Abstract
Schizophrenia is a highly debilitating mental disorder that affects −1% of the general population, yet it continues to be poorly understood. Recent studies have identified variations in several genes that are associated with this disorder in diverse populations, including those that encode neuregulin 1 (NRG1) and its receptor ErbB4. The past few years have witnessed exciting progress in our knowledge of NRG1 and ErbB4 functions and the biological basis of the increased risk for schizophrenia that is potentially conferred by polymorphisms in the two genes. An improved understanding of the mechanisms by which altered function of NRG1 and ErbB4 contributes to schizophrenia might eventually lead to the development of more effective therapeutics.
Schizophrenia is a severe and disabling mental disorder that is characterized by chronic positive symptoms (hallucinations, delusions and thought disorders), negative symptoms (social withdrawal, apathy and emotional blunting) and cognitive deficits. Although schizophrenia is a highly prevalent CNS disorder, it continues to be one of the least understood, primarily owing to its lack of pathological hallmarks. Most, if not all, commonly prescribed antipsychotics are anti-dopaminergic, and their use has been based on the ‘classical’ dopamine hypothesis, which posits that it is the hyperactivity of dopaminergic transmission that causes positive symptoms1. However, current antipsychotics are only modestly effective treatments for the cognitive dysfunction and negative symptoms that are associated with schizophrenia. Moreover, studies on the mechanism of action of antipsychotics have not been particularly informative about the pathogenesis of the disease2.
Recent genetic studies have provided insight into the possible aetiological mechanisms of this devastating disorder. Schizophrenia has a significant genetic component, and several genes have been associated with the disorder in diverse populations3. In particular, the identification of polymorphisms in the genes that encode neuregulin 1 (NRG1) and its receptor ErbB4 has provided a useful starting point from which to better dissect the pathogenic mechanisms of schizophrenia. The past few years have witnessed major progress in our understanding of NRG1 function in neurodevelopment, neurotransmission and synaptic plasticity and of the potential pathological basis of the increased risk that is conferred by polymorphisms in NRG1 and ERBB4. In this Review we briefly discuss the basic signalling machinery of NRG1, review recent findings on the roles of NRG1 and ErbB signalling during development and synaptic plasticity, and explore the implications for the pathophysiology of schizophrenia.
NRG1 and ErbB4 signalling
NRG1
NRG1 is a trophic factor that contains an epidermal growth factor (EGF)-like domain that signals by stimulating ErbB receptor tyrosine kinases. It belongs to a family of growth factors that are encoded by four individual genes (NRG1−4), of which NRG1 is the best-characterized. Presumably owing to the use of distinct 5' flanking regulatory elements and alternative splicing, NRG1 generates six types of protein (I−VI) and at least 31 isoforms (based on analysis of human expressed sequence tags4, 5, 6, 7, 8) (Fig. 1a); these isoforms include Neu differentiation factor (NDF), heregulin, glial growth factor (GGF) and acetylcholine-receptor-inducing activity (ARIA)9, 10, 11, 12, 13, 14, 15. Each of the protein types has a distinct amino-terminal region. The EGF-like domain is located in the membrane-proximal region of the extracellular domain that is necessary and sufficient for activation of the ErbB receptor tyrosine kinases. NRG1 isoforms differ in their levels and patterns of expression in various tissues, including in the brain7, 16. Mice that carry mutations that inactivate particular isoforms show distinct changes in neural development7, 17, 18, suggesting that the different NRG1 isoforms have different functions. Interestingly, some NRG1 isoforms are abnormally expressed in patients with schizophrenia.
Fig. 1.
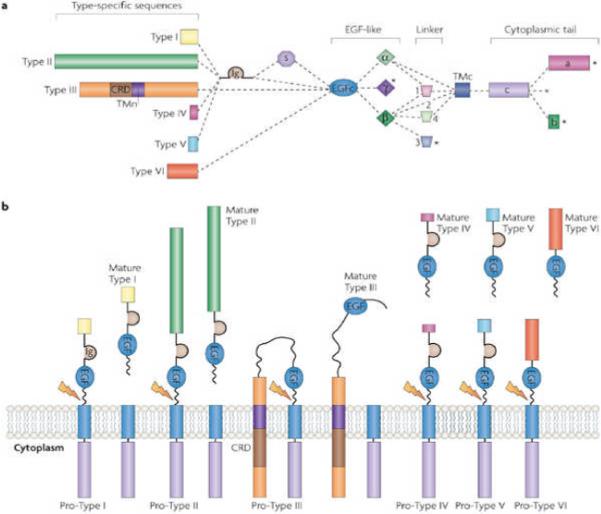
a | The six types of neuregulin 1 (NRG1) isoforms are classified according to their distinct amino-terminal sequences. In the type III isoforms, this sequence contains a cysteine-rich domain (CRD) that has a transmembrane domain (TMn). All six types of NRG1 isoforms have an epidermal growth factor (EGF)-like domain. Types I, II, IV and V have an immunoglobulin (Ig)-like domain between the N-terminal sequence and the EGF domain, with or without the spacer region (S), whereas the N-terminal-specific region of types III and VI is connected directly to the EGF domain. Variants are also generated by splicing in the linker regions and the C-terminal regions. Between the two regions is a C-terminal transmembrane domain (TMc). b | Most NRG1 isoforms are synthesized as transmembrane precursor polypeptides (pro-NRG1s) with the EGF domain located in the extracellular region, but in Type III NRG1 both the N- and the C-terminal regions are located inside the cell. Cleavage by tumour necrosis factor-α converting enzyme, β-site of amyloid precursor protein cleaving enzyme or meltrin β(indicated by the lightning arrow) generates mature NRG1s that are soluble, except in the case of Type III NRG1, which is thought to function in a manner that requires cell contact. The processing of Type IV, Type V and Type VI pro-NRG1s is less well-characterized but is thought to resemble that of Type I and Type II.
Most NRG1 isoforms are synthesized as membrane-anchored precursors, called pro-NRG1s, with the EGF domain positioned outside of the cell (Fig. 1b). Pro-NRG1s undergo proteolytic cleavage at the juxtamembrane region that lies on the carboxy-terminal side of the EGF-like domain. This leads to the release of diffusible, mature NRG1, except in the case of Type III NRG1. The cleavage is catalysed by three type I transmembrane proteases: tumour necrosis factor-α converting enzyme (TACE, also known as ADAM17)19, 20, β-site of amyloid precursor protein cleaving enzyme (BACE, also known as memapsin 2)21, 22 and meltrin beta (also known as ADAM19)23. Some NRG1 isoforms are synthesized without a transmembrane domain and are thus directly released into the extracellular space5. The expression and processing of pro-NRG1 are tightly regulated both temporally and spatially, as well as by neuronal activity24, 25, 26, 27.
ErbB receptor tyrosine kinases
NRG1 acts by stimulating a family of single-transmembrane receptor tyrosine kinases called ErbB proteins28. ErbB proteins have homology with the EGF receptor (EGFR, also known as ErbB1) (Fig. 2). ErbB4 is the only autonomous NRG1-specific ErbB that can both interact with the ligand and become activated by it as a tyrosine kinase. ErbB2, by contrast, functions as a co-receptor by forming heterodimers with other, ligand-bound ErbBs29. ErbB3 can bind to NRG1, but its homodimers are catalytically inactive, indicating that its kinase function is impaired30. EGFR does not bind to NRG1, but it can form heterodimers with ErbB4 (see below). Among the ErbB proteins, ErbB4 is the best-characterized for its function in the CNS. There is no evidence that ERBB2 and ERBB3 are susceptibility genes for schizophrenia and, unlike Erbb4-mutant mice, mice that carried mutations in Erbb2 and Erbb3 did not produce behaviours that were characteristic of schizophrenia31. Thus, we review NRG1 signalling and function with a focus on ErbB4.
Fig. 2.
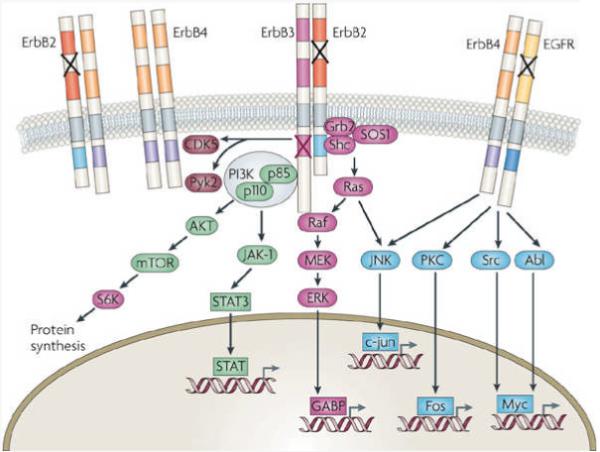
ErbB proteins are type I transmembrane receptor tyrosine kinases. Each has an extracellular region containing two extracellular cysteine-rich domains, a transmembrane domain, a short intracellular juxtamembrane region, a tyrosine kinase domain and a carboxy-terminal tail. In response to neuregulin 1 (NRG1) stimulation, ErbB proteins become dimerized to form homo- and heterodimers, such as ErbB2–ErbB3, ErbB4–ErbB4, ErbB2–ErbB4 and ErbB4–epidermal growth factor receptor (EGFR). ErbB2 does not bind to NRG1 (indicated by the black crosses) but has an active kinase domain; ErbB3 binds to NRG1 but has an impaired tyrosine kinase domain (indicated by the purple cross). Therefore, ErbB2 and ErbB3 need to form heterodimers with each other or with ErbB4 to be functional, whereas ErbB4 homodimers can bind to NRG1 and become activated. Activation of the tyrosine kinase domains leads to auto- and trans-phosphorylation of the intracellular domains, generating docking sites for the adaptor proteins Grb2 and Shc, which activate the Raf–MEK–ERK pathway, and for the p85 subunit of PI3K (indicated in the figure for the ErbB2–ErbB3 heterodimer), which activates the PI3K pathway and subsequently mTOR-dependent protein synthesis. NRG1 activates CDK5, which is also stimulated by neuronal activity36. EGFR does not bind to NRG1. Its downstream pathways include ones that involve JNK, Src, Abl, and PKC in addition to ones that involve ERK and PI3K/Akt.
Canonical forward signalling
In canonical forward signalling, NRG1-induced ErbB dimerization activates the ErbB kinase domain, resulting in auto- and trans-phosphorylation of the intracellular domains. This process seems to require ErbB endocytosis32, 33, 34. The phosphorylated tyrosine residues serve as docking sites for phosphopeptide-binding adaptor proteins or enzymes (Fig. 2). The Raf–MEK–ERK and PI3K–Akt–S6K pathways are frequently activated by the NRG1-induced stimulation of ErbB receptor homo- or heterodimers (Fig. 2). Other downstream kinases include c-Abl, JNK, CDK5, Fyn and Pyk2 (Refs 35,36,37,38). Similar to NRG1 transcripts, ERBB4 transcripts can be alternatively spliced to generate four ErbB4 isoforms that trigger distinct signalling cascades (Fig. 3). Ultimately, NRG1 activates specific transcriptional and translational programmes and thus has various long-term effects.
Fig. 3.
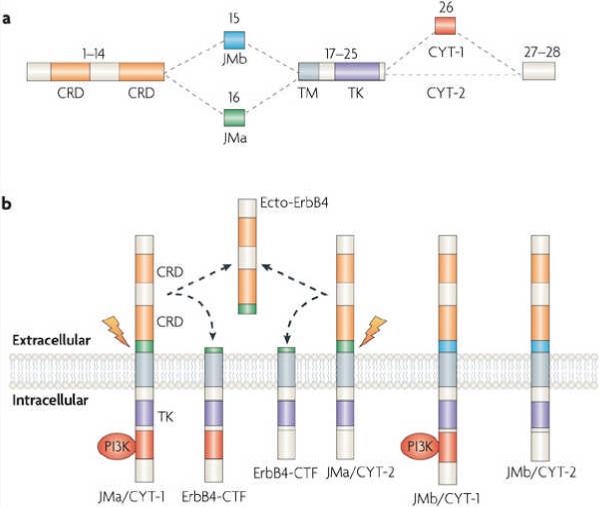
a | Inclusion of exon 26 in the intracellular domain of ErbB4 generates CYT-1, whereas its exclusion gives rise to CYT-2 (Refs 188,189). The extracellular juxtamembrane region of ErbB4 is encoded by either exon 16 or exon 15, to generate JMa or JMb, respectively. Different combinations of CYT-1, CYT-2, JMa and JMb thus create 4 isoforms of ErbB4. Exon numbers are shown on the top of corresponding domain structures. b | CYT-1 contains the motif that binds to the p85 subunit of PI3K and thus activates this kinase. Thus, although both isoforms are coupled to the Raf–MEK–ERK pathway, CYT-1 but not CYT-2 activates PI3K and subsequently Akt188, 189, 190. Both JMa and JMb can be activated by neuregulin 1 (NRG1) to initiate canonical signalling, but only JMa can be cleaved by tumour necrosis factor-α converting enzyme188, 191, 192 (indicated by the lightning arrow) to generate a soluble extracellular polypeptide, ecto-ErbB4 (which contains the NRG1 binding site), and a carboxy-terminal fragment (ErbB4-CTF). CRD, cysteine-rich domain; TK, tyrosine kinase domain; TM, transmembrane domain.
NRG1 signalling is mediated by heterodimers of ErbB2–ErbB3, ErbB2–ErbB4 and ErbB3–ErbB4 and by the homodimer ErbB4–ErbB4 (Fig. 2). ErbB4 can also form a heterodimer with EGFR. NRG1 stimulation can thus mediate signalling pathways that are typically associated with the EGFR, including the activation of Src family kinases, PLCγ, JNK and Abl37. However, the functional significance of ErbB4–EGFR heterodimers in the CNS remains unclear.
