Abstract
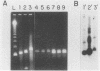
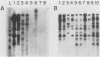
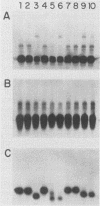
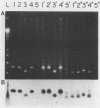
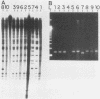
The human cytomegalovirus (HCMV) a-sequence (a-seq) is located in the joining region between the long (L) and short (S) unique sequences of the virus (L-S junction), and this hypervariable junction has been used to differentiate HCMV strains. The purpose of this study was to investigate whether there are differences among strains of human cytomegalovirus which could be characterized by polymerase chain reaction (PCR) amplification of the a-seq of HCMV DNA and to compare a PCR method of strain differentiation with conventional restriction fragment length polymorphism (RFLP) methodology by using HCMV junction probes. Laboratory strains of HCMV and viral isolates from individuals with HCMV infection were characterized by using both RFLPs and PCR. The PCR assay amplified regions in the major immediate-early gene (IE-1), the 64/65-kDa matrix phosphoprotein (pp65), and the a-seq of the L-S junction region. HCMV laboratory strains Towne, AD169, and Davis were distinguishable, in terms of size of the amplified product, when analyzed by PCR with primers specific for the a-seq but were indistinguishable by using PCR targeted to IE-1 and pp65 sequences. When this technique was applied to a characterization of isolates from individuals with HCMV infection, selected isolates could be readily distinguished. In addition, when the a-seq PCR product was analyzed with restriction enzyme digestion for the presence of specific sequences, these DNA differences were confirmed. PCR analysis across the variable a-seq of HCMV demonstrated differences among strains which were confirmed by RFLP in 38 of 40 isolates analyzed. The most informative restriction enzyme sites in the a-seq for distinguishing HCMV isolates were those of MnlI and BssHII. This indicates that the a-seq of HCMV is heterogeneous among wild strains, and PCR of the a-seq of HCMV is a practical way to characterize differences in strains of HCMV.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S. P. Cytomegalovirus and child day care. Evidence for an increased infection rate among day-care workers. N Engl J Med. 1989 Nov 9;321(19):1290–1296. doi: 10.1056/NEJM198911093211903. [DOI] [PubMed] [Google Scholar]
- Adler S. P. The molecular epidemiology of cytomegalovirus transmission among children attending a day care center. J Infect Dis. 1985 Oct;152(4):760–768. doi: 10.1093/infdis/152.4.760. [DOI] [PubMed] [Google Scholar]
- Akrigg A., Wilkinson G. W., Oram J. D. The structure of the major immediate early gene of human cytomegalovirus strain AD169. Virus Res. 1985 Mar;2(2):107–121. doi: 10.1016/0168-1702(85)90242-4. [DOI] [PubMed] [Google Scholar]
- Cassol S. A., Poon M. C., Pal R., Naylor M. J., Culver-James J., Bowen T. J., Russell J. A., Krawetz S. A., Pon R. T., Hoar D. I. Primer-mediated enzymatic amplification of cytomegalovirus (CMV) DNA. Application to the early diagnosis of CMV infection in marrow transplant recipients. J Clin Invest. 1989 Apr;83(4):1109–1115. doi: 10.1172/JCI113990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler S. H., Handsfield H. H., McDougall J. K. Isolation of multiple strains of cytomegalovirus from women attending a clinic for sexually transmitted disease. J Infect Dis. 1987 Apr;155(4):655–660. doi: 10.1093/infdis/155.4.655. [DOI] [PubMed] [Google Scholar]
- Chandler S. H., McDougall J. K. Comparison of restriction site polymorphisms among clinical isolates and laboratory strains of human cytomegalovirus. J Gen Virol. 1986 Oct;67(Pt 10):2179–2192. doi: 10.1099/0022-1317-67-10-2179. [DOI] [PubMed] [Google Scholar]
