Abstract
Soft tissue augmentation with temporary dermal fillers is a continuously growing field, supported by the ongoing development and advances in technology and biocompatibility of the products marketed. The longer lasting, less immunogenic and thus more convenient hyaluronic acid (HA) fillers are encompassing by far the biggest share of the temporary dermal filler market. Since the approval of the first HA filler, Restylane®, there are at least 10 HA fillers that have been approved by the FDA. Not all of the approved HA fillers are available on the market, and many more are coming. The Juvéderm™ product line (Allergan, Irvine, CA), consisting of Juvéderm™ Plus and Juvéderm™ Ultra Plus, was approved by the FDA in 2006. Juvéderm™ is a bacterium-derived nonanimal stabilized HA. Juvéderm™ Ultra and Ultra Plus are smooth, malleable gels with a homologous consistency that use a new technology called “Hylacross™ technology”. They have a high concentration of cross-linked HAs, which accounts for its longevity. Juvéderm™ Ultra Plus is used for volumizing and correcting deeper folds, whereas Juvéderm™ Ultra is best for contouring and volumizing medium depth facial wrinkles and lip augmentation. Various studies have shown the superiority of the HA filler products compared with collagen fillers for duration, volume needed, and patient satisfaction. Restylane®, Perlane®, and Juvéderm™ are currently the most popular dermal fillers used in the United States.
Keywords: hyaluronic acid gel, Juvéderm™, facial wrinkles, facial folds
Dermal fillers have become an integral part of any aesthetic physician’s intervention. The growing importance of the temporary dermal filler industry is reflected by the increasing growth in demand during the past years and a multitude of new products, which have come to market. According to the American Academy of Aesthetic Plastic Surgeons, 1,448,716 people received hyaluronic acid (HA) injections by plastic surgeons in 2007. This number does not, however, reflect all the procedures performed, as it does not include the procedures performed by dermatologists or other physicians.
The first dermal fillers used in the 1980s were animal-derived collagen fillers (Zyplast® and Zyderm®; Allergan, formerly Inamed). However, the need for products with longer clinical duration and no requirements for prior skin allergy testing lead to the development of the HA fillers. Of the two biologic fillers currently used – collagen and HAs – HAs have become the new gold standard, and have almost replaced collagen fillers (Cosmetic Surgery National Data Bank Statistics 2005). This is explained by the advantages of HAs over collagen, such as its longer duration (6–12 months compared with 2–4 months), no request for skin testing, fewer allergic side effects, and better pliability.
A third group of dermal fillers currently used are synthetic fillers, such as Sculptra® (Dermik Laboratories, Sanofi-Aventis, Bridgewater, NJ), Radiesse® (BioForm Medical, San Mateo, CA), and Artefill® (Artes Medical, Inc., San Diego, CA).
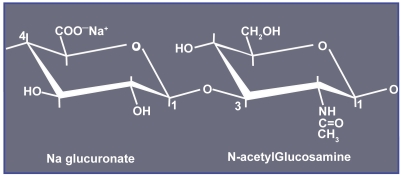
HA or hyaluronan is a naturally occurring linear polysaccharide (Figure 1). It can be found in skin, connective, epithelial, and neural tissues. It is ubiquitous across all species and does not require skin allergy testing prior to injection, which makes it very convenient for daily use. This glycosaminoglycan has the ability to bind 1,000 times its volume in water, which makes it the perfect substance for adding volume to the skin. In humans, the amount of naturally occurring HA in the skin decreases with age, which plays an important role in the development of the aging features and wrinkle formation, resulting in decreased tissue elasticity and hydration. Unmodified, natural HA has a half-life of approximately 24 hours before it is enzymatically broken down and metabolized in the liver into byproducts, water and carbon dioxide (Duranti et al 1998). In the skin, HA is broken down by hyaluronidase and by free radicals. Supplementation with oral antioxidants theoretically will increase the duration of HA fillers, but this has not been proven. The naturally occurring break down of HA by hyaluronidase depicts an important feature of the HA fillers as well as a major advantage over the collagen fillers, namely, rarely occurring areas of excess fullness, too superficial placement of the filler, or overcorrection can easily be dissolved or improved by intralesional injection of hylauronidase.