Non-canonical forward signalling
In non-canonical forward signalling, the juxtamembrane-a (JMa) isoform of ErbB4 (Fig. 3) is first cleaved by TACE to release a soluble extracellular peptide that contains the NRG1 binding site (ecto-ErbB4) (Fig. 4). The remaining membrane-anchored 80 kDa fragment (that is, ErbB4-CTF) is further cleaved in its transmembrane domain by presenilin-dependent γ-secretase to release the ErbB4 intracellular domain (ErbB4-ICD)39, 40, which has been shown to translocate to the nucleus and to regulate transcription41 (Fig. 4). Interestingly, ErbB4 isoforms exhibit distinct tissue-specific and brain-region-specific patterns of expression. Moreover, there is a reduction of JMa ErbB4 in neuronal precursor cells at late stages of embryonic development, providing a potential mechanism for late-onset astrogenesis41, 42.
Fig. 4.
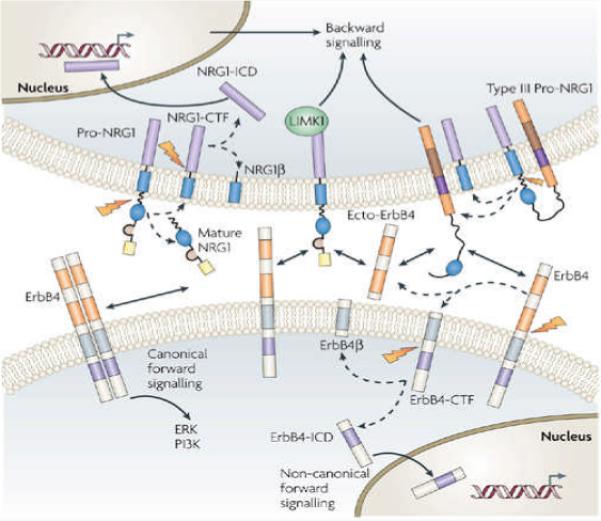
In non-canonical forward signalling (bottom cell, right-hand pathway), the carboxy-terminal fragment (ErbB4-CTF) is cleaved by γ-secretase to produce ErbB4-intracellular domain (ErbB4-ICD), which can translocate to the nucleus to regulate gene expression. When it is overexpressed in transfected cells, ErbB4-ICD interacts with several transcriptional regulators, including Eto2, STAT5, Mdm2 and YAP, to mediate the transcriptional activation or repression of heterologous promoters (not shown)193, 194, 195, 196, 197, 198. This interaction might require the phosphorylation of either ErbB4-ICD or the transcriptional regulator and/or the kinase activity of the ICD domain. For example, neuregulin 1 (NRG1) stimulation promotes the association of ErbB4-ICD with TAB2, an adaptor protein, in an ErbB4 kinase-domain-dependent manner41. TAB2 also interacts with the nuclear receptor co-repressor, N-CoR, to form a ternary complex that, upon translocation into the nucleus, represses the transcription of genes that are required for the differentiation of neural precursor cells into astrocytes. Backward signalling (top cell) by pro-NRG1 can proceed by two mechanisms. First, the C-terminal fragment of pro-NRG1 (NRG1-CTF), which is generated by extracellular cleavage, can be cleaved again by γ-secretase to generate NRG1-intracellular domain (NRG1-ICD), which can relocate into the nucleus to regulate gene transcription (left-hand pathway). Second, ErbB4 or ecto-ErbB4, which is released by extracellular cleavage, can serve as a ligand for pro-NRG1 or Type III NRG1, which function as receptors (right-hand pathway). It is unknown whether this interaction alters the phosphorylation of pro-NRG1 itself or whether it alters the activation of an intracellular kinase or phosphatase in pro-NRG1-expressing cells. Precisely how the signals are transduced also remains unknown. The cytoplasmic tail of pro-NRG1 interacts with the non-receptor protein kinase LIM kinase 1 (LIMK1)199, 200. This kinase has been shown to regulate actin dynamics in many cell types, including neurons. Interestingly, NRG1-ICD is required for NRG1 function in vivo201. Treatment of Type III NRG1-expressing neurons with a mixture of ecto-ErbB2 and ecto-ErbB4 promotes neuronal survival in vitro and alters the expression of several apoptotic genes24 (not shown). Canonical forward signalling (bottom cell, left-hand pathway) is explained in detail in Fig. 2.
NRG1–ErbB4 interactions also mediate both short- and long-range attraction for tangentially migrating interneurons: ErbB4 is expressed in a subpopulation of interneurons that migrate tangentially towards the cortex through a permissive corridor that expresses Type III NRG1 (Refs 58,59). Furthermore, the migration of interneurons from the medial ganglionic eminence towards the developing cortex might be mediated by a diffusible source of immunoglobulin (Ig)-domain-containing Type I or Type II NRG1. Loss of ErbB4 perturbs interneuron migration, leading to an altered number of GABAergic interneurons in the postnatal cortex59 (Fig. 5b).
Fig. 5.
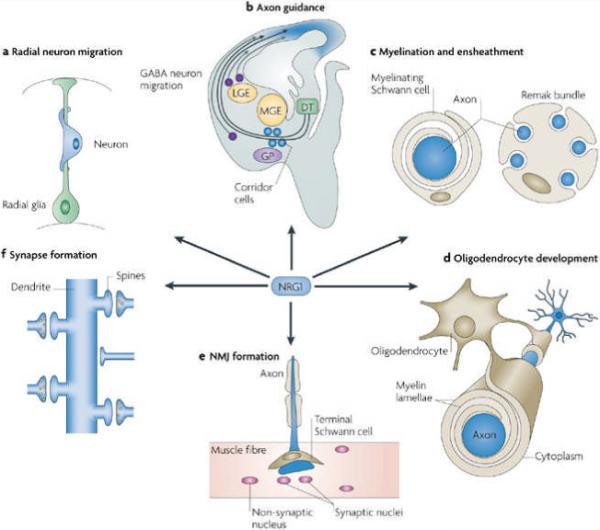
a | Neuregulin 1 (NRG1) (indicated by blue shading) is released from neurons to promote the formation and maintenance of radial glial cells, which are necessary for the radial migration of neurons from ventricular zones to the pial surface. b | Tangential migration of GABA (γ-aminobutyric acid)-ergic interneurons requires NRG1 in the cortical region; thalamocortical axon navigation through the diencephalon requires corridor cells that express NRG1. c | Myelination and ensheathment of peripheral nerves are controlled by the amounts of NRG1 produced in substrate axons. d | NRG1 from axons might regulate oligodendrocyte development and myelination of axons in the CNS. e | NRG1 is necessary for the formation of neuromuscular junctions (NMJs), probably through effects on terminal Schwann cell differentiation and survival. f | NRG1 stimulates CNS synapse formation. This panel shows typical excitatory synapses, formed between glutamatergic terminals and spines. DT, dorsal thalamus; GP, globus pallidus; LGE, lateral ganglionic eminence; MGE, medial ganglionic eminence. Part b modified, with permission, from Ref. 69 © (2006) Elsevier Science. Part d modified, with permission, from Ref. 201 © (2005) Macmillan Publishers Ltd.
In the adult rodent brain, interneurons in the olfactory bulb are replaced by neural progenitor cells that migrate into the bulb from the subventricular zone through the rostral migratory stream60. This process is in part regulated by ErbB4 that is expressed in neuroblasts in the subventricular zone and in the rostral migratory stream, as Erbb4-deficient mice exhibit altered neuroblast chain organization and migration as well as deficits in the placement and differentiation of olfactory bulb interneurons61.
Axon guidance
Recombinant NRG1 stimulates neurite outgrowth in multiple populations of primary neurons, including hippocampal neurons62, retinal neurons63, cerebellar granule cells64 and thalamic neurons65. In addition, NRG–ErbB-signalling-induced neurite outgrowth has been observed in neural cell lines66, 67, 68. Owing to the embryonic lethality of deleting the Nrg1 gene, the exact function of NRG1 in axon guidance is unclear18, 51, 52.
A recent study provided evidence that the thalamocortical axon (TCA) projection is regulated by NRG1 and ErbB4 (Ref. 65) (Fig. 5b). TCAs convey sensory and motor inputs to the cerebral cortex. They originate in the dorsal thalamus, run rostrally towards the telencephalon, make a sharp turn in a dorsal direction to enter the mantle region of the medial ganglionic eminence, and then advance through the striatum to finally reach the developing cortex69. TCA development depends on the migration of a population of lateral-ganglionic-eminence-derived interneurons towards the diencephalon, where the neurons form a permissive corridor for later-arriving TCAs. GABAergic neurons (or corridor cells) between the medial ganglionic eminence and the globus pallidus express cysteine-rich-domain-containing NRG1 (CRD-NRG1), which is thought to serve as a permissive cue that activates ErbB4 in TCAs, allowing growth into the developing telencephalon. In addition, diffusible Ig-NRG1 in the ventral and lateral pallidum serves as a long-range attractant that facilitates TCA projection through the dorsal striatum and into the cortex65. These observations demonstrate that the two isoforms of NRG1 cooperate in the guidance of TCAs.
Glial cell development, axon myelination and axon ensheathment
In the nervous system, specialized glial cells (Schwann cells in the PNS and oligodendrocytes in the CNS) extend plasma-membrane processes that wrap axons with a multilamellar membranous myelin sheath. Myelination increases the conduction velocity of action potentials and is a tightly controlled developmental process. Type III NRG1 has emerged as a key axon-derived regulator at virtually every stage of Schwann cell development70, 71, 72, 73, 74 (Fig. 5c).
NRG1 induces the commitment of neural crest cells to the gliogenic fate75, 76 and promotes their proliferation15, 51, 57, 77, 78, 79 and migration along axons51, 80. It also promotes Schwann cell proliferation and differentiation79, 81, 82. Disrupting NRG1 signalling by ablating all NRG1 isoforms, the Type III isoform or one of its receptors (ErbB2 and ErbB3) leads to an almost complete loss of Schwann cells and the sensory and motor neurons that they support49, 52, 71, 83, 84, 85. Recent studies have suggested that the level of Type III NRG1 in the axon is a key instructive signal for myelination72. Small axons (usually ones that are less than 1 micrometre in diameter) contain insufficient amounts of NRG1 to induce Schwann cell myelination and, consequently, they become ensheathed but remain unmyelinated, whereas myelinated axons are usually large and express higher levels of NRG1 (Refs 73,74) (Fig. 5c). Whether NRG1 signalling affects internode length or axonal calibre awaits further investigation. In one study, NRG1 signalling was implicated in the elaboration of myelin thickness and internodal length, as well as in determining the axonal calibre of myelinating Schwann cells86. However, heterozygous Nrg1+/− mice did not display an abnormal axonal size distribution, although they did have significant hypomyelination73. Conversely, for non-myelinating Schwann cells NRG1 serves as a pro-survival signal but inhibits proliferation87.
The role of NRG1 in myelination in the CNS has not been well-characterized. NRG1 is thought to serve as an axon-derived signal for oligodendrocyte development (Fig. 5d). In vitro studies showed that NRG1 promotes oligodendrocyte proliferation and survival88, 89, 90, 91. NRG1 is expressed in the subventricular zone of the rat brain at the critical time for oligodendrocyte differentiation, and it enhances the development of oligodendrocytes from bipotential (O2A) glial progenitor cells91. Neural tube explants prepared from Nrg1-knockout mice failed to produce oligodendrocytes in vitro; this effect could be rescued by exogenous NRG1 (Ref. 92). Moreover, disruption of NRG1 signalling suppressed oligodendrocyte differentiation from progenitor cells93. Furthermore, mice that expressed DN-ErbB4 showed reduced myelin thickness and a slower conduction velocity in their CNS axons compared with wild-type mice94. However, the reduction in ErbB signalling did not alter the number of myelinated and unmyelinated axons in the optic nerve and the corpus callosum, suggesting that NRG1 is required for the terminal differentiation of oligodendrocytes. This notion is supported by studies of Erbb2-null mice in which oligodendrocyte development is halted at the pre-oligodendroblast stage95. Although ErbB3 is expressed in oligodendrocytes, it is dispensable for their development96, presumably because of expression of autonomous ErbB4.
Synapse formation
NRG1 signalling also has a role in synapse formation. Indeed, ARIA (an Ig-domain-containing NRG1) was identified and purified on the basis of its ability to stimulate the synthesis of the acetylcholine receptor (AChR)11, 12. Furthermore, inhibition of ErbB kinase activity or of its downstream kinases ERK, JNK and CDK5 attenuates NRG1-induced expression of AChR in muscle cells35, 36, 97, 98, 99, 100. Nrg1 heterozygous-mutant mice exhibited a decreased postsynaptic AChR density and a reduced reliability of neuromuscular transmission101. However, conditional-mutant mice lacking both ErbB2 and ErbB4 in their skeletal muscle form neuromuscular junctions that are structurally and functionally normal in many respects102. These data indicate that the NRG1–ErbB signalling pathway in muscle might be dispensable for postsynaptic development. Alternatively, NRG1 might signal to muscle cells indirectly through Schwann cells to regulate the formation of neuromuscular junctions51, 85, 103, 104 (Fig. 5e). In addition, Type I and Type II but not Type III NRG1s are required for proprioceptive-afferent-evoked induction of muscle spindle differentiation105.
In the CNS, Type III NRG1 influences the expression level of neuronal AChRs, whereas Type I and Type II isoforms alter the expression of GABAA receptors64, 106, 107, 108. Alterations in the levels and profiles of various glutamate receptors (NMDA (N-methyl-D-aspartate) and AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) receptors) have been associated with signalling stimulated by Type I, Type II and Type III isoforms38, 109. Nrg1+/− mice show a decrease in the number of functional NMDA receptors in their forebrain110. Therefore, there might be differential regulation of glutamate receptors depending on the isoforms that are expressed.
