairment, axonal atrophy, and synaptic loss are ameliorated by M1, A metabolite of protopanaxadiol-type saponins. Neuropsychopharmacology. 2004;29:860–868. doi: 10.1038/sj.npp.1300388. [DOI] [PubMed] [Google Scholar]
- Tsai SK, Hung LM, Fu YT, Cheng H, Nien MW, Liu HY, Zhang FB, Huang SS. Resveratrol neuroprotective effects during focal cerebral ischemia injury via nitric oxide mechanism in rats. J Vasc Surg. 2007;46:346–353. doi: 10.1016/j.jvs.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Vafeiadou K, Vauzour D, Spencer JP. Neuroinflammation and its modulation by flavonoids. Endocr Metab Immune Disord Drug Targets. 2007;7:211–224. doi: 10.2174/187153007781662521. [DOI] [PubMed] [Google Scholar]
- Vauzour D, Vafeiadou K, Corona G, Pollard SE, Tzounis X, Spencer JP. Champagne wine polyphenols protect primary cortical neurons against peroxynitrite-induced injury. J Agric Food Chem. 2007;55:2854–2860. doi: 10.1021/jf063304z. [DOI] [PubMed] [Google Scholar]
- Voko Z, Hollander M, Hofman A, Koudstaal PJ, Breteler MM. Dietary antioxidants and the risk of ischemic stroke: the Rotterdam Study. Neurology. 2003;61:1273–1275. doi: 10.1212/01.wnl.0000090458.67821.a3. [DOI] [PubMed] [Google Scholar]
- Wang CN, Chi CW, Lin YL, Chen CF, Shiao YJ. The neuroprotective effects of phytoestrogens on amyloid beta protein-induced toxicity are mediated by abrogating the activation of caspase cascade in rat cortical neurons. J Biol Chem. 2001;276:5287–5295. doi: 10.1074/jbc.M006406200. [DOI] [PubMed] [Google Scholar]
- Wang J, Ho L, Zhao Z, et al. Moderate consumption of Cabernet Sauvignon attenuates Abeta neuropathology in a mouse model of Alzheimer's disease. Faseb J. 2006a;20:2313–2320. doi: 10.1096/fj.06-6281com. [DOI] [PubMed] [Google Scholar]
- Wang Q, Simonyi A, Li W, Sisk BA, Miller RL, Macdonald RS, Lubahn DE, Sun GY, Sun AY. Dietary grape supplement ameliorates cerebral ischemia-induced neuronal death in gerbils. Mol Nutr Food Res. 2005a;49:443–451. doi: 10.1002/mnfr.200500019. [DOI] [PubMed] [Google Scholar]
- Wang Q, Smith RE, Luchtefeld R, Sun AY, Simonyi A, Luo R, Sun GY. Bioavailabity of apocynin through its conversion to glycoconjugate but not to diapocynin. Phytomedicine. 2008;15:496–503. doi: 10.1016/j.phymed.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Sun AY, Simonyi A, et al. Neuroprotective mechanisms of curcumin against cerebral ischemia-induced neuronal apoptosis and behavioral deficits. J Neurosci Res. 2005b;82:138–148. doi: 10.1002/jnr.20610. [DOI] [PubMed] [Google Scholar]
- Wang Q, Tompkins KD, Simonyi A, Korthuis RJ, Sun AY, Sun GY. Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res. 2006b;1090:182–189. doi: 10.1016/j.brainres.2006.03.060. [DOI] [PubMed] [Google Scholar]
- Wang Q, Xu J, Rottinghaus GE, Simonyi A, Lubahn D, Sun GY, Sun AY. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Res. 2002;958:439–447. doi: 10.1016/s0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- Wang Q, Yu S, Simonyi A, Sun GY, Sun AY. Kainic acid-mediated excitotoxicity as a model for neurodegeneration. Mol Neurobiol. 2005c;31:3–16. doi: 10.1385/MN:31:1-3:003. [DOI] [PubMed] [Google Scholar]