Figure 1.
Structure of hyaluronic acid.
Features that differentiate the various HA fillers are particle size, the type of crosslinking agent used, the degree of crosslinking, the percentage of cross-linked HA, the amount of free (unmodified) HA present, and G′ (elastic modulus). All these physical and chemical attributes will influence the clinical characteristics of each filler, such as clinical indication, ease of injection, degree of tissue filling, longevity, clinical appearance, and side effects.
Currently 6 temporary HA fillers are FDA approved and on the market in the US: Restylane® (Medicis, Scottsdale, AZ), Perlane® (Medicis, Scottsdale, AZ), Prevelle Silk® (Mentor Corp., Santa Barbara, CA), Hylaform Plus® (Allergan, Irvine, CA), Anika® (Anika Therapeutics, Inc., MA), and Juvéderm™ (Allergan, Inc., Irvine, CA) (Table 1). Hylaform® and Captique® are no longer on the market in the US.
Table 1.
Hyaluronic acid (HA) fillers: crosslinking agents and concentration of HA
| Product | Captique | Hylaform | Juvéderm Ultra and Ultra Plus | Puragen | Prevelle | Restylane and Perlane |
|---|---|---|---|---|---|---|
| Crosslinking agent | DVS | DVS | BDDE | DEO | DVS | BDDE |
| Concentration | 4.5–6.5 mg/mL | 4.5–6.5 mg/mL | 24 mg/mL | 20 mg/mL | 4.5–6.5 mg/mL | 20 mg/mL |
Abbreviations: BDDE, 1,4-butanediol diglycidyl ether; DVS, divinyl sulphone; DEO, 2, 7, 8-diepoxyoctane.
Juvéderm™ (Allergan, Inc., Irvine, CA), which is also known as Hydrafill, was approved by the FDA in June 2006 for the correction of moderate to severe facial wrinkles and folds. Juvéderm™ filler agents have been on the market in European countries and Canada since 2003 (marketed as Juvéderm™ by the Corneal Group and by Allergan, formerly Inamed, and in some countries as Hydra Fill® by Allergan, formerly Inamed). The Juvéderm™ line comprises various products, such as Juvéderm™ 18, Juvéderm™ 24, Juvéderm™ 24 HV, Juvéderm™ 30, and Juvéderm™ 30 HV, of which only Juvéderm™ 24 HV (also known as Juvéderm™ Ultra) and Juvéderm™ 30 HV (also known as Juvéderm™ Ultra Plus) are available on the US market. The various products in the line differ in the concentration of HA as well as the amount and regularity of crosslinking.
Particle size and sizing technology
Juvéderm™ is derived from Streptococcus equi and manufactured by a bacterial fermentation process. Juvéderm™ is produced by a proprietary manufacturing process referred to as “Hylacross technology”, which refers to the fact that Juvéderm is not “sized” in contrast to the other HA fillers (Prevelle Silk®, Restylane®, Perlane®) which use sizing technology. “Sizing” is the process by which crosslinked HA is pushed through a specially sized screen and broken into pieces. The medium size pieces of HA are made into Restylane® while the larger ones are made into Perlane®. It is unknown what effect the sizing technology or the Hylacross technology have on a filler’s performance, or if they offer any benefit in the efficacy of the product. Many claims about the benefits of Hylacross technology have been made without scientific substantiation. For example, Smith (2007) noted a difference of the homologous Juvéderm™ gel fillers compared to fillers with gel particle suspensions, mentioned in his publication on the practical use of Juvéderm™. He claimed that after injection, the Juvéderm™ filler remained in the area where it was injected because of its cohesive nature and high viscosity, and did not flow away from the injection point. Also, in his opinion, Juvéderm fills more precisely and more efficiently. He stated that gel-particle fillers in contrast seem to flow away from the injection point, causing filling of unintended areas and waste of product. It is important to realize that this is one person’s opinion and has not been substantiated by scientific research. To the authors’ knowledge, no studies have scientifically characterized the diffusion and spread of the various HA fillers.