Recently, postsynaptic ErbB4 signalling has been shown to control the activity-dependent maturation of excitatory synapses111: synaptic activity triggers NRG1–ErbB4 signalling, which recruits or stabilizes ErbB4 at the synapse in a PSD95-dependent manner and stabilizes synaptic AMPA receptors. Interruption of NRG1–ErbB4 signalling causes the destabilization of synaptic AMPA receptors and spine structure, leading to the impairment of plasticity and, eventually, to the loss of spines and NMDA receptors.
NRG1 in synaptic plasticity and neuronal survival
NRG1 and ErbB4 are expressed in multiple regions in the adult brain58, 112, 113. Notably, the expression of NRG1 isoforms seems to be lamina-specific and largely non-overlapping in the cortex: Type I and Type II isoforms are expressed in layers 2, 3 and 6b; Type III isoforms are expressed in layer 5. Type I, Type II and Type III isoforms are also all expressed in the reticular nucleus of the thalamus, in the piriform cortex and throughout the hippocampus. By contrast, ErbB4 transcripts are detectable in cortical layers 2−6b and are present at high levels in regions where interneurons are enriched, including the medial habenula, the reticular nucleus of the thalamus and in the intercalated masses of the amygdala58, 112, 113. These findings suggest additional functions of NRG1–ErbB4 signalling in the mature nervous system. Indeed, NRG1 regulates both excitatory and inhibitory synaptic transmission in the adult brain. These observations are exciting because abnormal neurotransmission and/or synaptic plasticity have been observed in both glutamatergic and GABAergic pathways in the schizophrenic brain114, 115, 116, 117.
Short-term plasticity: glutamatergic transmission
ErbB4 interacts and co-localizes with PSD95, a postsynaptic scaffold protein that is essential for the assembly and function of glutamatergic synapses118, 119. This interaction enhances NRG-dependent intracellular signalling118. Further studies have indicated that ErbB4 is localized in anatomically defined PSDs in the brain120 (Fig. 6a). The targeting of ErbB4 to the PSD suggested that NRG1 might have a role in synaptic transmission or plasticity at excitatory synapses. Indeed, in collaboration with Salter and colleagues, we showed that bath application of NRG1 blocked tetanus-induced long-term potentiation (LTP) at Schaffer collateral/CA1 synapses in hippocampal slices within 20 minutes118, suggesting that protein synthesis might not be involved. The effect of NRG1 on LTP requires ErbB4, as both pharmacological inhibition and genetic ablation of ErbB4 prevented it121. When NRG1 was applied soon after LTP induction by theta-burst stimulation, it had a depotentiating effect in the hippocampus122. However, this effect was lost if NRG1 was added 50 min after induction122. These observations are in good agreement with the notion that NRG1–ErbB4 signalling suppresses both the induction and the expression of LTP.
Fig. 6.
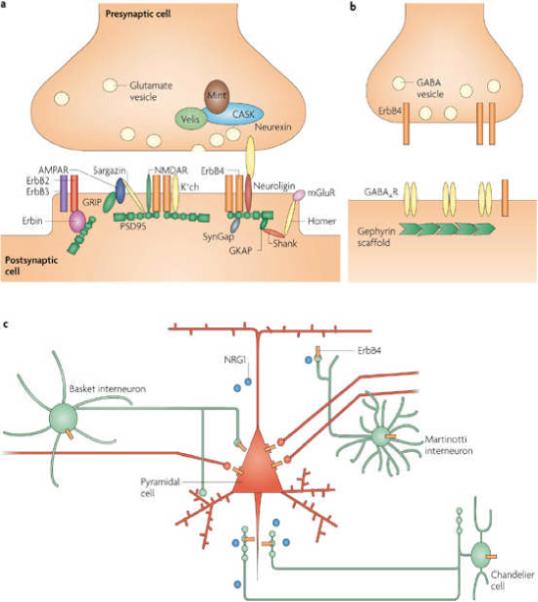
a | ErbB4 interacts specifically with the first and second PDZ domains of postsynaptic density (PSD) protein 95 (PSD95), a scaffold protein, and is localized in the PSD of excitatory synapses. The interaction with PSD95 enhances neuregulin 1 (NRG1) signalling, presumably by increasing ErbB4 homodimerization118. NRG1, by activating ErbB4, suppresses long-term potentiation induction and expression38, 118, 120, 121, 122. The mechanisms that underlie this effect remain unclear. Through PSD95, ErbB4 signalling might regulate the properties of NMDA (N-methyl-D-aspartate) receptors (NMDARs), AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) receptors (AMPARs) and K+ channels (K+ ch)202. Through the GKAP–Shank–Homer complex, ErbB4 signalling might be involved in regulating the function of metabotropic glutamate receptors (mGluRs). PSD95 might also recruit ErbB4 to the neuroligin–neurexin complex that is essential for synapse formation203. ErbB2, on the other hand, interacts with erbin, a protein that contains multiple leucine-rich domains and a PDZ domain204, 205. This interaction has been implicated in regulating NRG1 signalling206, 207. b | ErbB4 is present in the presynaptic terminals of GABA (γ-aminobutyric acid)-ergic interneurons112. NRG1 stimulates presynaptic ErbB4 to enhance activity-dependent GABA release through mechanisms that have yet to be identified. c | A working hypothesis for how NRG1 might regulate pyramidal neuron activity. The output of pyramidal neurons in the prefrontal cortex (PFC) is regulated by excitatory glutamatergic neurons (shown in red) and various inhibitory GABAergic interneurons (shown in green). NRG1 regulates glutamatergic transmission and/or plasticity by activating PSD-localized ErbB4. There are at least three types of GABAergic interneurons in the PFC. Wide-arbor basket cells target the somata and proximal dendrites of pyramidal neurons and adjust the integrated synaptic response. Chandelier cells (or axon-targeting interneurons) terminate at or near the axon hillock of pyramidal neurons, forming vertical arrays of terminals termed ‘cartridges’, to regulate the generation and timing of action potentials. Conversely, Martinotti cells terminate on distal dendrites of pyramidal cells to influence the dendritic processing and integration of synaptic inputs185, 208, 209, 210, 211. By controlling activity-dependent GABA release, NRG1 might repress the activity of pyramidal neurons. Part c modified, with permission, from Ref. 185 © (2005) Macmillan Publishers Ltd.
Studies of Nrg1(ΔEGF)+/− mice, which have a heterozygous deletion of the NRG1 EGF domain, suggest that the effect of NRG1 on LTP depends on the level of endogenous NRG1 (Ref. 38). Theta-burst-induced but not tetanus-induced LTP was deficient in hippocampal slices of adult mutant mice, suggesting that developmental disruption of NRG1 signalling impairs neurotransmission in adult animals. Intriguingly, at low doses NRG1 increased both tetanus- and theta-burst-induced LTP in hippocampal slices from adult Nrg1(ΔEGF)+/− mice, but higher doses of NRG1 suppressed LTP38. These observations suggest that the regulation of synaptic plasticity by NRG1 might depend on an initial NRG1 activity that is controlled by local concentrations of NRG1 isoforms, levels of ErbB kinases and neuronal activity that regulates NRG1 expression. An ‘inverted-U model’ has been proposed that might explain why both low and high levels of NRG1–ErbB activity could impair synaptic function123.
The mechanism by which NRG1 regulates LTP remains unclear. Basal synaptic transmission at the Schaffer collateral/CA1 synapses in hippocampal slices from adult mice was not affected by bath application of NRG1 (Refs 38,118,122,124), by acute inhibition of ErbB4 (Ref. 121) or by Erbb4−/− and Nrg1+/− mutation38, 121. NRG1 does not alter paired-pulse facilitation ratios118, 121, 122, 124, suggesting that NRG1 has no effect on short-term plasticity and acts through a postsynaptic mechanism. Moreover, the effect of NRG1 on LTP is observed in the presence of the GABAA-receptor blockers bicuculine and picrotoxin38, 121, 122 and thus cannot be explained by changes in GABA transmission (see below). A recent study reported that NRG1 depression of LTP at CA1 synapses is independent of GABAergic inhibition124. The activity of NMDA receptors is subject to modulation by tyrosine phosphorylation125, 126, 127, but the NRG1-induced suppression of LTP could not be attributed to an effect on basal NMDA currents because NRG1 did not alter basal NMDA-receptor-mediated synaptic responses in hippocampal slices118, 122, 124. These results might suggest that NRG1–ErbB4 signalling acts at a point downstream of NMDA receptor stimulation. Intriguingly, NRG1 has been shown to downregulate AMPA receptor currents and reduce surface glutamate-receptor-1-containing AMPA receptors in dissociated hippocampal neurons122.
Valuable insight has been provided by studies that used loss-of-function approaches. In the hippocampus of Nrg1(ΔTM)+/− (a heterozygous deletion of the NRG1 transmembrane domain) and Erbb4−/− mice, the expression of NMDA receptors is decreased and Tyr 1472 of the NMDA receptor subunit NR2B, a site that is key to NMDA receptors’ channel property, is hypophosphorylated38. Inhibition of NRG1–ErbB4 signalling in hippocampal slices from postnatal mice destabilizes synaptic AMPA receptors and leads to the loss of synaptic NMDA currents and spines111. Tyrosine phosphorylation of NR2B was reduced in the hippocampus of Nrg1(ΔTM)+/− and Erbb4−/− mice38, but tyrosine phosphorylation of the NR1, NR2A and NR2B subunits was not changed in NRG1-treated neurons under conditions in which NMDA currents were inhibited32. Therefore, the question of whether these mechanisms contribute to the rapid NRG1 regulation of synaptic transmission or synaptic plasticity warrants further investigation. The reduction in NMDA receptor levels in the hippocampus of the mutant mice38 is unlikely to occur after brief NRG1 stimulation that suppresses LTP.
It is worth pointing out that the regulation of neurotransmission by NRG1 might vary between brain regions. For example, NRG1 administration decreased NMDA-receptor-mediated excitatory postsynaptic currents in slices of prefrontal cortex (PFC), an area in which altered activity has been implicated in schizophrenia, and reduced whole-cell NMDA receptor currents in acutely isolated PFC pyramidal neurons32 by elevating intracellular Ca2+ and stimulating ERK. This resulted in enhanced actin depolymerization and subsequent internalization of NMDA receptors32. Moreover, NRG1 depressed entorhinal CA1 synaptic transmission but increased the dentate field excitatory-postsynaptic-potential response to entorhinal cortical stimulation in anaesthetized rats128. These observations indicate that the role of NRG1 in regulating synaptic plasticity might be more complex than was previously thought. More studies are needed to figure out whether the reported differential effects were due to variation in NRG1 (isoforms, timing and local concentration), to the activation of other ErbB kinases, to variation in the age of the animals from which brain slices were cultured and/or to other experimental particulars129.
Short-term plasticity: GABAergic transmission
Cognitive processes such as working memory and executive function are mediated by the PFC. The functions of the PFC are executed by pyramidal neurons, and the activity of these neurons is intricately controlled by inhibitory and excitatory synaptic inputs. Abnormal GABAergic and glutamatergic transmission in this area has been implicated in schizophrenia and is thought to contribute to the cognitive deficits that are associated with the disorder115. Despite the fact that ErbB4 is prominently expressed in interneurons113, 118, most studies have focused on its role in pyramidal cells. We showed that there was ErbB4 immunoreactivity at the terminals of GABAergic neurons that seemed to innervate pyramidal neurons in the PFC112 (Fig. 6b). Biochemical and electrophysiological studies have shown that exogenous NRG1 stimulates GABA release in response to depolarization, with no discernible effect on basal release112. This effect of NRG1 was blocked by an ErbB4 inhibitor and did not occur in cortical slices from Erbb4-mutant mice. Intriguingly, treatment with ecto-ErbB4 or inhibition of NRG1–ErbB4 signalling attenuated activity-dependent GABA release, indicating that GABA transmission is determined by the level of NRG1–ErbB4 signalling112.
Glutamatergic activity is known to increase GABAergic transmission130; it is therefore possible that the NRG1 regulation of evoked GABA release might be mediated by a glutamatergic mechanism. However, the NRG1-mediated enhancement of GABA release was not attenuated by inhibitors of NMDA and AMPA receptors112. Therefore, it is likely that NRG1 regulates GABA release by directly activating ErbB4 receptors on presynaptic terminals. This notion is supported by the observation that NRG1 increases depolarization-evoked GABA release from synaptosomes, which are isolated from any neural networks, and decreases paired-pulse ratios of evoked inhibitory postsynaptic potentials (IPSCs) in response to two consecutive stimulations, suggesting that NRG1 might facilitate vesicle release that is evoked by neuronal activation112. These results identify a novel function for NRG1–ErbB4 signalling: NRG1, through activity-dependent GABA release, regulates signal integration by pyramidal neurons. The final output of pyramidal neurons depends on glutamatergic and GABAergic inputs that are both regulated by NRG1. This function of NRG1 could have implications for working-memory deficits in patients with schizophrenia and in people with neurological disorders such as epilepsy112 (Fig. 6c).
Long-term plasticity
It is widely accepted that long-term plasticity, such as late-phase LTP, requires new protein synthesis. There is evidence to support the notion that NRG1 regulates long-term plasticity in the brain. First, NRG1 has been shown to stimulate the expression of receptors for key neurotransmitters, including glutamate, GABA and ACh64, 106, 107, 108, 109, 131; it could thus potentially regulate excitatory and/or inhibitory neurotransmission. Importantly, protein synthesis is necessary for the NRG1-mediated increase of GABA-induced currents in cerebellar granule cells64, the decrease of miniature IPSC amplitudes in CA1 hippocampal neurons108 and the increase in the peak amplitude of the ACh-induced current in hippocampal GABAergic neurons106. In addition, NRG1 stimulates the expression of Ca2+-activated K+ channels in parasympathetic neurons132, 133. Considering the distinct synaptic localization of ErbB4, it is tempting to speculate that NRG1 might regulate local protein synthesis at synapses. Finally, expression and processing of NRG1 are regulated by neuronal activity24, 25, 26, 27, so the increased neurotransmission that is induced by NRG1 and the subsequent production of more NRG1 are likely to contribute to long-term synaptic plasticity.