- Chou S. W. Acquisition of donor strains of cytomegalovirus by renal-transplant recipients. N Engl J Med. 1986 May 29;314(22):1418–1423. doi: 10.1056/NEJM198605293142205. [DOI] [PubMed] [Google Scholar]
- Chou S. W. Differentiation of cytomegalovirus strains by restriction analysis of DNA sequences amplified from clinical specimens. J Infect Dis. 1990 Sep;162(3):738–742. doi: 10.1093/infdis/162.3.738. [DOI] [PubMed] [Google Scholar]
- Chou S. W. Reactivation and recombination of multiple cytomegalovirus strains from individual organ donors. J Infect Dis. 1989 Jul;160(1):11–15. doi: 10.1093/infdis/160.1.11. [DOI] [PubMed] [Google Scholar]
- Collier A. C., Chandler S. H., Handsfield H. H., Corey L., McDougall J. K. Identification of multiple strains of cytomegalovirus in homosexual men. J Infect Dis. 1989 Jan;159(1):123–126. doi: 10.1093/infdis/159.1.123. [DOI] [PubMed] [Google Scholar]
- Demmler G. J., Buffone G. J., Schimbor C. M., May R. A. Detection of cytomegalovirus in urine from newborns by using polymerase chain reaction DNA amplification. J Infect Dis. 1988 Dec;158(6):1177–1184. doi: 10.1093/infdis/158.6.1177. [DOI] [PubMed] [Google Scholar]
- Drew W. L., Sweet E. S., Miner R. C., Mocarski E. S. Multiple infections by cytomegalovirus in patients with acquired immunodeficiency syndrome: documentation by Southern blot hybridization. J Infect Dis. 1984 Dec;150(6):952–953. doi: 10.1093/infdis/150.6.952. [DOI] [PubMed] [Google Scholar]
- Hsia K., Spector D. H., Lawrie J., Spector S. A. Enzymatic amplification of human cytomegalovirus sequences by polymerase chain reaction. J Clin Microbiol. 1989 Aug;27(8):1802–1809. doi: 10.1128/jcm.27.8.1802-1809.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S., Alford C. A., Reynolds D. W., Stagno S., Pass R. F. Molecular epidemiology of cytomegalovirus infections in women and their infants. N Engl J Med. 1980 Oct 23;303(17):958–962. doi: 10.1056/NEJM198010233031702. [DOI] [PubMed] [Google Scholar]
- Jiwa N. M., Van Gemert G. W., Raap A. K., Van de Rijke F. M., Mulder A., Lens P. F., Salimans M. M., Zwaan F. E., Van Dorp W., Van der Ploeg M. Rapid detection of human cytomegalovirus DNA in peripheral blood leukocytes of viremic transplant recipients by the polymerase chain reaction. Transplantation. 1989 Jul;48(1):72–76. doi: 10.1097/00007890-198907000-00017. [DOI] [PubMed] [Google Scholar]
- Kemble G. W., Mocarski E. S. A host cell protein binds to a highly conserved sequence element (pac-2) within the cytomegalovirus a sequence. J Virol. 1989 Nov;63(11):4715–4728. doi: 10.1128/jvi.63.11.4715-4728.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick B. A., Huang E. S. Human cytomegalovirus genome: partial denaturation map and organization of genome sequences. J Virol. 1977 Oct;24(1):261–276. doi: 10.1128/jvi.24.1.261-276.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarski E. S., Liu A. C., Spaete R. R. Structure and variability of the a sequence in the genome of human cytomegalovirus (Towne strain). J Gen Virol. 1987 Aug;68(Pt 8):2223–2230. doi: 10.1099/0022-1317-68-8-2223. [DOI] [PubMed] [Google Scholar]
- Mocarski E. S., Roizman B. Structure and role of the herpes simplex virus DNA termini in inversion, circularization and generation of virion DNA. Cell. 1982 Nov;31(1):89–97. doi: 10.1016/0092-8674(82)90408-1. [DOI] [PubMed] [Google Scholar]