HA concentration
The amount of HA in a product may contribute to its stiffness and longevity. Theoretically, the higher the amount of HA in the product, the stiffer it is and the longer it will last. However, not all of the HA in the product is crosslinked so one must take into account the overall percentage of cross-linking (how much of the HA is crosslinked) and the degree of crosslinking (is the HA molecule completely or partially crosslinked). Often, uncrosslinked HA is added to a filler product to increase its ease of injection as it functions as a lubricant. Both Juvéderm™ Ultra and Ultra Plus consist of 24 mg/mL of HA. Juvéderm™ Ultra is 9% crosslinked while Juvéderm™ Ultra Plus is 11% crosslinked (Table 2).
Table 2.
Percentage crosslinking in Juvéderm™ products
| Juvéderm 30 | Juvéderm 24 HV (Ultra) | Juvéderm 30 HV (Ultra Plus) | |
|---|---|---|---|
| Concentration | 24 mg/g | 24 mg/g | 24 mg/g |
| Crosslinking rate | 9% | 9% | 11% |
Type of crosslinking agent used
The crosslinking agent used in Juvéderm™ is 1,4-butanediol diglycidyl ether (BDDE). Other fillers are cross-linked with different crosslinking agents, such as divinyl sulphone (DVS) for Prevelle®, Captique®, and Hylaform®. Puragen® is cross-linked with 2, 7, 8-diepoxyoctane (DEO), which forms both ether and ester crosslinks. Crosslinking quality has to be in the right balance to maintain both duration and the biocompatibility of the HA filler. Each crosslinking agent has characteristics that affect the performance of the filler.
Elastic modules
The stiffness or G′ (pronounced G prime) of a product is one of the most important considerations. G′ is a measurement of gel hardness. It is obtained when a gel is placed on a plate. A second plate is placed over the gel and a lateral force is applied. The measurement of resistance to deformation is known as the elastic modulus or the G′. Together with the cohesivity of the product, G′ values could be used to determine the appropriate placement of an HA dermal filler. For example more robust products (higher G′ values and higher cohesivities) such as Juvéderm™ Ultra Plus and Perlane®, should be used in deeper lines, such as nasolabial folds and marionette lines, as well as to lift the lateral brow, to correct the nasal bridge, to give the ear lobe youthful volume, to evert the nipples, and to raise the nasal tip. More fluid products such as Juvéderm™ Ultra and Restylane® are more suited to be used over large areas such as the cheekbones and cheeks. Low G′ products such as Hylaform® and Prevelle Silk® are necessary in areas that require a softer agent, such as the body of the lip or the tear trough. As new products reach the market, knowing the G′ will help practitioners match fillers with indications.
Recommended injection sites
In contrast to Juvéderm™ Ultra, Juvéderm™ Ultra Plus has a higher proportion (11%) of crosslinked HA, which makes Ultra Plus more viscous. Consequently, Ultra Plus is more suitable for adding volume and correcting the deeper facial grooves and furrows, whereas Juvéderm™ Ultra is best suited for contouring and volumizing facial wrinkles and folds (Figures 2 and 3) (FDA 2006). Juvéderm™ Ultra and Ultra Plus can be grouped in the medium range of product stiffness, which makes them suitable for the use in any wrinkles, moderate or deep, as well as scar correction. Both Juvéderm™ products contain unmodified or uncross-linked, free HA. Unmodified HA is included as a lubricant to help decrease extrusion force and make injection easier. Juvéderm™ Ultra is injected into the mid-dermis via 30-gauge needle while Juvéderm™ Ultra Plus is implanted deeper via a 27-gauge needle. It is important to tightly attach the needles to the Luer-lock syringe to prevent detachment during injections. Various techniques of injection can be used with Juvéderm™, including serial puncture and tunneling. As Juvéderm™ is not completely hydrated with water in the syringe, and HA is well known to be able to bind 1,000 times its weight in water, Juvéderm™ will absorb water after injections and thus slightly expand within 24 hours after correction. The patients can thus be informed, that the effect will be “even better” 24 hours after the injection. However, it is important to consider this feature in clinical practice, especially when injecting the body of the lips, therefore one should always undercorrect to allow for expansion. Restylane® and Puragen® are also not completely hydrated in the syringe, whereas Captique®, Hylaform®, and Prevelle® are completely hydrated and will not expand after injection.