NRG1 and ErbB signalling in neuronal survival
In vitro studies indicate that NRG1 can be neurotrophic and neuroprotective for cortical neurons134, motor neurons135, dopaminergic neurons136, cochlear sensory neurons137 and PC12 cells138; it also protects neurons following ischaemia139, 140, 141. How this effect relates to schizophrenia awaits further investigation.
NRG1 and ErbB4 as susceptibility genes
Mutations in NRG1 and ERBB4 that are associated with schizophrenia
Schizophrenia has a strong genetic component, and several susceptibility genes for schizophrenia have been identified3. Meta-analyses of whole-genome linkage scans identified 8p as a susceptibility locus for the disorder142, 143. Extensive fine-mapping of the 8p locus and haplotype-association analysis of affected families in Iceland narrowed the region to 8p12−8p21, and NRG1, which lies in this region, was identified as a candidate gene for schizophrenia110 (Fig. 7a). The original ‘core at-risk haplotype’ (hereafter referred to as the ‘deCODE haplotype’) consisted of five single-nucleotide polymorphisms (SNPs) (SNP8NRG221132, SNP8NRG221533, SNP8NRG241930, SNP8NRG243177 and SNP8NRG433E1006) and two microsatellites (478B14−848 and 420M9−1395) in a region spanning the 5' end of NRG1 and extending into the second intron. The association of SNP8NRG221533 and two other SNPs, rs3924999 and rs2954041, in NRG1 was soon reported in an independent study of Chinese Han schizophrenia family trios (consisting of the father, the mother and the affected offspring)144. The genetic association between NRG1 and schizophrenia has been confirmed in follow-up studies in multiple populations in Scotland, Ireland, the United Kingdom, the Netherlands, Korea and China145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155. Most of the 80 schizophrenia-associated SNPs are localized to the 5' region110, 146, 149, 152, 153, 154, 155 and 3' region149, 150, 152, 155, 156 of NRG1. Evidence suggests that the 5' SNPs regulate NRG1 expression in patients with schizophrenia157, 158, 159. The risk allele SNP8NRG243177, which is part of the original deCODE haplotype, is associated with decreased activation of frontal and temporal lobe regions, increased development of psychotic symptoms and decreased premorbid IQ160. Together, these results provide strong evidence that NRG1 is a schizophrenia-susceptibility gene in many populations, although studies of Japanese, Irish and Spanish cohorts showed poor association161, 162, 163.
Fig. 7.
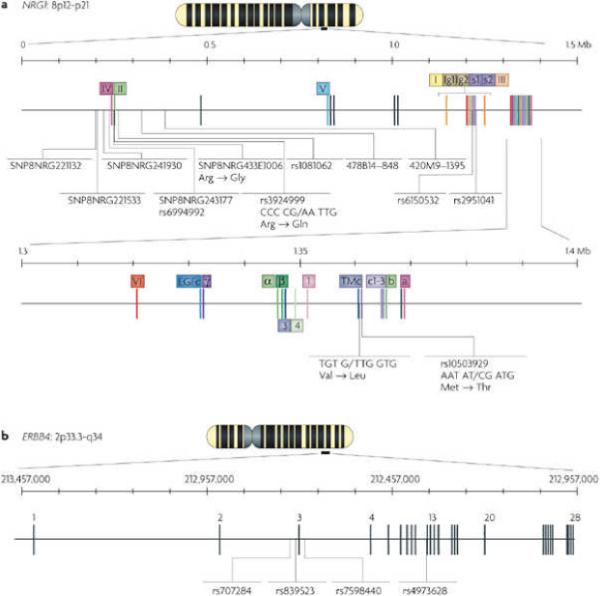
a | The neuregulin 1 (NRG1) gene is located in a 1.5 Mb region of DNA, at 8p12−8p21. Roman numerals indicate the type-specific exons. The original deCODE hyplotype including the SNPs SNP8NRG221132, SNP8NRG221533, SNP8NRG241930, SNP8NRG243177 and SNP8NRG433E1006 and two microsatellites (478B14−848 and 420M9−1395) is shown. Exons for individual domains of NRG1 are colour matched with the domain structure in Fig. 1a. b | The 1.15 Mb region of the ERBB4 gene, at 2q33.3−2q34. The SNPs are mainly clustered around exon 3 and in front of exon 13.
Of note are SNPs that generate changes in the coding region of NRG1. A missense mutation (Val to Leu) was identified in the transmembrane region of the NRG1 protein164. The change from Val to Leu is a conservative mutation that lengthens the aliphatic side chain of the amino acid by only a single hydrocarbon. The functional implication of this NRG1 mutation remains unclear. Interestingly, the rs3924999 mutation (Arg to Gln) in the Type-II-NRG1-specific region is significantly associated with deficits in pre-pulse inhibition, a measure of attentional processes and sensorimotor gating165 (although the rs10503929 mutation (Met to Thr) was not associated with changes in prepulse inhibition165). The rs3924999 mutation is also associated with an increased score on the Perceptual Aberration Scale (PAS) but not on the Schizotypal personality Questionnaire (SPQ)166. These results suggest that the rs3924999 mutation might have a role in causing the prepulse inhibition deficits and schizotypal personality in patients with schizophrenia.
ERBB4, which spans 1.15 Mb on chromosome 2q34, has also been suggested to be a susceptibility gene for schizophrenia in populations of Ashkenazi Jews, Caucasians and African Americans155, 158, 159, 167, 168 (Fig. 7b). This gene was among several that were identified as being disrupted by microdeletions or microduplications in patients with schizophrenia169. In a case-control association analysis of Ashkenazi Jews, three SNPs of ERBB4 showed a highly significant association with the disease158. A 46 kb core at-risk haplotype comprising three SNPs (rs707284, rs839523 and rs7598440) that surround exon 3 was identified by allele, genotype and haplotype frequency analysis158. A separate mutation screen identified an additional 15 SNPs in ERBB4 that are associated with schizophrenia167. The SNP in intron 12, rs4673628 (intervening sequence 12−15 C>T), shows a significant genetic interaction with the original NRG1 ‘Icelandic’ schizophrenia-associated haplotype. The nature of the interaction seems to be an excess of rs4673628 heterozygotes among patients with schizophrenia who carry the Icelandic NRG1 risk haplotype. One open question is how the mutations might be pathogenic. A recent study indicated that abnormal expression of the CYT-1 isoform of ERBB4 (Fig. 3) might be caused by mutations at rs4673628, at the three intronic risk SNPs surrounding exon 3 and at a core-risk haplotype159. This would implicate abnormal PI3K/Akt signalling in the pathologic mechanisms.
Behavioural studies of mice with hypomorphic NRG1 and ERBB4
It has been challenging to generate convincing animal models for abnormal behaviours that are unique to humans, such as the positive and negative symptoms in schizophrenia170. In rodents, positive symptoms are thought to be modelled by hyperactivity in response to novelty and hypersensitivity to psychostimulants, whereas negative symptoms are modelled by impaired social interaction and anhedonia. By contrast, cognitive deficits are not uniquely human and can thus be measured in rodents.
Studies of Nrg1- and Erbb4-mutant mice have provided support for the potential role of mutations in these genes as risk factors for schizophrenia. Nrg1- and Erbb4-hypomorphic or conditional-knockout mice showed ‘schizophrenic-like’ deficits, whereas Erbb2- and Erbb3- heterozygote-null mice seem to be behaviourally normal31, 171. Nrg1(ΔEGF)+/− and Nrg1(ΔTM)+/− mice are generally hyperactive in a number of tests, including the novel open-field test and the alternating-Y maze31, 110, 172. Interestingly, the hyperactivity of Nrg1 hypomorphs can be reversed by clozapine, an atypical antipsychotic that is used to treat schizophrenia, at a non-sedating dose. Hypomorphs of Nrg1(ΔIg)+/− mice (which have a heterozygous deletion in the Ig domain) are impaired in latent inhibition but exhibit normal activity in open-field and running-wheel tests in comparison with control littermates173. However, they display behaviours that are thought to indicate a schizophrenia-like phenotype, such as clozapine-induced suppression of open-field and running-wheel activity. Mice with an Erbb4 mutation that was generated specifically in the brain did not seem to have altered motor behaviour174. However, a more detailed analysis of these mutant mice revealed that they were more active than control animals at the initial stage of the behavioural evaluation but became less active than controls in longer, more comprehensive evaluations at older ages171. Deletion of the gene that encodes the NRG1-cleavage enzyme BACE generates similar ‘schizophrenic’ phenotypes to those that are observed in Nrg1+/− mice175, supporting the model that disruption of NRG1 signalling might participate in the pathogenesis of schizophrenia.
Recently, Nrg1-hypomorphic animals were shown to exhibit a selective disruption in their behavioural response to social novelty and increased aggression towards a conspecific172. These mice, however, were apparently normal in measures of emotionality/anxiety, spatial learning and working memory. The relatively mild phenotypes of Nrg1 heterozygous mutants might be due to functional redundancy supplied by NRG2 and/or NRG3, mutations in the genes of which have been associated with schizophrenia155. Finally, transgenic female mice expressing DN-ErbB4 in hypothalamic astrocytes exhibited delayed onset of puberty and reproductive development176. These observations demonstrate that Nrg1- and Erbb4-mutant mice exhibit behaviours that are similar to those of established rodent models of schizophrenia177.
NRG1 hypotheses of schizophrenia
Consistent with the ‘abnormal neural development’ model of schizophrenia44, 115, 178, NRG1–ErbB signalling is evidently involved in important processes of brain development. Loss of function of NRG1 or ErbB4 or perturbation of NRG1 signalling can cause deficits in the migration of pyramidal and GABAergic neurons, neurite outgrowth and axon projection, the myelination of axons and synapse formation. The resulting anatomical abnormalities could underlie the altered neurotransmission and cortical function that leads to psychotic symptoms and cognitive impairments. In addition, NRG1 has acute effects on both glutamatergic and GABAergic pathways, and it thus contributes to a second mode of action. Evidence from Nrg1-hypomorphic and Erbb4-mutant mice supports the idea that mutations in NRG1 and ERBB4 have a role in the aetiology of schizophrenia.
It is worth pointing out that most of the genetic variations or SNPs in both NRG1 and ERBB4 are either intronic or synonymous exonic substitutions or are located in 5' or 3' non-coding regions8. It therefore remains unclear how these changes affect disease susceptibility. One plausible hypothesis is that the genetic variations are regulatory and that they thus affect disease susceptibility by altering the expression or splicing of NRG1 and ERBB4 or by altering NRG1 or ErbB4 mRNA stability. Recent studies have provided some evidence for this hypothesis. For example, brain samples from patients with schizophrenia showed increased mRNA for Type I NRG1 (Refs 157,158,179) and abnormal expression of ErbBs158, 180, 181. Specifically, mRNA levels of Type I NRG1 were elevated both in the PFC and in the hippocampus157, 171. However, mRNAs of the JMa/CYT-1 isoform of ErbB4 were upregulated in the PFC but not in the hippocampus158, 159, suggesting that changes in the expression of ERBB4 isoforms are not secondary to NRG1 abnormalities and do not result from the general effects of illness state or medication. Whether NRG1 and ErbB4 protein levels are also altered has been a subject of controversy182, although a recent study showed that NRG1-ICD and ErbB4 protein levels were increased in the PFC of patients with schizophrenia183. This might be due to differences in the study participants’ ages or to differences in the experimental particulars, such as the antibodies that were used.
Gain-of-function and hypoglutamatergic function in schizophrenia
Altered ErbB4 levels might change the balance of ErbB4 homo- and heterodimers and their downstream signalling pathways. Thus, an increase in the CYT-1 isoform of ErbB4 would be expected to stimulate PI3K (alterations in which have previously been implicated in schizophrenia184) and subsequently Akt. Indeed, evidence of increased NRG1 signalling and/or function was found in the PFC of patients with schizophrenia182, including an increase in the PSD95–ErbB4 interaction that can further enhance NRG1 signalling118, activation of both ERK and Akt, and NRG1-induced suppression of NMDA receptor activation. These findings are exciting and, together with studies of NRG1 and ErbB4 expression in the schizophrenia brain, provide important links between the susceptibility genes and schizophrenic pathology. Based on the limited results, we propose a gain-of-function hypothesis of NRG1–ErbB4 signalling as a potential mechanism in the pathogenesis of schizophrenia: increased expression of Type I NRG1 and CYT-1 ErbB4 and/or increased NRG1 signalling in the PFC stimulates GABA transmission, which is anticipated to reduce the firing rate of glutamate-dependent pyramidal neurons. This would lead to hypofunction of the glutamatergic pathway, consistent with the reduced glutamatergic transmission and plasticity that is found in the brains of patients with schizophrenia114, 116, 117.
The activity of glutamic acid decarboxylase (GAD) and the staining of parvalbumin, a Ca2+-binding protein that is expressed in a subset of GABAergic interneurons, are reduced in the PFC of patients with schizophrenia185. In view of the newly identified function of NRG1–ErbB4 signalling as a potentiator of GABAergic transmission, it is possible that elevated NRG1–ErbB4 expression and signalling compensate for reduced GAD activity. Conversely, GAD activity and parvalbumin expression might be repressed in an effort to compensate for elevated NRG1–ErbB4 expression and signalling. Settling this uncertainty will require a thorough assessment of the changes in GAD activity, parvalbumin staining and the relative expression and activity of distinct NRG1–ErbB4 isoforms in schizophrenia patients of different ages.