- Wang X, Su B, Perry G, Smith MA, Zhu X. Insights into amyloid-beta-induced mitochondrial dysfunction in Alzheimer disease. Free radical biology & medicine. 2007;43:1569–1573. doi: 10.1016/j.freeradbiomed.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chang CF, Chou J, Chen HL, Deng X, Harvey BK, Cadet JL, Bickford PC. Dietary supplementation with blueberries, spinach, or spirulina reduces ischemic brain damage. Experimental neurology. 2005d;193:75–84. doi: 10.1016/j.expneurol.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Weinreb O, Amit T, Youdim MB. The application of proteomics for studying the neurorescue activity of the polyphenol (−)-epigallocatechin-3-gallate. Archives of biochemistry and biophysics. 2008;476:152–160. doi: 10.1016/j.abb.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Weinreb O, Mandel S, Amit T, Youdim MB. Neurological mechanisms of green tea polyphenols in Alzheimer's and Parkinson's diseases. J Nutr Biochem. 2004;15:506–516. doi: 10.1016/j.jnutbio.2004.05.002. [DOI] [PubMed] [Google Scholar]
- West T, Atzeva M, Holtzman DM. Pomegranate polyphenols and resveratrol protect the neonatal brain against hypoxic-ischemic injury. Developmental neuroscience. 2007;29:363–372. doi: 10.1159/000105477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Ying Z, Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Experimental neurology. 2006;197:309–317. doi: 10.1016/j.expneurol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Wu DC, Teismann P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu XF, Block ML, Zhang W, Qin L, Wilson B, Zhang WQ, Veronesi B, Hong JS. The role of microglia in paraquat-induced dopaminergic neurotoxicity. Antioxidants & redox signaling. 2005;7:654–661. doi: 10.1089/ars.2005.7.654. [DOI] [PubMed] [Google Scholar]
- Yang F, Lim GP, Begum AN, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- Yang W, Sun AY. Paraquat-induced free radical reaction in mouse brain microsomes. Neurochemical research. 1998a;23:47–53. doi: 10.1023/a:1022497319548. [DOI] [PubMed] [Google Scholar]
- Yang WL, Sun AY. Paraquat-induced cell death in PC12 cells. Neurochemical research. 1998b;23:1387–1394. doi: 10.1023/a:1020750706762. [DOI] [PubMed] [Google Scholar]
- Yi H, Akao Y, Maruyama W, Chen K, Shih J, Naoi M. Type A monoamine oxidase is the target of an endogenous dopaminergic neurotoxin, N-methyl(R)salsolinol, leading to apoptosis in SH-SY5Y cells. Journal of neurochemistry. 2006;96:541–549. doi: 10.1111/j.1471-4159.2005.03573.x. [DOI] [PubMed] [Google Scholar]
- Youdim KA, Joseph JA. A possible emerging role of phytochemicals in improving age-related neurological dysfunctions: a multiplicity of effects. Free radical biology & medicine. 2001;30:583–594. doi: 10.1016/s0891-5849(00)00510-4. [DOI] [PubMed] [Google Scholar]
- Zbarsky V, Datla KP, Parkar S, Rai DK, Aruoma OI, Dexter DT. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-OHDA model of Parkinson's disease. Free radical research. 2005;39:1119–1125. doi: 10.1080/10715760500233113. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang T, Qin L, Gao HM, Wilson B, Ali SF, Zhang W, Hong JS, Liu B. Neuroprotective effect of dextromethorphan in the MPTP Parkinson's disease model: role of NADPH oxidase. Faseb J. 2004;18:589–591. doi: 10.1096/fj.03-0983fje. [DOI] [PubMed] [Google Scholar]
- Zhu D, Lai Y, Shelat PB, Hu C, Sun GY, Lee JC. Phospholipases A2 mediate amyloid-beta peptide-induced mitochondrial dysfunction. J Neurosci. 2006;26:11111–11119. doi: 10.1523/JNEUROSCI.3505-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]