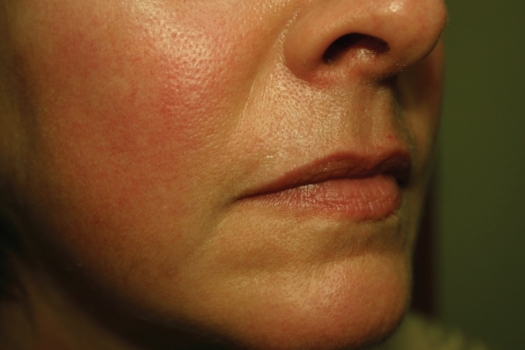
Figure 2.
Before Juvedérm™ to the Marionette lines.
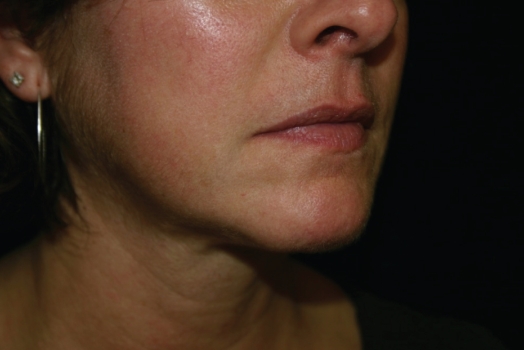
Figure 3.
24 hours after Juvedérm™ Ultra to right Marionette line.
The longevity of Juvéderm™ Ultra is about 6–9 months and Ultra Plus may last up to 12 months, which is similar to Restylane® and Perlane®. Captique® and Prevelle Silk® are thought to last 4–6 months and the duration of Puragen® is unknown at the time of publication of this article. Both Juvéderm™ products are packaged in 0.8-mL syringes as a clear gel and are stored at room temperature with a shelf-life of 24 months. The adverse-event profile of Juvéderm™ is mild and transient. As with all HA products, Juvéderm™ can cause erythema, swelling, and bruising after implantation. As Juvéderm™ Ultra and Ultra Plus lack an anesthetic, patients do feel pain during injection. Therefore, a topical anesthetic or a nerve block can be used to minimize discomfort.
Both Juvéderm™ Ultra and Ultra Plus have been approved for use in the nasolabial folds. In the authors’ experience, both products may also be used off-label for lip augmentation, for the correction of marionette folds, prejugal sulci, and as volume fillers for atrophy and acne scars. Furthermore, Juvéderm™ can be placed in the tear trough area, but extra care is necessary, due to the proximity to the eye with the risk of the needle popping off, thus one should inject very slowly with only moderate extrusion force. The needle is more likely to pop off when the syringe is almost empty so inject the tear trough area with a new syringe and save the last part of the syringe for less dangerous areas such as the nasolabial folds. Too superficial injections of Juvéderm™ can result in a bluish hue. Juvéderm™ Ultra can easily be placed in the vermillion border or the body of the lip. Again, one should be cautious as not to over-inject the vermillion border due to postponed expansion of the product.
As Juvéderm™ has been on the US marked only since late 2006, only a few publications have assessed the various characteristics of the Juvéderm™ products. These are reviewed below.