Conclusions and perspectives
Recent studies have provided compelling evidence that the NRG1–ErbB4 signalling pathway is involved in the pathogenesis of schizophrenia. Novel functions of NRG1 and ErbB4 have been identified in both neural development and synaptic plasticity. These advances have also raised many questions. Schizophrenia is most commonly viewed as a disorder of development44. Can a dysfunction in the NRG1–ErbB4-mediated regulation of neurotransmission be a mechanism of schizophrenia? The effect of NRG1 on both excitatory and inhibitory neurotransmission in adult-brain slices, including those of Nrg1-mutant mice, suggests that alterations in the NRG1–ErbB4 signalling pathway might result in dysfunctional regulation of synaptic plasticity in an already abnormally developed mature brain. Future studies will be facilitated by animal models that control NRG1–ErbB signalling in space and time to dissect developmental and post-developmental mechanisms. In particular, gain-of-function models that increase NRG1–ErbB4 signalling in the adult brain to mimic pathological findings in patients might be useful.
In addition to NRG1 and ERBB4, many other genes have been associated with schizophrenia, including those that encode catechol-O-methyl transferase (COMT), dysbindin (DTNBP1), regulator of G-protein signalling 4 (RGS4), disrupted-in-schizophrenia 1 (DISC1) and metabotropic glutamate receptor 3 (GRM3, also known as MGLUR3)3, 186. NRG1 activates various intracellular pathways. Does it regulate the expression of these susceptibility genes and/or their function? Conversely, is NRG1–ErbB signalling regulated by any of these genes? These are questions that remain to be addressed in the near future. The answers to these questions, however, could be complicated by recent studies that suggest that NRG2, NRG3 and EGFR might also serve as risk factors for schizophrenia — all of these proteins can stimulate ErbB kinases155. Nevertheless, these are exciting times for schizophrenia research. We anticipate that an improved understanding of the pathogenic mechanisms that are associated with the identified disease-associated genes might eventually lead to the development of more effective therapeutics.
Acknowledgements
We thank C. Lai, M. Salter, B. Li, G. Pitcher and C. Bergson for critical reading of the Review, the anonymous reviewers for their comments and critiques, X. Liu, Y. Tao, X. Li and A. Ting for comments and suggestions, and X. Liu and X. Cao for figure preparation. The work of the authors’ laboratories was supported in part by grants from the National Institute of Mental Health, the National Institute for Neurological Disorders and Stroke and the National Alliance for Research on Schizophrenia and Depression.
References
- 1.Carlsson A, Lindqvist M. Effect of chlorpromazine or haloperidol on formation of 3methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol. Toxicol. 1963;20:140–144. doi: 10.1111/j.1600-0773.1963.tb01730.x. [DOI] [PubMed] [Google Scholar]
- 2.Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J. Psychopharmacol. 1999;13:358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- 3.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol. Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 4.Tan W, et al. Molecular cloning of a brain-specific, developmentally regulated neuregulin 1 (NRG1) isoform and identification of a functional promoter variant associated with schizophrenia. J. Biol. Chem. 2007;282:24343–24351. doi: 10.1074/jbc.M702953200. [DOI] [PubMed] [Google Scholar]
- 5.Falls DL. Neuregulins: functions, forms, and signalling strategies. Exp. Cell Res. 2003;284:14–30. doi: 10.1016/s0014-4827(02)00102-7. [DOI] [PubMed] [Google Scholar]
- 6.Steinthorsdottir V, et al. Multiple novel transcription initiation sites for NRG1. Gene. 2004;342:97–105. doi: 10.1016/j.gene.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Meyer D, et al. Isoform-specific expression and function of neuregulin. Development. 1997;124:3575–3586. doi: 10.1242/dev.124.18.3575. [This study was the first to show that NRG1 isoforms have distinct functions in neural development. Type I NRG1 is required for the generation of neural-crest-derived neurons in the cranial ganglia, whereas Type III NRG1 has an important role in glial-cell development] [DOI] [PubMed] [Google Scholar]
- 8.Harrison PJ, Law AJ. Neuregulin 1 and schizophrenia: genetics, gene expression, and neurobiology. Biol. Psychiatry. 2006;60:132–140. doi: 10.1016/j.biopsych.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Peles E, et al. Isolation of the Neu/HER-2 stimulatory ligand: a 44 kd glycoprotein that induces differentiation of mammary tumour cells. Cell. 1992;69:205–216. doi: 10.1016/0092-8674(92)90131-u. [DOI] [PubMed] [Google Scholar]
- 10.Holmes WE, et al. Identification of heregulin, a specific activator of p185erbB2. Science. 1992;256:1205–1210. doi: 10.1126/science.256.5060.1205. [DOI] [PubMed] [Google Scholar]
- 11.Jessell TM, Siegel RE, Fischbach GD. Induction of acetylcholine receptors on cultured skeletal muscle by a factor extracted from brain and spinal cord. Proc. Natl Acad. Sci. USA. 1979;76:5397–5401. doi: 10.1073/pnas.76.10.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falls DL, Rosen KM, Corfas G, Lane WS, Fischbach GD. ARIA, a protein that stimulates acetylcholine receptor synthesis, is a member of the Neu ligand family. Cell. 1993;72:801–815. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- 13.Raff MC, Abney E, Brockes JP, Hornby-Smith A. Schwann cell growth factors. Cell. 1978;15:813–822. doi: 10.1016/0092-8674(78)90266-0. [DOI] [PubMed] [Google Scholar]
- 14.Lemke GE, Brockes JP. Identification and purification of glial growth factor. J. Neurosci. 1984;4:75–83. doi: 10.1523/JNEUROSCI.04-01-00075.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marchionni MA, et al. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature. 1993;362:312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- 16.Carraway KL, et al. Neuregulin-2, a new ligand of ErbB3/ErbB4-receptor tyrosine kinase. Nature. 1997;387:512–516. doi: 10.1038/387512a0. [DOI] [PubMed] [Google Scholar]
- 17.Fischbach GD, Rosen KM. ARIA: a neuromuscular junction neuregulin. Annu. Rev. Neurosci. 1997;20:429–458. doi: 10.1146/annurev.neuro.20.1.429. [DOI] [PubMed] [Google Scholar]
- 18.Kramer R, et al. Neuregulins with an Ig-like domain are essential for mouse myocardial and neuronal development. Proc. Natl Acad. Sci. USA. 1996;93:4833–4838. doi: 10.1073/pnas.93.10.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loeb JA, Susanto ET, Fischbach GD. The neuregulin precursor proARIA is processed to ARIA after expression on the cell surface by a protein kinase C-enhanced mechanism. Mol. Cell. Neurosci. 1998;11:77–91. doi: 10.1006/mcne.1998.0676. [DOI] [PubMed] [Google Scholar]
- 20.Montero JC, et al. The extracellular linker of pro-neuregulin-α2c is required for efficient sorting and juxtacrine function. Mol. Biol. Cell. 2007;18:380–393. doi: 10.1091/mbc.E06-06-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu X, et al. Bace1 modulates myelination in the central and peripheral nervous system. Nature Neurosci. 2006;9:1520–1525. doi: 10.1038/nn1797. [DOI] [PubMed] [Google Scholar]
- 22.Willem M, et al. Control of peripheral nerve myelination by the β-secretase BACE1. Science. 2006;314:664–666. doi: 10.1126/science.1132341. [DOI] [PubMed] [Google Scholar]
- 23.Yokozeki T, et al. Meltrin β(ADAM19) mediates ectodomain shedding of Neuregulin β1 in the Golgi apparatus: fluorescence correlation spectroscopic observation of the dynamics of ectodomain shedding in living cells. Genes Cells. 2007;12:329–343. doi: 10.1111/j.1365-2443.2007.01060.x. [DOI] [PubMed] [Google Scholar]
- 24.Bao J, Wolpowitz D, Role LW, Talmage DA. Back signalling by the Nrg-1 intracellular domain. J. Cell Biol. 2003;161:1133–1141. doi: 10.1083/jcb.200212085. [This was the first report to indicate a role for NRG1 in backward signalling. Binding of the extracellular domains of ErbB receptors and NRG1 elicits proteolytic release and the translocation of NRG1-ICD to the nucleus] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eilam R, Pinkas-Kramarski R, Ratzkin BJ, Segal M, Yarden Y. Activity-dependent regulation of Neu differentiation factor/neuregulin expression in rat brain. Proc. Natl Acad. Sci. USA. 1998;95:1888–1893. doi: 10.1073/pnas.95.4.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han B, Fischbach GD. Processing of ARIA and release from isolated nerve terminals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1999;354:411–416. doi: 10.1098/rstb.1999.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozaki M, Itoh K, Miyakawa Y, Kishida H, Hashikawa T. Protein processing and releases of neuregulin-1 are regulated in an activity-dependent manner. J. Neurochem. 2004;91:176–188. doi: 10.1111/j.1471-4159.2004.02719.x. [DOI] [PubMed] [Google Scholar]
- 28.Bublil EM, Yarden Y. The EGF receptor family: spearheading a merger of signalling and therapeutics. Curr. Opin. Cell Biol. 2007;19:124–134. doi: 10.1016/j.ceb.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Tzahar E, et al. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol. Cell. Biol. 1996;16:5276–5287. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guy PM, Platko JV, Cantley LC, Cerione RA, Carraway KL. Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proc. Natl Acad. Sci. USA. 1994;91:8132–8136. doi: 10.1073/pnas.91.17.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerlai R, Pisacane P, Erickson S. Heregulin, but not ErbB2 or ErbB3, heterozygous mutant mice exhibit hyperactivity in multiple behavioural tasks. Behav. Brain Res. 2000;109:219–227. doi: 10.1016/s0166-4328(99)00175-8. [This report was the first to link abnormal NRG1 signalling to behavioural deficits. Nrg1 hypomorphs, but not Erbb2 or Erbb3 hypomorphs, showed consistent hyperactivity in various behavioural tests] [DOI] [PubMed] [Google Scholar]
- 32.Gu Z, Jiang Q, Fu AK, Ip NY, Yan Z. Regulation of NMDA receptors by neuregulin signalling in PFC. J. Neurosci. 2005;25:4974–4984. doi: 10.1523/JNEUROSCI.1086-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Tao YM, Woo RS, Xiong WC, Mei L. Stimulated ErbB4 internalization is necessary for neuregulin signalling in neurons. Biochem. Biophys. Res. Commun. 2007;354:505–510. doi: 10.1016/j.bbrc.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang XL, Huang YZ, Xiong WC, Mei L. Neuregulin-induced expression of the acetylcholine receptor requires endocytosis of ErbB receptors. Mol. Cell. Neurosci. 2005;28:335–346. doi: 10.1016/j.mcn.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Si J, Wang Q, Mei L. Essential roles of c-JUN and c-JUN N-terminal kinase (JNK) in neuregulin-increased expression of the acetylcholine receptor ε-subunit. J. Neurosci. 1999;19:8489–8508. doi: 10.1523/JNEUROSCI.19-19-08498.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu AK, et al. Cdk5 is involved in neuregulin-induced AChR expression at the neuromuscular junction. Nature Neurosci. 2001;4:374–381. doi: 10.1038/86019. [DOI] [PubMed] [Google Scholar]
- 37.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nature Rev. Mol. Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 38.Bjarnadottir M, et al. Neuregulin1 (NRG1) signalling through Fyn modulates NMDA receptor phosphorylation: differential synaptic function in NRG1+/−knock-outs compared with wild-type mice. J. Neurosci. 2007;27:4519–4529. doi: 10.1523/JNEUROSCI.4314-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ni CY, Murphy MP, Golde TE, Carpenter G. γ-secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–2181. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 40.Lee HJ, et al. Presenilin-dependent γ-secretase-like intramembrane cleavage of ErbB4. J. Biol. Chem. 2002;277:6318–6323. doi: 10.1074/jbc.M110371200. [DOI] [PubMed] [Google Scholar]
- 41.Sardi SP, Murtie J, Koirala S, Patten BA, Corfas G. Presenilin-dependent ErbB4 nuclear signalling regulates the timing of astrogenesis in the developing brain. Cell. 2006;127:185–197. doi: 10.1016/j.cell.2006.07.037. [This report showed evidence that ErbB4-ICD, which is released by presenilin-dependent cleavage, regulates gene transcription and cell fate by forming a complex with other regulators] [DOI] [PubMed] [Google Scholar]
- 42.Fox IJ, Kornblum HI. Developmental profile of ErbB receptors in murine central nervous system: implications for functional interactions. J. Neurosci. Res. 2005;79:584–597. doi: 10.1002/jnr.20381. [DOI] [PubMed] [Google Scholar]
- 43.Bao J, et al. Activity-dependent transcription regulation of PSD-95 by neuregulin-1 and Eos. Nature Neurosci. 2004;7:1250–1258. doi: 10.1038/nn1342. [DOI] [PubMed] [Google Scholar]
- 44.Harrison PJ. Schizophrenia: a disorder of neurodevelopment? Curr. Opin. Neurobiol. 1997;7:285–289. doi: 10.1016/s0959-4388(97)80018-9. [DOI] [PubMed] [Google Scholar]
- 45.Corfas G, Rosen KM, Aratake H, Krauss R, Fischbach GD. Differential expression of ARIA isoforms in the rat brain. Neuron. 1995;14:103–115. doi: 10.1016/0896-6273(95)90244-9. [DOI] [PubMed] [Google Scholar]