- Rüger B., Klages S., Walla B., Albrecht J., Fleckenstein B., Tomlinson P., Barrell B. Primary structure and transcription of the genes coding for the two virion phosphoproteins pp65 and pp71 of human cytomegalovirus. J Virol. 1987 Feb;61(2):446–453. doi: 10.1128/jvi.61.2.446-453.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt G. M., Kovacs A., Zaia J. A., Horak D. A., Blume K. G., Nademanee A. P., O'Donnell M. R., Snyder D. S., Forman S. J. Ganciclovir/immunoglobulin combination therapy for the treatment of human cytomegalovirus-associated interstitial pneumonia in bone marrow allograft recipients. Transplantation. 1988 Dec;46(6):905–907. [PubMed] [Google Scholar]
- Shibata D., Martin W. J., Appleman M. D., Causey D. M., Leedom J. M., Arnheim N. Detection of cytomegalovirus DNA in peripheral blood of patients infected with human immunodeficiency virus. J Infect Dis. 1988 Dec;158(6):1185–1192. doi: 10.1093/infdis/158.6.1185. [DOI] [PubMed] [Google Scholar]
- Spaete R. R., Frenkel N. The herpes simplex virus amplicon: a new eucaryotic defective-virus cloning-amplifying vector. Cell. 1982 Aug;30(1):295–304. doi: 10.1016/0092-8674(82)90035-6. [DOI] [PubMed] [Google Scholar]
- Spaete R. R., Frenkel N. The herpes simplex virus amplicon: analyses of cis-acting replication functions. Proc Natl Acad Sci U S A. 1985 Feb;82(3):694–698. doi: 10.1073/pnas.82.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaete R. R., Mocarski E. S. The alpha sequence of the cytomegalovirus genome functions as a cleavage/packaging signal for herpes simplex virus defective genomes. J Virol. 1985 Jun;54(3):817–824. doi: 10.1128/jvi.54.3.817-824.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. H., Hock L., Tamashiro J. C. Cleavage maps for human cytomegalovirus DNA strain AD169 for restriction endonucleases EcoRI, BglII, and HindIII. J Virol. 1982 May;42(2):558–582. doi: 10.1128/jvi.42.2.558-582.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector S. A., Hirata K. K., Newman T. R. Identification of multiple cytomegalovirus strains in homosexual men with acquired immunodeficiency syndrome. J Infect Dis. 1984 Dec;150(6):953–956. doi: 10.1093/infdis/150.6.953. [DOI] [PubMed] [Google Scholar]
- Spector S. A., Neuman T. R., Hirata K. K. Rapid determination of molecular relatedness of isolates of human cytomegalovirus. J Infect Dis. 1985 Oct;152(4):755–759. doi: 10.1093/infdis/152.4.755. [DOI] [PubMed] [Google Scholar]
- Spector S. A. Transmission of cytomegalovirus among infants in hospital documented by restriction-endonuclease-digestion analyses. Lancet. 1983 Feb 19;1(8321):378–381. doi: 10.1016/s0140-6736(83)91499-x. [DOI] [PubMed] [Google Scholar]
- Stow N. D., McMonagle E. C., Davison A. J. Fragments from both termini of the herpes simplex virus type 1 genome contain signals required for the encapsidation of viral DNA. Nucleic Acids Res. 1983 Dec 10;11(23):8205–8220. doi: 10.1093/nar/11.23.8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamashiro J. C., Filpula D., Friedmann T., Spector D. H. Structure of the heterogeneous L-S junction region of human cytomegalovirus strain AD169 DNA. J Virol. 1984 Nov;52(2):541–548. doi: 10.1128/jvi.52.2.541-548.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlazny D. A., Frenkel N. Replication of herpes simplex virus DNA: localization of replication recognition signals within defective virus genomes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):742–746. doi: 10.1073/pnas.78.2.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfert C. M., Huang E. S., Stagno S. Restriction endonuclease analysis of cytomegalovirus deoxyribonucleic acid as an epidemiologic tool. Pediatrics. 1982 Nov;70(5):717–721. [PubMed] [Google Scholar]
- Zaia J. A., Forman S. J., Gallagher M. T., Vanderwal-Urbina E., Blume K. G. Prolonged human cytomegalovirus viremia following bone marrow transplantation. Transplantation. 1984 Mar;37(3):315–317. doi: 10.1097/00007890-198403000-00021. [DOI] [PubMed] [Google Scholar]