Review of publications
In the pivotal trial that led to FDA approval of Juvéderm™, Baumann et al (2007) compared the safety and effectiveness of 3 types of smooth-gel HA dermal fillers vs cross-linked collagen in the treatment of NLF in 439 subjects in a multicenter, double-masked, randomized, within-subject study. The subjects randomly received one of three types of smooth-gel HA dermal filler in one NLF and cross-linked bovine collagen in the other. The three different smooth-gel HAs used were J30 (Juvéderm™ 30), 24 HV (Juvéderm™ Ultra), or 30 HV (Juvéderm™ Ultra Plus), of which only the latter two are currently marketed in the US. The cross-linked bovine collagen filler used was Zyplast® (Allergan, formerly Inamed). The NLFs were to be filled to full correction (100% of the defect), and not overcorrected, and a maximum of 3 treatments – first treatment and up to 2 touch-ups at roughly 2-week intervals – were allowed to achieve optimal correction. NLF severity was assessed using the5-pointWrinkle Assessment Scale (WAS), with 0 = none (no wrinkle); 1 = mild (shallow, just perceptible wrinkle); 2 = moderate (moderately deep wrinkle); 3 = severe (deep wrinkle, well-defined edges but not overlapping); 4 = extreme (very deep wrinkle, redundant fold with overlapping skin). The results showed that all three dermal fillers proved longer-lasting clinical corrections than bovine collagen. Twenty-four weeks after the last treatment, 90% of subjects treated with 30 HV (Juvéderm™ Ultra Plus) dermal filler retained a clinically significant improvement, 88% treated with 24 HV (Juvéderm™ Ultra) and 81% with J30 dermal filler. The bovine collagen–treated NLFs showed clearly shorter longevity with lasting improvement after 24 weeks ranging from 36% to 45%. In addition to its superior longevity, the injection volume for HA dermal fillers proved to be lower (median, 1.6 mL) compared with bovine collagen (median, 2.0 mL), representing an additional important advantage for the patient in treatment costs and comfort. The only treatment-related adverse events observed were localized site reactions in the area of injection, which were mild to moderate in severity and did not differ between any filler type. In decreasing percentage those were injection site induration, erythema, edema, pain, nodule formation, bruising, discoloration, and pruritus; they lasted no more than 7 days. The preferred filler by the patients used was 24 HV (Juvéderm™ Ultra) with 88%, followed by 84% for 30 HV (Juvéderm™ Ultra Plus) and 78% for J30; the majority of subjects preferred HA fillers to the collagen fillers.
In an almost identical study design (Pinsky et al 2007), the safety and effectiveness of Juvéderm™ dermal fillers compared to Zyplast® bovine collagen for the correction of nasolabial folds (NLFs) was assessed in a multicenter, double-blind, randomized, within-subject controlled trial. 292 subjects were randomly treated with Juvéderm™ Ultra or Juvéderm™ Ultra Plus in one NLF and Zyplast® bovine collagen in the other NLF. The treating investigators were instructed to fill each NLF to full correction (100% of the defect), but not to overcorrect. A maximum of 3 treatments – first treatment and up to 2 touch-ups at roughly 2-week intervals – were allowed to achieve optimal correction. An average injection volume of 1.5 mL (2 syringes) of Juvéderm™ dermal filler was used for initial treatment and 0.7 mL (1 syringe) for repeat treatment. NLF severity was assessed using the 5-point Wrinkle Assessment Scale (WAS), and a validated photographic guide. After 6 month subjects showed a clinically significant mean level of improvement for the NLFs treated with Juvéderm™ Ultra or Juvéderm™ Ultra Plus, but not for NLFs treated with Zyplast®, supporting the above stated findings by showing a longer longevity for Juvéderm™ Ultra and Ultra Plus than for Zyplast®. The mean level of improvement was still clinically significant for subjects who returned for a follow-up treatment beyond 9 months, with the proportion of NLFs still showing clinically significant improvement in 75% with Juvéderm™ Ultra and 81% with Juvéderm™ Ultra Plus. Juvéderm™ Ultra Plus was shown to last even 12 months or longer. Again, the frequency and severity of treatment site reactions (eg, erythema, induration, pain, edema, nodule formation, bruising, pruritus, and discoloration) were mild or moderate and were similar for all fillers. The authors concluded, that due to its superior longevity, individuals treated with these Juvéderm™ dermal fillers may require to repeat treatments less frequently than those treated with bovine collagen fillers, and that less product will be needed at repeat treatments.