- 46.Steiner H, Blum M, Kitai ST, Fedi P. Differential expression of ErbB3 and ErbB4 neuregulin receptors in dopamine neurons and forebrain areas of the adult rat. Exp. Neurol. 1999;159:494–503. doi: 10.1006/exnr.1999.7163. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y, Ford BD, Mann MA, Fischbach GD. Neuregulin-1 increases the proliferation of neuronal progenitors from embryonic neural stem cells. Dev. Biol. 2005;283:437–445. doi: 10.1016/j.ydbio.2005.04.038. [DOI] [PubMed] [Google Scholar]
- 48.Gassmann M, et al. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–394. doi: 10.1038/378390a0. [This report was the first to demonstrate that ErbB4 is an essential in vivo regulator of the development of the CNS] [DOI] [PubMed] [Google Scholar]
- 49.Lee KF, et al. Requirement for neuregulin receptor erbB2 in neural and cardiac development. Nature. 1995;378:394–398. doi: 10.1038/378394a0. [DOI] [PubMed] [Google Scholar]
- 50.Erickson SL, et al. ErbB3 is required for normal cerebellar and cardiac development: a comparison with ErbB2-and heregulin-deficient mice. Development. 1997;124:4999–5011. doi: 10.1242/dev.124.24.4999. [DOI] [PubMed] [Google Scholar]
- 51.Wolpowitz D, et al. Cysteine-rich domain isoforms of the Neuregulin-1 gene are required for maintenance of peripheral synapses. Neuron. 2000;25:79–91. doi: 10.1016/s0896-6273(00)80873-9. [DOI] [PubMed] [Google Scholar]
- 52.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 53.Golding JP, Trainor P, Krumlauf R, Gassmann M. Defects in pathfinding by cranial neural crest cells in mice lacking the neuregulin receptor ErbB4. Nature Cell Biol. 2000;2:103–109. doi: 10.1038/35000058. [DOI] [PubMed] [Google Scholar]
- 54.Rio C, Rieff HI, Qi P, Khurana TS, Corfas G. Neuregulin and erbB receptors play a critical role in neuronal migration. Neuron. 1997;19:39–50. doi: 10.1016/s0896-6273(00)80346-3. [DOI] [PubMed] [Google Scholar]
- 55.Anton ES, Marchionni MA, Lee KF, Rakic P. Role of GGF/neuregulin signalling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development. 1997;124:3501–3510. doi: 10.1242/dev.124.18.3501. [DOI] [PubMed] [Google Scholar]
- 56.Gierdalski M, Sardi SP, Corfas G, Juliano SL. Endogenous neuregulin restores radial glia in a (ferret) model of cortical dysplasia. J. Neurosci. 2005;25:8498–8504. doi: 10.1523/JNEUROSCI.1476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schmid RS, et al. Neuregulin 1-erbB2 signalling is required for the establishment of radial glia and their transformation into astrocytes in cerebral cortex. Proc. Natl Acad. Sci. USA. 2003;100:4251–4256. doi: 10.1073/pnas.0630496100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yau HJ, Wang HF, Lai C, Liu FC. Neural development of the neuregulin receptor ErbB4 in the cerebral cortex and the hippocampus: preferential expression by interneurons tangentially migrating from the ganglionic eminences. Cereb. Cortex. 2003;13:252–264. doi: 10.1093/cercor/13.3.252. [DOI] [PubMed] [Google Scholar]
- 59.Flames N, et al. Short- and long-range attraction of cortical GABAergic interneurons by neuregulin-1. Neuron. 2004;44:251–261. doi: 10.1016/j.neuron.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 60.Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- 61.Anton ES, et al. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nature Neurosci. 2004;7:1319–1328. doi: 10.1038/nn1345. [DOI] [PubMed] [Google Scholar]
- 62.Gerecke KM, Wyss JM, Carroll SL. Neuregulin-1 β induces neurite extension and arborization in cultured hippocampal neurons. Mol. Cell. Neurosci. 2004;27:379–393. doi: 10.1016/j.mcn.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 63.Bermingham-McDonogh O, McCabe KL, Reh TA. Effects of GGF/neuregulins on neuronal survival and neurite outgrowth correlate with erbB2/neu expression in developing rat retina. Development. 1996;122:1427–1438. doi: 10.1242/dev.122.5.1427. [DOI] [PubMed] [Google Scholar]
- 64.Rieff HI, et al. Neuregulin induces GABAA receptor subunit expression and neurite outgrowth in cerebellar granule cells. J. Neurosci. 1999;19:10757–10766. doi: 10.1523/JNEUROSCI.19-24-10757.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lopez-Bendito G, et al. Tangential neuronal migration controls axon guidance: a role for neuregulin-1 in thalamocortical axon navigation. Cell. 2006;125:127–142. doi: 10.1016/j.cell.2006.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gamett DC, Cerione RA. Oncogenically activated or ligand-stimulated neu kinase stimulates neurite outgrowth in PC12 cells. FEBS Lett. 1994;351:335–339. doi: 10.1016/0014-5793(94)00855-8. [DOI] [PubMed] [Google Scholar]
- 67.Vaskovsky A, Lupowitz Z, Erlich S, Pinkas-Kramarski R. ErbB-4 activation promotes neurite outgrowth in PC12 cells. J. Neurochem. 2000;74:979–987. doi: 10.1046/j.1471-4159.2000.0740979.x. [DOI] [PubMed] [Google Scholar]
- 68.Pinkas-Kramarski R, et al. Differential expression of NDF/neuregulin receptors ErbB-3 and ErbB-4 and involvement in inhibition of neuronal differentiation. Oncogene. 1997;15:2803–2815. doi: 10.1038/sj.onc.1201466. [DOI] [PubMed] [Google Scholar]
- 69.Maroof AM, Anderson SA. Off on a tangent: thalamocortical axons traverse a permissive corridor across the basal telencephalon. Neuron. 2006;50:185–188. doi: 10.1016/j.neuron.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 70.Ho WH, Armanini MP, Nuijens A, Phillips HS, Osheroff PL. Sensory and motor neuron-derived factor. A novel heregulin variant highly expressed in sensory and motor neurons. J. Biol. Chem. 1995;270:14523–14532. doi: 10.1074/jbc.270.24.14523. [DOI] [PubMed] [Google Scholar]
- 71.Garratt AN, Britsch S, Birchmeier C. Neuregulin, a factor with many functions in the life of a schwann cell. Bioessays. 2000;22:987–996. doi: 10.1002/1521-1878(200011)22:11<987::AID-BIES5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 72.Nave KA, Salzer JL. Axonal regulation of myelination by neuregulin 1. Curr. Opin. Neurobiol. 2006;16:492–500. doi: 10.1016/j.conb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 73.Michailov GV, et al. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- 74.Taveggia C, et al. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shah NM, Marchionni MA, Isaacs I, Stroobant P, Anderson DJ. Glial growth factor restricts mammalian neural crest stem cells to a glial fate. Cell. 1994;77:349–360. doi: 10.1016/0092-8674(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 76.Lai C, Feng L. Implication of γ-secretase in neuregulin-induced maturation of oligodendrocytes. Biochem. Biophys. Res. Commun. 2004;314:535–542. doi: 10.1016/j.bbrc.2003.12.131. [DOI] [PubMed] [Google Scholar]
- 77.Dong Z, et al. Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation, and maturation of rat Schwann cell precursors. Neuron. 1995;15:585–596. doi: 10.1016/0896-6273(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 78.Grinspan JB, Marchionni MA, Reeves M, Coulaloglou M, Scherer SS. Axonal interactions regulate Schwann cell apoptosis in developing peripheral nerve: neuregulin receptors and the role of neuregulins. J. Neurosci. 1996;16:6107–6118. doi: 10.1523/JNEUROSCI.16-19-06107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Winseck AK, et al. In vivo analysis of Schwann cell programmed cell death in the embryonic chick: regulation by axons and glial growth factor. J. Neurosci. 2002;22:4509–4521. doi: 10.1523/JNEUROSCI.22-11-04509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mahanthappa NK, Anton ES, Matthew WD. Glial growth factor 2, a soluble neuregulin, directly increases Schwann cell motility and indirectly promotes neurite outgrowth. J. Neurosci. 1996;16:4673–4683. doi: 10.1523/JNEUROSCI.16-15-04673.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scherer SS, et al. Connexin32 is a myelin-related protein in the PNS and CNS. J. Neurosci. 1995;15:8281–8294. doi: 10.1523/JNEUROSCI.15-12-08281.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Trachtenberg JT, Thompson WJ. Schwann cell apoptosis at developing neuromuscular junctions is regulated by glial growth factor. Nature. 1996;379:174–177. doi: 10.1038/379174a0. [DOI] [PubMed] [Google Scholar]
- 83.Adlkofer K, Lai C. Role of neuregulins in glial cell development. Glia. 2000;29:104–111. doi: 10.1002/(sici)1098-1136(20000115)29:2<104::aid-glia2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 84.Riethmacher D, et al. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature. 1997;389:725–730. doi: 10.1038/39593. [DOI] [PubMed] [Google Scholar]
- 85.Woldeyesus MT, et al. Peripheral nervous system defects in erbB2 mutants following genetic rescue of heart development. Genes Dev. 1999;13:2538–2548. doi: 10.1101/gad.13.19.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen S, et al. Neuregulin 1-erbB signalling is necessary for normal myelination and sensory function. J. Neurosci. 2006;26:3079–3086. doi: 10.1523/JNEUROSCI.3785-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen S, et al. Disruption of ErbB receptor signalling in adult non-myelinating Schwann cells causes progressive sensory loss. Nature Neurosci. 2003;6:1186–1193. doi: 10.1038/nn1139. [DOI] [PubMed] [Google Scholar]
- 88.Fernandez PA, et al. Evidence that axon-derived neuregulin promotes oligodendrocyte survival in the developing rat optic nerve. Neuron. 2000;28:81–90. doi: 10.1016/s0896-6273(00)00087-8. [DOI] [PubMed] [Google Scholar]
- 89.Canoll PD, et al. GGF/neuregulin is a neuronal signal that promotes the proliferation and survival and inhibits the differentiation of oligodendrocyte progenitors. Neuron. 1996;17:229–243. doi: 10.1016/s0896-6273(00)80155-5. [DOI] [PubMed] [Google Scholar]
- 90.Flores AI, et al. Akt-mediated survival of oligodendrocytes induced by neuregulins. J. Neurosci. 2000;20:7622–7630. doi: 10.1523/JNEUROSCI.20-20-07622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vartanian T, Corfas G, Li Y, Fischbach GD, Stefansson K. A role for the acetylcholine receptor-inducing protein ARIA in oligodendrocyte development. Proc. Natl Acad. Sci. USA. 1994;91:11626–11630. doi: 10.1073/pnas.91.24.11626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vartanian T, Fischbach G, Miller R. Failure of spinal cord oligodendrocyte development in mice lacking neuregulin. Proc. Natl Acad. Sci. USA. 1999;96:731–735. doi: 10.1073/pnas.96.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim JY, Sun Q, Oglesbee M, Yoon SO. The role of ErbB2 signalling in the onset of terminal differentiation of oligodendrocytes in vivo. J. Neurosci. 2003;23:5561–5571. doi: 10.1523/JNEUROSCI.23-13-05561.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roy K, et al. Loss of erbB signalling in oligodendrocytes alters myelin and dopaminergic function, a potential mechanism for neuropsychiatric disorders. Proc. Natl Acad. Sci. USA. 2007;104:8131–8136. doi: 10.1073/pnas.0702157104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Park SK, Solomon D, Vartanian T. Growth factor control of CNS myelination. Dev. Neurosci. 2001;23:327–337. doi: 10.1159/000048716. [DOI] [PubMed] [Google Scholar]
- 96.Schmucker J, et al. erbB3 is dispensable for oligodendrocyte development in vitro and in vivo. Glia. 2003;44:67–75. doi: 10.1002/glia.10275. [DOI] [PubMed] [Google Scholar]
- 97.Si J, Miller DS, Mei L. Identification of an element required for acetylcholine receptor-inducing activity (ARIA)-induced expression of the acetylcholine receptor epsilon subunit gene. J. Biol. Chem. 1997;272:10367–10371. doi: 10.1074/jbc.272.16.10367. [DOI] [PubMed] [Google Scholar]
- 98.Si J, Luo Z, Mei L. Induction of acetylcholine receptor gene expression by ARIA requires activation of mitogen-activated protein kinase. J. Biol. Chem. 1996;271:19752–19759. doi: 10.1074/jbc.271.33.19752. [DOI] [PubMed] [Google Scholar]
- 99.Altiok N, Altiok S, Changeux JP. Heregulin-stimulated acetylcholine receptor gene expression in muscle: requirement for MAP kinase and evidence for a parallel inhibitory pathway independent of electrical activity. EMBO J. 1997;16:717–725. doi: 10.1093/emboj/16.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tansey MG, Chu GC, Merlie JP. ARIA/HRG regulates AChR epsilon subunit gene expression at the neuromuscular synapse via activation of phosphatidylinositol 3-kinase and Ras/MAPK pathway. J. Cell Biol. 1996;134:465–476. doi: 10.1083/jcb.134.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sandrock AW, et al. Maintenance of acetylcholine receptor number by neuregulins at the neuromuscular junction in vivo. Science. 1997;276:599–603. doi: 10.1126/science.276.5312.599. [DOI] [PubMed] [Google Scholar]
- 102.Escher P, et al. Synapses form in skeletal muscles lacking neuregulin receptors. Science. 2005;308:1920–1923. doi: 10.1126/science.1108258. [DOI] [PubMed] [Google Scholar]
- 103.Lin W, et al. Aberrant development of motor axons and neuromuscular synapses in erbB2-deficient mice. Proc. Natl Acad. Sci. USA. 2000;97:1299–1304. doi: 10.1073/pnas.97.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morris JK, et al. Rescue of the cardiac defect in ErbB2 mutant mice reveals essential roles of ErbB2 in peripheral nervous system development. Neuron. 1999;23:273–283. doi: 10.1016/s0896-6273(00)80779-5. [DOI] [PubMed] [Google Scholar]
- 105.Hippenmeyer S, et al. A role for neuregulin1 signalling in muscle spindle differentiation. Neuron. 2002;36:1035–1049. doi: 10.1016/s0896-6273(02)01101-7. [DOI] [PubMed] [Google Scholar]
- 106.Liu Y, Ford B, Mann MA, Fischbach GD. Neuregulins increase α7 nicotinic acetylcholine receptors and enhance excitatory synaptic transmission in GABAergic interneurons of the hippocampus. J. Neurosci. 2001;21:5660–5669. doi: 10.1523/JNEUROSCI.21-15-05660.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yang X, Kuo Y, Devay P, Yu C, Role L. A cysteine-rich isoform of neuregulin controls the level of expression of neuronal nicotinic receptor channels during synaptogenesis. Neuron. 1998;20:255–270. doi: 10.1016/s0896-6273(00)80454-7. [DOI] [PubMed] [Google Scholar]
- 108.Okada M, Corfas G. Neuregulin1 downregulates postsynaptic GABAA receptors at the hippocampal inhibitory synapse. Hippocampus. 2004;14:337–344. doi: 10.1002/hipo.10185. [DOI] [PubMed] [Google Scholar]
- 109.Ozaki M, Sasner M, Yano R, Lu HS, Buonanno A. Neuregulin-β induces expression of an NMDA-receptor subunit. Nature. 1997;390:691–694. doi: 10.1038/37795. [DOI] [PubMed] [Google Scholar]
- 110.Stefansson H, et al. Neuregulin 1 and susceptibility to schizophrenia. Am. J. Hum. Genet. 2002;71:877–892. doi: 10.1086/342734. [This was the first report to identify SNPs and microsatellites in NRG1 as being associated with schizophrenia] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor ErbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [In this study, the roles of NRG1 and ErbB4 in the formation and maturation of glutamatergic synapses were investigated by gain and loss of function in cultured postnatal hippocampal slices. Evidence was shown that NRG1–ErbB4 signalling is essential for synapse maturation and plasticity] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Woo RS, et al. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [This paper provided evidence that NRG1 enhances activity-dependent GABA release through ErbB4 that is present in GABAergic terminals] [DOI] [PubMed] [Google Scholar]
- 113.Lai C, Lemke G. An extended family of protein-tyrosine kinase genes differentially expressed in the vertebrate nervous system. Neuron. 1991;6:691–704. doi: 10.1016/0896-6273(91)90167-x. [DOI] [PubMed] [Google Scholar]
- 114.Harrison PJ. The hippocampus in schizophrenia: a review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl.) 2004;174:151–162. doi: 10.1007/s00213-003-1761-y. [DOI] [PubMed] [Google Scholar]
- 115.Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of γ-aminobutyric acid and glutamate alterations. Arch. Neurol. 2006;63:1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- 116.Moghaddam B. Targeting metabotropic glutamate receptors for treatment of the cognitive symptoms of schizophrenia. Psychopharmacology (Berl.) 2004;174:39–44. doi: 10.1007/s00213-004-1792-z. [DOI] [PubMed] [Google Scholar]
- 117.Tsai G, Coyle JT. Glutamatergic mechanisms in schizophrenia. Annu. Rev. Pharmacol. Toxicol. 2002;42:165–179. doi: 10.1146/annurev.pharmtox.42.082701.160735. [DOI] [PubMed] [Google Scholar]
- 118.Huang YZ, et al. Regulation of neuregulin signalling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26:443–455. doi: 10.1016/s0896-6273(00)81176-9. [This paper and reference 119 were the first to show that ErbB4 interacts and colocalizes with PSD95 in cultured neurons. In addition, this paper provided the first evidence that NRG1 suppresses LTP in the hippocampus and that interaction with PSD95 facilitates NRG1 signalling] [DOI] [PubMed] [Google Scholar]
- 119.Garcia RAG, Vasudevan K, Buonanno A. The neuregulin receptor ErbB4 interacts with PDZ-containing proteins at neuronal synapses. Proc. Natl Acad. Sci. USA. 2000;97:3596–3601. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ma L, et al. Ligand-dependent recruitment of the ErbB4 signalling complex into neuronal lipid rafts. J. Neurosci. 2003;23:3164–3175. doi: 10.1523/JNEUROSCI.23-08-03164.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pitcher GM, Beggs S, Woo RS, Mei L, Salter MW. ErbB4 is a suppressor of long-term potentiation in the adult hippocampus. Neuroreport. 2008;19:139–143. doi: 10.1097/WNR.0b013e3282f3da10. [This paper used pharmacological and genetic approaches to demonstrate the essential role of ErbB4 in the suppression of LTP by NRG1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kwon OB, Longart M, Vullhorst D, Hoffman DA, Buonanno A. Neuregulin-1 reverses long-term potentiation at CA1 hippocampal synapses. J. Neurosci. 2005;25:9378–9383. doi: 10.1523/JNEUROSCI.2100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Role LW, Talmage DA. Neurobiology: new order for thought disorders. Nature. 2007;448:263–265. doi: 10.1038/448263a. [DOI] [PubMed] [Google Scholar]
- 124.Iyengar SS, Mott DD. Neuregulin blocks synaptic strengthening after epileptiform activity in the rat hippocampus. Brain Res. 2008 Feb 29; doi: 10.1016/j.brainres.2008.02.045. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 125.Wang YT, Salter MW. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- 126.Yu XM, Askalan R, Keil GJ, Salter MW. NMDA channel regulation by channel-associated protein tyrosine kinase Src. Science. 1997;275:674–678. doi: 10.1126/science.275.5300.674. [DOI] [PubMed] [Google Scholar]
- 127.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 128.Roysommuti S, Carroll SL, Wyss JM. Neuregulin-1 β modulates in vivo entorhinal-hippocampal synaptic transmission in adult rats. Neuroscience. 2003;121:779–785. doi: 10.1016/s0306-4522(03)00503-7. [DOI] [PubMed] [Google Scholar]
- 129.Fischbach GD. NRG1 and synaptic function in the CNS. Neuron. 2007;54:495–497. doi: 10.1016/j.neuron.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 130.Belan PV, Kostyuk PG. Glutamate-receptor-induced modulation of GABAergic synaptic transmission in the hippocampus. Pflugers Arch. 2002;444:26–37. doi: 10.1007/s00424-002-0783-3. [DOI] [PubMed] [Google Scholar]
- 131.Xie F, Raetzman LT, Siegel RE. Neuregulin induces GABAA receptor β2 subunit expression in cultured rat cerebellar granule neurons by activating multiple signalling pathways. J. Neurochem. 2004;90:1521–1529. doi: 10.1111/j.1471-4159.2004.02685.x. [DOI] [PubMed] [Google Scholar]
- 132.Cameron JS, Dryer L, Dryer SE. β-neuregulin-1 is required for the in vivo development of functional Ca2+-activated K+ channels in parasympathetic neurons. Proc. Natl Acad. Sci. USA. 2001;98:2832–2836. doi: 10.1073/pnas.041394098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Subramony P, Dryer SE. Neuregulins stimulate the functional expression of Ca2+-activated K+ channels in developing chicken parasympathetic neurons. Proc. Natl Acad. Sci. USA. 1997;94:5934–5938. doi: 10.1073/pnas.94.11.5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li BS, et al. Cyclin-dependent kinase-5 is involved in neuregulin-dependent activation of phosphatidylinositol 3-kinase and Akt activity mediating neuronal survival. J. Biol. Chem. 2003;278:35702–35709. doi: 10.1074/jbc.M302004200. [DOI] [PubMed] [Google Scholar]
- 135.Ricart K, et al. Interactions between β-neuregulin and neurotrophins in motor neuron apoptosis. J. Neurochem. 2006;97:222–233. doi: 10.1111/j.1471-4159.2006.03739.x. [DOI] [PubMed] [Google Scholar]
- 136.Zhang L, et al. Neurotrophic and neuroprotective effects of the neuregulin glial growth factor-2 on dopaminergic neurons in rat primary midbrain cultures. J. Neurochem. 2004;91:1358–1368. doi: 10.1111/j.1471-4159.2004.02817.x. [DOI] [PubMed] [Google Scholar]
- 137.Stankovic K, et al. Survival of adult spiral ganglion neurons requires erbB receptor signalling in the inner ear. J. Neurosci. 2004;24:8651–8661. doi: 10.1523/JNEUROSCI.0733-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Goldshmit Y, Erlich S, Pinkas-Kramarski R. Neuregulin rescues PC12-ErbB4 cells from cell death induced by H2O2. Regulation of reactive oxygen species levels by phosphatidylinositol 3-kinase. J. Biol. Chem. 2001;276:46379–46385. doi: 10.1074/jbc.M105637200. [DOI] [PubMed] [Google Scholar]
- 139.Li Y, et al. Neuroprotection by neuregulin-1 in a rat model of permanent focal cerebral ischemia. Brain Res. 2007;1184:277–283. doi: 10.1016/j.brainres.2007.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shyu WC, et al. Neuregulin-1 reduces ischemia-induced brain damage in rats. Neurobiol. Aging. 2004;25:935–944. doi: 10.1016/j.neurobiolaging.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 141.Croslan DR, et al. Neuroprotective effects of neuregulin-1 on B35 neuronal cells following ischemia. Brain Res. 2008 Mar 4; doi: 10.1016/j.brainres.2008.02.059. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Badner JA, Gershon ES. Meta-analysis of whole-genome linkage scans of bipolar disorder and schizophrenia. Mol. Psychiatry. 2002;7:405–411. doi: 10.1038/sj.mp.4001012. [DOI] [PubMed] [Google Scholar]
- 143.Lewis CM, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: schizophrenia. Am. J. Hum. Genet. 2003;73:34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yang JZ, et al. Association study of neuregulin 1 gene with schizophrenia. Mol. Psychiatry. 2003;8:706–709. doi: 10.1038/sj.mp.4001377. [Using the polymerase-chain-reaction-based restriction-fragment length polymorphism method and denaturing high-performance liquid chromatography, this independent study identified two additional NRG1 SNPs that are associated with schizophrenia in 246 Chinese Han schizophrenic family trios] [DOI] [PubMed] [Google Scholar]
- 145.Kim JW, et al. Linkage and association of schizophrenia with genetic variations in the locus of neuregulin 1 in Korean population. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006;141:281–286. doi: 10.1002/ajmg.b.30209. [DOI] [PubMed] [Google Scholar]
- 146.Hall D, Gogos JA, Karayiorgou M. The contribution of three strong candidate schizophrenia susceptibility genes in demographically distinct populations. Genes Brain Behav. 2004;3:240–248. doi: 10.1111/j.1601-183X.2004.00078.x. [DOI] [PubMed] [Google Scholar]
- 147.Stefansson H, et al. Association of neuregulin 1 with schizophrenia confirmed in a Scottish population. Am. J. Hum. Genet. 2003;72:83–87. doi: 10.1086/345442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Williams NM, et al. Support for genetic variation in neuregulin 1 and susceptibility to schizophrenia. Mol. Psychiatry. 2003;8:485–487. doi: 10.1038/sj.mp.4001348. [DOI] [PubMed] [Google Scholar]
- 149.Bakker SC, et al. Neuregulin 1: genetic support for schizophrenia subtypes. Mol. Psychiatry. 2004;9:1061–1063. doi: 10.1038/sj.mp.4001564. [DOI] [PubMed] [Google Scholar]
- 150.Li T, et al. Identification of a novel neuregulin 1 at-risk haplotype in Han schizophrenia Chinese patients, but no association with the Icelandic/Scottish risk haplotype. Mol. Psychiatry. 2004;9:698–704. doi: 10.1038/sj.mp.4001485. [DOI] [PubMed] [Google Scholar]
- 151.Tang JX, et al. Polymorphisms within 5' end of the Neuregulin 1 gene are genetically associated with schizophrenia in the Chinese population. Mol. Psychiatry. 2004;9:11–12. doi: 10.1038/sj.mp.4001436. [DOI] [PubMed] [Google Scholar]
- 152.Petryshen TL, et al. Support for involvement of neuregulin 1 in schizophrenia pathophysiology. Mol. Psychiatry. 2005;10:366–374, 328. doi: 10.1038/sj.mp.4001608. [DOI] [PubMed] [Google Scholar]
- 153.Corvin AP, et al. Confirmation and refinement of an ‘at-risk’ haplotype for schizophrenia suggests the EST cluster, Hs.97362, as a potential susceptibility gene at the Neuregulin-1 locus. Mol. Psychiatry. 2004;9:208–213. doi: 10.1038/sj.mp.4001412. [DOI] [PubMed] [Google Scholar]
- 154.Zhao X, et al. A case control and family based association study of the neuregulin1 gene and schizophrenia. J. Med. Genet. 2004;41:31–34. doi: 10.1136/jmg.2003.014977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Benzel I, et al. Interactions among genes in the ErbB-Neuregulin signalling network are associated with increased susceptibility to schizophrenia. Behav. Brain Funct. 2007;3:31. doi: 10.1186/1744-9081-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lachman HM, et al. Analysis of polymorphisms in AT-rich domains of neuregulin 1 gene in schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006;141:102–109. doi: 10.1002/ajmg.b.30242. [DOI] [PubMed] [Google Scholar]