The results of the above mentioned studies are supported by a recent study by Lupo et al which compared Juvéderm™ Ultra Plus HA filler with Zyplast® bovine collagen in a multicenter, double-blind, randomized, within-subject, controlled study (Lupo et al 2008). In a split face mode, severe NLFs of 87 subjects were treated, one side with Juvéderm™ Ultra Plus and the other side with Zyplast®. In the study population all Fitzpatrick skin types were represented, 36% having darker skin types (Fitzpatrick types IV through VI). Up to two touch-up treatments were allowed at 2-week intervals. Effectiveness was assessed using the validated, static, 5-point Wrinkle Assessment Scale (WAS) with a photographic guide. The Juvéderm™ Ultra Plus filler showed significantly better NLF severity scores compared to Zyplast® at each follow-up time point from 4 to 24 weeks. At 24-week follow-up clinically significant correction of NLF treated with Juvéderm™ were shown in 96% compared with 41% Zyderm®. The clinical correction with Juvéderm™ Ultra Plus remained high, whereas the scores for Zyplast® nearly returned to baseline over the period of 24 weeks. At 24 weeks, the mean improvement was still 1.7 with the Juvéderm™ Ultra Plus product but only 0.5 with bovine collagen. Longevity was shown by maintenance of the clinical correction for 1 year or more in 81% of NLFs treated with Juvéderm™. The median volume of Juvéderm™ required was 0.7 mL (one syringe), significantly less than for Zyplast® (1.6 mL). For the initial treatment, the median volume of Juvéderm™ Ultra Plus injected was 2 syringes (1.6 mL), and less than 1 syringe (0.7 mL) for the retreatment at after 6–9 months.
Treatment site reactions were similar for Juvéderm™ Ultra Plus and Zyplast® and were similar to those in the above-mentioned trials. As for patient satisfaction, most subjects preferred Juvéderm™ Ultra Plus (85%) versus collagen (10%); 5% showed no preference.
In summary, all three studies above show superior longevity of the HA Juvéderm™ fillers compared to bovine collagen fillers. Juvéderm™ Ultra Plus was shown to exert longer lasting clinical results than Juvéderm™ Ultra. Initial treatments required roughly two syringes of Juvéderm™, and retreatments required only one syringe. Volumes required for collagen were higher throughout the studies. Treatment site reactions and side effects were similar for all fillers, HA and collagen, and were always short in duration (less than 7 days) and mild in severity. Most patients seem to prefer Juvéderm™ fillers to bovine collagen.
Juvéderm™ Ultra Plus was compared with Radiesse® in an European study by Moers-Capri et al (2007) that compared the hydroxylapatite filler with two HA fillers for the treatment of the nasolabial folds. The objective of this multicenter, blinded, randomized trial was to compare patient satisfaction, efficacy and durability of the various fillers. A total of 205 patients were randomized into 3 arms, receiving Radiesse® (CaHA gel), Juvéderm™ Ultra, or Perlane®. After the first treatment a touch-up was performed 4 months later and patients were followed up at 8, and 12 months, without any additional touch-ups. The injections were performed with a 27-gauge needle into the mid to deep dermis. At 8 months follow-up, NLFs treated with CaHA gel were significantly more improved, than with any HA, as assessed by Global Aesthetic Improvement Scale (GAIS) (Narins et al 2003). Moreover, the volumes used for CaHA gel were lower than for the HAs. In the patient satisfaction CaHA consistently scored highest, and Juvéderm™ scored lowest, even lower than Perlane®.