- 157.Law AJ, et al. Neuregulin 1 transcripts are differentially expressed in schizophrenia and regulated by 5' SNPs associated with the disease. Proc. Natl Acad. Sci. USA. 2006;103:6747–6752. doi: 10.1073/pnas.0602002103. [In this study, Type I NRG1 mRNA was found to be upregulated in the hippocampus of patients with schizophrenia, suggesting that the association of NRG1 with schizophrenia might be mediated by altered expression of the gene] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Silberberg G, Darvasi A, Pinkas-Kramarski R, Navon R. The involvement of ErbB4 with schizophrenia: association and expression studies. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006;141:142–148. doi: 10.1002/ajmg.b.30275. [This paper compared the expression of ErbB4 isoforms in the dorsolateral prefrontal cortex (DLPFC) of controls and patients with schizophrenia and showed that the CYT-1 and JMa isoforms are overexpressed at the mRNA level in the DLPFC of schizophrenia patients] [DOI] [PubMed] [Google Scholar]
- 159.Law AJ, Kleinman JE, Weinberger DR, Weickert CS. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum. Mol. Genet. 2007;16:129–141. doi: 10.1093/hmg/ddl449. [This paper studied the expression of ErbB4 isoforms in the DLPFC and hippocampus of controls and patients with schizophrenia. The CYT-1 and JMa ErbB4 isoforms were increased in the DLPFC but not the hippocampus of the patients. This observation suggests that dysregulation of the splice-variant-specific expression of ErbB4 might be region-specific] [DOI] [PubMed] [Google Scholar]
- 160.Hall J, et al. A neuregulin 1 variant associated with abnormal cortical function and psychotic symptoms. Nature Neurosci. 2006;9:1477–1478. doi: 10.1038/nn1795. [DOI] [PubMed] [Google Scholar]
- 161.Thiselton DL, et al. No evidence for linkage or association of neuregulin-1 (NRG1) with disease in the Irish study of high-density schizophrenia families (ISHDSF). Mol. Psychiatry. 2004;9:777–783. doi: 10.1038/sj.mp.4001530. [DOI] [PubMed] [Google Scholar]
- 162.Iwata N, et al. No association with the neuregulin 1 haplotype to Japanese schizophrenia. Mol. Psychiatry. 2004;9:126–127. doi: 10.1038/sj.mp.4001456. [DOI] [PubMed] [Google Scholar]
- 163.Rosa A, et al. Family-based association study of neuregulin-1 gene and psychosis in a Spanish sample. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007;144:954–957. doi: 10.1002/ajmg.b.30511. [DOI] [PubMed] [Google Scholar]
- 164.Walss-Bass C, et al. A novel missense mutation in the transmembrane domain of neuregulin 1 is associated with schizophrenia. Biol. Psychiatry. 2006;60:548–553. doi: 10.1016/j.biopsych.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 165.Hong LE, Wonodi I, Stine OC, Mitchell BD, Thaker GK. Evidence of missense mutations on the neuregulin 1 gene affecting function of prepulse inhibition. Biol. Psychiatry. 2007;63:17–23. doi: 10.1016/j.biopsych.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lin HF, et al. Neuregulin 1 gene and variations in perceptual aberration of schizotypal personality in adolescents. Psychol. Med. 2005;35:1589–1598. doi: 10.1017/S0033291705005957. [DOI] [PubMed] [Google Scholar]
- 167.Norton N, et al. Evidence that interaction between neuregulin 1 and its receptor erbB4 increases susceptibility to schizophrenia. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006;141:96–101. doi: 10.1002/ajmg.b.30236. [DOI] [PubMed] [Google Scholar]
- 168.Nicodemus KK, et al. Further evidence for association between ErbB4 and schizophrenia and influence on cognitive intermediate phenotypes in healthy controls. Mol. Psychiatry. 2006;11:1062–1065. doi: 10.1038/sj.mp.4001878. [DOI] [PubMed] [Google Scholar]
- 169.Walsh T, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [This study identified ERBB4 as one of the genes that is disrupted by the microdeletions and microduplications that are associated with schizophrenia] [DOI] [PubMed] [Google Scholar]
- 170.Arguello PA, Gogos JA. Modeling madness in mice: one piece at a time. Neuron. 2006;52:179–196. doi: 10.1016/j.neuron.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 171.Golub MS, Germann SL, Lloyd KC. Behavioral characteristics of a nervous system-specific erbB4 knock-out mouse. Behav. Brain Res. 2004;153:159–170. doi: 10.1016/j.bbr.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 172.O'Tuathaigh CM, et al. Phenotypic characterization of spatial cognition and social behaviour in mice with ‘knockout’ of the schizophrenia risk gene neuregulin 1. Neuroscience. 2007;147:18–27. doi: 10.1016/j.neuroscience.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 173.Rimer M, Barrett DW, Maldonado MA, Vock VM, Gonzalez-Lima F. Neuregulin-1 immunoglobulin-like domain mutant mice: clozapine sensitivity and impaired latent inhibition. Neuroreport. 2005;16:271–275. doi: 10.1097/00001756-200502280-00014. [DOI] [PubMed] [Google Scholar]
- 174.Thuret S, et al. The neuregulin receptor, ErbB4, is not required for normal development and adult maintenance of the substantia nigra pars compacta. J. Neurochem. 2004;91:1302–1311. doi: 10.1111/j.1471-4159.2004.02809.x. [DOI] [PubMed] [Google Scholar]
- 175.Savonenko AV, et al. Alteration of BACE1-dependent NRG1/ErbB4 signalling and schizophrenia-like phenotypes in BACE1-null mice. Proc. Natl Acad. Sci. USA. 2008;105:5585–5590. doi: 10.1073/pnas.0710373105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Prevot V, et al. Normal female sexual development requires neuregulin-erbB receptor signalling in hypothalamic astrocytes. J. Neurosci. 2003;23:230–239. doi: 10.1523/JNEUROSCI.23-01-00230.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lipska BK. Using animal models to test a neurodevelopmental hypothesis of schizophrenia. J. Psychiatry Neurosci. 2004;29:282–286. [PMC free article] [PubMed] [Google Scholar]
- 178.Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu. Rev. Neurosci. 2002;25:409–432. doi: 10.1146/annurev.neuro.25.112701.142754. [DOI] [PubMed] [Google Scholar]
- 179.Hashimoto R, et al. Expression analysis of neuregulin-1 in the dorsolateral PFC in schizophrenia. Mol. Psychiatry. 2004;9:299–307. doi: 10.1038/sj.mp.4001434. [This study was the first to show that Type I NRG1 expression is increased in the DLPFC of patients with schizophrenia and positively correlates with antipsychotic medication dosage] [DOI] [PubMed] [Google Scholar]
- 180.Hakak Y, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc. Natl Acad. Sci. USA. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Sugai T, et al. Prefrontal abnormality of schizophrenia revealed by DNA microarray: impact on glial and neurotrophic gene expression. Ann. NY Acad. Sci. 2004;1025:84–91. doi: 10.1196/annals.1316.011. [DOI] [PubMed] [Google Scholar]
- 182.Hahn CG, et al. Altered neuregulin 1-erbB4 signalling contributes to NMDA receptor hypofunction in schizophrenia. Nature Med. 2006;12:824–828. doi: 10.1038/nm1418. [This paper showed that there is a marked increase in NRG1-induced activation of ErbB4, ERK and Akt and in the ErbB4–PSD95 interaction in the PFC in schizophrenia, accompanied by a reduction in tyrosine phosphorylation of NMDAR2A in response to NMDA stimulation. These findings suggest that enhanced NRG1 signalling might contribute to NMDA hypofunction in schizophrenia] [DOI] [PubMed] [Google Scholar]
- 183.Chong VZ, et al. Elevated neuregulin-1 and ErbB4 protein in the PFC of schizophrenic patients. Schizophr. Res. 2008;100:270–280. doi: 10.1016/j.schres.2007.12.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kalkman HO. The role of the phosphatidylinositide 3-kinase-protein kinase B pathway in schizophrenia. Pharmacol. Ther. 2006;110:117–134. doi: 10.1016/j.pharmthera.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 185.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nature Rev. Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 186.Norton N, Williams HJ, Owen MJ. An update on the genetics of schizophrenia. Curr. Opin. Psychiatry. 2006;19:158–164. doi: 10.1097/01.yco.0000214341.52249.59. [DOI] [PubMed] [Google Scholar]
- 187.Elenius K, et al. A novel juxtamembrane domain isoform of HER4/ErbB4. Isoform-specific tissue distribution and differential processing in response to phorbol ester. J. Biol. Chem. 1997;272:26761–26768. doi: 10.1074/jbc.272.42.26761. [DOI] [PubMed] [Google Scholar]
- 188.Sawyer C, Hiles I, Page M, Crompton M, Dean C. Two erbB-4 transcripts are expressed in normal breast and in most breast cancers. Oncogene. 1998;17:919–924. doi: 10.1038/sj.onc.1202015. [DOI] [PubMed] [Google Scholar]
- 189.Junttila TT, Sundvall M, Maatta JA, Elenius K. Erbb4 and its isoforms: selective regulation of growth factor responses by naturally occurring receptor variants. Trends Cardiovasc. Med. 2000;10:304–310. doi: 10.1016/s1050-1738(01)00065-2. [DOI] [PubMed] [Google Scholar]
- 190.Rio C, Buxbaum JD, Peschon JJ, Corfas G. Tumour necrosis factor-α-converting enzyme is required for cleavage of erbB4/HER4. J. Biol. Chem. 2000;275:10379–10387. doi: 10.1074/jbc.275.14.10379. [DOI] [PubMed] [Google Scholar]
- 191.Cheng QC, Tikhomirov O, Zhou W, Carpenter G. Ectodomain cleavage of ErbB-4: characterization of the cleavage site and m80 fragment. J. Biol. Chem. 2003;278:38421–38427. doi: 10.1074/jbc.M302111200. [DOI] [PubMed] [Google Scholar]
- 192.Arasada RR, Carpenter G. Secretase-dependent tyrosine phosphorylation of Mdm2 by the ErbB-4 intracellular domain fragment. J. Biol. Chem. 2005;280:30783–30787. doi: 10.1074/jbc.M506057200. [DOI] [PubMed] [Google Scholar]
- 193.Feng SM, et al. The HER4 cytoplasmic domain, but not its C terminus, inhibits mammary cell proliferation. Mol. Endocrinol. 2007;21:1861–1876. doi: 10.1210/me.2006-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Linggi B, Carpenter G. ErbB-4 s80 intracellular domain abrogates ETO2-dependent transcriptional repression. J. Biol. Chem. 2006;281:25373–25380. doi: 10.1074/jbc.M603998200. [DOI] [PubMed] [Google Scholar]
- 195.Omerovic J, et al. Ligand-regulated association of ErbB-4 to the transcriptional co-activator YAP65 controls transcription at the nuclear level. Exp. Cell Res. 2004;294:469–479. doi: 10.1016/j.yexcr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 196.Vidal GA, Naresh A, Marrero L, Jones FE. Presenilin-dependent γ-secretase processing regulates multiple ERBB4/HER4 activities. J. Biol. Chem. 2005;280:19777–19783. doi: 10.1074/jbc.M412457200. [DOI] [PubMed] [Google Scholar]
- 197.Komuro A, Nagai M, Navin NE, Sudol M. WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. J. Biol. Chem. 2003;278:33334–33341. doi: 10.1074/jbc.M305597200. [DOI] [PubMed] [Google Scholar]
- 198.Wang JY, Wigston DJ, Rees HD, Levey AI, Falls DL. LIM kinase 1 accumulates in presynaptic terminals during synapse maturation. J. Comp. Neurol. 2000;416:319–334. doi: 10.1002/(sici)1096-9861(20000117)416:3<319::aid-cne4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 199.Wang JY, Frenzel KE, Wen D, Falls DL. Transmembrane neuregulins interact with LIM kinase 1, a cytoplasmic protein kinase implicated in development of visuospatial cognition. J. Biol. Chem. 1998;273:20525–20534. doi: 10.1074/jbc.273.32.20525. [DOI] [PubMed] [Google Scholar]
- 200.Liu X, et al. Domain-specific gene disruption reveals critical regulation of neuregulin signalling by its cytoplasmic tail. Proc. Natl Acad. Sci. USA. 1998;95:13024–13029. doi: 10.1073/pnas.95.22.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sherman DL, Brophy PJ. Mechanisms of axon ensheathment and myelin growth. Nature Rev. Neurosci. 2005;6:683–690. doi: 10.1038/nrn1743. [DOI] [PubMed] [Google Scholar]
- 202.Kim E, Sheng M. PDZ domain proteins of synapses. Nature Rev. Neurosci. 2004;5:771–781. doi: 10.1038/nrn1517. [DOI] [PubMed] [Google Scholar]
- 203.Craig AM, Kang Y. Neurexin-neuroligin signalling in synapse development. Curr. Opin. Neurobiol. 2007;17:43–52. doi: 10.1016/j.conb.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Huang YZ, Wang Q, Xiong WC, Mei L. Erbin is a protein concentrated at postsynaptic membranes that interacts with PSD-95. J. Biol. Chem. 2001;276:19318–19326. doi: 10.1074/jbc.M100494200. [DOI] [PubMed] [Google Scholar]
- 205.Borg JP, et al. ERBIN: a basolateral PDZ protein that interacts with the mammalian ERBB2/HER2 receptor. Nature Cell Biol. 2000;2:407–414. doi: 10.1038/35017038. [DOI] [PubMed] [Google Scholar]
- 206.Huang YZ, Zang M, Xiong WC, Luo Z, Mei L. Erbin suppresses the MAP kinase pathway. J. Biol. Chem. 2003;278:1108–1114. doi: 10.1074/jbc.M205413200. [DOI] [PubMed] [Google Scholar]
- 207.Dai P, Xiong WC, Mei L. Erbin inhibits RAF activation by disrupting the sur-8-Ras-Raf complex. J. Biol. Chem. 2006;281:927–933. doi: 10.1074/jbc.M507360200. [DOI] [PubMed] [Google Scholar]
- 208.Markram H, et al. Interneurons of the neocortical inhibitory system. Nature Rev. Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 209.Gupta A, Wang Y, Markram H. Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science. 2000;287:273–278. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- 210.Kawaguchi Y, Kubota Y. Neurochemical features and synaptic connections of large physiologically-identified GABAergic cells in the rat frontal cortex. Neuroscience. 1998;85:677–701. doi: 10.1016/s0306-4522(97)00685-4. [DOI] [PubMed] [Google Scholar]
- 211.Zaitsev AV, et al. Localization of calcium-binding proteins in physiologically and morphologically characterized interneurons of monkey dorsolateral PFC. Cereb. Cortex. 2005;15:1178–1186. doi: 10.1093/cercor/bhh218. [DOI] [PubMed] [Google Scholar]