There are many factors to be understood, in order to know which HA filler to use (Table 3). Because no peer-reviewed scientific publications have reviewed the above-mentioned properties, it is impossible at this point to know how important these various characteristics are in choosing a filler. More data need to be collected to properly understand if, for example, sizing technology makes a difference or if ester bonds last longer than ether bonds. These distinctions will become clearer and more important as more HA fillers reach the market and more data are collected.
Table 3.
Factors to consider when choosing an hyaluronic acid (HA) filler
| Concentration of HA |
| Cost |
| Choices of syringe size |
| Degree of crosslinking |
| Design of the syringe |
| Duration of correction |
| G prime (stiffness) |
| How much of the HA is crosslinked vs uncrosslinked |
| Hydration level of product in the syringe |
| Presence of lidocaine |
| Required needle size for injection |
| Sizing technology |
| Type of crosslinking technology used |
Footnotes
Disclosures
None of the authors has any conflicts to disclose.
References
- Cosmetic Surgery National Data Bank Statistics. Los Alamitos (CA): American Society of Aesthetic Plastic Surgery; 2005. [Accessed 2006 Jun 7]. [online] URL: http://www.surgery.org/download/2005stats.pdf. [DOI] [PubMed] [Google Scholar]
- Duranti F, Salti G, Bovani B, et al. Injectable hyaluronic acid gel for soft-tissue augmentation: A clinical and histological study. Dermatol Surg. 1998;24:1317. doi: 10.1111/j.1524-4725.1998.tb00007.x. [DOI] [PubMed] [Google Scholar]
- Smith KC. Practical use of Juvéderm: early experience. Plast Reconstr Surg. 2007;120(Suppl):67S–73S. doi: 10.1097/01.prs.0000285107.16836.16. [DOI] [PubMed] [Google Scholar]
- [FDA] Food and Drug Administration. Summary of safety and effectiveness data. Rockville (MD): 2006. [Accessed 2006 Aug 23]. [online] URL: http://www.fda.gov/cdrh/pdf5/p050047b.pdf. [Google Scholar]
- Baumann LS, Shamban AT, Lupo MP, et al. Juvéderm vs Zyplast, Nasolabial Fold Study Group. Comparison of smooth-gel hyaluronic acid dermal fillers with cross-linked bovine collagen: a multicenter, double-masked, randomized, within-subject study. Dermatol Surg. 2007;33(Suppl 2):S128–35. doi: 10.1111/j.1524-4725.2007.33352.x. [DOI] [PubMed] [Google Scholar]
- Pinsky MA, Thomas JA, Murphy, et al. Juvedérm injectable gel: A multicenter, double-blind, randomized study of safety and effectiveness [poster]. American Society for Aesthetic Plastic Surgery Annual Meeting; New York, NY. April 19–24; 2007. [DOI] [PubMed] [Google Scholar]
- Lupo MP, Smith SR, Thomas JA, et al. Effectiveness of Juvéderm Ultra Plus dermal filler in the treatment of severe nasolabial folds. Plast Reconstr Surg. 2008;121:289–97. doi: 10.1097/01.prs.0000294968.76862.83. [DOI] [PubMed] [Google Scholar]
- Moers-Carpi M, Vogt S, Santos BM, et al. A multicenter, randomized trial comparing calcium hydroxylapatite to two hyaluronic acids for treatment of nasolabial folds. Dermatol Surg. 2007;33(Suppl 2):S144–51. doi: 10.1111/j.1524-4725.2007.33354.x. [DOI] [PubMed] [Google Scholar]
- Narins RS, Brandt F, Leyden J, et al. A randomized, double-blind, multicenter comparison of the efficacy and tolerability of Restylane versus Zyplast for the correction of nasolabial folds. Dermatol Surg. 2003;29:588–95. doi: 10.1046/j.1524-4725.2003.29150.x. [DOI] [PubMed] [Google Scholar]